Abstract
Despite advances in the treatment and mitigation of critical illness caused by infection with SARS-CoV-2, millions of survivors have a devastating, post-acute infection syndrome known as long COVID. A large proportion of patients with long COVID have nervous system dysfunction, which is also seen in the distinct but overlapping condition of post-intensive care syndrome (PICS), putting survivors of COVID-19-related critical illness at high risk of long-lasting morbidity affecting multiple organ systems and, as a result, engendering measurable deficits in quality of life and productivity. In this Series paper, we discuss neurological, cognitive, and psychiatric sequelae in patients who have survived critical illness due to COVID-19. We review current knowledge of the epidemiology and pathophysiology of persistent neuropsychological impairments, and outline potential preventive strategies based on safe, evidence-based approaches to the management of pain, agitation, delirium, anticoagulation, and ventilator weaning during critical illness. We highlight priorities for current and future research, including possible therapeutic approaches, and offer considerations for health services to address the escalating health burden of long COVID.
Introduction
Since the emergence of SARS-CoV-2 in late 2019, there have been more than 750 million reported cases of COVID-19 worldwide, and nearly 7 million deaths.1 In the USA alone, there have been more than 100 million cases and more than 1 million deaths.1 The development of novel vaccines and effective therapeutic approaches have helped to reduce mortality in well-resourced countries.2 However, despite these advances, millions of patients who survive acute disease have a devastating range of post-acute sequelae of SARS-CoV-2 infection, known as long COVID. Studies in the USA and Europe have shown that more than two-thirds of patients admitted to hospital with COVID-19 report incomplete recovery months after their index hospitalisation, with evidence of new or newly recognised impairments that affect quality of life and employment, which can result in financial difficulties for patients and their families.3–5
The most frequently reported symptoms of long COVID fall into two major categories: cardiopulmonary (eg, dyspnoea, cough, atypical chest pain, and autonomic instability) or neuropsychological (including neurological, cognitive, and psychiatric sequelae such as memory loss, executive dysfunction, depression, anxiety, severe fatigue, and sleep disruption, referred to here as neuro-long COVID or neuro-LC).6 Cardiopulmonary sequelae are easy to conceptualise for clinicians because of the obvious mechanistic links to acute pulmonary infection by SARS-CoV-2. By contrast, mechanisms by which acute infection might lead to diverse neuropsychological sequelae are less intuitive. Additionally, these neuropsychological sequelae are highly variable, have been reported in severity that does not always match the seriousness of acute disease, and are difficult to observe objectively. As a result, despite overwhelming scientific data to support long COVID as an organic post-acute infection syndrome7,8 and similarities to the growing list of other syndromes that occur after acute infection—such as myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and long Lyme (also known as chronic Lyme disease or post-treatment Lyme disease syndrome)—patients have struggled to find recognition of their persistent neuropsychological injury by the medical profession.9 The same is true for intensive care unit (ICU) survivors of critical illness who have post-intensive care syndrome (PICS), an often debilitating collection of symptoms including physical weakness, cognitive dysfunction, and psychological distress that can persist for months or years after critical illness.10,11 Intensivists, pulmonologists, and other ICU caregivers need to be aware of the combined risks of long COVID (including neuro-LC) and PICS that put survivors of severe COVID-19 in a place of double jeopardy as they attempt to navigate survival despite often not meeting diagnostic criteria for a specific disease. Strategies for mitigation should start with treatment during the acute phase of SARS-CoV-2 infection. Furthermore, as part of efforts to reduce the burden of neuro-LC, critical care personnel in the ICU need to anticipate long-term outcomes for patients, and providers in post-ICU clinics need to recognise and address both neuro-LC and PICS. An improved understanding of the pathophysiological mechanisms underlying neuro-LC will be key to the development of preventive and therapeutic strategies.
This is the second paper in a Lancet Respiratory Medicine Series about the post-acute sequelae of COVID-19. The first paper12 in the Series focuses on respiratory sequelae of COVID-19, addressing lung injury and the origins of breathlessness in hospitalised and non-hospitalised patients with COVID-19, and outlining considerations for the long-term care of patients. The third paper13 focuses on the burden of multisystem sequelae of COVID-related critical illness, describing effects on multiple organ systems and potential pathophysiological mechanisms. We provide an overview of what is known about the clinical features, causes, and underlying mechanisms of neurological, cognitive, and psychiatric sequelae of COVID-19-related critical illness, including sequelae recognised as components of PICS. We review recommendations for optimum acute care (with a view to limiting long-term deleterious health effects), consider pathways of long-term care and recovery, and highlight priorities for research.
Epidemiology of long COVID
To support an informed understanding of the impacts, potential mechanisms, and optimum management of neuro-LC, we begin with a brief overview of long COVID, the parent condition under which neuro-LC occurs. Clinicians and researchers have yet to reach consensus on a standard definition of long COVID, although efforts are underway to effectively classify the disorder on the basis of symptom clusters distinct from those in individuals who were never infected with SARS-CoV-2.14 Whereas some current definitions focus on symptoms that persist after initial infection, others incorporate new symptoms that can develop after the original illness and persist for a prolonged period. Table 1 compares the recent definitions of long COVID from the US Centers for Disease Control and Prevention (CDC),15,16 the World Health Organization (WHO),17 and the UK National Institute for Health and Care Excellence (NICE).18 The absence of a standardised or pathophysiological definition of long COVID syndrome and highly variable investigations based on patient self-reporting, often without control groups, have resulted in substantial variability in epidemiological reports. In the USA, estimates suggest that more than 10–15 million adults have long COVID,19 representing 6·2% of the total number of SARS-CoV-2 infections in 2020 and 2021. Davis and colleagues7 weighted the evidence and conservatively settled on an estimate of 10% of infected individuals, which is probably an underestimate given the number of unreported cases. Under this parent condition of long COVID, the proportion of people with neuro-LC is not well established but this remains a common and disabling manifestation of the syndrome.
Table 1:
Definitions of long COVID
| US Centers for Disease Control and Prevention (CDC)15,16 | World Health Organization (WHO)17 | UK National Institute for Health and Care Excellence (NICE)18 | |
|---|---|---|---|
|
| |||
| Primary terms used | Long COVID | Post COVID-19 condition | Ongoing symptomatic COVID-19 or post-COVID-19 syndrome |
| Current definition | Signs, symptoms, and conditions that last 4 weeks or more after acute infection | New-onset or persistent symptoms in individuals with a history of probable or confirmed SARS-CoV-2 infection, usually 3 months after the onset of COVID-19, that last for at least 2 months and cannot be explained by an alternative diagnosis; no minimum number of symptoms is required for the diagnosis; a different definition might be applicable for children | Ongoing symptomatic COVID-19 defined as signs and symptoms that last 4–12 weeks after the onset of acute symptoms; post-COVID-19 syndrome defined as signs and symptoms that develop during or after an infection consistent with COVID-19, continue for more than 3 months, and cannot be explained by an alternative diagnosis |
| Plans to review and revise | Ongoing research initiatives (eg, the NIH RECOVER Initiative) are designed to clarify the clinical spectrum, phenotypes, and duration of long COVID; the CDC National Health Interview Survey 2022 and 2023 contains questions on ME/CFS, a closely related syndrome, and will examine the connections among ME/CFS, long COVID, and other chronic conditions; NASEM has an active committee that is examining the working definitions of long COVID | The definition is expected to change as new evidence emerges and knowledge of the sequelae of COVID-19 continues to evolve | Recommendations for future research include a focus on the clinical course of post-COVID-19 syndrome and the presentation of post-COVID-19 syndrome in children, young people, pregnant women, and older people |
ME/CFS=myalgic encephalomyelitis/chronic fatigue syndrome. NASEM=National Academies of Sciences, Engineering, and Medicine. NIH=National Institutes of Health. RECOVER=Researching COVID to Enhance Recovery.
Neurological, cognitive, and psychiatric sequelae
Persistent neurological deficits that last for months or years after acute COVID-19 have been reported since the early stages of the pandemic (table 2; figure 1).20–38 As shown in a 2021 survey of more than 3700 people who had confirmed or suspected COVID-19,39 1700 respondents (45·2%) required a reduced work schedule after SARS-CoV-2 infection, and an additional 839 (22·3%) were not working at the time of the survey due to illness, attesting to the potential societal impact of cognitive and physical impairments. Many patients with neurological symptoms meet the diagnostic criteria for or have symptoms consistent with ME/CFS.40,41 ME/CFS is a chronic, multisystem disease affecting about 20 million people worldwide that manifests as chronic fatigue, post-exertional malaise, and cognitive impairment.42 Standard diagnostic tests typically return normal results and affected people are often undiagnosed or misdiagnosed with other conditions, such as depression. In many cases, ME/CFS worsens with physical or mental activity, including cognitive behavioural therapy and graded exercise therapy.42 Estimates of the economic impact of COVID-19 indicated that if the societal burden of ME/CFS is extrapolated to the nearly 86 million documented survivors of COVID-19 in the USA (as of June, 2022) and considering a range of 5–20% for those survivors with long COVID, the annual medical costs could be tens of billions of dollars, and lost income and annual economic impact (excluding costs of disability services, social services, and lost caregiver income) in the order of hundreds of billions of dollars.40,41 The public health burden of long COVID is estimated to be the largest seen from an emerging disease in the past 100 years.43
Table 2:
Neurological, cognitive, and psychiatric complications and related symptoms after SARS-CoV-2 infection
| Symptoms | Examples of related diagnoses | Possible mechanisms | |
|---|---|---|---|
|
| |||
| Impaired memory and cognition | Brain fog, trouble concentrating, word-finding difficulty | Alzheimer’s disease, mild cognitive impairment, COVID-19-associated encephalitis | Global brain atrophy20 and atrophy of structures related to memory and cognition (eg, parahippocampal gyrus); hippocampal hypometabolism;21 autoimmune encephalitis22 |
| Disorders of equilibrium | Dizziness, imbalance | Vertigo, orthostasis | Cerebellar atrophy and hypometabolism;21 secondary cardiopulmonary insufficiency (eg, due to decreased respiratory efficiency and increased metabolic requirement) |
| Constitutional disorders | Fatigue, headache | Chronic fatigue syndrome, migraine, tension-type headache | Residual systemic inflammatory state23 leading to increased tissue energy consumption; impaired tissue oxygenation (eg, due to functional anaemia, small vessel endothelial dysfunction impairing capillary blood flow, or impaired trans-alveolar O2 transport); inadequate sleep efficiency; impaired CO2 clearance and mild metabolic acidosis; increased intracranial pressure; subclinical meningeal inflammation; dysregulated pain receptor physiology |
| Disorders of the peripheral nerves or neuromuscular junction | Limb weakness, paraesthesia, facial palsy, difficulty swallowing | Guillain-Barré syndrome, peripheral neuropathy, dysautonomia, Bell’s palsy | Axonal degeneration, multifocal demyelination, or small fibre neuropathy24 |
| Seizures | Brief, paroxysmal alterations in behaviour, staring spells with loss of awareness, convulsions | Focal onset epilepsy, multisystem inflammatory syndrome in children, encephalitis | Post-infection inflammatory cascade affecting focal brain areas25 (eg, mesial temporal regions) |
| Mental health disorders | Mood changes (depression, anxiety), hallucinations, delusions, catatonia | Major depressive disorder, stress and adjustment disorders, anxiety disorders, psychotic disorders | Modulation of affective information processing via alterations in olfactory cortical firing;26 structural changes in brain regions responsible for emotional processing (eg, anterior cingulate cortex, orbitofrontal cortex, ventral striatum, amygdala);20 decreased psychological resilience (premorbid or context-specific)27 |
| Sensory disorders | Altered smell and taste, hearing abnormalities, or vision changes | Meniere’s disease, anosmia, dysgeusia, tinnitus | Prolonged chemokine induction, microglial activation, and suppression of neuronal transcriptome in olfactory bulb and olfactory epithelium;28 dysfunctional sensory integration due to loss of function in parietal association cortex;20 direct viral invasion of inner ear29 |
| Extrapyramidal and movement disorders | Abnormal involuntary movements, tremor, bradykinesia, dystonia, myoclonus | Parkinsonism, chorea, dystonia | Inflammatory cytokines, microglial activation or molecular mimicry leading to structural or functional damage to the basal ganglia30,31 |
| Cerebrovascular disorders | Sudden-onset weakness, sensory changes, or vision deficits | Ischaemic stroke, transient ischaemic attack, haemorrhagic stroke, cerebral venous thrombosis | Hypercoagulable state;32 microvascular endothelial dysfunction33 |
| Myelopathies | Paraplegia, incontinence, pain | Transverse myelitis, acute disseminated encephalomyelitis, spinal epidural abscess, spinal cord ischaemia | Autoimmune activation due to molecular mimicry;34 coagulopathy, arterial thrombosis, or abscess formation35 |
Composite outcomes are shown with symptoms, related diagnoses, and possible underlying mechanisms.
Figure 1: Potential mechanistic pathways and therapeutic interventions for neuro-LC.
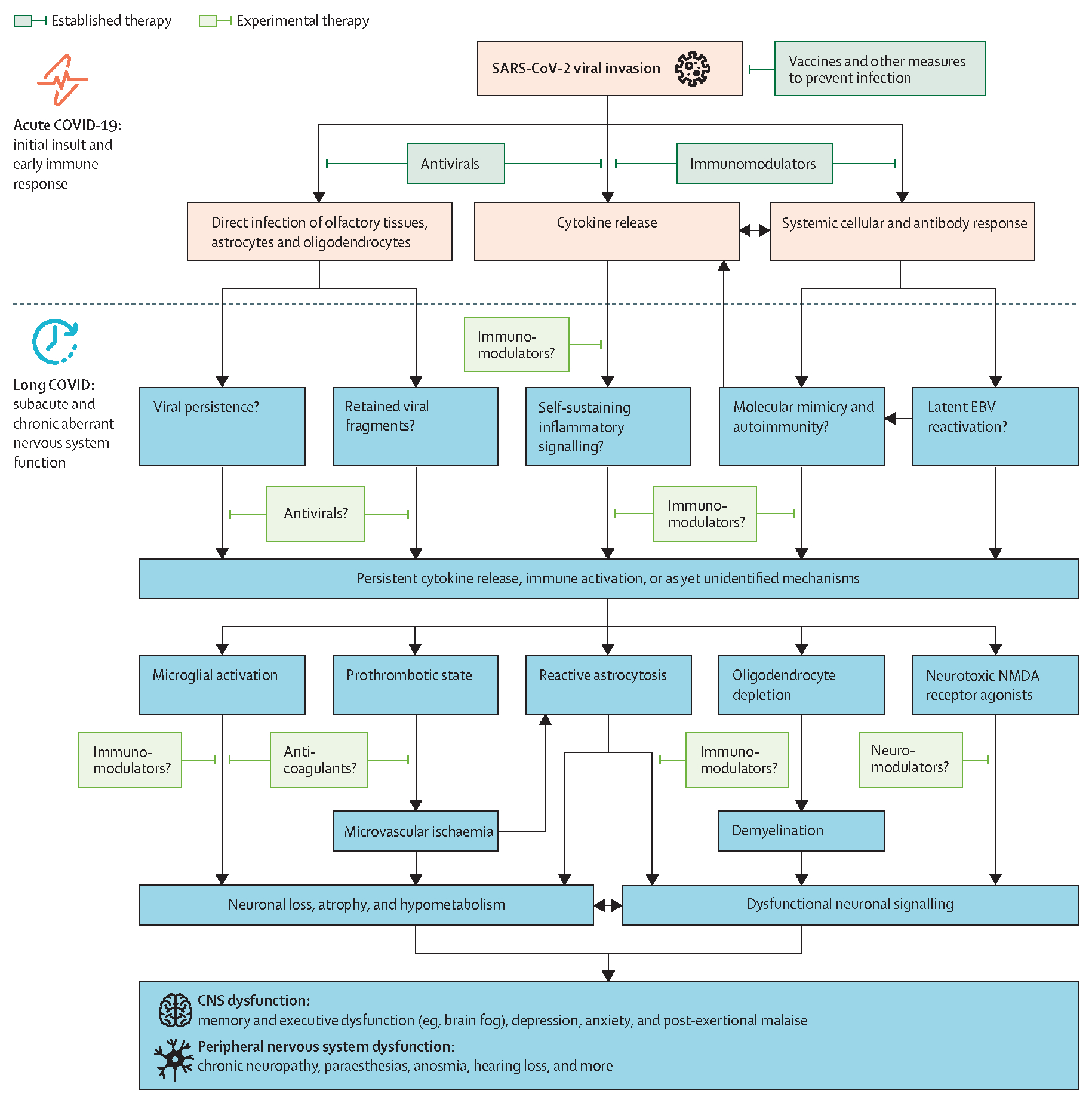
An informed approach to mitigating and reversing neuro-LC symptoms will require a detailed understanding of its pathogenesis. The diagram summarises current understanding of potential mechanistic pathways of neuro-LC; many of the pathobiological features overlap with those of the post-intensive care syndrome. Dark green rectangles indicate therapies shown to be effective for acute COVID-19. Lighter green rectangles indicate novel therapeutics that are being studied to prevent or ameliorate neuro-LC. Persistent systemic and neuroinflammatory processes extending beyond the acute infectious stage are a unifying pathway for neuro-LC, although additional, unidentified mechanisms probably exist. Work in other neurological diseases such as stroke, multiple sclerosis, and Guillain-Barré syndrome indicates that this inflammatory cascade, if left unchecked, can lead to neuronal loss, grey and white matter atrophy, hypometabolism, and associated dysfunctional neuronal signalling in the CNS and peripheral nervous system. EBV=Epstein-Barr virus. Neuro-LC=neurological, cognitive, and psychiatric sequelae of COVID-19 (neuro-long COVID). NMDA=N-methyl-D-aspartate.
Neuro-LC in survivors of COVID-19 has been reported up to 1 year after acute illness. In one of the largest studies to date, Xu and colleagues44 used the national health-care databases of the US Department of Veterans Affairs to build a cohort of about 150 000 individuals with COVID-19 and estimated the risk of incident neurological disorders in this cohort compared with more than 5 million contemporary and historical uninfected controls, in the 12 months after an acute infection. They obtained demographic data, outpatient-encounter and inpatient-encounter data (including diagnosis and procedures), and inpatient and outpatient medications. Outcomes were based on International Classification of Diseases 10th Revision (ICD-10) codes and prescription medications. Most composite outcomes reported in patients 1 year after a positive test for SARS-CoV-2 were significantly higher than those in uninfected controls, with about a 40% higher risk of having some manifestation of neuro-LC (hazard ratio [HR] 1·43, 95% CI 1·38–1·47).44 These included cerebrovascular disorders, which contributed to higher occurrence of ischaemic and haemorrhagic stroke and transient ischaemic attack, and diagnoses of neurodegenerative disease such as Alzheimer’s disease, Parkinson’s-like disease, and peripheral nervous system changes. The risks were evident in all subgroups, which were based on age, race, sex, smoking status, and the presence or absence of obesity, diabetes, chronic kidney disease, hyperlipidaemia, hypertension, or immune dysfunction. Given the relatively homogeneous population of predominantly White males in this study, the relative risk of neuro-LC in those at greater risk of Alzheimer’s disease (including women and African Americans) or of other related neurodegenerative disorders deserves further study.
Using the same US Department of Veterans Affairs database, Xie and colleagues45 evaluated mental health outcomes in patients with COVID-19 and found an increased risk of incident anxiety disorders (HR 1·35, 95% CI 1·30–1·39), depressive disorders (1·39, 1·34–1·43), and stress and adjustment disorders (1·38, 1·34–1·43) at 30 days after COVID-19 compared with contemporary uninfected controls.45 There was a greater than 50% increased risk of the use of prescription antidepressants, benzodiazepines, and opioids, with a 25–30% increased risk of opioid and other substance use disorders.45
To our knowledge, no studies have reported the natural history of neuro-LC symptoms specific to ICU survivors. In the prospective long COVID ComPaRe cohort (La Communauté de Patients pour la Recherche), of 968 patients with ongoing symptoms 2 months after confirmed COVID-19, the probability of persistent long COVID symptoms 1 year after symptom onset was 84·9% (95% CI 79·8–90·4).46 Some symptoms (eg, paraesthesia) increased in prevalence over time, while others (eg, loss of smell) decreased in prevalence, and still others, (eg, word-finding difficulty) did not substantially change over time. Similar pattern was reported in another survey-based cohort: the prevalence of brain fog (a term used to describe symptoms of confusion and difficulty thinking clearly or concentrating) and memory issues was relatively stable over a period of 28 weeks, whereas imbalance and olfactory or gustatory dysfunction decreased in prevalence over the same period.39
Overall, these striking increases in morbidity risks are motivating further work to understand underlying mechanisms and establish optimum management of neuro-LC, which we explore in the following sections.
Pathophysiological models of neuro-LC
Early autopsy studies of critically ill patients with COVID-19 did not show significant viral invasion of neurons,47,48 leading to the initial belief that there was little involvement of the brain in SARS-CoV-2 infection. However, studies did indicate that patients had pronounced inflammatory changes and microglial activation.49,50 Furthermore, systemic inflammation has been associated specifically with cognitive impairment in patients with neuro-LC in cohort studies such as the Post-hospitalisation COVID-19 study (PHOSP-COVID).51 Although the virus does not appear to persist in neurons, SARS-CoV-2 readily infects and activates astrocytes and microglial cells.52 Glial cell activation is the hallmark of neuroinflammation and can result in regional brain atrophy, a finding reported in neuro-LC and associated with cognitive deficits.20,53 Patients with COVID-19, compared with control participants without a history of infection, show significant reductions in grey matter thickness in the orbitofrontal cortex and parahippocampal gyrus and in global brain size, in addition to increased tissue damage in areas associated with the olfactory cortex.20 These areas, collectively referred to as the limbic system, are important for memory and emotion processing. Subtle tissue damage in these regions might be one of the driving factors for cognitive and psychiatric impairments in COVID-19 survivors. Similarly, patients with neuro-LC have reduced metabolic activity in the parahippocampal gyrus and thalamus,53 areas important for contextual associations and relaying sensory and motor information across disparate brain regions. The thalamus also strongly influences arousal, circadian rhythms, and pain perception, which can be disrupted in patients with neuro-LC.53 Notably, hypometabolism and chronic neuroinflammation are important clinical manifestations of mild cognitive impairment, Alzheimer’s disease, and dementias of other causes.54–56 The similarities and differences between neuro-LC, mild cognitive impairment, and Alzheimer’s disease—with overlapping collections of symptoms—will help to direct future studies of potential therapeutic interventions for neuro-LC. Table 2 illustrates common symptoms of neuro-LC and potential underlying mechanisms.
Persistent inflammation, triggered by initial SARS-CoV-2 infection and propagated by ongoing immune activation affecting these regions (possibly through persistent viral reservoirs and antigenic stimulation, or targeting of an endogenous antigen in the case of molecular mimicry and autoimmunity34), is thus a leading candidate for the underlying mechanism of a diverse array of neuro-LC symptoms (figure 1). Additionally, a high proportion of critically ill patients with COVID-19 show Epstein-Barr virus and cytomegalovirus reactivations, although it is unclear whether these reactivations predispose to hyperinflammation.57
Preclinical data show that SARS-CoV-2 infection, in contrast to influenza virus, leads to persistent immune cell activation and production of inflammatory cytokines in the olfactory bulb and epithelium.28 These changes were accompanied by signs of elevated compulsiveness or anxiety-like behaviours 1 month after infection in a hamster model. Similar findings were observed in postmortem olfactory tissues of individuals who died at least 1 month after resolution of acute COVID-19.28 Fernández-Castañeda and colleagues58 modelled mild respiratory COVID-19 in a mouse expressing the viralentry receptor for SARS-CoV-2—human angiotensin-converting enzyme 2 (ACE2)—by delivering SARS-CoV-2 intranasally. They found signs of neuro inflammation with elevated concentrations of chemokines in the cerebrospinal fluid and serum. These changes led to activation of microglia in subcortical and hippocampal white matter regions, with distinct effects on specific neural cell populations. The activation of microglia appeared to be mediated by persistently elevated concentrations of C-C motif chemokine 11 (CCL11), which has been associated with ageing and inhibition of neurogenesis. In a small human study (n=63), higher concentrations of serum CCL11 were identified in participants with neuro-LC than in those with long COVID without cognitive symptoms.58,59
Potentially modifiable risk factors for neuro-LC
Although direct viral tropism with SARS-CoV-2 was initially hypothesised to contribute to neuro-LC symptoms, it seems more likely that patients are predisposed to clinical sequelae through indirect effects of infection, disease, and clinical care, such as persistent inflammation or viral load, clotting abnormalities, low blood oxygen, exposure to sedative and analgesic drugs, isolation, and immobility. Identification of modifiable risk factors that could be targeted to reduce the burden of neuro-LC is a priority for research and clinical practice.
Clotting abnormalities
Prolonged viral presence, hypoxia, and inflammatory responses are hypothesised to lead to persistent endothelial damage, extensive vascular endotheliitis, and thrombosis, which put patients at risk of neuro-LC.58,60 In studies that included proteomics and fluorescence microscopy, plasma samples from patients with long COVID contained large anomalous (amyloid) deposits (microclots).32,60 In support of clotting abnormalities as a possible risk factor for neuro-LC are large registry studies of patients who were hospitalised or admitted to the ICU with COVID-19, which have found rates of venous thromboembolism and pulmonary embolism in the range of 1–3% at 3 months after discharge.61,62 Studies evaluating biomarker profiles that might be associated with increased thrombosis among survivors of COVID-19 hospitalisation have identified very high serum concentrations of ferritin, D-dimer, and C-reactive protein,63 high erythrocyte sedimentation rate,63 large anomalous (amyloid) deposits (microclots32 resistant to fibrinolysis), and an increase in plasma factor VIII and plasminogen activator inhibitor 1 concentrations related to the continuous activation of endothelial cells,64 which might explain, partly, the hypercoagulable and hypofibrinolytic states that could predispose patients to neuro-LC.
Hypoxia
Patients with COVID-19 often have severe hypoxaemia, although many do not report any discomfort or obvious increased work of breathing as an early manifestation.65,66 This so-called happy hypoxaemia early in the course of SARS-CoV-2 infection is caused primarily by ventilation–perfusion (V/Q) mismatch as a result of adequate pulmonary arterial blood flow to poorly ventilated alveoli, secondary to the relative failure of the hypoxic pulmonary vasoconstriction mechanism during SARS-CoV-2 infection.67 Endothelial injury and a hypercoagulable state are also emerging as a central hallmark of COVID-19 pathogenesis.66,67 The hypercoagulable state leads to further deterioration in V/Q mismatch and lung tissue damage. Diffusing capacity of the lungs can be impaired, leading to a raised alveolar–arterial oxygen gradient (PAO2 - PaO2). Prolonged hypoxaemia increases the risk of cerebral compromise, particularly in so-called watershed regions of the brain (areas of the frontal and parietal cortex at the margins of distribution of the major vascular structures). Chronic intermittent hypoxia causes changes in signalling pathways at the neuronal synapse,68 which might contribute to the increased risk of cognitive deficits that many patients have even months after acute COVID-19.
Sedation and analgesia
Early reports during the pandemic advocated neuromuscular blockade and deep sedation to treat patients with COVID-19-associated acute respiratory distress syndrome (ARDS). Patient factors such as increased patient–ventilator dyssynchrony, need for higher positive end-expiratory pressures, agitation, and the decision to prone patients might also have contributed to the desire for deeper sedation. The COVID-D cohort study69 of adult patients with COVID-19 who received care in the ICU included 2088 patients from 69 sites in 14 countries. 1827 (87·5%) received invasive mechanical ventilation, with 1337 (64·0%) receiving benzodiazepines for a median of 7 days (IQR 4–12) and 1481 (70·9%) receiving propofol for a median of 7 days (4–11). 1704 (81·6%) of 2088 patients were in a coma for a median of 10 days (IQR 6–15) and 1147 (54·9%) had delirium for a median of 3 days (2–6). Thus, a substantial proportion of patients had acute brain organ dysfunction for almost 2 weeks. Benzodiazepine sedative infusions were associated with a higher risk of delirium (odds ratio [OR] 1·59, 95% CI 1·33–1·91). Spontaneous awakening and breathing trials were performed in only a quarter of eligible patients. Thus, sedation practices during the pandemic lost the gains made over the previous two decades, during which benzodiazepine use had reduced (to <5% of patients receiving invasive mechanical ventilation), duration of acute brain dysfunction had reduced (to a reported median of <5 days), and spontaneous awakening and breathing trials were established as standards of care.70–72 This increased use of sedation portends a substantial risk for the development of neuro-LC, given that delirium is one of the strongest potentially modifiable risk factors for long-term cognitive impairment after critical illness.73 Depth of sedation and use of benzodiazepine medications have also been associated with worse symptoms of post-traumatic stress disorder and psychological sequelae.74,75 Regardless of the indication, prolonged sedation is therefore not without consequences,76 and understanding of the association between sedation and post-acute sequelae of critical illness provides an opportunity to improve care for patients in the future.
Isolation
Critically ill patients with COVID-19 might have been uniquely affected by social isolation resulting from restricted visitation in most hospitals during the pandemic. In the COVID-D study,69 family visitation occurred on less than 20% of eligible days; however, when (virtual or in-person) visitation was allowed, the risk of delirium the following day significantly decreased (OR 0·73, 95% CI 0·63–0·84). Family presence in the ICU has been associated with decreased anxiety, reduced length of stay, and increased sense of security, satisfaction, and quality of care among patients. These findings suggest that social isolation might have acute and long-lasting effects as part of neuro-LC (cognitive and psychiatric) after acute COVID-19.
Mitigating neuro-LC and supporting patients in recovery
Over the past 36 months of the COVID-19 pandemic, much has been learned about the pathophysiological underpinnings of SARS-CoV-2 and the host response to infection, early and late clinical manifestations, and best treatment strategies during acute illness. Furthermore, population-based studies have shown the beneficial effects of vaccination77–79 and early antiviral therapy (nirmatrelvir)80,81 in reducing long COVID symptoms (including neuro-LC). Unfortunately, early in the pandemic, many key management strategies developed during the past two decades on the basis of high-quality research in critically ill populations were abandoned with the misguided notion that COVID-19 pneumonia, ARDS, and critical illness reflect a completely different entity requiring unique management strategies. However, this has not proved to be the case, with more similarities than differences between COVID-19-related critical illness and critical illness due to other causes. As we learn more about the lasting impact of SARS-CoV-2 in the form of long COVID, tested management strategies during critical illness need to be reinstated, and an appreciation of and commitment to essential support systems for patients are needed to prevent an emerging epidemic of neuro-LC. We propose some actionable steps, based on available evidence and expert recommendation, which might help to ameliorate neuro-LC.
Anticoagulation in critically ill patients
The COVID-19 Treatment Guidelines (updated in April, 2023)82 recommend that patients with COVID-19 who previously received anticoagulant or antiplatelet therapies for underlying conditions should continue these medications unless significant bleeding develops or other contraindications are present. For patients admitted to the ICU, prophylaxis with low-molecular-weight heparin (LMWH) or unfractionated heparin (UFH) is preferred over oral anticoagulants, and LMWH is preferred over UFH. Venous thromboembolism prophylaxis should not be continued after hospital discharge for patients with COVID-19 unless they have another indication or are deemed to be high risk, and an individualised approach should be used to identify high-risk hospitalised patients with COVID-19 for extended thromboprophylaxis with a direct oral anticoagulant. For critically ill patients with COVID-19 who have rapid deterioration of pulmonary, cardiac, or neurological function or sudden, localised loss of peripheral perfusion, the COVID-19 Treatment Guidelines Panel recommends evaluating patients for thromboembolic disease and treating therapeutically if indicated. When diagnostic imaging is not possible, patients who are highly suspected to have thromboembolic disease should be started on therapeutic anticoagulation as per standard institutional protocols. This guidance extends to patients who require extracorporeal membrane oxygenation (ECMO) or continuous renal replacement therapy, or those who have thrombosis related to catheters or ECMO filters. Although the guidelines are based on expert opinion and founded in evidence-based standards for venous thromboembolism prophylaxis, we expect efficacy studies to confirm their applicability to patients with COVID-19 and show an association with decreased risk of adverse neurological outcomes—in particular, cerebrovascular sequelae. Ongoing studies include, among others, the global FREEDOM COVID-19 Anticoagulation Trial (NCT04512079) and the ANTIcoagulation in Severe COVID-19 Patients (ANTICOVID) study (NCT04808882).
Protocolised support of mechanically ventilated patients
When possible, health-care providers should adhere to current guidelines83,84 for the practice of mechanical ventilation, especially in patients with COVID-19. These guidelines recommend limited use of neuromuscular blockade, avoidance of continuous infusions of benzodiazepines, targeting of light levels of sedation, frequent awakening and breathing trials, and early mobilisation. These practices improve short-term outcomes but might also reduce the risk of PICS,85 which afflicts a high proportion of survivors of acute respiratory failure10,86,87 and has a symptom profile largely overlapping that of neuro-LC.88 This guideline is in line with earlier recommendations from Fan and colleagues89 to adhere to evidence-based practices and manage mechanical ventilation and the associated needs of individual patients with COVID-19 as patients without the disease would be managed, including the use of lung-protective strategies that have been established in randomised trials over the past two decades. As the ability to care for patients who have COVID-19 improves with better strategies to combat the virus and its accompanying sequelae, and as health-care systems prepare for the next wave of COVID-19 and other emerging pandemics, ICU practitioners must move away from deep sedation and strive for lighter levels of sedation when patient-related factors and logistical issues permit its safe use, to mitigate PICS and promote optimum recovery of body and mind after acute disease.
The ABCDEF (A2F) bundle and Prevention and Management of Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption (PADIS) guidelines84 for adult patients in the ICU provide a framework for best practice to help to free patients from mechanical ventilation faster and to reduce delirium, and potentially to reduce the downstream impact of neuro-LC. On the basis of high-impact randomised controlled trials (RCTs) over the past two decades, the A2F bundle has now been tested in clinical practice, outside the setting of an RCT, and was associated with significant improvement in mortality, time on mechanical ventilation, and length of hospital stay.90 The main tenets of the A2F bundle include assessment and management of pain, both spontaneous awakening and breathing trials, choice of sedatives, delirium assessment and management, early mobility and exercise, and family empowerment and engagement. The protocolised practices of the A2F bundle have been well validated, with substantial evidence showing beneficial effects on outcomes in the ICU survivorship population. Future studies examining best implementation practices to decrease the risk of neuro-LC will be a crucial step towards improved outcomes in survivors of COVID-19.
A: Assessment and management of pain
All critically ill patients should be evaluated for pain using validated pain assessment tools.91–93 An analgesia-first approach should be used, in which pain is addressed before sedating a patient.94
B: Both spontaneous awakening and breathing trials
Critically ill, mechanically ventilated patients should be lightly sedated if agitated, after pain has been addressed. Additionally, patients should be screened daily to assess suitability for spontaneous awakening trials (SATs) and undergo a SAT if they pass the screen.72,84 Similarly, patients should undergo a daily spontaneous breathing trial (SBT) screen and trial if they pass the screen.72,84 A coordinated approach to SATs and SBTs has been shown to improve ventilator-free days (14·7 vs 11·6 days; mean difference 3·1 days, 95% CI 0·7–5·6; p=0·02), time in the ICU (median 9·1 days vs 12·9 days; p=0·01), and mortality at any instant during the year after enrolment (HR 0·68, 95% CI 0·50–0·92; p=0·01) compared with SATs and SBTs used in isolation.72
C: Choice of sedatives
If patients require sedation due to agitation or patient–ventilator dyssynchrony, non-benzodiazepine sedatives such as propofol or dexmedetomidine should be used.70 These agents are equally efficacious, with similar outcomes with regard to delirium, time on mechanical ventilation, ICU length of stay, and mortality as seen in mechanically ventilated patients with and without sepsis.70,95 Furthermore, conduct of SATs can minimise the duration of sedation and cumulative sedation use, in addition to reducing time on mechanical ventilation, ICU length of stay, and mortality.72
D: Delirium assessment and management
All patients should be evaluated for delirium using a validated instrument, such as the Confusion Assessment Method for the ICU (CAM-ICU)96,97 or the Intensive Care Delirium Screening Checklist.98 In patients diagnosed with delirium, a thorough assessment should be done to investigate potential causes of delirium. Antipsychotic medications can be used to control dangerous agitation temporarily, although data are clear that these medications do not prevent or treat delirium.71,99
E: Early mobility and exercise
Critically ill patients should be screened daily and engage in early mobilisation to allow faster weaning from mechanical ventilation and functional independence at hospital discharge.100,101 In a recent RCT of 200 mechanically ventilated patients who were recruited before the emergence of COVID-19,85 early mobilisation resulted in lower rates of cognitive impairment (24% vs 43%; absolute difference −19·2%, 95% CI −32·1 to −6·3; p=0·004) at 1 year after hospital discharge. ICU-acquired weakness was also less prevalent at 1 year among participants who received early mobilisation.
F: Family empowerment and engagement
When possible, families should be allowed to visit patients (preferably in person but at least virtually) to avoid social isolation102 and possibly reduce the risk of delirium.69 Protocolised and intentional family engagement and empowerment might also facilitate patient-centred care by informing the health-care team’s understanding of the patient’s individual psychosocial context, goals, and preferences.
Future directions
Ongoing efforts to develop a consensus definition of long COVID and neuro-LC, with attention to phenotypic subtypes, will inform the design of future observational and interventional studies on this subject. A better understanding of the natural history and progression of symptom clusters beyond 1 year after acute infection is also needed in survivors of COVID-19 in general, and particularly in survivors of COVID-19-related critical illness. At present, there are no specific, targeted preventive or therapeutic treatments for neuro-LC, and the development of safe and effective interventions is a priority for future research. As we have discussed, neurological, cognitive, and psychiatric sequelae of SARS-CoV-2 infection are probably driven by neuroinflammation, suggesting that specific inflammatory pathways are rational targets to combat these devastating consequences of COVID-19. Dexamethasone is now part of most acute COVID-19 management protocols to reduce inflammation and, given its proven benefit in other acute neurological conditions, the efficacy of dexamethasone as a preventive treatment against neuro-LC could be explored. Janus kinase (JAK) inhibitors such as baricitinib improve survival in acute COVID-19103,104 and are another potential treatment modality that warrants further study in long COVID. JAK inhibitors reduce inflammation by blocking the inflammatory JAK signalling cascade that is activated when the cytokine interleukin-6 binds to a cell-surface receptor.105 Ruloxitinib, a JAK inhibitor related to baricitinib, has been shown to restore functional immune responses and reduce viral reservoir size in people living with HIV,106 and to reduce histopathological features of HIV encephalitis in a mouse model.107 Similarly, neuroinflammation mediated by the JAK/STAT pathway has been implicated in the development of neurodegenerative diseases such as Parkinson’s disease.108 Baricitinib has been shown to improve cognitive function in mice with chronic neuroinflammation due to HIV infection,109 but it has not been studied in humans to treat cognitive or physical impairments related to long COVID. Because persistent inflammation and immune activation from viral antigenic stimulation are likely contributors to the symptomatology of long COVID, baricitinib represents a potential therapeutic option, especially for neuro-LC.
Identifying biomarker profiles and clinical profiles that are associated with neuro-LC is another area of study that might help to identify patients at risk and target preventive and therapeutic interventions. Although chemokines such as CCL11 have been identified in individuals with mild SARS-CoV-2 infection and were associated with impaired hippocampal neurogenesis,58 the mechanisms by which these changes lead to worse symptoms after SARS-CoV-2 infection, compared with influenza, have yet to be clarified.
Post-ICU support systems will need to expand ICU recovery clinics and build a survivorship community to meet the needs of patients with neuro-LC and PICS (figure 2). Many patients with long COVID meet the diagnostic criteria for ME/CFS or have symptoms consistent with ME/CFS,40,41 which can worsen with physical or mental activity.42 Predictive biomarkers that can guide clinical care will be essential to distinguish patients who are likely to benefit from established rehabilitation therapies from those unlikely to benefit or at risk of harm.
Figure 2: Developing a survivorship community for patients with long COVID and PICS.

Post-ICU support systems need to be strengthened to provide patients with cognitive and physical rehabilitation, easier connections to the health-care community through referrals from post-ICU clinics and dedicated social workers, and primary care physicians who recognise the manifestations of neuro-LC. Many patients with neurological symptoms meet diagnostic criteria for or have symptoms consistent with ME/CFS. Discerning these symptoms in the management of patients with neuro-LC is imperative because individuals with ME/CFS might need to apply rigorous pacing techniques to avoid cognitive and physical distress exacerbated by therapy. (A) A 32-year-old father, husband, firefighter, and emergency medical technician developed mild acute COVID-19 in October, 2021, despite being fully vaccinated. The patient was healthy, fully functional, and employed before infection, but showed symptoms consistent with cognitive impairment, post-traumatic stress disorder, and precipitous hearing loss after the acute disease, with resultant loss of employment. He had hearing evaluated as part of employment screening in 2014; the picture on the left, taken 8 months after acute COVID-19, shows hearing loss since the previous evaluation. This profound sensorineural hearing impairment as part of long COVID, which necessitated bilateral hearing aids, appears to be permanent. The picture on the right shows the patient in June, 2023, 1 year and 8 months after the acute disease; he has now received the first of two planned cochlear implants and he receives social security disability funds that make up about 50% of his previous income. (B) A support group of individuals with long COVID, facilitated by a neuropsychologist, at the Critical Illness, Brain Dysfunction, and Survivorship Center, Vanderbilt University Medical Center, Nashville, TN, USA. Patients in these groups have reported that the community saved them from near-certain suicide. We urge the medical community to create a safe place for patients with long COVID to have community in recovery. (C) Patients with long COVID need multidisciplinary clinical care and a support group of like-minded individuals, which should be a priority for long COVID and post-ICU centres worldwide to address the substantial burden of neuro-LC. The panel lists key components of the survivorship clinic and survivorship support group at the authors’ institution. ICU=intensive care unit. ME/CFS=myalgic encephalomyelitis/chronic fatigue syndrome. Neuro-LC=neurological, cognitive, and psychiatric sequelae of COVID-19 (neuro-long COVID). PICS=post-intensive care syndrome.
Finally, the critical care community, which has lost tremendous resources in experienced nurses and other health-care professionals during the course of the pandemic, with invaluable institutional memory, remains in a period of deeply needed recovery. Investment and a commitment to understanding and responding to the experiences and needs of health-care workers are required. Priorities include studies of long-term workforce trends arising from the pandemic; measures to ensure the safety of health-care workers (eg, availability of vaccines and personal protective equipment); education and mitigation of psychological stress among health-care workers; improved support for reasonable working hours, staffing patterns, and wages and benefits; and development of a sustained pipeline of trained professionals to manage the ever-increasing demands of work in the ICU.
Conclusions
Neuro-LC, a subset of clinical manifestations within the larger context of long COVID, threatens to be a devastating epidemic resulting from the COVID-19 pandemic that could affect patients’ quality of life for years to come. Symptoms that persist or emerge after COVID-19-related critical illness—the overlapping syndromes of PICS and neuro-LC—could add to the burden of neurological, cognitive, and psychiatric sequelae of COVID-19. Although advances in understanding of the pathophysiological underpinnings of SARS-CoV-2 infection and the host response will help to delineate new treatment modalities, adherence to best-practice guidelines is likely to help in mitigating neuro-LC in the interim. Systemic and organ-level inflammation and viral reservoirs are the leading hypotheses for the mechanistic underpinnings of long COVID in general and neuro-LC in particular. Future studies investigating the use of inflammatory modulators and antiviral agents, such as the STOP-PASC trial of nirmatrelvir plus ritonavir for the treatment of long COVID (NCT05576662), will be important in the search for strategies to mitigate the devastating effects of this collection of disorders in survivors of COVID-19-related critical illness.
Key messages.
Vaccines, antivirals, and anti-inflammatory medications reduce the morbidity and mortality associated with acute COVID-19, but millions of patients go on to have a devastating subacute and chronic illness known as long COVID, even after mild acute disease
Many survivors of critical illness have neurological and psychological features of PICS plus long COVID for months or years after hospitalisation for acute COVID-19, including cognitive impairments, ME/CFS, anxiety, depression, post-traumatic stress disorder, peripheral nerve symptoms, and new cerebrovascular disease; we refer to this collection of post-acute sequelae as neuro-long COVID or neuro-LC
Neuro-LC is a burgeoning public health crisis, affecting millions of individuals and society at large, with detrimental effects on quality of life, ability to remain in the workforce, and financial and mental wellbeing of patients and caregivers
Key management strategies that were based on high-quality research and widely used in the ICU before the emergence of COVID-19 were abandoned during the early stages of the pandemic; a change in ICU culture and practice is needed to reduce known risk factors for PICS and mitigate the development of neuro-LC by re-establishing strict adherence to lung-protective ventilation and evidence-based safety checklists such as the ABCDEF bundle, which promotes management of pain and delirium, minimisation of sedation, and early mobilisation
Clinics and support groups dedicated to long COVID and PICS are needed to provide care, support, and a community of like-minded individuals for patients and caregivers to address the current and escalating burden of neuro-LC
Studies to understand the phenotypic subtypes, natural history, and biomarker profiles of long COVID and neuro-LC will need to go hand in hand with research efforts to develop targeted preventive and therapeutic interventions to reduce the burden of neuro-LC in survivors of COVID-19-related critical illness
ICU=intensive care unit. ME/CFS=myalgic encephalomyelitis/chronic fatigue syndrome. PICS=post-intensive care syndrome.
Search strategy and selection criteria.
We searched PubMed for articles published from Jan 1, 2020, to April 30, 2023, using the terms (“long COVID” OR “post-COVID” OR “post-acute sequelae of COVID” OR “PASC COVID”), (“critical illness” OR “critical care” OR “ICU” OR “intensive care”), and (“neurocognitive” OR “cognitive impairment” OR “mental illness” OR “mental health” OR “mood disorder” OR “depression” OR “anxiety” OR “brain fog” OR “neuropsychology”). Additional relevant articles were identified through searches of reference lists of selected articles and the authors’ personal files. Only full articles published in English were considered. Papers were selected on the basis of relevance to the aims of this Series paper, with a particular focus on pathophysiology and available or potential future strategies to mitigate neurological, cognitive, and psychiatric sequelae of COVID-19-related critical illness.
Acknowledgments
We thank Seth Kiehl for sharing his graphic arts expertise in the preparation of figure 1. SWR is supported by US National Institutes of Health (NIH) grant K23AG072030 and by a Brain & Behavior Research Foundation Young Investigator award (#30322). FEH and JAB are supported by NIH grant RF1 AG075341, and JEW by NIH grant R01GM120484.
Footnotes
Declaration of interests
EWE and SWR have submitted a grant proposal to study the effects of baricitinib on long COVID. The other authors declare no competing interests.
This is the second in a Series of three papers about the post-acute sequelae of COVID-19 All papers in the Series are available at www.thelancet.com/series/post-acute-sequelae-of-COVID-19
Contributor Information
Pratik Pandharipande, Department of Anesthesiology, Division of Anesthesiology Critical Care Medicine, Vanderbilt University Medical Center, Nashville, TN, USA; Critical Illness, Brain Dysfunction, and Survivorship (CIBS) Center, Vanderbilt University Medical Center, Nashville, TN, USA.
Shawniqua Williams Roberson, Departments of Neurology and Biomedical Engineering, Vanderbilt University, Nashville, TN, USA; Critical Illness, Brain Dysfunction, and Survivorship (CIBS) Center, Vanderbilt University Medical Center, Nashville, TN, USA.
Fiona E Harrison, Department of Medicine, Division of Diabetes, Endocrinology and Metabolism, Vanderbilt University Medical Center, Nashville TN; Critical Illness, Brain Dysfunction, and Survivorship (CIBS) Center, Vanderbilt University Medical Center, Nashville, TN, USA.
Jo Ellen Wilson, Department of Psychiatry and Behavioral Sciences, Vanderbilt University Medical Center, Nashville TN; Critical Illness, Brain Dysfunction, and Survivorship (CIBS) Center, Vanderbilt University Medical Center, Nashville, TN, USA; Tennessee Valley Veteran’s Affairs Geriatric Research Education Clinical Center, VA Tennessee Valley Healthcare System, Nashville, TN, USA.
Julie A Bastarache, Department of Medicine, Division of Allergy, Pulmonary and Critical Care Medicine, Vanderbilt University Medical Center, Nashville TN; Critical Illness, Brain Dysfunction, and Survivorship (CIBS) Center, Vanderbilt University Medical Center, Nashville, TN, USA.
E Wesley Ely, Department of Medicine, Division of Allergy, Pulmonary and Critical Care Medicine, Vanderbilt University Medical Center, Nashville TN; Critical Illness, Brain Dysfunction, and Survivorship (CIBS) Center, Vanderbilt University Medical Center, Nashville, TN, USA; Tennessee Valley Veteran’s Affairs Geriatric Research Education Clinical Center, VA Tennessee Valley Healthcare System, Nashville, TN, USA.
References
- 1.WHO. WHO Coronavirus (COVID-19) Dashboard. 2023. https://covid19.who.int (accessed July 1, 2023).
- 2.Suthar AB, Wang J, Seffren V, Wiegand RE, Griffing S, Zell E. Public health impact of covid-19 vaccines in the US: observational study. BMJ 2022; 377: e069317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Admon AJ, Iwashyna TJ, Kamphuis LA, et al. Assessment of symptom, disability, and financial trajectories in patients hospitalized for COVID-19 at 6 months. JAMA Netw Open 2023; 6: e2255795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evans RA, McAuley H, Harrison EM, et al. Physical, cognitive, and mental health impacts of COVID-19 after hospitalisation (PHOSP-COVID): a UK multicentre, prospective cohort study. Lancet Respir Med 2021; 9: 1275–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsuzuki S, Miyazato Y, Terada M, Morioka S, Ohmagari N, Beutels P. Impact of long-COVID on health-related quality of life in Japanese COVID-19 patients. Health Qual Life Outcomes 2022; 20: 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taquet M, Sillett R, Zhu L, et al. Neurological and psychiatric risk trajectories after SARS-CoV-2 infection: an analysis of 2-year retrospective cohort studies including 1 284 437 patients. Lancet Psychiatry 2022; 9: 815–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis HE, McCorkell L, Vogel JM, Topol EJ. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol 2023; 21: 133–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iwasaki A, Putrino D. Why we need a deeper understanding of the pathophysiology of long COVID. Lancet Infect Dis 2023; 23: 393–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choutka J, Jansari V, Hornig M, Iwasaki A. Unexplained post-acute infection syndromes. Nat Med 2022; 28: 911–23. [DOI] [PubMed] [Google Scholar]
- 10.Marra A, Pandharipande PP, Girard TD, et al. Co-occurrence of post-intensive care syndrome problems among 406 survivors of critical illness. Crit Care Med 2018; 46: 1393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liou A, Schweickert WD, Files DC, Bakhru RN. A survey to assess primary care physician awareness of complications following critical illness. J Intensive Care Med 2023; published online March 27. 10.1177/08850666231164303. [DOI] [PubMed] [Google Scholar]
- 12.Singh SJ, Baldwin MM, Daynes E, et al. Respiratory sequelae of COVID-19: pulmonary and extrapulmonary origins, and approaches to clinical care and rehabilitation. Lancet Respir Med 2023; published online May 19. 10.1016/S2213-2600(23)00159-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parotto M, Gyöngyösi M, Howe K, et al. Post-acute sequelae of COVID-19: understanding and addressing the burden of multisystem manifestations. Lancet Respir Med 2023; published online July 17. 10.1016/S2213-2600(23)00239-4. [DOI] [PubMed] [Google Scholar]
- 14.Thaweethai T, Jolley SE, Karlson EW, et al. Development of a definition of postacute sequelae of SARS-CoV-2 infection. JAMA 2023; 329: 1934–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Department of Health and Human Services. What is long COVID? 2020. https://www.covid.gov/longcovid/definitions (accessed June 11, 2023).
- 16.Department of Health and Human Services. National research action plan on long COVID. August 2022. https://www.covid.gov/assets/files/National-Research-Action-Plan-on-Long-COVID-08012022.pdf (accessed July 1, 2023).
- 17.WHO. A clinical case definition of post COVID-19 condition by a Delphi consensus. Oct 6, 2021. https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1 (accessed July 1, 2023).
- 18.National Institute for Health and Care Excellence (NICE), Scottish Intercollegiate Guidelines Network (SIGN), and Royal College of General Practitioners (RCGP). COVID-19 rapid guideline: managing the long-term effects of COVID-19. Nov 3, 2022. https://www.nice.org.uk/guidance/ng188/resources/covid19-rapid-guideline-managing-the-longterm-effects-of-covid19-pdf-51035515742 (accessed July 1, 2023).
- 19.Wulf Hanson S, Abbafati C, Aerts JG, et al. Estimated global proportions of individuals with persistent fatigue, cognitive, and respiratory symptom clusters following symptomatic COVID-19 in 2020 and 2021. JAMA 2022; 328: 1604–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Douaud G, Lee S, Alfaro-Almagro F, et al. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature 2022; 604: 697–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guedj E, Campion JY, Dudouet P, et al. 18F-FDG brain PET hypometabolism in patients with long COVID. Eur J Nucl Med Mol Imaging 2021; 48: 2823–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siow I, Lee KS, Zhang JJY, Saffari SE, Ng A. Encephalitis as a neurological complication of COVID-19: a systematic review and meta-analysis of incidence, outcomes, and predictors. Eur J Neurol 2021; 28: 3491–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rutkowska-Zapała M, Suski M, Szatanek R, et al. Human monocyte subsets exhibit divergent angiotensin I-converting activity. Clin Exp Immunol 2015; 181: 126–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oaklander AL, Mills AJ, Kelley M, et al. Peripheral neuropathy evaluations of patients with prolonged long COVID. Neurol Neuroimmunol Neuroinflamm 2022; 9: e1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balcom EF, Nath A, Power C. Acute and chronic neurological disorders in COVID-19: potential mechanisms of disease. Brain 2021; 144: 3576–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soudry Y, Lemogne C, Malinvaud D, Consoli SM, Bonfils P. Olfactory system and emotion: common substrates. Eur Ann Otorhinolaryngol Head Neck Dis 2011; 128: 18–23. [DOI] [PubMed] [Google Scholar]
- 27.Wang S, Quan L, Chavarro JE, et al. Associations of depression, anxiety, worry, perceived stress, and loneliness prior to infection with risk of post-COVID-19 conditions. JAMA Psychiatry 2022; 79: 1081–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frere JJ, Serafini RA, Pryce KD, et al. SARS-CoV-2 infection in hamsters and humans results in lasting and unique systemic perturbations after recovery. Sci Transl Med 2022; 14: eabq3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeong M, Ocwieja KE, Han D, et al. Direct SARS-CoV-2 infection of the human inner ear may underlie COVID-19-associated audiovestibular dysfunction. Commun Med (Lond) 2021; 1: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Limphaibool N, Iwanowski P, Holstad MJV, Kobylarek D, Kozubski W. Infectious etiologies of parkinsonism: pathomechanisms and clinical implications. Front Neurol 2019; 10: 652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Merello M, Bhatia KP, Obeso JA. SARS-CoV-2 and the risk of Parkinson’s disease: facts and fantasy. Lancet Neurol 2021; 20: 94–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pretorius E, Vlok M, Venter C, et al. Persistent clotting protein pathology in long COVID/post-acute sequelae of COVID-19 (PASC) is accompanied by increased levels of antiplasmin. Cardiovasc Diabetol 2021; 20: 172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Castanares-Zapatero D, Chalon P, Kohn L, et al. Pathophysiology and mechanism of long COVID: a comprehensive review. Ann Med 2022; 54: 1473–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nunez-Castilla J, Stebliankin V, Baral P, et al. Potential autoimmunity resulting from molecular mimicry between SARS-CoV-2 spike and human proteins. Viruses 2022; 14: 1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sampogna G, Tessitore N, Bianconi T, et al. Spinal cord dysfunction after COVID-19 infection. Spinal Cord Ser Cases 2020; 6: 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bull-Otterson L, Baca S, Saydah S, et al. Post-COVID conditions among adult COVID-19 survivors aged 18–64 and ≥65 years—United States, March 2020–November 2021. MMWR Morb Mortal Wkly Rep 2022; 71: 713–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ballering AV, van Zon SKR, Olde Hartman TC, Rosmalen JGM. Persistence of somatic symptoms after COVID-19 in the Netherlands: an observational cohort study. Lancet 2022; 400: 452–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Y-H, Chen Y, Wang Q-H, et al. One-year trajectory of cognitive changes in older survivors of COVID-19 in Wuhan, China: a longitudinal cohort study. JAMA Neurol 2022; 79: 509–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davis HE, Assaf GS, McCorkell L, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine 2021; 38: 101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mirin AA. A preliminary estimate of the economic impact of long COVID in the United States. Fatigue 2022; 10: 190–199. [Google Scholar]
- 41.Cutler DM. The costs of long COVID. JAMA Health Forum 2022; 3: e221809. [DOI] [PubMed] [Google Scholar]
- 42.Committee on the Diagnostic Criteria for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome, Board on the Health of Select Populations, Institute of Medicine. Beyond myalgic encephalomyelitis/chronic fatigue syndrome: redefining an illness. Washington, DC, USA: National Academies Press, 2015. [PubMed] [Google Scholar]
- 43.Levine RL. Addressing the long-term effects of COVID-19. JAMA 2022; 328: 823–24. [DOI] [PubMed] [Google Scholar]
- 44.Xu E, Xie Y, Al-Aly Z. Long-term neurologic outcomes of COVID-19. Nat Med 2022; 28: 2406–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xie Y, Xu E, Al-Aly Z. Risks of mental health outcomes in people with covid-19: cohort study. BMJ 2022; 376: e068993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tran VT, Porcher R, Pane I, Ravaud P. Course of post COVID-19 disease symptoms over time in the ComPaRe long COVID prospective e-cohort. Nat Commun 2022; 13: 1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matschke J, Lütgehetmann M, Hagel C, et al. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol 2020; 19: 919–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Solomon IH, Normandin E, Bhattacharyya S, et al. Neuropathological features of Covid-19. N Engl J Med 2020; 383: 989–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peluso MJ, Deitchman AN, Torres L, et al. Long-term SARS-CoV-2-specific immune and inflammatory responses in individuals recovering from COVID-19 with and without post-acute symptoms. Cell Rep 2021; 36: 109518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peluso MJ, Lu S, Tang AF, et al. Markers of immune activation and inflammation in individuals with postacute sequelae of severe acute respiratory syndrome coronavirus 2 infection. J Infect Dis 2021; 224: 1839–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.PHOSP-COVID Collaborative Group. Clinical characteristics with inflammation profiling of long COVID and association with 1-year recovery following hospitalisation in the UK: a prospective observational study. Lancet Respir Med 2022; 10: 761–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Andrews MG, Mukhtar T, Eze UC, et al. Tropism of SARS-CoV-2 for human cortical astrocytes. Proc Natl Acad Sci USA 2022; 119: e2122236119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sollini M, Morbelli S, Ciccarelli M, et al. Long COVID hallmarks on [18F]FDG-PET/CT: a case-control study. Eur J Nucl Med Mol Imaging 2021; 48: 3187–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Groot C, Risacher SL, Chen JQA, et al. Differential trajectories of hypometabolism across cognitively-defined Alzheimer’s disease subgroups. Neuroimage Clin 2021; 31: 102725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stage EC Jr, Svaldi D, Phillips M, et al. Neurodegenerative changes in early- and late-onset cognitive impairment with and without brain amyloidosis. Alzheimers Res Ther 2020; 12: 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Whitson HE, Colton C, El Khoury J, et al. Infection and inflammation: new perspectives on Alzheimer’s disease. Brain Behav Immun Health 2022; 22: 100462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Klein J, Wood J, Jaycox J, et al. Distinguishing features of long COVID identified through immune profiling. medRxiv 2022; published online Aug 10. 10.1101/2022.08.09.22278592 (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fernández-Castañeda A, Lu P, Geraghty AC, et al. Mild respiratory COVID can cause multi-lineage neural cell and myelin dysregulation. Cell 2022; 185: 2452–68.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Venkataramani V, Winkler F. Cognitive deficits in long Covid-19. N Engl J Med 2022; 387: 1813–15. [DOI] [PubMed] [Google Scholar]
- 60.Monje M, Iwasaki A. The neurobiology of long COVID. Neuron 2022; 110: 3484–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Giannis D, Allen SL, Tsang J, et al. Postdischarge thromboembolic outcomes and mortality of hospitalized patients with COVID-19: the CORE-19 registry. Blood 2021; 137: 2838–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Patell R, Bogue T, Koshy A, et al. Postdischarge thrombosis and hemorrhage in patients with COVID-19. Blood 2020; 136: 1342–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pasini E, Corsetti G, Romano C, et al. Serum metabolic profile in patients with long-Covid (PASC) syndrome: clinical implications. Front Med (Lausanne) 2021; 8: 714426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.von Meijenfeldt FA, Havervall S, Adelmeijer J, et al. Sustained prothrombotic changes in COVID-19 patients 4 months after hospital discharge. Blood Adv 2021; 5: 756–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dhont S, Derom E, Van Braeckel E, Depuydt P, Lambrecht BN. The pathophysiology of happy hypoxemia in COVID-19. Respir Res 2020; 21: 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thachil J Hypoxia—an overlooked trigger for thrombosis in COVID-19 and other critically ill patients. J Thromb Haemost 2020; 18: 3109–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lang M, Som A, Mendoza DP, et al. Hypoxaemia related to COVID-19: vascular and perfusion abnormalities on dual-energy CT. Lancet Infect Dis 2020; 20: 1365–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mukandala G, Tynan R, Lanigan S, O’Connor JJ. The effects of hypoxia and inflammation on synaptic signaling in the CNS. Brain Sci 2016; 6: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pun BT, Badenes R, Heras La Calle G, et al. Prevalence and risk factors for delirium in critically ill patients with COVID-19 (COVID-D): a multicentre cohort study. Lancet Respir Med 2021; 9: 239–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hughes CG, Mailloux PT, Devlin JW, et al. Dexmedetomidine or propofol for sedation in mechanically ventilated adults with sepsis. N Engl J Med 2021; 384: 1424–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Girard TD, Exline MC, Carson SS, et al. Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med 2018; 379: 2506–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet 2008; 371: 126–34. [DOI] [PubMed] [Google Scholar]
- 73.Pandharipande PP, Girard TD, Jackson JC, et al. Long-term cognitive impairment after critical illness. N Engl J Med 2013; 369: 1306–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Girard TD, Shintani AK, Jackson JC, et al. Risk factors for posttraumatic stress disorder symptoms following critical illness requiring mechanical ventilation: a prospective cohort study. Crit Care 2007; 11: R28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jones C, Griffiths RD, Humphris G, Skirrow PM. Memory, delusions, and the development of acute posttraumatic stress disorder-related symptoms after intensive care. Crit Care Med 2001; 29: 573–80. [DOI] [PubMed] [Google Scholar]
- 76.Shehabi Y, Chan L, Kadiman S, et al. for the Sedation Practice in Intensive Care Evaluation (SPICE) Study Group investigators. Sedation depth and long-term mortality in mechanically ventilated critically ill adults: a prospective longitudinal multicentre cohort study. Intensive Care Med 2013; 39: 910–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ayoubkhani D, Bermingham C, Pouwels KB, et al. Trajectory of long covid symptoms after covid-19 vaccination: community based cohort study. BMJ 2022; 377: e069676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Azzolini E, Levi R, Sarti R, et al. Association between BNT162b2 vaccination and long COVID after infections not requiring hospitalization in health care workers. JAMA 2022; 328: 676–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Notarte KI, Catahay JA, Velasco JV, et al. Impact of COVID-19 vaccination on the risk of developing long-COVID and on existing long-COVID symptoms: a systematic review. EClinicalMedicine 2022; 53: 101624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Peluso MJ, Anglin K, Durstenfeld MS, et al. Effect of oral nirmatrelvir on long COVID symptoms: 4 cases and rationale for systematic studies. Pathog Immun 2022; 7: 95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xie Y, Choi T, Al-Aly Z. Nirmatrelvir and the risk of post-acute sequelae of COVID-19. medRxiv 2022; published online Nov 5. 10.1101/2022.11.03.22281783 (preprint). [DOI] [Google Scholar]
- 82.National Institutes of Health. Coronavirus disease 2019 (COVID-19) treatment guidelines. 2023. https://www.covid19treatmentguidelines.nih.gov/ (accessed July 1, 2023). [PubMed]
- 83.Alhazzani W, Møller MH, Arabi YM, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19). Intensive Care Med 2020; 46: 854–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Devlin JW, Skrobik Y, Gélinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med 2018; 46: e825–73. [DOI] [PubMed] [Google Scholar]
- 85.Patel BK, Wolfe KS, Patel SB, et al. Effect of early mobilisation on long-term cognitive impairment in critical illness in the USA: a randomised controlled trial. Lancet Respir Med 2023; 11: 563–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Needham DM, Davidson J, Cohen H, et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders’ conference. Crit Care Med 2012; 40: 502–09. [DOI] [PubMed] [Google Scholar]
- 87.Elliott D, Davidson JE, Harvey MA, et al. Exploring the scope of post-intensive care syndrome therapy and care: engagement of non-critical care providers and survivors in a second stakeholders meeting. Crit Care Med 2014; 42: 2518–26. [DOI] [PubMed] [Google Scholar]
- 88.Hodgson CL, Higgins AM, Bailey MJ, et al. Comparison of 6-month outcomes of survivors of COVID-19 versus non-COVID-19 critical illness. Am J Respir Crit Care Med 2022; 205: 1159–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fan E, Beitler JR, Brochard L, et al. COVID-19-associated acute respiratory distress syndrome: is a different approach to management warranted? Lancet Respir Med 2020; 8: 816–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pun BT, Balas MC, Barnes-Daly MA, et al. Caring for critically ill patients with the ABCDEF bundle: results of the ICU Liberation Collaborative in over 15,000 adults. Crit Care Med 2019; 47: 3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Payen JF, Bosson JL, Chanques G, Mantz J, Labarere J, DOLOREA Investigators. Pain assessment is associated with decreased duration of mechanical ventilation in the intensive care unit: a post hoc analysis of the DOLOREA study. Anesthesiology 2009; 111: 1308–16. [DOI] [PubMed] [Google Scholar]
- 92.Gélinas C, Fillion L, Puntillo KA, Viens C, Fortier M. Validation of the critical-care pain observation tool in adult patients. Am J Crit Care 2006; 15: 420–27. [PubMed] [Google Scholar]
- 93.Payen JF, Bru O, Bosson JL, et al. Assessing pain in critically ill sedated patients by using a behavioral pain scale. Crit Care Med 2001; 29: 2258–63. [DOI] [PubMed] [Google Scholar]
- 94.Strøm T, Martinussen T, Toft P. A protocol of no sedation for critically ill patients receiving mechanical ventilation: a randomised trial. Lancet 2010; 375: 475–80. [DOI] [PubMed] [Google Scholar]
- 95.Shehabi Y, Howe BD, Bellomo R, et al. , for the ANZICS Clinical Trials Group and the SPICE III Investigators. Early sedation with dexmedetomidine in critically ill patients. N Engl J Med 2019; 380: 2506–17. [DOI] [PubMed] [Google Scholar]
- 96.Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA 2001; 286: 2703–10. [DOI] [PubMed] [Google Scholar]
- 97.Ely EW, Margolin R, Francis J, et al. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU). Crit Care Med 2001; 29: 1370–79. [DOI] [PubMed] [Google Scholar]
- 98.Bergeron N, Dubois MJ, Dumont M, Dial S, Skrobik Y. Intensive Care Delirium Screening Checklist: evaluation of a new screening tool. Intensive Care Med 2001; 27: 859–64. [DOI] [PubMed] [Google Scholar]
- 99.van den Boogaard M, Slooter AJC, Brüggemann RJM, et al. Effect of haloperidol on survival among critically ill adults with a high risk of delirium: the REDUCE randomized clinical trial. JAMA 2018; 319: 680–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet 2009; 373: 1874–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schaller SJ, Anstey M, Blobner M, et al. Early, goal-directed mobilisation in the surgical intensive care unit: a randomised controlled trial. Lancet 2016; 388: 1377–88. [DOI] [PubMed] [Google Scholar]
- 102.Rosa RG, Falavigna M, da Silva DB, et al. Effect of flexible family visitation on delirium among patients in the intensive care unit: the ICU Visits randomized clinical trial. JAMA 2019; 322: 216–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Marconi VC, Ramanan AV, de Bono S, et al. , for the COV-BARRIER Study Group. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial. Lancet Respir Med 2021; 9: 1407–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ely EW, Ramanan AV, Kartman CE, et al. , for the COV-BARRIER Study Group. Efficacy and safety of baricitinib plus standard of care for the treatment of critically ill hospitalised adults with COVID-19 on invasive mechanical ventilation or extracorporeal membrane oxygenation: an exploratory, randomised, placebo-controlled trial. Lancet Respir Med 2022; 10: 327–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bronte V, Ugel S, Tinazzi E, et al. Baricitinib restrains the immune dysregulation in patients with severe COVID-19. J Clin Invest 2020; 130: 6409–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Marconi VC, Moser C, Gavegnano C, et al. Randomized trial of ruxolitinib in antiretroviral-treated adults with human immunodeficiency virus. Clin Infect Dis 2022; 74: 95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Haile WB, Gavegnano C, Tao S, Jiang Y, Schinazi RF, Tyor WR. The Janus kinase inhibitor ruxolitinib reduces HIV replication in human macrophages and ameliorates HIV encephalitis in a murine model. Neurobiol Dis 2016; 92: 137–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lashgari NA, Roudsari NM, Momtaz S, Sathyapalan T, Abdolghaffari AH, Sahebkar A. The involvement of JAK/STAT signaling pathway in the treatment of Parkinson’s disease. J Neuroimmunol 2021; 361: 577758. [DOI] [PubMed] [Google Scholar]
- 109.Gavegnano C, Haile WB, Hurwitz S, et al. Baricitinib reverses HIV-associated neurocognitive disorders in a SCID mouse model and reservoir seeding in vitro. J Neuroinflammation 2019; 16: 182. [DOI] [PMC free article] [PubMed] [Google Scholar]


