Abstract
The groESL operon is under complex regulation in Caulobacter crescentus. In addition to strong induction after exposure to heat shock, under physiological growth conditions, its expression is subject to cell cycle control. Transcription and translation of the groE genes occur primarily in predivisional cells, with very low levels of expression in stalked cells. The regulatory region of groESL contains both a ς32-like promoter and a CIRCE element. Overexpression of C. crescentus ς32 gives rise to higher levels of GroEL and increased levels of the groESL transcript coming from the ς32-like promoter. Site-directed mutagenesis in CIRCE has indicated a negative role for this cis-acting element in the expression of groESL only at normal growth temperatures, with a minor effect on heat shock induction. Furthermore, groESL-lacZ transcription fusions carrying mutations in CIRCE are no longer cell cycle regulated. Analysis of an hrcA null strain, carrying a disruption in the gene encoding the putative repressor that binds to the CIRCE element, shows constitutive synthesis of GroEL throughout the Caulobacter cell cycle. These results indicate a negative role for the hrcA gene product and the CIRCE element in the temporal control of the groESL operon.
The heat shock response is a universal phenomenon by which all living cells, when exposed to temperatures higher than their normal physiological temperatures, induce the synthesis of a group of proteins generally known as the heat shock proteins (Hsps).
In prokaryotes, the best-studied mechanism of heat shock induction is that of the gram-negative bacterium Escherichia coli. In this organism, the heat shock response is positively regulated at the level of transcription by the alternate sigma factor ς32, encoded by the rpoH gene, whose levels increase drastically (about 20-fold) during the first few minutes after heat shock due to derepression of its translation and to a transient increase in its half-life (for a review, see reference 32). The level and activity of ς32 are negatively regulated in E. coli by the products of the heat shock genes dnaK, dnaJ, and grpE. DnaK, DnaJ, and GrpE cooperate to present ς32 to the FtsH protease, which degrades the heat shock sigma factor (14, 26), whereas DnaK and DnaJ work together to inhibit ς32 activity in heat shock gene transcription by preventing its binding to the RNA polymerase core and GrpE partially reverses this inhibition (9).
In contrast to E. coli, no evidence for involvement of ς32-like factors in heat shock gene expression was found in gram-positive bacteria, such as Bacillus subtilis (13) and Clostridium acetobutylicum (19). However, a highly conserved inverted repeat (IR) sequence was detected in front of some of the major heat shock genes (dnaK and groE), which was proposed to substitute for the ς32 regulation of the heat shock response in gram-positive bacteria. It soon became apparent that this inverted repeat sequence, also known as the CIRCE element (named CIRCE for controlling inverted repeat of chaperone expression), was more widespread than initially imagined, being identified in front of the groESL operons of several gram-negative bacteria, including those presenting ς32-like promoters (2, 24).
The role of the CIRCE element has been investigated, and evidence indicates that it functions as an operator site to which a repressor binds (24, 31, 33). The protein coded by orf39, the first gene in the dnaK operon of B. subtilis, with homologs found in several other bacteria and recently renamed hrcA (22, 23), was found to bind the IR at the DNA level and to serve as the repressor in B. subtilis (31).
In the gram-negative bacterium Caulobacter crescentus, several heat shock-inducible genes have been characterized and they all present ς32-like promoters (2, 11, 22). The gene encoding the ς32 homolog of C. crescentus has been isolated, and one of its promoters (P2) aligns with the ς32 consensus sequence, with transcription from this promoter increasing dramatically during heat shock (21, 29). Recently, in vitro transcription assays using C. crescentus Eς32 RNA polymerase holoenzyme and in vivo studies using rpoH-lacZ transcription fusions have confirmed the identity of P2 as a ς32-dependent promoter (30). The levels of ς32 increase transiently during heat shock in C. crescentus, and the increased transcription of rpoH seems to account for the induction of ς32 levels (21, 30). This mode of regulation for C. crescentus ς32 differs from that of its E. coli counterpart, whose complex regulatory region does not include a ς32-dependent promoter (7).
The C. crescentus groESL operon has been characterized and shown to be subject to a dual type of control (2). Besides being heat shock inducible, its expression is cell cycle regulated during growth at normal temperatures. The results of primer extension analysis suggested the presence of two putative promoters regulating the expression of groESL, with only one (P2) presenting characteristics of a ς32-transcribed promoter and being induced by heat shock. Furthermore, a CIRCE element was found downstream of P2, suggesting that both a ς32 promoter and the IR element regulate the expression of groESL in Caulobacter (2).
In this report, we confirm the ς32-dependent expression of groESL by overexpressing the C. crescentus heat shock sigma factor using a multicopy plasmid and showing that the increase in ς32 levels results in an increase in the amount of GroEL and an increase in the amount of the transcript coming from the ς32-like promoter. Furthermore, the role of the CIRCE element in the regulation of the Caulobacter groESL operon was investigated by using site-directed mutagenesis to obtain mutations in this cis-acting element. The groE regulatory regions containing each of these mutations were fused to a promoterless lacZ gene, and expression of β-galactosidase was analyzed in C. crescentus cells harboring these transcription fusions after heat shock and throughout the cell cycle at normal temperatures. A C. crescentus strain with a disruption in the hrcA gene (22) was also investigated for GroEL expression. Data obtained indicated that HrcA and the CIRCE element are involved in cell cycle control of the groESL operon.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The synchronizable C. crescentus NA1000 (8) and LS2293 (hrcA) (22) strains were grown in PYE (5) medium or in modified minimal M2 glucose medium (15). Cultures were synchronized by centrifugation through a Ludox gradient using a modified version (6) of the procedure of Evinger and Agabian (8). E. coli TG-1 was used for phage propagation and cloning, and S17-1 was used as the donor strain in conjugations with C. crescentus. Plasmid placZ/290 (10) contains a promoterless lacZ gene and was used for transcriptional fusions with the C. crescentus groESL regulatory region. Plasmids pTrc-His B (Invitrogen) and pPROEX-1 (Gibco-BRL) were used for overexpressing C. crescentus GroEL and DnaK proteins, respectively, in E. coli. Plasmid pAR33 (21) is a derivative of the multicopy plasmid pBBR1 (1) carrying the C. crescentus rpoH gene coding for ς32 and its promoter region. Plasmid pCS225 contains a lacZ transcription fusion with the promoter of the xylX gene (17), and cells carrying this plasmid were grown in PYE medium containing 0.1% xylose.
Site-directed mutagenesis of the groESL regulatory regions and construction of lacZ transcription fusions.
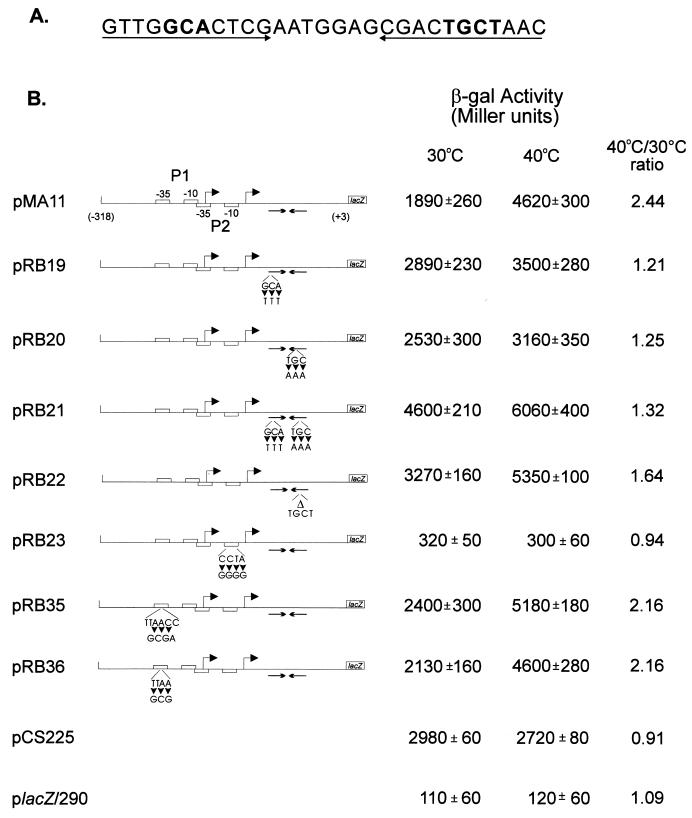
Site-directed mutagenesis was performed by the method of Kunkel et al. (16), using a DNA fragment containing the regulatory region of the groESL operon (2) cloned in M13mp19 as the template. The synthetic oligonucleotides ML3 (5′-CCAGCGTCGTTGTTTCTCGAATGGAGC-3′), LRC (5′-GAATGGAGCGACAAATAACAGTCCCGC-3′), LDR (5′-GAATGGAGCGACAACAGTCCCGC-3′), MH10 (5′-CGAGGGCCGGCGGGGGACTCACCAGCG-3′), GP135 (5′-GAAGCGCTCCCCGCGAGCGCCCGAAAA-3′), and GP136 (5′-GAAGCTCCCCGCGCCGCGCCCGAA-3′) were used to obtain the site-directed mutations. The underlined bases correspond to the changes introduced in the wild-type sequence. The DNA fragments containing the mutations introduced by using ML3, LRC, LDR, MH10, GP135, and GP136 were then ligated to placZ/290, giving rise to transcription fusions pRB19, pRB20, pRB22, pRB23, pRB35, and pRB36, respectively (for the location of each mutation, see Fig. 3). Transcription fusion pRB21 was obtained by doing a second round of site-directed mutagenesis, using construct pRB19 as the template and the synthetic oligonucleotide LRC. Plasmids containing these transcription fusions were introduced into C. crescentus by conjugation with an E. coli donor (S17-1). β-Galactosidase activity in mid-log-phase cultures was measured by the method of Miller (18).
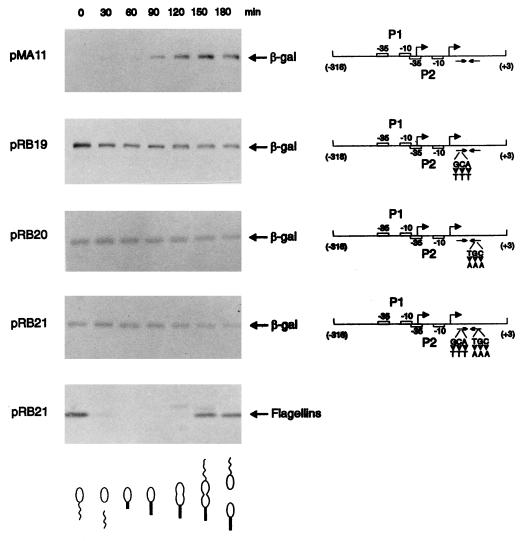
FIG. 3.
Changes in transcriptional activity due to mutations in the groESL regulatory region. (A) Nucleotide sequence of the inverted repeat (CIRCE element) located downstream of heat shock promoter P2. Nucleotides shown in bold type have been mutated. (B) Schematic representations of the fragments cloned into the transcription reporter vector placZ/290 and the corresponding β-galactosidase (β-gal) activity of each construct. Site-directed mutations were carried out by the method of Kunkel et al. (16), with M13mp19 containing the groESL regulatory region, as described in Materials and Methods. The arrowheads indicate base changes, and the triangle indicates a four-base deletion. All mutant constructs and the control plasmids were assayed for β-galactosidase activity (18) in mid-log-phase, plasmid-bearing C. crescentus NA1000 cultures either grown at 30°C or after 1 h of incubation at 40°C. pCS225 is a transcription fusion containing the promoter region of the xylX gene, required for growth on xylose, fused to the lacZ gene in placZ/290 (17). The values of β-galactosidase activity and the corresponding standard deviations represent the averages of six independent assays.
Western blots.
Aliquots of C. crescentus cultures at 30°C or after heat shock at 42°C for different lengths of time were collected, and total protein was extracted as previously described (2). The protein extracts were resolved by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis, and the proteins were transferred to nitrocellulose membranes by the method of Towbin et al. (27). Analysis of the membranes was performed as previously described (2), except for the use of specific polyclonal antisera anti-GroEL or anti-DnaK and an enhanced chemiluminescence kit (Amersham). The anti-GroEL antiserum was obtained from a rabbit immunized with a fusion protein overexpressed in E. coli, corresponding to about 36 kDa of the carboxy-terminal portion of C. crescentus GroEL containing an N-terminal histidine tag from the expression vector pTrc-His B (Invitrogen). The anti-DnaK antiserum was obtained essentially the same way, but using expression vector pPROEX-1 (Gibco-BRL) and a DNA fragment encoding 65 kDa of the carboxy-terminal portion of C. crescentus DnaK.
Immunoprecipitation.
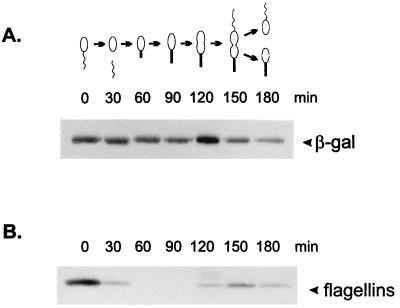
The temporal control of groE-lacZ transcription fusions and GroEL synthesis was investigated in synchronized cultures by pulse-labelling aliquots of cells at different times of the cycle with [35S]methionine for 5 min and immunoprecipitation of the labelled proteins using either anti-β-galactosidase (Sigma) or anti-GroEL antiserum, as previously described (12). As a control of synchronization, antisera against C. crescentus flagellins were also used. Similarly, the time course of heat shock induction of β-galactosidase synthesis in groE-lacZ transcription fusions was investigated by pulse-labelling mid-log-phase cultures with [35S]methionine for 2 min and immunoprecipitation of the labelled proteins using anti-β-galactosidase antiserum.
Primer extension assay.
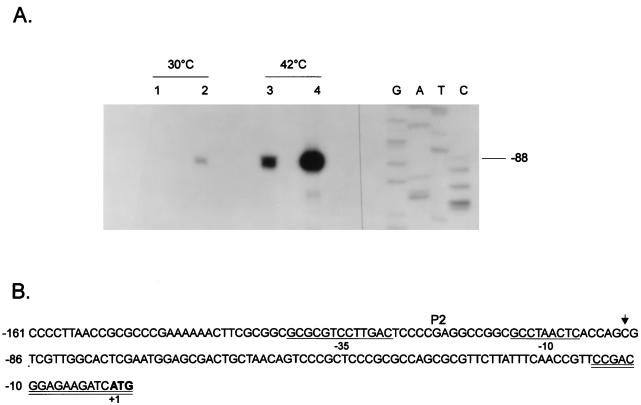
Mapping of the transcriptional start sites was carried out by a primer extension assay. A synthetic oligonucleotide (18-mer) complementary to nucleotides −15 to +3 (see Fig. 2B) was 5′ end labelled with [γ-32P]ATP and polynucleotide kinase and hybridized to 50 μg of total RNA isolated from C. crescentus cells grown at 30°C or after a 15-min exposure to 42°C. Annealing was carried out at 50°C for at least 4 h in 25 μl of 100 mM piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES) buffer (pH 7.0) containing 1 M NaCl and 5 mM EDTA. The nucleic acids were ethanol precipitated and resuspended in 50 μl of 25 mM Tris-HCl (pH 8.3) containing 3 mM MgCl2, 75 mM KCl, 1 mM dithiothreitol, 40 U of RNase inhibitor, and 1 mM (each) dATP, dCTP, dGTP, and dTTP. The annealed primer was extended at 37°C for 90 min using 300 U of Moloney murine leukemia virus reverse transcriptase (Amersham). RNA was digested for 30 min at 37°C by the addition of 1 μg of RNase A, and the extended products were analyzed by electrophoresis on denaturing sequencing gels followed by autoradiography.
FIG. 2.
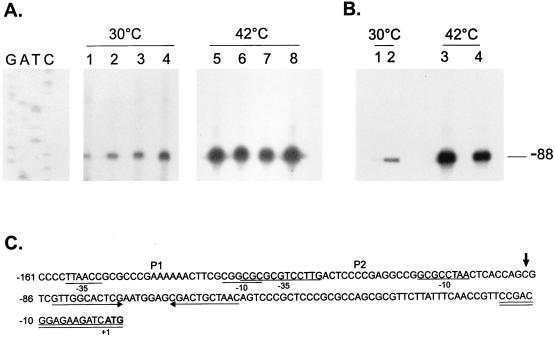
Increased groESL mRNA levels in a ς32-overexpressing C. crescentus strain. (A) An 18-nucleotide primer complementary to nucleotides −15 to +3 of the 5′ region of the groESL operon was 5′ end labelled and hybridized to 50 μg of total RNA isolated from NA1000 cells carrying (lanes 2 and 4) or not carrying (lanes 1 and 3) the plasmid pAR33 (high-copy-number plasmid containing C. crescentus rpoH gene) grown at 30°C or exposed to 42°C for 15 min. The hybrids were then extended by using reverse transcriptase, and the extension products were resolved by denaturing gel electrophoresis and autoradiography. (B) Sequence of the 5′ region of the groESL operon. The transcription start site is shown by an arrow over the nucleotide sequence. The putative −10 and −35 regions of the P2 promoter are underlined. The ATG of the initiator methionine is shown in bold type. The oligonucleotide used in primer extension experiments is complementary to the sequence underlined twice.
RESULTS
C. crescentus groESL operon expression is controlled by ς32.
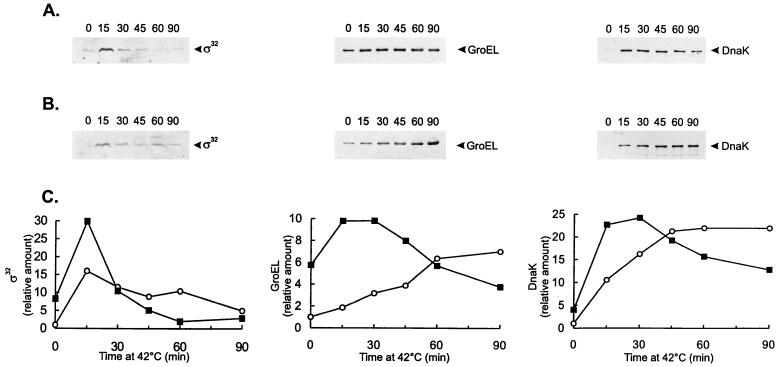
To demonstrate that the C. crescentus ς32 homolog regulates expression of the groESL operon, we analyzed a C. crescentus strain carrying a high-copy-number plasmid (pAR33) containing the ς32 gene and its promoter region (21). For cells grown at 30°C, the levels of ς32 in NA1000 cells harboring pAR33 were investigated by Western blotting using antiserum against the E. coli ς32 and shown to be about eightfold higher than those in NA1000 cells lacking the plasmid (Fig. 1). After heat shock, ς32 levels were twofold higher in the strain carrying pAR33 than in the control strain (Fig. 1). Since in vitro transcription studies have shown that the purified C. crescentus ς32 protein in the presence of RNA polymerase core enzyme specifically recognizes the heat shock-regulated promoter P1 of the C. crescentus dnaK gene (29), we investigated the levels of DnaK protein in the ς32-overexpressing strain. As shown in Fig. 1, the amount of DnaK in this strain was about fivefold higher in cells growing at 30°C than in the wild-type strain. During the first 30 min after heat shock at 42°C, DnaK protein levels were also higher in the ς32-overexpressing strain (about twofold). After that, the amount of DnaK became equal to or lower than that in the control strain subjected to heat shock, probably reflecting the fact that the levels of ς32 decrease faster in the overexpressing strain under these conditions (Fig. 1).
FIG. 1.
(A and B) Western blot analyses of GroEL and DnaK in C. crescentus strains containing different levels of ς32. NA1000 cells carrying (A) or not carrying (B) plasmid pAR33 (high-copy-number plasmid containing the C. crescentus rpoH gene) were grown to mid-log phase at 30°C or shifted to 42°C for the indicated times (minutes). Protein extracts were prepared and separated by SDS-polyacrylamide gel electrophoresis. The proteins were transferred to nitrocellulose membranes and probed with antibody to C. crescentus GroEL or C. crescentus DnaK and antibody against E. coli ς32, as described in Materials and Methods. Bound antigen was visualized by chemiluminescence and recorded on X-ray film. Equal amounts of protein were applied to each lane. (C) The relative levels of ς32, GroEL, and DnaK, determined by densitometry scanning of the films, for strains NA1000 (○) and NA1000 carrying pAR33 (▪) are shown.
The amount of GroEL was similarly investigated in the ς32-overexpressing strain, and as shown in Fig. 1, for cultures grown at 30°C, GroEL levels were sixfold higher in this strain than in the control. After heat shock, GroEL levels were higher (two- to fivefold) in the strain harboring pAR33 up to 45 min of exposure to 42°C. For longer periods of heat shock, as observed for DnaK, the amount of GroEL was determined to be lower in the ς32-overexpressing strain.
To investigate if the increase in the amount of GroEL was due to increased transcription of the groESL operon, primer extension experiments were performed by using an 18-nucleotide synthetic oligomer complementary to nucleotides −15 to +3 (Fig. 2) and RNA was isolated from cells of NA1000 strain carrying or not carrying the multicopy plasmid pAR33, grown at 30°C or after 15 min at 42°C, as described in Materials and Methods. As shown in Fig. 2, the amounts of transcript initiating at the ς32-like promoter were about 10-fold higher in ς32-overexpressing cells growing at 30°C and about 7-fold higher when the cells were subjected to a 15-min heat shock at 42°C compared to cells with normal levels of ς32, as determined by densitometry scanning of the autoradiogram (not shown).
Analysis of transcriptional fusions containing mutations in the CIRCE element.
In order to investigate the role of the CIRCE element in expression of the C. crescentus groESL operon, mutations in either one or both arms of the inverted repeat were constructed by site-directed mutagenesis and the mutated groESL regulatory regions were subcloned into the transcription reporter vector placZ/290. As can be observed in Fig. 3, when three bases were changed on either the left arm or the right arm of the inverted repeat, as in transcription fusions pRB19 and pRB20, higher levels of β-galactosidase activity were observed for cells grown at 30°C. These levels were about 50% higher than those observed in the wild-type construct pMA11. In both cases, however, the levels of β-galactosidase activity still increased after exposure of the cells to 40°C for 1 h. When both arms were mutated, reconstructing the dyad symmetry of the inverted repeat but with a different nucleotide sequence, as in pRB21, the levels of β-galactosidase activity were even higher at 30°C, reaching 240% of the value obtained for the wild-type construct pMA11, and here also β-galactosidase levels increased still further after exposure of the cells to 40°C, reaching levels 30% higher than those observed after heat shock with the wild-type transcription fusion pMA11. Similar results were obtained when four bases were deleted in the right arm of the inverted repeat, as in transcription fusion pRB22. These results indicate that it is not the formation of a putative stem-loop structure that regulates expression of groESL, since in all four mutants, such a structure could still be formed, as determined by using the FOLDRNA program of the Genetics Computer Group package (not shown). Instead, the data suggest that a protein or proteins probably bind to the inverted repeat in a sequence-specific manner, negatively regulating expression of groESL at normal temperatures.
Figure 3 also shows that nucleotide changes in the putative −10 region of the heat shock promoter P2 lead to very low levels of β-galactosidase activity, similar to the values obtained with the vector alone. Mutations in the −35 region of the P1 promoter, however, do not result in lower levels of transcriptional activity, since the values of β-galactosidase activity determined for pRB35 and pRB36 were found to be higher than that of the wild-type construct either at 30°C or after 1 h at 40°C. These results seem to indicate that if P1 is a real promoter, it does not contribute significantly to transcription of the groESL operon, either at 30°C or during heat shock. For a control, we used the xylX-lacZ transcription fusion where the promoter region of the xylX gene, which is required for growth on xylose and is not heat shock inducible, was subcloned into placZ/290, giving rise to plasmid pCS225 (17). We observed that β-galactosidase activity in this case does not increase after heat shock (Fig. 3).
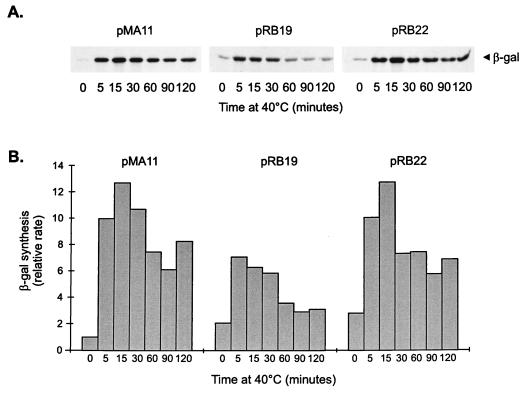
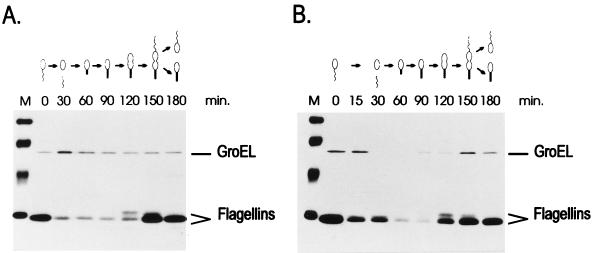
To confirm that the promoter constructs containing mutations in the CIRCE element are still heat shock inducible, the rate of synthesis of β-galactosidase was determined in cells harboring pMA11, pRB19, or pRB22, after different lengths of exposure to 40°C. As shown in Fig. 4, the rate of β-galactosidase synthesis increases significantly after heat shock in cells harboring each of the three constructs. In the case of pRB22, for instance, the pattern of heat shock induction of β-galactosidase synthesis is similar to that observed for the wild-type construct pMA11. In cells harboring pRB19, the rate of β-galactosidase synthesis presents a lower but significant level of induction (3.5-fold) after heat shock. The fact that the amount of induction observed with pRB19 does not reach wild-type levels could be due to a decrease in the half-life of the mRNA produced during heat shock, which is suggested by an apparently faster turnoff of the response in this case.
FIG. 4.
Time course of heat shock induction of β-galactosidase (β-gal) synthesis in different groESL-lacZ transcription fusions. (A) C. crescentus NA1000 cells harboring transcription fusion pMA11, pRB19, or pRB22 were subjected to heat shock at 40°C, and aliquots of cells were pulse-labelled with [35S]methionine for 2 min at the indicated times. Cells were then harvested by centrifugation, and protein extracts were immunoprecipitated with anti-β-galactosidase antibody to determine the rate of β-galactosidase synthesis before and during heat shock. (B) Relative rates of β-galactosidase synthesis determined by densitometry scanning of the autoradiograms.
Role of the CIRCE element in cell cycle control of groESL expression.
We had previously shown that sequences upstream of the heat shock promoter (P2) were not necessary for cell cycle control of the groESL operon (2). Since the ς32 protein was shown to be synthesized constitutively throughout the cell cycle at 30°C (21), we decided to investigate the role of the CIRCE element in groESL temporal control. Synthesis of β-galactosidase directed by the groESL regulatory region carrying mutations in the CIRCE element was analyzed throughout the C. crescentus cell cycle. To measure the rate of β-galactosidase synthesis, samples of synchronized NA1000 cultures harboring the groESL-lacZ transcription fusion pMA11, pRB19, pRB20, or pRB21 were pulse-labelled with [35S]methionine at different times during the cell cycle, and extracts of these cells were immunoprecipitated with anti-β-galactosidase antibody.
Figure 5 shows that the rate of β-galactosidase synthesis, which is temporally controlled in the wild-type construct (pMA11), becomes constant throughout the cell cycle when the CIRCE element carries a mutation in the left arm (pRB19), the right arm (pRB20), or in both arms of the inverted repeat (pRB21). As an internal control for cell synchrony, the rate of synthesis of the flagellins was determined in all experiments. In Fig. 5, we present the experiment done with cells harboring the pRB21 construct, showing the normal pattern of flagellin synthesis. These results indicate that groESL-lacZ transcription fusions carrying mutations in the CIRCE element do not present cell cycle control of β-galactosidase synthesis.
FIG. 5.
Transcription fusions containing mutations in the CIRCE element lose cell cycle control. Synchronized cultures of C. crescentus NA1000 harboring different transcription fusions were pulse-labelled with [35S]methionine at the indicated times during the cell cycle. Extracts of these cells were then immunoprecipitated with anti-β-galactosidase antibody, as described in Materials and Methods, to analyze the rate of β-galactosidase synthesis as a function of the cell cycle. A schematic representation of each transcription fusion is shown on the right. pMA11 is the wild-type construct; pRB19 and pRB20 carry mutations in the left arm and the right arm of the IR, respectively; pRB21 has mutations in both arms of the IR. As an internal control for cell synchrony, flagellin synthesis was determined throughout the cycle with the pRB21 construct. The drawings at the bottom of the figure are schematic representations of the C. crescentus cell cycle corresponding to the time indicated in each lane.
Disruption of the C. crescentus hrcA gene leads to constitutive expression of groESL at physiological temperatures.
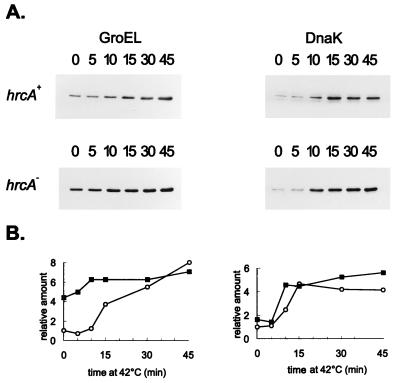
The C. crescentus hrcA gene encodes a protein with homologs reported for several other bacteria (22, 25) and was shown both in Caulobacter and in B. subtilis to be involved in the regulation of heat shock operons containing the CIRCE element (22, 31, 33). We have previously shown that in C. crescentus disruption of the hrcA gene increased transcription of the groESL operon ninefold at physiological temperatures with no significant effect on transcription after heat shock (22). This effect on transcription was not observed in the dnaKJ operon of Caulobacter, which does not present a CIRCE element in its regulatory region (2, 11, 22). Higher levels of groESL mRNA observed in cells of the hrcA mutant strain growing at normal temperatures are accompanied by an increase in the levels of GroEL protein, which were observed in Western blots using anti-GroEL antibody. As shown in Fig. 6, hrcA null mutant cells growing at 30°C present increased levels of GroEL (about fourfold) compared to those of the wild-type strain at the same temperature. The amount of GroEL still increased after heat shock, reaching similar levels in both mutant and wild-type cells after 45 min at 42°C, compatible with the data on transcription. Consistent with the fact that no increase in dnaKJ transcription is observed in hrcA mutant cells growing at 30°C compared to wild-type cells (22), no increase in the levels of DnaK was observed when the same Western blot was analyzed with anti-DnaK antibody (Fig. 6).
FIG. 6.
Effect of HrcA on the level of GroEL. Cells of C. crescentus NA1000 (hrcA+) or LS2293, which presents a disruption of the hrcA gene, were grown to mid-log phase at 30°C or shifted to 42°C for the times (minutes) indicated. Protein extracts were prepared and separated by SDS-polyacrylamide gel electrophoresis. After transfer of the proteins to nitrocellulose membranes, the blots were probed with antibody to GroEL or to DnaK, as described in Materials and Methods. Equal amounts of protein were applied to all lanes, and hrcA+ and hrcA samples were loaded on the same gel and processed together. The relative levels of GroEL and DnaK, determined by densitometry scanning of the films, are shown below the blots; hrcA+ (○) and hrcA (▪).
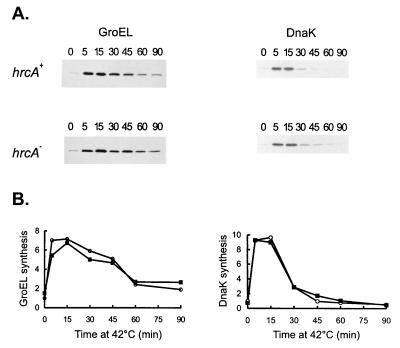
To confirm that GroEL synthesis is induced after heat shock in the hrcA null strain, wild-type and mutant cells were pulse-labelled with [35S]methionine after different times of exposure to 42°C, and extracts of these cells were immunoprecipitated with anti-GroEL antibody. As shown in Fig. 7, the rates of GroEL synthesis increase to similar extents in the hrcA null strain (fivefold) and the wild-type strain (sevenfold) during heat shock. As a control, the rate of DnaK synthesis was also investigated by immunoprecipitating the same cell extracts with the anti-DnaK antibody (Fig. 7). The increase in the rate of DnaK synthesis during heat shock was about ninefold in both the wild type and the hrcA null strain. These results indicate that C. crescentus HrcA negatively regulates expression of the groESL operon at normal temperatures, with no significant effect on expression during heat shock.
FIG. 7.
Effects of HrcA on GroEL and DnaK syntheses during heat shock. Cells of C. crescentus NA1000 (hrcA+) or LS2293 (hrcA) were subjected to heat shock at 40°C, and aliquots of cells were pulse-labelled with [35S]methionine for 2 min at the indicated times (in minutes). Cells were then harvested by centrifugation, and protein extracts were immunoprecipitated with anti-GroEL or anti-DnaK antibodies. The relative rates of GroEL and DnaK syntheses were determined by densitometry scanning of the autoradiograms. Symbols: ○, hrcA+; ▪, hrcA.
To investigate whether hrcA disruption affected temporal expression of the groESL operon, cultures of strain LS2293 were synchronized and synthesis of GroEL was analyzed throughout the cell cycle. Aliquots of cells at various stages of the cycle were pulse-labelled with [35S]methionine, cell extracts were prepared and the proteins were immunoprecipitated with anti-GroEL antiserum. As shown in Fig. 8, the rate of GroEL synthesis in the hrcA mutant strain is not controlled by the cell cycle, in contrast to the differential synthesis of GroEL observed in the hrcA+ strain NA1000. As an internal control of cell synchrony, the flagellins were also immunoprecipitated by adding antiflagellin antisera to the same samples, as described in Materials and Methods. As apparent in Fig. 8, there is no difference in the pattern of flagellin synthesis for the hrcA and hrcA+ strains, indicating that loss of temporal control is specific for the groESL operon. Furthermore, cell synchrony, as observed through light microscopy, was exactly as in the wild-type cells.
FIG. 8.
GroEL synthesis is constitutive in the hrcA null strain. Synchronized cultures of C. crescentus NA1000 (B) or LS2293 containing a disruption of the hrcA gene (A) were pulse-labelled with [35S]methionine at the indicated times during the cell cycle. Extracts of these cells were then immunoprecipitated with antisera against C. crescentus GroEL and antisera against C. crescentus flagellins, as described in Materials and Methods, to analyze GroEL and flagellin (as a control) synthesis as a function of the cell cycle. The drawings at the top of the figure are schematic representations of the C. crescentus cell cycle corresponding to the time indicated in each lane. M lanes contain 14C-labelled molecular mass markers (Amersham) in descending order: 97,400 Da, 66,000 Da, 46,000 Da, and 30,000 Da.
To confirm that the effect of hrcA disruption on GroEL expression is at the level of transcription, we looked at β-galactosidase synthesis in synchronized cultures of strain LS2293 harboring the wild-type transcription fusion pMA11. As shown in Fig. 9, the rate of β-galactosidase synthesis is no longer cell cycle controlled in the hrcA null strain, indicating that transcription directed by the groESL regulatory region becomes constitutive in the absence of HrcA protein.
FIG. 9.
Transcription directed by groESL regulatory region is constitutive in the hrcA null strain. Synchronized cultures of C. crescentus hrcA null strain (LS2293) harboring the wild-type groESL-lacZ transcription fusion pMA11 were pulse-labelled with [35S]methionine at the indicated times during the cell cycle. Extracts of these cells were then immunoprecipitated with anti-β-galactosidase antibody, as described in Materials and Methods, to determine the rate of β-galactosidase (β-gal) synthesis as a function of the cell cycle. As a control of cell synchrony, the rate of flagellin synthesis was determined throughout the cycle.
Enhanced transcription in CIRCE and hrcA mutants is from the ς32 promoter.
In order to investigate from which promoter the increased transcription directed by the groESL regulatory region was occurring in the hrcA null strain and in the CIRCE element mutants, primer extension experiments were carried out with an 18-nucleotide synthetic oligomer complementary to nucleotides −15 to +3 (Fig. 10C) and RNA isolated from cells of different mutant strains grown at 30°C or subjected to heat shock at 42°C for 30 min, as described in Materials and Methods. As shown in Fig. 10, a single transcription start site was observed in all mutant strains, which coincides with the initiation site highly induced during heat shock, indicating that in all cases enhanced transcription was occurring from the ς32 promoter (P2). The fact that no transcript originating from the putative P1 promoter was observed in these experiments, in conjunction with the data using site-directed mutagenesis (Fig. 3), strongly suggests that P1 might not be a true promoter.
FIG. 10.
Primer extension mapping of the transcription start sites in hrcA and CIRCE mutants. (A) An 18-nucleotide primer complementary to nucleotides −15 to +3 was 5′ end labelled and hybridized to 50 μg of total RNA isolated from NA1000 cells harboring transcription fusion pMA11 (lanes 1 and 5), pRB19 (lanes 2 and 6), pRB20 (lanes 3 and 7), or pRB21 (lanes 4 and 8) grown at 30°C or exposed to 42°C for 30 min. The hybrids were then extended by using reverse transcriptase, and the extension products were resolved by denaturing gel electrophoresis and autoradiography. (B) The same procedure as described above for panel A was performed except that the RNA used was isolated from NA1000 (lanes 1 and 3) or LS2293 cells (lanes 2 and 4) grown at 30°C or after a 15-min exposure to 42°C. The sequencing ladder shown in panel A was generated by using the same 18-residue oligonucleotide as the primer and the plasmid pUC19 containing the groESL regulatory region as the template. (C) Sequence of the 5′ region of the groESL operon. The transcription start site is shown by an arrow over the nucleotide sequence. The putative −10 and −35 regions of the P1 and P2 promoters are underlined. The CIRCE element is depicted by arrows under the nucleotide sequence. The oligonucleotide used in primer extension experiments is complementary to the sequence underlined twice. The ATG of the initiator methionine is shown in bold type.
Even though the primer extension product coming from the chromosomal groESL operon and from the plasmid-borne fusion have the same size, the data in Fig. 10 clearly show that there is enhanced transcription occurring from the heat-inducible promoter in the CIRCE mutants and the hrcA null strain, and no other transcription initiation site is observed in the mutant strains.
DISCUSSION
The initial description of a highly conserved inverted repeat sequence, the CIRCE element, in front of the dnaKJ and groESL operons of the gram-positive bacteria B. subtilis and C. acetobutylicum, in association with a vegetative ς70 promoter, led to the idea that this inverted repeat was substituting for a ς32 promoter and therefore would be found only when ς32 was absent. Nevertheless, it soon became clear that the CIRCE element is quite widespread, being found in the dnaK and/or groE operons of numerous phylogenetically distant bacteria (24), including bacterial species containing ς32-mediated heat shock regulation (2, 20, 25). In particular, this report presents evidence of an operon controlled by a ς32 promoter and the CIRCE element combined: the C. crescentus groESL operon.
Previous characterization of the groESL regulatory region had indicated the existence of two putative promoters, P1 and P2, corresponding to two transcriptional start sites, determined in primer extension experiments (2). Only P2 was highly induced during heat shock and presented −10 and −35 sequences conforming to the consensus sequence of ς32 promoters. In the present study, we show that mutations in the putative −35 region of P1 do not affect expression of the groESL-lacZ transcription fusion, either in cells growing at 30°C or during heat shock. Furthermore, primer extension experiments under conditions somewhat different from those previously used (2) did not confirm the transcription initiation site corresponding to the P1 promoter. Together these results indicate that P1 might not be a real promoter. We also showed that a mutation in the putative −10 region of the P2 promoter gave rise to very low levels of β-galactosidase activity, which were close to the levels determined for the reporter vector alone, indicating that the heat shock-inducible promoter P2 is essential for transcriptional activity, at physiological temperatures and during heat shock. Furthermore, the observation that cells harboring extra copies of the C. crescentus ς32 gene presented a significant increase in GroEL levels (sixfold at 30°C), similar to that observed for the DnaK protein whose gene has been shown by in vitro transcription studies to be ς32 dependent (29), and the results of primer extension experiments showing that the amount of the transcript initiating at the ς32-like promoter is increased in the ς32-overexpressing strain corroborate the hypothesis that P2 is a ς32 promoter.
Besides the ς32-like promoter, the groESL operon has a highly conserved CIRCE element located downstream of P2 (2). In C. crescentus, only the groESL operon, not the dnaKJ operon, contains the CIRCE element, (2, 3). A third heat shock operon was recently isolated in C. crescentus (22); this operon encodes the protein GrpE, which works in conjunction with DnaK, DnaJ, and HrcA, which is the putative repressor that binds to the CIRCE element. The hrcA-grpE operon regulatory region contains a ς32 promoter but no CIRCE element (22).
Consistent with the presence of the CIRCE element in the regulatory region of the groESL operon, its expression is altered in a hrcA null mutant. Higher groESL mRNA levels were observed in hrcA mutant cells growing at physiological temperatures than in hrcA+ cells (22). As expected, no differences were detected in dnaKJ mRNA levels in hrcA and hrcA+ cells. In addition, no significant differences were observed in mRNA levels of both operons during heat shock when hrcA+ and hrcA cells were compared, suggesting a role for hrcA only at normal growth temperatures.
In the present report, we complemented these studies by looking at expression at the protein level. In agreement with a negative regulatory role for hrcA in CIRCE-containing operons, GroEL protein levels were observed to be fourfold higher in hrcA mutant cells than in hrcA+ cells growing at physiological temperatures, whereas no significant differences were observed in DnaK protein levels in both strains. GroEL levels increased further after heat shock, reaching similar levels in hrcA+ and hrcA cells after 30 min of exposure to 42°C. In addition, we showed that the rate of GroEL synthesis increases during heat shock to the same extent in the hrcA null mutant (threefold) and in wild-type cells (fourfold). These results are compatible with a repressor role for HrcA in cells growing at physiological temperatures.
Furthermore, we show here that point mutations in the CIRCE element located in the left arm, the right arm, or both arms of the inverted repeat, as well as a four-base deletion in the right arm, give rise to higher levels of expression of groESL-lacZ transcription fusions (50 to 240% higher) in cells growing at 30°C, with no significant effect on expression in cells subjected to heat shock. The data presented also indicated that HrcA binds to the CIRCE element in a sequence-specific manner.
The experiments with the hrcA null mutant and the site-directed mutations in the inverted repeat suggested a role for CIRCE and HrcA under non-heat shock conditions, and indeed the cell cycle-regulated expression groESL observed at physiological temperatures (2) is lost when either the CIRCE element is mutated or when the hrcA gene product is not present. Moreover, enhanced transcription observed at physiological temperatures in hrcA and CIRCE mutants was shown to occur from the ς32-like promoter.
How the CIRCE element and the product of the hrcA gene control temporal expression of the groESL operon remains to be determined. The rate of C. crescentus hrcA transcription, determined by using a hrcA-lacZ transcription fusion, did not show significant variations during the cell cycle (22). The levels of HrcA protein, however, have not been determined. A possible cell cycle-dependent posttranslational activation or inactivation of HrcA should also be investigated. For instance, the cell cycle-regulated expression of Caulobacter late flagellar genes is due to a temporally controlled posttranslational activation of the transcriptional regulator FlbD (28). A similar type of control could be occurring with HrcA.
The reason why groESL expression is under cell cycle control in C. crescentus is not known. However, the roles of GroEL and GroES in the assembly of multiprotein complexes and the increase in GroEL levels observed in predivisional cells (2), coincident with the time of flagellum biogenesis, suggest a possible explanation for cell cycle control of groESL expression. The description of an E. coli groEL mutant with a defect in motility strengthens this hypothesis (4). A similar study with the characterization of C. crescentus groEL mutants is presently being undertaken to investigate a possible role for GroEL in flagellum assembly.
ACKNOWLEDGMENTS
This investigation was supported by grants to S.L.G. from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq-PADCT). R.L.B. is a FAPESP predoctoral fellow, and M.A. is a CNPq predoctoral fellow. S.L.G. was partially supported by CNPq.
We thank L. Shapiro for the antiflagellin antiserum, A. C. Reisenauer for plasmid pAR33, R. C. Roberts for C. crescentus LS2293, U. Jenal for plasmid pCS225, M. V. Marques for critical reading of the manuscript, and Elisety de Andrade Silva for manuscript preparation.
REFERENCES
- 1.Antoine R, Locht C. Isolation and molecular characterization of a novel broad-host-range plasmid from Bordetella bronchiseptica with sequence similarities to plasmids from gram-positive organisms. Mol Microbiol. 1992;6:1785–1799. doi: 10.1111/j.1365-2958.1992.tb01351.x. [DOI] [PubMed] [Google Scholar]
- 2.Avedissian M, Gomes S L. Expression of the groESL operon is cell cycle controlled in Caulobacter crescentus. Mol Microbiol. 1996;19:79–89. doi: 10.1046/j.1365-2958.1996.347879.x. [DOI] [PubMed] [Google Scholar]
- 3.Avedissian M, Lessing D, Gober J W, Shapiro L, Gomes S L. Regulation of the Caulobacter crescentus dnaKJ operon. J Bacteriol. 1995;177:3479–3484. doi: 10.1128/jb.177.12.3479-3484.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burnett B P, Horwich A L, Low K B. A carboxy-terminal deletion impairs the assembly of GroEL and confers a pleiotropic phenotype in Escherichia coli K-12. J Bacteriol. 1994;176:6980–6985. doi: 10.1128/jb.176.22.6980-6985.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Contreras I, Shapiro L, Henry S. Membrane phospholipid composition of Caulobacter crescentus. J Bacteriol. 1987;135:1130–1136. doi: 10.1128/jb.135.3.1130-1136.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dingwall A, Gober J W, Shapiro L. Identification of a Caulobacter basal body structural gene and a cis-acting site required for activation of transcription. J Bacteriol. 1990;172:6066–6076. doi: 10.1128/jb.172.10.6066-6076.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erickson J W, Vaughn V, Walter W A, Neidhardt F C, Gross C A. Regulation of the promoters and transcripts of rpoH, the Escherichia coli heat shock regulatory gene. Genes Dev. 1987;1:419–432. doi: 10.1101/gad.1.5.419. [DOI] [PubMed] [Google Scholar]
- 8.Evinger M, Agabian N. Envelope-associated nucleoid from Caulobacter crescentus stalked and swarmer cells. J Bacteriol. 1977;132:294–301. doi: 10.1128/jb.132.1.294-301.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gamer J, Multhaup G, Tomoyasu T, McCarty J S, Rudiger S, Schonfeld H J, Schirra C, Bujard H, Bukau B. A cycle of binding and release of the DnaK, DnaJ and GrpE chaperones regulate activity of the Escherichia coli heat shock transcription factor sigma 32. EMBO J. 1996;15:607–617. [PMC free article] [PubMed] [Google Scholar]
- 10.Gober J W, Shapiro L. A developmentally regulated Caulobacter flagellar promoter is activated by 3′ enhancer and IHF binding elements. Mol Biol Cell. 1992;3:913–916. doi: 10.1091/mbc.3.8.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomes S L, Gober J W, Shapiro L. Expression of the Caulobacter heat shock gene dnaK is developmentally controlled during growth at normal temperatures. J Bacteriol. 1990;172:3051–3059. doi: 10.1128/jb.172.6.3051-3059.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gomes S L, Shapiro L. Differential expression and position of chemotaxis methylation proteins in Caulobacter. J Mol Biol. 1984;77:551–568. doi: 10.1016/0022-2836(84)90238-9. [DOI] [PubMed] [Google Scholar]
- 13.Hecker M, Schumann W, Völker V. Heat-shock and general stress response in Bacillus subtilis. Mol Microbiol. 1996;19:417–428. doi: 10.1046/j.1365-2958.1996.396932.x. [DOI] [PubMed] [Google Scholar]
- 14.Herman C, Thévenet D, D’Ari R, Bouloc P. Degradation of ς32, the heat shock regulator in Escherichia coli, is governed by HflB. Proc Natl Acad Sci USA. 1995;92:3516–3520. doi: 10.1073/pnas.92.8.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson R C, Ely B. Isolation of spontaneously derived mutants of Caulobacter crescentus. Genetics. 1977;86:25–32. doi: 10.1093/genetics/86.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kunkel T A, Roberts J D, Zakour R A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- 17.Meisenzahl A C, Shapiro L, Jenal U. Isolation and characterization of a xylose-dependent promoter from Caulobacter crescentus. J Bacteriol. 1997;179:592–600. doi: 10.1128/jb.179.3.592-600.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 19.Narberhaus F, Bahl H. Cloning, sequencing, and molecular analysis of the groESL operon of Clostridium acetobutylicum. J Bacteriol. 1992;174:3282–3289. doi: 10.1128/jb.174.10.3282-3289.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Narberhaus F, Weiglhofer W, Fischer H-M, Hennecke H. The Bradyrhizobium japonicum rpoH1 gene encoding a ς32-like protein is part of a unique heat shock gene cluster together with groESL1 and three small heat shock genes. J Bacteriol. 1996;178:5337–5346. doi: 10.1128/jb.178.18.5337-5346.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reisenauer A C, Mohr C D, Shapiro L. Regulation of a heat shock ς32 homolog in Caulobacter crescentus. J Bacteriol. 1996;178:1919–1927. doi: 10.1128/jb.178.7.1919-1927.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roberts R C, Toochinda C, Avedissian M, Baldini R L, Gomes S L, Shapiro L. Identification of a Caulobacter crescentus operon encoding hrcA, involved in negatively regulating heat-inducible transcription, and the chaperone gene grpE. J Bacteriol. 1996;178:1829–1841. doi: 10.1128/jb.178.7.1829-1841.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schulz A, Schumann W. hrcA, the first gene of the Bacillus subtilis dnaK operon encodes a negative regulator of class I heat shock genes. J Bacteriol. 1996;178:1088–1093. doi: 10.1128/jb.178.4.1088-1093.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Segal G, Ron E Z. Heat shock activation of the groESL operon of Agrobacterium tumefaciens and the regulatory roles of the inverted repeat. J Bacteriol. 1996;178:3634–3640. doi: 10.1128/jb.178.12.3634-3640.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Segal G, Ron E Z. Regulation and organization of the groE and dnaK operons in Eubacteria. FEMS Microbiol Lett. 1996;138:1–10. doi: 10.1111/j.1574-6968.1996.tb08126.x. [DOI] [PubMed] [Google Scholar]
- 26.Tomoyasu T, Gamer J, Bukau B, Kanemori M, Mori H, Rutman A J, Oppenheim A B, Yura T, Yamanaka K, Niki H, Hiraga S, Ogura T. Escherichia coli FtsH is a membrane-bound ATP-dependent protease which degrades the heat-shock transcription factor ς32. EMBO J. 1995;14:2551–2560. doi: 10.1002/j.1460-2075.1995.tb07253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wingrove J A, Mangan E K, Gober J W. Spatial and temporal phosphorylation of a transcriptional activator regulates pole-specific gene expression in Caulobacter. Genes Dev. 1993;7:1979–1992. doi: 10.1101/gad.7.10.1979. [DOI] [PubMed] [Google Scholar]
- 29.Wu J, Newton A. Isolation, identification, and transcriptional specificity of the heat shock sigma factor ς32 from Caulobacter crescentus. J Bacteriol. 1996;178:2094–2101. doi: 10.1128/jb.178.7.2094-2101.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu J, Newton A. The Caulobacter heat shock sigma factor gene rpoH is positively autoregulated from a ς32-dependent promoter. J Bacteriol. 1997;179:514–521. doi: 10.1128/jb.179.2.514-521.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yuan G, Wong S-L. Isolation and characterization of Bacillus subtilis groE regulatory mutants: evidence for orf39 in the dnaK operon as a repressor gene in regulating the expression of both groE and dnaK. J Bacteriol. 1995;177:6462–6468. doi: 10.1128/jb.177.22.6462-6468.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yura T, Nagai H, Mori H. Regulation of the heat-shock response in Bacteria. Annu Rev Microbiol. 1993;47:321–350. doi: 10.1146/annurev.mi.47.100193.001541. [DOI] [PubMed] [Google Scholar]
- 33.Zuber V, Schumann W. CIRCE, a novel heat shock element involved in regulation of heat shock operon dnaK of Bacillus subtilis. J Bacteriol. 1994;176:1359–1363. doi: 10.1128/jb.176.5.1359-1363.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]