Abstract
Background: Influenza virus infection leads to acute pulmonary injury and acute respiratory distress syndrome (ARDS). The Radiographic Assessment of Lung Edema (RALE) score has been proposed as a reliable tool for the evaluation of the opacity of chest X-rays (CXRs). This study aimed to examine the RALE scores and outcomes in patients with influenza-associated ARDS. Methods: Patients who were newly diagnosed with influenza-associated ARDS from December 2015 to March 2016 were enrolled. Two independent reviewers scored the CXRs obtained on the day of ICU admission and on days 2 and 7 after intensive care unit (ICU) admission. Results: During the study, 47 patients had influenza-associated ARDS. Five died within 7 days of ICU admission. Of the remaining 42, non-survivors (N = 12) had higher Sequential Organ Failure Assessment scores (SOFA) at ICU admission and higher day 7 RALE scores than survivors (N = 30). The day 7 RALE score independently related to late in-hospital mortality (aOR = 1.121, 95% CI: 1.014–1.240, p = 0.025). Conclusions: The RALE score for the evaluation of opacity on CXRs is a highly reproducible tool. Moreover, RALE score on day 7 was an independent predictor of late in-hospital mortality in patients with influenza-associated ARDS.
Keywords: influenza, Radiographic Assessment of Lung Edema score, acute respiratory distress syndrome, chest X-ray
1. Introduction
Influenza, a communicable ailment, is brought on by the invasion of either the influenza A virus or influenza B virus. Additionally, there are known subtypes, influenza C virus and influenza D virus [1]. It spreads rapidly, particularly during the winter season, and it is prevalent worldwide. Nearly annual influenza epidemics result in global deaths of more than 500,000 per year [2]. The symptoms associated with being infected with the influenza virus can vary greatly, ranging from mild symptoms of upper respiratory tract infection to severe cases of pneumonia and acute respiratory distress syndrome (ARDS), which can be life-threatening. These severe outcomes may result from the influenza virus infection itself or from secondary infections caused by bacteria, other viruses, or fungi [3,4,5,6].
ARDS is a diffuse pulmonary alveolar and endothelial injury secondary to the inflammatory process. This syndrome manifests as acute hypoxemia and requires mechanical ventilation and oxygen for life support [7]. Noncardiogenic pulmonary edema is a critical characteristic of both the pathogenesis and prognosis of ARDS [8], but the current methods for quantifying the severity of pulmonary edema are invasive (e.g., right cardiac catheterization) or time consuming (e.g., computed tomographic imaging). The Radiographic Assessment of Lung Edema (RALE) score is a proposed method for evaluating the density and extent of opacities on chest radiographs in patients diagnosed with ARDS; it can be utilized to assess both the degree of pulmonary edema and the severity of ARDS [9,10,11,12,13,14]. Jabaudon et al. reported that changes in the RALE score during the first 3 days after admission to the intensive care unit (ICU) are linked to the survival rate of patients who have ARDS [10]. Nevertheless, the relationship between the RALE score at a later stage and the in-hospital mortality rate of patients with influenza-induced ARDS has yet to be established.
The objective of this retrospective study was to investigate the associations of serial RALE scores and clinical outcomes with the diagnosis of influenza-associated ARDS. It also aimed to determine the best RALE scores across different lengths of ICU stay for determining late in-hospital mortality.
2. Materials and Methods
2.1. Study Design and Patient Selection
This retrospective observational study was conducted at a 3000-bed comprehensive tertiary medical center. The Institutional Ethical Review Board of the hospital approved the study. The IRB granted a waiver for informed consent owing to the retrospective nature of the observational study. The study identified patients who received a new diagnosis of influenza-associated ARDS between December 2015 and March 2016. The following are the exclusion criteria: (1) a diagnosis of virology-proven influenza that was not confirmed by either rapid influenza diagnostic test or reverse-transcription polymerase chain reaction (RT-PCR), (2) age < 18 years, (3) death within 7 days after ICU admission, and (4) incomplete data. The study participants were segregated into two distinct groups for analysis. The first group comprised patients who were discharged alive from the hospital, also named as the survival group. The second group consisted of patients who died during hospitalization, referred to as the nonsurvival group.
2.2. Definitions of ARDS and RALE Score
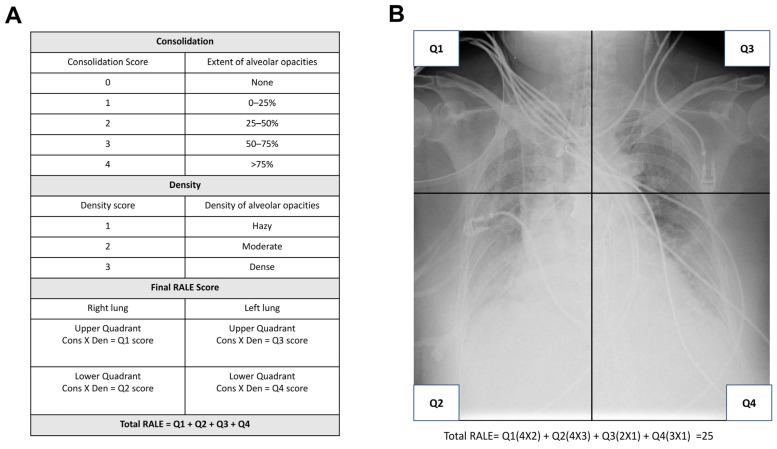
Throughout the study period, ARDS evaluation was conducted following the Berlin definition, outlined as: (1) acute onset of respiratory distress within one week, (2) the presence of opacities in bilateral lungs as confirmed by imaging studies, which cannot be entirely attributed to cardiogenic pulmonary edema, and (3) hypoxemia identified by the ratio of arterial oxygen partial pressure to the fraction of inspired oxygen (PaO2/FiO2) equal to or less than 300, when applying positive end-expiratory pressure or continuous positive airway pressure of 5 cmH2O or greater. The details of the RALE score have been described in previous publications [9,15,16] and are illustrated in Figure A1. To determine the RALE score, the radiograph’s consolidation and density scores were assessed for each quadrant (i.e., upper/lower right and upper/lower left quadrants) and were added together. The consolidation scores were determined by evaluating the degree of opacities in each quadrant and assigned scores based on the extent of involvement. This entailed assigning scores to each quadrant based on the extent of consolidation and density observed, with the following criteria: none (0 points), <25% (1 point), 25–50% (2 points), 50–75% (3 points), and >75% (4 points). Additionally, the opacity density was assessed for each quadrant and given a score of hazy (1 point), moderate (2 points), or dense (3 points). Two independent pulmonologists (HCS and CCC) scored the chest X-rays (CXRs) obtained on the day of ICU admission (day 0) and on days 2 and 7 following ICU admission.
2.3. Data Collection and Severity Evaluation
Data on CXRs, demographic characteristics, and preexisting comorbidities were extracted from both medical charts and electronic medical records. Disease severity was assessed using several scoring systems, including the Acute Physiology and Chronic Health Evaluation (APACHE) II score, Sequential Organ Failure Assessment (SOFA) score, Pneumonia Severity Index (PSI), and CURB-65 score, all of which were determined on the day of admission to ICU. The results of the laboratory tests at ICU admission, medications including osteltamivir, corticosteroid, vasopressors, and sedative agents, and the application of renal replacement therapy and extracorporeal membrane oxygenation (ECMO) support were also obtained and analyzed.
2.4. Outcome Evaluation
The present study assessed several outcomes, including the duration of ICU and hospital stays, mechanical ventilation, and all-cause mortality rate at the time of discharge. Interobserver agreement was also assessed for the RALE score calculation. All patients were monitored from admission to either death or discharge.
2.5. Statistical Analysis
The reliability of the RALE scores between independent reviewers was evaluated by computing the average-measures intraclass correlation coefficient (ICC) using a two-way random consistency model for the scores on day 0 (i.e., ICU admission day), as well as for days 2 and 7 after ICU admission. The agreement between independent reviewers was visualized using Bland–Altman plots. The Mann–Whitney U test was used to analyze continuous variables, which were presented as median values along with the interquartile range (IQR). On the other hand, categorical variables were analyzed using the chi-square test and reported as counts and percentages. We conducted a multivariate logistic regression analysis to identify independent factors related to in-hospital mortality, considering variables with a p-value less than 0.2. The resulting odds ratios (ORs) with 95% confidence intervals (CIs) were calculated. A significance level of p < 0.05 (two-tailed) was used for all statistical analyses. The software packages used for data analysis were SPSS Statistics for Windows/Macintosh version 25.0 (IBM, Armonk, NY, USA) and MedCalc version 20.215.
3. Results
3.1. Patient Characteristics
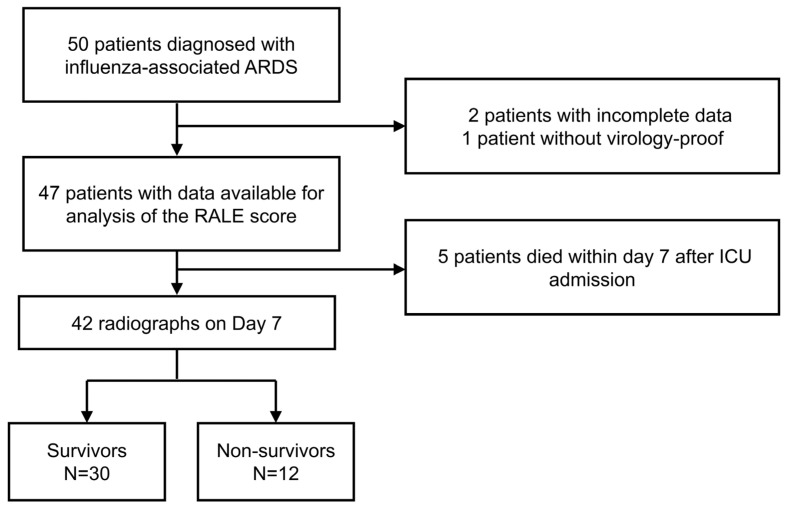
Fifty patients were admitted with the diagnosis of influenza-associated ARDS. Based on the exclusion criteria, a total of eight patients were ineligible and therefore excluded from the study (Figure 1). The 42 remaining patients with a median age of 65 years (IQR: 55–79) were enrolled. Among the 42 patients enrolled in the current study, influenza was diagnosed in three individuals via bronchoalveolar lavage, two through sputum analysis, while the remaining cases were identified using nasopharyngeal specimens. As a whole group, the durations of mechanical ventilation, ICU stay, and hospitalization were 17 (IQR: 11–31), 19 (IQR: 12–26), and 28 (IQR: 17–51) days, respectively. At ICU admission, the median SOFA and APACHE II scores were 7 (IQR: 5–8) and 23 (IQR: 16–30), respectively. During ICU admission, two (4.8%) patients received new renal replacement therapy, while 14 (33.3%) of the patients received ECMO support.
Figure 1.
Flow of patients with ARDS who were included in the RALE score analysis.
3.2. Demographic and Baseline Clinical Data
Table 1 presents the demographic characteristics and baseline clinical variables for the 42 enrolled patients. Of these patients, 30 (71.4%) survived and were subsequently discharged from the hospital. Notably, the nonsurvival group had a higher SOFA score on admission compared to the survival group (p = 0.049). However, no significant differences were observed between the two groups regarding demographics and other baseline clinical variables.
Table 1.
Demographic characteristics and baseline clinical data of the two study groups.
| Survivors (n = 30) |
Non-Survivors (n = 12) |
p Value | |
|---|---|---|---|
| Age (years) | 65 [55–80] | 65 [56–81] | 0.933 |
| Male sex | 22 (73) | 10 (83) | 0.492 |
| BMI (kg/m2) | 23 [21–27] | 24 [21–27] | 0.933 |
| Comorbidities | |||
| Diabetes mellitus | 12 (40) | 5 (42) | 0.921 |
| Cardiovascular disease | 19 (63) | 6 (50) | 0.426 |
| Chronic kidney disease | 4 (13) | 1 (8) | 0.651 |
| At ICU admission | |||
| WBC (103/mm3) | 8.4 [6.9–14.0] | 7.6 [5.1–11.5] | 0.411 |
| Albumin (g/dL) | 2.9 [2.4–3.1] | 2.6 [2.4–2.8] | 0.228 |
| CRP (mg/dL) | 21.4 [6.3–30.0] | 12.3 [8.2–15.0] | 0.344 |
| Lactate (mg/dL) | 13.8 [8.2–19.8] | 14.7 [10.6–20.4] | 0.616 |
| Creatine kinase (U/L) | 144.0 [67.0–484.5] | 186.5 [49.0–313.0] | 0.954 |
| ALT (IU/L) | 28.5 [17.4–40.3] | 42.0 [20.3–90.8] | 0.200 |
| Severity | |||
| CURB-65 | 3.0 [1.0–4.0] | 2.5 [2.0–3.8] | 0.830 |
| PSI | 114.5 [97.0–148.8] | 126.0 [124.0–144.8] | 0.140 |
| SOFA | 7.0 [4.0–8.0] | 7.5 [6.3–12.8] | 0.049 |
| APACHEII | 20.5 [15.0–29.3] | 25.5 [20.5–30.0] | 0.112 |
| RALE score | |||
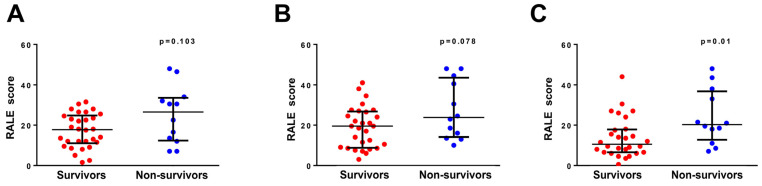
| Day 0 | 17.8 [11.0–24.8] | 26.5 [12.4–33.5] | 0.103 |
| Day 2 | 19.5 [8.8–26.8] | 23.8 [14.1–43.5] | 0.078 |
| Day 7 | 9.5 [6.3–17.8] | 20.3 [12.8–36.8] | 0.010 |
BMI, body mass index; WBC, white blood cell; CRP, C-reactive protein; ALT, alanine aminotransferase; PSI, Pneumonia Severity Index; APCHEII, Acute Physiology and Chronic Health Evaluation II; SOFA, Sequential Organ Failure Assessment score; ICU, intensive care unit; Day 0, at ICU admission. Continuous data are expressed as median with interquartile range [IQR] and are compared by Mann–Whitney U test. Categorical variables are expressed as number of patients (%) and are compared by chi-square test. A p value of <0.05 is considered statistically significant.
3.3. Interobserver Agreement for RALE Scores
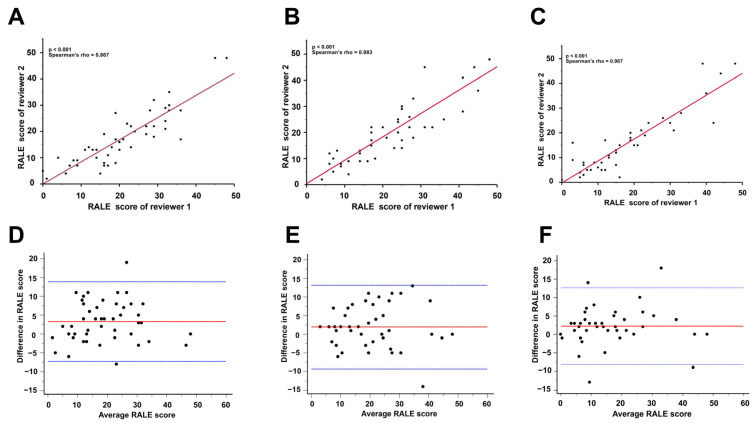
The RALE scores of the two observers were compared, and the results are shown in Figure A2. The ICCs for RALE scores were excellent at ICU admission (ICC = 0.872, 95% CI = 0.781–0.927), on day 2 (ICC = 0.884, 95% CI = 0.798–0.934), and on day 7 after ICU admission (ICC = 0.908, 95% CI = 0.834–0.950), indicating a high degree of agreement between the observers (Figure A2A–C). Bland–Altman plots also showed a strong agreement across the range of RALE scores (Figure A2D–F).
3.4. Clinical Complications and Hospital Outcomes
Table 2 compares the clinical complications and hospital outcomes between the two groups. The nonsurvival group demonstrated a higher incidence of bacteremia among the patients (p = 0.026) and vasopressor use (p = 0.014) than the patients in the survival group. However, no significant differences in the duration of invasive mechanical ventilation (p = 0.503), length of ICU stay (p = 0.062), and length of hospitalization (p = 0.944) were observed between these two groups.
Table 2.
Comparisons of clinical complications, treatment, and hospital outcomes between the two study groups.
| Survivors (n = 30) |
Non-Survivors (n = 12) |
p Value | |
|---|---|---|---|
| Complications | |||
| Bacteremia | 2 (7) | 1 (33) | 0.026 |
| Treatments | |||
| IMV | 27 (90) | 12 (100) | 0.256 |
| Corticosteroid use | 16 (53) | 8 (67) | 0.430 |
| Vasopressors use | 10 (33) | 9 (75) | 0.014 |
| Outcomes | |||
| ICU days | 18 [9–22] | 26 [16–38] | 0.062 |
| Hospitalization days | 28 [16–55] | 30 [18–48] | 0.944 |
| IMV days | 16 [11–31] | 18 [12–38] | 0.503 |
HAP, hospital acquired pneumonia; IMV, invasive mechanical ventilation; NIPPV, noninvasive positive pressure ventilators; ICU, intensive care unit. Continuous data were expressed as median with interquartile range [IQR] and were compared by Mann–Whitney U test. Categorical variables were expressed as number of patients (%) and were compared by chi-square test. A p value of <0.05 was considered statistically significant.
3.5. RALE Score and Survival
Figure 2 shows the comparisons of RALE scores on days 0, 2, and 7. No significant differences in RALE scores were observed at ICU admission (p = 0.103, Figure 2A) and on day 2 after ICU admission (p = 0.078, Figure 2B). However, compared with the survival group, the nonsurvival group had higher RALE score at day 7 after ICU admission (p = 0.01).
Figure 2.
RALE score and survival status. RALE score at ICU admission (A), on day 2 after ICU admission (B), and on day 7 after ICU admission (C) in the survival and nonsurvival groups. The nonsurvival group has a higher RALE score than the survival group on day 7 after ICU admission (p = 0.010).
3.6. Independent Predicting Factor for In-Hospital Mortality
Variables associated with in-hospital mortality (p < 0.2) were subjected to multivariate logistic regression analysis to investigate whether the RALE score is an independent predictor for in-hospital mortality. The results after adjustments for the SOFA score, APACHEII score, PSI at admission, vasopressors use, and bacteremia development revealed that the RALE scores at days 0 and 2 were not associated with in-hospital mortality (Table A1 and Table A2). However, the RALE scores at day 7 are an independent predictor for in-hospital mortality (aOR = 1.121, 95% CI: 1.014–1.240, p = 0.025, Table 3).
Table 3.
Multivariate logistic regression analyses of Day 7 RALE and other factors associated with death.
| aOR (95% CI) | p Value | |
|---|---|---|
| Initial SOFA | 1.068 (0.795–1.435) | 0.660 |
| Vasopressors | 1.606 (0.205–12.573) | 0.652 |
| Bacteremia | 9.669 (0.587–159.133) | 0.112 |
| APACHE II | 0.974 (0.831–1.143) | 0.750 |
| PSI | 1.027 (0.991–1.065) | 0.144 |
| Day 7 RALE | 1.121 (1.014–1.240) | 0.025 |
Initial SOFA: Sequential Organ Failure Assessment Score at the first day of ICU admission; APCHEII, Acute Physiology and Chronic Health Evaluation II; PSI, Pneumonia Severity Index; Day 7, at Day 7 after ICU admission; OR, odds ratio; CI, confidence interval. aOR, odds ratio after adjustment for other confounding factors. A p value of <0.05 was considered statistically significant.
4. Discussion
Influenza-associated ARDS is a complex and severe disease that requires critically supportive care in the ICU. Considering the findings of the current study, the RALE score is reproducible. Furthermore, higher RALE scores at day 7 after ICU admission were independently associated with in-hospital mortality.
The CXR was systematically scored to quantify the severity of pulmonary edema and to obtain a RALE score [9]. Our study verified the reproducibility of the RALE score among diverse patient cohorts and independent reviewers, as evidenced by the high intraclass correlation coefficient (ICC) obtained, as well as in the original study that introduced the RALE score [9]. The RALE scoring system is dependent on physician interpretation of standard chest radiographs, which enhances its practicality and ease of use as a tool for quantifying the extent of pulmonary edema. This radiographic scoring has been found to be associated with the severity and clinical outcomes of ARDS [9,10,11,12]. However, an association was not observed between the RALE scores of the baseline (at ICU admission) CXR and in-hospital mortality. It is possible that the initial radiographic evaluation of pulmonary edema may not be sufficient to capture extrapulmonary organ damage in patients with ARDS. Studies that assessed the RALE score in patients with ARDS from causes other than influenza had conflicting results in terms of the prognostic capacity of the baseline RALE score [10,11,17]. This finding supports the absence of association between the baseline RALE score and in-hospital mortality [11,17].
The RALE scores on day 7 after ICU admission were independently associated with in-hospital mortality, supporting previous findings that the dynamic change in RALE score rather than the baseline RALE has an association with morality [11,12]. This phenomenon can also be attributed to the causes of influenza-associated ARDS. For the pathogenesis of influenza-associated ARDS, influenza virus primarily targets epithelial cells, and because of viral infection, epithelial cells may produce cytokines that stimulate the recruitment of leukocytes and activation of adjacent endothelial cells. The activation of endothelial cells and infiltration of leucocytes further exacerbate inflammation, contributing to the development of ARDS. Additionally, influenza infection may occur in conjunction with or be followed by secondary bacterial infection, which can also trigger ARDS [18,19]. The RALE scores on day 7 after ICU admission may have a better ability to capture secondary bacterial pneumonia than baseline RALE score, resulting in a stronger association with in-hospital mortality.
Past experiences, such as the outbreaks of severe acute respiratory syndrome [20], middle east respiratory syndrome [21], and coronavirus disease 2019 [22], have demonstrated that clusters of viral pneumonia that manifest within a brief period can serve as a critical signal of an outbreak or pandemic. Therefore, there is a pressing need for a fast, precise, and economical method for identifying viral pneumonia. Chest X-ray (CXR) is an effective tool for the prompt detection of outbreaks caused by novel viruses [23]. The favorable findings in influenza present that the RALE score may be a potential tool for the outcome prediction of viral pneumonia.
Our study had several strengths. First, patients recruited in the current study all underwent chest X-rays on the designated study day, allowing for the calculation of the RALE score. Secondly, the RALE score was shown to be both feasible and reliable, with low variability observed between the scorers. Thirdly, pulmonologists are able to calculate the RALE score at bedside without invasive procedures, and this invaluable information can assist pulmonologists in promptly treating ARDS patients. Finally, to our knowledge, this study was the first to investigate the association of RALE score with the severity and prognosis of influenza-associated ARDS. Several limitations must be acknowledged in this study. First, this study is a single-center study with a small sample size, in which only 42 cases were included in the final analysis. Second, this study is a retrospective cohort study, which may involve some missing medical data. Third, not all patients with influenza were diagnosed via RT-PCR. Some patients were enrolled via rapid influenza diagnostic test, which may cause false-positive results. Finally, our study focused solely on patients diagnosed with influenza-induced ARDS who required ICU admission. Therefore, the generalizability of our findings to patients with less severe cases of influenza remains uncertain. For better understanding of the relationship of RALE score and influenza-associated ARDS, a well-designed prospective clinical study is necessary.
5. Conclusions
In conclusion, the RALE score offers a reliable means of evaluating the degree of radiographic edema and is easily implemented in clinical practice. The results show that the RALE score of baseline CXR was not associated with in-hospital mortality. However, the RALE scores on day 7 after ICU admission were an independent predictor of in-hospital mortality.
Acknowledgments
The authors thank all the clinical and nursing staff who took care of the patients with influenza in Taipei Veterans General Hospital.
Appendix A
Figure A1.
(A) Consolidation and density scoring in the RALE score. (B) Calculation of the RALE score of the illustrative CXR. Firstly, the CXR is divided into four quadrants defined by drawing a horizontal line from the first branch of the left main bronchus with the spinal column separating the left and right lungs. The consolidation extent and density of each quadrant were scored separately and multiplied for each quadrant, and the total RALE score was obtained through the summation of all quadrant scores (0–48).
Figure A2.
Agreement between two independent reviewers for RALE score. Scatter plots (A–C) and Bland–Altmann plots (D–F) showing the agreement between two independent reviewers for the RALE scores at ICU admission (n = 47, (A,D)), on day 2 after ICU admission (n = 45, (B,E)), and on day 7 after ICU admission (n = 41, (C,F)). Intraclass correlation coefficients (ICCs) were calculated to assess the agreement between the two reviewers. In scatter plots (A–C), the red lines mean regression lines. In Bland–Altmann plots (D–F), the red horizontal lines represent the mean difference, and the blue dotted lines represent the limits of agreement (the mean difference ± 1.96 standard deviation of differences). ICC was excellent at ICU admission (ICC = 0.872, 95% CI = 0.781–0.927, p < 0.001), on day 2 after ICU admission (ICC = 0.884, 95% CI = 0.798–0.934, p < 0.001), and on day 7 after ICU admission (ICC = 0.908, 95% CI = 0.834–0.950, p < 0.001).
Appendix B
Table A1.
Multivariate logistic regression analyses of Day 0 RALE and other factors associated with death.
| aOR (95% CI) | p Value | |
|---|---|---|
| Initial SOFA | 1.082 (0.822–1.423) | 0.576 |
| Vasopressors | 3.004 (0.465–19.424) | 0.248 |
| Bacteremia | 2.771 (0.287–26.786) | 0.379 |
| APACHE II | 1.010 (0.865–1.178) | 0.903 |
| PSI | 1.009 (0.980–1.038) | 0.543 |
| Day 0 RALE | 1.045 (0.954–1.145) | 0.340 |
Initial SOFA: Sequential Organ Failure Assessment Score at the first day of ICU admission; APCHEII, Acute Physiology and Chronic Health Evaluation II; PSI, Pneumonia Severity Index; Day 0, at the day of ICU admission; OR, odds ratio; CI, confidence interval. aOR, odds ratio after adjustment for other confounding factors. A p value of <0.05 was considered statistically significant.
Table A2.
Multivariate logistic regression analyses of Day 2 RALE and other factors associated with death.
| aOR (95% CI) | p Value | |
|---|---|---|
| Initial SOFA | 1.090 (0.833–1.427) | 0.529 |
| Vasopressors | 2.218 (0.261–18.863) | 0.466 |
| Bacteremia | 3.249 (0.303–34.886) | 0.330 |
| APACHE II | 0.998 (0.850–1.173) | 0.985 |
| PSI | 1.012 (0.982–1.044) | 0.432 |
| Day 2 RALE | 1.043 (0.955–1.139) | 0.352 |
Initial SOFA: Sequential Organ Failure Assessment Score at the first day of ICU admission; APCHEII, Acute Physiology and Chronic Health Evaluation II; PSI, Pneumonia Severity Index; Day 2, at the Day 2 after ICU admission; OR, odds ratio; CI, confidence interval. aOR, odds ratio after adjustment for other confounding factors. A p value of <0.05 was considered statistically significant.
Author Contributions
Conceptualization: H.-C.S., C.-C.C., W.-K.Y. and K.-Y.Y.; Data curation: H.-C.S., C.-C.C. and W.-C.C.; Formal analysis: H.-C.S., W.-K.Y. and K.-Y.Y.; Methodology: H.-C.S., W.-K.Y. and K.-Y.Y.; Project administration: W.-K.Y. and K.-Y.Y.; Supervision: W.-K.Y., K.-Y.Y. and Y.-M.C.; Writing of the original draft: H.-C.S. and W.-K.Y.; Writing, review, and editing: H.-C.S., W.-K.Y. and K.-Y.Y. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This retrospective study was performed in accordance with the Declaration of Helsinki and approved by the Institutional Ethical Review Board of Taipei Veterans General Hospital (No. 2021-07-028BC).
Informed Consent Statement
Informed consent was waived by the IRB due to the retrospective nature of the study.
Data Availability Statement
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by grants from the National Science and Technology Council (Taiwan, MOST 109-2314-B-010-051-MY3, NSTC 112-2314-B-A49-040, K.-Y.Y.; 112-2314-B-075-022, W.-K.Y.; 112-2314-B-075-050, W.-C.C.), Taipei Veterans General Hospital—National Yang-Ming University Excellent Scientist Cultivation Program (107-V-A-001, W.-K.Y.), and Taipei Veterans General Hospital (V112C-068 and V112D65-003-MY2-1, K.-Y.Y.; V111A-012, H.-C.S.; V112B-031 and V111B-024, W.-C.C.; V111C-043 and V112C-193, W.-K.Y.). This work was also supported by grants from the Ministry of Education, Higher Education SPROUT Project for Cancer Progression Research Center (111W31101) and the Cancer and Immunology Research Center (112W31101).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Krammer F., Smith G.J.D., Fouchier R.A.M., Peiris M. Influenza. Nat. Rev. Dis. Primers. 2018;4:3. doi: 10.1038/s41572-018-0002-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fiers W., De Filette M., Birkett A., Neirynck S., Jou W.M. A “universal” human influenza A vaccine. Virus Res. 2004;103:173–176. doi: 10.1016/j.virusres.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 3.Brundage J.F., Shanks G.D. Deaths from bacterial pneumonia during 1918-19 influenza pandemic. Emerg. Infect. Dis. 2008;14:1193–1199. doi: 10.3201/eid1408.071313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramsey C., Kumar A. H1N1: Viral pneumonia as a cause of acute respiratory distress syndrome. Curr. Opin. Crit. Care. 2011;17:64–71. doi: 10.1097/MCC.0b013e3283427259. [DOI] [PubMed] [Google Scholar]
- 5.Salazar F., Bignell E., Brown G.D., Cook P.C., Warris A. Pathogenesis of respiratory viral and fungal coinfections. Clin. Microbiol. Rev. 2022;35:e0009421. doi: 10.1128/CMR.00094-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goka E., Vallely P., Mutton K., Klapper P. Influenza A viruses dual and multiple infections with other respiratory viruses and risk of hospitalisation and mortality. Influenza Other Respir. Viruses. 2013;7:1079–1087. doi: 10.1111/irv.12020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.ARDS Definition Task Force. Ranieri V.M., Rubenfeld G.D., Thompson B.T., Ferguson N.D., Caldwell E., Fan E., Camporota L., Slutsky A.S. Acute respiratory distress syndrome: The Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 8.Matthay M.A., Ware L.B., Zimmerman G.A. The acute respiratory distress syndrome. J. Clin. Investig. 2012;122:2731–2740. doi: 10.1172/JCI60331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Warren M.A., Zhao Z., Koyama T., Bastarache J.A., Shaver C.M., Semler M.W., Rice T.W., Matthay M.A., Calfee C.S., Ware L.B. Severity scoring of lung oedema on the chest radiograph is associated with clinical outcomes in ARDS. Thorax. 2018;73:840–846. doi: 10.1136/thoraxjnl-2017-211280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jabaudon M., Audard J., Pereira B., Jaber S., Lefrant J.Y., Blondonnet R., Godet T., Futier E., Lambert C., Bazin J.E., et al. Early changes over time in the radiographic assessment of lung edema score are associated with survival in ARDS. Chest. 2020;158:2394–2403. doi: 10.1016/j.chest.2020.06.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kotok D., Yang L., Evankovich J.W., Bain W., Dunlap D.G., Shah F., Zhang Y., Manatakis D.V., Benos P.V., Barbash I.J., et al. The evolution of radiographic edema in ARDS and its association with clinical outcomes: A prospective cohort study in adult patients. J. Crit. Care. 2020;56:222–228. doi: 10.1016/j.jcrc.2020.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Todur P., Srikant N., Prakash P. Correlation of oxygenation and radiographic assessment of lung edema (RALE) score to lung ultrasound score (LUS) in acute respiratory distress syndrome (ARDS) patients in the intensive care unit. Can. J. Respir. Ther. 2021;57:53–59. doi: 10.29390/cjrt-2020-063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Yousif N., Komanduri S., Qurashi H., Korzhuk A., Lawal H.O., Abourizk N., Schaefer C., Mitchell K.J., Dietz C.M., Hughes E.K., et al. Radiographic assessment of lung edema (RALE) scores are highly reproducible and prognostic of clinical outcomes for inpatients with COVID-19. medRxiv. 2022 doi: 10.1101/2022.06.10.22276249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taniguchi H., Ohya A., Yamagata H., Iwashita M., Abe T., Takeuchi I. Prolonged mechanical ventilation in patients with severe COVID-19 is associated with serial modified-lung ultrasound scores: A single-centre cohort study. PLoS ONE. 2022;17:e0271391. doi: 10.1371/journal.pone.0271391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zimatore C., Pisani L., Lippolis V., Warren M.A., Calfee C.S., Ware L.B., Algera A.G., Smit M.R., Grasso S., Schultz M.J. Accuracy of the radiographic assessment of lung edema score for the siagnosis of ARDS. Front. Physiol. 2021;12:672823. doi: 10.3389/fphys.2021.672823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Voigt I., Mighali M., Manda D., Aurich P., Bruder O. Radiographic assessment of lung edema (RALE) score is associated with clinical outcomes in patients with refractory cardiogenic shock and refractory cardiac arrest after percutaneous implantation of extracorporeal life support. Intern. Emerg. Med. 2022;17:1463–1470. doi: 10.1007/s11739-022-02937-7. [DOI] [PubMed] [Google Scholar]
- 17.Valk C.M.A., Zimatore C., Mazzinari G., Pierrakos C., Sivakorn C., Dechsanga J., Grasso S., Beenen L., Bos L.D.J., Paulus F., et al. The prognostic capacity of the radiographic assessment for lung edema score in patients with COVID-19 acute respiratory distress syndrome-An international multicenter observational study. Front. Med. 2021;8:772056. doi: 10.3389/fmed.2021.772056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Short K.R., Kroeze E., Fouchier R.A.M., Kuiken T. Pathogenesis of influenza-induced acute respiratory distress syndrome. Lancet Infect. Dis. 2014;14:57–69. doi: 10.1016/S1473-3099(13)70286-X. [DOI] [PubMed] [Google Scholar]
- 19.Kalil A.C., Thomas P.G. Influenza virus-related critical illness: Pathophysiology and epidemiology. Crit. Care. 2019;23:258. doi: 10.1186/s13054-019-2539-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vijayanand P., Wilkins E., Woodhead M. Severe acute respiratory syndrome (SARS): A review. Clin. Med. 2004;4:152–160. doi: 10.7861/clinmedicine.4-2-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arabi Y.M., Balkhy H.H., Hayden F.G., Bouchama A., Luke T., Baillie J.K., Al-Omari A., Hajeer A.H., Senga M., Denison M.R., et al. Middle East Respiratory Syndrome. N. Engl. J. Med. 2017;376:584–594. doi: 10.1056/NEJMsr1408795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen H., Guo J., Wang C., Luo F., Yu X., Zhang W., Li J., Zhao D., Xu D., Gong Q., et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: A retrospective review of medical records. Lancet. 2020;395:809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang J., Xie Y., Pang G., Liao Z., Verjans J., Li W., Sun Z., He J., Li Y., Shen C., et al. Viral Pneumonia Screening on Chest X-rays Using Confidence-Aware Anomaly Detection. IEEE Trans. Med. Imaging. 2021;40:879–890. doi: 10.1109/TMI.2020.3040950. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.