ABSTRACT
Yoga is an ancient wisdom comprising a multitude of physical and mental practices that are aimed toward a state of optimum physical, mental, social, and spiritual health. Neuropathic pain (NP) is caused by a lesion or disease of the somatosensory nervous system that is often unresponsive to currently available modes of treatment, portending an inferior quality of life for patients. This systematic review and meta-analysis aim to investigate the effect and the potential role of yoga in NP syndromes. PubMed, Scopus, Elton Bryson Stephens Company (EBSCO), and Cochrane Library were screened for randomized controlled trials (RCTs) assessing the effects of yoga in patients on NP. Usual care, no treatment, or any active treatments were acceptable as control interventions. Primary outcome measures were objective or subjective assessment measures of pain intensity. For each outcome, standardized mean differences and 95% confidence intervals (CIs) were calculated. A total of four studies were included for qualitative synthesis. Meta-analysis of three studies revealed an overall effect (Z) in the favor of yoga as an intervention for NP, when compared to controls, although the effect was not statistically significant (three RCTs; Z = 1.10 [P = 0.27]; Heterogeneity: τ2 = 0.37; χ2 = 27.78, df = 2 [P < 0.00001]; I2 = 93%). This review divulged the overall favorable effect of yoga in NP, although it was not statistically significant. It highlights the promising role of yoga on pain intensity and quality of life in NP syndromes while showing that yoga has the advantage of being an inexpensive and easily accessible mode of therapy. Extensive research on the efficiency and safety of yoga must be conducted using robust RCTs with rigorous methodologies.
Keywords: Central sensitization, multiple sclerosis, neuropathy, pain matrix, pranayama, trigeminal neuralgia
Introduction
Neuropathic pain
Neuropathic pain (NP) is frequently described as a shooting or scorching type of pain caused by a somatosensory system lesion or disease, comprising peripheral fibers (Aβ, Aδ, and C fibers) and central neurons. NP can be peripheral or central, with peripheral NP including trigeminal neuralgia, post-herpetic, nerve injury, post-amputation pain, painful polyneuropathy (diabetes mellitus), and radiculopathy, while central NP includes spinal cord injury, post-stroke pain, and multiple sclerosis-related pain.[1] NP appears to be more difficult to treat than many other types of chronic pain and affects 7–10% of the general population.[1] Patients with NP have significantly lower health-related quality of life (HR-QOL) due to pain-related interference, functional distress, decreased capacity to work, and lowered mobility thus affecting productivity and work efficiency.[2] Furthermore, spouses of NP patients have been demonstrated to suffer from negative social repercussions connected to NP.[2]
The current armamentarium of drugs available for management of NP include topical anesthetics, opioids, serotonin–norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), and gabapentinoids.[3] The latter two drugs are alternative second-line options in case of nonsatisfactory results from the former. Despite the availability of these drugs, it can be difficult to treat patients with NP, which can be attributed to multiple reasons. First, the efficacy of these drugs is poor, which is masked by the limited quality of evidence. The number needed to treat (NNT) (pain reduction by at least 50%, NNT) varies from 3.6 for the TCAs to 6.4 for the SNRIs, with equivalent NNT for pregabalin, 7.7; gabapentin, 7.2; strong opioids, 4.3; and weak opioids, 11.7.[3] Second, the occurrence of adverse effects of the drug limits their use as well as hinders dose-escalation in clinical practice.[3] This leads to poor compliance by patients and hesitation to consume the drug. Finally, due to the low efficacy of treatments, patients are often initiated on multiple drug therapies, which increases the pill burden, amounting to polypharmacy and high costs of therapy.[4] Increased drug prescriptions, visits to healthcare providers, morbidity from the pain, and the underlying condition add up to the already deteriorating HR-QOL. Given the limited long-term efficacy of available treatment options and the poor quality of life observed in NP patients, there is a significant need to discover therapies that have a holistic approach involving techniques that act not only at the physical dimensions but also at the mental and emotional components, therefore providing a chance at the sustained and long-term benefit.
Yoga as an efficient treatment modality for neuropathic pain
Yoga is a nearly 4,000-year-old spiritual discipline[5] with origins in India.[6] Yoga is most often associated with physical postures (asanas), breathing exercises (pranayama), and meditation (dhyana).[7] Previous research demonstrates the beneficial effects of yoga on a variety of pain outcomes.[8-12] The physical components of yoga cause a decrease in inflammatory mediators,[13] while yoga practice also activates the endogenous opioid mechanism, which reduces pain symptoms.[14]
Yoga and mindfulness help improve and sustain physical and emotional health, by turning down the hypothalamic–pituitary–adrenal (HPA) axis and the sympathetic nervous system. Meditation and pranayama can help people deal with the emotional aspects of pain, effectively reducing anxiety and depression and improving the perceived quality of life.[15] Meditation has been shown to increase cognitive control and may cause better pain tolerance in experimentally induced pain as well as pathological NP. Yoga and meditation act on peripheral nerves as well as cause the correction of aberrant neuroplasticity in the higher-order cortical areas. Despite the compelling evidence supporting yoga’s use as part of a multidisciplinary approach to help people with chronic pain,[8-12,16,17] there seems a dearth of robust studies investigating the effects and mechanisms of yoga among patients of NP.
Rationale behind this systematic review/meta-analysis
NP frequently does not react well to typical pain medications and occasionally worsens rather than improves with time. It can cause major disability in certain persons. The intricacy of neuropathic symptoms, poor results, and challenging treatment decisions all appear to contribute to the burden of chronic NP. Despite hurdles, advances in understanding the pathophysiology of NP are stimulating the development of new diagnostic tools and customized therapies, emphasizing the importance of a multidisciplinary approach to NP management.[1] Since, earlier studies have examined yoga’s impact on a range of pain problems and revealed a positive impact on pain outcomes in chronic headache,[8] fibromyalgia,[9] low back pain,[10] menstrual pain,[11] and labor pain,[12] it is imperative to search and evaluate the current evidence on yoga and meditation as an adjunct therapy in managing NP.
Materials and Methods
Study design
Randomized controlled trials (RCTs) in the English language published in peer-reviewed journals were included.
Participants/Population
Adult populations (18 years or older) of either sex with non-compressive NP (not amenable to surgical treatment) secondary to trigeminal neuralgia, multiple sclerosis, post-stroke discomfort, or diabetic neuropathy of any duration and severity were included. Studies involving patients under 18 years of age with fibromyalgia, cancer, migraine, headaches (primary, secondary, or tension headaches), headaches caused by tumors, headache-related emergencies, or NP caused by nerve compression (amenable to any kind of surgery) were deemed ineligible.
Intervention/Exposure
Studies evaluating the effect of yoga interventions on change in pain-related outcomes were included. Studies employing alternative and complementary treatment modalities other than yoga, such as mindfulness-based stress reduction, acupressure, acupuncture, Tai-chi, qigong, music therapy, reiki, etc., were excluded. Yoga was defined as “a mind–body intervention that includes any of the components of gentle stretching (asana), breathing control (pranayama), and meditation.”
Comparator/Control interventions
Studies utilizing all forms of control group interventions (standard care, alternate treatment modalities, waitlist controls, etc.) or no intervention/waitlist were included, provided they were RCTs.
Outcomes
Studies measuring the change in outcomes of pain using objective or subjective assessment measures including questionnaires and tools like the visual analog scale (VAS), numeric rating scale (NRS), pain bothersome on 11-point NRS, McGill Pain Questionnaire, the VAS relating to total comfort and sensation of Pain, etc., were included.
Search terms and databases
Using Boolean search operators (AND/OR), a comprehensive search was conducted on the electronic databases of PubMed, PubMed Central, Scopus, EBSCO Essentials, and Cochrane Library Sources for the following terms: trigeminal neuralgia, post-stroke pain, diabetic neuropathy, multiple sclerosis, NP, nerve pain, neuropathy, post-herpetic neuralgia, multiple sclerosis, yoga, meditation, yoga nidra, asana, pranayama, and mantra. The scope of searches was restricted to titles, abstracts, and keywords of research articles published in English from the beginning of time until October 2022. The final searches were conducted on October 2, 2022.
Study selection procedures
All search results were compiled together and uploaded to the Rayyan online automation tool for duplicate detection.[18] After removing duplicate results, five co-authors independently screened the remaining studies for potential reports worthy of inclusion. The studies were divided and distributed among the co-authors along with a screening checklist form created on the online platform “Google Sheets” to be filled out simultaneously, ensuring foolproof screening prior to reaching a conclusion. It was ensured that every study was screened to meet the aforementioned eligibility criteria based on the Population, Intervention, Comparator and Outcome (PICO) of the original review question. Any conflicts that arose during the screening procedure were discussed with the supervisor and resolved by mutual consensus. After the screening process was completed, the sixth co-author rechecked each study to ensure there were no mistakes.
Data extraction
For data extraction, basic characteristic details like authors, journal, year, country, study design, sample size, and population characteristics and demographics were collected from each of the included studies. Methodological details reported included intervention type and duration, sampling method, mode of randomization and allocation concealment, participant or assessor blinding, number and types of outcomes assessed, type of analysis performed, and time points of outcome measurement for each study.
Summary measures
The mean and standard deviation (SD) were used to report descriptive variables of population demographics and outcome variables across studies. P value, mean difference, and confidence intervals (CIs) were reported (where available) to illustrate comparisons in outcome variables of pain and other physiological and psychological assessments.
Meta-analysis
RevMan software from Cochrane[19] was used to perform the meta-analysis and to generate the Forest plot. Initially, we checked the data provided in the previous studies and decided to use the mean differences and standard deviations of the differences among the different intervention groups and control groups. The calculations were done according to the formulas given in the Cochrane Handbook.[20] Heterogeneity was tested using the I2 statistic, with I2 values over 50% indicating strong heterogeneity. Tau2 was used to determine how much heterogeneity was explained by sub-group differences.
Risk of bias (RoB) assessment
The risk of bias (RoB) for individual studies was assessed by investigating individual studies for randomization process, deviations from the intended interventions, missing outcome data, measurement of the outcome, and selective reporting of results. The RoB report was prepared on the above five domains using the revised RoB2 tool[21] by two co-authors, and after discussing with a senior researcher, to identify any discrepancies.
Results
Inclusion process of the selected studies
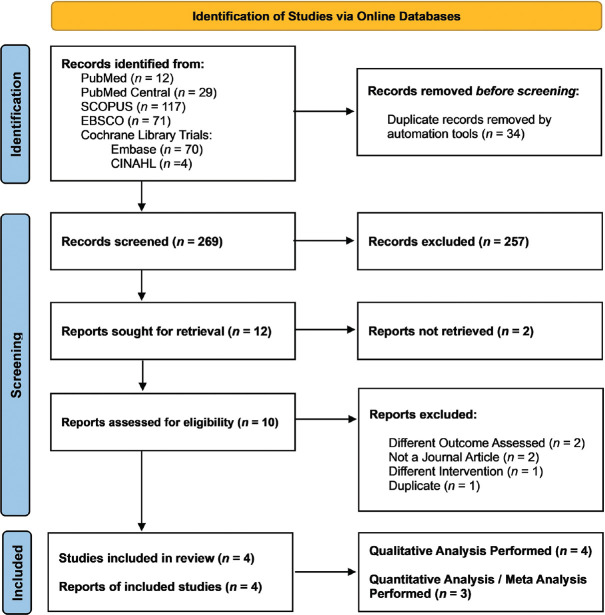
The searches conducted in the electronic databases yielded a total of 303 results. The PRISMA flowchart[22] in Figure 1 depicts the number of search results returned by each database. There were 34 duplicate studies among these articles, which were eliminated. According to inclusion and exclusion criteria, 257 studies of the 269 remaining studies were excluded during hand screening by co-authors, based on information in the titles and abstracts, and only 12 were considered for in-depth analysis. During inclusion evaluation two studies assessed outcomes other than pain,[23,24] one study was a poster presentation,[25] one was a study protocol,[26] one employed an intervention other than yoga,[27] one was a duplicate,[28] and two other studies could not be retrieved.[17,29] Consequently, eight of the twelve selected studies were excluded, leaving only four for qualitative analysis.[28,30-32] Only three of the final four studies were chosen for quantitative analysis.[30-32] One study[28] was not taken in the quantitative analysis because the outcome measures were not continuous data in contrast to the other three studies.
Figure 1.
PRISMA Flow Diagram (PRISMA - Preferred Reporting Items for Systematic Reviews and Meta-Analyses): Depicting the number of search results returned by each database
General characteristics of included studies
All four included studies utilized yogic intervention and evaluated a total of 285 participants.[28,30-32] Two studies[28,30] included populations of females with multiple sclerosis with a mean age of 31.6[28] and 30 years,[30] respectively. One study had older female participants with diabetic neuropathy having a mean age of 62.9 years.[32] Half of the participants in the fourth study had myofascial pain, and the other half had trigeminal neuralgia.[31] Table 1 shows detailed information on the characteristics of individual studies.
Table 1.
Basic Characteristics of the Included Studies
| Study (Authors, Year, and Country) | Population Characteristics | Randomization; Allocation Concealment and Blinding | Sample Size; Group Allocation and Dropouts (if any) | Intervention Types (Experimental, Control, and/or Comparator) | Intervention Duration and Follow-Up Period | Outcome Measure used for Assessing Pain | Reviewers’ Remarks |
|---|---|---|---|---|---|---|---|
| Dehkordi AH et al. (2016, Iran)[28] | Women diagnosed with multiple sclerosis using McDonald diagnostic criteria of MS. Mean age=30 years. | Systematic randomization; no information on allocation concealment; data analysts were blinded to participants’ groups. | Total enrolled (n=60): Experimental (n=30); Control (n=30). Total analyzed (n=40); Dropouts (n=20, 10 from each group). | Experimental Group: Yoga (physical practices, breathing practices, and meditation); Control Group: No intervention | Three months (60-to-70-minute sessions per week). No information given about follow-up. | Bayer’s numerical scale | There were 10 dropouts from each group, but intention-to-treat analysis was not implemented. |
| Doulatabad SN et al. (2013, Iran)[30] | Women between 18 and 45 years of age with at least 2 years of MS, and the agility to practice yoga. Mean age=31.6 years. | No information provided | Experimental (n=30); Control (n=30); Total (n=60) | Experimental group: Yoga (physical practices, breathing practices, and meditation); Control group: No intervention | Three months (eight 60-to-90-minute sessions per month) Outcomes assessed after one month. No Follow-up. | Likert scale as part of the MSQoL-54 standard questionnaires | Intervention was provided for 3 months; but the outcomes were assessed at the baseline and after one month of intervention. |
| Bhalla K et al. (2019, India)[31] | Each group of 20 participants included 10 with myofascial pain and 10 with trigeminal neuralgia. No information provided for age and gender of participants. Trigeminal neuralgia population group was included in review, and myofascial pain-related group was excluded as the mechanisms of myofascial pain are related to muscular atrophy[33] | No information provided | n=60 participants allocated to three equal groups. Control Group (A) (n=20); Experimental Group (B) (n=20); Comparator Group (C) (n=20). | Control group (A) was only given pharmacotherapy treatment for pain, Experimental group (B) was given yoga and naturopathy along with pharmacotherapy. Comparator Group (C) was given yoga and naturopathy intervention without the medication. The experimental group for this review was as group B and the comparator/control group was group A. | Three months (duration and frequency of yoga and meditation sessions were not mentioned). Regular follow-ups were conducted at every 10 days starting from the baseline. | Visual analog scale | Outcomes were assessed for all the participants during regular follow-ups, but analysis was done only for the baseline and the ninth follow-up, i.e., after 3 months of enrollment. |
| Hussain N et al. (2019, Pakistan)[32] | Elderly females diagnosed with Type 1 or Type 2 diabetes for >12 months with diabetic neuropathy and age >55 years. Mean age=62.9 years. | Computer generated randomization; no information provided for allocation concealment and blinding | Total enrolled (n=119); Dropouts (n=14); Total analyzed (n=105): Experimental (n=37); Control (n=32); Comparator (n=36). | Experimental Group: Progressive relaxation meditation; Comparator Group: Mindfulness-based Meditation; Control Group: Control meditation. Progressive Relaxation Meditation was considered as experimental intervention since mindfulness-based meditation does not meet intervention eligibility of yoga. | Two months (twice a week for 30 minutes per session). Follow-ups at every 4 weeks duration from the baseline. | BPI) modified for painful diabetic peripheral neuropathy (BPI-DPN Q4) | The intervention duration was 2 months; but outcomes were assessed at baseline and after 3 months of intervention. There were 14 dropouts, but Intention-to-treat analysis was not implemented. |
All four studies evaluated pain-related outcomes using a variety of methods, including the Likert scale as part of the MSQoL-54 standard questionnaire,[30] the VAS,[31] the brief pain inventory (BPI) modified for painful diabetic peripheral neuropathy (BPI-DPN Q4),[32] and the Bayer numerical scale.[28] Two studies allocated their 60 participants into two groups.[28,30] One study allocated 60 individuals into three equal groups,[31] whereas another study divided 105 participants into three groups.[32] Table 2 provides details on specific yogic interventions for individual studies.
Table 2.
Details of specific yogic interventions used in the included studies
| Study Authors | Details of Yoga Intervention of the experimental group |
|---|---|
| Dehkordi AH et al.[28] | Yoga practice included Hatha yoga, which has three basic components, postures (asanas), breathing techniques (pranayama), and meditation (dhyana). Each session ended with a 10-minute of deep relaxation. |
| Doulatabad SN et al.[30] | Forty minutes of slow-motion exercising, breathing exercises, mental control meditation exercise, and 10–15 minutes of corpse pose (supine rest) at the beginning and at the end. |
| Bhalla K et al.[31] | Yoga practices included breathing practices like “Surya Anulom Vilom, Surya Bhedna, Nadi Shodhana, Bhastrika” and physical postures like “Shavasan, Vajrasana, Vakrasana, Balasan and Tadasan,” and meditation. Type of meditation is not mentioned. Naturopathy practices included acupuncture, facial massage therapy, hot and cold therapy, and facial exercises. |
| Hussain N et al.[32] | Progressive relaxation meditation consisted of 5 minutes of sitting quietly, 23 minutes of progressive muscle relaxation, and 2–3 minutes of awakening. |
Risk of bias for included studies
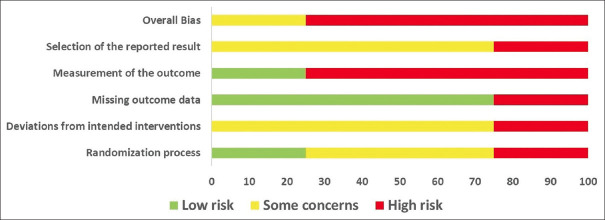
The following section reports the RoB assessments of all four studies included, elaborating on the potential biases arising due to the randomization process, deviations from the intended interventions, missing outcome data, measurement of outcome, and selective reporting of results. Figures 2 and 3 depict the RoB for individual studies.
Figure 2.
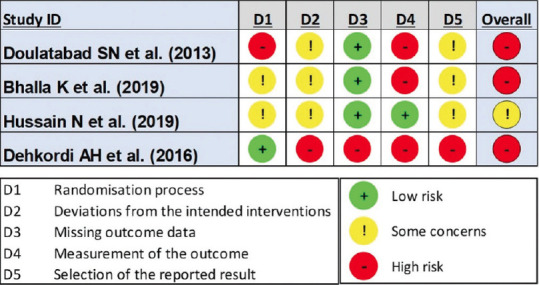
Risk of Bias Assessment for Individual Randomized Controlled Trials as per ROB-2 Tool
Figure 3.
Risk of Bias Assessment for all four Randomized Controlled Trails, Depicted as Percentage for the Domains of ROB-2 Tool
-
1.1.1.
RoB due to Randomization Process: One study[30] had a high RoB, two[31,32] had some concerns, and another[28] had a low RoB. Reasons included a lack of information on the randomization process,[30,31] allocation concealment,[28,30-32] and possible differences between groups at baseline.[30] Since the intervention was yoga, participant blinding could not have been done for any of the studies.
-
1.1.2.
RoB due to Deviations from Intended Interventions: Three studies[30-32] were evaluated and found to have some concerns raising unclear RoB due to lack of protocols, no prior information regarding the authors’ intention for the intervention, and lack of outcome data reported for the dropouts. One study[28] showed high risk as the number of participants and intervention groups varied when cross-checked with the study protocol available online.
-
1.1.3.
RoB due to Missing Outcome Data: Two studies[30,31] had no dropouts at all, while one[32] had dropouts evenly distributed across the groups, thus showing a low RoB for missing outcome data. However, the fourth study[28] did not provide any information regarding the data for dropped-out participants, thus putting it in the high-risk category.
-
1.1.4.
RoB due to Measurement of the Outcome: All four studies used validated and quantifiable measures for assessing participants’ level of pain, but they were all subjective measures. Moreover, considering that the control group was either not given any intervention[28,30] or a pharmacological drug,[31] making it highly susceptible for the outcome assessment at the participant’s end to be influenced by the knowledge of the intervention received, except for one study utilizing similar behavioral interventions,[32] making it fall under the low-risk category.
-
1.1.5.
RoB due to Selection of the Reported Results: Assessor blinding was only implemented in one study;[28] however, deviations were found in the number of intended outcomes measured, along with a lack of clarity on the analysis performed and the authors’ categorical representation for data-related outcomes, thus raising high concerns for the RoB.[28] The remaining three studies showed some concerns, such as a lack of intention-to-treat analysis where required,[32] and a lack of appropriate use of statistical tests to compare outcomes between groups.[30,31]
-
1.1.6.
Overall RoB: Based on the above assessments, it was estimated that three of the four studies showed a high RoB overall,[28,30,31] while one study showed some concerns,[32] thus making the present set of evidence low in methodological quality and susceptible to biases.
Pain response to yoga
Pain was significantly reduced (P < 0.05) in all the studies within the interventional group. Compared to the control group, pain was significantly reduced in only one of the four included studies (P < 0.05).[28] Notably, in this study,[28] the data were reported as categorical variables using counts and percentages (reported as very low, low, moderate, and severe).
Other significant findings of yoga intervention
Other significant findings (P < 0.05) as compared to baseline in yoga intervention groups included stress,[31] anxiety,[28] physiological indices (blood pressure, respiration, pulse rate, and temperature),[28] Patient Global Impression of Change (PGIC),[32] and patient satisfaction with treatment.[32] Significant improvements in comparison to the control group included fatigue intensity.[28] In one study QOL significantly changed between groups[32] while in others just within the experimental group.[30,31] In the study by Bhalla et al.,[31] all three outcome measures, namely QOL, stress, and pain demonstrated better results in the control group, where only medication was prescribed, compared to the interventional group, where medication, yoga, and naturopathy were all provided. In the study by Hussain et al.,[32] the comparator group Mindfulness - Meditation (MM) showed a more significant (P < 0.01) reduction of pain than Progressive Muscle Relaxation (PMR) (P < 0.05) when compared to the control group.
Meta-analysis
One study[28] was not taken in the quantitative analysis because the outcome measures were not continuous data in contrast to the other three studies. In two[30,31] out of three studies, the standard deviations of the difference were not provided. Hence, we chose to use the imputation method described in the Cochrane document.[20] One of the studies[32] was chosen to calculate the correlation coefficient because this study provided both the difference of means and standard deviation between pre- and post-intervention in both the experimental and control groups. The overall direction of the effect is in favor of yoga as an intervention, as compared to the control groups (overall effect: Z =1.10 [P = 0.27]).
The heterogeneity is very high (heterogeneity: τ2 = 0.37; χ2 = 27.78; df = 2 [P < 0.00001]; I² = 93%) because in one of the studies,[31] the change of mean scores in the control group (treated with carbamazepine, an established treatment for NP) is larger than that of the experimental group (given both yoga intervention and pharmacotherapy). In the other two studies,[30,32] the experimental groups have a larger effect as compared to controls. The overall effect (Z) favors yoga as an intervention over the control; however, this result is not statistically significant (overall effect: Z = 1.10 [P = 0.27]). Table 3 shows the details on statistical computations of the meta-analysis, while Figure 4 depicts the Forest plot.
Table 3.
Statistical Computations for the Meta-Analysis of Included Studies
| Study | Experimental | Control | Weight | Mean Difference | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| Mean | Standard Deviation | Total | Mean | Standard Deviation | Total | IV, Random, 95% CI | ||
| Bhalla et al. 2019 (Myofascial Pain)[31] | 0 | 0 | 0 | 0 | 0 | 0 | Not estimable | |
| Bhalla et al. 2019 (Trigeminal Neuralgia)[31] | 6.09 | 1.28 | 10 | 7.5 | 0.84 | 10 | 24.20% | −1.41 [−2.36, −0.46] |
| Doulatabad et al. 2013[30] | 1 | 0.96 | 30 | 0.1 | 0.1 | 30 | 36.50% | 0.90 [0.55, 1.25] |
| Hussain et al. 2019[32] | 0.6 | 0.1 | 32 | −0.5 | 0.2 | 37 | 39.40% | 1.10 [1.03, 1.17] |
| Total (95%) | 72 | 77 | 100% | 0.42 [−0.33, 1.17] | ||||
| Heterogeneity: τ2=0.37; χ2=27.78; df=2 (P<0.00001); I2=93% | ||||||||
| Test of overall effect: Z=1.10 (P=0.27) | ||||||||
Figure 4.
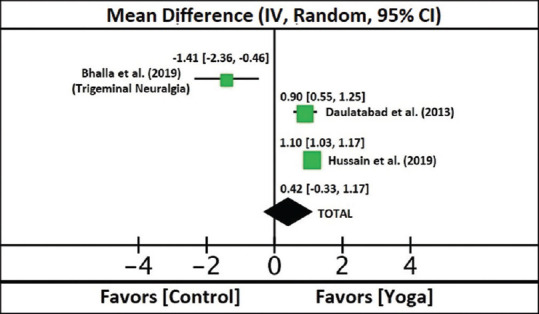
Forest Plot for the Three Estimable Randomized Controlled Trials
Discussion
The purpose of this review was to assess the evidence for the effectiveness of yoga interventions for NP. A meta-analysis combining results from all the trials divulged the overall favorable effect of yoga on NP, although the effect was not statistically significant, and great heterogeneity was observed between the trials assessed in our study. The etiology of NP among the subjects included in our study were trigeminal neuralgia, multiple sclerosis, and diabetic neuropathy. Improvement in pain intensity was observed after 3 months of yogic intervention in three studies with one or two sessions per week, each ranging from 60 to 80 minutes.[28,31,32] The studies also revealed collateral benefits of yogic interventions such as improvement in stress, anxiety, fatigue, quality of life, and patient satisfaction from therapy.[28,31,32] It is worth mentioning that patients diagnosed with multiple sclerosis reported the maximum improvement in pain intensity.
Our study retrieved only a limited number of small trials due to the paucity of available literature. Only yoga-centric studies were included in our review for conceptual clarity, although there is emerging evidence in favor of other mind–body interventions like Tai-chi, acupressure, Mindfulness based stress reduction (MBSR), etc., but further research is required in that field. Included studies lacked methodological quality leading to high RoB, mainly observed due to randomization process, selection of the reported result, and measurement of outcome. In order to reduce impediments to external validity of findings, it is suggested that future studies should carefully evaluate study’s participants’ characteristics and use a well-defined “dose” of yoga, similar to the FITT principle (frequency, intensity, time, and type of exercise) used in physical activity assessment, which is common to sports medicine research.[34] Alongside, it is equally important to standardize training for both the treatment and placebo providers, using standardized tools for outcome assessments and implementing proper randomization and allocation concealment methods to minimize bias. The studies included in our systematic review utilized different batteries to assess pain; therefore, a dose–response relationship could not be established, which is important to generalize suitable treatment to combat pain. This emphasizes the need for introduction of standardized scales for pain assessment in future studies, which can help provide further objective evidence regarding the various yoga techniques and the individual role of each in alleviating pain. Further rigorous methodological and high-quality RCTs are needed to further understand and confirm the effects of standardized yoga programs directed toward the treatment of NP. The generalizability of the findings is limited due to the number of small trials and lack of studies evaluating the role of yoga in various other forms of neuropathy. Heterogeneity between the studies also affects generalizability of results due to various aspects of yogic interventions used. For example, one study used only meditation technique while others used all three aspects of yoga (physical postures, breathing, and meditation/relaxation). Moreover, co-interventions given along with yoga, like in the case of one study[31] using naturopathy practices along with yoga, also affect generalizability. Although our study did not reveal any significant benefit from yoga in NP, it is worth noting that this outcome was based on analyses of three studies only, among which a beneficial role was divulged by two. This presents a serious challenge to generalizing yoga techniques’ impact on NP. No follow-up evaluations were done in any of the included investigations; hence, no long-term impacts could be found.
To the best of our knowledge, this is the first meta-analysis to explore the benefits of yoga on NP syndromes. Past research shows yoga’s positive impact on a range of pain outcomes in headache,[8] fibromyalgia,[9] low back pain,[10] menstrual pain,[11] and labor pain.[12] We can conclude from our study that introduction of yoga as an alternative therapeutic approach for the management of chronic NP may be a safe and possibly effective method that can be explored through well-designed RCTs. The various yoga techniques that have demonstrated to be beneficial in NP include physical postures, breathing exercises, meditation, and progressive muscle relaxation.[28,30,31] The evolution of our understanding of yoga is still in its early stages and future trials need to be devised to precisely unravel the pertinent role of this ancient art in our management of NP. Yoga needs to be explored further, not only as a complementary therapy but as a primary treatment too. It has been well documented that, in addition to physical pain, patients with chronic NP suffer from mental health problems as well. As a result, polypharmacy is rampant for nonsteroidal anti-inflammatory drugs and other medications among the masses, causing adverse events and complications like chronic kidney diseases due to chronic use. This affects the patient’s finances as well as their health, especially in countries like India that are still developing. Our review provides a strong basis for future studies and suggests that yoga exercises could prove to be a safe, cost-effective therapy for the growing public health issue of neuropathies. As feedback from these results, future studies could be better designed, keeping in mind the RoB, and can incorporate more tools and techniques for the quantification of yoga and meditation. For instances, functional neuroimaging, which is noninvasive and relatively easier to study, could be utilized.
Conclusion
Yoga, a well-accepted ancient technique of bodily healing, is a promising therapy in NP secondary to multiple sclerosis, diabetes, and trigeminal neuralgia. It may have a positive impact on pain intensity and quality of life in NP syndromes. However, further research using robust RCTs is warranted to investigate and quantify the efficacy and safety of yoga using functional neuroimaging tools for establishing yoga as a clinical therapeutic modality for NP management.
Other information
This review was registered prospectively with PROSPERO on October 14, 2022 under registration number CRD42022364381. The protocol is publicly available online[35] and no amends were made to the same after registration. This project was not funded by any grants of any kind, and the institutional ethics permission was not required for the same. The authors declare that there are no competing interests among them, and no conflict of interest exists, financial, or otherwise. The template data collection form, data extracted from included studies, data used for analyses, and the analytic code can be made available by the authors upon reasonable request.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Colloca L, Ludman T, Bouhassira D, Baron R, Dickenson AH, Yarnitsky D, et al. Neuropathic pain. Nat Rev Dis Primer. 2017;3:17002. doi: 10.1038/nrdp.2017.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O´Connor AB. Neuropathic pain:Quality-of-life impact, costs and cost effectiveness of therapy. Pharmacoeconomics. 2009;27:95–112. doi: 10.2165/00019053-200927020-00002. [DOI] [PubMed] [Google Scholar]
- 3.Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, et al. Pharmacotherapy for neuropathic pain in adults:A systematic review and meta-analysis. Lancet Neurol. 2015;14:162–73. doi: 10.1016/S1474-4422(14)70251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Langley PC, Van Litsenburg C, Cappelleri JC, Carroll D. The burden associated with neuropathic pain in Western Europe. J Med Econ. 2013;16:85–95. doi: 10.3111/13696998.2012.729548. [DOI] [PubMed] [Google Scholar]
- 5.DiStasio SA. Integrating yoga into cancer care. Clin J Oncol Nurs. 2008;12:125–30. doi: 10.1188/08.CJON.125-130. [DOI] [PubMed] [Google Scholar]
- 6.Riley D. Hatha yoga and the treatment of illness. Altern Ther Health Med. 2004;10:20–1. [PubMed] [Google Scholar]
- 7.Wren AA, Wright MA, Carson JW, Keefe FJ. Yoga for persistent pain:New findings and directions for an ancient practice. Pain. 2011;152:477–80. doi: 10.1016/j.pain.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anheyer D, Klose P, Lauche R, Saha FJ, Cramer H. Yoga for Treating Headaches:A systematic review and meta-analysis. J Gen Intern Med. 2020;35:846–54. doi: 10.1007/s11606-019-05413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bravo C, Skjaerven LH, Guitard Sein-Echaluce L, Catalan-Matamoros D. Effectiveness of movement and body awareness therapies in patients with fibromyalgia:A systematic review and meta-analysis. Eur J Phys Rehabil Med. 2019;55:646–57. doi: 10.23736/S1973-9087.19.05291-2. [DOI] [PubMed] [Google Scholar]
- 10.Wieland LS, Skoetz N, Pilkington K, Vempati R, D'Adamo CR, Berman BM. Yoga treatment for chronic non-specific low back pain. Cochrane Database Syst Rev. 2017;1:CD010671. doi: 10.1002/14651858.CD010671.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim SD. Yoga for menstrual pain in primary dysmenorrhea:A meta-analysis of randomized controlled trials. Complement Ther Clin Pract. 2019;36:94–9. doi: 10.1016/j.ctcp.2019.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Bolanthakodi C, Raghunandan C, Saili A, Mondal S, Saxena P. Prenatal Yoga:Effects on alleviation of labor pain and birth outcomes. J Altern Complement Med N Y N. 2018;24:1181–8. doi: 10.1089/acm.2018.0079. [DOI] [PubMed] [Google Scholar]
- 13.Gupta S, Gautam S, Kumar U, Arora T, Dada R. Potential role of yoga intervention in the management of chronic non-malignant pain. Evid Based Complement Alternat Med. 2022;5448671:5448671. doi: 10.1155/2022/5448671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Esch T, Winkler J, Auwärter V, Gnann H, Huber R, Schmidt S. Neurobiological aspects of mindfulness in pain autoregulation:Unexpected results from a randomized-controlled trial and possible implications for meditation research. Front Hum Neurosci. 2017;10:674. doi: 10.3389/fnhum.2016.00674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vallath N. Perspectives on Yoga inputs in the management of chronic pain. Indian J Palliat Care. 2010;16:1. doi: 10.4103/0973-1075.63127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ross A, Thomas S. The health benefits of yoga and exercise:A review of comparison studies. J Altern Complement Med. 2010;16:3–12. doi: 10.1089/acm.2009.0044. [DOI] [PubMed] [Google Scholar]
- 17.Dunne J, Chih HJ, Begley A, Daly A, Gerlach R, Schütze R, et al. A randomised controlled trial to test the feasibility of online mindfulness programs for people with multiple sclerosis. Mult Scler Relat Disord. 2021;48:102728. doi: 10.1016/j.msard.2020.102728. [DOI] [PubMed] [Google Scholar]
- 18.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan —A web and mobile app for systematic reviews. Syst Rev. 2016;5:210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Review Manager (RevMan). Version 5.4. The Cochrane Collaboration; 2020. [[Last accessed on 2023 May 07]]. Available from: https://revman.cochrane.org/
- 20.Higgins J, Green S. 16.1. 3.2 Imputing standard deviations for changes from baseline. Cochrane Handbook for Systematic Reviews of Interventions. 2011. [[Last accessed on 2023 May 07]]. Available from: https://handbook-5-1.cochrane.org/chapter_16/16_1_3_2_imputing_standard_deviations_for_changes_from_baseline.htm .
- 21.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2:A revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 22.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement:An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patil SS, Raghuram N, Singh A, Rajesh SK, Ahmed S, Hongasandra N. A prospective study on type-2 diabetic complications and efficacy of integrated yoga:A pan India 2017. Ann Neurosci. 2021;28:21–8. doi: 10.1177/09727531211016271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Razazian N, Yavari Z, Farnia V, Azizi A, Kordavani L, Bahmani DS, et al. Exercising impacts on fatigue, depression, and paresthesia in female patients with multiple sclerosis. Med Sci Sports Exerc. 2016;48:796–803. doi: 10.1249/MSS.0000000000000834. [DOI] [PubMed] [Google Scholar]
- 25.Nishant S, Madanmohan T, Das AK, Ramkumar T, Senthilkumar S. Effect of 12 Week yoga therapy as a lifestyle intervention in patients of type 2 diabetes mellitus with distal symmetric polyneuropathy. Indian Journal of Physiology and Pharmacology - Compliment Altern Med APPICON. 2011;55:64. [Google Scholar]
- 26.Cavalera C, Pagnini F, Rovaris M, Mendozzi L, Pugnetti L, Garegnani M. A telemedicine meditation intervention for people with multiple sclerosis and their caregivers:Study protocol for a randomized controlled trial. Trials. 2016;17:4. doi: 10.1186/s13063-015-1136-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meize-Grochowski R, Shuster R, Boursaw B, DuVal M, Murray-Krezan C, Schrader R, et al. Mindfulness meditation in older adults with postherpetic neuralgia:A randomized controlled pilot study. Geriatr Nurs. 2015;36:154–60. doi: 10.1016/j.gerinurse.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dehkordi AH, Jivad N, Solati K. Effects of yoga on physiological indices, Anxiety and social functioning in multiple sclerosis patients:A randomized trial. J Clin Diagnos Res. 2016;10:VC01–5. doi: 10.7860/JCDR/2016/18204.7916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yildirim P, Gultekin A. The effect of a stretch and strength-based yoga exercise program on patients with neuropathic pain due to lumbar disc herniation. Spine (Phila Pa 1976) 2022;47:711–9. doi: 10.1097/BRS.0000000000004316. [DOI] [PubMed] [Google Scholar]
- 30.Doulatabad SN, Nooreyan K, Doulatabad AN, Noubandegani ZM. The effects of pranayama, hatha and raja yoga on physical pain and the quality of life of women with multiple sclerosis. Afr J Tradit Complement Altern Med. 2013;10:49–52. [PMC free article] [PubMed] [Google Scholar]
- 31.Bhalla K, Kamarthi N, Malik SS, Goel S, Gupta S. Comparison of conventional pharmacological therapy and holistic approaches (Naturopathy and Yoga) in the management of chronic orofacial pain:A randomized controlled study. J Indian Acad Oral Med Radiol. 2019;31:29–35. [Google Scholar]
- 32.Hussain N, Said ASA. Mindfulness-based meditation versus progressive relaxation meditation:Impact on chronic pain in older female patients with diabetic neuropathy. J Evid Based Integr Med. 2019;24:2515690X19876599. doi: 10.1177/2515690X19876599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jafri MS. Mechanisms of myofascial pain. Int Sch Res Notices. 2014;2014:523924. doi: 10.1155/2014/523924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barisic A, Leatherdale ST, Kreiger N. Importance of frequency, intensity, time and type (FITT) in physical activity assessment for epidemiological research. Can J Public Health. 2011;102:174–5. doi: 10.1007/BF03404889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bhardwaj P, Pathania M, Ahuja N, Parchani A, Singh S, Sethi D. Yoga as an adjunct therapy in the management of neuropathic pain: A systematic review & meta-analysis. PROSPERO International prospective register of systematic reviews. 2022. [[Last accessed on 2023 May 07]]. CRD42022364381. Available from: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022364381 .