Abstract
A gene (comC) essential for natural transformation was identified in Acinetobacter sp. strain BD413. ComC has a typical leader sequence and is similar to different type IV pilus assembly factors. A comC mutant (T308) is not able to bind or take up DNA but exhibits a piliation phenotype indistinguishable from the transformation wild type as revealed by electron microscopy.
Although natural transformation is a broadly distributed property among gram-negative soil bacteria, hardly anything is known about the components involved in DNA uptake and their assembly into the presumptive complex structures involved in DNA binding and uptake and the regulation of competence induction. Acinetobacter spp. are ubiquitous in terrestrial and aquatic environments and have been the subject of many studies of the genetics of their broad catabolic capabilities (3–5). At least two Acinetobacter sp. strains are highly competent for natural transformation (1, 11). We chose a miniencapsulated mutant of the highly competent Acinetobacter sp. strain BD4 (11), designated Acinetobacter sp. strain BD413, as a model microorganism to study natural transformation in gram-negative soil bacteria. We recently reported on the generation by random kanamycin marker insertion of five Acinetobacter mutants completely defective in natural transformation from the highly transformable Acinetobacter sp. strain ADP239, a pobA (the p-hydroxybenzoate hydroxylase gene) mutant of strain BD413. Complementation studies of one such mutant, T205, led to the identification of the competence factor ComP (14). To identify additional components of the natural transformation system in BD413 and to gain further insights into the biogenesis and the structure of the transformation system, we analyzed another transformation-defective mutant, T308.
Characterization of mutant T308.
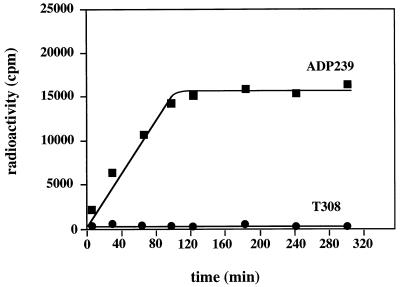
The strains and plasmids used in this study are shown in Table 1. Bacteria were grown in Luria-Bertani medium or in mineral medium (14). We previously reported a high proficiency of DNA repair in mutant T308 and therefore excluded the possibility that the transformation defect of T308 was the result of a RecA dysfunction (14). To characterize the mutant phenotype in more detail, we compared the DNA binding and uptake of ADP239 (the transformation wild type) and T308 essentially as described previously (14). These studies revealed that T308 exhibited a 66%-reduced level of DNase-sensitive DNA. It is further evident from Fig. 1 that ADP239 took up DNA at a linear rate, reaching a maximum of 16,700 cpm after 120 min. In contrast, mutant T308 was completely defective in DNA uptake.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant genotype or phenotype | Source or reference |
|---|---|---|
| Acinetobacter sp. | ||
| BD413 | Miniencapsulated mutant of strain BD4 | 11 |
| ADP239 | Spontaneous pobA mutant of BD413 | 7 |
| Transformation-defective mutants of Acinetobacter sp. strain BD413 | 14 | |
| T205 | pobA comP::nptII Kmr | |
| T308 | pobA comC::nptII Kmr | |
| E. coli S17-1 | thi-1 proA hsdR17(rK− mK+) recA1 RP4-2(Tcs::Mu) (Kms::Tn7) | 21 |
| Plasmids | ||
| pBluescriptII KS | Apr | Stratagene |
| pRK415 | Tcrlacp/o | 13 |
| pUC4K | Apr Kmr | 22 |
| pRT308-2 | pRK415 harboring the marker insertion and flanking regions from mutant T308; Tcr Kmr | This study |
| pRT308 | pRK415 containing the insert of pRT308-2 devoid of the Kmr cassette; Tcr | This study |
| pCL20 | pRK415 harboring a 20.1-kb fragment of Acinetobacter sp. strain BD413 DNA containing comC; Tcr | This study |
| pRK1 | BamHI-XbaI subclone of pRT308; Tcr | This study |
| pRK2 | SstI-BamHI subclone of pRT308; Tcr | This study |
| pRK3 | HindIII-XbaI subclone of pRT308; Tcr | This study |
| pRK9 | ClaI-XbaI subclone of pRT308; Tcr | This study |
| pSE9 | 4.6-kb ClaI-XbaI insert of pRK9 in pBluescriptII KS | This study |
| pZR408 | 2.7-kb SstI-AccI insert in pUC18 containing pobA | 3 |
FIG. 1.
DNA uptake by Acinetobacter sp. strain ADP239 and the transformation-defective mutant strain T308. Competent cells (10 ml each; 109 cells ml−1) of strain ADP239 and mutant strain T308 were incubated at 30°C in mineral medium with 40 ng of α-35S-dATP-radiolabeled DNA per ml. At the time intervals indicated, 0.5-ml samples were taken, and the radioactivity of the cells was determined as described previously (14).
Cloning of T308 mutant allele and regeneration of T308 wild-type allele.
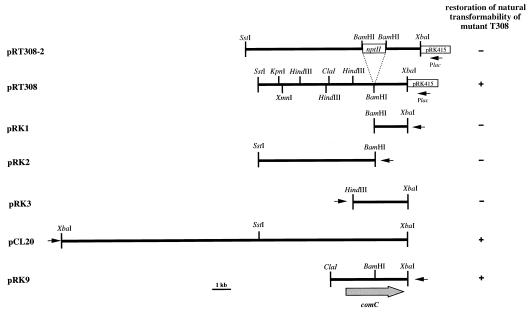
All molecular procedures were standard techniques (20). Transformations of strain ADP239 and spot matings of the transformation-deficient recipients with recombinant Escherichia coli S17-1 donor cells were performed as described recently (14). The mutated chromosomal locus from T308 was recovered on a 9.8-kb SstI-XbaI fragment (pRT308-2 [Fig. 2]). Transformation of the wild-type strain, ADP239, with this 9.8-kb SstI-XbaI fragment gave rise to mutants which had phenotypes indistinguishable from that of mutant T308. This result shows that pRT308-2 carries all of the sequence information required to generate the transformation-defective phenotype of the mutant T308.
FIG. 2.
Physical map of the DNA fragment carrying the T308 mutant locus and localization of the BD413 competence factor, comC (large arrow), by subcloning and complementation analysis. Plasmids are named on the left. The direction of transcription from the lac promoter is indicated by the small arrows. Transformability was tested by demanding acquisition of the pobA wild-type allele from BD413 DNA during growth of ADP239 (pobA mutant) transconjugants carrying pRT308, pRK1, pRK2, pRK3, pRK9, or pCL20, with p-hydroxybenzoate as the sole carbon source. −, transformability not restored; +, transformability restored.
Southern hybridization experiments strongly suggest that the mutation in T308 is the result of an integration event giving rise to gene disruption. Thus, the deletion of the Kmr marker from the recovered mutant locus should generate the wild-type allele. Deletion of the internal 1.3-kb BamHI fragment carrying the nptII gene resulted in plasmid pRT308, carrying a 8.5-kb SstI-XbaI insert (Fig. 2). pRT308 was introduced into mutant T308 by conjugation via E. coli S17-1. All transconjugants tested were able to take up DNA by natural transformation, with transformation frequencies of 3.9 × 10−4 to 4.1 × 10−4 transformants (based on viable counts) in the presence of saturating DNA concentrations. These frequencies are comparable to wild-type transformation frequencies, which is evidence for a complete restoration of the wild-type transformation phenotype by pRT308. All transconjugants still exhibited the nptII-encoded Kmr phenotype, and plasmid pRT308 could be recovered from all of the transconjugants examined, which indicates that the transformation deficiency of T308 was complemented by providing the recombinant plasmid pRT308 in trans. Furthermore, these results indicate that the insertion of the nptII marker gene into the genome of mutant T308 does not cause any polar effects on the genes that may be located downstream of the marker-affected mutant locus. A variety of derivatives of the 8.5-kb SstI-XbaI fragment were constructed and tested for their ability to complement the mutation in T308. These studies identified the 4.6-kb ClaI-XbaI fragment (pRK9) as the smallest fragment able to restore the wild-type transformation phenotype (Fig. 2). To confirm that the fragment generated from the recovered mutant locus is identical to wild-type DNA, the wild-type gene was cloned from XbaI-digested chromosomal DNA of strain BD413 into pRK415. Plasmid pCL20 contained a 20.1-kb XbaI fragment and was found to restore the wild-type transformation phenotype of mutant T308 (Fig. 2). The results of restriction analysis, Southern hybridizations, and DNA sequencing revealed that the insert in pRK9 is identical to the DNA cloned from the wild type. These results show that the comC disruption in mutant T308 by nptII marker insertion was caused by an allelic replacement recombination of contiguous DNA fragments flanking the nptII gene, which resulted in a marker gene insertion without causing any deletion or duplication events.
Identification and characterization of comC, a gene complementing the transformation defect of mutant T308.
The 4.6-kb ClaI-XbaI fragment of pRK9 was inserted into pBluescriptII KS, yielding pSE9 (Table 1), which was subjected to unidirectional deletions. The complete nucleotide sequence of the ClaI-XbaI fragment (4,584 bp) in pSE9 was determined for both strands, and sequence analysis revealed one complete open reading frame, designated comC, of 3,624 bp extending from nucleotide positions 717 (ATG) to 4340, spanning the BamHI site used to generate mutant T308 by marker insertion. comC is preceded by a well-conserved and well-placed Shine-Dalgarno sequence. The complementation of the transformation-defective mutant T308 was found to be independent of the insert orientation with respect to the lac promoter, as shown for complementation with pRK9 in Fig. 2. This indicates that comC is expressed under the control of its native promoter. A conserved ς70 promoter sequence (TTGCGAN20TATTAA) was found within a region 141 to 172 bp upstream of comC. The 39.4% G+C content of comC is within the characteristic range of 37 to 45% for coding regions in Acinetobacter species, and the codon usage was much like that found in previously sequenced Acinetobacter genes (12, 24).
Features of ComC and similarity to type IV pilus assembly and adhesion factors.
ComC contains 1,208 amino acids (aa), with a calculated molecular mass of 132 kDa. The N terminus exhibits structural features characteristic of the three domains of signal peptides (23): (i) a positively charged N terminus (Lys 8 and Arg 10), (ii) a hydrophobic core (Ala 11 to Ala 18), and (iii) a C-terminal domain containing small neutral residues (Lys 21 to Thr 24). These findings suggest that ComC is located, and acting, at the cell surface.
Database searches and sequence alignments revealed that ComC exhibits similarities to proteins involved in the assembly of type IV pili in pathogenic bacteria, such as PilC (1,037 aa; 21% identity) (19), PilC1 (1,038 aa; 22% identity), and PilC2 (1,048 aa; 22% identity) of Neisseria meningitidis (15); PilC1 (1,044 aa; 22% identity) and PilC2 (1,050 aa; 21% identity) of Neisseria gonorrhoeae (10); and PilY1 (1,161 aa; 21% identity) of Pseudomonas aeruginosa (2). The similarities of ComC to the type IV pilus biogenesis factors are in the same range as the similarities found for PilC2 to PilY1 and are not restricted to a certain region, whereas the similarities displayed by PilC2 to PilY1 are restricted to the C terminus. ComC and its homologs are also of similar size and possess large regions of hydrophilic amino acids.
ComC is not essential for the biogenesis of pilus fibers and twitching motility.
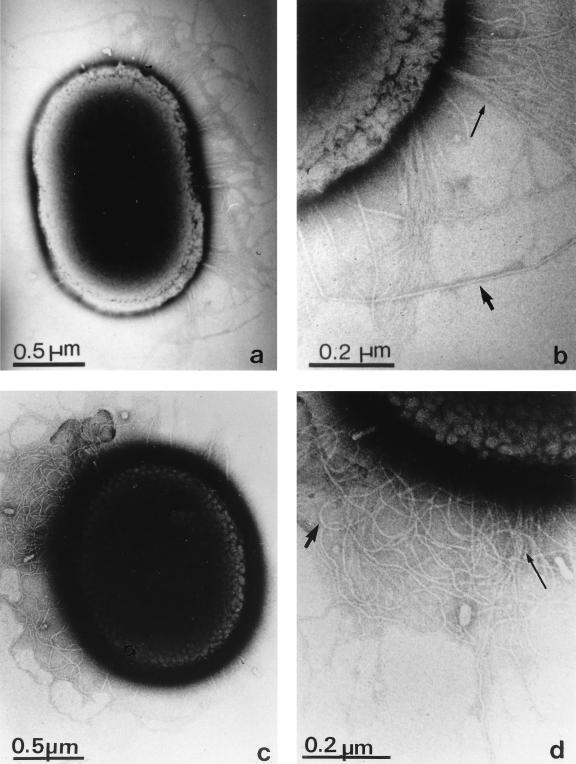
PilC1 and PilC2 of N. gonorrhoeae and the recently identified PilC of N. meningitidis exhibit dual functions in type IV pilus biogenesis and in transformation (16, 19). To address the question of whether ComC also displays such a dual function, the ultrastructures of ADP239, mutant T308, and transconjugant cells restored to natural transformation were analyzed. Electron micrographs confirmed our recent finding that cells of strain ADP239 possess two types of fimbriae (14): thin ones with a diameter of 3.5 nm, which appear in bundles, and thicker isolated fimbriae with a diameter of about 6 nm. The piliation phenotype of the transformation-defective mutant T308 was indistinguishable from the piliation of ADP239 cells (Fig. 3), indicating that ComC is not essential for the biogenesis of pili in Acinetobacter sp. strain BD413. The thick pili of Acinetobacter sp. strains have been found to mediate a special kind of surface translocation, termed twitching motility (8). Since the presence of thick pili does not preclude a functional defect of the pili, such as a defect in twitching, T308 was analyzed for its ability to perform twitching motility, which was monitored by the appearance of spreading zones along the central streak of growth of the cells on agar plates as described previously (14). These studies revealed that T308 was not impaired in twitching (data not shown) and therefore provide substantial evidence that the transformation factor ComC is not essential for pilus biogenesis.
FIG. 3.
Demonstration of intact pili on the surface of strain ADP239 and the comC mutant strain T308. Electron micrographs were taken of phosphotungstic acid-stained cells grown to the logarithmic phase in mineral medium with succinate as a carbon source. Representative samples are shown of thick pili (short arrows) and bundles of thin pili (long arrows) on the surfaces of strain ADP239 (a and b) and of mutant T308 (c and d).
Conclusions.
The PilC of N. gonorrhoeae and PilY1 of P. aeruginosa have been localized in both the outer membrane and the fimbrial fractions (2, 6), and PilC was found especially in the fimbrial tip of N. gonorrhoeae (18), which is also suggested for PilY1 of P. aeruginosa (2). The similarity of ComC to PilC and PilY1 and the DNA binding and uptake studies suggest that ComC is exported and acts in a cell surface protein complex required for DNA binding and uptake in Acinetobacter sp. strain BD413. Analogous to the function once predicted for the multifunctional gonococcal PilC2, ComC might represent a basement protein or a molecular usher, required for the correct subunit presentation of a growing oligomeric structure mediating DNA transfer through the outer membrane. Homologous sets of pil-like genes are found not only in DNA transfer systems but also in bacterial protein secretion systems (for a review, see reference 9). These homologies might reflect a common scheme of macromolecular transport.
Nucleotide sequence accession number.
The sequence of the open reading frame designated comC has been deposited in the GenBank database under accession no. AF027189.
Acknowledgments
This work was supported by grant AV 9/4-1 from the Deutsche Forschungsgemeinschaft.
We are indebted to G. Gottschalk, Göttingen, for stimulating discussions and generous support. We are grateful to M. Madkour and F. Mayer, Göttingen, for help with the electron microscopy studies. Thanks are due to V. Müller, Munich, for suggestions concerning the transport studies.
REFERENCES
- 1.Ahlquist E F, Fewson C A, Ritchie D A, Podmore J, Rowell V. Competence for genetic transformation in Acinetobacter calcoaceticus NCIB8250. FEMS Microbiol Lett. 1980;7:107–109. [Google Scholar]
- 2.Alm R A, Hallinan J P, Watson A A, Mattick J S. Fimbrial biogenesis genes of Pseudomonas aeruginosa: pilW and pilX increase the similarity of type 4 fimbriae to the GSP protein-secretion systems and pilY1 encodes a gonococcal PilC homologue. Mol Microbiol. 1996;22:161–173. doi: 10.1111/j.1365-2958.1996.tb02665.x. [DOI] [PubMed] [Google Scholar]
- 3.Averhoff B, Gregg-Jolly L, Elsemore D, Ornston L N. Genetic analysis of supraoperonic clustering by use of natural transformation in Acinetobacter calcoaceticus. J Bacteriol. 1992;174:200–204. doi: 10.1128/jb.174.1.200-204.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DiMarco A A, Averhoff B, Kim E E, Ornston L N. Evolutionary divergence of pobA, the structural gene encoding p-hydroxybenzoate hydroxylase in an Acinetobacter calcoaceticus strain well-suited for genetic analysis. Gene. 1993;125:25–33. doi: 10.1016/0378-1119(93)90741-k. [DOI] [PubMed] [Google Scholar]
- 5.DiMarco A A, Averhoff B, Ornston L N. Identification of the transcriptional activator pobR and characterization of its role in the expression of pobA, the structural gene for p-hydroxybenzoate hydroxylase in Acinetobacter calcoaceticus. J Bacteriol. 1993;175:4499–4506. doi: 10.1128/jb.175.14.4499-4506.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fussenegger M, Rudel T, Barten R, Ryll R, Meyer T F. Transformation competence and type-4 pilus biogenesis in Neisseria gonorrhoeae—a review. Gene. 1997;192:125–134. doi: 10.1016/s0378-1119(97)00038-3. [DOI] [PubMed] [Google Scholar]
- 7.Hartnett G B, Averhoff B, Ornston L N. Selection of Acinetobacter calcoaceticus mutants deficient in the p-hydroxybenzoate hydroxylase gene (pobA), a member of a supraoperonic cluster. J Bacteriol. 1990;172:6160–6161. doi: 10.1128/jb.172.10.6160-6161.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henrichsen J, Blom J. Correlation between twitching motility and possession of polar fimbriae in Acinetobacter calcoaceticus. Acta Pathol Microbiol Scand Sect B. 1975;83:103–115. doi: 10.1111/j.1699-0463.1975.tb00078.x. [DOI] [PubMed] [Google Scholar]
- 9.Hobbs M, Mattick J S. Common components in the assembly of type 4 fimbriae, DNA transfer systems, filamentous phage and protein-secretion apparatus: a general system for the formation of surface-associated protein complexes. Mol Microbiol. 1993;10:233–243. doi: 10.1111/j.1365-2958.1993.tb01949.x. [DOI] [PubMed] [Google Scholar]
- 10.Jonsson A B, Rahman M, Normark S. Pilus biogenesis gene, pilC, of Neisseria gonorrhoeae: pilC1 and pilC2 are each part of a larger duplication of the gonococcal genome and share upstream and downstream homologous sequences with opa and pil loci. Microbiology. 1995;14:2367–2377. doi: 10.1099/13500872-141-10-2367. [DOI] [PubMed] [Google Scholar]
- 11.Juni E, Janik A. Transformation of Acinetobacter calcoaceticus (Bacterium anitratum) J Bacteriol. 1969;98:281–288. doi: 10.1128/jb.98.1.281-288.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaplan J B, Goncharoff P, Seibold A M, Nichols B P. Nucleotide sequence of the Acinetobacter calcoaceticus trpGDC gene cluster. Mol Biol Evol. 1984;1:456–472. doi: 10.1093/oxfordjournals.molbev.a040331. [DOI] [PubMed] [Google Scholar]
- 13.Keen N T, Tamaki S, Kobayashi D, Trollinger D. Improved broad-host-range plasmids for DNA cloning in Gram-negative bacteria. Gene. 1988;70:191–197. doi: 10.1016/0378-1119(88)90117-5. [DOI] [PubMed] [Google Scholar]
- 14.Porstendörfer D, Drotschmann U, Averhoff B. A novel competence gene, comP, is essential for natural transformation of Acinetobacter sp. strain BD413. Appl Environ Microbiol. 1997;63:4150–4157. doi: 10.1128/aem.63.11.4150-4157.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rahman M, Kallstrom H, Normark S, Jonsson A B. PilC of pathogenic Neisseria is associated with the bacterial cell surface. Mol Microbiol. 1997;25:11–25. doi: 10.1046/j.1365-2958.1997.4601823.x. [DOI] [PubMed] [Google Scholar]
- 16.Rudel T, Facius D, Barten R, Scheuerpflug I, Nonnenmacher E, Meyer T F. Role of pili and the phase-variable PilC protein in natural competence for transformation of Neisseria gonorrhoeae. Proc Natl Acad Sci USA. 1995;92:7986–7990. doi: 10.1073/pnas.92.17.7986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rudel T, Boxberger H-J, Meyer T F. Pilus biogenesis and epithelial cell adherence of Neisseria gonorrhoeae pilC double knock-out mutants. Mol Microbiol. 1995;17:1057–1071. doi: 10.1111/j.1365-2958.1995.mmi_17061057.x. [DOI] [PubMed] [Google Scholar]
- 18.Rudel T, Scheuerpflug I, Meyer T F. Neisseria PilC protein identified as type-4 pilus tip-located adhesin. Nature. 1995;373:357–359. doi: 10.1038/373357a0. [DOI] [PubMed] [Google Scholar]
- 19.Ryll R, Rudel T, Scheuerpflug I, Barten R, Meyer T F. PilC of Neisseria meningitidis is involved in class II pilus formation and restores pilus assembly, natural transformation competence and adherence to epithelial cells in PilC-deficient gonococci. Mol Microbiol. 1997;23:879–892. doi: 10.1046/j.1365-2958.1997.2631630.x. [DOI] [PubMed] [Google Scholar]
- 20.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 21.Simon R, Priefer U, Pühler A. A broad host-range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram-negative bacteria. Bio/Technology. 1983;1:784–791. [Google Scholar]
- 22.Vieira J, Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982;19:259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- 23.von Heijne G. Signals for protein targeting into and across membranes. In: Maddy A H, Harris J R, editors. Subcellular biochemistry. New York, N.Y: Plenum Press; 1994. pp. 1–19. [DOI] [PubMed] [Google Scholar]
- 24.White P J, Hunter I S, Fewson C A. Codon usage in Acinetobacter structural genes. In: Towner K, Bergogne-Berezin E, Fewson C A, editors. The biology of Acinetobacter. FEMS Symposia Series. New York, N.Y: Plenum Press; 1991. pp. 251–258. [Google Scholar]