Abstract
Background:
Primary care physicians are crucial in identifying SARS-CoV-2 infection and transferring suspected cases since they are on the front lines of health care. Micronutrients are used as an adjunctive treatment for viral respiratory infections. Because there is currently no effective antiviral therapy for COVID-19, micronutrients such as iron, zinc, and vitamin D may be important for the survival of critically ill patients.
Objective:
To establish and emphasize a relationship between iron, zinc, and vitamin D to COVID-19.
Materials and Methods:
PubMed database was used for articles selection. All relevant articles to our review with the topics regarding the use of iron, zinc and vitamin D in COVID-19 patients. We excluded other articles, which are not related to this field and did not match inclusion criteria. The data extracted according to specific form and double reviewed by the group members.
Results:
The search of the mentioned database returned a total of 3614 studies that were included for title screening. 2910 of them were included for abstract screening, which lead to the exclusion of 1064 articles. The remaining 1846 publications full texts were reviewed. The full-text revision led to the exclusion of 1812 studies, and 34 were enrolled for final data extraction.
Conclusion:
This study raised the idea of employing zinc, iron, and vitamin D as ingredients to either protect SARS-CoV-2 patients or to speed up recovery, decrease symptoms severity and decrease mortality rates.
Keywords: COVID-19, iron, micronutrients, SARS-CoV-2, vitamin D, zinc
Introduction
COVID-19 in 2020 brought challenges to the public health system with an emerging virus with respiratory contagion called SARS-CoV-2. COVID-19 is characterized by the symptoms of viral pneumonia, such as fever, fatigue, dry cough, and lymphopenia. Patients have reported comorbidities such as diabetes, cardiovascular disease, liver disease, kidney disease, and malignant tumors. This disease also affects physical activity, sedentary action, and psychological emotion.[1]
The goal of primary health care is to offer all residents access to affordable, high-quality medical treatment. A well-integrated primary health care and public health system is crucial for a coordinated response. Growing evidence of the influence of primary care physicians on health promotion shows that they play a crucial role in epidemic management.[2]
Primary care has a crucial role to play in managing ambulatory COVID-19 and identifying patients who are more likely to experience severe symptoms so that outpatient monoclonal antibodies can be started or patients can be admitted to the hospital. Systematic solutions and extra resources are crucial for addressing the new primary care difficulties brought about by the introduction of the vaccination, the accompanying hesitation, and social hurdles. Primary care has the power to go over vaccine obstacles and engage with hard-to-reach people since it is the trusted source for health care and has new tools to reach out into the community.[3]
The concern of today’s communities is to find a way to prevent or treat COVID-19 and reduce its symptoms in the patients. However, the genetic mutations and more resistant strains of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerge; the designed vaccines and adjuvant therapies would potentially control the symptoms and severity of COVID-19.[4]
An appropriate diet and good nutritional status are essential for an optimal immune response to prevent infections. On the other hand, a poor diet and deficiency of these nutrients will increase the disease burden. Evidence proposes that nutrients are involved in the development of COVID-19.[5]
Micronutrients are used as an adjunctive treatment for viral respiratory infections. Because there is currently no effective antiviral therapy for COVID-19, adjuvant intervention may be important for the survival of critically ill patients.[4] It has been indicated that some nutrients, including vitamin D, magnesium, and zinc are essential in the modulation of the immune system and interferon (IFN) signaling pathway.[3]
The well-known essential role of vitamins, minerals, metalloids, and other micronutrients in many biological, biochemical, and molecular processes along with in vivo studies demonstrating a significant role of several micronutrients in COVID-19, led clinicians use micronutrients as a promising adjuvant therapy against SARS-CoV-2 severe pneumonia.[5]
Recent reviews agree that micronutrients play a crucial role in COVID-19 progression, prognosis, and survival, as in multiple other viral infections that primarily affect the respiratory tract. The already studied nutritional interventions for SARS-CoV and MERS-CoV infections and the underlying mechanism via which micronutrients inhibit these viruses are encouraging, as SARS-CoV-2 shares a 79.5% sequence identity with SARS-CoV and 50% with MERS-CoV.[4]
Materials and Methods
Sample and study groups
Exploratory research with a quantitative approach is included in this Integrative Literature Review (ILR). ILR is a method for gathering previously published studies with the goal of synthesizing evidence on a topic; it is widely used in the health sciences to find healthcare methods and determine innovations, allowing for the application of evidence-based services, ensuring quality, and promoting patient safety. It comprises six steps that must be completed in order: Explanation of the study issue; inclusion and exclusion criteria; sample definition; evaluation of included studies; findings interpretation; and presentation of the ILR synthesis.
The PICO (Patient, Intervention, Comparison, and Outcome) technique is commonly used in evidence-based practice, and it was utilized to develop the research question. The PICO strategy advises that problems observed in clinical practice, research, and teaching be divided into four elements: Patient, Intervention, and Treatment, Outcome, Comparison (PICO). The construction of these pieces allows for more flexibility in the design made to answer to the problem at hand.
The papers that answered the research question were reviewed in depth after searching and defining the sample. Following data collection, the information was grouped in a table, allowing the profile of the articles to be described and the main points were highlighted.
PubMed central Information Service was chosen as the search databases for the publications used within the study, as they are high-quality sources. PubMed being one of the largest digital libraries on the internet developed by the National Center for Biotechnology Information (NCBI), which is a part of the United States National Library of Medicine. The founded articles were screened by titles and reviewing the abstracts.
Inclusion criteria: The articles were selected based on the relevance to the project, which should include one of the following topics; zinc, iron, vitamin D, COVID-19, SARS-CoV-2. Studies included in PubMed central database, published less than a year ago, and English language were included.
Exclusion criteria: All articles that were not signed in PubMed central database, studies that do not have one of these topics as their primary end, or repeated studies, studies published more than one year, and studies in language other than English were excluded. Review articles as well as studies published more than a year ago were excluded.
Statistical analysis
No software was utilized to analyze the data. The data were extracted based on specific form that contains (title of the publication, author’s name, objective, summary, results, and outcomes). Double revision of each member’s outcomes was applied to ensure the validity and minimize the mistakes. During article selection, studies were doubled-reviewed, and their results to assure that we enroll the studies related to the objective of our study, and to avoid or minimize errors in the results.
Results
Figure 1 shows the selection and identification of studies. The search of the mentioned databases returned a total of 3614 studies that were included for title screening. 2910 of them were included for abstract screening, which lead to the exclusion of 1064 articles. The remaining 1846 publications full texts were reviewed. The full-text revision led to the exclusion of 1812 studies, and 34 were enrolled for final data extraction [Tables 1-3].
Figure 1.
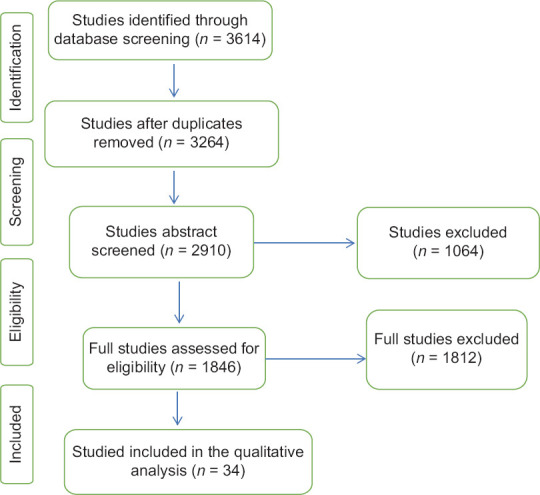
Prisma chart for included studies. The included studies had different study designs
Table 1.
Author, year of publication, study design, micronutrient (iron), and study outcomes
| Author, year | Study design | Micronutrients | Study participants | Outcomes |
|---|---|---|---|---|
| Uta, M., et al. (2022)[6] | A case-control study | Iron Supplementation | 446 expectant mothers. 95 pregnant women were found to have COVID-19, which is markedly more common in people with iron-deficient anemia. | In COVID-19 patients, anemia was much more common and was closely connected to puerperal infection, emergency c-sections, and small for gestational age. It is advised to supplement these two’s nutritional intake in order to promote the newborn’s healthy development and growth and prevent a host of problems during pregnancy in the case of a COVID-19 pandemic. |
| Bastin, A., et al. (2021)[7] | A case-control study | Iron Chelator and Iron Supplement | 147 patients with a positive PCR test result and 39 normal individuals | It is possible to think of using iron chelators to lower iron intake as a therapeutic objective. Measuring Fe and ferritin is also helpful for identifying the condition and gauging its severity. |
| Zhao, K., et al. (2020)[8] | A Retrospective Study | Iron | 50 patients in hospitals with COVID-19 confirmed. The impact of serum iron on COVID-19 severity and death was assessed. | The COVID-19 patients had serum iron insufficiency, which was found. Serum iron levels were closely associated with the severity and mortality of the illness. In COVID-19 patients, low serum iron concentration was an independent risk factor for death. |
| Campione E., et al. (2021)[9] | A clinical trial | Lactoferrin | Three sets of 92 mild-to-moderate COVID-19 patients (67/92) and asymptomatic (25/92) patients were assembled. 32 hospitalized patients got just standard-of-care (SOC) treatment; 28 patients in home-based isolation received no medication; and 32 patients (14 hospitalized and 18 in home-based isolation) received only oral and intranasal liposomal bLf. 32 healthy, untreated, COVID-19 negative participants were also added for additional analysis. | A substantial reduction in serum ferritin, IL-6, and D-dimers levels was seen in individuals receiving bLf treatment. Negative incidents were not reported. These findings prompted us to hypothesize that bLf might play a role in the treatment of mild-to-moderate COVID-19 individuals who are asymptomatic. |
| Tojo K., et al. (2021)[10] | Two-center observational study | Iron | 136 adult hospitalized COVID-19 patients | Serum iron levels and RF severity are inversely correlated in COVID-19 hospitalized patients. Higher blood iron levels are linked to the emergence of severe RF, suggesting that a deficient response to reduced serum iron may be a COVID-19 aggravating factor. |
| Algahtani F. D., et al. (2021)[11] | A randomized, prospective, interventional pilot study | Lactoferrin | 54 patients with COVID-19 who had mild to moderate symptoms that were confirmed in the lab. Only participants in the treatment group received lactoferrin at various doses for seven days; participants in the control group received the approved Egyptian COVID-19 management protocol. | The results prevent drawing any firm conclusions about its potential advantages as a support therapy. |
| Rosa L., et al. (2021)[12] | A retrospective study | Lactoferrin | 121 patients. | Lf is safe and well-tolerated by all patients, making it a potential crucial adjunctive therapy in the fight against SARS-CoV-2 infection. |
Table 3.
Author, year of publication, study design, micronutrient (Zinc) and study outcomes
| Author, year | Study design | Micronutrients | Study participants | Outcomes |
|---|---|---|---|---|
| Szarpak, L. et al. (2021)[27] | A meta-analysis | Zinc | 1474 patients included in 4 studies | Zinc supplementation did not have any beneficial impact on the course of COVID-19 evaluated as survival to hospital discharge and in-hospital mortality. The zinc-supplemented group had longer hospital stay despite shorter ICU stay. |
| Tabatabaeizadeh S. A. (2022)[28] | A meta-analysis | Zinc | 1506 participants in case and control groups were included in meta-analysis. | zinc supplementation is associated with a lower mortality rate in COVID-19 patients. Zinc supplementation could be considered as a simple way and cost benefit approach for reduction of mortality in COVID-19 patients. |
| Razeghi Jahromi, S., et al. (2021)[29] | An observational study | Zinc | 84 patients diagnosed with COVID-19 presenting to the emergency ward | Results suggest that increasing levels of Zn were accompanied by a decrease in serum CRP level. However, the significant association between Zn and disease severity was lost after adjusting for confounding factors. |
| Arrieta, F., et al. (2021)[30] | A retrospective study | Zinc | Thirty-five people with COVID-19 in need of PN were studied | Serum zinc concentrations during PN support were inversely associated with length of hospital stay but not with mortality. |
| Singh, S., et al. (2021)[31] | Cross-sectional study | Zinc | The study analyzed COVID-19 mortality and incidence (case) data from 23 socially similar European populations with comparable confounders (population: 522.47 million; experiencing up to >150-fold difference in death rates) | results suggest a positive correlation between populations’ Zn-sufficiency status and COVID-19 mortality as well as incidence. The observed association is contrary to what would be expected if Zn sufficiency was protective in COVID-19. |
| Razeghi Jahromi, S., et al. (2021)[32] | An observational study | Zinc | 84 COVID-19 patients | Results suggest that increasing levels of Zn was accompanied by a decrease in serum CRP level. However, the significant association between Zn, and disease severity was lost after adjusting for confounding factors. |
| Verschelden, G., et al. (2021)[33] | An observational study | Zinc | 139 eligible patients were included | Zinc concentration is significantly correlated with only the length of hospital, but not with mortality or morbidity. As such, findings do not support the role of zinc as a robust prognostic marker among hospitalized COVID-19 patients who in our cohort presented a high prevalence of zinc deficiency. |
| Al Sulaiman, K., et al. (2021)[34] | A two-center propensity-score matched study | Zinc | 164 patients were included, 82 patients received zinc. | The use of zinc sulfate as an additional treatment in critically ill COVID-19 patients may improve survival. Furthermore, zinc supplementation may have a protective effect on the kidneys. |
| Ekemen Keleş, Y., et al. (2022)[35] | A prospective observational study | Zinc | 100 children with confirmed COVID-19 and 269 children in the control group participated in the study. | Fasting status, diurnal fluctuation, exercise, and sex are just a few of the variables that might affect serum zinc levels, which can reflect the zinc status of the population as a whole rather than the individual. Given that individuals with COVID-19 and low serum zinc levels were more likely to be hospitalized, it is likely that these patients need a thorough evaluation of their living conditions. |
| Gordon, A. M., and Hardigan P. C. (2021)[36] | An ambulatory, interventional, prospective, single-blind study | Zinc | 104 participants | Zinc is a relatively inexpensive mineral nutrient that is an effective prophylactic agent to prevent and mitigate the potentially deadly symptomatic SARS-CoV-2 infection. |
| Balboni, E., et al. (2022)[37] | A systematic review | Zinc | four randomized clinical trials using zinc supplementation | studies on the effects of zinc supplementation did not confirm efficacy |
| Ali, N., et al. (2021)[38] | A retrospective study | Zinc | Asian and European population | there is not enough evidence on the association between individual zinc status and COVID-19 infections and mortality. |
| Hunter, J., et al. (2021)[39] | A systematic review and meta-analysis of randomized controlled trials | Zinc | 28 RCTs with 5446 participants were included | Zinc may help to avoid the signs and symptoms of and lessen the duration of respiratory tract infections. Some people may find their tolerance is limited by minor unpleasant effects. Different zinc formulations and dosages did not appear to have comparable efficacy or effectiveness. |
Table 1 provides information on the severity of symptoms, prognosis, and use of iron supplements in COVD-19 cases. The use of iron during pregnancy to prevent problems during pregnancy in the COVID-19 pandemic context was validated by a study[6] on pregnant women. Serum iron levels were highly linked with the disease’s severity and mortality.[8] A therapeutic objective was reportedly to use iron chelators to lower iron intake.[7] While the results of one trial prevented drawing any firm conclusions regarding lactoferrin’s potential benefits as a support therapy,[11] it was discovered to be a significant supplemental treatment in the fight against SARS-CoV-2 infection.[9,12]
Table 2 shows data regarding vitamin D supplementation use and serum iron level in severity of symptoms and prognosis of COVD-19 cases. Vitamin D status seems to be strongly associated with COVID-19 clinical severity[13,14,15,16,17,18,19,20,21,22,23,24,25,26] as it shortened hospital stay and decreased mortality in COVID-19 cases.[13,16-18,21,25,26] Vitamin D was also associated with better COVID-19 outcomes.[15,18,20,26]
Table 2.
Author, year of publication, study design, micronutrient (Vit D), and study outcomes
| Author, year | Study design | Micronutrients | Study participants | Outcomes |
|---|---|---|---|---|
| Gönen, M. S., et al. (2021)[13] | A retrospective then a prospective study | Vit D | Retrospective data analysis was used in the study to examine 867 COVID-19 cases. After then, a prospective study with 210 cases and 23 healthy subjects was carried out. 95 of the 163 instances that received vitamin D supplements underwent a 14-day follow-up. | Vitamin D treatment shortened hospital stay and decreased mortality in COVID-19 cases, even in the existence of comorbidities. Vitamin D supplementation is effective on various target parameters; therefore, it is essential for COVID-19 treatment. |
| Bania, A., et al. (2022)[14] | A Systematic Review | Calcifediol | 11 Studies | Future research should concentrate on calcifediol, which is given to COVID-19 patients after diagnosis and is by far the most promising treatment. |
| Oristrell, J., et al. (2022)[15] | A population-based, cohort study | Cholecalciferol and calcifediol | We compared propensity score-matched untreated controls to all people under the age of 18 residing in Barcelona-Central Catalonia (n=4.6 million) who had cholecalciferol or calcifediol supplements. | Patients receiving cholecalciferol or calcifediol supplements and reaching serum 25OHD levels below 30 ng/ml had improved COVID-19 outcomes. |
| Shah, K., et al. (2022)[16] | Systematic reviews and meta-analysis | Vit D | 10 Studies | Vitamin D supplementation is effective in reducing COVID-19 severity. Hence vitamin D should be recommended as an adjuvant therapy for COVID-19. |
| Hosseini, B., et al. (2022)[17] | A Systematic Review and Meta-Analysis | Vit D | Primary prevention is covered in five studies (one RCT, four NRISs), secondary prevention is covered in five (two RCTs, three NRISs), and tertiary prevention is covered in thirteen (six RCTs, seven NRISs). | While vitamin D supplementation exhibited protective effects against death and ICU admission in COVID-19 patients, it had no discernible effect on the chance of infection with COVID-19. |
| Varikasuvu, S. R., et al. (2022)[18] | A systematic review and meta-analysis | Vit D | A total of 6 RCTs with 551 COVID-19 patients were included. | When all the results from all RCTs were combined, vitamin D consumption was linked to a statistically significant decline in the frequencies of COVID-19-related incidents. The relative risk of ICU admission and death outcomes following vitamin D treatment, however, showed no meaningful difference. |
| Gallelli, L., et al. (2021)[19] | A multicenter real practice study | Vit D | 117 subjects | In order to treat male patients with an acute COVID-19 infection, 1,25(OH) 2D3 may be administered. |
| Rawat, D., et al. (2021)[20] | A systematic review and meta-analysis” | Vit D | Total 5 studies (3 RCTs and 2 Quasi-experimental) including n=467 patients were included. | No significant difference with vitamin-D supplementation on major health-related outcomes in COVID-19. Well-designed RCTs are required addressing this topic. |
| Ben-Eltriki, M., et al. (2021)[21] | A Meta-Analysis of Observational Studies | Vit D | Twenty-four observational studies containing 3637 participants were included | The study reported a potential increased risk of developing severe COVID-19 infection among patients with low vitamin D levels. |
| Nimavat, N., et al. (2021)[22] | A case-control study at a tertiary care hospital | Vit D | Total 156 cases and 204 controls were enrolled in the study | Vitamin D status appears to be strongly associated with COVID-19 clinical severity. |
| Parant, F., et al. (2022)[23] | An observational retrospective cohort | Vit D | 228 older hospitalized patients during the first wave of the COVID-19 pandemic. | VitD supplementation taken during the 3 months preceding the infection onset may have a protective effect on the development of severe COVID-19 forms in older adults. |
| Dror, A. A., et al. (2022)[24] | A retrospective study | 25- hydroxyvitamin D3 | 1176 patients admitted with COVID-19 were categorized according to disease severity and level of 25(OH) D. | Among hospitalized COVID-19 patients, pre-infection deficiency of vitamin D was associated with increased disease severity and mortality. |
| Ma, W., et al. (2022)[25] | Prospective study | Vit D | Among 39,315 participants, 1768 reported a positive test for SARS-CoV-2 infection. | study provides suggestive evidence on the association between higher predicted circulating 25(OH) D concentrations and a lower risk of SARS-CoV-2 infection. Greater intake of vitamin D supplements was associated with a lower risk of hospitalization. Our data also support an association between exposure to UV-B or UV-A, independently of vitamin D and SARS-CoV-2 infection |
| Lin, L. Y., et al. (2022)[26] | A cohort study using UK Biobank | Vit D | 307,512 participants | The study found no evidence of an association between historical vitamin D status and hospitalization or mortality due to COVID-19, along with inconsistent results for any association between vitamin D and diagnosis of COVID-19. |
Table 3 provides information on blood iron levels, zinc supplementation, and the severity of symptoms in COVD-19 cases. A decreased mortality rate in COVID-19 patients, the prevention of symptoms of respiratory tract infections, and a shorter incubation period have all been linked to zinc supplementation.[28,34,35,39] Others, however, reported improved symptoms without any changes to the mortality rate.[30,33] Unexpected findings from one investigation indicate a relationship between COVID-19 incidence and mortality and populations’ Zn sufficient level.[31] Insufficient data prevented two trials from confirming efficacy in COVID-19 cases.[37,38] According to one study,[27] zinc had no positive effects on the progression of COVID-19.
Discussion
SARS-CoV-2 infection rates with newer variations are still devastating most of the world after more than a year of the COVID-19 pandemic. High positive patient numbers are overwhelming the world’s healthcare systems. Many hospitalized patients’ COVID-19 mortality is caused by silent hypoxia, which is followed by rapid deterioration and, in some circumstances, septic shock. Understanding the interactions and linkages with human host components during pathogenesis and immune evasion tactics is urgently needed. Except for the Omicron variety, which requires a recent booster, acquired immunity through vaccination or earlier infection is currently sufficient to protect against the new SARS-CoV-2 variations. The severity of illness presentations and viral loads in new strains have both increased.[40]
Micronutrients include vitamins, minerals, trace elements, and other substances that affect the frequency and function of innate and adaptive immune cells, the production and development of virus-specific antibodies, the production of pro- and anti-inflammatory cytokines, the oxidative burst reaction, and other processes. These processes are involved in initiating, maintaining, and regulating host immune reactions to virus infections.[41]
The pathophysiology of COVID-19 is being linked more and more to iron excess. Indeed, a number of the symptoms of COVID-19, including inflammation, hypercoagulation, hyperferritinemia, and immunological dysfunction, are also evocative of iron excess. Even while iron is necessary for all living cells, it can be harmful because it contributes to the production of reactive oxygen species, which makes free unbound iron, which results from iron dysregulation and overload, particularly reactive (ROS).[42,43] Activating either acute or chronic inflammatory processes, which are linked to a number of clinical diseases, ROS interact with and destroy cellular lipids, nucleic acids, and proteins. Furthermore, ferroptosis, a recently identified non-apoptotic cell death, is directly caused by iron-catalyzed lipid degradation.[44] Unlike apoptosis, ferroptosis is immunogenic and, in addition to causing increased cell death, also encourages a number of inflammatory-related reactions. In therapeutic situations defined by iron overload, iron chelators have been shown to be generally safe and to protect patients.[42,45] Additionally, iron chelators’ antiviral properties are well supported by a wealth of evidence. In addition, the naturally occurring iron chelator lactoferrin (Lf) has anti-inflammatory and immunomodulatory properties. It can bind to a number of coronavirus receptors, preventing the entry of the virus into host cells. Thus, during the current COVID-19 pandemic, iron chelators may be highly helpful.[42,46]
Although there is a lot of info regarding the relationship between a patient’s zinc status and viral and respiratory tract infections, study evidence regarding COVID-19 is still lacking. However, it can be assumed based on previous reports and is discussed in this perspective, which focuses on how zinc supplementation can rebalance the immune system. Particularly, there has not been much discussion of the effect of zinc in virally produced vascular problems.[47] It is interesting to note that the majority of the COVID-19 risk groupings identified are also groups that have been linked to zinc deficiency.[48] Zinc deficiency can likely be added to the factors that predispose people to infection and the harmful progression of COVID-19 because zinc is necessary to maintain natural tissue barriers like the respiratory epithelium, preventing pathogen entry, for a balanced function of the immune system and the redox system, and for a healthy function of the immune system. Last but not least, it may be considered that administering zinc is advantageous for the majority of the population, particularly those with poor zinc status, due to its direct antiviral characteristics.[49,50]
Zinc’s capacity to prevent viral replication has been shown in numerous research. The common cold (typically a coronavirus variety), respiratory syncytial virus infections, cytomegalovirus infections, and herpes labialis are among these viruses. More crucially, zinc can stop the development of the SARS coronavirus (SARS-CoV) and the equine arteritis virus (EAV) in laboratory-grown cells. Zinc may help in the later stages of an infection by reducing the effects of immune system dysregulation, inflammation, and oxidative stress brought on by hypoxia.[51-53] Zinc controls immunological response, is anti-inflammatory, aids in the recovery of patients with severe pneumonia, and protects against hypoxia-induced ischemia reperfusion injury, according to numerous studies. Therefore, it has been suggested that zinc supplementation would be advantageous for COVID-19 patients given the possibility that zinc can target various pathways involved in the intricate pathophysiology of COVID-19 infection.[54-56]
Traditional research has connected vitamin D to healthy bones and calcium absorption. However, over the past ten years, it has become increasingly clear how important vitamin D is in controlling immune function and inflammation. Despite this important function, vitamin D deficiency is still common, affecting about 25% of US and Australian populations, as well as close to 40% of Canadian and European populations, with 25-hydroxyvitamin D (25(OH)D) levels below 50 nmol/L (the standard threshold for defining vitamin D deficiency).[57]
Vitamin D may play a complex and evolving function in the management or treatment of COVID-19. Vitamin D has the ability to affect the severity and results of COVID-19 via influencing a variety of immune system functions. SARS-CoV-2 infections cause the downregulation of ACE2, which results in a toxic buildup of Ang II and ultimately causes ARDS. The interactions between SARS-CoV-2 and the RAAS have been shown to be mitigated by vitamin D.[58] Vitamin D is able to stimulate the vasorelaxant ACE2/Ang-(1-7)/Mas receptor axis, which protects against acute lung injury and ARDS, as a negative endocrine regulator on the RAAS. By attaching to the VDR and blocking the production of renin-producing enzymes and proteins, vitamin D inhibits the biosynthesis of renin. It has also been demonstrated that vitamin D promotes ACE2 expression. There is data that suggests that differing expressions of ACE2, which generate a heightened and more robust immunological response in females, may be the reason for the differential presentation of COVID-19 between males and females (higher likelihood of ICU admissions and death in males).[59,60] At this point, it is established that ACE2 is expressed in higher amounts in males than in females, and the effect of ACE2 expression on COVID-19 severity is assessed.[61]
Conclusion
By rendering the virus effects and functioning as indicators for the severity, this study raised the idea of employing zinc, iron, and vitamin D as ingredients to either protect SARS-CoV-2 patients or to speed up recovery, decrease symptoms severity, and decrease mortality rates. Although there were some studies did not support the beneficial effects of these supplements in COVID-19 cases and some few others reported negative effects of these supplements, the vast majority of studies recommended their usage in COVID-19 management. Large-scale studies with larger sample size are recommended in this field.
Key points
Primary care physicians are crucial in identifying SARS-CoV-2 infection and transferring suspected cases since they are on the front lines of health care. However, a primary care physician’s knowledge and attitude of their duty can affect how well they function.
Iron supplementations induce better prognosis and prevention of COVID-19 infection.
Zinc supplementations induce better prognosis and prevention of COVID-19 infection.
Vit D supplementations induce better prognosis and prevention of COVID-19 infection.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Guan WJ, Liang WH, Zhao Y, Liang HR, Chen ZS, Li YM, et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: A nationwide analysis. Eur Respir J. 2020;55:2000547. doi: 10.1183/13993003.00547-2020. doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zeber JE, Khanna N. Primary care responses to the COVID-19 pandemic. Fam Pract. 2021;38(Suppl 1):i1–2. doi: 10.1093/fampra/cmab087. doi: 10.1093/fampra/cmab087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vanderlind WM, Rabinovitz BB, Miao IY, Oberlin LE, Bueno-Castellano C, Fridman C, et al. A systematic review of neuropsychological and psychiatric sequalae of COVID-19: Implications for treatment. Curr Opin Psychiatry. 2021;34:420–33. doi: 10.1097/YCO.0000000000000713. doi: 10.1097/YCO.0000000000000713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pechlivanidou E, Vlachakis D, Tsarouhas K, Panidis D, Tsitsimpikou C, Darviri C, et al. The prognostic role of micronutrient status and supplements in COVID-19 outcomes: A systematic review. Food Chem Toxicol. 2022;162:112901. doi: 10.1016/j.fct.2022.112901. doi: 10.1016/j.fct.2022.112901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pedrosa LFC, Barros ANAB, Leite-Lais L. Nutritional risk of vitamin D, vitamin C, zinc, and selenium deficiency on risk and clinical outcomes of COVID-19: A narrative review. Clin Nutr ESPEN. 2022;47:9–27. doi: 10.1016/j.clnesp.2021.11.003. doi: 10.1016/j.clnesp.2021.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uta M, Neamtu R, Bernad E, Mocanu AG, Gluhovschi A, Popescu A, et al. The influence of nutritional supplementation for iron deficiency anemia on pregnancies associated with SARS-CoV-2 infection. Nutrients. 2022;14:836. doi: 10.3390/nu14040836. doi: 10.3390/nu14040836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bastin A, Shiri H, Zanganeh S, Fooladi S, Momeni Moghaddam MA, Mehrabani M, et al. Iron chelator or iron supplement consumption in COVID-19?The role of iron with severity infection. Biol Trace Elem Res. 2022;200:4571–81. doi: 10.1007/s12011-021-03048-8. doi: 10.1007/s12011-021-03048-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao K, Huang J, Dai D, Feng Y, Liu L, Nie S. Serum iron level as a potential predictor of coronavirus disease 2019 severity and mortality: A retrospective study. Open Forum Infect Dis. 2020;7:ofaa250. doi: 10.1093/ofid/ofaa250. doi: 10.1093/ofid/ofaa250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campione E, Lanna C, Cosio T, Rosa L, Conte MP, Iacovelli F, et al. Lactoferrin as antiviral treatment in COVID-19 management: Preliminary evidence. Int J Environ Res Public Health. 2021;18:10985. doi: 10.3390/ijerph182010985. doi: 10.3390/ijerph182010985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tojo K, Sugawara Y, Oi Y, Ogawa F, Higurashi T, Yoshimura Y, et al. The U-shaped association of serum iron level with disease severity in adult hospitalized patients with COVID-19. Sci Rep. 2021;11:13431. doi: 10.1038/s41598-021-92921-6. doi: 10.1038/s41598-021-92921-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Algahtani FD, Elabbasy MT, Samak MA, Adeboye AA, Yusuf RA, Ghoniem ME. The prospect of lactoferrin use as adjunctive agent in management of SARS-CoV-2 patients: A randomized pilot study. Medicina (Kaunas) 2021;57:842. doi: 10.3390/medicina57080842. doi: 10.3390/medicina57080842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosa L, Tripepi G, Naldi E, Aimati M, Santangeli S, Venditto F, et al. Ambulatory COVID-19 patients treated with lactoferrin as a supplementary antiviral agent: A preliminary study. J Clin Med. 2021;10:4276. doi: 10.3390/jcm10184276. doi: 10.3390/jcm10184276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gönen MS, Alaylıoğlu M, Durcan E, Özdemir Y, Şahin S, Konukoğlu D, et al. Rapid and effective vitamin D supplementation may present better clinical outcomes in COVID-19 (SARS-CoV-2) patients by altering serum INOS1, IL1B, IFNg, cathelicidin-LL37, and ICAM1. Nutrients. 2021;13:4047. doi: 10.3390/nu13114047. doi: 10.3390/nu13114047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bania A, Pitsikakis K, Mavrovounis G, Mermiri M, Beltsios ET, Adamou A, et al. Therapeutic vitamin D supplementation following COVID-19 diagnosis: Where do we stand?-A systematic review. J Pers Med. 2022;12:419. doi: 10.3390/jpm12030419. doi: 10.3390/jpm12030419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oristrell J, Oliva JC, Casado E, Subirana I, Domínguez D, Toloba A, et al. Vitamin D supplementation and COVID-19 risk: A population-based, cohort study. J Endocrinol Invest. 2022;45:167–79. doi: 10.1007/s40618-021-01639-9. doi: 10.1007/s40618-021-01639-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah K, Varna VP, Sharma U, Mavalankar D. Does vitamin D supplementation reduce COVID-19 severity?A systematic review. QJM. 2022;115:665–72. doi: 10.1093/qjmed/hcac040. doi: 10.1093/qjmed/hcac040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hosseini B, El Abd A, Ducharme FM. Effects of vitamin D supplementation on COVID-19 related outcomes: A systematic review and meta-analysis. Nutrients. 2022;14:2134. doi: 10.3390/nu14102134. doi: 10.3390/nu14102134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varikasuvu SR, Thangappazham B, Vykunta A, Duggina P, Manne M, Raj H, et al. COVID-19 and vitamin D (Co-VIVID study): A systematic review and meta-analysis of randomized controlled trials. Expert Rev Anti Infect Ther. 2022;20:907–13. doi: 10.1080/14787210.2022.2035217. doi: 10.1080/14787210.2022.2035217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gallelli L, Mannino GC, Luciani F, de Sire A, Mancuso E, Gangemi P, et al. Vitamin D serum levels in subjects tested for SARS-CoV-2: What are the differences among acute, healed, and negative COVID-19 patients?A multicenter real-practice study. Nutrients. 2021;13:3932. doi: 10.3390/nu13113932. doi: 10.3390/nu13113932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rawat D, Roy A, Maitra S, Shankar V, Khanna P, Baidya DK. “Vitamin D supplementation and COVID-19 treatment: A systematic review and meta-analysis”. Diabetes Metab Syndr. 2021;15:102189. doi: 10.1016/j.dsx.2021.102189. doi: 10.1016/j.dsx.2021.102189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ben-Eltriki M, Hopefl R, Wright JM, Deb S. Association between Vitamin D status and risk of developing severe COVID-19 infection: A meta-analysis of observational studies. J Am Nutr Assoc. 2022;41:679–89. doi: 10.1080/07315724.2021.1951891. doi: 10.1080/07315724.2021.1951891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nimavat N, Singh S, Singh P, Singh SK, Sinha N. Vitamin D deficiency and COVID-19: A case-control study at a tertiary care hospital in India. Ann Med Surg (Lond) 2021;68:102661. doi: 10.1016/j.amsu.2021.102661. doi: 10.1016/j.amsu.2021.102661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parant F, Bouloy J, Haesebaert J, Bendim'red L, Goldet K, Vanhems P, et al. Vitamin D and COVID-19 severity in hospitalized older patients: Potential benefit of prehospital vitamin D supplementation. Nutrients. 2022;14:1641. doi: 10.3390/nu14081641. doi: 10.3390/nu14081641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dror AA, Morozov N, Daoud A, Namir Y, Yakir O, Shachar Y, et al. Pre-infection 25-hydroxyvitamin D3 levels and association with severity of COVID-19 illness. PLoS One. 2022;17:e0263069. doi: 10.1371/journal.pone.0263069. doi: 10.1371/journal.pone.0263069. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Ma W, Nguyen LH, Yue Y, Ding M, Drew DA, Wang K, et al. Associations between predicted vitamin D status, vitamin D intake, and risk of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and coronavirus disease 2019 (COVID-19) severity. Am J Clin Nutr. 2022;115:1123–33. doi: 10.1093/ajcn/nqab389. doi: 10.1093/ajcn/nqab389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin LY, Mulick A, Mathur R, Smeeth L, Warren-Gash C, Langan SM. The association between vitamin D status and COVID-19 in England: A cohort study using UK Biobank. PLoS One. 2022;17:e0269064. doi: 10.1371/journal.pone.0269064. doi: 10.1371/journal.pone.0269064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Szarpak L, Pruc M, Gasecka A, Jaguszewski MJ, Michalski T, Peacock FW, et al. Should we supplement zinc in COVID-19 patients?Evidence from a meta-analysis. Pol Arch Intern Med. 2021;131:802–7. doi: 10.20452/pamw.16048. doi: 10.20452/pamw.16048. [DOI] [PubMed] [Google Scholar]
- 28.Tabatabaeizadeh SA. Zinc supplementation and COVID-19 mortality: A meta-analysis. Eur J Med Res. 2022;27:70. doi: 10.1186/s40001-022-00694-z. doi: 10.1186/s40001-022-00694-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Razeghi Jahromi S, Moradi Tabriz H, Togha M, Ariyanfar S, Ghorbani Z, Naeeni S, et al. The correlation between serum selenium, zinc, and COVID-19 severity: An observational study. BMC Infect Dis. 2021;21:899. doi: 10.1186/s12879-021-06617-3. doi: 10.1186/s12879-021-06617-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.da Costa BT, Araújo GRL, da Silva Júnior RT, Santos LKS, Lima de Souza Gonçalves V, Lima DBA, et al. Effects of nutrients on immunomodulation in patients with severe COVID-19: Current knowledge. World J Crit Care Med. 2022;11:201–18. doi: 10.5492/wjccm.v11.i4.201. doi: 10.5492/wjccm.v11.i4.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh S, Diwaker A, Singh BP, Singh RK. Nutritional immunity, zinc sufficiency, and COVID-19 mortality in socially similar European populations. Front Immunol. 2021;12:699389. doi: 10.3389/fimmu.2021.699389. doi: 10.3389/fimmu.2021.699389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Razeghi Jahromi S, Moradi Tabriz H, Togha M, Ariyanfar S, Ghorbani Z, Naeeni S, et al. The correlation between serum selenium, zinc, and COVID-19 severity: An observational study. BMC Infect Dis. 2021;21:899. doi: 10.1186/s12879-021-06617-3. doi: 10.1186/s12879-021-06617-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verschelden G, Noeparast M, Noparast M, Goossens MC, Lauwers M, Cotton F, et al. Plasma zinc status and hyperinflammatory syndrome in hospitalized COVID-19 patients: An observational study. Int Immunopharmacol. 2021;100:108163. doi: 10.1016/j.intimp.2021.108163. doi: 10.1016/j.intimp.2021.108163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Al Sulaiman K, Aljuhani O, Al Shaya AI, Kharbosh A, Kensara R, Al Guwairy A, et al. Evaluation of zinc sulfate as an adjunctive therapy in COVID-19 critically ill patients: A two center propensity-score matched study. Crit Care. 2021;25:363. doi: 10.1186/s13054-021-03785-1. doi: 10.1186/s13054-021-03785-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ekemen KeleşY, Yılmaz Çiftdoğan D, Çolak A, Kara Aksay A, Üstündag G, Şahin A, et al. Serum zinc levels in pediatric patients with COVID-19. Eur J Pediatr. 2022;181:1575–84. doi: 10.1007/s00431-021-04348-w. doi: 10.1007/s00431-021-04348-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gordon AM, Hardigan PC. A case-control study for the effectiveness of oral zinc in the prevention and mitigation of COVID-19. Front Med (Lausanne) 2021;8:756707. doi: 10.3389/fmed.2021.756707. doi: 10.3389/fmed.2021.756707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Balboni E, Zagnoli F, Filippini T, Fairweather-Tait SJ, Vinceti M. Zinc and selenium supplementation in COVID-19 prevention and treatment: A systematic review of the experimental studies. J Trace Elem Med Biol. 2022;71:126956. doi: 10.1016/j.jtemb.2022.126956. doi: 10.1016/j.jtemb.2022.126956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ali N, Fariha KA, Islam F, Mohanto NC, Ahmad I, Hosen MJ, et al. Assessment of the role of zinc in the prevention of COVID-19 infections and mortality: A retrospective study in the Asian and European population. J Med Virol. 2021;93:4326–33. doi: 10.1002/jmv.26932. doi: 10.1002/jmv.26932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hunter J, Arentz S, Goldenberg J, Yang G, Beardsley J, Myers SP, et al. Zinc for the prevention or treatment of acute viral respiratory tract infections in adults: A rapid systematic review and meta-analysis of randomised controlled trials. BMJ Open. 2021;11:e047474. doi: 10.1136/bmjopen-2020-047474. doi: 10.1136/bmjopen-2020-047474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gupta Y, Maciorowski D, Medernach B, Becker DP, Durvasula R, Libertin CR, et al. Iron dysregulation in COVID-19 and reciprocal evolution of SARS-CoV-2: Natura nihil frustra facit. J Cell Biochem. 2022;123:601–19. doi: 10.1002/jcb.30207. doi: 10.1002/jcb.30207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gombart AF, Pierre A, Maggini S. A review of micronutrients and the immune system-working in harmony to reduce the risk of infection. Nutrients. 2020;12:236. doi: 10.3390/nu12010236. doi: 10.3390/nu12010236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Habib HM, Ibrahim S, Zaim A, Ibrahim WH. The role of iron in the pathogenesis of COVID-19 and possible treatment with lactoferrin and other iron chelators. Biomed Pharmacother. 2021;136:111228. doi: 10.1016/j.biopha.2021.111228. doi: 10.1016/j.biopha.2021.111228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cassat JE, Skaar EP. Iron in infection and immunity. Cell Host Microbe. 2013;13:509–19. doi: 10.1016/j.chom.2013.04.010. doi: 10.1016/j.chom.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rainey NE, Moustapha A, Saric A, Nicolas G, Sureau F, Petit PX. Iron chelation by curcumin suppresses both curcumin-induced autophagy and cell death together with iron overload neoplastic transformation. Cell Death Discov. 2019;5:150. doi: 10.1038/s41420-019-0234-y. doi: 10.1038/s41420-019-0234-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cavezzi A, Troiani E, Corrao S. COVID-19: Hemoglobin, iron, and hypoxia beyond inflammation. A narrative review. Clin Pract. 2020;10:1271. doi: 10.4081/cp.2020.1271. doi: 10.4081/cp.2020.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Edeas M, Saleh J, Peyssonnaux C. Iron: Innocent bystander or vicious culprit in COVID-19 pathogenesis? Int J Infect Dis. 2020;97:303–5. doi: 10.1016/j.ijid.2020.05.110. doi: 10.1016/j.ijid.2020.05.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wessels I, Rolles B, Rink L. The potential impact of zinc supplementation on COVID-19 pathogenesis. Front Immunol. 2020;11:1712. doi: 10.3389/fimmu.2020.01712. doi: 10.3389/fimmu.2020.01712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wessels I, Rink L. Micronutrients in autoimmune diseases: Possible therapeutic benefits of zinc and vitamin D. J Nutr Biochem. 2020;77:108240. doi: 10.1016/j.jnutbio.2019.108240. doi: 10.1016/j.jnutbio.2019.108240. [DOI] [PubMed] [Google Scholar]
- 49.Read SA, Obeid S, Ahlenstiel C, Ahlenstiel G. The role of zinc in antiviral immunity. Adv Nutr. 2019;10:696–710. doi: 10.1093/advances/nmz013. doi: 10.1093/advances/nmz013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Skalny AV, Rink L, Ajsuvakova OP, Aschner M, Gritsenko VA, Alekseenko SI, et al. Zinc and respiratory tract infections: Perspectives for COVID-19 (Review) Int J Mol Med. 2020;46:17–26. doi: 10.3892/ijmm.2020.4575. doi: 10.3892/ijmm.2020.4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hulisz D. Efficacy of zinc against common cold viruses: An overview. J Am Pharm Assoc (2003) 2004;44:594–603. doi: 10.1331/1544-3191.44.5.594.Hulisz. doi: 10.1331/1544-3191.44.5.594.hulisz. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suara RO, Crowe JE., Jr Effect of zinc salts on respiratory syncytial virus replication. Antimicrob Agents Chemother. 2004;48:783–90. doi: 10.1128/AAC.48.3.783-790.2004. doi: 10.1128/AAC.48.3.783-790.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.te Velthuis AJ, van den Worm SH, Sims AC, Baric RS, Snijder EJ, van Hemert MJ. Zn (2+) inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010;6:e1001176. doi: 10.1371/journal.ppat.1001176. doi: 10.1371/journal.ppat.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Berg K, Bolt G, Andersen H, Owen TC. Zinc potentiates the antiviral action of human IFN-alpha tenfold. J Interferon Cytokine Res. 2001;21:471–4. doi: 10.1089/10799900152434330. doi: 10.1089/10799900152434330. [DOI] [PubMed] [Google Scholar]
- 55.Haase H, Rink L. Multiple impacts of zinc on immune function. Metallomics. 2014;6:1175–80. doi: 10.1039/c3mt00353a. doi: 10.1039/c3mt00353a. [DOI] [PubMed] [Google Scholar]
- 56.Jayawardena R, Sooriyaarachchi P, Chourdakis M, Jeewandara C, Ranasinghe P. Enhancing immunity in viral infections, with special emphasis on COVID-19: A review. Diabetes Metab Syndr. 2020;14:367–82. doi: 10.1016/j.dsx.2020.04.015. doi: 10.1016/j.dsx.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ghelani D, Alesi S, Mousa A. Vitamin D and COVID-19: An overview of recent evidence. Int J Mol Sci. 2021;22:10559. doi: 10.3390/ijms221910559. doi: 10.3390/ijms221910559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Karahan S, Katkat F. Impact of serum 25(OH) vitamin D level on mortality in patients with COVID-19 in Turkey. J Nutr Health Aging. 2021;25:189–96. doi: 10.1007/s12603-020-1479-0. doi: 10.1007/s12603-020-1479-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Giménez VMM, Sanz RL, Marón FJM, Ferder L, Manucha W. Vitamin D-RAAS connection: An integrative standpoint into cardiovascular and neuroinflammatory disorders. Curr Protein Pept Sci. 2020;21:948–54. doi: 10.2174/1389203721666200606220719. doi: 10.2174/1389203721666200606220719. [DOI] [PubMed] [Google Scholar]
- 60.Xu J, Yang J, Chen J, Luo Q, Zhang Q, Zhang H. Vitamin D alleviates lipopolysaccharide-induced acute lung injury via regulation of the renin-angiotensin system. Mol Med Rep. 2017;16:7432–8. doi: 10.3892/mmr.2017.7546. doi: 10.3892/mmr.2017.7546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ambrosino I, Barbagelata E, Ortona E, Ruggieri A, Massiah G, Giannico OV, et al. Gender differences in patients with COVID-19: A narrative review. Monaldi Arch Chest Dis. 2020;90 doi: 10.4081/monaldi.2020.1389. doi: 10.4081/monaldi.2020.1389. [DOI] [PubMed] [Google Scholar]


