Abstract
Critical limb ischemia incidence and prevalence have increased over the years. However, there are no successful treatments to improve quality of life and to reduce the risk of cardiovascular and limb events in these patients. Advanced regenerative therapies have focused their interest on the generation of new blood vessels to repair tissue damage through the use of stem cells. One of the most promising sources of stem cells with high potential in cell-based therapy is adipose-derived stem cells (ASCs). ASCs are adult mesenchymal stem cells that are relatively abundant and ubiquitous and are characterized by a multilineage capacity and low immunogenicity. The proangiogenic benefits of ASCs may be ascribed to: (a) paracrine secretion of proangiogenic molecules that may stimulate angiogenesis; (b) secretion of microvesicles/exosomes that are also considered as a novel therapeutic prospect for treating ischemic diseases; and (c) their differentiation capability toward endothelial cells (ECs). Although we know the proangiogenic effects of ASCs, the therapeutic efficacy of ASCs after transplantation in peripheral artery diseases patients is still relatively low. In this review, we evidence the potential therapeutic use of ASCs in ischemic regenerative medicine. We also highlight the main challenges in the differentiation of these cells into functional ECs. However, significant efforts are still needed to ascertain relevant transcription factors, intracellular signaling and interlinking pathways in endothelial differentiation.
Keywords: peripheral artery disease, critical limb ischemia, adipose-derived stem cells, cell therapy, angiogenesis, ASC differentiation
1. Introduction
Since the last century, cardiovascular diseases (CVDs) have been the leading cause of death and are also a major contributor to premature death, disability, health care expenditures, and lost productivity globally [1,2,3]. CVDs are a set of heterogeneous chronic heart and circulatory system disorders that are asymptomatic; only advanced disease causes multifactorial symptoms, and gradually evolves throughout life [4]. This group of disorders includes coronary heart disease, cerebrovascular disease, peripheral artery disease (PAD) and other vascular conditions [2].
PAD is a vascular disease caused mainly by atherosclerosis. It is defined as the narrowing and obstruction of the blood flow of major systemic arteries other than those of cerebral and coronary circulations [5]. Symptomatic clinical manifestations of PAD include intermittent claudication (IC) and critical limb ischemia (CLI), which is the most severe manifestation. Patients with CLI have a major risk of tissue loss, amputation, cardiovascular events and mortality [5,6,7,8]. Treatment for patients with CLI is usually directed toward limiting the consequences of systemic atherosclerosis, such as is the case with myocardial infarction or stroke. Therefore, therapies such as statins, angiotensin converting-enzyme inhibitors or angiotensin-receptor blockers, and antiplatelet agents are frequently used [9,10]. All patients with ischemic ulcers have to receive wound care and other medical approaches to promote healing and reduce pain. Of note, no medical therapies are effective in improving perfusion to the lower extremity in patients with PAD. Surgical and catheter-based revascularization are the preferred approaches for CLI and should be considered the first treatment option. In correctly selected patients, either modality can result in preservation of life and limb of ≥75% at 1 year. However, although numbers vary greatly from center to center, many patients with CLI are poor candidates for revascularization or have no option for revascularization at all. Even after a successful revascularization, the graft can fail, or stenosis can reoccur after catheter-based treatment. Clearly, a need exists to develop new treatment strategies for patients with CLI who are not candidates for revascularization [11,12].
The concept of therapeutic angiogenesis has emerged as an investigational approach to identify angiogenic agents to promote the development of collateral vascular networks in ischemic tissues and subsequently increase perfusion [13]. Angiogenesis is the development of new blood vessels from the preexisting ones. These remodeling and growth of new vessels can be achieved by gene therapy, protein therapy and cell therapy. Cell-based therapy is focused on the use of stem cells due to their unlimited therapeutic potential [14]. Stem cells are resident cells in the bone marrow, where three different populations co-exist: endothelial progenitor cells, bone-marrow mesenchymal stem cells and hematopoietic stem cells. Stem cells can also be found in tissues (also referred as somatic stem cells or adult stem cells) as in white adipose tissue, skeletal muscle and myocardium.
Stem cells exhibit self-renewal capacity and can differentiate into multiple cell phenotypes that offer therapeutic solutions for many diseases [15]. As such, a novel therapeutic option is the delivery of autologous or allogenic adult stem cells into ischemic tissue to prevent tissue damage in the affected area [16]. One of the most promising sources of stem cell populations with high potential in clinical medicine is adipose-derived stem cells (ASCs). ASCs are adult mesenchymal stem cells isolated from white adipose tissue that exhibit different interesting properties. Numerous reports underline the capacity of ASCs to differentiate into other cell types, including endothelial cells (ECs) [17,18]. This multilineage capacity together with their abundance and low immunogenicity lead to the supposition that these cells have a prominent role in cell-based therapy [15]. ASCs have been deeply studied in the field of neovascularization. ASCs secrete multiple angiogenic and antiapoptotic biological molecules, such as growth factors [19], cytokines [15], microvesicles (MVs) and proangiogenic molecules, that participate in the formation of neovascular-like structures and interact with microvascular ECs [20]. The ability of ASCs to differentiate into ECs has been described as a potential mechanism for the ASC-mediated pro-angiogenic benefits. However, key transcription factors, intracellular signaling, and interlinking pathways that promote ASC differentiation toward specific lineages or cell fates are still under investigation.
Hence, the aim of this review is to rigorously evaluate the relevant scientific literature for conclusive evidence that ASCs can differentiate into functional ECs and contribute to vascular repair and clinical improvement in CLI.
2. Peripheral Artery Disease
The aorta, the main artery of our body, is bifurcated into two arteries, which at the point of the leg are branched into several arteries, but with one main artery supplying blood flow to the distal leg. The formation of an atherosclerotic plaque disrupts blood flow and results in vessel restriction or occlusion. There is an interruption/cessation of the vessel metabolic demands that leads to limb ischemia. The formation of reactive oxygen species results in cell dysfunction, or even cell death, of blood vessel-forming cells and perpetuates tissue ischemia [7,21]. PAD is the pathological condition that results in the obstruction of vessels and obliteration of arterial blood flow, limiting blood supply to organs other than the heart [7,21,22]. PAD is a common disease; it is the third leading cause of atherosclerotic cardiovascular morbidity and often remains unrecognized and underdiagnosed. Traditional CVDs risk factors are associated with the risk of developing PAD: age, cigarette smoking, diabetes mellitus, hypertension, hypercholesterolemia, and sedentary state. All these major atherosclerotic risk factors are associated with a 2- to 4-fold increased risk of PAD [7,22]. PAD is classified as: (a) asymptomatic, where the lack of noticeable symptoms puts patients at risk of morbidity and mortality; or (b) symptomatic, where the degree of manifestation ranges from intermittent claudication to CLI. Intermittent claudication is a primarily quality-of-life disease characterized by the occurrence of leg pain, arching, cramping, or fatigue induced by exercise that can be relieved by resting. Meanwhile, as the disease worsens, the aggravation of the lesion leads to CLI, and it is characterized by chronic ischemic rest pain present for at least 2 weeks with or without ulcers or gangrene [7,21].
3. Critical Limb Ischemia
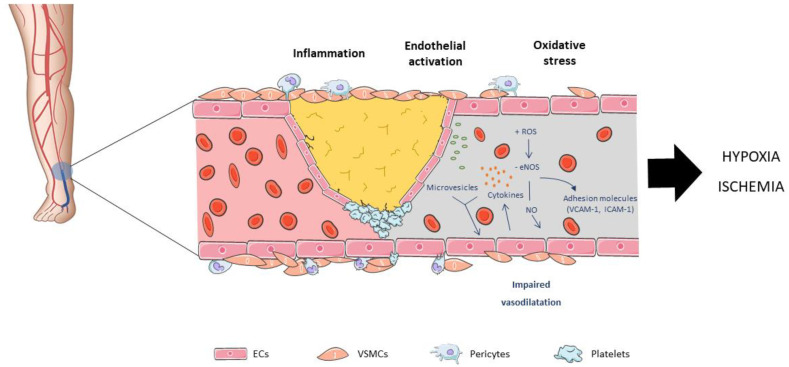
CLI represents the most advanced form and end stage of PAD, which results in the occlusion of the arteries, leading to hypoxia and ischemia of the skeletal muscle. It is a highly morbid disease with a diminished health-related quality of life associated with a high risk of tissue loss, limb amputation and a precursor of cardiovascular events (Figure 1).
Figure 1.
Peripheral arterial disease (PAD) is mainly caused by atherosclerosis. The earliest event is endothelium activation by adherence of mononuclear cells to endothelial cells (ECs), followed by an inflammatory cascade, the formation of fatty streaks and leading to atheroma plaque formation. Endothelial dysfunction entails a change in the synthesis and secretion of different substances and expression of diverse endothelial genes. Among them, monocyte chemoattractant protein-1, platelet-derived growth factors (PDGFs), platelet adhesion molecule-1, or endothelial nitric oxide synthase (eNOS). In the presence of hyperlipidemia, hypertension, smoking or diabetes, there is an increased oxidative stress, which promotes the synthesis of pro-atherogenic molecules such as cytokines and chemokines, interleukins, adhesion molecules, leading to an inhibition of eNOS activity, and consequently a reduction in nitric oxide bioavailability.
The goals of medical management for CLI are not only to improve quality of life but also to reduce the risk of cardiovascular and major limb events. It includes aggressive risk factor modification, including lifestyle modification, smoking cessation, antithrombotic and antihypertensive therapy and lipid-lowering therapy [7,22,23]. However, bypass vascular surgery and endovascular interventions are necessary to restore vascular function and structure and revascularization is the optimal treatment for CLI to increase limb perfusion. Even after successful revascularization procedures, residual microvascular disease may well limit the effectiveness of the interventions [24]. Moreover, a significant proportion of patients (up to 1 of 3) cannot benefit from this intervention because size and severity of the lesion, which finally leads to limb amputation or palliation [22,23]. There is a necessity to formulate novel treatment approaches for individuals diagnosed with CLI who are ineligible for revascularization.
In the last decades, angiogenesis has appeared as a hope for these patients. This complex process leads to new blood vessel formation and acts as a compensatory mechanism in response to ischemic diseases. It increases oxygen and nutrients supplies to tissue in order to protect their function.
4. Therapeutic Angiogenesis
Efficient and simultaneous transport of gases, liquids, nutrients, signaling molecules and circulating cells to organs is necessary to meet the metabolic demands of active cells [24,25]. Homeostasis is maintained by complex and highly branched tubular networks of vessels integrating the vascular system. The arterial wall is a three-layered structure composed of the tunica intima, media and adventitia and finally surrounded by perivascular adipose tissue. The tunica intima, which is in intimate contact with perfusing blood is composed by a single layer of endothelial cells (ECs). Vascular smooth muscle cells (VSMCs), collagens and elastic fibers are the main components of the tunica media. Tunica adventitia is composed of connective tissue, progenitor cells, fibroblasts, adipocytes, nerves and vasa vasorum [26,27]. All these cell types and protein matrices are essential for maintaining stable vascular structure and function. Under pathological conditions, ischemic tissue has a low oxygen supply, free radical production, and cellular response, which induce EC dysfunction and inflammatory response. Regeneration of the vasculature requires transcriptional signaling that enhances vascular repair and remodeling, by the activation of transcription factors, such as hypoxia-inducible factor-1 [22,24]. This transcription factor stimulates the release of angiogenic factors, cytokines and other signals to induce existing capillary EC proliferation, migration, and invasion of the host stroma toward the source of angiogenic stimuli to restore the microvasculature [28]. Vascular growth can occur in three different forms that together contribute to a high level of organization and maturation of the vascular network and, also, contribute to tissue repair and remodeling in ischemic vascular diseases [25,28,29]. Angiogenesis, arteriogenesis and vasculogenesis contribute to neovascularization, as they are part of the same process and complement each other. These cellular processes are driven by partially overlapping cellular and molecular pathways [28]. Angiogenesis is defined as the sprouting of new capillaries from existing vessels, which involves temporally and spatially regulated changes in gene expression [25]. In adults, most vasculature is quiescent, and angiogenesis is required for tissue repair or remodeling. The arteriogenesis process consists on the stabilization of primitive vessels into the capillary network through the generation of an extracellular matrix by mural cells and the activation of ECs downstream signaling pathways and cytokines that mediate vessel remodeling. Adult vasculogenesis is another process of neovascularization where endothelial progenitor cells are mobilized from the bone marrow to the endothelium, differentiate into ECs, and contribute to microvasculature restoration via paracrine functions [21,28]. The interplay of these three neovascularization processes is important for restoring limb function after ischemia. However, these processes are disrupted in PAD fundamentally due to microvascular EC dysfunction [25].
The concept of ‘therapeutic angiogenesis’ emerged approximately two decades ago as an investigational approach for patients with ischemic lesions who did not undergo revascularization. This technique is based on the remodeling and growth of new vessels in the ischemic region to alleviate hypoxic damage to organs and tissues [30]. Regenerative medicine therapies include gene therapy, protein therapy and cell-based therapy. Protein therapy is based on the administration of angiogenic growth factors, such as vascular endothelial growth factor (VEGF) or fibroblast growth factor (FGF), at a specific site of interest to promote angiogenesis and collateral artery formation. However, the short half-life of these proteins, the high dosages needed, the repeated injections, and the protection from proteolytic degradation have led to investigations on gene or cell-based therapy [21,23,30]. Gene therapy consists of the introduction of pro-angiogenic genes into ischemic sites using nonviral or viral vectors. These vectors are integrated into the host chromosome, resulting in expression of angiogenic genes even after cell division. However, an overproduction of a certain angiogenic factor could inhibit blood vessel formation. Thus, it is required a precise balance between different signals [30]. Initially, the first agent transfected was VEGF isoform 165 and 121. Few years later, other pro-angiogenic factors such as FGF, hepatocyte growth factor, platelet-derived growth factor, prokineticin 2, angiogenic factor with G-patch and Forkhead-associated domain 1, human telomerase reverse transcriptase (hTERT), and some microRNAs, also showed promising results in promoting angiogenesis in animal models [13,21,31]. The objective is to maintain the angiogenic activity of the gene transcribed molecules at a specific site of interest [32]. Cell-based therapy refers to the transfer of autologous or allogenic cells, which can be genetically engineered or manipulated and can be administered topically or as injectables, infusions, bioscaffolds or scaffold-free systems. The selection of potential candidate cells is based on their capability to self-renew and differentiate into blood-vessel-associated cells or organ-specific cell types, or secretion of pro-angiogenic growth factors. Several stem cells had been identified, isolated and applied in clinical trials. However, some challenges have to be overcome: acquiring enough cells, in vivo viability and integration with the host tissue [33]. The cells that have been used in clinical trials of cell-based therapies in PAD include bone marrow mononuclear cells bone marrow mesenchymal stem cells, granulocyte colony stimulating factor-mobilized peripheral blood mononuclear cells, endothelial progenitor cells, and granulocyte colony stimulating factor monotherapy [13]. Moreover, cell-based therapy is used in multiple therapeutic areas such as regenerative medicine, immunotherapy, and cancer therapy, and it combines stem cell- and non-stem-cell-based unicellular or multicellular therapies [34]. To date, cell-based therapy in the field of regenerative medicine involve either the administration of differentiated stem cells into vascular cells or the induction of angiogenic growth factor expression through paracrine signaling exerted by the respective cell [21,23].
5. Stem Cells: Adipose Tissue-Derived Stem Cells
Stem cells are an undifferentiated cell population with the ability to extensively proliferate, replicate themselves (clonate) and differentiate into different cell lineages. Stem cells are found in different tissues with different levels of differentiation (pluripotent, multipotent, and tissue-resident stem cells). These different potencies make them candidates for cell-based therapy (Table 1) [35].
After vascular injury, there is a process which entails ECs proliferation and migration. However, it has been reported that in response to vascular injury or dysfunction, stem progenitor cells are homed to the lesion area where they can differentiate into vascular ECs, VSMCs and inflammatory cells contributing to revascularization. Moreover, it has also been reported that there are vascular stem progenitor cells residing within the structure of the vessel wall that can differentiate into several types of vascular cells and promote angiogenesis [26,36]. These stem progenitor cells include endothelial progenitor cells, smooth muscle progenitor cells, mesenchymal stem cells and pericytes. Mesenchymal stem cells are multipotent non-hematopoietic stromal cells, that can be isolated from various adult tissues, that are considered to provide structural support to the organs [37]. These cells are considered one of the most important cell types for cell-based therapy, not only for their multilineage differentiation capacity, but also for their active secretome, that can promote autocrine and paracrine signaling contributing to angiogenesis, and to cross-talk with resident stem cells [34]. Within mesenchymal stem cells, ASCs are a multipotent mesenchymal stem cell population, relatively abundant that can be easily isolated in large quantities from adipose tissue [38,39,40,41].
Table 1.
Studies using stem cells to treat different diseases.
| Cell Source | Disease | Type of Study | Reference |
|---|---|---|---|
| Human embryonic stem cells | Diabetes mellitus | In Vivo | Kroon et al. [42] |
| Human adipose tissue-derived mesenchymal stem cells | Type 1 diabetes mellitus | Clinical trial | Trivedi et al. [43] |
| Mouse adipose tissue-derived stem cells | Type 2 diabetes mellitus | In Vivo | Wang et al. [44] |
| Hematopoietic stem cell transplantation | Chronic myeloid leukemia | Clinical trial | Hackanson and Waller et al. [45] |
| Bone marrow-derived mesenchymal stem cells | Liver failure | In Vivo | Kuo et al. [46] |
| Human embryonic stem cells | Pulmonary fibrosis | In Vivo | Banerjee et al. [47] |
| Autologous hematopoietic stem cells | Refractory Crohn’s disease | Clinical trial | Cassinotti et al. [48] Oyama et al. [49] |
| Autologous non-myeloablative hemopoietic stem cells | Multiple sclerosis | Clinical trial | Burt et al. [50] |
| Mouse embryonic stem cells | Parkinson disease | In Vivo | Björklund et al. [51] |
| Rat autologous adipose tissue-derived stem cells | Wound healing | In Vivo | Zhou et al. [52] |
| Allogenic adipose tissue-derived mesenchymal stem cells | Acute ischemic stroke | Clinical trial | De Celis-Ruiz et al. [53] |
| Human adipose-derived mesenchymal stem cells exosomes | Atherosclerosis | In Vitro and in vivo | Yu et al. [54] Xing et al. [55] |
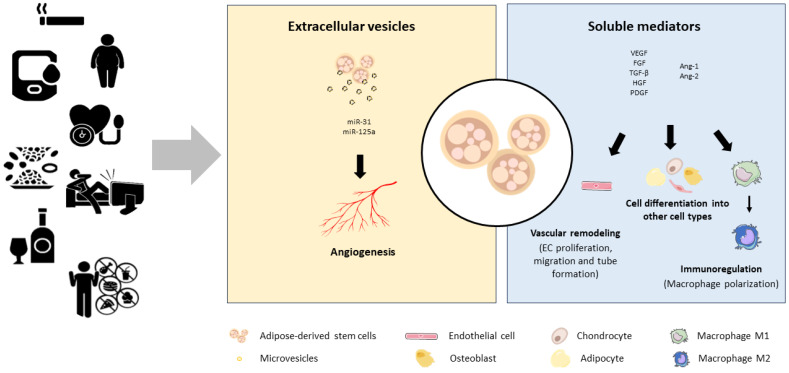
The International Federation for Adipose Therapeutics and Sciences and the International Society for Cell and Gene Therapy established a minimum criterion for the characterization of ASCs: plastic adhesion, expression of different surface antigens and the in vitro potency to differentiate into pre-adipocyte, chondrocyte, and osteoblasts [56]. There is a common consensus that these cells do not express unique surface markers but do express surface antigens of mesenchymal stem cells. Immunophenotypically, ASCs can be identified by the presence (CD90, CD44, CD29, CD105, CD13, CD34, CD73, CD166, CD10, CD49e and CD59) and absence (CD31, CD45, CD14, CD11b, CD19, CD56 and CD146) of several surface markers. However, the expression of some cell surface markers remains controversial. For instance, some data sustains that ASCs can be distinguished from bone marrow stem cells by the lack of CD106 surface antigen expression [57,58]; and other surface marker, CD34, is present in freshly isolated ASCs but its expression disappears or remains at low levels after several passages [58,59]. But even though ASCs change progressively their phenotype with passages in vitro, their multilineage differentiation and proliferation capacity is retained [60]. Moreover, these cells do no express type II human leukocyte antigen and thus do not induce immunological rejection after allogenic transplantation and are exempt from ethical implications [26,37,61] as it is the case with embryonic stem cells. For this reason, these adult mesenchymal stem cells have been investigated and used in a wide variety of fields due to their commitment to differentiate into many cell types. There is reported information on their use in cardiovascular diseases, metabolic diseases, skeletal tissue regeneration or wound healing and skin aging, among others. Currently, there are more than 100 ASC clinical trials registered in the Clinical Trials database [62]. However, their secretome, microvesicles and differentiation capacity into the endothelial lineage has attracted the interest of researchers (Figure 2).
Figure 2.
ASC autocrine and paracrine signaling. ASCs secrete many significant autocrine and paracrine proteins, such as soluble growth factors (bFGF, VEGF), microvesicles enriched with miRNAs (miRNA-31, miRNA-126, miRNA-125a) and immunomodulatory molecules that influence immediate blood capillary environment and induce ASC differentiation into multiple cell lineages. These pro-angiogeneic benefits are modified by cardiovascular risk factors such as hypertension, obesity, diabetes, dyslipidemia, smoking, physical inactivity, alcohol misuse or unhealthy diet.
6. The Importance of ASCs Secretome and Its Effects on Cellular Mechanisms
ASCs secrete multiple bioactive molecules involved in cellular differentiation, migration, proliferation and autocrine and paracrine signaling [20]. The secretome of ASCs is composed by pro- and anti-inflammatory cytokines and chemokines [15], growth factors [19], cytosolic components, and extracellular vesicles, among others [63,64].
A notable mechanism associated with the development of collateral vascular networks and increased perfusion after ASCs transplantation is the secretion of multiple autocrine and paracrine angiogenic growth factors [33,65,66]. Growth factors, such as vascular endothelial growth factor (VEGF), basic-fibroblast growth factor (bFGF) or transforming growth factor (TGF)-, bind to their receptors in the cell membrane of ECs and lead to cellular activities such as endothelial growth, migration, and tube formation [63,67].
Apart from soluble growth factors, further investigations by Kang et al. recognized a new mechanism of cell–cell communication. These investigations evidenced that ASCs release MVs that can directly promote angiogenesis in vitro and in vivo. MVs are vesicles that are enclosed by a phospholipid bilayer membrane, contain natural signaling molecules and act as an intercellular communicators [27]. In vivo experiments have revealed that ASC-derived MVs (ASCs-MVs) promote tube formation and significantly up-regulate the expression of growth factors and receptors on ECs [68]. These MVs are enriched with miRNAs, in particular miRNA-31, promoting migration and tube formation in recipient ECs. These pro-angiogenic effects were also observed in mouse Matrigel plug assays, which revealed that ASC-MVs induce functional vasculature formation [64]. Other investigations revealed that ASC-MVs are enriched with miRNA-125a, which promotes the formation of endothelial tip cells by repressing the angiogenic inhibitor delta-like 4 [69].
Angiogenic capacity of ASCs may be modified by different factors. ASCs may be obtained from different source of adipose tissue, subcutaneous or visceral adipose tissue. It has been reported that the metabolic differences between both depots affect ASCs properties such as proliferation, differentiation and apoptosis, as well as gene expression patterns. ASCs from the subcutaneous adipose tissue have a greater capacity to proliferate and differentiate, whereas ASCs from visceral adipose tissue express related to lipid metabolism [41,70,71]. In addition, cardiovascular risk factors also negatively modified their properties. Ferrer et al. demonstrated that ASCs pluripotency, self-renewal capacity, differentiation and angiogenic properties were modified by type 2 diabetes mellitus [72]. Obesity also impairs ASCs properties in both depots, specifically it affects genes related to stemness, lineage commitment and inflammation. ASCs from obese patients compared to ASCs derived from nonobese patients show lower proliferation, differentiation and proangiogenic capacities, overall negatively modifying ASCs regenerative capacity [41,73,74]. Diabetes mellitus is one of the main risk factors of PAD, increasing the risk of lower limb amputation. Autologous ASCs from these patients have their proangiogenic properties impaired, so allogenic ASCs are required to induce vascular remodeling.
As ASCs are an ideal cell source for angiogenic therapy and autologous cells have limitations, it is needed to increase their angiogenic potency in the presence of cardiovascular risk factors.
7. ASCs Differentiation into an Endothelial-like Phenotype to Increase Their Angiogenic Potential
To direct ASCs differentiation potential into endothelial lineage-specific pattern, in vitro studies are centered on manipulating the components of endothelial growth medium (EGM). EGM is usually supplemented with two angiogenic growth factors: VEGF and bFGF, both implicated in the differentiation pathway into EC-like cells. Several studies indicated that these factors are two plausible factors to induce endothelial characteristics of ASCs. VEGF is a 40 kDa extremely potent heterodimeric glycoprotein with pro-angiogenic functions. In humans, VEGF family involves several members, amongst which it must be outlined the role of VEGF-A [75,76]. This growth factor actively participates in the regulation of the angiogenesis process. It contributes to the revascularization process by the mobilization and recruitment of endothelial and hematopoietic stem cells. These stem cells express the tyrosine kinase cell receptors VEGFRs, receptors expressed predominantly on vascular ECs. The angiogenic factor VEGF binds to VEGFRs increasing vascular permeability and the migration, proliferation, and differentiation of ECs thus contributing to angiogenesis [75,76,77]. After 10 days of stimulation, ASCs change their morphology towards a EC-like morphology [78]. In addition, to confirm the induction of functional ECs in vitro, the studies observed the formation of capillary-like structures in Matrigel-coated coverslips after treatment with VEGF [18,78]. Commitment toward an endothelial lineage was further determined by significant increases in EC-specific markers such as CD31, von Willebrand Factor, CD144, and eNOS [18,77,78]. A synergistic effect of VEGF and FGF has also been proposed. ASCs cultured in medium containing FGF and VEGF show increased EC markers PECAM-1, CD34, VE-cadherin, and eNOS, as opposed to ASCs cultured with FGF or VEGF alone [18,78]. These results suggest a co-stimulatory effect of FGF and VEGF necessary to elicit robust ASCs differentiation into ECs.
Further information has been gathered in studies using bFGF, a 18kDa protein expressed in various cell types. bFGF has been shown to induce angiogenesis, wound healing and vascular remodeling [79,80]. The inhibition of FGF signaling in ASCs significantly reduced mRNA and protein expression of EC markers and the inhibition capacity to subsequently form capillary-like structures on Matrigel [66,78]. All these results suggest FGF as a critical inducing factor in ASCs differentiation into ECs.
Several studies have shown the angiogenic potential of ASCs differentiated into EC-lineages as EC substitutes in vascular remodeling [81]. In these studies, ASCs cultured in specific medium for differentiation into an endothelial phenotype are subcutaneously injected into mouse models of hind limb ischemia. Subsequently, the histologic architecture, microvascular formation, capillary density, perfusion [17,82] and vascular gene expression [18] have been evaluated. The main results of these in vivo experiments showed that ASCs differentiate into the endothelial lineage and participate in blood vessel formation. It has been demonstrated by a marked increase in the blood flow as well as in the capillary density in the ischemic hind limb of nude mice [17,18,82], and interestingly ASCs can be incorporated into the new vasculature [18]. Moreover, neovascularization was confirmed by increased expression levels of angiogenic genes [81].
8. Signaling Pathways Involved in ASCs Differentiation into an Endothelial-like Phenotype
To determine the signaling pathways that regulate ASCs differentiation into ECs by EGM, the involvement of different cellular pathways has been explored. Cao et al. reported the importance of the PI3K pathway, one of the major pathways for EC proliferation and survival [18]. This signaling pathway is highly conserved and stimulates the phosphorylation of Akt. The activation of this protein plays a central role in numerous cellular functions including cell metabolism, growth, proliferation and survival, protein synthesis, transcription, and apoptosis. Also, it plays a central role in angiogenesis [18,83]. The results revealed that PI3K inhibition completely blocked ASCs-endothelial differentiation. It is important to note that this finding suggests that the endothelial phenotype acquisition is dependent on the PI3K signaling [18]. EGM, that is supplemented with several growth factors, such as VEGF, epidermal growth factor, insulin-like growth factor 1 and/or bFGF, stimulates the activation of the PI3K signaling pathway. Co-incubation of ASCs with an Akt pathway inhibitor, results in a complete abrogation of ASCs differentiation toward ECs as well as cell sprouting [16]. These findings are in accordance with the results of Cao et al. They identified VEGF/PI3K/Akt as a key signaling pathway for ASCs differentiation into ECs [18]. In addition, it has been demonstrated that the paracrine effects of FGF are also mediated by the PI3K/Akt pathway, which inhibits the activity of target molecules by phosphorylation [84].
Interestingly, Almalki et al. reported that silencing matrix metalloproteinase (MMP)-2 and MMP-14 contribute to promote an ASC-endothelial like phenotype and function after treatment with endothelial basal medium containing VEGF [77,85]. VEGF induces ASCs differentiation into ECs via the activation of VEGFR2; hence, the inhibitory effect of the MMPs could be due to the cleavage of this receptor and the inhibition of the endothelial differentiation pathway. In addition, their findings suggests that VEGF activates VEGFR2, thus activating the MAPK/ERK signaling pathway to promote and induce the transcription of EC markers during ASCs differentiation into ECs [85].
On the other hand, miRNAs are short non-coding RNA molecules involved in the regulation of several cellular processes. MiRNA-126 has been identified as an endothelial-specific and highly expressed miRNA [86,87]. It is involved in the regulation of endothelial migration, cytoskeleton reorganization, capillary network stability, cell survival and apoptosis [87]. Furthermore, studies on miRNA-126 demonstrated that this miRNA is necessary for the maintenance of vascular integrity [86,88]. Xie et al. observed that during ASCs differentiation into ECs by EGM, miRNA-126 expression gradually increases as well as the expression of vascular endothelial cell markers [86]. Interestingly, Arderiu et al. focused their research in the interaction between ECs and ASCs through their secretome. They found that microvascular ECs produce and secrete bFGF, which regulates ASCs differentiation into ECs. It down-regulates miRNA-145 expression in ASCs [89] and promotes angiogenic properties and vascular network formation through targeting V-ets avian erythroblastosis virus E26 oncogene homolog 1 (ETS1), a transcription factor necessary for the maintenance of endothelial integrity and capillary formation. The intracellular signaling pathway is involved [84] (Figure 3).
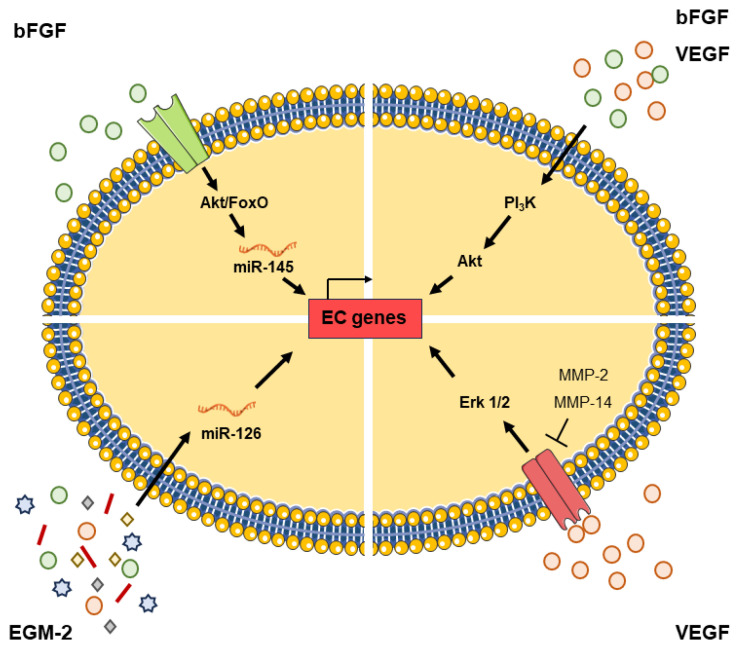
Figure 3.
Transcriptional regulation in ASCs differentiation into ECs. ASCs endothelial differentiation into EC-like cells by the acquisition of EC phenotype is regulated by several signaling pathways, transcription factors and microRNAs. Growth factors, present in endothelial cell growth medium or secreted by ECs, interact with their receptors in ASCs. Thus, it activates PI3K and MAPK signaling pathways, and regulates the transcription of EC-specific cell markers, such as CD31, von Willebrand Factor, CD144, PECAM-1, CD34, and eNOS. Moreover, these factors can also regulate the expression of microRNAs (miRNA-145 and miRNA-126), which interact with transcription factors that in turn induce the up-regulation of EC markers.
9. Other Cells with Angiogenic Potential
Monocytes are a heterogeneous population of mononuclear cells responsible for the control and clearance of infections. In addition, it has been shown that monocytes also play an intricate role in different biological functions being one of them angiogenesis [90,91]. The initiation of neovascularization in ischemic areas is related to the infiltration and activation of inflammatory cells within hypoxic areas [28,92,93]. Activated ECs release molecules that recruit monocytes from circulation to the ischemic tissue and subsequently promote postnatal neovascularization [91]. Recruited monocytes stimulate EC functions by releasing proangiogenic mediators, but at the same time, the interaction between monocytes and ECs results in cross-modulation of angiogenesis. The proangiogenic environment induces monocytes to transdifferentiate into endothelial cell-like cells and acquire endothelial features [90,91,94].
We have contributed to clarify this interaction of endothelial cells and monocytes to promote angiogenesis [90,91,94,95]. We demonstrated that monocytes have a paracrine cross-talk with microvascular ECs with release of extracellular microvesicles (EVs) [94]. In addition, this cross-talk induces ECs to release tissue factor-rich MVs, which induces monocytes transdifferentiation into EC-like cells ready to form newly release of EVs. Therefore, there is a positive feedback between monocytes and microvascular ECs that induces angiogenesis in ischemic zones, contributing to ischemic tissue repair [91,95]. Activated ECs also release EVs containing high levels of miRNA-126, which are transferred to monocytes and while it does not promote EC-like cell differentiation it has effects on several components of angiogenic pathways [90].
10. Conclusions and Future Perspectives
The field of therapeutic angiogenesis was met with great excitement in the late 1990s and appeared as a radical new paradigm for treating ischemic diseases. The enthusiasm generated by those early studies was dampened by disappointing results. In recent years, stem cell transplantation has been recognized as a new technique with therapeutic angiogenic effects on ischemic diseases. However, the presence of cardiovascular risk factors and metabolic disorders in patients with PAD seems to negatively influence the effects of adult stem cells, discouraging their autologous use in the clinic. The use of allogenic stem cells from healthy donors may overcome these limitations. Different studies have proven the capacity of ASCs and their secretome as an attractive vehicle for cell-based therapeutics. The modulation of different molecular mechanism increases the therapeutic efficiency of ASCs and suggests a potential strategy to elevate their angiogenic effect for the treatment of ischemic diseases. Therefore, direct cell differentiation is a promising direction in the cell-based therapy and regenerative medicine fields, especially in ischemic tissue regeneration by therapeutic angiogenesis. Taking this into consideration, further studies are required to ascertain and differentially characterize the roles and beneficial effects of the different autocrine and paracrine regulators of angiogenesis and neovessel formation in ASC differentiation into ECs.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
L.B. declares to have acted as SAB member of Sanofi, Novo Nordisk and MSD and to have founded the Spin-off Ivastatin Therapeutics S (all unrelated to this work). The remaining authors have nothing to disclose.
Funding Statement
This work supported by Spanish Ministry of Economy and Competitiveness of Science and Agencia Estatal de Investigación (AEI/10.13039/501100011033-[PID2019-107160RB-I00] to L.B.); Red Española de Terapias Avanzadas (TERAV-RD21/0017/0013 to L.B. and G.A.); Centro de Investigación Biomedica en Red Cardiovascular (CIBERCV-CB16/11/00411 to L.B. and G.A.); and Instituto de Salud Carlos III (ISCIII) (PI20/01517 to G.A.), cofounded by Fondo Europeo de Desarrollo Regional (FEDER) “Una Manera de Hacer Europa”. We thank the Generalitat of Catalunya (Secretaria d’Universitats i Recerca, Departament d’Economia i Coneixement, 2021 SGR 01006).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Eid M.A., Mehta K., Barnes J.A., Wanken Z., Columbo J.A., Stone D.H., Goodney P., Mayo Smith M. The Global Burden of Peripheral Artery Disease. J. Vasc. Surg. 2023;77:1119–1126.e1. doi: 10.1016/j.jvs.2022.12.015. [DOI] [PubMed] [Google Scholar]
- 2.Teo K.K., Rafiq T. Cardiovascular Risk Factors and Prevention: A Perspective From Developing Countries. Can. J. Cardiol. 2021;37:733–743. doi: 10.1016/j.cjca.2021.02.009. [DOI] [PubMed] [Google Scholar]
- 3.Vaduganathan M., Mensah G.A., Turco J.V., Fuster V., Roth G.A. The Global Burden of Cardiovascular Diseases and Risk: A Compass for Future Health. J. Am. Coll. Cardiol. 2022;80:2361–2371. doi: 10.1016/j.jacc.2022.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Francula-Zaninovic S., Nola I.A. Management of Measurable Variable Cardiovascular Disease’ Risk Factors. Curr. Cardiol. Rev. 2018;14:153–163. doi: 10.2174/1573403X14666180222102312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conte S.M., Vale P.R. Peripheral Arterial Disease. Heart Lung Circ. 2018;27:427–432. doi: 10.1016/j.hlc.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 6.Belch J.J.F., Topol E.J., Agnelli G., Bertrand M., Califf R.M., Clement D.L., Creager M.A., Easton J.D., Gavin J.R., Greenland P., et al. Critical Issues in Peripheral Arterial Disease Detection and Management: A Call to Action. Arch. Intern. Med. 2003;163:884–892. doi: 10.1001/archinte.163.8.884. [DOI] [PubMed] [Google Scholar]
- 7.Campia U., Gerhard-Herman M., Piazza G., Goldhaber S.Z. Peripheral Artery Disease: Past, Present, and Future. Am. J. Med. 2019;132:1133–1141. doi: 10.1016/j.amjmed.2019.04.043. [DOI] [PubMed] [Google Scholar]
- 8.Aday A.W., Matsushita K. Epidemiology of Peripheral Artery Disease and Polyvascular Disease. Circ. Res. 2021;128:1818–1832. doi: 10.1161/CIRCRESAHA.121.318535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sunner S.S., Welsh R.C., Bainey K.R. Medical Management of Peripheral Arterial Disease: Deciphering the Intricacies of Therapeutic Options. CJC Open. 2021;3:936–949. doi: 10.1016/j.cjco.2021.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holder T.A., Gutierrez J.A., Aday A.W. Medical Management of Peripheral Artery Disease. Cardiol. Clin. 2021;39:471–482. doi: 10.1016/j.ccl.2021.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hankey G.J., Norman P.E., Eikelboom J.W. Medical Treatment of Peripheral Arterial Disease. JAMA. 2006;295:547–553. doi: 10.1001/jama.295.5.547. [DOI] [PubMed] [Google Scholar]
- 12.Jin H., Quesada C., Aliabouzar M., Kripfgans O.D., Franceschi R.T., Liu J., Putnam A.J., Fabiilli M.L. Release of Basic Fibroblast Growth Factor from Acoustically-Responsive Scaffolds Promotes Therapeutic Angiogenesis in the Hind Limb Ischemia Model. J. Control. Release. 2021;338:773–783. doi: 10.1016/j.jconrel.2021.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shimamura M., Nakagami H., Koriyama H., Morishita R. Gene Therapy and Cell-Based Therapies for Therapeutic Angiogenesis in Peripheral Artery Disease. BioMed Res. Int. 2013;2013:186215. doi: 10.1155/2013/186215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Szöke K., BeckstrØm K.J., Brinchmann J.E. Human Adipose Tissue as a Source of Cells with Angiogenic Potential. Cell Transplant. 2012;21:235–250. doi: 10.3727/096368911X580518. [DOI] [PubMed] [Google Scholar]
- 15.Si Z., Wang X., Sun C., Kang Y., Xu J., Wang X., Hui Y. Adipose-Derived Stem Cells: Sources, Potency, and Implications for Regenerative Therapies. Biomed. Pharmacother. 2019;114:108765. doi: 10.1016/j.biopha.2019.108765. [DOI] [PubMed] [Google Scholar]
- 16.Bekhite M.M., Finkensieper A., Rebhan J., Huse S., Schultze-Mosgau S., Figulla H.R., Sauer H., Wartenberg M. Hypoxia, Leptin, and Vascular Endothelial Growth Factor Stimulate Vascular Endothelial Cell Differentiation of Human Adipose Tissue-Derived Stem Cells. Stem Cells Dev. 2014;23:333–351. doi: 10.1089/scd.2013.0268. [DOI] [PubMed] [Google Scholar]
- 17.Miranville A., Heeschen C., Sengenès C., Curat C.A., Busse R., Bouloumié A. Improvement of Postnatal Neovascularization by Human Adipose Tissue-Derived Stem Cells. Circulation. 2004;110:349–355. doi: 10.1161/01.CIR.0000135466.16823.D0. [DOI] [PubMed] [Google Scholar]
- 18.Cao Y., Sun Z., Liao L., Meng Y., Han Q., Zhao R.C. Human Adipose Tissue-Derived Stem Cells Differentiate into Endothelial Cells In Vitro and Improve Postnatal Neovascularization In Vivo. Biochem. Biophys. Res. Commun. 2005;332:370–379. doi: 10.1016/j.bbrc.2005.04.135. [DOI] [PubMed] [Google Scholar]
- 19.Rehman J., Traktuev D., Li J., Merfeld-Clauss S., Temm-Grove C.J., Bovenkerk J.E., Pell C.L., Johnstone B.H., Considine R.V., March K.L. Secretion of Angiogenic and Antiapoptotic Factors by Human Adipose Stromal Cells. Circulation. 2004;109:1292–1298. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 20.Bachmann S., Jennewein M., Bubel M., Guthörl S., Pohlemann T., Oberringer M. Interacting Adipose-Derived Stem Cells and Microvascular Endothelial Cells Provide a Beneficial Milieu for Soft Tissue Healing. Mol. Biol. Rep. 2020;47:111–122. doi: 10.1007/s11033-019-05112-y. [DOI] [PubMed] [Google Scholar]
- 21.Han J., Luo L., Marcelina O., Kasim V., Wu S. Therapeutic Angiogenesis-Based Strategy for Peripheral Artery Disease. Theranostics. 2022;12:5015–5033. doi: 10.7150/thno.74785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cooke J.P., Meng S. Vascular Regeneration in Peripheral Artery Disease. Arterioscler. Thromb. Vasc. Biol. 2020;40:1627–1634. doi: 10.1161/ATVBAHA.120.312862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Conte M.S., Bradbury A.W., Kolh P., White J.V., Dick F., Fitridge R., Mills J.L., Ricco J.B., Suresh K.R., Murad M.H., et al. Global Vascular Guidelines on the Management of Chronic Limb-Threatening Ischemia. J. Vasc. Surg. 2019;69:3S–125S.e40. doi: 10.1016/j.jvs.2019.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Annex B.H., Cooke J.P. New Directions in Therapeutic Angiogenesis and Arteriogenesis in Peripheral Arterial Disease. Circ. Res. 2021;128:1944–1957. doi: 10.1161/CIRCRESAHA.121.318266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adams R.H., Alitalo K. Molecular Regulation of Angiogenesis and Lymphangiogenesis. Nat. Rev. Mol. Cell Biol. 2007;8:464–478. doi: 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- 26.Jiang L., Sun X., Deng J., Hu Y., Xu Q. Different Roles of Stem/Progenitor Cells in Vascular Remodeling. Antioxid. Redox Signal. 2021;35:192–203. doi: 10.1089/ars.2020.8199. [DOI] [PubMed] [Google Scholar]
- 27.Ye C., Zheng F., Wu N., Zhu G., Li X. Extracellular Vesicles in Vascular Remodeling. Acta Pharmacol. Sin. 2022;43:2191–2201. doi: 10.1038/s41401-021-00846-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silvestre J.S., Mallat Z., Tedgui A., Lévy B.I. Post-Ischaemic Neovascularization and Inflammation. Cardiovasc. Res. 2008;78:242–249. doi: 10.1093/cvr/cvn027. [DOI] [PubMed] [Google Scholar]
- 29.Carmeliet P. Angiogenesis in Health and Disease. Int. J. Biochem. 1993;25:1344. doi: 10.1038/nm0603-653. [DOI] [Google Scholar]
- 30.Chu H., Wang Y. Therapeutic Angiogenesis: Controlled Delivery of Angiogenic Factors. Ther. Deliv. 2013;44:2144–2151. doi: 10.4155/tde.12.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Attanasio S., Snell J. Therapeutic Angiogenesis in the Management of Critical Limb Ischemia: Current Concepts and Review. Cardiol. Rev. 2009;17:115–120. doi: 10.1097/CRD.0b013e318199e9b7. [DOI] [PubMed] [Google Scholar]
- 32.Mitsos S., Katsanos K., Koletsis E., Kagadis G.C., Anastasiou N., Diamantopoulos A., Karnabatidis D., Dougenis D. Therapeutic Angiogenesis for Myocardial Ischemia Revisited: Basic Biological Concepts and Focus on Latest Clinical Trials. Angiogenesis. 2012;15:1–22. doi: 10.1007/s10456-011-9240-2. [DOI] [PubMed] [Google Scholar]
- 33.Nakagami H., Morishita R., Maeda K., Kikuchi Y., Ogihara T., Kaneda Y. Adipose Tissue-Derived Stromal Cells as a Novel Option for Regenerative Cell Therapy. J. Atheroscler. Thromb. 2006;13:77–81. doi: 10.5551/jat.13.77. [DOI] [PubMed] [Google Scholar]
- 34.El-Kadiry A.E.H., Rafei M., Shammaa R. Cell Therapy: Types, Regulation, and Clinical Benefits. Front. Med. 2021;8:756029. doi: 10.3389/fmed.2021.756029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kolios G., Moodley Y. Introduction to Stem Cells and Regenerative Medicine. Respiration. 2012;85:3–10. doi: 10.1159/000345615. [DOI] [PubMed] [Google Scholar]
- 36.Tao J., Cao X., Yu B., Qu A. Vascular Stem/Progenitor Cells in Vessel Injury and Repair. Front. Cardiovasc. Med. 2022;9:845070. doi: 10.3389/fcvm.2022.845070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zomer H.D., Vidane A.S., Gonçalves N.N., Ambrósio C.E. Mesenchymal and Induced Pluripotent Stem Cells: General Insights and Clinical Perspectives. Stem Cells Cloning. 2015;8:125. doi: 10.1016/S1525-0016(16)34271-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Ugarte D.A., Morizono K., Elbarbary A., Alfonso Z., Zuk P.A., Zhu M., Dragoo J.L., Ashjian P., Thomas B., Benhaim P., et al. Comparison of Multi-Lineage Cells from Human Adipose Tissue and Bone Marrow. Cells Tissues Organs. 2003;174:101–109. doi: 10.1159/000071150. [DOI] [PubMed] [Google Scholar]
- 39.Fraser J.K., Wulur I., Alfonso Z., Hedrick M.H. Fat Tissue: An Underappreciated Source of Stem Cells for Biotechnology. Trends Biotechnol. 2006;24:150–154. doi: 10.1016/j.tibtech.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 40.Hassanshahi A., Hassanshahi M., Khabbazi S., Hosseini-Khah Z., Peymanfar Y., Ghalamkari S., Su Y.W., Xian C.J. Adipose-Derived Stem Cells for Wound Healing. J. Cell. Physiol. 2019;234:7903–7914. doi: 10.1002/jcp.27922. [DOI] [PubMed] [Google Scholar]
- 41.Arderiu G., Lambert C., Ballesta C., Moscatiello F., Vilahur G., Badimon L. Cardiovascular Risk Factors and Differential Transcriptomic Profile of the Subcutaneous and Visceral Adipose Tissue and Their Resident Stem Cells. Cells. 2020;9:2235. doi: 10.3390/cells9102235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kroon E., Martinson L.A., Kadoya K., Bang A.G., Kelly O.G., Eliazer S., Young H., Richardson M., Smart N.G., Cunningham J., et al. Pancreatic Endoderm Derived from Human Embryonic Stem Cells Generates Glucose-Responsive Insulin-Secreting Cells in Vivo. Nat. Biotechnol. 2008;26:443–452. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- 43.Trivedi H.L., Vanikar A.V., Thakker U., Firoze A., Dave S.D., Patel C.N., Patel J.V., Bhargava A.B., Shankar V. Human Adipose Tissue-Derived Mesenchymal Stem Cells Combined with Hematopoietic Stem Cell Transplantation Synthesize Insulin. Transplant. Proc. 2008;40:1135–1139. doi: 10.1016/j.transproceed.2008.03.113. [DOI] [PubMed] [Google Scholar]
- 44.Wang M., Song L., Strange C., Dong X., Wang H. Therapeutic Effects of Adipose Stem Cells from Diabetic Mice for the Treatment of Type 2 Diabetes. Mol. Ther. 2018;26:1921–1930. doi: 10.1016/j.ymthe.2018.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hackanson B., Waller C.F. Long-Term Follow-up of Patients with Chronic Myeloid Leukemia Having Received Autologous Stem Cell Transplantation. Ann. Hematol. 2011;90:395–399. doi: 10.1007/s00277-010-1094-y. [DOI] [PubMed] [Google Scholar]
- 46.Kuo T.K., Hung S.P., Chuang C.H., Chen C.T., Shih Y.R.V., Fang S.C.Y., Yang V.W., Lee O.K. Stem Cell Therapy for Liver Disease: Parameters Governing the Success of Using Bone Marrow Mesenchymal Stem Cells. Gastroenterology. 2008;134:2111. doi: 10.1053/j.gastro.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Banerjee E.R., Laflamme M.A., Papayannopoulou T., Kahn M., Murry C.E., Henderson W.R. Human Embryonic Stem Cells Differentiated to Lung Lineage-Specific Cells Ameliorate Pulmonary Fibrosis in a Xenograft Transplant Mouse Model. PLoS ONE. 2012;7:e33165. doi: 10.1371/journal.pone.0033165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cassinotti A., Annaloro C., Ardizzone S., Onida F., Della Volpe A., Clerici M., Usardi P., Greco S., Maconi G., Bianchi Porro G., et al. Autologous Haematopoietic Stem Cell Transplantation without CD34+ Cell Selection in Refractory Crohn’s Disease. Gut. 2008;57:211–217. doi: 10.1136/gut.2007.128694. [DOI] [PubMed] [Google Scholar]
- 49.Oyama Y., Craig R.M., Traynor A.E., Quigley K., Statkute L., Halverson A., Brush M., Verda L., Kowalska B., Krosnjar N., et al. Autologous Hematopoietic Stem Cell Transplantation in Patients with Refractory Crohn’s Disease. Gastroenterology. 2005;128:552–563. doi: 10.1053/j.gastro.2004.11.051. [DOI] [PubMed] [Google Scholar]
- 50.Burt R.K., Loh Y., Cohen B., Stefosky D., Balabanov R., Katsamakis G., Oyama Y., Russell E.J., Stern J., Muraro P., et al. Autologous Non-Myeloablative Haemopoietic Stem Cell Transplantation in Relapsing-Remitting Multiple Sclerosis: A Phase I/II Study. Lancet Neurol. 2009;8:244–253. doi: 10.1016/S1474-4422(09)70017-1. [DOI] [PubMed] [Google Scholar]
- 51.Björklund L.M., Sánchez-Pernaute R., Chung S., Andersson T., Chen I.Y.C., McNaught K.S.P., Brownell A.L., Jenkins B.G., Wahlestedt C., Kim K.S., et al. From the Cover: Embryonic Stem Cells Develop into Functional Dopaminergic Neurons after Transplantation in a Parkinson Rat Model. Proc. Natl. Acad. Sci. USA. 2002;99:2344. doi: 10.1073/pnas.022438099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou X., Ning K., Ling B., Chen X., Cheng H., Lu B., Gao Z., Xu J. Multiple Injections of Autologous Adipose-Derived Stem Cells Accelerate the Burn Wound Healing Process and Promote Blood Vessel Regeneration in a Rat Model. Stem Cells Dev. 2019;28:1463–1472. doi: 10.1089/scd.2019.0113. [DOI] [PubMed] [Google Scholar]
- 53.de Celis-Ruiz E., Fuentes B., Alonso de Leciñana M., Gutiérrez-Fernández M., Borobia A.M., Gutiérrez-Zúñiga R., Ruiz-Ares G., Otero-Ortega L., Laso-García F., Gómez-de Frutos M.C., et al. Final Results of Allogeneic Adipose Tissue-Derived Mesenchymal Stem Cells in Acute Ischemic Stroke (AMASCIS): A Phase II, Randomized, Double-Blind, Placebo-Controlled, Single-Center, Pilot Clinical Trial. Cell Transplant. 2022;31:09636897221083863. doi: 10.1177/09636897221083863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yu C., Tang W., Lu R., Tao Y., Ren T., Gao Y. Human Adipose-Derived Mesenchymal Stem Cells Promote Lymphocyte Apoptosis and Alleviate Atherosclerosis via MiR-125b-1-3p/BCL11B Signal Axis. Ann. Palliat. Med. 2021;10:2123–2133. doi: 10.21037/apm-21-49. [DOI] [PubMed] [Google Scholar]
- 55.Xing X., Li Z., Yang X., Li M., Liu C., Pang Y., Zhang L., Li X., Liu G., Xiao Y. Adipose-Derived Mesenchymal Stem Cells-Derived Exosome-Mediated MicroRNA-342-5p Protects Endothelial Cells against Atherosclerosis. Aging. 2020;12:3880–3898. doi: 10.18632/aging.102857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bourin P., Bunnell B.A., Casteilla L., Dominici M., Katz A.J., March K.L., Redl H., Rubin J.P. Stromal Cells from the Adipose Tissue-Derived Stromal Vascular Fraction and Culture Expanded Adipose Tissue-Derived Stromal/Stem Cells: A Joint Statement of the International Federation for Adipose Therapeutics (IFATS) and Science and the International S. Cytotherapy. 2014;15:5. doi: 10.1016/j.jcyt.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mieczkowska A., Schumacher A., Filipowicz N., Wardowska A., Zieliński M., Madanecki P., Nowicka E., Langa P., Deptuła M., Zieliński J., et al. Immunophenotyping and Transcriptional Profiling of in Vitro Cultured Human Adipose Tissue Derived Stem Cells. Sci. Rep. 2018;8:11339. doi: 10.1038/s41598-018-29477-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dubey N.K., Mishra V.K., Dubey R., Deng Y.H., Tsai F.C., Deng W.P. Revisiting the Advances in Isolation, Characterization and Secretome of Adipose-Derived Stromal/Stem Cells. Int. J. Mol. Sci. 2018;19:2200. doi: 10.3390/ijms19082200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Suga H., Matsumoto D., Eto H., Inoue K., Aoi N., Kato H., Araki J., Yoshimura K. Functional Implications of CD34 Expression in Human Adipose-Derived Stem/Progenitor Cells. Stem Cells Dev. 2009;18:1201–1209. doi: 10.1089/scd.2009.0003. [DOI] [PubMed] [Google Scholar]
- 60.Yang Y., Chen X.H., Li F.G., Chen Y.X., Gu L.Q., Zhu J.K., Li P. In Vitro Induction of Human Adipose-Derived Stem Cells into Lymphatic Endothelial-like Cells. Cell. Reprogram. 2015;17:69–76. doi: 10.1089/cell.2014.0043. [DOI] [PubMed] [Google Scholar]
- 61.Mildmay-White A., Khan W. Cell Surface Markers on Adipose-Derived Stem Cells: A Systematic Review. Curr. Stem Cell Res. Ther. 2017;12:484–492. doi: 10.2174/1574888X11666160429122133. [DOI] [PubMed] [Google Scholar]
- 62.ClinicalTrials.Gov. [(accessed on 7 July 2023)]; Available online: https://clinicaltrials.gov/
- 63.Trzyna A., Banaś-Ząbczyk A. Adipose-Derived Stem Cells Secretome and Its Potential Application in “Stem Cell-Free Therapy”. Biomolecules. 2021;11:878. doi: 10.3390/biom11060878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kang T., Jones T.M., Naddell C., Bacanamwo M., Calvert J.W., Thompson W.E., Bond V.C., Chen Y.E., Liu D. Adipose-Derived Stem Cells Induce Angiogenesis via Microvesicle Transport of MiRNA-31. Stem Cells Transl. Med. 2016;5:440–450. doi: 10.5966/sctm.2015-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Madonna R., Geng Y.J., De Caterina R. Adipose Tissue-Derived Stem Cells: Characterization and Potential for Cardiovascular Repair. Arterioscler. Thromb. Vasc. Biol. 2009;29:1723–1729. doi: 10.1161/ATVBAHA.109.187179. [DOI] [PubMed] [Google Scholar]
- 66.Ning H., Liu G., Lin G., Yang R., Lue T.F., Lin C.S. Fibroblast Growth Factor 2 Promotes Endothelial Differentiation of Adipose Tissue-Derived Stem Cell. J. Sex. Med. 2009;6:967–979. doi: 10.1111/j.1743-6109.2008.01172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hutchings G., Janowicz K., Moncrieff L., Dompe C., Strauss E., Kocherova I., Nawrocki M.J., Kruszyna Ł., Wasiatycz G., Antosik P., et al. The Proliferation and Differentiation of Adipose-Derived Stem Cells in Neovascularization and Angiogenesis. Int. J. Mol. Sci. 2020;21:3790. doi: 10.3390/ijms21113790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ren S., Chen J., Duscher D., Liu Y., Guo G., Kang Y., Xiong H., Zhan P., Wang Y., Wang C., et al. Microvesicles from Human Adipose Stem Cells Promote Wound Healing by Optimizing Cellular Functions via AKT and ERK Signaling Pathways. Stem Cell Res. Ther. 2019;10:47. doi: 10.1186/s13287-019-1152-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liang X., Zhang L., Wang S., Han Q., Zhao R.C. Exosomes Secreted by Mesenchymal Stem Cells Promote Endothelial Cell Angiogenesis by Transferring MiR-125a. J. Cell Sci. 2016;129:2182–2189. doi: 10.1242/jcs.170373. [DOI] [PubMed] [Google Scholar]
- 70.Badimon L., Oñate B., Vilahur G. Adipose-Derived Mesenchymal Stem Cells and Their Reparative Potential in Ischemic Heart Disease. Rev. Esp. Cardiol. 2015;68:599–611. doi: 10.1016/j.recesp.2015.02.025. [DOI] [PubMed] [Google Scholar]
- 71.Lambert C., Arderiu G., Bejar M.T., Crespo J., Baldellou M., Juan-Babot O., Badimon L. Stem Cells from Human Cardiac Adipose Tissue Depots Show Different Gene Expression and Functional Capacities. Stem Cell Res. Ther. 2019;10:361. doi: 10.1186/s13287-019-1460-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ferrer-Lorente R., Bejar M.T., Tous M., Vilahur G., Badimon L. Systems Biology Approach to Identify Alterations in the Stem Cell Reservoir of Subcutaneous Adipose Tissue in a Rat Model of Diabetes: Effects on Differentiation Potential and Function. Diabetologia. 2014;57:246–256. doi: 10.1007/s00125-013-3081-z. [DOI] [PubMed] [Google Scholar]
- 73.Oñate B., Vilahur G., Ferrer-Lorente R., Ybarra J., Díez-Caballero A., Ballesta-López C., Moscatiello F., Herrero J., Badimon L. The Subcutaneous Adipose Tissue Reservoir of Functionally Active Stem Cells Is Reduced in Obese Patients. FASEB J. 2012;26:4327–4336. doi: 10.1096/fj.12-207217. [DOI] [PubMed] [Google Scholar]
- 74.Oñate B., Vilahur G., Camino-López S., Díez-Caballero A., Ballesta-López C., Ybarra J., Moscatiello F., Herrero J., Badimon L. Stem Cells Isolated from Adipose Tissue of Obese Patients Show Changes in Their Transcriptomic Profile That Indicate Loss in Stemcellness and Increased Commitment to an Adipocyte-like Phenotype. BMC Genom. 2013;14:625. doi: 10.1186/1471-2164-14-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Melincovici C.S., Boşca A.B., Şuşman S., Mărginean M., Mihu C., Istrate M., Moldovan I.M., Roman A.L., Mihu C.M. Vascular Endothelial Growth Factor (VEGF)—Key Factor in Normal and Pathological Angiogenesis. Rom. J. Morphol. Embryol. 2018;59:455–467. [PubMed] [Google Scholar]
- 76.Tang J., Wang J., Kong X., Yang J., Guo L., Zheng F., Zhang L., Huang Y., Wan Y. Vascular Endothelial Growth Factor Promotes Cardiac Stem Cell Migration via the PI3K/Akt Pathway. Exp. Cell Res. 2009;315:3521–3531. doi: 10.1016/j.yexcr.2009.09.026. [DOI] [PubMed] [Google Scholar]
- 77.Almalki S.G., Valle Y.L., Agrawal D.K. MMP-2 and MMP-14 Silencing Inhibits VEGFR2 Cleavage and Induces the Differentiation of Porcine Adipose-Derived Mesenchymal Stem Cells to Endothelial Cells. Stem Cells Transl. Med. 2017;6:1385–1398. doi: 10.1002/sctm.16-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Khan S., Villalobos M.A., Choron R.L., Chang S., Brown S.A., Carpenter J.P., Tulenko T.N., Zhang P. Fibroblast Growth Factor and Vascular Endothelial Growth Factor Play a Critical Role in Endotheliogenesis from Human Adipose-Derived Stem Cells. J. Vasc. Surg. 2017;65:1483–1492. doi: 10.1016/j.jvs.2016.04.034. [DOI] [PubMed] [Google Scholar]
- 79.Cherubino M., Marra K.G. Adipose-Derived Stem Cells for Soft Tissue Reconstruction. Regen. Med. 2009;4:109–117. doi: 10.2217/17460751.4.1.109. [DOI] [PubMed] [Google Scholar]
- 80.Yu P.J., Ferrari G., Galloway A.C., Mignatti P., Pintucci G. Basic Fibroblast Growth Factor (FGF-2): The High Molecular Weight Forms Come of Age. J. Cell. Biochem. 2007;100:1100–1108. doi: 10.1002/jcb.21116. [DOI] [PubMed] [Google Scholar]
- 81.Harris W.M., Plastini M., Kappy N., Ortiz T., Chang S., Brown S., Carpenter J.P., Zhang P. Endothelial Differentiated Adipose-Derived Stem Cells Improvement of Survival and Neovascularization in Fat Transplantation. Aesthetic Surg. J. 2019;39:220–232. doi: 10.1093/asj/sjy130. [DOI] [PubMed] [Google Scholar]
- 82.Planat-Benard V., Silvestre J.S., Cousin B., André M., Nibbelink M., Tamarat R., Clergue M., Manneville C., Saillan-Barreau C., Duriez M., et al. Plasticity of Human Adipose Lineage Cells Toward Endothelial Cells: Physiological and Therapeutic Perspectives. Circulation. 2004;109:656–663. doi: 10.1161/01.CIR.0000114522.38265.61. [DOI] [PubMed] [Google Scholar]
- 83.Hemmings B.A., Restuccia D.F. PI3K-PKB/Akt Pathway. Cold Spring Harb. Perspect. Med. 2012;4:a011189. doi: 10.1101/cshperspect.a011189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Arderiu G., Peña E., Aledo R., Juan-Babot O., Crespo J., Vilahur G., Oñate B., Moscatiello F., Badimon L. MicroRNA-145 Regulates the Differentiation of Adipose Stem Cells toward Microvascular Endothelial Cells and Promotes Angiogenesis. Circ. Res. 2019;125:74–89. doi: 10.1161/CIRCRESAHA.118.314290. [DOI] [PubMed] [Google Scholar]
- 85.Almalki S.G., Agrawal D.K. ERK Signaling Is Required for VEGF-A/VEGFR2-Induced Differentiation of Porcine Adipose-Derived Mesenchymal Stem Cells into Endothelial Cells. Stem Cell Res. Ther. 2017;8:113. doi: 10.1186/s13287-017-0568-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xie H., Wang F., Chen X., Ying H. MiR-126 Is Essential for Endothelial Phenotype Expression during Endothelial Differentiation in Adipose-Derived Stem Cells. Mol. Med. Rep. 2018;17:442–446. doi: 10.3892/mmr.2017.7915. [DOI] [PubMed] [Google Scholar]
- 87.Qu M.-J., Pan J.-J., Shi X.-J., Zhang Z.-J., Tang Y.-H., Yang G.-Y. MicroRNA-126 Is a Prospective Target for Vascular Disease. Neuroimmunol. Neuroinflamm. 2018;5:10. doi: 10.20517/2347-8659.2018.01. [DOI] [Google Scholar]
- 88.Fish J.E., Santoro M.M., Morton S.U., Yu S., Yeh R.F., Wythe J.D., Ivey K.N., Bruneau B.G., Stainier D.Y.R., Srivastava D. MiR-126 Regulates Angiogenic Signaling and Vascular Integrity. Dev. Cell. 2008;15:272–284. doi: 10.1016/j.devcel.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xue Y.N., Yan Y., Chen Z.Z., Chen J., Tang F.J., Xie H.Q., Tang S.J., Cao K., Zhou X., Wang A.J., et al. LncRNA TUG1 Regulates FGF1 to Enhance Endothelial Differentiation of Adipose-Derived Stem Cells by Sponging MiR-143. J. Cell. Biochem. 2019;120:19087–19097. doi: 10.1002/jcb.29232. [DOI] [PubMed] [Google Scholar]
- 90.Arderiu G., Peña E., Civit-Urgell A., Badimon L. Endothelium-Released Microvesicles Transport MiR-126 That Induces Proangiogenic Reprogramming in Monocytes. Front. Immunol. 2022;13:836662. doi: 10.3389/fimmu.2022.836662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Arderiu G., Peña E., Badimon L. Ischaemic Tissue Released Microvesicles Induce Monocyte Reprogramming and Increase Tissue Repair by a Tissue Factor-Dependent Mechanism. Cardiovasc. Res. 2021;118:2354–2366. doi: 10.1093/cvr/cvab266. [DOI] [PubMed] [Google Scholar]
- 92.Capoccia B.J., Gregory A.D., Link D.C. Recruitment of the Inflammatory Subset of Monocytes to Sites of Ischemia Induces Angiogenesis in a Monocyte Chemoattractant Protein-1-Dependent Fashion. J. Leukoc. Biol. 2008;84:760–768. doi: 10.1189/jlb.1107756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sanberg P.R., Park D.H., Kuzmin-Nichols N., Cruz E., Hossne N.A., Buffolo E., Willing A.E. Monocyte Transplantation for Neural and Cardiovascular Ischemia Repair. J. Cell. Mol. Med. 2010;14:553. doi: 10.1111/j.1582-4934.2009.00903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Arderiu G., Espinosa S., Peña E., Aledo R., Badimon L. Monocyte-Secreted Wnt5a Interacts with FZD 5 in Microvascular Endothelial Cells and Induces Angiogenesis through Tissue Factor Signaling. J. Mol. Cell Biol. 2014;6:380–393. doi: 10.1093/jmcb/mju036. [DOI] [PubMed] [Google Scholar]
- 95.Arderiu G., Espinosa S., Peña E., Crespo J., Aledo R., Bogdanov V.Y., Badimon L. Tissue Factor Variants Induce Monocyte Transformation and Transdifferentiation into Endothelial Cell-like Cells. J. Thromb. Haemost. 2017;15:1689–1703. doi: 10.1111/jth.13751. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.