Abstract
Background: Pretreatment CT Perfusion (CTP) parameters serve as reliable surrogates of collateral status (CS). In this study, we aim to assess the relationship between the novel compensation index (CI, Tmax > 4 s/Tmax > 6 s) and already established CTP collateral markers, namely cerebral blood volume (CBV) index and Hypoperfusion Intensity Ratio (HIR), with the reference standard American Society of Interventional and Therapeutic Neuroradiology (ASITN) collateral score (CS) on DSA. Methods: In this retrospective study, inclusion criteria were the following: (a) CT angiography confirmed anterior circulation large vessel occlusion from 9 January 2017 to 10 January 2023; (b) diagnostic CT perfusion; and (c) underwent mechanical thrombectomy with documented DSA-CS. Student t-test, Mann–Whitney-U-test and Chi-square test were used to assess differences. Spearman’s rank correlation and logistic regression analysis were used to assess associations. p ≤ 0.05 was considered significant. Results: In total, 223 patients (mean age: 67.8 ± 15.8, 56% female) met our inclusion criteria. The CI (ρ = 0.37, p < 0.001) and HIR (ρ = −0.29, p < 0.001) significantly correlated with DSA-CS. Whereas the CBV Index (ρ = 0.1, p > 0.05) did not correlate with DSA-CS. On multivariate logistic regression analysis taking into account age, sex, ASPECTS, tPA, premorbid mRS, NIH stroke scale, prior history of TIA, stroke, atrial fibrillation, diabetes mellitus, hyperlipidemia, heart disease and hypertension, only CI was not found to be independently associated with DSA-CS (adjusted OR = 1.387, 95% CI: 1.09–1.77, p < 0.01). Conclusion: CI demonstrates a stronger correlation with DSA-CS compared to both the HIR and CBV Index where it may show promise as an additional quantitative pretreatment CS biomarker.
Keywords: acute ischemic stroke, compensation index, collateral status, CBV index, hypoperfusion intensity ratio, HIR
1. Background
Collateral status (CS) is an excellent biomarker associated with ischemic core growth and success of reperfusion in patients with anterior circulation acute ischemic stroke caused by large vessel occlusion (AIS-LVO) [1,2,3,4,5].
Pretreatment CT perfusion (CTP) parameters of the hypoperfusion intensity ratio (HIR) and cerebral blood volume (CBV) index are validated using pretreatment imaging surrogates of CS [6,7,8,9,10,11]. The CBV index is defined as the average CBV in Tmax > 6 s region compared to the average CBV in normal brain tissue [12]. Hypoperfusion intensity ratio (HIR) was defined as the volume of tissue with Tmax > 10 s divided by the volume of tissue with Tmax > 6 s [13]. Both the CBV Index and HIR parameters have each shown a correlation with infarct growth and with clinical outcomes following mechanical thrombectomy (MT) [14,15,16,17,18,19].
Although these CTP markers of CS have been previously validated, none take into account the volume of tissue with a perfusion delay of Tmax > 4 s, which is thought to represent benign oligemia [20]. The volume of benign oligemia directly relates to autoregulation by vasodilation, which is dependent on the patient’s CS [20]. We postulate that Tmax > 4 s may also be a marker of CS where patients with higher Tmax > 4 s and lower Tmax > 6 s volumes are considered a favorable compensatory response through collateral routes. We therefore present the compensation index (CI) as the volume of tissue with Tmax > 4 s divided by the volume of tissue with Tmax > 6 s as a novel quantitative pretreatment CS biomarker.
In this study, we aimed to investigate the relationship between the CBV index, HIR and CI as pretreatment CTP markers of CS with the reference standard ASITN CS. We hypothesize that the CI correlates as well as existing markers of CTP collateral status CBV index and HIR with CS on DSA.
2. Methods
2.1. Study Design
We performed a retrospective analysis of prospectively maintained stroke databases, and we identified consecutive patients from two comprehensive stroke centers from 29 July 2019 to 10 January 2023 who met our inclusion criteria. This study was approved through the Johns Hopkins Institutional Review Board (IRB00269637) and follows the STROBE checklist guidelines as an observational study [21].
2.2. Study Participants
The inclusion criteria for this study were the following: (a) MT triage within 24 h of symptom onset or last known well; (b) diagnostically adequate multimodal pretreatment CT imaging including noncontrast CT (NCCT), CT angiography (CTA) and CTP; (c) AIS due to a CTA confirmed large vessel occlusion of proximal supraclinoid ICA, ICA terminus, MCA occlusion, specifically including M1 and proximal M2 segments of the MCA [22]; and (d) who underwent MT and had recorded ASITN CS [23].
This study was conducted in accordance with the Declaration of Helsinki and the Health Insurance Portability and Accountability Act (HIPAA). Informed consent was waived by the institutional review boards given the retrospective study design. The decisions to administer IV thrombolysis and/or perform MT were made on an individual basis based on consensus stroke team evaluation per institutional protocols.
2.3. Data Collection
Baseline and clinical data were collected through electronic records and stroke center databases for each patient including demographics, site of occlusion, laterality of occlusion, TOAST classification [24] and baseline CTP parameters at first presentation.
2.4. CTP Image Acquisition
Whole brain pretreatment CTP was performed on the Siemens Somatom Force (Erlangen, Germany) with the following parameters: 70 kVP, 200 effective mAs, rotation time 0.25 s, average acquisition time 60 s, collimation 48 × 1.2 mm, pitch value 0.7, 4D range 114 mm × 1.5 s. CTP images are then automatically processed using RAPID, a commercial FDA-approved AI software (iSchemaView, Menlo Park, CA, USA) for generating quantitative perfusion maps.
2.5. Image Analysis
All the CTPs were assessed by board-certified neuroradiologists with 9 years of working experience for diagnostic adequacy of the CTPs, where only those deemed diagnostic adequate were included in the study. CTP images were then post-processed using RAPID commercial software (IschemaView, Menlo Park, CA, USA) for generating Tmax and CBV maps, from which the CBV Index, HIR, Tmax > 4 s, and Tmax > 6 s were calculated. CI was defined as the ratio of the Tmax > 4 s volume divided by the Tmax > 6 s volume.
The compensation index (CI) was calculated as the volume of tissue with Tmax > 4 s divided by the volume of tissue with Tmax > 6 s as a novel quantitative pretreatment CS biomarker.
The ASITN CS was independently assessed by a board-certified neuroradiologist and the performing neurointerventionalist. Any discrepancies were resolved based on a consensus review. ASITN grades included the following: Grade 0, no collaterals visible to the ischemic region; Grade 1, slow collaterals to the periphery with persisting defect; Grade 2, rapid collaterals to the periphery with persisting defect; Grade 3, slow-but-complete collateral flow to the ischemic territory; and Grade 4, rapid and complete collateral flow to the ischemic territory [23].
2.6. Statistical Analysis
The objective of this study is to assess the association between CI, CBV Index, HIR and DSA CS. Descriptive statistics were used to summarize patient data. Categorical data was described using contingency tables including counts and percentages; continuous variables were summarized with mean (± Standard Deviation) or median (range). A student t-test was used in the data analysis for continuous variables, the Mann–Whitney U test was used in the data analysis for ordinal data and the Chi-square test was used for categorical data.
Spearman correlation analysis was used to assess the correlation between CI, CBV Index and HIR with CS based on DSA CS. Statistically significant analysis was described as p < 0.05, p < 0.01 and p < 0.001.
A logistic model was used to estimate the odds ratio between CI, CBV Index, and HIR when the ASITN CS score was dichotomized as a binary variable (0–2 versus 3–5). In the multivariate data analysis, the adjusted odds ratio between CI, CBV Index and HIR with DSA CS score was estimated using a multiple logistic regression model, adjusting for any potential confounding variables. p ≤ 0.05 was considered significant.
3. Results
A total of 223 consecutive patients (mean age: 67.77 ± 15.76, 56.1% female) met our inclusion criteria. In total, 80 patients (35.9%) received intravenous tissue plasminogen activator administered (IV tPA).
Of 223 patients, 162 (72.7%) had M1 segment occlusion, 60 (19.3%) had proximal M2 segment occlusion and 16 (7.2%) had ICA supraclinoid segment occlusion. Patient demographic and stroke treatment details are presented in Table 1.
Table 1.
Demographics of study participants.
| Study Demographics | Total (n = 223) | DSA ASITN CS (0–2) (n = 151) | DSA ASITN CS (3–4) (n = 72) |
p Value |
|---|---|---|---|---|
| Age in Years (mean ± standard deviation) | 67.77 ± 15.76 | 68.17 ± 15.90 | 66.93 ± 15.53 | 0.583 |
| Sex (Numbers (%)) | 0.636 | |||
| Female | 125 (56.05%) | 83 (54.97%) | 42 (58.33%) | |
| Male | 98 (43.95%) | 68 (45.03%) | 30 (41.67%) | |
| Race (Numbers (%)) | 0.268 | |||
| African American | 90 (40.36%) | 62 (41.06%) | 28 (38.89%) | |
| Caucasian | 117 (52.47%) | 78 (51.66%) | 39 (54.17%) | |
| Asian | 7 (3.14%) | 3 (1.99%) | 4 (5.56%) | |
| Other | 9 (4.04%) | 8 (5.30%) | 1 (1.39%) | |
| Pertinent Details at Presentation | ||||
| Prior history of stroke or Transient Ischemic Attack (Numbers (%)) | 44 (19.73%) | 29 (19.21%) | 15 (20.83%) | 0.775 |
| Admission NIH Stroke Scale (mean ± standard deviation) | 15.65 ± 6.82 | 16.30 ± 6.82 | 14.29 ± 6.66 | 0.040 * |
| Premorbid modified Rankin score (mRS) (mean ± standard deviation) | 0.63 ± 1.07 | 0.55 ± 1.02 | 0.80 ± 1.14 | 0.105 |
| Admission Alberta stroke program early CT score (ASPECTS) (mean ± standard deviation) | 8.64 ± 1.86 | 8.43 ± 2.03 | 9.07 ± 1.30 | 0.005 * |
| Intravenous tissue plasminogen activator administered (IV tPA) (Numbers (%)) | 80 (35.87%) | 51 (33.77%) | 29 (40.28%) | 0.344 |
| Segment Occlusion (Number (%)) | <0.001 * | |||
| Supraclinoid Internal Carotid Artery | 16 (7.17%) | 14 (9.27%) | 2 (2.78%) | |
| Middle Cerebral Artery, M1 segment | 162 (72.65%) | 122 (80.79%) | 40 (55.56%) | |
| Middle Cerebral Artery, Proximal M2 segment | 60 (19.3%) | 24 (11.3%) | 36 (36.4%) | |
| Relevant Time Parameters | ||||
| Door to CT time (in minutes, mean ± standard deviation) | 61.13 ± 144.10 | 65.72 ± 169.41 | 52.05 ± 74.02 | 0.645 |
| Door-to-groin puncture time (in minutes, mean ± standard deviation) | 211.50 ± 208.26 | 212.30 ± 224.78 | 209.59 ± 166.50 | 0.959 |
| CT-to-groin puncture time (in minutes, mean ± standard deviation) | 174.68 ± 174.67 | 172.60 ± 180.94 | 179.50 ± 163.16 | 0.878 |
| Door to recanalization time (in minutes, mean ± standard deviation) | 404.06 ± 419.30 | 346.49 ± 335.381 | 542.77 ± 559.01 | 0.065 |
| CT Perfusion Parameters | ||||
| Compensation Index (CI) | 2.24 ± 1.39 | 2.03 ± 1.04 | 2.67 ± 1.85 | p = 0.001 |
| Hypoperfusion Intensity Ratio (HIR) | 0.38 ± 0.22 | 0.41 ± 0.21 | 0.32 ± 0.22 | p < 0.05 |
| Cerebral Blood Volume (CBV) Index | 0.79 ± 0.16 | 0.78 ± 0.15 | 0.80 ± 0.16 | p = 0.42 |
Statistically significant difference was assessed using unpaired student t-test for continuous variables and Chi-square test for categorical variables. Statistically significant results are highlighted with asterisks *.
4. Spearman Correlation Analyses
4.1. CI and ASITN Collateral Score
Distribution of CI in patients with good and poor CS defined by ASITN CS is illustrated in Table 1.
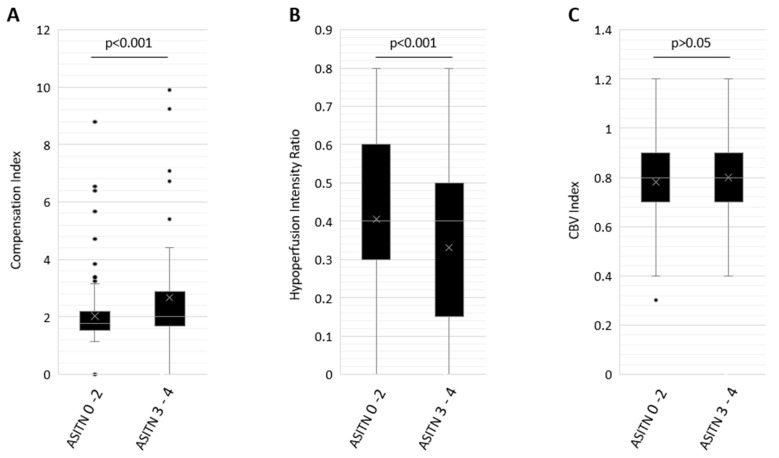
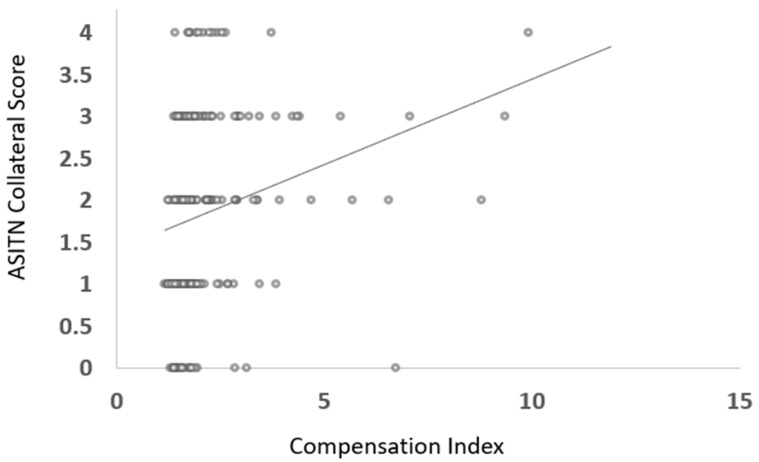
Mean CI in patients with good DSA CS was 2.67 ± 1.85 and with poor DSA CS was 2.03 ± 1.04, p < 0.001 (Figure 1). A modest, significant positive correlation was observed between the compensation index and ASITN CS (ρ = 0.37, p < 0.001) (Figure 2).
Figure 1.
Box and whisker plot distribution of Compensation Index (CI, (A)), Cerebral Blood Volume Index (CBV Index, (B)) and Hypoperfusion Index (HIR, (C)) in patients with poor DSA CS of 0–2 and robust DSA CS of 3 or greater. Box plot represents median and interquartile range, whereas whiskers represent minimum and maximum values.
Figure 2.
Scatter plot illustrating distribution of compensation index and ASITN collateral score in patients with anterior circulation large vessel occlusion.
Univariable logistic regression analysis to assess the association of CI with good DSA CS showed OR of 1.39 (95% CI: 1.10–1.74, p < 0.001). On multivariate logistic regression analysis taking into account sex, age, ASPECTS, tPA, premorbid mRS, NIH stroke scale, prior history of TIA, stroke, atrial fibrillation, diabetes mellitus, hyperlipidemia, heart disease and hypertension, CI was found to be independently associated with DSA CS (adjusted OR = 1.387, 95% CI: 1.09–1.77, p < 0.01 (Table 2).
Table 2.
Logistic regression model including Compensation Index (CI) in predicting good collateral status as defined by DSA ASITN CS of 3 or greater.
| Variables | Univariate Analysis Unadjusted Odds Ratio (OR (95% Confidence Interval)) | Multivariate Logistic Regression Analysis | |||
|---|---|---|---|---|---|
| Adjusted OR | 95% Confidence Interval | p Value | |||
| Lower | Upper | ||||
| Compensation Index (CI) | 1.39 (1.10–1.74) | 1.387 | 1.090 | 1.766 | 0.008 |
| Age | 0.99 (0.98–1.01) | 0.989 | 0.966 | 1.013 | 0.366 |
| Sex | 0.80 (0.49–1.30) | 0.932 | 0.493 | 1.761 | 0.827 |
| Race | 0.99 (0.72–1.40) | 0.917 | 0.579 | 1.453 | 0.714 |
| Hypertension | 0.74 (0.41–1.27) | 0.625 | 0.289 | 1.354 | 0.234 |
| Hyperlipidemia | 1.05 (0.65–1.70) | 1.136 | 0.599 | 2.155 | 0.696 |
| Diabetes Mellitus | 1.16 (0.68–2.00) | 1.159 | 0.555 | 2.423 | 0.694 |
| Heart Disease | 1.01 (0.63–1.64) | 0.914 | 0.446 | 1.873 | 0.806 |
| Atrial Fibrillation | 1.17 (0.72–1.91) | 1.548 | 0.740 | 3.236 | 0.245 |
| Prior Stroke or Transient Ischemic Attack | 1.06 (0.59–1.91) | 1.048 | 0.486 | 2.258 | 0.906 |
| Intravenous Tissue Plasminogen Activator Administered (IV tPA) | 1.24 (0.76–2.04) | 1.298 | 0.676 | 2.492 | 0.433 |
| Admission National Institute of Health (NIH) Stroke Scale | 0.96 (0.93–0.99) | 0.957 | 0.912 | 1.005 | 0.076 |
| Premorbid Modified Rankin Score (mRS) | 1.17 (0.94–1.46) | 1.312 | 0.963 | 1.787 | 0.085 |
| Admission Alberta Stroke Program Early CT Score (ASPECTS) | 1.25 (1.06–1.45) | 1.143 | 0.937 | 1.395 | 0.188 |
4.2. HIR and ASITN Collateral Score
Distribution of HIR in patients with good and poor CS defined by ASITN CS is illustrated in Table 1.
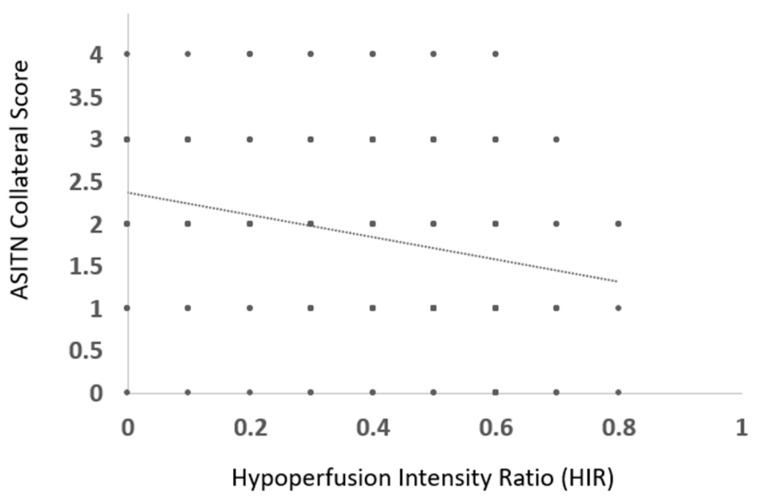
Mean HIR in patients with good DSA CS was 0.32 ± 0.22 and with poor DSA CS was 0.41 ± 0.21, p < 0.05 (Figure 1). A weak, significant negative correlation was observed between HIR and ASITN CS (ρ = −0.29, p < 0.001) (Figure 3).
Figure 3.
Scatter plot illustrating distribution of HIR with ASITN collateral score in patients with anterior circulation large vessel occlusion.
Univariable logistic regression analysis to assess for association of HIR with good DSA CS showed OR of 3.37 (95% CI: 2.0–5.65, p < 0.05). On multivariate logistic regression analysis taking into account sex, age, ASPECTS, tPA, premorbid mRS, NIH stroke scale, prior history of TIA, stroke, atrial fibrillation, diabetes mellitus, hyperlipidemia, heart disease and hypertension, HIR was not found to be independently associated with DSA CS (adjusted OR = 0.38, p = 0.22, 95% CI: 0.08–1.76) (Table 3).
Table 3.
Logistic regression model including Hypoperfusion Intensity Ratio (HIR) in predicting good collateral status as defined by DSA ASITN CS of 3 or greater.
| Variables | Univariate Analysis Unadjusted Odds Ratio (OR (95% Confidence Interval)) | Multivariate Logistic Regression Analysis | |||
|---|---|---|---|---|---|
| Adjusted OR | 95% Confidence Interval | p Value | |||
| Lower | Upper | ||||
| Hypoperfusion Intensity Ratio (HIR) | 3.37 (2.0–5.65) | 0.378 | 0.081 | 1.757 | 0.215 |
| Age | 0.99 (0.98–1.01) | 0.990 | 0.967 | 1.013 | 0.383 |
| Sex | 0.80 (0.49–1.30) | 0.957 | 0.504 | 1.816 | 0.893 |
| Race | 0.99 (0.72–1.40) | 0.869 | 0.553 | 1.365 | 0.542 |
| Hypertension | 0.74 (0.41–1.27) | 0.626 | 0.293 | 1.339 | 0.227 |
| Hyperlipidemia | 1.05 (0.65–1.70) | 1.140 | 0.608 | 2.139 | 0.683 |
| Diabetes Mellitus | 1.16 (0.68–2.00) | 1.077 | 0.523 | 2.219 | 0.840 |
| Heart Disease | 1.01 (0.63–1.64) | 0.885 | 0.435 | 1.801 | 0.736 |
| Atrial Fibrillation | 1.17 (0.72–1.91) | 1.588 | 0.773 | 3.262 | 0.208 |
| Prior Stroke or Transient Ischemic Attack | 1.06 (0.59–1.91) | 1.030 | 0.481 | 2.205 | 0.940 |
| Intravenous Tissue Plasminogen Activator Administered (IV tPA) | 1.24 (0.76–2.04) | 1.163 | 0.610 | 2.217 | 0.646 |
| Admission National Institute of Health (NIH) Stroke Scale | 0.96 (0.93–0.99) | 0.960 | 0.914 | 1.009 | 0.108 |
| Premorbid Modified Rankin Score (mRS) | 1.17 (0.94–1.46) | 1.308 | 0.964 | 1.775 | 0.085 |
| Admission Alberta Stroke Program Early CT Score (ASPECTS) | 1.25 (1.06–1.45) | 1.168 | 0.959 | 1.423 | 0.122 |
4.3. CBV Index and ASITN Collateral Score
The distribution of the CBV Index in patients with good and poor CS defined by ASITN CS is illustrated in Table 1.
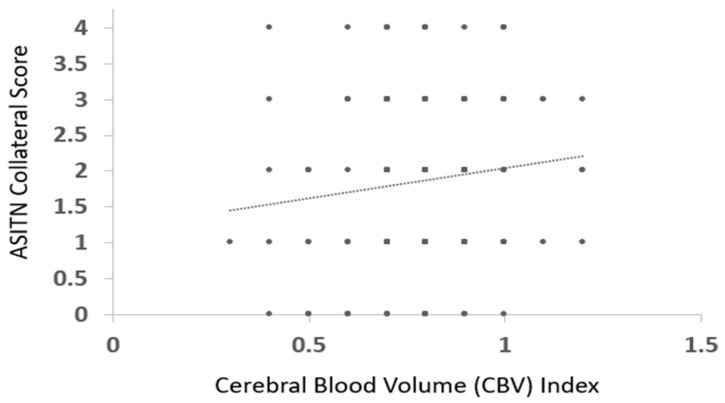
Mean CBV Index in patients with good DSA CS was 0.80 ± 0.16, and with poor DSA CS was 0.78 ± 0.15, p > 0.05 (Figure 1). No significant correlation was observed between the CBV index and ASITN CS (ρ = 0.1, p > 0.05) (Figure 4).
Figure 4.
Scatter plot illustrating distribution of CBV index with ASITN collateral score in patients with anterior circulation large vessel occlusion.
Univariable logistic regression analysis to assess the association of the CBV Index with good DSA CS showed an OR of 2.09 (95% CI: 0.36–12.25, p = 0.42).
5. Discussion
Our study demonstrates that CI better correlates with the reference standard DSA CS than the HIR and CBV index. Furthermore, when accounting for confounders in multivariable regression analysis, only the CI was independently associated with DSA CS. This is the first study exploring associations of CI, as a baseline CTP CS parameter, with DSA CS.
Good CS is associated with higher chances of successful recanalization following MT, better penumbra salvage and favorable functional outcomes [16,25,26,27,28,29,30,31,32,33]. Hence, assessing CS before treatment can aid in prognostication for AIS-LVO patients. Subjective assessment of CTA data has a wide range of inter- and intra-user agreement [34,35,36], making it critical to use more objective markers in decision making. Prior studies have validated certain pretreatment quantitative CTP parameters as surrogates of CS and predictors of clinical outcomes [37,38,39,40,41,42,43,44,45,46,47].
Of the previously described quantitative CTP CS parameters, HIR has been the most studied. Several prior studies have demonstrated that HIR can serve as a reliable CS marker [19,48,49]. HIR correlation with ASITN CS is well established, with studies showing a statistically significant correlation. The degree of correlation has been reported over a range of modest association (coefficient (r) = −0.33) [48] to moderate association (coefficient (r = −0.76) [19,49]. Our correlation was similar to Guenego et al. [48], whereas it was lower than what was reported by Kurmann et al. [19] and Ai et al. [49]. The wide range of variation in the correlation coefficient is multifactorial. First, the patient selection is different across different studies. Kurmann et al. [19] included M1 and M2 segment occlusions, Ai et al. [49] included all patients who underwent MT and Guenego et al. [48] included only M1 occlusions, whereas, in our cohort, we report all patients with anterior circulation large vessel occlusion. Second, the sample size ranged from 30 to 115 patients; thus, ours has the largest sample size of 223.
CBV index has also shown a good correlation in predicting the infarct growth, and clinical and functional outcomes following MT in AIS-LVO patients [16,50]. However, to date, no studies have directly evaluated the relationship between the CBV index and ASITN CS. In our study, we did not find any significant correlation between the CBV index and ASITN CS. This could stem from the fact that, by definition, the CBV index takes into account venous collaterals. Whereas ASITN CS measured following intra-arterial contrast injection is heavily dependent on the arterial flow, and may not be imaged long enough through the venous phase to capture that portion of the collateral cascade, this may factor out the venous collaterals in CS estimation [26,51].
In our study, we present a novel quantitative pretreatment CTP biomarker of CS with the CI, which utilizes Tmax > 4 s volume. Tmax > 4 s volume is thought to mainly represent benign oligemia or tissue that is hypoperfused but will not go onto infarct in the absence of intervention [20]. However, we postulate that the Tmax > 4 s volume can also reflect a component of CS. We assert that CI, as a ratio of Tmax > 4 s volume divided by Tmax > 6 s volume, is a surrogate for collateral-based compensatory response where a higher ratio represents a more favorable profile. We surmise that the difference in volume representing benign oligemia to penumbra likely relates to autoregulation, driven by vasodilation from collateral routes. This vasodilatory response would require a robust collateral supply that prevents the already hypoperfused tissue from reaching critical levels [20].
There are several limitations of this study to acknowledge. First, this study is hypothesis generating with the use of a convenience sample that was retrospectively reviewed from our prospectively collected database. Second, our analysis is restricted to the use of one commercial software platform and two comprehensive stroke centers. Third, there is a modest inter- and intra-observer agreement of ASITN CS [52]. Our study is, however, strengthened by our sample size of 223 derived from our prospectively maintained databases.
Our study lays the foundation for larger, more robust studies further assessing CI as an imaging biomarker of CS. Future studies may evaluate the relationship between CI and risk of post-procedural hemorrhagic transformation and clinical outcomes. For instance, with several recent trials showing the efficacy of MT with large cores [53,54,55,56], assessment of CS in this subpopulation may provide additional value, particularly in regard to rapid infarct growth.
Future studies are needed to expand our understanding of the adjunct role of CI with other similar pretreatment CTP-based markers in clinical evaluation and decision making in patients with AIS-LVO.
Acknowledgments
This study is supported by the Johns Hopkins University Department of Radiology Physician Scientist Incubator Program (RAD-PSI) to VSY and the Johns Hopkins School of Medicine Physician Scientist Scholar Program to DAL.
Abbreviations
| AIS | Acute ischemic stroke |
| CI | Compensation Index |
| CS | Collateral status |
| CBV | Cerebral blood volume |
| DWI | Diffusion-weighted imaging |
| HIR | Hypoperfusion intensity ratio |
| LVO | Large vessel occlusion |
| mRS | Modified Rankin score |
| MT | Mechanical thrombectomy |
Author Contributions
Methodology, M.K., M.H., R.X., L.L., J.C., A.A.D., A.G., M.W., F.G., V.U., J.H., K.N., A.T.R., G.W.A. and J.J.H.; Formal analysis, S.W.; Resources, C.G.; Writing—original draft, D.A.L. and A.B.B.; Writing—review & editing, V.S.Y.; Supervision, V.S.Y. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was approved through the Johns Hopkins Institutional Review Board (IRB00269637).
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
Greg Albers, Jeremy Heit and Vivek Yedavalli are consultants for Rapid (iSchemaView, Menlo Park, CA, USA). The remaining authors declare no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Xu Y., Guo S., Jiang H., Han H., Sun J., Wu X. Collateral Status and Clinical Outcomes after Mechanical Thrombectomy in Patients with Anterior Circulation Occlusion. J. Healthc. Eng. 2022;2022:7796700. doi: 10.1155/2022/7796700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gensicke H., Al-Ajlan F., Fladt J., Campbell B.C., Majoie C.B., Bracard S., Hill M.D., Muir K.W., Demchuk A., Román L.S., et al. Comparison of Three Scores of Collateral Status for Their Association With Clinical Outcome: The HERMES Collaboration. Stroke. 2022;53:3548–3556. doi: 10.1161/STROKEAHA.122.039717. [DOI] [PubMed] [Google Scholar]
- 3.Sperti M., Arba F., Acerbi A., Busto G., Fainardi E., Sarti C. Determinants of cerebral collateral circulation in acute ischemic stroke due to large vessel occlusion. Front. Neurol. 2023;14:1181001. doi: 10.3389/fneur.2023.1181001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peisker T., Vaško P., Mikulenka P., Lauer D., Kožnar B., Sulženko J., Roháč F., Kučera D., Girsa D., Kremeňová K., et al. Clinical and radiological factors predicting stroke outcome after successful mechanical intervention in anterior circulation. Eur. Heart J. Suppl. 2022;24((Suppl. B)):B48–B52. doi: 10.1093/eurheartjsupp/suac010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y., Wang J., Qiu J., Li W., Sun X., Zhao Y., Liu X., Zhao Z., Liu L., Nguyen T.N., et al. Association between collaterals, cerebral circulation time and outcome after thrombectomy of stroke. Ann. Clin. Transl. Neurol. 2023;10:266–275. doi: 10.1002/acn3.51718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prasetya H., Tolhuisen M.L., Koopman M.S., Kappelhof M., Meijer F.J.A., Yo L.S.F., Nijeholt G.J.L., van Zwam W.H., van der Lugt A., Roos Y.B.W.E.M., et al. Value of CT Perfusion for Collateral Status Assessment in Patients with Acute Ischemic Stroke. Diagnostics. 2022;12:3014. doi: 10.3390/diagnostics12123014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin L., Chen C., Tian H., Bivard A., Spratt N., Levi C.R., Parsons M.W. Perfusion Computed Tomography Accurately Quantifies Collateral Flow After Acute Ischemic Stroke. Stroke. 2020;51:1006–1009. doi: 10.1161/STROKEAHA.119.028284. [DOI] [PubMed] [Google Scholar]
- 8.Shi F., Gong X., Liu C., Zeng Q., Zhang M., Chen Z., Yan S., Lou M. Acute Stroke: Prognostic Value of Quantitative Collateral Assessment at Perfusion CT. Radiology. 2019;290:760–768. doi: 10.1148/radiol.2019181510. [DOI] [PubMed] [Google Scholar]
- 9.Nael K., Sakai Y., Larson J., Goldstein J., Deutsch J., Awad A.J., Pawha P., Aggarwal A., Fifi J., Deleacy R., et al. CT Perfusion collateral index in assessment of collaterals in acute ischemic stroke with delayed presentation: Comparison to single phase CTA. J. Neuroradiol. 2022;49:198–204. doi: 10.1016/j.neurad.2021.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Shuaib A., Butcher K., Mohammad A.A., Saqqur M., Liebeskind D.S. Collateral blood vessels in acute ischaemic stroke: A potential therapeutic target. Lancet Neurol. 2011;10:909–921. doi: 10.1016/S1474-4422(11)70195-8. [DOI] [PubMed] [Google Scholar]
- 11.Singer O.C., Berkefeld J., Nolte C.H., Bohner G., Reich A., Wiesmann M., Groeschel K., Boor S., Neumann-Haefelin T., Hofmann E., et al. Collateral vessels in proximal middle cerebral artery occlusion: The ENDOSTROKE study. Radiology. 2015;274:851–858. doi: 10.1148/radiol.14140951. [DOI] [PubMed] [Google Scholar]
- 12.Karamchandani R.R., Strong D., Rhoten J.B., Prasad T., Selig J., Defilipp G., Asimos A.W. Cerebral blood volume index as a predictor of functional independence after basilar artery thrombectomy. J. Neuroimaging. 2022;32:171–178. doi: 10.1111/jon.12933. [DOI] [PubMed] [Google Scholar]
- 13.Wang C.M., Chang Y.M., Sung P.S., Chen C.H. Hypoperfusion Index Ratio as a Surrogate of Collateral Scoring on CT Angiogram in Large Vessel Stroke. J. Clin. Med. 2021;10:1296. doi: 10.3390/jcm10061296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arenillas J.F., Cortijo E., García-Bermejo P., Levy E.I., Jahan R., Liebeskind D., Goyal M., Saver J.L., Albers G.W. Relative cerebral blood volume is associated with collateral status and infarct growth in stroke patients in SWIFT PRIME. J. Cereb. Blood Flow Metab. 2018;38:1839–1847. doi: 10.1177/0271678X17740293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guenego A., Mlynash M., Christensen S., Kemp S., Heit J.J., Lansberg M.G., Albers G.W. Hypoperfusion ratio predicts infarct growth during transfer for thrombectomy. Ann. Neurol. 2018;84:616–620. doi: 10.1002/ana.25320. [DOI] [PubMed] [Google Scholar]
- 16.Rao V.L., Mlynash M., Christensen S., Yennu A., Kemp S., Zaharchuk G., Heit J.J., Marks M.P., Lansberg M.G., Albers G.W. Collateral status contributes to differences between observed and predicted 24-h infarct volumes in DEFUSE 3. J. Cereb. Blood Flow Metab. 2020;40:1966–1974. doi: 10.1177/0271678X20918816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elijovich L., Goyal N., Mainali S., Hoit D., Arthur A.S., Whitehead M., Choudhri A.F. CTA collateral score predicts infarct volume and clinical outcome after endovascular therapy for acute ischemic stroke: A retrospective chart review. J. Neurointerv. Surg. 2016;8:559–562. doi: 10.1136/neurintsurg-2015-011731. [DOI] [PubMed] [Google Scholar]
- 18.Kimmel E.R., Al Kasab S., Harvey J.B., Bathla G., Ortega-Gutierrez S., Toth G., Jaksich E.M., Sheharyar A., Roa J., Hasan D.M., et al. Absence of Collaterals is Associated with Larger Infarct Volume and Worse Outcome in Patients with Large Vessel Occlusion and Mild Symptoms. J. Stroke Cerebrovasc. Dis. 2019;28:1987–1992. doi: 10.1016/j.jstrokecerebrovasdis.2019.03.032. [DOI] [PubMed] [Google Scholar]
- 19.Kurmann C., Kaesmacher J., Pilgram-Pastor S., Piechowiak E., Scutelnic A., Heldner M., Dobrocky T., Gralla J., Mordasini P. Correlation of Collateral Scores Derived from Whole-Brain Time-Resolved Flat Panel Detector Imaging in Acute Ischemic Stroke. AJNR Am. J. Neuroradiol. 2022;43:1627–1632. doi: 10.3174/ajnr.A7657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamalian S., Kamalian S., Konstas A., Maas M., Payabvash S., Pomerantz S., Schaefer P., Furie K., González R., Lev M. CT perfusion mean transit time maps optimally distinguish benign oligemia from true “at-risk” ischemic penumbra, but thresholds vary by postprocessing technique. AJNR Am. J. Neuroradiol. 2012;33:545–549. doi: 10.3174/ajnr.A2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simera I., Moher D., Hoey J., Schulz K.F., Altman D.G. A catalogue of reporting guidelines for health research. Eur. J. Clin. Investig. 2010;40:35–53. doi: 10.1111/j.1365-2362.2009.02234.x. [DOI] [PubMed] [Google Scholar]
- 22.Waqas M., Mokin M., Primiani C.T., Gong A.D., Rai H.H., Chin F., Rai A.T., Levy E.I., Siddiqui A.H. Large Vessel Occlusion in Acute Ischemic Stroke Patients: A Dual-Center Estimate Based on a Broad Definition of Occlusion Site. J. Stroke Cerebrovasc. Dis. 2020;29:104504. doi: 10.1016/j.jstrokecerebrovasdis.2019.104504. [DOI] [PubMed] [Google Scholar]
- 23.Higashida R.T., Furlan A.J., Roberts H., Tomsick T., Connors B., Barr J., Dillon W., Warach S., Broderick J., Tilley B., et al. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke. 2003;34:e109–e137. doi: 10.1161/01.STR.0000082721.62796.09. [DOI] [PubMed] [Google Scholar]
- 24.Adams H.P., Jr., Bendixen B.H., Kappelle L.J., Biller J., Love B.B., Gordon D.L., Marsh E.E., 3rd Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.STR.24.1.35. [DOI] [PubMed] [Google Scholar]
- 25.Liu L., Ding J., Leng X., Pu Y., Huang L.-A., Xu A., Wong K.S.L., Wang X., Wang Y. Guidelines for evaluation and management of cerebral collateral circulation in ischaemic stroke 2017. Stroke Vasc. Neurol. 2018;3:117–130. doi: 10.1136/svn-2017-000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liebeskind D.S., Tomsick T.A., Foster L.D., Yeatts S.D., Carrozzella J., Demchuk A.M., Jovin T.G., Khatri P., von Kummer R., Sugg R.M., et al. Collaterals at angiography and outcomes in the Interventional Management of Stroke (IMS) III trial. Stroke. 2014;45:759–764. doi: 10.1161/STROKEAHA.113.004072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liebeskind D.S. Collateral lessons from recent acute ischemic stroke trials. Neurol. Res. 2014;36:397–402. doi: 10.1179/1743132814Y.0000000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marks M.P., Lansberg M.G., Mlynash M., Olivot J.-M., Straka M., Kemp S., McTaggart R., Inoue M., Zaharchuk G., Bammer R., et al. Effect of collateral blood flow on patients undergoing endovascular therapy for acute ischemic stroke. Stroke. 2014;45:1035–1039. doi: 10.1161/STROKEAHA.113.004085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bang O.Y., Saver J.L., Buck B.H., Alger J.R., Starkman S., Ovbiagele B., Kim D., Jahan R., Duckwiler G.R., Yoon S.R., et al. Impact of collateral flow on tissue fate in acute ischaemic stroke. J. Neurol. Neurosurg. Psychiatry. 2008;79:625–629. doi: 10.1136/jnnp.2007.132100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khunte M., Wu X., Avery E.W., Gandhi D., Payabvash S., Matouk C., Heit J.J., Wintermark M., Albers G.W., Sanelli P., et al. Impact of collateral flow on cost-effectiveness of endovascular thrombectomy. J. Neurosurg. 2022;137:1801–1810. doi: 10.3171/2022.2.JNS212887. [DOI] [PubMed] [Google Scholar]
- 31.Murray N.M., Culbertson C.J., Wolman D.N., Mlynash M., Lansberg M.G. Hypoperfusion Intensity Ratio Predicts Malignant Edema and Functional Outcome in Large-Vessel Occlusive Stroke with Poor Revascularization. Neurocrit. Care. 2021;35:79–86. doi: 10.1007/s12028-020-01152-6. [DOI] [PubMed] [Google Scholar]
- 32.Fainardi E., Busto G., Rosi A., Scola E., Casetta I., Bernardoni A., Saletti A., Arba F., Nencini P., Limbucci N., et al. Tmax Volumes Predict Final Infarct Size and Functional Outcome in Ischemic Stroke Patients Receiving Endovascular Treatment. Ann. Neurol. 2022;91:878–888. doi: 10.1002/ana.26354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Monteiro A., Cortez G.M., Greco E., Aghaebrahim A., Sauvageau E., Hanel R.A. Hypoperfusion intensity ratio for refinement of elderly patient selection for endovascular thrombectomy. J. Neurointerv. Surg. 2022;14:242–247. doi: 10.1136/neurintsurg-2020-017218. [DOI] [PubMed] [Google Scholar]
- 34.Schaefer P.W., Souza L., Kamalian S., Hirsch J.A., Yoo A.J., Kamalian S., Gonzalez R.G., Lev M.H. Limited reliability of computed tomographic perfusion acute infarct volume measurements compared with diffusion-weighted imaging in anterior circulation stroke. Stroke. 2015;46:419–424. doi: 10.1161/STROKEAHA.114.007117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chu Y., Ma G., Xu X.Q., Lu S.-S., Cao Y.-Z., Shi H.-B., Liu S., Wu F.-Y. Total and regional ASPECT score for non-contrast CT, CT angiography, and CT perfusion: Inter-rater agreement and its association with the final infarction in acute ischemic stroke patients. Acta Radiol. 2022;63:1093–1101. doi: 10.1177/02841851211029080. [DOI] [PubMed] [Google Scholar]
- 36.Zussman B., Jabbour P., Talekar K., Gorniak R., Flanders A.E. Sources of variability in computed tomography perfusion: Implications for acute stroke management. Neurosurg. Focus. 2011;30:E8. doi: 10.3171/2011.3.FOCUS1136. [DOI] [PubMed] [Google Scholar]
- 37.Vagal A., Aviv R., Sucharew H., Reddy M., Hou Q., Michel P., Jovin T., Tomsick T., Wintermark M., Khatri P. Collateral Clock Is More Important than Time Clock for Tissue Fate. Stroke. 2018;49:2102–2107. doi: 10.1161/STROKEAHA.118.021484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bang O.Y., Goyal M., Liebeskind D.S. Collateral Circulation in Ischemic Stroke: Assessment Tools and Therapeutic Strategies. Stroke. 2015;46:3302–3309. doi: 10.1161/STROKEAHA.115.010508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Uniken Venema S.M., Dankbaar J.W., van der Lugt A., Dippel D.W.J., van der Worp H.B. Cerebral Collateral Circulation in the Era of Reperfusion Therapies for Acute Ischemic Stroke. Stroke. 2022;53:3222–3234. doi: 10.1161/STROKEAHA.121.037869. [DOI] [PubMed] [Google Scholar]
- 40.Liebeskind D.S., Jahan R., Nogueira R.G., Zaidat O.O., Saver J.L., Investigators S. Impact of collaterals on successful revascularization in Solitaire FR with the intention for thrombectomy. Stroke. 2014;45:2036–2040. doi: 10.1161/STROKEAHA.114.004781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Menon B.K., Qazi E., Nambiar V., Foster L.D., Yeatts S.D., Liebeskind D., Jovin T.G., Goyal M., Hill M.D., Tomsick T.A., et al. Differential Effect of Baseline Computed Tomographic Angiography Collaterals on Clinical Outcome in Patients Enrolled in the Interventional Management of Stroke III Trial. Stroke. 2015;46:1239–1244. doi: 10.1161/STROKEAHA.115.009009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guenego A., Farouki Y., Mine B., Bonnet T., Hulscher F., Wang M., Elens S., Suarez J.V., Jodaitis L., Ligot N., et al. Hypoperfusion Intensity Ratio Predicts Infarct Growth After Successful Thrombectomy for Distal Medium Vessel Occlusion. Clin. Neuroradiol. 2022;32:849–856. doi: 10.1007/s00062-022-01141-6. [DOI] [PubMed] [Google Scholar]
- 43.Wan Z., Meng Z., Xie S., Fang J., Li L., Chen Z., Liu J., Jiang G. Correlation between Hypoperfusion Intensity Ratio and Functional Outcome in Large-Vessel Occlusion Acute Ischemic Stroke: Comparison with Multi-Phase CT Angiography. J. Clin. Med. 2022;11:5274. doi: 10.3390/jcm11185274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guenego A., Marcellus D.G., Martin B.W., Christensen S., Albers G.W., Lansberg M.G., Marks M.P., Wintermark M., Heit J.J. Hypoperfusion Intensity Ratio Is Correlated With Patient Eligibility for Thrombectomy. Stroke. 2019;50:917–922. doi: 10.1161/STROKEAHA.118.024134. [DOI] [PubMed] [Google Scholar]
- 45.Winkelmeier L., Heit J.J., Adusumilli G., Geest V., Christensen S., Kniep H., van Horn N., Steffen P., Bechstein M., Sporns P., et al. Hypoperfusion Intensity Ratio Is Correlated With the Risk of Parenchymal Hematoma After Endovascular Stroke Treatment. Stroke. 2023;54:135–143. doi: 10.1161/STROKEAHA.122.040540. [DOI] [PubMed] [Google Scholar]
- 46.Wu R.R., Lu S.S., Cao Y.Z., Xu X.-Q., Jia Z.-Y., Zhao L.-B., Liu S., Shi H.-B., Wu F.-Y. Hypoperfusion intensity ratio correlates with clinical outcome of endovascular thrombectomy in acute ischaemic stroke patients with late therapeutic window. Clin. Radiol. 2022;77:570–576. doi: 10.1016/j.crad.2022.04.003. [DOI] [PubMed] [Google Scholar]
- 47.Imaoka Y., Shindo S., Miura M., Terasaki T., Mukasa A., Todaka T. Hypoperfusion intensity ratio and CBV index as predictive parameters to identify underlying intracranial atherosclerotic stenosis in endovascular thrombectomy. J. Neuroradiol. 2023;50:424–430. doi: 10.1016/j.neurad.2022.10.005. [DOI] [PubMed] [Google Scholar]
- 48.Guenego A., Fahed R., Albers G.W., Kuraitis G., Sussman E.S., Martin B.W., Marcellus D.G., Olivot J., Marks M.P., Lansberg M.G., et al. Hypoperfusion intensity ratio correlates with angiographic collaterals in acute ischaemic stroke with M1 occlusion. Eur. J. Neurol. 2020;27:864–870. doi: 10.1111/ene.14181. [DOI] [PubMed] [Google Scholar]
- 49.Ai Z., Jiang L., Zhao B., Su H., Yin X., Chen Y.C. Hypoperfusion Intensity Ratio Correlates with Angiographic Collaterals and Infarct Growth in Acute Stroke with Thrombectomy. Curr. Med. Imaging. 2023;19:1561–1569. doi: 10.2174/1573405619666230123142657. [DOI] [PubMed] [Google Scholar]
- 50.Li B.H., Wang J.H., Yang S., Wang D.-Z., Zhang Q., Cheng X.-D., Yu N.-W., Guo F.-Q. Cerebral blood volume index may be a predictor of independent outcome of thrombectomy in stroke patients with low ASPECTS. J. Clin. Neurosci. 2022;103:188–192. doi: 10.1016/j.jocn.2022.07.006. [DOI] [PubMed] [Google Scholar]
- 51.Maguida G., Shuaib A. Collateral Circulation in Ischemic Stroke: An Updated Review. J. Stroke. 2023;25:179–198. doi: 10.5853/jos.2022.02936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ben Hassen W., Malley C., Boulouis G., Clarençon F., Bartolini B., Bourcier R., Régent C.R., Bricout N., Labeyrie M.A., Gentric J.C., et al. Inter- and intraobserver reliability for angiographic leptomeningeal collateral flow assessment by the American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology (ASITN/SIR) scale. J. Neurointerv. Surg. 2019;11:338–341. doi: 10.1136/neurintsurg-2018-014185. [DOI] [PubMed] [Google Scholar]
- 53.Bendszus M., Bonekamp S., Berge E., Boutitie F., Brouwer P., Gizewski E., Krajina A., Pierot L., Randall G., Simonsen C.Z., et al. A randomized controlled trial to test efficacy and safety of thrombectomy in stroke with extended lesion and extended time window. Int. J. Stroke. 2019;14:87–93. doi: 10.1177/1747493018798558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sarraj A., Hassan A.E., Abraham M.G., Ortega-Gutierrez S., Kasner S.E., Hussain M.S., Chen M., Blackburn S., Sitton C.W., Churilov L., et al. Trial of Endovascular Thrombectomy for Large Ischemic Strokes. N. Engl. J. Med. 2023;388:1259–1271. doi: 10.1056/NEJMoa2214403. [DOI] [PubMed] [Google Scholar]
- 55.Yoshimura S., Sakai N., Yamagami H., Uchida K., Beppu M., Toyoda K., Matsumaru Y., Matsumoto Y., Kimura K., Takeuchi M., et al. Endovascular Therapy for Acute Stroke with a Large Ischemic Region. N. Engl. J. Med. 2022;386:1303–1313. doi: 10.1056/NEJMoa2118191. [DOI] [PubMed] [Google Scholar]
- 56.Huo X., Ma G., Tong X., Zhang X., Pan Y., Nguyen T.N., Yuan G., Han H., Chen W., Wei M., et al. Trial of Endovascular Therapy for Acute Ischemic Stroke with Large Infarct. N. Engl. J. Med. 2023;388:1272–1283. doi: 10.1056/NEJMoa2213379. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are contained within the article.