Abstract
Introduction: Spinal cord injury (SCI) is a significant and transforming event, with an estimated annual incidence of 40 cases per million individuals in North America. Considering the significance of accurate diagnosis and effective therapy in managing SCI, Machine Learning (ML) and Robot-Assisted Gait Training (RAGT) technologies hold promise for enhancing optimal practices and elevating the quality of care. This study aims to determine the impact of the ML and RAGT techniques employed on the outcome results of SCI. Methods: We reviewed four databases, including PubMed, Scopus, ScienceDirect, and the Cochrane Central Register of Controlled Trials (CENTRAL), until 20 August 2023. The keywords used in this study encompassed the following: a comprehensive search was executed on research exclusively published in the English language: machine learning, robotics, and spinal cord injury. Results: A comprehensive search was conducted across four databases, identifying 2367 articles following rigorous data filtering. The results of the odd ratio (OR) and confidence interval (CI) of 95% for the ASIA Impairment Scale, or AIS grade A, were 0.093 (0.011–0.754, p = 0.026), for AIS grade B, 0.875 (0.395–1.939, p = 0.743), for AIS grade C, 3.626 (1.556–8.449, p = 0.003), and for AIS grade D, 8.496 (1.394–51.768, p = 0.020). The robotic group exhibited a notable reduction in AS (95% CI = −0.239 to −0.045, p = 0.004) and MAS (95% CI = −3.657 to −1.066, p ≤ 0.001) measures. This study also investigated spasticity and walking ability, which are significant. Conclusions: The ML approach exhibited enhanced precision in forecasting AIS result scores. Implementing RAGT has been shown to impact spasticity reduction and improve walking ability.
Keywords: spinal cord injury, machine learning, robot-assisted gait training
1. Introduction
Spinal cord injury (SCI) is a significant and transforming event, with an estimated annual incidence of 40 cases per million individuals in North America [1]. Following the occurrence of an injury, there are physiological repercussions that impact several body systems and are frequently accompanied by a substantial risk of mortality. Previous studies have reported inconsistent findings about the rates of in-hospital mortality, which have been shown to range from 3% to 13%. Similarly, the 1 year mortality rates after SCI have been estimated to range from 5% to 10% [2,3,4,5]. Numerous research studies have previously elucidated predictive features and algorithms for evaluating the probability of death after SCI. However, there is currently a shortage of prognostic instruments tailored specifically to the SCI patient cohort that may be conveniently employed in a clinical environment [2,5,6,7,8]. Apart from its utility in informing clinical decision-making and facilitating patient and family talks, a predictive tool may also serve as a valuable instrument in clinical research by accounting for the possible influence of distinct patient and injury variables on the mortality risk of study participants.
Considering the significance of accurate diagnosis and effective therapy in managing SCI, Machine Learning (ML) and Robot-Assisted Gait Training (RAGT) technologies hold promise for enhancing optimal practices and elevating the quality of care. The numerical value is provided by the user [9,10]. ML is often regarded as the most promising field within the domain of artificial intelligence (AI). It encompasses using algorithms to automatically generate predictions or outputs by analyzing the attributes of given inputs [11]. ML possesses inherent advantages in processing large datasets compared to traditional statistical approaches. They exhibit greater precision and reproducibility than conventional models and even skilled operators. ML can potentially uncover nuanced information that may not be perceptible to the human eye in specific image-related activities [11,12]. The user’s text is too short to be rewritten academically. In the current era characterized by large-scale datasets, ML techniques can significantly enhance diagnostic accuracy and prognosis [13].
Clinicians consistently face challenges in rehabilitating patients to improve pain management, reduce stiffness, and increase walking capacity. The application of RAGT in rehabilitation has experienced increased prevalence due to its ability to transcend the constraints imposed by the extent of an individual’s muscle paralysis. The provision of recurrent and functional task training by RAGT has been found to elicit increased activity in the sensorimotor cortex (specifically, S1 and S2) and the cerebellar areas [14,15]. The convergence of advancements in fundamental neuroscience and technology innovation has presented neurosurgery with distinctive prospects for utilizing ML and RAGT in research and clinical settings to enhance patient care [16].
Moreover, using customized or precision medicine in the context of patients with SCI seems beneficial in customizing expectations and treatment strategies, considering the intrinsic diversity observed within this group in terms of outcomes, functional prognosis, and the rehabilitation process [17,18,19]. This study comprehensively aims to examine the impact of ML on predicting AIS score outcomes and RAGT on rehabilitation outcomes. The focus was on research endeavors to enhance therapeutic advancements and develop predictive models.
2. Material and Methods
2.1. Search Strategy
The PRISMA [20] systematically reviewed four databases, including PubMed, Scopus, ScienceDirect, and the Cochrane Central Register of Controlled Trials (CENTRAL), until 20 August 2023. The MeSH phrases and keywords used in this study encompassed the following: a comprehensive search was executed on research exclusively published in the English language: machine learning, robotics, and spinal cord injury. The reference lists of the published works were examined to identify potential areas for further investigation. In instances where duplicate studies were identified, preference has been given to studies with larger sample sizes. Each study produced the subsequent findings: (1) the initial name and year of publication; (2) the nation and total sample; (3) type of study design; (4) level of injury; (5) ASIA Impairment Scale (AIS) grade; (6) intervention; and (7) outcome.
2.2. Data Selection
Three reviewers (D.P.W.W., S.M., and T.G.B.M.) independently performed the selection. The conflict among the first three reviewers was settled by the establishment of a consensus by the fourth and fifth reviewers. Exclusion of studies occurred in cases where essential outcome measures were absent or not assessed. The included papers should be: (1) a paper that investigated ML and RAGT; (2) research given information on ML and RAGT as well as outcome status; (3) studies that provide the computation data for the calculation of the total sample, mean, and standard deviation (SD); and (4) a full-text article. The protocol for this review was registered in PROSPERO, with the registration number CRD42023464103. The publication was subsequently developed according to PRISMA principles.
2.3. Data Extraction
The relevant data were extracted using a pre-established Google Sheets Excel Online form by two reviewers (D.P.W.W. and S.W.) who worked independently. Any discrepancies were identified and resolved through consensus with a senior reviewer (S.M.). When data was absent or doubts arose, we initiated electronic correspondence with the authors via email to acquire the necessary data.
2.4. Risk of Bias
Two authors (D.P.W.W. and S.M.) independently evaluated the bias quality of the chosen randomized controlled trials (RCTs) using the Cochrane risk of bias assessment methodology [20]. Two authors have used the Newcastle Ottawa Scale (NOS) [21] to evaluate the chosen articles’ methodological quality independently. We divided the articles’ overall quality into moderate (4–6) and high (7–9). Any potential conflicts were effectively resolved by open dialogue and the attainment of mutual agreement facilitated by the involvement of the third author (S.W.).
2.5. Statistical Analysis
RCTs and non-RCTs were categorized into separate groups and subjected to individual studies afterward. The treatment impact was analyzed using Comprehensive Meta-Analysis (CMA) version 3 through statistical analysis. The mean differences (MD), odds ratio (OR), and 95% CI were computed for outcome measures. We use a random effect model for the analysis. The application of a random effect model offers distinct advantages over a fixed effect model due to its ability to effectively capture the entirety of the population under study.
3. Results
3.1. Search Results and Study Characteristics
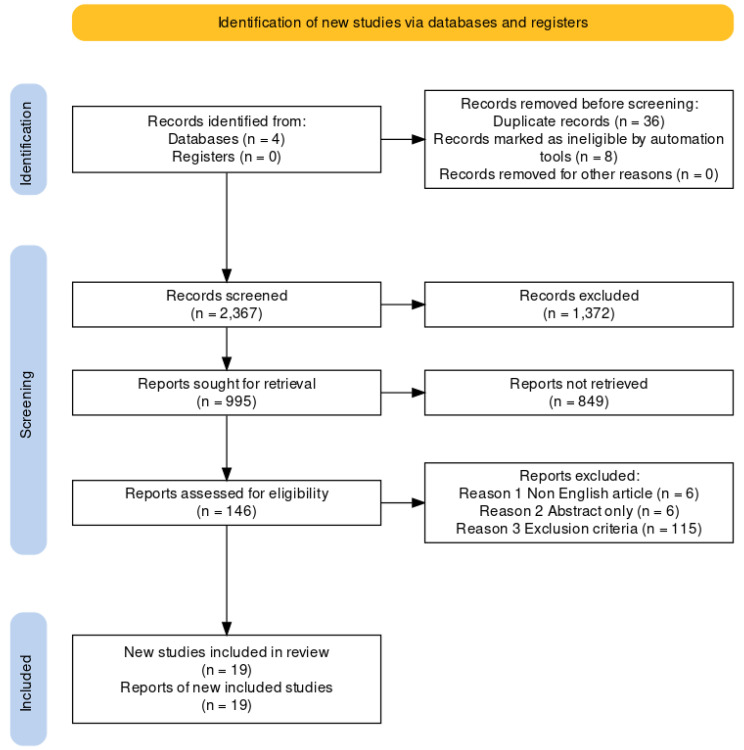
A comprehensive search was conducted across four databases, identifying 2367 articles following rigorous data filtering. A cumulative sum of 127 papers was deemed ineligible for inclusion in the study due to their failure to match the predetermined criteria for research inclusion in Figure 1. Ultimately, a total of 19 publications were selected for further research. The combined sample size of the papers included in this study was 1508 patients. The sample comprised 16 publications utilizing ML techniques and three articles using RAGT techniques. The articles were sourced from various nations, including the USA, Canada, Japan, Italy, Spain, Republic of Korea, and Switzerland.
Figure 1.
The flowchart depicts the process of selecting studies.
The analysis incorporated both RCT and non-RCT study designs. The outcomes examined in the RAGT group encompassed measures such as the Ashworth Scale (AS), Modified Ashworth Scale (MAS), Visual Analog Scale (VAS), Lower Extremity Motor Score (LEMS), 6 Minute Walk Test (6MWT), 10 Meter Walk Test (10MWT), and Timed Up and Go Test (TUG). In the context of the ML group, an examination was conducted on the AIS grade result. The highest marks obtained from the collection of 13 articles are AIS C and D. The OR (95% CI) analysis was employed for the ML group. In contrast, the mean differences (MD) were utilized for the RAGT group. The features of the studies are presented in Table 1 and Table 2.
Table 1.
RAGT concise overview of the chosen papers’ key characteristics and bias risk.
| Author, Year | Country | Total Sample (TS) | Study Design | Intervention | Outcome | Level of Injury | NOS |
|---|---|---|---|---|---|---|---|
| Hornby 2005 [22] | USA | 30 | RCT | The utilization of robotic assistance in BWSTT and therapist-assisted BWSTT. The intervention involved engaging in overground ambulation via a mobile suspension device for three 30 min weekly sessions over 8 weeks. | LEMS, 6MWT | AIS B, C, and D. The level of damage is located above the tenth thoracic vertebra (T10). | - |
| Wirz 2005 [23] | USA | 20 | Single group | The Lokomat (DGO) intervention consisted of an 8 week duration, with participants attending three to five sessions per week, each lasting 45 min. | AS, LEMS, 6MWT, 10MWT, and TUG | AIS C and D. The level of injury is at L1 or above. | 7 |
| Field 2011 [24] | USA | 64 | RCT | The participants engaged in a training regimen for 12 weeks, with a frequency of 5 days per week. The training program encompassed four distinct modalities, namely treadmill-based training with manual help (TM), treadmill-based training with stimulation (TS), overground training with motivation (OG), and treadmill-based training with robotic assistance (LR). | LEMS | AIS C and D. The level of damage is located at or above the tenth thoracic vertebra (T10). | - |
| Alcobendas 2012 [25] | Spain | 75 | RCT | The study consisted of a total of 40 sessions conducted over 8 weeks. Each session lasted for approximately 1 h and involved a Lokomat group intervention. Specifically, participants spent 30 min utilizing the Lokomat device within each session, followed by an additional 30 min of normal physical treatment. The overground group implemented a standardized biological treatment protocol for one hour. | VAS, LEMS, 6MWT, and 10MWT | AIS C and D. The range of injuries observed in the individual spans from the second cervical vertebra (C2) to the twelfth thoracic vertebra (T12). | - |
| Aach 2014 [26] | Germany | 8 | Pre-post experimental design | HAL had been used for 90 days, with a frequency of five weekly sessions. | LEMS, 6MWT, 10MWT, and TUG | ASIA A. Degree of damage: T8 to L2 | 7 |
| del-Ama 2014 [27] | Switzerland | 3 | Pilot study | The Kinesis system was implemented during the first week, whereas no intervention was delivered the following week. | AS, VAS, 6MWT, and 10MWT | AIS A and D. Injuries impact the spinal levels encompassing L1 and L2. | 8 |
| Labruyère 2014 [28] | Switzerland | 9 | RCT | The first group underwent 16 sessions of RAGT using the Lokomat device, followed by an additional 16 strength training sessions. Group 2 received the intervention in reverse order. | VAS, LEMS, and 10MWT | AIS C and D. The extent of the injury ranges from the fourth cervical vertebra (C4) to the eleventh thoracic vertebra (T11). | - |
| Niu 2014 [29] | USA | 40 | RCT | The experimental group underwent twelve 1 h Lokomat training sessions over one month, whereas the control group did not receive any interventions. | TUG | AIS B, C, and D. The level of damage is located above the tenth thoracic vertebra (T10). | - |
| Shin 2014 [30] | South Korea | 53 | RCT | In four weeks, the RAGT group received three 40 min sessions per week of RAGT in addition to regular physiotherapy. The conventional group received physiotherapy twice daily, five days per week. | LEMS | AIS D. The level of injury is classified as upper motor neuron (UMN) involvement. | - |
| Varoqui 2014 [31] | USA | 30 | RCT | The Lokomat group participated in three weekly sessions for four weeks, each lasting one hour. The control group, on the other hand, did not receive any intervention. | 6MWT, 10MWT, TUG | AIS C and D. The level of damage is located above the tenth thoracic vertebra (T10). | - |
| Duffell 2015 [32] | USA | 56 | RCT | The study involved allocating participants with an incomplete SCI into three groups: a control group receiving no intervention, a group receiving Lokomat intervention, and a group receiving tizanidine intervention. | TUG | AIS C and D The level of damage is located above the tenth thoracic vertebra (T10). | - |
| Lam 2015 [33] | Canada | 15 | RCT | The Lokomat-assisted BWSTT intervention was conducted for 45 min, three times a week, for three months. | 6MWT, 10MWT | AIS C and D. Exclusion criteria encompassed individuals with lower motoneuron damage or lesion levels than T11. | - |
| Stampacchia 2016 [34] | Italy | 21 | Single group | The robotic exoskeleton (Ekso GT) exercise lasted approximately 40 min. | MAS, VAS | AIS A, B, and D. The observed lesions were located at the low cervical level (C7), dorsal level, and high lumbar level (L1–L2). | 7 |
| Mazzoleni 2017 [35] | Italy | 7 | Single group | The study consisted of 20 sessions, with a frequency of three sessions per week. The first set of sessions utilized a FES cycling system called Pegaso. It was followed by another group of 20 sessions, again with a frequency of three sessions per week, where participants used an overground robotic exoskeleton called the Ekso GT. | MAS, VAS, 6MWT, 10MWT, and TUG | AIS A. Injury severity: T4–T12 | 7 |
| Watanabe 2019 [36] | Japan | 2 | Case report | HAL has been used 3–4 times weekly for eight sessions. It is performed with regular physical therapy, each lasting approximately 20–30 min. | MAS, LEMS | AIS C and D Injury severity: T8–T10, L1 | 7 |
| Wirz 2017 [37] | USA | 21 | RCT | The intervention group received a training duration of 50 min per session, while the control group had a training duration of 25 min per session using the Lokomat device. Both groups underwent training sessions 3–5 days per week for a total period of 8 weeks. | 10MWT | AIS B and C. C4 to T12 are affected. | - |
AS: Ashworth Scale; MAS: Modified Ashworth Scale; VAS: Visual Analog Scale; 6MWT: 6 Minute Walk Test; 10MWT: 10 Meter Walking Test; LEMS: Lower Extremity Motor Score; TUG: Timed Up And Go Test; BWSTT: Body Weight-Supported Treadmill Training; HAL: Hybrid Assistive Limb; FES: Functional Electrical Stimulation; AIS: ASIA Impairment Scale; SCI: Spinal Cord Injury; NOS: Newcastle Ottawa Scale.
Table 2.
ML concise overview of the chosen papers’ key characteristics and bias risk.
| Author, Year | Country | Total Sample (TS) | Study Design | Intervention | Outcome | AIS Grade | NOS |
|---|---|---|---|---|---|---|---|
| DeVries 2019 [38] | Canada | 862 | Retrospective | The comparison of unsupervised MLA and LR, utilizing comprehensive neurological data for total admission, did not reveal any clinically significant disparities in functional prediction compared to previous models. | The F1-score has been demonstrated to possess greater reliability in evaluating algorithms than the area under the operating curve. | AIS A, B, C, and D | 8 |
| Torres 2021 [39] | USA | 118 | Retrospective | A similar network has been developed among patients to predict neurological recovery following spinal cord damage, focusing on MAP recorded before surgery. | The findings from the network analysis indicate that deviations from the optimal MAP range, either in the form of hypotension or hypertension, during surgical procedures are correlated with a reduced probability of achieving neurological recovery. | AIS A, B, C, D, and E | 8 |
| Agarwal 2022 [40] | USA | 74 | Retrospective | This study uses a deep-tree-based machine learning approach to evaluate the impact of intraoperative MAP and vasopressor administration on enhancing neurological outcomes in individuals with acute spinal cord injury. | An association between a MAP ranging from 80 to 96 mmHg and enhanced neurological function has been observed. Conversely, 93 min or more spent outside the MAP range of 76 to 104 mmHg had been associated with a worse outcome. | AIS A, B, C, D, and E | 7 |
MLA: Machine Learning Algorithms; LR: Logistic Regression; MAP: Mean Arterial Pressure; NOS: Newcastle Ottawa Scale.
3.2. Bias Assessment
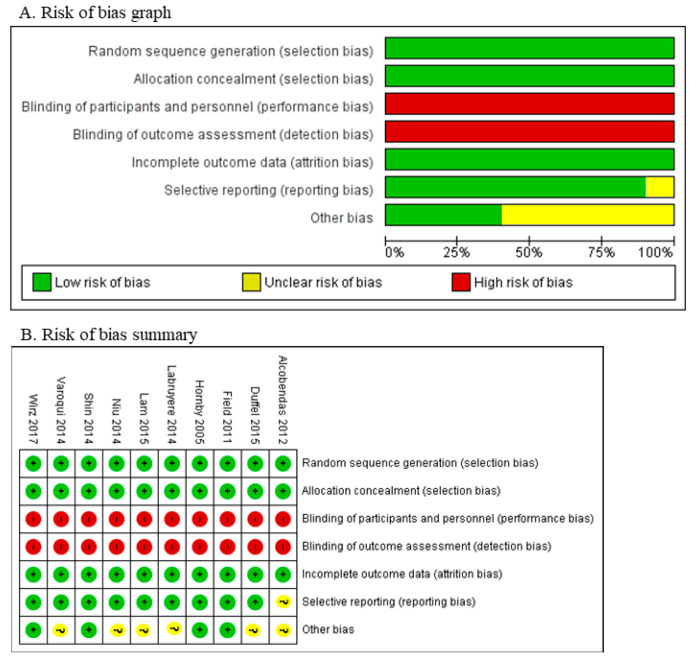
All studies considered in the analysis demonstrate a minimal likelihood of selection bias. The existence of a wide range of rehabilitation procedures in numerous research studies has led to a significant occurrence of performance and detection bias. All research investigations demonstrate a low-risk level of attrition and reporting bias. Several studies exhibit a lack of clarity concerning potential biases, including issues related to loss of follow-up in Figure 2, Table 1 and Table 2.
Figure 2.
The risk of bias include the study are Alcobendas et al., 2012 [25]; Duffel et al., 2015 [32]; Field et al., 2011 [24]; Hornby et al., 2005 [22]; Labruyère et al., 2014 [28]; Lam et al., 2015 [33]; Niu et al., 2014 [29]; Shin et al., 2014 [30]; Varoqui et al., 2014 [31]; Wirz et al., 2017 [37].
3.3. Analysis of the ML Group
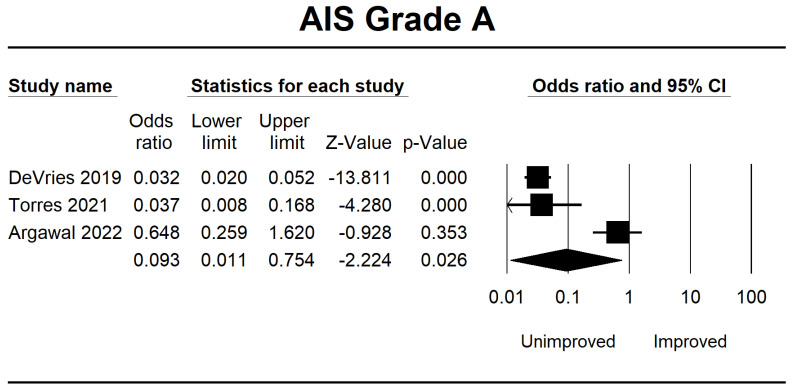
The analysis of all AIS grades A, B, C, and D included three RCT articles that fulfilled the inclusion criteria. We gathered data on the ability of ML to forecast outcomes based on the AIS score upon the patient’s initial hospital admission. We categorized these results into two groups: unimproved and improved in the AIS score. According to the findings of a meta-analysis, predicting using ML in SCI patients with AIS grade A does not improve their condition after re-evaluation following therapy. It may happen due to the presence of a complete injury. The results of the OR CI 95% for AIS grade A were 0.093 (0.011–0.754, p = 0.026).
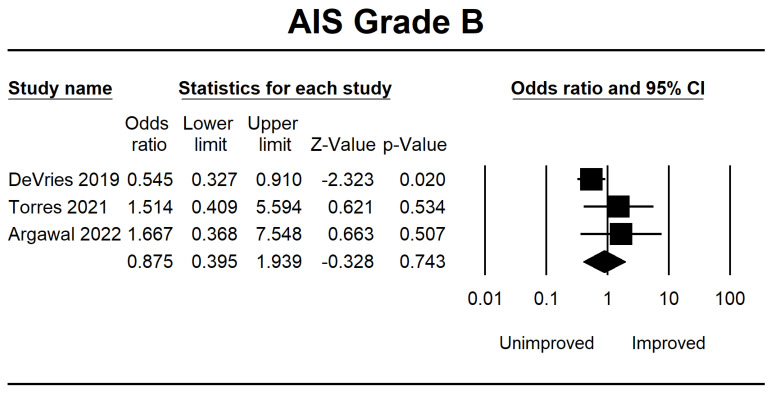
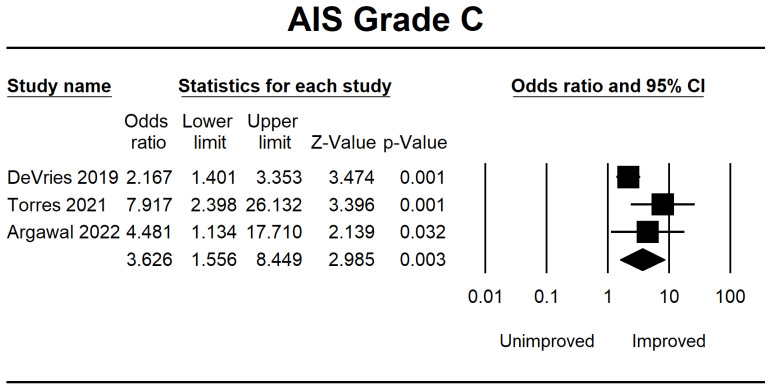
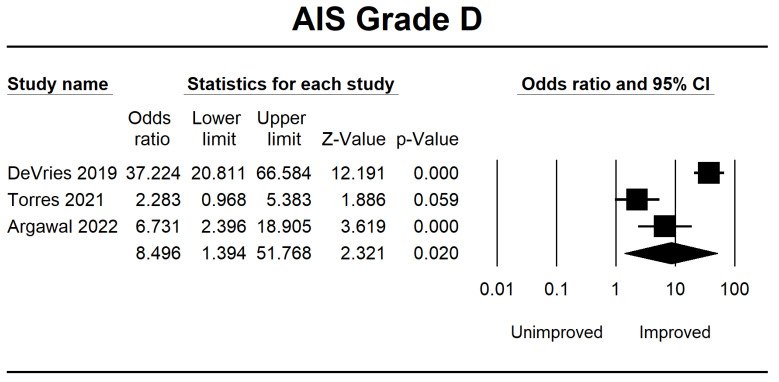
Meanwhile, in AIS B, several patients demonstrated progress in the forest plot, but this outcome is because there is no significant difference of 0.875 (0.395–1.939, p = 0.743). Considerable improvement in AIS grade C was 3.626 (1.556–8.449, p = 0.003), and AIS grade D was 8.496 (1.394–51.768, p = 0.020). The final result is shown in Figure 3, Figure 4, Figure 5 and Figure 6.
Figure 3.
Forest plot of AIS grade A using OR ratio analysis between unimproved and improved prediction groups. The square box represents the point estimate for the respective study, while the horizontal line is the 95% CI. The diamonds represent pooled results. DeVries et al., 2009 [38]; Torres et al., 2021 [39]; Agarwal et al., 2022 [40].
Figure 4.
Forest plot of AIS grade B using OR ratio analysis between unimproved and improved prediction groups. The square box represents the point estimate for the respective study, while the horizontal line is the 95% CI. The diamonds represent pooled results. DeVries et al., 2009 [38]; Torres et al., 2021 [39]; Agarwal et al., 2022 [40].
Figure 5.
Forest plot of AIS grade C using OR ratio analysis between unimproved and improved prediction groups. The square box represents the point estimate for the respective study, while the horizontal line is the 95% CI. The diamonds represent pooled results. DeVries et al., 2009 [38]; Torres et al., 2021 [39]; Agarwal et al., 2022 [40].
Figure 6.
Forest plot of AIS grade D using OR ratio analysis between unimproved and improved prediction groups. The square box represents the point estimate for the respective study, while the horizontal line is the 95% CI. The diamonds represent pooled results. DeVries et al., 2009 [38]; Torres et al., 2021 [39]; Agarwal et al., 2022 [40].
3.4. Analysis of the RAGT Group
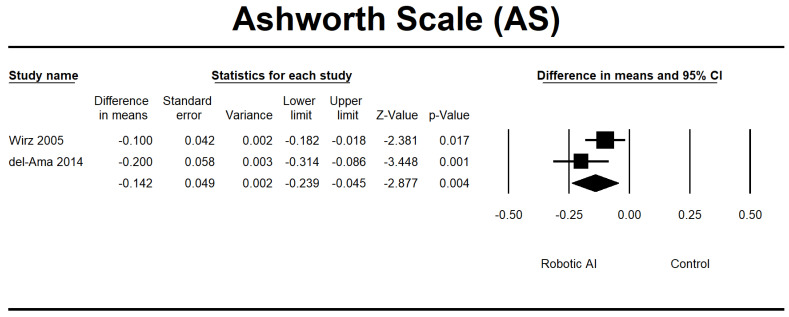
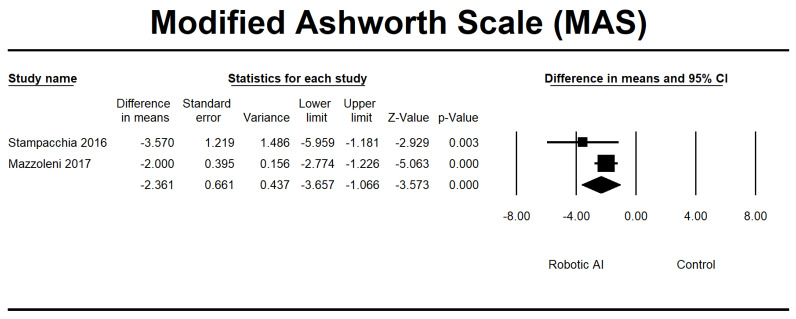
Four RCTs [23,28,30,32] were conducted to evaluate the effects of interventions on spasticity. In the conducted investigations, all participants’ spasticity levels were categorized as mild, as indicated by a MAS score ranging from 0 to 2. Furthermore, it was noted that there were no significant alterations in spasticity levels following the implementation of RAGT. The robotic group exhibited a notable reduction in AS (95% CI = −0.239 to −0.045, p = 0.004) and MAS (95% CI = −3.657 to −1.066, p ≤ 0.001) measures. The pooled MD using MAS and AS was −2.149 and −0.142, respectively (Figure 7 and Figure 8).
Figure 7.
Forest plot of AS using standardized mean difference analysis between robotic and control groups. The square box represents the mean differences for the respective study, while the horizontal line is the 95% CI. The diamonds represent pooled results. Wirz et al., 2005 [23]; del-Ama et al., 2014 [27].
Figure 8.
Forest plot of MAS using standardized mean difference analysis between robotic and control groups. The square box represents the mean differences for the respective study, while the horizontal line is the 95% CI. The diamonds represent pooled results. Stampacchia et al., 2016 [34]; Mazzoleni et al., 2017 [35].
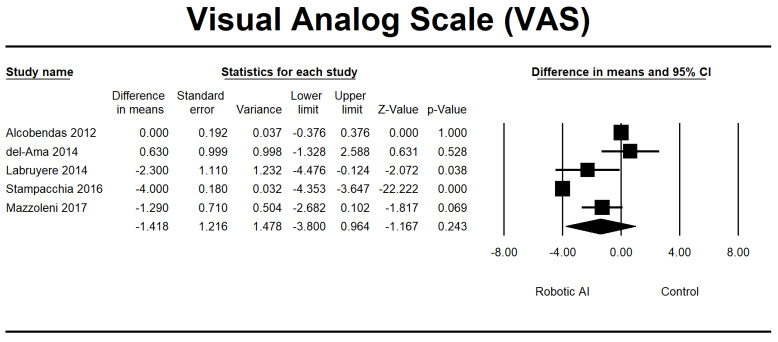
We also analyzed the pain parameter using the VAS variable. The findings from the analysis of the primary outcomes of pain following RAGT are depicted in Figure 9, consisting of two RCTs [28,32] and three non-RCTs [22,29,37]. Despite the observed trend indicating a potential reduction in pain in the robotic group, there was no statistically significant difference between the robotic and control groups. This lack of significance was consistent in the analysis (p = 0.243). The pooled MD was −1.418. The studies included in the research reported various pain levels, varying from mild to moderate.
Figure 9.
Forest plot of VAS using standardized mean difference analysis between robotic and control groups. The square box represents the mean differences for the respective study, while the horizontal line is the 95% CI. The diamonds represent pooled results. Alcobendas et al., 2012 [25]; del-Ama et al., 2014 [27]; Labruyère et al., 2014 [28]; Stampacchia et al., 2016 [34]; Mazzoleni et al., 2017 [35].
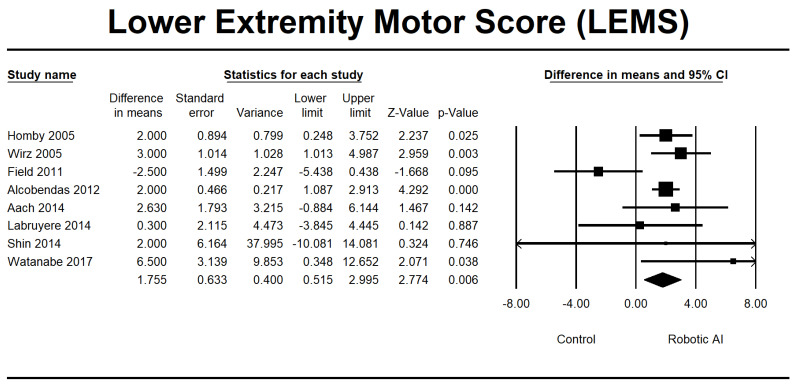
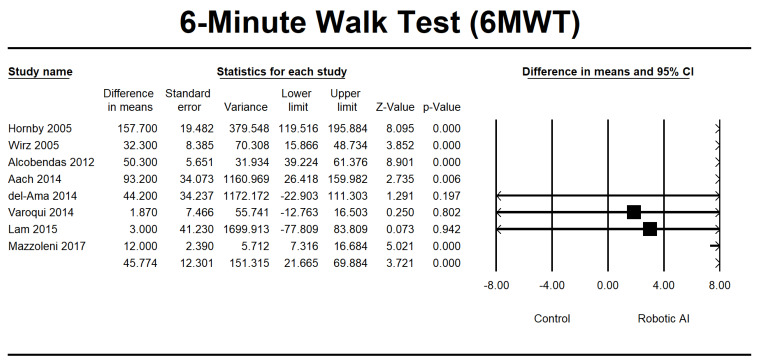
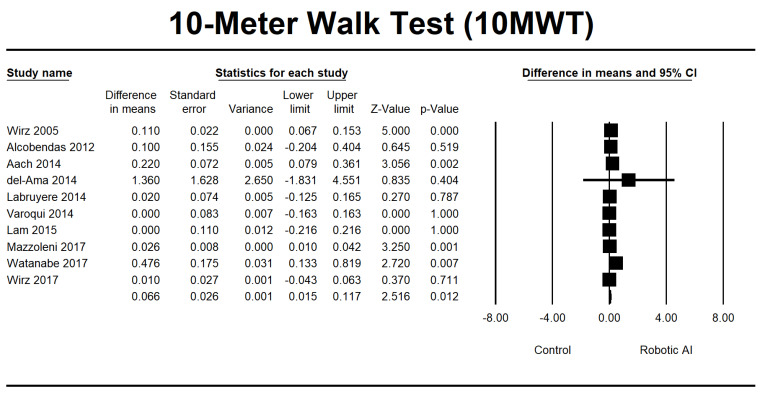
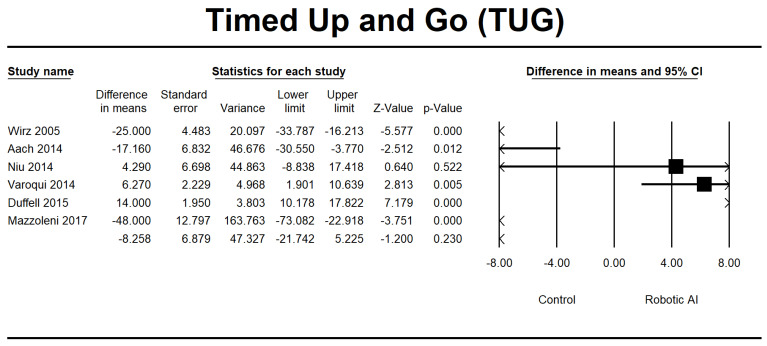
This study investigated walking ability by combining the LEMS, 6MWT, 10MWT, and TUG group analyses. In the LEMS analysis, we found five RCTs [22,24,25,28,29] and three non-RCTs [23,26,37] with statistically significant beneficial effects in favor of the robotic group (95% [CI] = 0.515 to 2.995, p ≤ 0.05). The mean difference is 1.755, as depicted in Figure 10. The 6MWT is a commonly used assessment tool in four RCTs [22,25,30,32], and four non-RCTs [23,26,27,34] were conducted to evaluate the 6MWT. Irrespective of the type of study design, there was a significant increase in walking distance in the group that received robotic assistance. The CI is 95% (21.665–69.884, p ≤ 0.001), with MD 45.774, as shown in Figure 11. The 10MWT comprises five RCTs [25,28,30,32,36] and five non-RCTs [23,26,27,34,37]. The 10MWT demonstrated a substantial improvement in the robotic group, as indicated by CI 95% 0.015–0.117, p = 0.012, with MD 0.066, as shown in Figure 12. The TUG study comprised a total of three RCTs [28,30,31]. The findings indicated a noteworthy enhancement in favor of the robotic group, with a CI of 95% −21.742 to 5.225, p = 0.230. The MD obtained by pooling the data using a random effects model was −8.258, as shown in Figure 13.
Figure 10.
Forest plot of LEMS using standardized mean difference analysis between robotic and control groups. The square box represents the mean differences for the respective study, while the horizontal line is the 95% CI. The diamonds represent pooled results. Hornby et al., 2005 [22]; Wirz et al., 2005 [23]; Field et al., 2011 [24]; Alcobendas et al., 2012 [25]; Aach et al., 2014 [26]; Labruyère et al., 2014 [28]; Shin et al., 2014 [30]; Watanabe et al., 2019 [36].
Figure 11.
Forest plot of 6MWT using standardized mean difference analysis between robotic and control groups. The square box represents the mean differences for the respective study, while the horizontal line is the 95% CI. The diamonds represent pooled results. Hornby et al., 2005 [22]; Wirz et al., 2005 [23]; Alcobendas et al., 2012 [25]; Aach et al., 2014 [26]; del-Ama et al., 2014 [27]; Varoqui et al., 2014 [31]; Lam et al., 2015 [33]; Mazzoleni et al., 2017 [35].
Figure 12.
Forest plot of 10MWT using standardized mean difference analysis between robotic and control groups. The square box represents the mean differences for the respective study, while the horizontal line is the 95% CI. The diamonds represent pooled results. Wirz et al., 2005 [23]; Alcobendas et al., 2012 [25]; Aach et al., 2014 [26]; del-Ama et al., 2014 [27]; Labruyère et al., 2014 [28]; Varoqui et al., 2014 [31]; Lam et al., 2015 [33]; Mazzoleni et al., 2017 [35]; Watanabe et al., 2019 [36]; Wirz et al., 2017 [37].
Figure 13.
Forest plot of TUG using standardized mean difference analysis between robotic and control groups. The square box represents the mean differences for the respective study, while the horizontal line is the 95% CI. The diamonds represent pooled results. Wirz et al., 2005 [23]; Aach et al., 2014 [26]; Niu et al., 2014 [29]; Varoqui et al., 2014 [31]; Duffel et al., 2015 [32]; Mazzoleni et al., 2017 [35].
4. Discussion
The findings demonstrated encouraging outcomes in forecasting the improvement of AIS. Clinicians have used the AIS to categorize SCI and assess the extent of recovery. It may involve documenting enhancements, such as an improvement in AIS grade or deteriorations [41]. Within the confines of a conventional clinical environment, the primary determinants recognized for the prognostication of SCI recovery encompass patient age, patient gender, duration of hospitalization, manner of hospital release, SCI classification, procedural timing, nature of procedure, and presence of comorbidities. The prognosis of SCI is typically assessed using bedside evaluation and MRI or through classic clinical analysis, such as the calculation of odds ratios [42]. Hence, it is possible to identify the factors that can predict the recovery of SCI by incorporating the initial AIS scores into ML algorithms. This framework leverages big data and precision medicine, serving as a valuable tool for clinicians to enhance the overall prognosis of SCI patients. The current ML study [43] demonstrates a higher test accuracy of 73.6% than the MRI accuracy of 71.4%. A few studies [43,44,45,46,47] collectively utilize a patient sample size that is one to two times larger and incorporates a comprehensive evaluation of feature importance. Furthermore, even considering all AIS grades and employing a far less complex model that can be readily implemented, the outcomes are generally similar or superior.
The reduction of spasticity can be attributed to various theoretical frameworks. The RAGT technique elicits rhythmic movements in the lower limbs and offers sensory input. Prior research has indicated that rhythmic passive exercise has the potential to cause the reorganization of spinal circuitry and reduce stiffness in individuals with SCI [12]. The potential impact of repetitive elements within a therapy program on the enhancement of spasticity and locomotor function through the stimulation of spinal locomotor centers has been suggested [48]. Repetitive functional task training, sometimes called RAGT, represents a form of intervention that involves repeating available tasks. The mechanisms above could explain the observed reduction in spasticity resulting from RAGT [48]. As previously mentioned, despite decreasing spasticity, RAGT improves the detection of rhythmic muscle activations.
Furthermore, it is worth considering the significance of weight bearing as a contributing component. RAGT offers assistance that enables individuals to apply load to their lower extremities while engaging in training activities. The application of weight bearing on the lower limbs and the subsequent increase in muscle activation can positively impact the recovery of lower extremity motor function in individuals with LEMS. Furthermore, the findings of this meta-analysis indicate that the 6MWT can enhance endurance levels without imposing the strain associated with deliberate muscular contractions [34]. As evidenced by the findings of the LEMS improvement results, enhanced lower extremity strength probably contributes to an augmentation in walking speed, as observed in the 10MWT variable [49].
5. Limitations
One constraint of the analysis is the methodology employed for data collection in non-RCTs, particularly in the context of informing predictive modeling. It is generally acknowledged that prospective approaches are more suitable for developing accurate predictive models. The existing body of literature on this subject is minimal and exhibits variability in both topic and research design, hindering the possibility of conducting a meta-analysis or facilitating direct comparisons. Undoubtedly, the degree of injury has been demonstrated as a fundamental determinant in predicting the long-term functional result. Ultimately, models must undergo external validation and be meticulously implemented before their utilization and dependence in clinical settings. Regrettably, the identified articles lacked specific descriptions of the symptoms exhibited by patients. Furthermore, most of the research evaluated treatment efficacy solely during a predetermined timeframe, impeding our ability to examine this aspect comprehensively. The limitations of our study include the potential for future research to explore and offer additional insights into the symptoms and follow-up duration.
6. Conclusions
The ML approach exhibited enhanced precision in forecasting AIS result scores. The implementation of RAGT has been shown to positively impact the reduction of spasticity and the improvement of walking ability. The implementation of RAGT has been proven to be beneficial in the normalization of muscle tone and enhancement of lower extremity function. The presence of variability among individuals with SCI presents a distinct and advantageous prospect for AI to facilitate desired results and evaluate risk within this specific group of patients.
Author Contributions
Participated in formulation and development of the study’s conceptualization and design, D.P.W.W., S.M. and S.W.; actively involved in the process of performing searches within databases and then extracting material that was deemed relevant and of interest, T.G.B.M. and R.M.R.; participated in the process of analyzing and interpreting data, D.P.W.W. and S.W.; manuscript—draft, S.M., S.W. and T.G.B.M. The article has been critically reviewed by R.M.R. and D.P.W.W. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
This study did not include generating or analyzing new data. Sharing data is neither relevant nor appropriate to this article’s subject matter.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Lee B.B., Cripps R.A., Fitzharris M., Wing P.C. The Global Map for Traumatic Spinal Cord Injury Epidemiology: Update 2011, Global Incidence Rate. Spinal Cord. 2014;52:110–116. doi: 10.1038/sc.2012.158. [DOI] [PubMed] [Google Scholar]
- 2.Fallah N., Noonan V.K., Waheed Z., Rivers C.S., Plashkes T., Bedi M., Etminan M., Thorogood N.P., Ailon T., Chan E., et al. Development of a Machine Learning Algorithm for Predicting In-Hospital and 1-Year Mortality after Traumatic Spinal Cord Injury. Spine J. 2022;22:329–336. doi: 10.1016/j.spinee.2021.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Varma A., Hill E.G., Nicholas J., Selassie A. Predictors of Early Mortality after Traumatic Spinal Cord Injury: A Population-Based Study. Spine. 2010;35:778–783. doi: 10.1097/BRS.0b013e3181ba1359. [DOI] [PubMed] [Google Scholar]
- 4.Chamberlain J.D., Meier S., Mader L., von Groote P.M., Brinkhof M.W.G. Mortality and Longevity after a Spinal Cord Injury: Systematic Review and Meta-Analysis. Neuroepidemiology. 2015;44:182–198. doi: 10.1159/000382079. [DOI] [PubMed] [Google Scholar]
- 5.Cao Y., Krause J.S., DiPiro N. Risk Factors for Mortality after Spinal Cord Injury in the USA. Spinal Cord. 2013;51:413–418. doi: 10.1038/sc.2013.2. [DOI] [PubMed] [Google Scholar]
- 6.Shibahashi K., Nishida M., Okura Y., Hamabe Y. Epidemiological State, Predictors of Early Mortality, and Predictive Models for Traumatic Spinal Cord Injury: A Multicenter Nationwide Cohort Study. Spine. 2019;44:479–487. doi: 10.1097/BRS.0000000000002871. [DOI] [PubMed] [Google Scholar]
- 7.Azarhomayoun A., Aghasi M., Mousavi N., Shokraneh F., Vaccaro A.R., Haj Mirzaian A., Derakhshan P., Rahimi-Movaghar V. Mortality Rate and Predicting Factors of Traumatic Thoracolumbar Spinal Cord Injury; A Systematic Review and Meta-Analysis. Bull. Emerg. Trauma. 2018;6:181–194. doi: 10.29252/beat-060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bank M., Gibbs K., Sison C., Kutub N., Papatheodorou A., Lee S., Stein A., Bloom O. Age and Other Risk Factors Influencing Long-Term Mortality in Patients With Traumatic Cervical Spine Fracture. Geriatr. Orthop. Surg. Rehabil. 2018;9:2151459318770882. doi: 10.1177/2151459318770882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raju B., Jumah F., Ashraf O., Narayan V., Gupta G., Sun H., Hilden P., Nanda A. Big Data, Machine Learning, and Artificial Intelligence: A Field Guide for Neurosurgeons. J. Neurosurg. 2020;135:373–383. doi: 10.3171/2020.5.JNS201288. [DOI] [PubMed] [Google Scholar]
- 10.Mesbah S., Ball T., Angeli C., Rejc E., Dietz N., Ugiliweneza B., Harkema S., Boakye M. Predictors of Volitional Motor Recovery with Epidural Stimulation in Individuals with Chronic Spinal Cord Injury. Brain A J. Neurol. 2021;144:420–433. doi: 10.1093/brain/awaa423. [DOI] [PubMed] [Google Scholar]
- 11.Jordan M.I., Mitchell T.M. Machine Learning: Trends, Perspectives, and Prospects. Science. 2015;349:255–260. doi: 10.1126/science.aaa8415. [DOI] [PubMed] [Google Scholar]
- 12.Chang M., Canseco J.A., Nicholson K.J., Patel N., Vaccaro A.R. The Role of Machine Learning in Spine Surgery: The Future Is Now. Front. Surg. 2020;7:54. doi: 10.3389/fsurg.2020.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galbusera F., Casaroli G., Bassani T. Artificial Intelligence and Machine Learning in Spine Research. JOR Spine. 2019;2:e1044. doi: 10.1002/jsp2.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sliwinski C., Nees T.A., Puttagunta R., Weidner N., Blesch A. Sensorimotor Activity Partially Ameliorates Pain and Reduces Nociceptive Fiber Density in the Chronically Injured Spinal Cord. J. Neurotrauma. 2018;35:2222–2238. doi: 10.1089/neu.2017.5431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Winchester P., McColl R., Querry R., Foreman N., Mosby J., Tansey K., Williamson J. Changes in Supraspinal Activation Patterns Following Robotic Locomotor Therapy in Motor-Incomplete Spinal Cord Injury. Neurorehabilit. Neural Repair. 2005;19:313–324. doi: 10.1177/1545968305281515. [DOI] [PubMed] [Google Scholar]
- 16.Obermeyer Z., Emanuel E.J. Predicting the Future—Big Data, Machine Learning, and Clinical Medicine. N. Engl. J. Med. 2016;375:1216–1219. doi: 10.1056/NEJMp1606181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khan O., Badhiwala J.H., Grasso G., Fehlings M.G. Use of Machine Learning and Artificial Intelligence to Drive Personalized Medicine Approaches for Spine Care. World Neurosurg. 2020;140:512–518. doi: 10.1016/j.wneu.2020.04.022. [DOI] [PubMed] [Google Scholar]
- 18.Dietz N., Wagers S., Harkema S.J., D’Amico J.M. Intrathecal and Oral Baclofen Use in Adults With Spinal Cord Injury: A Systematic Review of Efficacy in Spasticity Reduction, Functional Changes, Dosing, and Adverse Events. Arch. Phys. Med. Rehabil. 2023;104:119–131. doi: 10.1016/j.apmr.2022.05.011. [DOI] [PubMed] [Google Scholar]
- 19.Ter Wengel P.V., Post M.W.M., Martin E., Stolwijk-Swuste J., Hosman A.J.F., Sadiqi S., Vandertop W.P., Öner F.C. Neurological Recovery after Traumatic Spinal Cord Injury: What Is Meaningful? A Patients’ and Physicians’ Perspective. Spinal Cord. 2020;58:865–872. doi: 10.1038/s41393-020-0436-4. [DOI] [PubMed] [Google Scholar]
- 20.Higgins J.P.T., Altman D.G. Cochrane Handbook for Systematic Reviews of Interventions. Wiley; Hoboken, NJ, USA: 2008. Assessing Risk of Bias in Included Studies; pp. 187–241. [Google Scholar]
- 21.Stang A. Critical Evaluation of the Newcastle-Ottawa Scale for the Assessment of the Quality of Nonrandomized Studies in Meta-Analyses. Eur. J. Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 22.Hornby T.G., Campbell D.D., Zemon D.H., Kahn J.H. Clinical and Quantitative Evaluation of Robotic-Assisted Treadmill Walking to Retrain Ambulation after Spinal Cord Injury. Top. Spinal Cord Inj. Rehabil. 2005;11:1–17. doi: 10.1310/14Q9-AD7M-FXX9-1G2J. [DOI] [Google Scholar]
- 23.Wirz M., Zemon D.H., Rupp R., Scheel A., Colombo G., Dietz V., Hornby T.G. Effectiveness of Automated Locomotor Training in Patients with Chronic Incomplete Spinal Cord Injury: A Multicenter Trial. Arch. Phys. Med. Rehabil. 2005;86:672–680. doi: 10.1016/j.apmr.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 24.Field-Fote E.C., Roach K.E. Influence of a Locomotor Training Approach on Walking Speed and Distance in People with Chronic Spinal Cord Injury: A Randomized Clinical Trial. Phys. Ther. 2011;91:48–60. doi: 10.2522/ptj.20090359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alcobendas-Maestro M., Esclarín-Ruz A., Casado-López R.M., Muñoz-González A., Pérez-Mateos G., González-Valdizán E., Martín J.L.R. Lokomat Robotic-Assisted Versus Overground Training Within 3 to 6 Months of Incomplete Spinal Cord Lesion: Randomized Controlled Trial. Neurorehabilit. Neural Repair. 2012;26:1058–1063. doi: 10.1177/1545968312448232. [DOI] [PubMed] [Google Scholar]
- 26.Aach M., Cruciger O., Sczesny-Kaiser M., Höffken O., Meindl R.C., Tegenthoff M., Schwenkreis P., Sankai Y., Schildhauer T.A. Voluntary Driven Exoskeleton as a New Tool for Rehabilitation in Chronic Spinal Cord Injury: A Pilot Study. Spine J. Off. J. North Am. Spine Soc. 2014;14:2847–2853. doi: 10.1016/j.spinee.2014.03.042. [DOI] [PubMed] [Google Scholar]
- 27.Del-Ama A.J., Gil-Agudo Á., Pons J.L., Moreno J.C. Hybrid Gait Training with an Overground Robot for People with Incomplete Spinal Cord Injury: A Pilot Study. Front. Hum. Neurosci. 2014;8:298. doi: 10.3389/fnhum.2014.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Labruyère R., Van Hedel H.J.A. Strength Training versus Robot-Assisted Gait Training after Incomplete Spinal Cord Injury: A Randomized Pilot Study in Patients Depending on Walking Assistance. J. NeuroEngineering Rehabil. 2014;11:4. doi: 10.1186/1743-0003-11-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niu X., Varoqui D., Kindig M., Mirbagheri M.M. Prediction of Gait Recovery in Spinal Cord Injured Individuals Trained with Robotic Gait Orthosis. J. Neuroeng. Rehabil. 2014;11:42. doi: 10.1186/1743-0003-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shin J.C., Kim J.Y., Park H.K., Kim N.Y. Effect of Robotic-Assisted Gait Training in Patients with Incomplete Spinal Cord Injury. Ann. Rehabil. Med. 2014;38:719–725. doi: 10.5535/arm.2014.38.6.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Varoqui D., Niu X., Mirbagheri M.M. Ankle Voluntary Movement Enhancement Following Robotic-Assisted Locomotor Training in Spinal Cord Injury. J. Neuroeng. Rehabil. 2014;11:46. doi: 10.1186/1743-0003-11-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duffell L.D., Brown G.L., Mirbagheri M.M. Interventions to Reduce Spasticity and Improve Function in People With Chronic Incomplete Spinal Cord Injury: Distinctions Revealed by Different Analytical Methods. Neurorehabilit. Neural Repair. 2015;29:566–576. doi: 10.1177/1545968314558601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lam T., Pauhl K., Ferguson A., Malik R.N., Krassioukov A., Eng J.J. Training with Robot-Applied Resistance in People with Motor-Incomplete Spinal Cord Injury: Pilot Study. J. Rehabil. Res. Dev. 2015;52:113–129. doi: 10.1682/JRRD.2014.03.0090. [DOI] [PubMed] [Google Scholar]
- 34.Stampacchia G., Rustici A., Bigazzi S., Gerini A., Tombini T., Mazzoleni S. Walking with a Powered Robotic Exoskeleton: Subjective Experience, Spasticity and Pain in Spinal Cord Injured Persons. NeuroRehabilitation. 2016;39:277–283. doi: 10.3233/NRE-161358. [DOI] [PubMed] [Google Scholar]
- 35.Mazzoleni S., Battini E., Rustici A., Stampacchia G. An Integrated Gait Rehabilitation Training Based on Functional Electrical Stimulation Cycling and Overground Robotic Exoskeleton in Complete Spinal Cord Injury Patients: Preliminary Results; Proceedings of the 2017 International Conference on Rehabilitation Robotics (ICORR); London, UK. 17–20 July 2017; Piscataway, NJ, USA: IEEE; pp. 289–293. [DOI] [PubMed] [Google Scholar]
- 36.Watanabe H., Marushima A., Kawamoto H., Kadone H., Ueno T., Shimizu Y., Endo A., Hada Y., Saotome K., Abe T., et al. Intensive Gait Treatment Using a Robot Suit Hybrid Assistive Limb in Acute Spinal Cord Infarction: Report of Two Cases. J. Spinal Cord Med. 2019;42:395–401. doi: 10.1080/10790268.2017.1372059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wirz M., MacH O., Maier D., Benito-Penalva J., Taylor J., Esclarin A., DIetz V. Effectiveness of Automated Locomotor Training in Patients with Acute Incomplete Spinal Cord Injury: A Randomized, Controlled, Multicenter Trial. J. Neurotrauma. 2017;34:1891–1896. doi: 10.1089/neu.2016.4643. [DOI] [PubMed] [Google Scholar]
- 38.DeVries Z., Hoda M., Rivers C.S., Maher A., Wai E., Moravek D., Stratton A., Kingwell S., Fallah N., Paquet J., et al. Development of an Unsupervised Machine Learning Algorithm for the Prognostication of Walking Ability in Spinal Cord Injury Patients. Spine J. 2020;20:213–224. doi: 10.1016/j.spinee.2019.09.007. [DOI] [PubMed] [Google Scholar]
- 39.Torres-Espín A., Haefeli J., Ehsanian R., Torres D., Almeida C.A., Huie J.R., Chou A., Morozov D., Sanderson N., Dirlikov B., et al. Topological Network Analysis of Patient Similarity for Precision Management of Acute Blood Pressure in Spinal Cord Injury. eLife. 2021;10:e68015. doi: 10.7554/eLife.68015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Agarwal N., Aabedi A.A., Torres-Espin A., Chou A., Wozny T.A., Mummaneni P.V., Burke J.F., Ferguson A.R., Kyritsis N., Dhall S.S., et al. Decision Tree–Based Machine Learning Analysis of Intraoperative Vasopressor Use to Optimize Neurological Improvement in Acute Spinal Cord Injury. Neurosurg. Focus. 2022;52:E9. doi: 10.3171/2022.1.FOCUS21743. [DOI] [PubMed] [Google Scholar]
- 41.Chay W., Kirshblum S. Predicting Outcomes After Spinal Cord Injury. Phys. Med. Rehabil. Clin. 2020;31:331–343. doi: 10.1016/j.pmr.2020.03.003. [DOI] [PubMed] [Google Scholar]
- 42.Burns A.S., Marino R.J., Flanders A.E., Flett H. Handbook of Clinical Neurology. Volume 109. Elsevier; Amsterdam, The Netherlands: 2012. Clinical Diagnosis and Prognosis Following Spinal Cord Injury; pp. 47–62. [DOI] [PubMed] [Google Scholar]
- 43.Okimatsu S., Maki S., Furuya T., Fujiyoshi T., Kitamura M., Inada T., Aramomi M., Yamauchi T., Miyamoto T., Inoue T., et al. Determining the Short-Term Neurological Prognosis for Acute Cervical Spinal Cord Injury Using Machine Learning. J. Clin. Neurosci. 2022;96:74–79. doi: 10.1016/j.jocn.2021.11.037. [DOI] [PubMed] [Google Scholar]
- 44.Inoue T., Ichikawa D., Ueno T., Cheong M., Inoue T., Whetstone W.D., Endo T., Nizuma K., Tominaga T. XGBoost, a Machine Learning Method, Predicts Neurological Recovery in Patients with Cervical Spinal Cord Injury. Neurotrauma Rep. 2020;1:8–16. doi: 10.1089/neur.2020.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chou A., Torres-Espin A., Kyritsis N., Huie J.R., Khatry S., Funk J., Hay J., Lofgreen A., Shah R., McCann C., et al. Expert-Augmented Automated Machine Learning Optimizes Hemodynamic Predictors of Spinal Cord Injury Outcome. PLoS ONE. 2022;17:e0265254. doi: 10.1371/journal.pone.0265254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fan G., Liu H., Yang S., Luo L., Wang L., Pang M., Liu B., Zhang L., Han L., Rong L. Discharge Prediction of Critical Patients with Spinal Cord Injury: A Machine Learning Study with 1485 Cases 2021. medRxiv. :2021. doi: 10.1101/2021.06.26.21259569. [DOI] [PubMed] [Google Scholar]
- 47.Buri M., Tanadini L.G., Hothorn T., Curt A. Unbiased Recursive Partitioning Enables Robust and Reliable Outcome Prediction in Acute Spinal Cord Injury. J. Neurotrauma. 2022;39:266–276. doi: 10.1089/neu.2020.7407. [DOI] [PubMed] [Google Scholar]
- 48.Dietz V., Sinkjaer T. Handbook of Clinical Neurology. Volume 109. Elsevier; Amsterdam, The Netherlands: 2012. Spasticity; pp. 197–211. [DOI] [PubMed] [Google Scholar]
- 49.Barbeau H., Danakas M., Arsenault B. The Effects of Locomotor Training in Spinal Cord Injured Subjects: A Preliminary Study. Restor. Neurol. Neurosci. 1993;5:81–84. doi: 10.3233/RNN-1993-5122. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study did not include generating or analyzing new data. Sharing data is neither relevant nor appropriate to this article’s subject matter.