Abstract
In enterobacteria, the methyl group of methionine is donated by 5-methyltetrahydrofolate that is synthesized from N5,10-methylenetetrahydrofolate by the 5,10-methylenetetrahydrofolate reductase. The Streptomyces lividans metF gene, which encodes 5,10-methylenetetrahydrofolate reductase, has been cloned. It encodes a protein of 307 amino acids with a deduced molecular mass of 33,271 Da. S1 exonuclease mapping of the transcription initiation site showed that the metF gene is expressed, forming a leaderless mRNA. A 13-bp tandem repeat located immediately upstream of the promoter region shows homology with the consensus MetR-binding sequence of Salmonella typhimurium. Expression of metF in multicopy plasmids in S. lividans resulted in accumulation of a 32-kDa protein, as shown by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Disruption of the metF gene led to methionine auxotrophy. Integration of the disrupting plasmid at the metF locus was confirmed by Southern hybridization in three randomly isolated transformants. The methionine auxotrophy was complemented by transformation of the auxotrophs with an undisrupted metF gene. These results indicate that the folate branch is essential for methionine biosynthesis in streptomycetes, as occurs in enterobacteria.
Methionine, an important amino acid in bacterial metabolism, acts as the initiator of protein synthesis and in protein elongation. In addition, some methionine derivatives (e.g., S-adenosylmethionine) serve as methyl donors for a variety of methylation steps in cells (C-1 metabolism). Very little is known about the genes for methionine biosynthesis in Streptomyces species despite the interest in this amino acid as a precursor of many metabolites containing methyl groups produced by actinomycetes (5, 22) and gram-negative bacteria (11). In Escherichia coli and Salmonella typhimurium, methionine biosynthesis is encoded by 12 scattered genes which form the met regulon (25, 27). This regulon consists of 10 biosynthetic genes (metA, metB, metC, metE, metF, metH, metK, metL, metQ, and metX), 2 regulatory genes (metJ and metR), and the methionyl-tRNA synthetase gene (metG).
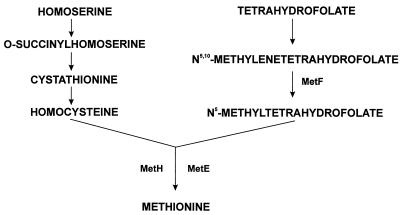
In enterobacteria, the last step in methionine biosynthesis is the methylation of homocysteine, which is catalyzed by either of two transmethylase enzymes, the metE and metH gene products (27) (Fig. 1). The methyl group transferred by these enzymes to homocysteine is donated by 5-methyltetrahydrofolate. This compound is synthesized from N5,10-methylenetetrahydrofolate by the 5,10-methylenetetrahydrofolate reductase (the metF gene product).
FIG. 1.
Biosynthetic pathway of methionine showing the formation of the homocysteine moiety from homoserine (left branch) and the origin of the methyl group from the folate branch (right branch). metF encodes the 5,10-methylenetetrahydrofolate reductase (MetF); MetE and MetH are alternative methyltransferases.
In E. coli and S. typhimurium, the methyl group of N5-methyltetrahydrofolate derives necessarily from N5,10-methylenetetrahydrofolate, an intermediate of the so-called folate branch of the methionine pathway (27). It is unclear, however, whether the same pathway occurs in Streptomyces species or other gram-positive bacteria. In this paper, we report the cloning and characterization of the Streptomyces lividans metF homolog and its involvement in methionine biosynthesis.
Total DNA of S. lividans 1326 was used for genomic library construction; the same strain was used as the host in gene disruption experiments. E. coli DH5α (13) was used for plasmid isolation and subcloning of DNA fragments, and E. coli WK6 was used for the isolation of single-strand DNA. All plasmid constructions used (Table 1) derive from pIJ2921 (17), pBluescript KS+ (Stratagene), pIJ699 (20), pGM7 (24), and pULVK99 (4).
TABLE 1.
Plasmids used in this study
| Plasmid | Relevant features | Source or reference |
|---|---|---|
| pIJ2921 | 2.7-kb E. coli plasmid; lacZ complementation; bla | 17 |
| pBluescript | 2.9-kb E. coli phagemid; lacZ complementation; bla (sequencing vector) | Stratagene |
| pIJ699 | 9.6-kb bifunctional E. coli-Streptomyces positive selection vector; tsr aph vph | 20 |
| pGM7 | 4.95-kb Streptomyces temperature-sensitive replication plasmid; tsr (gene disruption vector) | 24 |
| pULVK99 | 7.8-kb bifunctional E. coli-Streptomyces positive selection vector; tsr kan | 4 |
| pMETF1 | pIJ2921 carrying a 10-kb PstI DNA fragment of S. lividans containing metF (original clone) | This work |
| pMETF50 | pIJ2921 carrying a 1.4-kb KpnI DNA fragment containing metF | This work |
| pMETF100 | pIJ2921 carrying a 255-bp SalI DNA fragment internal to metF | This work |
| pMETF150 | pIJ699 carrying a 1.4-kb BglII DNA fragment derived from pMETF50 and containing metF | This work |
| pMETF200 | pGM7 carrying a 0.3-kb BglII DNA fragment derived from pMETF100 and containing the 255-bp SalI DNA fragment internal to metF | This work |
| pVKK-metF | pULVK99 carrying a 1.4-kb BglII DNA fragment derived from pMETF50 and containing metF | This work |
Streptomyces strains were grown on solid MEY (16) or R2YE (32) medium or in YEME with 34% sucrose for dispersed growth in liquid cultures (16) and were supplemented with thiostrepton (25 μg/ml for solid media and 5 μg/ml for liquid media) when required. For overexpression of the metF gene, S. lividans(pMETF150) was cultured in minimal NMMP medium without Casamino Acids and with 0.5% glucose as a carbon source (16) and supplemented with thiostrepton (5 μg/ml). E. coli strains were grown in Luria broth or Luria agar medium supplemented with ampicillin (100 μg/ml) when required.
S. lividans total DNA, total RNA, and plasmids were isolated as described by Hopwood et al. (16). E. coli plasmids were isolated as reported by Kieser (19) or by the boiling method of Holmes and Quigley (15). All restriction endonuclease digestions, ligations, and DNA manipulations were performed by standard protocols (28) under conditions recommended by the manufacturers (Boehringer, Mannheim, Germany; Promega, Madison, Wis.; and Fermentas AB, Vilnius, Lithuania).
Cloning and sequencing of the S. lividans metF gene.
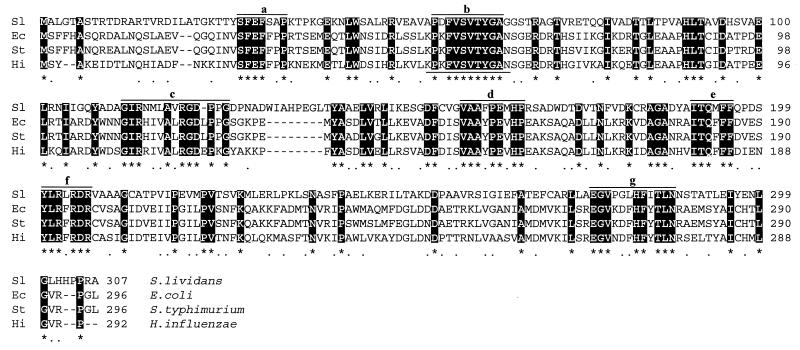
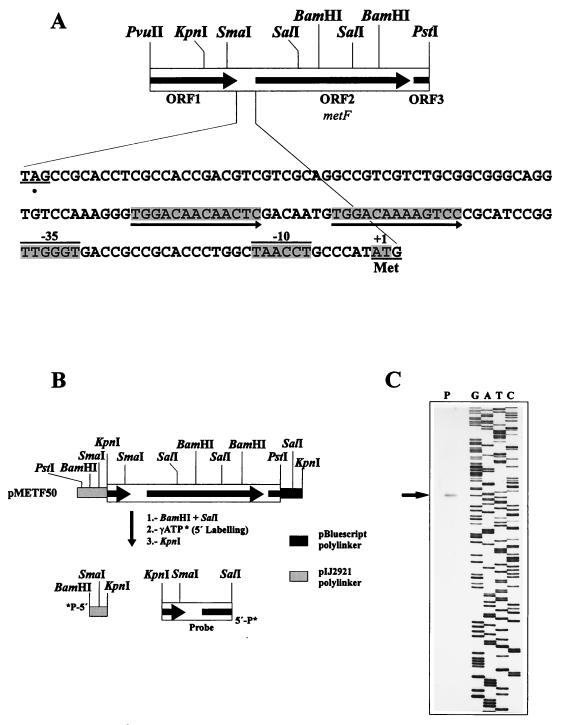
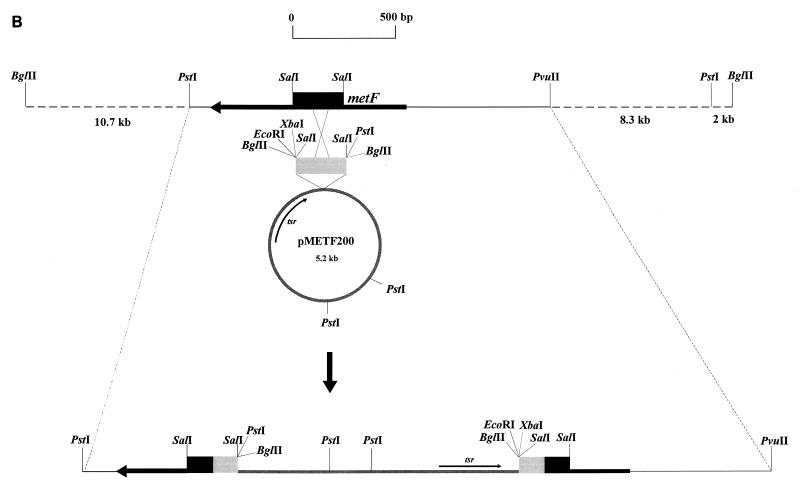
S. lividans total DNA was digested with PstI, and the resulting fragments were analyzed by Southern hybridization with a degenerate 36-mer oligonucleotide, 5′-AAGCCHAAGTTCGTHTCHGTHACHTACGGHGCHAAC-3′ (where H is G or C), as a probe designed according to a conserved amino acid motif present in the homologous proteins from E. coli and other enterobacteria (see Fig. 3). A 10-kb PstI DNA band that hybridized with the 32P-labelled probe was detected. PstI DNA fragments with similar sizes (9.5 to 10.5 kb) were isolated from an agarose gel run under the same conditions. These fragments were ligated to dephosphorylated PstI-digested plasmid pIJ2921, and the ligation mixture was used to transform E. coli DH5α. The transformants were analyzed by colony hybridization with the same 32P-labelled 36-mer oligonucleotide. A strongly hybridizing clone that harbored a recombinant pIJ2921-derived plasmid with a 10-kb PstI insert of DNA from S. lividans was isolated. By restriction analysis and Southern hybridization experiments, the oligonucleotide hybridizing sequence was located to a 1.7-kb PvuII-PstI fragment (Fig. 2A).
FIG. 3.
Alignment of the amino acid sequences of the methylenetetrahydrofolate reductases of S. lividans (AJ001630), E. coli (P00394), S. typhimurium (P11003), and H. influenzae (P45208) by using the CLUSTAL program. Conserved amino acids are in white-on-black type. Motifs a to g are sequences conserved in all tetrahydrofolate reductases. The amino acid sequence used for constructing the degenerate probe is underlined.
FIG. 2.
(A) Physical map of the 1.7-kb PvuII-PstI DNA region of S. lividans containing the metF gene (ORF2). The first in-frame ATG codon of ORF2 is underlined and labelled “Met.” The transcription start point is shaded and labelled +1, and the −10 and −35 boxes of the promoter region are indicated. The 13-bp direct repeat (putative MetR-binding site) is underlined with arrows. (B) Strategy for high-resolution S1 nuclease protection studies of the transcription start point. (C) Protected band (arrow) on S1 mapping experiments.
The nucleotide sequence of this DNA fragment revealed the presence of three open reading frames (ORF), two of which (ORF1 and ORF3) were incomplete. Compared with proteins in the SWISS-PROT database, the product of ORF1 displayed high homology with the Bacillus subtilis thiC gene product, which is involved in thiamine biosynthesis (36), and the product of ORF3 showed homology with a hypothetical 10.2-kDa B. subtilis membrane protein. The protein encoded by ORF2 showed strong homology with MetF proteins of E. coli (34.5% identical amino acids), S. typhimurium (33.8% identity), and Haemophilus influenzae (34.6% identity) (Fig. 3). The metF gene product (5,10-methylenetetrahydrofolate reductase) is involved in the folate branch of the methionine biosynthesis pathway. The gene encoded by ORF2 was tentatively designated metF.
Close analysis of the known bacterial 5,10-methylenetetrahydrofolate reductases revealed seven conserved motifs (Fig. 3) that may be involved in the catalytic activity of these enzymes.
Promoter region of the metF gene.
The S. lividans metF gene has a G+C content of 69% and codes for a putative 307-amino-acid protein. Sequence analysis of the metF upstream region revealed the presence of a putative promoter, showing −10 and −35 boxes (Fig. 2A) similar to the consensus sequences reported for Streptomyces promoters (31). To confirm the presence of the promoter and to identify the transcription initiation site, high-resolution S1 mapping was carried out with a 590-bp KpnI-SalI DNA fragment labelled with 32P at the 5′ end of SalI (Fig. 2B), as described by Fernández-Abalos et al. (9). A 283- or 284-bp protected DNA fragment was observed, which gives a transcription start site for the metF gene located at an adenine or thymine coinciding with the first nucleotide of the first in-frame ATG codon of ORF2; this result is in good agreement with the expected site based on the putative −10 region and indicates that this gene has a leaderless promoter (Fig. 2C) (18). No obvious ribosome binding site sequence was detected upstream from the translation initiation codon. This is also the case for other Streptomyces genes in which the transcription start point is at or near the translation initiation point (1).
The intergenic region, between the thiC and metF genes, contains a 13-bp tandem repeat, TGGACAACAACTC, located immediately upstream from the −35 box of the metF promoter that shows homology with the MetR-binding consensus sequence of S. typhimurium (5′-TGAANN[T/A]NNTTCA-3′ (33, 34). MetR belongs to the LysR family of bacterial activator proteins (14) and takes part in the positive regulation of metE, metF, and metH in S. typhimurium (6, 35). This sequence may be the equivalent MetR-binding motif in gram-positive bacteria to that reported in Salmonella species (see “Regulation of metF expression,” below).
Expression of metF in S. lividans results in accumulation of a 32-kDa protein.
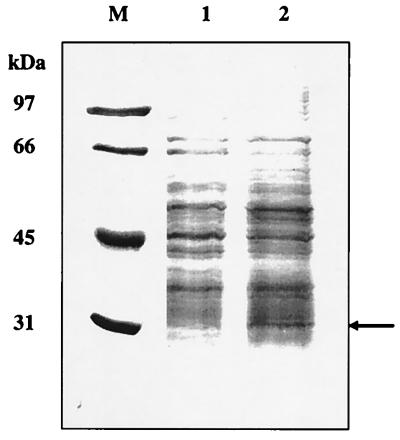
The S. lividans metF gene codes for a protein with a deduced molecular mass of 33,271 Da. To confirm that metF was expressed in S. lividans, a 1.4-kb KpnI fragment containing the metF gene and its promoter was subcloned in pIJ699 (a multicopy Streptomyces plasmid), creating plasmid pMETF150. S. lividans(pMETF150) transformants were cultured in liquid minimal medium containing ammonium sulfate as the only nitrogen source, since the metF gene is known to be regulated negatively in E. coli and S. typhimurium by the presence of methionine and vitamin B12 in the medium (21, 23). As shown in Fig. 4, the S. lividans(pMETF150) crude extract contains a protein band revealed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) of increased intensity, with a molecular mass of 32 kDa, which agrees with the mass estimated for the deduced metF gene product. The intensity of the protein band is not very high despite the increased copy number (50 to 300 copies per cell, since pMETF150 contains the pIJ101 replicon) (16), suggesting that expression of the metF promoter is strictly regulated.
FIG. 4.
SDS-PAGE (12% polyacrylamide) of crude extracts of S. lividans(pIJ699) (lane 1) and S. lividans(pMETF150) (lane 2). Cultures were grown in NMMP medium (16) supplemented with thiostrepton (5 μg/ml). Lane M, SDS-PAGE molecular weight standards (low range; Bio-Rad). Sizes are indicated on the left. The overexpressed protein is indicated with an arrow.
Disruption of metF leads to methionine auxotrophy.
In order to study the role of metF gene product in methionine biosynthesis, we inactivated metF by gene disruption. To achieve this, a 255-bp SalI fragment, internal to the metF gene (previously converted to BglII ends), was subcloned in vector pGM7, a Streptomyces plasmid with a temperature-sensitive replicon (24). The resulting integrative plasmid (pMETF200) was transformed into S. lividans protoplasts, and the transformants were incubated at 39°C (a nonpermissive temperature) to eliminate autonomously replicating plasmids. After 3 days of incubation, 106 cells were plated on MEY medium supplemented with thiostrepton. About 0.01% of the original S. lividans(pMETF200) cells were able to grow on this medium. These colonies were replicated to thiostrepton minimal medium. About 10% of them did not grow on this medium, suggesting that they were putative methionine auxotrophs in which homologous recombination had disrupted the coding region of the chromosomal metF gene. The rest of the colonies were able to grow and represented either spontaneous thiostrepton-resistant mutants or incompletely cured colonies.
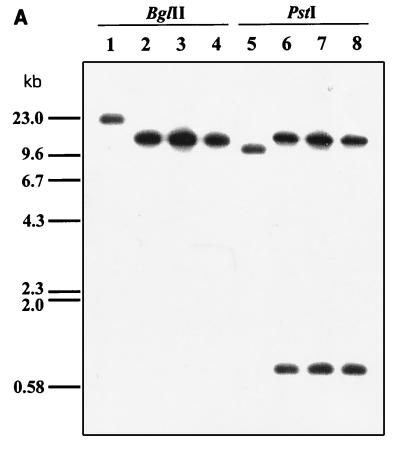
Homologous integration was verified by Southern hybridization experiments using the 255-bp SalI fragment internal to metF as a probe. Total DNA from three randomly isolated putative methionine auxotrophs (MD1 to MD3) was digested with BglII and PstI. Results of the Southern hybridization experiment are shown in Fig. 5A. The three putative auxotrophs gave the same DNA hybridization pattern, which was clearly different from the control. The integration mechanism is shown in Fig. 5B. Further confirmation of the homologous integration was achieved by plasmid rescue. Total DNA from one of the mutant strains (MD2) was isolated and digested with XbaI and EcoRI (two enzymes which do not cut the metF gene). The DNA fragments obtained were religated and transformed into S. lividans. Restriction analysis of the plasmids derived from the thiostrepton-resistant colonies revealed the presence of the pGM7 plasmid plus part of the metF gene and a DNA fragment located downstream from metF in the chromosome.
FIG. 5.
(A) Disruption of the S. lividans metF gene, as shown by hybridization with a 255-bp SalI probe internal to metF. Lanes: 1 and 5, undisrupted S. lividans 1326 (control); 2 and 6, S. lividans MD1 (met); 3 and 7, S. lividans MD2 (met); 4 and 8, S. lividans MD3 (met). Total DNA of each strain was digested with BglII (lanes 1 to 4) or PstI (lanes 5 to 8). (B) Disruption of metF by integration of pMETF200; tsr, thiostrepton resistance gene (used as a marker).
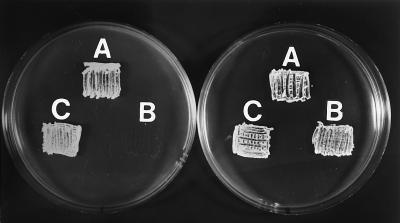
Spores from one of the mutants, S. lividans MD1 metF, were plated on minimal medium with or without methionine. The mutant was able to grow only in the medium supplemented with methionine (Fig. 6). Introduction of the pVKK-metF plasmid, which contains the undisrupted metF gene, restored the ability of the MD1 mutant to grow on minimal medium without methionine.
FIG. 6.
Growth in Streptomyces minimal medium of the parental strain S. lividans 1326 (A), the disrupted S. lividans MD1 (B), and a transformant of S. lividans MD1 with plasmid pVKK-metF (C). The righthand plate was supplemented with l-methionine (50 μg/ml).
Role of MetF in the methionine pathway.
The E. coli metF gene product is involved in the folate branch of the methionine biosynthetic pathway (27). It catalyzes the reduction of N5,10-methylenetetrahydrofolate to N5-methyltetrahydrofolate, which in turn gives its methyl group to homocysteine in order to form methionine, in a reaction catalyzed by either a vitamin B12-dependent methyltransferase (the metH gene product) or a vitamin B12-independent methyltransferase (the metE gene product).
Genes homologous to metF have been found in other gram-negative bacteria, such as S. typhimurium (30) and H. influenzae (10). However, this is the first time that a metF homolog has been found in a gram-positive bacterium. Its involvement in methionine biosynthesis has been proved by gene disruption that resulted in methionine auxotrophy. Growth of the disrupted mutant was restored by transformation with an undisrupted metF-containing plasmid (pVKK-metF). These results indicate that the folate branch is essential to provide the methyl group of methionine in actinomycetes. Synthesis of the folic acid moiety by the formyl tetrahydrofolate synthetase has been reported recently in Streptococcus mutans, another gram-positive bacterium (7).
Regulation of metF expression.
Expression of the E. coli and S. typhimurium metF genes is negatively controlled by two mechanisms (2, 26, 27). One of these mechanisms uses the metJ gene product as a repressor and S-adenosylmethionine as a corepressor (8, 29). The other uses the metH gene product as a repressor and vitamin B12 as a corepressor (12, 23).
In addition, a positive regulatory mechanism in S. typhimurium in which the metR gene product modulates expression of metE, metF, and metH has been described (6, 35). In S. typhimurium, three putative MetR-binding sites are required for MetR-mediated regulation of metF (6). The consensus MetR-binding sequence has been identified as 5′-TGAANN(T/A)NNTTCA-3′ (33, 34). The S. lividans metF gene has a 13-bp tandem repeat upstream from its coding region that shows homology with this consensus sequence, suggesting that a similar positive regulation of metF occurs in S. lividans.
metF belongs to the group of Streptomyces genes containing a leaderless mRNA (1, 18). These promoters lack a standard Shine-Dalgarno sequence. Shine-Dalgarno sequences (typically located 5 to 13 nucleotides upstream of translational start codons) are complementary to the anti-Shine–Dalgarno sequences found near the 3′ termini of 16S rRNAs (3). Translation of leaderless mRNAs in the absence of conventional Shine-Dalgarno sequences starts by interaction of the 30S ribosome subunit with the AUG codon as it emerges from the RNA polymerase, thus coupling transcription and translation (18). The efficiency of translation of metF and other leaderless promoters is a subject of great interest.
Nucleotide sequence accession number.
The nucleotide sequence of the S. lividans metF gene has been deposited in GenBank under accession no. AJ001630.
Acknowledgments
This work was supported by a grant from the CICYT (BIO97-0650-CO2-02).
We thank W. Wohlleben and A. Pühler (Bielefeld, Germany) for providing plasmid pGM7.
REFERENCES
- 1.Anné J, Van Mellaert L. Streptomyces lividans as host for heterologous protein production. FEMS Microbiol Lett. 1993;114:121–128. doi: 10.1111/j.1574-6968.1993.tb06561.x. [DOI] [PubMed] [Google Scholar]
- 2.Belfaiza J, Parsot C, Martel A, Buothier de la Tour C, Margarita D, Cohen G N, Saint-Girons I. Evolution in the biosynthesis pathways: two enzymes catalyzing consecutive steps in methionine biosynthesis originate from a common ancestor and possess a similar regulatory region. Proc Natl Acad Sci USA. 1986;83:867–871. doi: 10.1073/pnas.83.4.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bibb M J, Cohen S N. Gene expression in Streptomyces: construction and application of promoter-probe plasmid vectors in Streptomyces lividans. Mol Gen Genet. 1982;187:265–277. doi: 10.1007/BF00331128. [DOI] [PubMed] [Google Scholar]
- 4.Chary, V. K., and J. F. Martín. Unpublished data.
- 5.Coque J J R, Enguita F J, Martín J F, Liras P. A two-protein component 7α-cephem-methoxylase encoded by two genes of the cephamycin C cluster converts cephalosporin C to 7-methoxycephalosporin C. J Bacteriol. 1995;177:2230–2235. doi: 10.1128/jb.177.8.2230-2235.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cowan J M, Urbanowski M L, Talmi M, Stauffer G V. Regulation of the Salmonella typhimurium metF gene by the MetR protein. J Bacteriol. 1993;175:5862–5866. doi: 10.1128/jb.175.18.5862-5866.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crowley P J, Gutiérrez J A, Hillman J D, Bleiweis A S. Genetic and physiologic analysis of a formyl-tetrahydrofolate synthetase mutant of Streptococcus mutans. J Bacteriol. 1997;179:1563–1572. doi: 10.1128/jb.179.5.1563-1572.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Emmett R M, Johnson J R. Control of metF gene expression in maxicell preparations of Escherichia coli K-12: reversible action of the metJ protein and effect of vitamin B12. J Bacteriol. 1986;168:1491–1494. doi: 10.1128/jb.168.3.1491-1494.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernández-Abalos J M, Sánchez P, Coll P M, Villanueva J R, Pérez P, Santamaría R I. Cloning and nucleotide sequence of celA1, an endo-β-1,4-glucanase-encoding gene from Streptomyces halstedii JM8. J Bacteriol. 1992;174:6368–6376. doi: 10.1128/jb.174.20.6368-6376.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fleischmann R D, Adams M D, White O, Clayton R A, Kirkness E F, Kerlavage A R, Bult C J, Tomb J-F, Dougherty B A, Merrick J M, McKenney K, Sutton G, Fitzhugh W, Fields C A, Gocayne J D, Scott J D, Shirley R, Liu L-I, Glodek A, Kelley J M, Weidman J F, Phillips C A, Spriggs T, Hedblom E, Cotton M D, Utterback T R, Hanna M C, Nguyen D T, Saudek D M, Brandon R C, Fine L D, Fritchman J L, Fuhrmann J L, Geoghagen N S M, Gnehm C L, McDonald L A, Small K V, Fraser C M, Smith H O, Venter J C. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science. 1995;269:496–512. doi: 10.1126/science.7542800. [DOI] [PubMed] [Google Scholar]
- 11.Geelen D, Leyman B, Mergaert P, Klarskov K, van Montagu M, Geremia R, Holsters M. NodS is an S-adenosyl-l-methionine-dependent methyltransferase that methylates chitooligosaccharides deacetylated at the non-reducing end. Mol Microbiol. 1995;17:387–397. doi: 10.1111/j.1365-2958.1995.mmi_17020387.x. [DOI] [PubMed] [Google Scholar]
- 12.Greene R C, Williams R D, Kung H-F, Spears C, Weissbach H. Effects of methionine and vitamin B12 on the activities of methionine biosynthetic enzymes in metJ mutants of Escherichia coli K-12. Arch Biochem Biophys. 1973;158:249–256. doi: 10.1016/0003-9861(73)90619-x. [DOI] [PubMed] [Google Scholar]
- 13.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 14.Henikoff S, Haughn G W, Calvo J M, Wallace J C. A large family of bacterial activator proteins. Proc Natl Acad Sci USA. 1988;85:6602–6606. doi: 10.1073/pnas.85.18.6602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holmes D S, Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981;114:193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- 16.Hopwood D A, Bibb M J, Chater K F, Kieser T, Bruton C J, Kieser H M, Lydiate D J, Smith C P, Ward J M, Schrempf H. Genetic manipulation of Streptomyces: a laboratory manual. Norwich, United Kingdom: John Innes Foundation; 1985. [Google Scholar]
- 17.Janssen G R, Bibb M J. Derivatives of pUC18 that have BglII sites flanking a modified multiple cloning site and that retain the ability to identify recombinant clones by visual screening of Escherichia coli colonies. Gene. 1993;124:133–134. doi: 10.1016/0378-1119(93)90774-w. [DOI] [PubMed] [Google Scholar]
- 18.Jones R L, III, Jaskula J C, Janssen G R. In vivo translational start site selection on leaderless mRNA transcribed from the Streptomyces fradiae aph gene. J Bacteriol. 1992;174:4753–4760. doi: 10.1128/jb.174.14.4753-4760.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kieser T. Factors affecting the isolation of ccc DNA from Streptomyces lividans and Escherichia coli. Plasmid. 1984;12:19–36. doi: 10.1016/0147-619x(84)90063-5. [DOI] [PubMed] [Google Scholar]
- 20.Kieser T, Melton R E. Plasmid pIJ699, a multicopy positive selection vector for Streptomyces. Gene. 1988;65:83–91. doi: 10.1016/0378-1119(88)90419-2. [DOI] [PubMed] [Google Scholar]
- 21.Kung H-F, Spears C, Greene R C, Weissbach H. Regulation of the terminal reactions in methionine biosynthesis by vitamin B12 and methionine. Arch Biochem Biophys. 1972;150:23–31. doi: 10.1016/0003-9861(72)90005-7. [DOI] [PubMed] [Google Scholar]
- 22.Martín J F, Liras P. Biosynthetic pathways of secondary metabolites in industrial microorganisms. In: Rehm H-J, Reed G, editors. Biotechnology. Vol. 1. Weinheim, Germany: Verlag Chemie GmbH; 1981. pp. 211–233. [Google Scholar]
- 23.Milner L, Whitfield C, Weissbach H. Effect of l-methionine and vitamin B12 on methionine biosynthesis in Escherichia coli. Arch Biochem Biophys. 1969;133:413–419. doi: 10.1016/0003-9861(69)90470-6. [DOI] [PubMed] [Google Scholar]
- 24.Muth G, Nußbaumer B, Wohlleben W, Pühler A. A vector system with temperature-sensitive replication for gene disruption and mutational cloning in Streptomyces. Mol Gen Genet. 1989;219:341–348. [Google Scholar]
- 25.Old I G, Phillips S E V, Stockley P G, Saint-Girons I. Regulation of methionine biosynthesis in the Enterobacteriaceae. Prog Biophys Mol Biol. 1991;56:145–185. doi: 10.1016/0079-6107(91)90012-h. [DOI] [PubMed] [Google Scholar]
- 26.Phillips S E V, Manfield I, Parsons I, Davidson B E, Rafferty J B, Somers W S, Margarita D, Cohen G N, Saint-Girons I, Stockley P G. Cooperative tandem binding of met repressor of Escherichia coli. Nature. 1989;341:711–715. doi: 10.1038/341711a0. [DOI] [PubMed] [Google Scholar]
- 27.Saint-Girons I, Parsot C, Zakin M M, Bârzu O, Cohen G N. Methionine biosynthesis in Enterobacteriaceae: biochemical, regulatory, and evolutionary aspects. Crit Rev Biochem. 1988;23:S1–S42. doi: 10.3109/10409238809083374. [DOI] [PubMed] [Google Scholar]
- 28.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 29.Shoeman R, Redfield B, Coleman T, Greene R C, Smith A A, Brot N, Weissbach H. Regulation of methionine synthesis in Escherichia coli: effect of metJ gene product and S-adenosyl-methionine on the expression of the metF gene. Proc Natl Acad Sci USA. 1985;82:3601–3605. doi: 10.1073/pnas.82.11.3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stauffer G V, Stauffer L T. Cloning and nucleotide sequence of the Salmonella typhimurium LT2 metF gene and its homology with the corresponding sequence of Escherichia coli. Mol Gen Genet. 1988;212:246–251. doi: 10.1007/BF00334692. [DOI] [PubMed] [Google Scholar]
- 31.Strohl W R. Compilation and analysis of DNA sequences associated with apparent streptomycetea promoters. Nucleic Acids Res. 1992;20:961–972. doi: 10.1093/nar/20.5.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thompson C J, Ward J M, Hopwood D A. DNA cloning in Streptomyces: resistance genes from antibiotic-producing species. Nature. 1980;286:525–527. doi: 10.1038/286525a0. [DOI] [PubMed] [Google Scholar]
- 33.Urbanowski M L, Stauffer G V. The control region of the metH gene of Salmonella typhimurium LT2: an atypical met promoter. Gene. 1988;73:193–200. doi: 10.1016/0378-1119(88)90325-3. [DOI] [PubMed] [Google Scholar]
- 34.Urbanowski M L, Stauffer G V. Genetic and biochemical analysis of the MetR activator-binding site in the metE metR control region of Salmonella typhimurium. J Bacteriol. 1989;171:5620–5629. doi: 10.1128/jb.171.10.5620-5629.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Urbanowski M L, Stauffer L T, Plamann L S, Stauffer G V. A new methionine locus, metR, encodes a trans-acting protein required for activation of the metE and metH genes in Escherichia coli and Salmonella typhimurium. J Bacteriol. 1987;169:1391–1397. doi: 10.1128/jb.169.4.1391-1397.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y, Taylor S V, Chiu H-J, Begley T P. Characterization of the Bacillus subtilis thiC operon involved in thiamine biosynthesis. J Bacteriol. 1997;179:3030–3035. doi: 10.1128/jb.179.9.3030-3035.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]