Abstract
(1) Background: studies have shown that some patients experience mental deterioration after bariatric surgery. (2) Methods: We examined whether the use of probiotics and improved eating habits can improve the mental health of people who suffered from mood disorders after bariatric surgery. We also analyzed patients’ mental states, eating habits and microbiota. (3) Results: Depressive symptoms were observed in 45% of 200 bariatric patients. After 5 weeks, we noted an improvement in patients’ mental functioning (reduction in BDI and HRSD), but it was not related to the probiotic used. The consumption of vegetables and whole grain cereals increased (DQI-I adequacy), the consumption of simple sugars and SFA decreased (moderation DQI-I), and the consumption of monounsaturated fatty acids increased it. In the feces of patients after RYGB, there was a significantly higher abundance of two members of the Muribaculaceae family, namely Veillonella and Roseburia, while those after SG had more Christensenellaceae R-7 group, Subdoligranulum, Oscillibacter, and UCG-005. (4) Conclusions: the noted differences in the composition of the gut microbiota (RYGB vs. SG) may be one of the determinants of the proper functioning of the gut–brain microbiota axis, although there is currently a need for further research into this topic using a larger group of patients and different probiotic doses.
Keywords: bariatric surgery, sleeve gastrectomy, roux-en-y gastric bypass, obesity, microbiota, gut–brain axis, beck scale, depression, diet, probiotics
1. Introduction
The benefits of bariatric surgery include improvements in psychosocial status, social relationships, and quality of life [1,2]. Symptoms of depression and anxiety decrease in the majority of patients 6–24 months after undergoing the bariatric procedure. However, they reappear in some patients after 36–60 months, sometimes even returning to pre-surgery levels [3]. Studies have shown that, compared to preoperative values, at 10 years after bariatric surgery (despite successful weight loss), patients’ mental statuses often deteriorate, including overall mental health, sense of control, and fear of intimacy [4]. The meta-analyses performed suggest that patients undergoing bariatric surgery have a higher risk of self-harm/suicide attempts than the control group, being matched in terms of age, gender, and BMI. The authors suggested that this may be related to various pre- and postoperative psychosocial, physiological, and medical factors [5,6].
One of the factors affecting mental state and mood is gut microbiota [7]. The diagnosis of mental disorders (including depression) is associated with the presence of dysbiosis [8]. In our previous study, we showed that bariatric surgery affects the gut microbiota, which may play an important role in the development of depressive and gastrointestinal symptoms [9]. Low fiber consumption and increased levels of fecal isobutyric acid may lead to intestinal inflammation. Alterations in the composition or functions of the microbiota in depressed patients may be related to inflammation, reduced production of short-chain fatty acids, impaired intestinal barrier integrity, and abnormalities in neurotransmitter, carbohydrate, and amino acid metabolic processes [10]. Psychobiotics are probiotics that have a beneficial effect on mental health [11]. Most systematic reviews and meta-analyses confirm the beneficial effects of psychobiotics on depressive symptoms in healthy people, as well as patients with major depressive disorder (MDD) and people with various concomitant psychiatric and somatic disorders [12,13]. The mechanism of action that drives psychobiotics is not well understood. It may be related to a reduction in blood levels of kynurenine [14,15], which may have neurotoxic and neurodegenerative effects [16]. Other mechanisms are related to urinary cortisol reduction, anti-inflammatory effects [13,17,18], or the increased production of brain-derived neurotrophic factor (BDNF), which correlates with antidepressant activity [19]. Supplementation with Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 strains reduces the level of somatization, anxiety, and the intensity of depressive behaviors and the hostility index, as well as plasma cortisol levels [20].
The nutritional status of an organism is also important for proper psychological functioning. For post-bariatric surgery patients, an energy-dense diet with large amounts of vegetables and fruits and restricting the consumption of highly processed products, saturated fatty acids (SFA), and simple sugars is recommended. In addition, consuming an adequate amount of protein is crucial, which is estimated to be 60–80 g/day. Dairy products, eggs, fish, lean meat, and the seeds of legumes are recommended as the main sources of protein. Equally important is the consumption of complex carbohydrates that provide fiber, as well as adequate vitamin and mineral intake [21,22,23,24]. Such a diet is known to be helpful for improving the composition of intestinal microbiota [25,26].
Unfortunately, despite these recommendations, protein, vitamin, and mineral deficiencies are diagnosed in many patients after bariatric surgery. The main causes are insufficient consumption of individual food components, the development of food aversion, a lack of recommended nutritional supplementation, decreased secretion of hydrochloric acid in the stomach, bypassing a part of the intestine that allows absorption of nutrients, and gastrointestinal concomitants [27]. Also, in patients suffering from depression, the concentrations of many micronutrients in the diet are low, as are those of omega-3 fatty acids [28]. It is known that vitamin B deficiency contributes to the development of hyperhomocysteinemia (found in some patients after bariatric surgery), which correlates with the development of depression, for example, through the generation of oxidative stress, the remodeling of the extracellular matrix of the brain and endothelial dysfunction, and the disruption of the integrity of the blood–brain barrier [29]. A low intake of omega-3 fatty acids in the diet, combined with a high intake of omega-6 fatty acids (a typical ratio in the Western diet), increases the concentrations of lipopolysaccharide (LPS) and LPS-binding protein (LBP) in the blood, increases intestinal permeability and, thus, increases the risk of developing depressive disorders [28].
To the best of our current knowledge, there are few scientific studies addressing the relationship between dietary changes, intestinal barrier functioning, and worsening mental status in patients after bariatric surgery. The aim of the study was to evaluate the efficacy of a five week probiotic therapy with multistrain probiotic preparation (Sanprobi Barrier) and the introduction of a balanced diet to improve the mental performance of patients ≥6 months after bariatric surgery who suffered from mood disorders. The multi-strain probiotic used in the previous study, showed both a reduction in depressive symptoms [30,31], and favorable effect on metabolic risk factors. Additionally, it improves integrity of the gut barrier [32,33,34] and can modify the influence of microbiota on biochemical, physiological and immunological parameters related to obesity and inflammation [35].
2. Materials and Methods
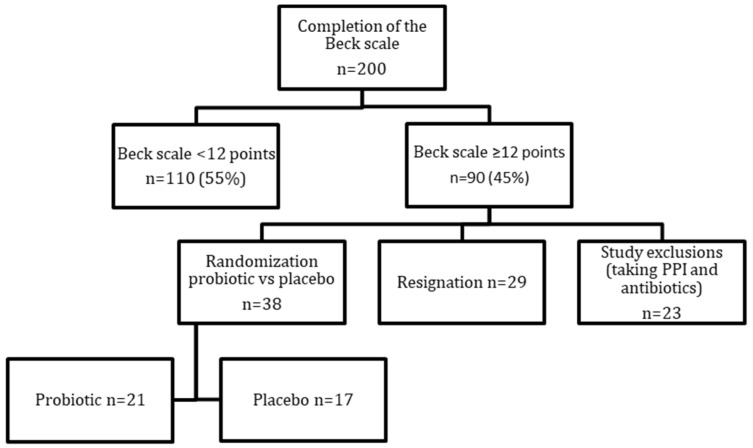
Two hundred patients who had undergone a Roux-en-Y gastric bypass (RYGB) or a sleeve gastrectomy (SG) at least 6 months before, completed the Beck Depression Inventory (a self-rating scale to screen for depression symptoms). Patients with a score of ≥12 points were eligible for the study [36,37,38]. Of the 90 subjects who scored ≥12 points, 23 subjects had exclusion criteria (taking antibiotics and proton pump inhibitors in the six months before testing), and 29 subjects declined further participation in the project. This allowed the initiation of further studies with a group of 38 patients. During the first study visit, biological material (blood, feces) was collected, and anthropometric measurements and a questionnaire test were performed. Diet was assessed using a food diary for the previous 72 h.
Patients were randomized and double-blinded into two groups, one of which received a probiotic or placebo for a period of 5 weeks:
Probiotic: Sanprobi Barrier (manufacturer: Sanprobi sp. z o. o. sp. k., Szczecin, Poland), consisting of Bifidobacterium bifidum W23, Bifidobacterium lactis W51, Bifidobacterium lactis W52, Lactobacillus acidophilus W37, Levilactobacillus brevis W63, Lacticaseibacillus casei W56, Ligilactobacillus salivarius W24, Lactococcus lactis W19, and Lactococcus lactis W58;
Placebo: corn starch, maltodextrins, vegetable protein;
Dosage: 4 capsules (2 × 109 CFU)/day (taken with a meal (2 capsules in the morning and 2 capsules in the evening);
The product is available commercially on the Polish market and its composition and dosage have been approved by the relevant health authorities.
Patients in both groups were educated about the principles of a healthy diet and were given a balanced meal plan tailored to the needs of patients after bariatric surgery [21]. After 5 weeks, patients were invited back for a follow-up visit, and the entire series of trials was repeated (Figure 1). All subjects were asked to bring probiotic/placebo blisters to the follow-up visit, which enabled the control of capsule taking. Additionally, a survey (food frequency questionnaire) was conducted asking about the degree of compliance with dietary recommendations and a 72 h food diary was collected.
Figure 1.
Study design. PPI—proton pump inhibitors.
2.1. Surgical Techniques
The sleeve gastrectomy was performed using the laparoscopic method. Approximately 80% of the stomach was removed along the greater curvature to form a new sleeve-like stomach, removing the fundus and body of the stomach. The remaining stomach had a capacity of about 150 mL. The Roux-en-Y Gastric Bypass was also performed laparoscopically. In this procedure, the stomach was transected creating a gastric pouch of approximately 20–30 mL, which was then anastomosed with mid-jejunum, creating the alimentary limb. The Roux-limb length was 75–150 cm [39,40,41].
2.2. Anthropometric Examinations
Body composition analysis was performed using a Jawon Medical ioi-353 analyzer (Jawon, Seongnam-si, Republic of Korea). Body height [cm] was measured using a metric stadiometer. Waist circumference [cm] and hip circumference [cm] were measured using a tape measure.
2.3. Survey Research—Mental State
The Beck Depression Inventory (BDI), a self-assessment questionnaire, was used to assess the occurrence and severity of depressive symptoms. The total score ranges from 0 to 63 points, with the higher the score, the higher the severity of depressive symptoms. ≥12 points were considered as the presence of depressive symptoms [36,37,38].
The Athenian Insomnia Scale is a scale consisting of 8 items that assess falling asleep, waking up at night and in the morning, total sleep time, sleep quality, well-being, psychophysical fitness, and daytime sleepiness. The total score ranged from 0 to 24 points, with a higher score representing poorer sleep quality. A score of ≥6 points was considered insomnia [42].
The Hamilton Depression Rating Scale (HDRS) was administered by an experienced psychiatrist. A 21-item version was used in the study, including items on guilt, suicidal ideation, circadian rhythm disturbances, agitation, anxiety, somatic symptoms, and psychomotor retardation. Responses are scored on a scale of 0–4 and 0–2. A score of ≥7 points indicates the presence of depression [43,44].
2.4. Eating Habits Assessment
A food frequency questionnaire (FFQ), consisting of a comprehensive list of foods and beverages with response categories indicating usual frequency of consumption over a period of time (never or almost never, once a month or less often, several times a month, several times a week, every day, several times a day) was used.
The consumption log for the last 72 h was evaluated using the Diet 5D program (Food and Nutrition Institute, Warsaw, Poland). Based on the data collected with the above tools, the International Diet Quality Index (DQI-I) was calculated, with a total score ranging from 0 to 100 points. The lower the score, the poorer the quality of the diet used. This indicator consists of four categories:
Variety—consumption of each food group (fish, meat, eggs, legumes, vegetables, fruits, cereals) and different sources of protein (fish, meat, eggs, legumes, dairy products) (0–20 points);
Adequacy—assessment of consumption of individual food components that need to be supplied to ensure a healthy diet and prevent malnutrition (vegetables, fruits, cereals, fiber, protein, iron, calcium, vitamin C) (0–40 points);
Moderation—assessing the consumption of dietary components that may require a reduction in daily intake due to an increased risk of developing chronic diseases (total fat, cholesterol, saturated fatty acids, sodium, low calorie foods) (0–30 points);
Overall dietary balance—assessment of the ratio of individual macronutrients and fatty acids (0–10 points) [45].
2.5. Laboratory Tests
Biological material was collected from patients at two time points—visit 1 and control (after 5 weeks). Venous blood was collected in EDTA tubes (Sarstedt, Bionovo, Legnica, Poland), centrifuged (3500 rpm for 10 min), and then the plasma and morphos fractions were separated in each eppendorf. Stool was collected using a stool sample kit (Kałszyk, Poland). The biological material was stored at −80 °C until laboratory analyses were performed.
2.6. Markers of the Intestinal Barrier Integrity
Fecal zonulin levels were determined by enzyme-linked immunosorbent assay (ELISA) (Immundiagnostik AG, Bensheim, Germany) according to the manufacturer’s protocol. Absorbance was measured with a spectrophotometer (Sunrise, Tecan, Männedor, Switzerland) at 450 nm.
The concentration of LPS and occludin in blood serum was determined using an ELISA assay (EIAAB SCIENCE INC, Wuhan, China), and that of LBP was determined using an ELISA assay (FineTest, Wuhan, China) according to the manufacturer’s protocols. In each case, absorbance was measured using a spectrophotometer (Sunrise, Tecan, Männedor, Switzerland) at 450 nm.
2.7. Sequencing Analysis of Bacterial 16S RNA Genes
DNA isolation from feces and sequencing of the V3–V4 regions of the 16S rDNA gene were performed using the Illumina MiSeq instrument (Illumina INC, San Diego, CA, USA) at the Institute of Clinical Molecular Biology, of the University of Kiel (Kiel, Germany) according to their own protocol. DNA was isolated using microcentrifuge columns with silica membrane. Extracted DNA was purified using an Agencourt AMPure®XP instrument (Beckman Coulter, Brea, CA, USA). Bacterial 16S RNA analysis was based on amplification of the V3–V4 regions of the 16S rRNA gene and was performed using the Metagenomic Library Construction Kit 16S (V3–V4) for Next Generation Sequencing (Takara Bio Inc., Kusatsu, Japan). Sequencing was performed using the Illumina MiSeq v3 2 × 250 bp Kit (Illumina Inc., San Diego, CA, USA).
2.8. Determination of Homocysteine and Vitamin D Levels in Blood Serum
The concentration of vitamin D metabolite 25 (OH) was determined by Diagnostyka SA with the automated chemiluminescence immunoassay method (CLIA) using the LIAISON XL device (DiaSorin, Vercelli, Italy). Serum homocysteine concentration was determined by ELISA (Bioassay Technology Laboratory, Shanghai, China) according to the manufacturer’s protocol. Absorbance was measured using a spectrophotometer (Sunrise, Tecan, Männedor, Switzerland) at 450 nm.
2.9. 16S rRNA Sequence Preprocessing
The sequencing was carried out using Illumina paired-end technology, specifically targeting the V3–V4 region of the 16S rRNA gene. To assess the quality of the raw sequencing data, a thorough initial quality screening was conducted using FastQC (version 0.12.1) and MultiQC (version 1.12) tools. Subsequently, all data preprocessing was executed using the QIIME 2 software [46]. We used the Divisive Amplicon Denoising Algorithm 2 (DADA2) [47], which is a widely used tool for denoising and quality filtering of amplicon-based sequencing data, such as 16S rRNA gene sequences. DADA2 employs a denoising algorithm to identify and correct errors within the sequencing reads. It models the error rate using the quality scores and uses this information to distinguish true biological variants (amplicon sequence variants, ASVs) from sequencing errors. After quality filtering, trimming, denoising, and chimera removal, DADA2 assigned 5685 unique ASV to each high-quality sequence and provided estimates of the abundance of each ASV within each sample (including a minimum frequency of 22.0, a 1st quartile at 23,939, a median frequency of 33,121.0, a 3rd quartile at 40,242.5, a maximum frequency of 78,764.0, and an overall mean frequency of 31,861.6). Three samples with the lowest abundance (22, 31, 43) were removed from downstream analysis. Furthermore, taxonomic classification was performed utilizing the QIIME2 implementation of VSEARCH in conjunction with the Silva 138–99 reference dataset of 16S rRNA gene sequences for taxonomic assignment. Prior to classification, an additional step involved filtering low-abundance features—the ASVs with a frequency (total abundance across samples) below 10 were excluded, allowing for a focus on robust and abundant sequences. ASVs assigned to Eukaryota, Archaea, mitochondria, and chloroplasts were excluded from the dataset due to their likely representation of contaminants or non-bacterial sequences. Lastly, the remaining ASVs were collapsed into six taxonomic levels, ranging from genus to phylum, to simplify the dataset for subsequent microbial community analysis, providing a comprehensive and refined dataset for downstream analysis.
2.10. Statistical Analysis
To compare baseline characteristics of patients, we utilized either the Wilcoxon test or Fisher test. To evaluate differences between treatment groups (Placebo versus Probiotic) concerning changes in clinical response variables from baseline to the end of the intervention, including Beck Depression Inventory (BDI), Hamilton Psychiatric Rating Scale for Depression (HRSD), Insomnia scale, and Diet Quality Index—International (DQI-I), we employed a mixed-effect linear model implemented in the lme4 R package (version 1.1–34) [48]. To provide more accurate and reliable estimates of the standard errors and degrees of freedom for the fixed effects (and more reliable p values), we used the Kenward–Roger approximation, as implemented in the lmerTest R package (version 3.1-3) [49]. To elucidate how changes in predictor variables influenced the response variables, accounting for both fixed and random effects, we used marginal effects in conjunction with predictor effect plots. Marginal effects, calculated using the marginaleffects R package (version 0.14.0), provide insight into the average change in the outcome variable for a specific predictor variable while holding all other variables constant, especially in models with multiple predictors, as they allow you to isolate the effect of one predictor variable while keeping others at fixed values. Predictor effect plots are graphical representations that show how the predicted values of the response variable change as a function of one or more predictor variables.
The analysis of gut microbiome data was conducted at baseline, where alpha-diversity measures were compared between groups using a mixed-effect model (as described above), considering the type of surgery. To test for significant differences in the composition of biological communities among groups, we applied Permutational Multivariate Analysis of Variance (PERMANOVA) implemented in the vegan R package (2.6-4) using the Bray–Curtis dissimilarity metric. Principal Coordinate Analysis (PCoA) was used for reducing the dimensionality of beta-diversity and representing it in two-dimensions.
In the analysis of genus-level data (with a 10% prevalence filtering), differential abundance analysis (DAA) was performed using five different methods: ALDEx2, ANCOMBC2, WilcoxTSS, WilcoxRarefied, and LEfSe. WilcoxTSS and WilcoxRarefied applied the Wilcoxon test to relative abundance data (TSS, total sum scaling) and rarefied data WilcoxRarefied (with sampling depth = 14,193), respectively. All statistical analyses were carried out using the R software (version 4.2.3, R Core Team (2022)) [50].
3. Results
3.1. Patients’ Characteristics
The characteristics of patients are presented in Table 1. There were no significant differences between the Placebo and Probiotic groups.
Table 1.
Characteristics of patients.
| Placebo | Probiotic | P | Q | |||
|---|---|---|---|---|---|---|
| n | Mean ± sd, n (%) | n | Mean ± sd, n (%) | |||
| age | 17 | 44.4 ± 10.4 | 21 | 44.9 ± 10.7 | 0.659 | 0.843 |
| Beck scale | 17 | 18.2 ± 7.1 | 21 | 18.8 ± 8.4 | 0.952 | 0.971 |
| Hamilton scale | 16 | 12.9 ± 4.6 | 17 | 13.3 ± 4.7 | 0.971 | 0.971 |
| Insomnia scale | 16 | 10.5 ± 4.8 | 17 | 10.1 ± 3.1 | 0.612 | 0.843 |
| Waist circumference (cm) | 17 | 100.6 ± 11.4 | 21 | 96.2 ± 12.7 | 0.277 | 0.665 |
| WHR | 17 | 0.86 ± 0.07 | 21 | 0.85 ± 0.09 | 0.702 | 0.843 |
| Weight (kg) | 17 | 93.2 ± 18.8 | 21 | 84.8 ± 15.5 | 0.168 | 0.589 |
| FFM (kg) | 17 | 59.3 ± 10.0 | 21 | 57.1 ± 10.4 | 0.463 | 0.788 |
| Fat mass (kg) | 17 | 33.9 + 11.5 | 21 | 29.1 ± 8.2 | 0.191 | 0.589 |
| BMI (kg/m2) | 17 | 32.2 ± 5.3 | 21 | 30.1 ± 4.5 | 0.127 | 0.589 |
| Weight at surgery day (kg) | 17 | 125.9 ± 24.2 | 21 | 114.5 ± 16.0 | 0.167 | 0.589 |
| Time after surgery (months) | 17 | 41.3 ± 41.8 | 21 | 28.4 ± 27.4 | 0.462 | 0.788 |
| LBP (ng/mL) | 17 | 551 ± 127 | 21 | 643 ± 236 | 0.127 | 0.589 |
| LPS (pg/mL) | 17 | 107.9 ± 33.4 | 21 | 97.9 ± 36.6 | 0.252 | 0.665 |
| Homocysteine (nmol/mL) | 17 | 8.4 ± 10.4 | 21 | 8.6 ± 8.8 | 0.411 | 0.788 |
| Zonulin (ng/mL) | 14 | 145.7 ± 84.6 | 15 | 128.9 ± 63.4 | 0.777 | 0.847 |
| Occludin (ng/mL) | 17 | 13.2 ± 3.4 | 21 | 14.1 ± 3.6 | 0.500 | 0.788 |
| Vitamin D (ng/mL) | 17 | 19.4 ±7.9 | 19 | 20.3 ± 6.5 | 0.318 | 0.694 |
| DQI_I (points) | 17 | 46.1 ± 9.7 | 21 | 48.6 ± 7.5 | 0.186 | 0.589 |
| Variety_DQI_I (points) | 17 | 9.6 ± 4.8 | 21 | 10.0 ± 4.9 | 0.677 | 0.843 |
| Adequacy_DQI_I (points) | 17 | 22.5 ± 3.6 | 21 | 22.7 ± 3.5 | 0.746 | 0.847 |
| Moderation_DQI_I (points) | 17 | 13.2 ± 4.8 | 21 | 15.3 ± 4.3 | 0.196 | 0.589 |
| Overall_balance_DQI_I (points) | 17 | 0.71 ± 1.99 | 21 | 0.57 ± 0.93 | 0.196 | 0.788 |
| Surgery type (RYGB/SG) | 17 | 4 (23.5)/13 (76.5) | 21 | 12 (57.1)/9 (42.9) | 0.052 | 0.589 |
sd—standard deviation, WHR—waist-to-hip ratio, FFM—fat-free mass, BMI—body mass index, LBP—lipopolysaccharide binding protein, LPS—lipopolysaccharide, DQI-I—diet quality index international, RYGB—Roux-en-Y gastric bypass, SG—sleeve gastrectomy.
3.2. Psychiatric Scales
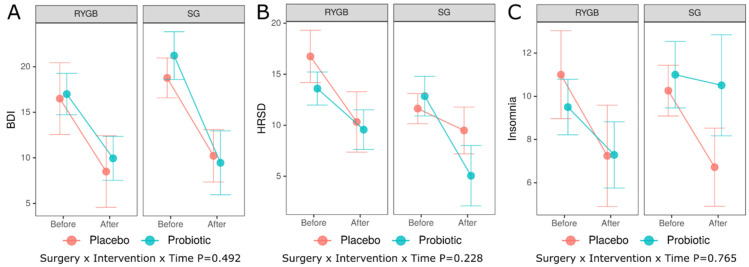
First, we examined differences between treatments groups (Placebo versus Probiotic) with respect to the average change from baseline to end of intervention in three response variables, i.e., Beck Depression Inventory (BDI), Hamilton Psychiatric Rating Scale for Depression (HRSD), and Insomnia scale, while accounting for the type of bariatric surgery (RYGB versus SG, Figure 2).
Figure 2.
Predictor effect plots summarizing the role of intervention and surgery type on predicted values of the BDI (A), HRSD (B), and Insomnia (C). BDI—beck depression inventory, HRSD—Hamilton psychiatric rating scale for depression, RYGB—Roux-en-Y gastric bypass, SG—sleeve gastrectomy. P—p value obtained from a general mixed-effects model for the three-way interaction, i.e., type of surgery (RYGB versus SG) by intervention (Placebo versus Probiotic) by time (Before versus After); BDI—Beck Depression Inventory, HRSD—Hamilton Psychiatric Rating Scale for Depression, RYGB—Roux-en-Y Gastric Bypass, SG—Sleeve Gastrectomy.
The lack of significant interaction between the type of surgery, intervention and time (p = 0.492, p = 0.228, p = 0.765 for BDI, HRSD and Insomnia, respectively) indicates that the average changes from baseline to end-point were not significantly different. The differences of predicted outcomes from baseline to end-point (Before versus After)—average marginal effects (AME) evaluated for all combinations of the two remaining categorical predictors (type of surgery and intervention) are summarized in Table 2. Although the changes over time were significant in some cases, especially for BDI, pairwise comparisons of these changes between Placebo and Probiotic groups did not reveal any significant differences.
Table 2.
Average marginal effects as a difference in predicted outcomes (Before versus After) for all combinations of levels of the categorical predictor (type of surgery and intervention) for psychiatric outcomes.
| Outcome | Surgery | Intervention | Est | SE | p | Estpairwise | SEpairwise | Ppairwise |
|---|---|---|---|---|---|---|---|---|
| BDI | RYGB | Placebo | −8.00 | 3.49 | 0.022 | −0.95 | 4.11 | 0.817 |
| Probiotic | −7.05 | 2.17 | 0.001 | |||||
| SG | Placebo | −8.55 | 2.69 | 0.002 | 3.22 | 4.25 | 0.449 | |
| Probiotic | −11.76 | 3.29 | <0.001 | |||||
| HRSD | RYGB | Placebo | −6.43 | 3.86 | 0.096 | −2.40 | 4.59 | 0.601 |
| Probiotic | −4.03 | 2.49 | 0.106 | |||||
| SG | Placebo | −2.15 | 2.71 | 0.428 | 5.66 | 4.42 | 0.201 | |
| Probiotic | −7.80 | 3.50 | 0.026 | |||||
| Insomnia | RYGB | Placebo | −3.76 | 2.92 | 0.199 | −1.54 | 3.48 | 0.658 |
| Probiotic | −2.21 | 1.89 | 0.242 | |||||
| SG | Placebo | −3.54 | 2.09 | 0.090 | −3.05 | 3.40 | 0.370 | |
| Probiotic | −0.49 | 2.68 | 0.855 |
Examined contrasts in all cases—Before versus After (After minus Before), BDI—Beck Depression Inventory, HRSD—Hamilton Psychiatric Rating Scale for Depression, RYGB—Roux-en-Y Gastric Bypass, SG—Sleeve Gastrectomy, Est—estimate of average marginal effect (AME), SE—standard error, p—p value for testing the significance of AME estimates, Estpairwise, SEpairwise, Ppairwise—estimates of AME, standard error and p value, respectively, for pairwise comparisons between Placebo and Probiotic group (Placebo minus Probiotic).
3.3. DQI-I and Its Subscales
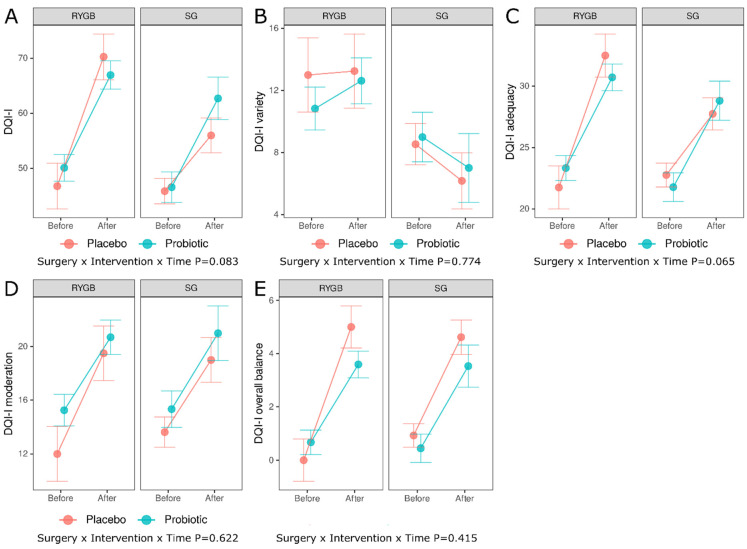
In addition to psychiatric outcomes, Diet Quality Index-International (DQI-I) and four subscales, namely variety, adequacy, moderation and overall balance, were also examined. Figure 3 and Table 3 shows all DQI-I related outcomes.
Figure 3.
Predictor effect plots summarizing the role of intervention and surgery type on predicted values of the DQI-I (A), DQI-I variety (B), DQI-I adequacy (C), DQI-I moderation (D), and DQI-I overall balance (E), RYGB—Roux-en-Y Gastric Bypass, SG—Sleeve Gastrectomy, DQI-I—diet quality index international.
Table 3.
Average marginal effects as a difference in predicted outcomes (Before versus After) for all combinations of levels of the categorical predictor (type of surgery and intervention) for DQI-I.
| Outcome | Surgery | Intervention | Est | SE | p | Estpairwise | SEpairwise | Ppairwise |
|---|---|---|---|---|---|---|---|---|
| DQI-I | RYGB | Placebo | 23.5 | 4.12 | <0.001 | 6.64 | 4.85 | 0.171 |
| Probiotic | 16.9 | 2.55 | <0.001 | |||||
| SG | Placebo | 10.1 | 3.14 | 0.001 | −6.02 | 4.95 | 0.224 | |
| Probiotic | 16.1 | 3.83 | <0.001 | |||||
| DQI-I variety |
RYGB | Placebo | 0.25 | 2.38 | 0.916 | −1.54 | 2.80 | 0.582 |
| Probiotic | 1.79 | 1.47 | 0.224 | |||||
| SG | Placebo | −2.36 | 1.81 | 0.192 | −0.37 | 2.86 | 0.897 | |
| Probiotic | −1.99 | 2.21 | 0.268 | |||||
| DQI-I adequacy |
RYGB | Placebo | 10.75 | 1.64 | <0.001 | 3.37 | 1.94 | 0.082 |
| Probiotic | 7.39 | 1.02 | <0.001 | |||||
| SG | Placebo | 4.98 | 1.26 | <0.001 | −2.06 | 1.99 | 0.300 | |
| Probiotic | 7.04 | 1.54 | <0.001 | |||||
| DQI-I moderation |
RYGB | Placebo | 7.50 | 2.89 | 0.009 | 2.05 | 3.38 | 0.544 |
| Probiotic | 5.45 | 1.75 | 0.002 | |||||
| SG | Placebo | 5.38 | 2.02 | 0.008 | −0.28 | 3.18 | 0.929 | |
| Probiotic | 5.67 | 2.46 | 0.021 | |||||
| DQI-I overall balance |
RYGB | Placebo | 5.00 | 1.09 | <0.001 | 2.08 | 1.27 | 0.103 |
| Probiotic | 2.92 | 0.66 | <0.001 | |||||
| SG | Placebo | 3.69 | 0.77 | <0.001 | 0.61 | 1.21 | 0.614 | |
| Probiotic | 3.08 | 0.94 | <0.001 |
RYGB—Roux-en-Y Gastric Bypass, SG—Sleeve Gastrectomy, DQI-I—diet quality index international, Est—estimate of average marginal effect (AME), SE—standard error, p—p value for testing the significance of AME estimates, Estpairwise, SEpairwise, Ppairwise—estimates of AME, standard error and p value, respectively, for pairwise comparisons between Placebo and Probiotic group (Placebo minus Probiotic).
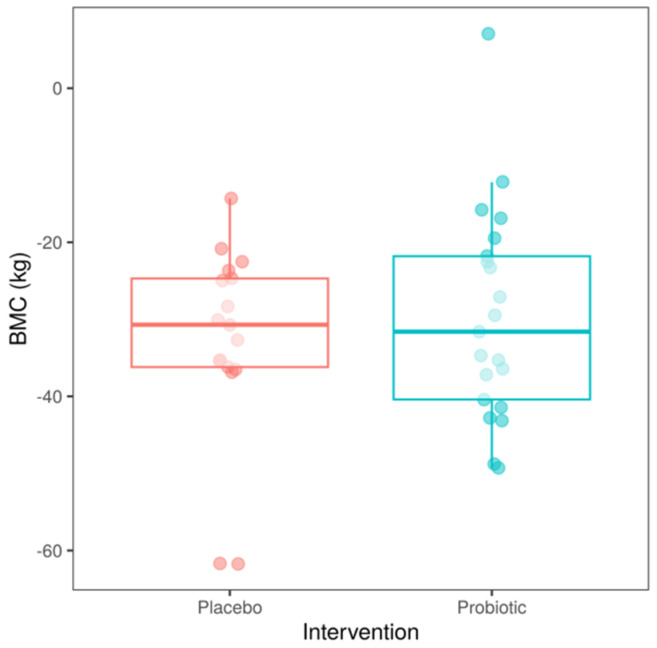
In the next stage, we investigated the effect of body mass change (BMC), in addition to intervention, to see if patients who had experienced a higher loss of body mass between the time of their surgery and the start of the trial had a distinct reaction to the probiotic intervention. BMC in patients receiving placebo and probiotic is shown in Figure 4. In subsequent analyses, we excluded three patients with extreme values (outliers, i.e., −61.7, −61.7 and 7.1 kg).
Figure 4.
Body mass change (BMC) between the time of patient’s surgery and the start of the study.
The BMC was computed as the difference between the patient’s body mass at the start of the study and the patient’s body mass at the time of surgery; hence, a decrease is indicated by a negative number, while an increase is indicated by a positive value; BMC did not differ between groups, p = 0.837 (Wilcoxon test).
3.4. Gut Microbiota
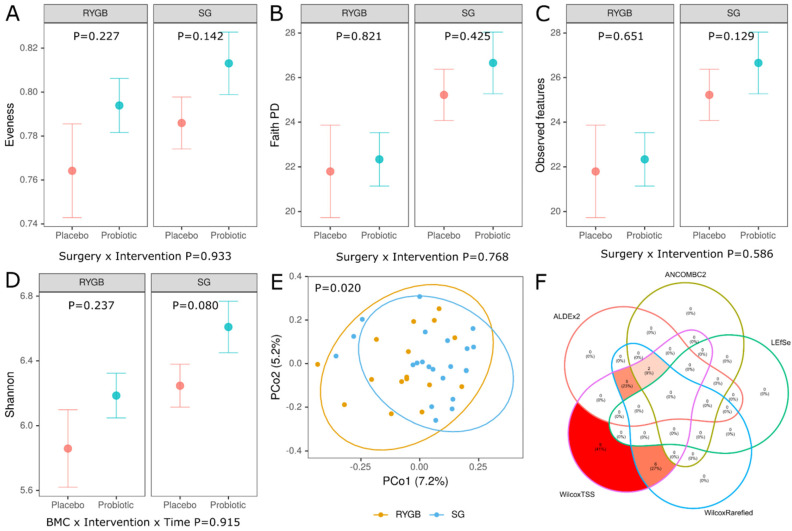
At baseline, alpha diversity did not differ between Placebo and Probiotic groups regardless of the type of bariatric surgery (Figure 5A–D). In contrast, we found a significant difference (PERMANOVA p = 0.020) in overall taxonomic composition between RYGB versus SG (but not between Placebo and Probiotic, PERMANOVA p = 0.777), as demonstrated in PCoA of Bray–Curtis distances (Figure 5E) which shows a separation between RYGB and SG. In line with this finding, there were numerous differences in the abundance of individual taxa between these groups and the results of differential abundance analysis (DAA) at the genus level using various methods are shown in Figure 5F. The Venn diagram illustrates a correspondence of 5 DAA methods and reveals that 7 genera, genus belonging to Muribaculaceae family (enriched in the RYGB), Christensenellaceae R-7 group (enriched in the SG), Veillonella (enriched in the RYGB), Subdoligranulum (enriched in the SG), Roseburia (enriched in the RYGB), Oscillibacter (enriched in the SG) and UCG-005 (enriched in the SG), were identified as differentially abundant between RYGB and SG in the ALDEx2, WilcoxTSS and Wilcox. When ANCOMBC2 method was considered, only two of them (genus belonging to Muribaculaceae family, Christensenellaceae R-7 group) remained differentially abundant. The taxa at higher levels of taxonomy were also found to be differentially abundant (Figure S1).
Figure 5.
Alpha-diversity, beta-diversity and differential abundance analysis at baseline. Alpha-diversity (A–D), beta-diversity using Bray–Curtis distance (E) and Venn diagram (F). Below plots in (A–D), p values are obtained from a general mixed-effects model for the two-way interaction, i.e., type of surgery (RYGB versus SG) by intervention (Placebo versus Probiotic) while p values in each facet refer to Placebo versus Probiotic comparison in the RYGB and SG group; (E)—Principal-coordinate analysis (PCoA) ordination plot based on Bray–Curtis distance metrics demonstrating significant grouping of samples (PERMANOVA F = 1.27, p = 0.020 RYGB versus SG); (F)—Venn diagram comparing 5 methods of differential abundance analysis at the genus level (10% prevalence filtered) (ALDEx2, ANCOMBC2, WilcoxTSS, WilcoxRarefied and LEfSe) [51,52,53], WilcoxTSS and WilcoxRarefied use Wilcoxon test on relative abundance data (TSS, total sum scaling) or rarefied data (sampling depth = 14,193), RYGB—Roux-en-Y Gastric Bypass, SG—Sleeve Gastrectomy.
4. Discussion
To the best of our knowledge, the study conducted is the first to address the possibility of reducing the severity of depressive disorders in people after bariatric surgery using diet and probiotic therapy.
Depressive symptoms were observed in 45% of 200 patients who underwent bariatric surgery. The presence of depressive disorders was also noted in other studies. Alsubaie et al. [54] showed that 30.4% of patients suffered from moderate or severe depression and 33% from moderate or severe anxiety disorders after bariatric surgery. In addition, the authors associated the occurrence of depression with young age, postoperative complications, and psychiatric symptoms that occurred 1 to 2 years after surgery [54]. It seems that disturbances in the function of the cerebral and intestinal axes may also have a significant influence on the deterioration of the mental state of patients after bariatric surgery [55].
At present, there are lack of data in the literature examining the relationship between changes in the microbiota and the development of depression after bariatric surgery. Finding such marker bacteria in the future could be a very valuable predictor of the development of depression. In a previous paper, we published results that indicate a role for the gut microbiota and inflammatory processes in the development of depressive disorders in patients after bariatric surgery [9]. Of note, the bariatric surgery itself significantly affects the composition of the microbiota [56]. In our study, we noted significant differences in the composition of the gut microbiota of patients undergoing RYGB and SG surgery. In the feces of patients after RYGB, there was a significantly higher abundance of Muribaculaceae family, Veillonella and Roseburia, while those after SG had more Christensenellaceae R-7 group, Subdoligranulum, Oscillibacter and UCG-005. Muribaculaceae produce enzymes that break down complex carbohydrates. In one study conducted in an animal model, the authors suggested that their higher abundance may be related to higher dietary fiber intake [57,58]. Although in another study, Muribaculaceae appear to be positively correlated with body weight and have been linked to the lean phenotype [59]. Interestingly, their numbers are also positively related to depressive and anxiety behaviors [60]. In contrast, the relative abundance of Veillonella was lower in studies involving participants with anxiety and depression [61]. Another study [62] found an association of thirteen microbial taxa, including genera Eggerthella, Subdoligranulum, Coprococcus, Sellimonas, Lachnoclostridium, Hungatella, Ruminococcaceae (UCG002, UCG003 and UCG005), LachnospiraceaeUCG001, Eubacterium ventriosum and Ruminococcusgauvreauiigroup, and family Ruminococcaceae with depressive symptoms. These bacteria are known to be involved in the synthesis of glutamate, butyrate, serotonin and gamma amino butyric acid (GABA), which are key neurotransmitters for depression. In addition, the number of Clostridiales also seems to be important for emotional functioning [63]. The number of Clostridiales (including Faecalibacterium, Roseburia, Lachnospira, Anaerostipes) is reduced in many mental disorders, which is associated with disturbances in amino acid and carbohydrate metabolism [63]. It is worth noting that Roseburia is one of the most important butyrate-producing bacteria, which is important for modulating the brain–gut axis [64,65]. The presence of Roseburia has been associated with good cognitive abilities [63,66] and increased insulin sensitivity [67], while insulin resistance is associated with high systemic branched-chain amino acid concentrations [68]. Excess consumption of valine, leucine, and isoleucine may lead to a decrease in brain tryptophan (a precursor of serotonin) concentration and thus to a decrease in brain serotonin concentration [69,70]. When the excess of branched-chain amino acids competes with tryptophan, the transport of this amino acid across the blood–brain barrier is less efficient [70,71]. The authors of one of the papers [63] suggested that the gut microbiota contribute (at least indirectly) to occurrence of mental disorders through low numbers of Clostridiales. This bacterial order, very important for the homeostasis of the organism, plays a significant role in the degradation of branched amino acids and prevents their increased concentration in the bloodstream (which could counteract the decrease in serotonin synthesis in the central nervous system) [63].
In our study, after 5 weeks, we noted an improvement in patients’ mental functioning (reduction in BDI and HRSD), but it was not related to the probiotic used (Figure 2). Effect of probiotics after bariatric surgery does not seem very significant. A systematic review by Cook et al. [72] also found no effect of probiotics on quality of life and weight loss after bariatric surgery. This may be due to significant changes in the composition of the microbiota after surgery, functional changes (e.g., food passage) of gastrointestinal tract, changes in body weight and dietary habits. The dosage, method and timing of probiotic use are adapted to the anatomically and functionally typical gastrointestinal tract. There are no data on the adhesion and other mechanisms of action of probiotics in the gastrointestinal tract undergoing surgery.
In our study, the most important factor that contributed to the reduction in the severity of depressive disorders may have been the dietary intervention. Consumption of vegetables and whole grain cereals increased (DQI-I adequacy), consumption of simple sugars and SFA decreased (moderation DQI-I), and consumption of monounsaturated fatty acids derived mainly from olive oil increased (overall balance DQI-I). A Western-style diet, characterized by the consumption of highly processed products with a high content of sugars and saturated fatty acids and, at the same time, a low consumption of vegetables, fruits and fiber, favors the development of microbiota disorders [73]. This type of diet predisposes to low microbiological diversity, increased intestinal barrier permeability, endotoxemia, and chronic low-intensity inflammation, which can contribute to the development of many diseases [7,73]. In addition, a diet with a high glycemic load increases the risk of developing mood disorders, fatigue, and severity of depression symptoms compared with diets based on a low glycemic load (especially in overweight or obese people) [74]. On the other hand, a diet rich in fruits, vegetables, nuts, legumes, and whole grains seems to have a beneficial effect on psychophysical performance [75]. This type of diet has been shown to be associated with a lower risk of MDD and anxiety disorders than in people following the Western diet [76]. In addition, the introduction of a Mediterranean diet has been associated with an improvement in depression symptoms in patients suffering from MDD with low diet quality [77].
The original composition of the microbiota may be important for the functioning of the intestinal barrier and the effectiveness of its sealing. It seems that RYGB has a greater impact on the gut microbiota than restrictive surgery [72]. This could be due to the fact that the anatomical changes induced by the RYGB procedure reduce the absorptive surface of the intestine and also result in the stomach being exposed to higher amounts of gastric acid. In addition, the passage of food through the gastrointestinal tract is accelerated, and the increased pH in the intestine alters its redox potential, which affects the increase in the number of aerobic and facultative aerobic microorganisms, i.e., Proteobecteria [78].
In our study, we noted no significant differences in parameters assessing intestinal barrier integrity (LPS, LBP, zonulin, occludin) between the group taking the probiotic and the placebo. However, the study by Clemente-Postigo et al. [75] showed that the concentration of LPS and LBP in the blood depended on the bariatric surgery performed and the previous blood glucose level of the patient. LPS and LBP concentrations decreased significantly (compared with preoperative values) on the ninetieth day after SG surgery. In contrast, LBP levels increased on the 15th day after bile duct exclusion and returned to preoperative levels on the 90th day after surgery. The values increased again on the 90th day after surgery [79]. In turn, Yang et al. [80] showed that LBP levels were related to BMI and high-sensitivity C-reactive protein, and that LBP levels decreased significantly one year after bariatric surgery compared with preoperative levels. The exact mechanisms leading to changes in LPS and LBP concentrations in the blood of patients after bariatric surgery are not known. They appear to be related to changes in gut microbiota composition, diet, and severity of inflammation [80,81].
Due to significant limitations, this study should be considered a pilot study. Small study groups, short time of intervention, high heterogeneity and difficulties in compliance were the biggest limitations. However, the topic addressed is very interesting and requires further well-designed clinical and mechanistic studies.
5. Conclusions
In our study, we noted a reduction in the severity of depressive symptoms, although it was not related to the probiotic therapy used. Of note, in patients who have undergone bariatric surgery, special attention should be paid to the proper balance of meals (as one of the elements contributing to the maintenance of mental health). In addition, the noted differences in the composition of the gut microbiota (RYGB vs. SG) may be one of the determinants of the functioning of the gut–brain microbiota axis, although there is currently a need for further research on this topic with a larger group of patients and different probiotic doses.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu15234905/s1, Figure S1: Gut microbiota—the different abundance of taxa at higher levels of taxonomy.
Author Contributions
Conceptualization, N.K. and E.S.; methodology, E.S. and I.Ł.; software, M.K., A.M.-V.K. and A.N.; validation, E.S., I.Ł. and M.W.; formal analysis, M.K.; investigation, N.K., K.K. and B.K.; resources, K.K. and B.K.; data curation, N.K. and E.S.; writing—original draft preparation, N.K., I.Ł., M.F. and E.S.; writing—review and editing, E.S.; visualization, M.K.; supervision, I.Ł. and M.K.; project administration, E.S.; funding acquisition, E.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Bioethics Committee of the Pomeranian Medical University in Szczecin (resolution no. KB-0012/40/17) on 27 February 2017.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
Authors Mariusz Kaczmarczyk and Igor Łoniewski were employed by the company Sanprobi sp. z o.o. sp. k. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding Statement
This research was funded by the Pomeranian Medical University in Szczecin, Poland (Project number: WNoZ 330-01/S/2022).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Major P., Matłok M., Pędziwiatr M., Migaczewski M., Budzyński P., Stanek M., Kisielewski M., Natkaniec M., Budzyński A. Quality of Life After Bariatric Surgery. Obes. Surg. 2015;25:1703–1710. doi: 10.1007/s11695-015-1601-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jumbe S., Bartlett C., Jumbe S.L., Meyrick J. The Effectiveness of Bariatric Surgery on Long Term Psychosocial Quality of Life —A Systematic Review. Obes. Res. Clin. Pract. 2016;10:225–242. doi: 10.1016/j.orcp.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 3.Spirou D., Raman J., Smith E. Psychological Outcomes Following Surgical and Endoscopic Bariatric Procedures: A Systematic Review. Obes. Rev. 2020;21:e12998. doi: 10.1111/obr.12998. [DOI] [PubMed] [Google Scholar]
- 4.Canetti L., Bachar E., Bonne O. Deterioration of Mental Health in Bariatric Surgery after 10 Years despite Successful Weight Loss. Eur. J. Clin. Nutr. 2016;70:17–22. doi: 10.1038/ejcn.2015.112. [DOI] [PubMed] [Google Scholar]
- 5.Castaneda D., Popov V.B., Wander P., Thompson C.C. Risk of Suicide and Self-Harm Is Increased After Bariatric Surgery-a Systematic Review and Meta-Analysis. Obes. Surg. 2019;29:322–333. doi: 10.1007/s11695-018-3493-4. [DOI] [PubMed] [Google Scholar]
- 6.Lim R.B.C., Zhang M.W.B., Ho R.C.M. Prevalence of All-Cause Mortality and Suicide among Bariatric Surgery Cohorts: A Meta-Analysis. Int. J. Environ. Res. Public Health. 2018;15:1519. doi: 10.3390/ijerph15071519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Madison A., Kiecolt-Glaser J.K. Stress, Depression, Diet, and the Gut Microbiota: Human–Bacteria Interactions at the Core of Psychoneuroimmunology and Nutrition. Curr. Opin. Behav. Sci. 2019;28:105–110. doi: 10.1016/j.cobeha.2019.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Limbana T., Khan F., Eskander N. Gut Microbiome and Depression: How Microbes Affect the Way We Think. Cureus. 2020;12:e9966. doi: 10.7759/cureus.9966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Komorniak N., Martynova-Van Kley A., Nalian A., Wroński M., Kaseja K., Kowalewski B., Kaźmierczak-Siedlecka K., Łoniewski I., Kaczmarczyk M., Podsiadło K., et al. Association between Fecal Microbiota, SCFA, Gut Integrity Markers and Depressive Symptoms in Patients Treated in the Past with Bariatric Surgery-The Cross-Sectional Study. Nutrients. 2022;14:5372. doi: 10.3390/nu14245372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flux M.C., Lowry C.A. Finding Intestinal Fortitude: Integrating the Microbiome into a Holistic View of Depression Mechanisms, Treatment, and Resilience. Neurobiol. Dis. 2020;135:104578. doi: 10.1016/j.nbd.2019.104578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dinan T.G., Stanton C., Cryan J.F. Psychobiotics: A Novel Class of Psychotropic. Biol. Psychiatry. 2013;74:720–726. doi: 10.1016/j.biopsych.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Sanada K., Nakajima S., Kurokawa S., Barceló-Soler A., Ikuse D., Hirata A., Yoshizawa A., Tomizawa Y., Salas-Valero M., Noda Y., et al. Gut Microbiota and Major Depressive Disorder: A Systematic Review and Meta-Analysis. J. Affect. Disord. 2020;266:1–13. doi: 10.1016/j.jad.2020.01.102. [DOI] [PubMed] [Google Scholar]
- 13.Misera A., Liśkiewicz P., Łoniewski I., Skonieczna-Żydecka K., Samochowiec J. Effect of Psychobiotics on Psychometric Tests and Inflammatory Markers in Major Depressive Disorder: Meta-Analysis of Randomized Controlled Trials with Meta-Regression. Pharmaceuticals. 2021;14:952. doi: 10.3390/ph14100952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kazemi A., Noorbala A.A., Azam K., Eskandari M.H., Djafarian K. Effect of Probiotic and Prebiotic vs Placebo on Psychological Outcomes in Patients with Major Depressive Disorder: A Randomized Clinical Trial. Clin. Nutr. 2019;38:522–528. doi: 10.1016/j.clnu.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 15.Rudzki L., Ostrowska L., Pawlak D., Małus A., Pawlak K., Waszkiewicz N., Szulc A. Probiotic Lactobacillus Plantarum 299v Decreases Kynurenine Concentration and Improves Cognitive Functions in Patients with Major Depression: A Double-Blind, Randomized, Placebo Controlled Study. Psychoneuroendocrinology. 2019;100:213–222. doi: 10.1016/j.psyneuen.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 16.Schwarcz R., Bruno J.P., Muchowski P.J., Wu H.-Q. Kynurenines in the Mammalian Brain: When Physiology Meets Pathology. Nat. Rev. Neurosci. 2012;13:465–477. doi: 10.1038/nrn3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akkasheh G., Kashani-Poor Z., Tajabadi-Ebrahimi M., Jafari P., Akbari H., Taghizadeh M., Memarzadeh M.R., Asemi Z., Esmaillzadeh A. Clinical and Metabolic Response to Probiotic Administration in Patients with Major Depressive Disorder: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrition. 2016;32:315–320. doi: 10.1016/j.nut.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Amirani E., Milajerdi A., Mirzaei H., Jamilian H., Mansournia M.A., Hallajzadeh J., Ghaderi A. The Effects of Probiotic Supplementation on Mental Health, Biomarkers of Inflammation and Oxidative Stress in Patients with Psychiatric Disorders: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Complement. Ther. Med. 2020;49:102361. doi: 10.1016/j.ctim.2020.102361. [DOI] [PubMed] [Google Scholar]
- 19.Heidarzadeh-Rad N., Gökmen-Özel H., Kazemi A., Almasi N., Djafarian K. Effects of a Psychobiotic Supplement on Serum Brain-Derived Neurotrophic Factor Levels in Depressive Patients: A Post Hoc Analysis of a Randomized Clinical Trial. J. Neurogastroenterol. Motil. 2020;26:486–495. doi: 10.5056/jnm20079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Messaoudi M., Lalonde R., Violle N., Javelot H., Desor D., Nejdi A., Bisson J.-F., Rougeot C., Pichelin M., Cazaubiel M., et al. Assessment of Psychotropic-like Properties of a Probiotic Formulation (Lactobacillus Helveticus R0052 and Bifidobacterium Longum R0175) in Rats and Human Subjects. Br. J. Nutr. 2011;105:755–764. doi: 10.1017/S0007114510004319. [DOI] [PubMed] [Google Scholar]
- 21.Mechanick J.I., Apovian C., Brethauer S., Timothy Garvey W., Joffe A.M., Kim J., Kushner R.F., Lindquist R., Pessah-Pollack R., Seger J., et al. Clinical Practice Guidelines for the Perioperative Nutrition, Metabolic, and Nonsurgical Support of Patients Undergoing Bariatric Procedures—2019 Update: Cosponsored by American Association of Clinical Endocrinologists/American College of Endocrinology, The Obesity Society, American Society for Metabolic and Bariatric Surgery, Obesity Medicine Association, and American Society of Anesthesiologists. Obesity. 2020;28:O1–O58. doi: 10.1002/oby.22719. [DOI] [PubMed] [Google Scholar]
- 22.Cambi M.P.C., Baretta G.A.P. Bariatric Diet Guide: Plate Model Template for Bariatric Surgery Patients. Arq. Bras. Cir. Dig. 2018;31:e1375. doi: 10.1590/0102-672020180001e1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tyson C.C., Nwankwo C., Lin P.-H., Svetkey L.P. The Dietary Approaches to Stop Hypertension (DASH) Eating Pattern in Special Populations. Curr. Hypertens. Rep. 2012;14:388–396. doi: 10.1007/s11906-012-0296-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sherf Dagan S., Goldenshluger A., Globus I., Schweiger C., Kessler Y., Kowen Sandbank G., Ben-Porat T., Sinai T. Nutritional Recommendations for Adult Bariatric Surgery Patients: Clinical Practice. Adv. Nutr. 2017;8:382–394. doi: 10.3945/an.116.014258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh R.K., Chang H.-W., Yan D., Lee K.M., Ucmak D., Wong K., Abrouk M., Farahnik B., Nakamura M., Zhu T.H., et al. Influence of Diet on the Gut Microbiome and Implications for Human Health. J. Transl. Med. 2017;15:73. doi: 10.1186/s12967-017-1175-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin P.-H., van Vliet S., Lin C.-Y., Svetkey L., Tyson C., Scialla J. Impact of the DASH Diet on Intestinal Permeability and Inflammation Markers. Curr. Dev. Nutr. 2020;4:542. doi: 10.1093/cdn/nzaa046_042. [DOI] [Google Scholar]
- 27.Steenackers N., Van der Schueren B., Mertens A., Lannoo M., Grauwet T., Augustijns P., Matthys C. Iron Deficiency after Bariatric Surgery: What Is the Real Problem? Proc. Nutr. Soc. 2018;77:445–455. doi: 10.1017/S0029665118000149. [DOI] [PubMed] [Google Scholar]
- 28.Bear T.L.K., Dalziel J.E., Coad J., Roy N.C., Butts C.A., Gopal P.K. The Role of the Gut Microbiota in Dietary Interventions for Depression and Anxiety. Adv. Nutr. 2020;11:890–907. doi: 10.1093/advances/nmaa016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Komorniak N., Szczuko M., Kowalewski B., Stachowska E. Nutritional Deficiencies, Bariatric Surgery, and Serum Homocysteine Level: Review of Current Literature. Obes. Surg. 2019;29:3735–3742. doi: 10.1007/s11695-019-04100-2. [DOI] [PubMed] [Google Scholar]
- 30.Steenbergen L., Sellaro R., van Hemert S., Bosch J.A., Colzato L.S. A Randomized Controlled Trial to Test the Effect of Multispecies Probiotics on Cognitive Reactivity to Sad Mood. Brain Behav. Immun. 2015;48:258–264. doi: 10.1016/j.bbi.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 31.Chahwan B., Kwan S., Isik A., van Hemert S., Burke C., Roberts L. Gut Feelings: A Randomised, Triple-Blind, Placebo-Controlled Trial of Probiotics for Depressive Symptoms. J. Affect. Disord. 2019;253:317–326. doi: 10.1016/j.jad.2019.04.097. [DOI] [PubMed] [Google Scholar]
- 32.Szulińska M., Łoniewski I., van Hemert S., Sobieska M., Bogdański P. Dose-Dependent Effects of Multispecies Probiotic Supplementation on the Lipopolysaccharide (LPS) Level and Cardiometabolic Profile in Obese Postmenopausal Women: A 12-Week Randomized Clinical Trial. Nutrients. 2018;10:773. doi: 10.3390/nu10060773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sabico S., Al-Mashharawi A., Al-Daghri N.M., Yakout S., Alnaami A.M., Alokail M.S., McTernan P.G. Effects of a Multi-Strain Probiotic Supplement for 12 Weeks in Circulating Endotoxin Levels and Cardiometabolic Profiles of Medication Naïve T2DM Patients: A Randomized Clinical Trial. J. Transl. Med. 2017;15:249. doi: 10.1186/s12967-017-1354-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sabico S., Al-Mashharawi A., Al-Daghri N.M., Wani K., Amer O.E., Hussain D.S., Ahmed Ansari M.G., Masoud M.S., Alokail M.S., McTernan P.G. Effects of a 6-Month Multi-Strain Probiotics Supplementation in Endotoxemic, Inflammatory and Cardiometabolic Status of T2DM Patients: A Randomized, Double-Blind, Placebo-Controlled Trial. Clin. Nutr. 2019;38:1561–1569. doi: 10.1016/j.clnu.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 35.Kaczmarczyk M., Szulińska M., Łoniewski I., Kręgielska-Narożna M., Skonieczna-Żydecka K., Kosciolek T., Bezshapkin V., Bogdański P. Treatment With Multi-Species Probiotics Changes the Functions, Not the Composition of Gut Microbiota in Postmenopausal Women With Obesity: A Randomized, Double-Blind, Placebo-Controlled Study. Front. Cell Infect. Microbiol. 2022;12:815798. doi: 10.3389/fcimb.2022.815798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Y.-P., Gorenstein C. Assessment of Depression in Medical Patients: A Systematic Review of the Utility of the Beck Depression Inventory-II. Clinics. 2013;68:1274–1287. doi: 10.6061/clinics/2013(09)15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kapci E.G., Uslu R., Turkcapar H., Karaoglan A. Beck Depression Inventory II: Evaluation of the Psychometric Properties and Cut-off Points in a Turkish Adult Population. Depress. Anxiety. 2008;25:E104–E110. doi: 10.1002/da.20371. [DOI] [PubMed] [Google Scholar]
- 38.Beck A.T., Ward C.H., Mendelson M., Mock J., Erbaugh J. An Inventory for Measuring Depression. Arch. Gen. Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 39.Pucci A., Batterham R.L. Mechanisms Underlying the Weight Loss Effects of RYGB and SG: Similar, yet Different. J. Endocrinol. Investig. 2019;42:117–128. doi: 10.1007/s40618-018-0892-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Benaiges D., Más-Lorenzo A., Goday A., Ramon J.M., Chillarón J.J., Pedro-Botet J., Roux J.A.F.-L. Laparoscopic Sleeve Gastrectomy: More than a Restrictive Bariatric Surgery Procedure? World J. Gastroenterol. 2015;21:11804–11814. doi: 10.3748/wjg.v21.i41.11804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schauer P.R., Ikramuddin S., Hamad G., Eid G.M., Mattar S., Cottam D., Ramanathan R., Gourash W. Laparoscopic Gastric Bypass Surgery: Current Technique. J. Laparoendosc. Adv. Surg. Tech. 2003;13:229–239. doi: 10.1089/109264203322333557. [DOI] [PubMed] [Google Scholar]
- 42.Sirajudeen M.S., Dilshad Manzar M., Alqahtani M., Alzhrani M., Albougami A., Somasekharan Pillai P., Spence D.W., Pandi-Perumal S.R. Psychometric Properties of the Athens Insomnia Scale in Occupational Computer Users. Healthcare. 2020;8:89. doi: 10.3390/healthcare8020089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hamilton M. A Rating Scale for Depression. J. Neurol. Neurosurg. Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zimmerman M., Martinez J.H., Young D., Chelminski I., Dalrymple K. Severity Classification on the Hamilton Depression Rating Scale. J. Affect. Disord. 2013;150:384–388. doi: 10.1016/j.jad.2013.04.028. [DOI] [PubMed] [Google Scholar]
- 45.Kim S., Haines P.S., Siega-Riz A.M., Popkin B.M. The Diet Quality Index-International (DQI-I) Provides an Effective Tool for Cross-National Comparison of Diet Quality as Illustrated by China and the United States. J. Nutr. 2003;133:3476–3484. doi: 10.1093/jn/133.11.3476. [DOI] [PubMed] [Google Scholar]
- 46.Bolyen E., Rideout J.R., Dillon M.R., Bokulich N.A., Abnet C.C., Al-Ghalith G.A., Alexander H., Alm E.J., Arumugam M., Asnicar F., et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019;37:852–857. doi: 10.1038/s41587-019-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Callahan B.J., McMurdie P.J., Rosen M.J., Han A.W., Johnson A.J.A., Holmes S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods. 2016;13:581–583. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bates D., Mächler M., Bolker B., Walker S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015;67:1–48. doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- 49.Kuznetsova A., Brockhoff P.B., Christensen R.H.B. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017;82:1–26. doi: 10.18637/jss.v082.i13. [DOI] [Google Scholar]
- 50.R Foundation for Statistical Computing . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2015. [(accessed on 24 August 2023)]. Available online: https://www.r-project.org/ [Google Scholar]
- 51.Fernandes A.D., Reid J.N., Macklaim J.M., McMurrough T.A., Edgell D.R., Gloor G.B. Unifying the Analysis of High-Throughput Sequencing Datasets: Characterizing RNA-Seq, 16S rRNA Gene Sequencing and Selective Growth Experiments by Compositional Data Analysis. Microbiome. 2014;2:15. doi: 10.1186/2049-2618-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin H., Peddada S.D. Analysis of Compositions of Microbiomes with Bias Correction. Nat. Commun. 2020;11:3514. doi: 10.1038/s41467-020-17041-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Segata N., Izard J., Waldron L., Gevers D., Miropolsky L., Garrett W.S., Huttenhower C. Metagenomic Biomarker Discovery and Explanation. Genome Biol. 2011;12:R60. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alsubaie S., Asiri G., Asiri E., Alqahtani F., Bredy G., Alshehri D. Depression and Anxiety on Post-Bariatric Surgery among Saudi Adults Residing in Abha, Asir Province, Saudi Arabia. Int. J. Med. Dev. Ctries. 2021;5:165–171. doi: 10.24911/IJMDC.51-1605799192. [DOI] [Google Scholar]
- 55.Brown R.M., Guerrero-Hreins E., Brown W.A., le Roux C.W., Sumithran P. Potential Gut–Brain Mechanisms behind Adverse Mental Health Outcomes of Bariatric Surgery. Nat. Rev. Endocrinol. 2021;17:549–559. doi: 10.1038/s41574-021-00520-2. [DOI] [PubMed] [Google Scholar]
- 56.Gentile J.K.A., Oliveira K.D., Pereira J.G., Tanaka D.Y., Guidini G.N., Cadona M.Z., Siriani-Ribeiro D.W., Perondini M.T. The Intestinal Microbiome in Patients Undergoing Bariatric Surgery: A Systematic Review. Arq. Bras. Cir. Dig. 2022;35:e1707. doi: 10.1590/0102-672020220002e1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang B., Kong Q., Li X., Zhao J., Zhang H., Chen W., Wang G. A High-Fat Diet Increases Gut Microbiota Biodiversity and Energy Expenditure Due to Nutrient Difference. Nutrients. 2020;12:3197. doi: 10.3390/nu12103197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barouei J., Bendiks Z., Martinic A., Mishchuk D., Heeney D., Hsieh Y.-H., Kieffer D., Zaragoza J., Martin R., Slupsky C., et al. Microbiota, Metabolome, and Immune Alterations in Obese Mice Fed a High-Fat Diet Containing Type 2 Resistant Starch. Mol. Nutr. Food Res. 2017;61:1700184. doi: 10.1002/mnfr.201700184. [DOI] [PubMed] [Google Scholar]
- 59.Liu Z., Coales I., Penney N., McDonald J.A.K., Phetcharaburanin J., Seyfried F., Li J.V. A Subset of Roux-En-Y Gastric Bypass Bacterial Consortium Colonizes the Gut of Nonsurgical Rats without Inducing Host-Microbe Metabolic Changes. mSystems. 2020;5:e01047-20. doi: 10.1128/mSystems.01047-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu X., Teng T., Li X., Fan L., Xiang Y., Jiang Y., Du K., Zhang Y., Zhou X., Xie P. Impact of Inosine on Chronic Unpredictable Mild Stress-Induced Depressive and Anxiety-Like Behaviors With the Alteration of Gut Microbiota. Front. Cell Infect. Microbiol. 2021;11:697640. doi: 10.3389/fcimb.2021.697640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhu J., Li M., Shao D., Ma S., Wei W. Altered Fecal Microbiota Signatures in Patients With Anxiety and Depression in the Gastrointestinal Cancer Screening: A Case-Control Study. Front. Psychiatry. 2021;12:757139. doi: 10.3389/fpsyt.2021.757139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Radjabzadeh D., Bosch J.A., Uitterlinden A.G., Zwinderman A.H., Ikram M.A., van Meurs J.B.J., Luik A.I., Nieuwdorp M., Lok A., van Duijn C.M., et al. Gut Microbiome-Wide Association Study of Depressive Symptoms. Nat. Commun. 2022;13:7128. doi: 10.1038/s41467-022-34502-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li J., Ma Y., Bao Z., Gui X., Li A.N., Yang Z., Li M.D. Clostridiales Are Predominant Microbes That Mediate Psychiatric Disorders. J. Psychiatr. Res. 2020;130:48–56. doi: 10.1016/j.jpsychires.2020.07.018. [DOI] [PubMed] [Google Scholar]
- 64.Silva Y.P., Bernardi A., Frozza R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020;11:25. doi: 10.3389/fendo.2020.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Louis P., Young P., Holtrop G., Flint H.J. Diversity of Human Colonic Butyrate-Producing Bacteria Revealed by Analysis of the Butyryl-CoA:Acetate CoA-Transferase Gene. Environ. Microbiol. 2010;12:304–314. doi: 10.1111/j.1462-2920.2009.02066.x. [DOI] [PubMed] [Google Scholar]
- 66.Dhiman R.K. Gut Microbiota and Hepatic Encephalopathy. Metab. Brain Dis. 2013;28:321–326. doi: 10.1007/s11011-013-9388-0. [DOI] [PubMed] [Google Scholar]
- 67.Vrieze A., Van Nood E., Holleman F., Salojärvi J., Kootte R.S., Bartelsman J.F.W.M., Dallinga-Thie G.M., Ackermans M.T., Serlie M.J., Oozeer R., et al. Transfer of Intestinal Microbiota from Lean Donors Increases Insulin Sensitivity in Individuals with Metabolic Syndrome. Gastroenterology. 2012;143:913–916.e7. doi: 10.1053/j.gastro.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 68.Wang T.J., Larson M.G., Vasan R.S., Cheng S., Rhee E.P., McCabe E., Lewis G.D., Fox C.S., Jacques P.F., Fernandez C., et al. Metabolite Profiles and the Risk of Developing Diabetes. Nat. Med. 2011;17:448–453. doi: 10.1038/nm.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Morris G., Berk M., Carvalho A., Caso J.R., Sanz Y., Walder K., Maes M. The Role of the Microbial Metabolites Including Tryptophan Catabolites and Short Chain Fatty Acids in the Pathophysiology of Immune-Inflammatory and Neuroimmune Disease. Mol. Neurobiol. 2017;54:4432–4451. doi: 10.1007/s12035-016-0004-2. [DOI] [PubMed] [Google Scholar]
- 70.Wessels A.G., Kluge H., Hirche F., Kiowski A., Schutkowski A., Corrent E., Bartelt J., König B., Stangl G.I. High Leucine Diets Stimulate Cerebral Branched-Chain Amino Acid Degradation and Modify Serotonin and Ketone Body Concentrations in a Pig Model. PLoS ONE. 2016;11:e0150376. doi: 10.1371/journal.pone.0150376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huang F., Wu X. Brain Neurotransmitter Modulation by Gut Microbiota in Anxiety and Depression. Front. Cell Dev. Biol. 2021;9:472. doi: 10.3389/fcell.2021.649103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cook J., Lehne C., Weiland A., Archid R., Ritze Y., Bauer K., Zipfel S., Penders J., Enck P., Mack I. Gut Microbiota, Probiotics and Psychological States and Behaviors after Bariatric Surgery—A Systematic Review of Their Interrelation. Nutrients. 2020;12:2396. doi: 10.3390/nu12082396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Statovci D., Aguilera M., MacSharry J., Melgar S. The Impact of Western Diet and Nutrients on the Microbiota and Immune Response at Mucosal Interfaces. Front. Immunol. 2017;8:838. doi: 10.3389/fimmu.2017.00838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Breymeyer K.L., Lampe J.W., McGregor B.A., Neuhouser M.L. Subjective Mood and Energy Levels of Healthy Weight and Overweight/Obese Healthy Adults on High-and Low-Glycemic Load Experimental Diets. Appetite. 2016;107:253–259. doi: 10.1016/j.appet.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Muñoz M.-A., Fíto M., Marrugat J., Covas M.-I., Schröder H., REGICOR and HERMES investigators Adherence to the Mediterranean Diet Is Associated with Better Mental and Physical Health. Br. J. Nutr. 2009;101:1821–1827. doi: 10.1017/S0007114508143598. [DOI] [PubMed] [Google Scholar]
- 76.Jacka F.N., Pasco J.A., Mykletun A., Williams L.J., Hodge A.M., O’Reilly S.L., Nicholson G.C., Kotowicz M.A., Berk M. Association of Western and Traditional Diets with Depression and Anxiety in Women. Am. J. Psychiatry. 2010;167:305–311. doi: 10.1176/appi.ajp.2009.09060881. [DOI] [PubMed] [Google Scholar]
- 77.Opie R.S., O’Neil A., Jacka F.N., Pizzinga J., Itsiopoulos C. A Modified Mediterranean Dietary Intervention for Adults with Major Depression: Dietary Protocol and Feasibility Data from the SMILES Trial. Nutr. Neurosci. 2018;21:487–501. doi: 10.1080/1028415X.2017.1312841. [DOI] [PubMed] [Google Scholar]
- 78.Seganfredo F.B., Blume C.A., Moehlecke M., Giongo A., Casagrande D.S., Spolidoro J.V.N., Padoin A.V., Schaan B.D., Mottin C.C. Weight-Loss Interventions and Gut Microbiota Changes in Overweight and Obese Patients: A Systematic Review. Obes. Rev. 2017;18:832–851. doi: 10.1111/obr.12541. [DOI] [PubMed] [Google Scholar]
- 79.Clemente-Postigo M., del Mar Roca-Rodriguez M., Camargo A., Ocaña-Wilhelmi L., Cardona F., Tinahones F.J. Lipopolysaccharide and Lipopolysaccharide-Binding Protein Levels and Their Relationship to Early Metabolic Improvement after Bariatric Surgery. Surg. Obes. Relat. Dis. 2015;11:933–939. doi: 10.1016/j.soard.2014.11.030. [DOI] [PubMed] [Google Scholar]
- 80.Yang P.-J., Lee W.-J., Tseng P.-H., Lee P.-H., Lin M.-T., Yang W.-S. Bariatric Surgery Decreased the Serum Level of an Endotoxin-Associated Marker: Lipopolysaccharide-Binding Protein. Surg. Obes. Relat. Dis. 2014;10:1182–1187. doi: 10.1016/j.soard.2014.02.022. [DOI] [PubMed] [Google Scholar]
- 81.Tuomi K., Logomarsino J.V. Bacterial Lipopolysaccharide, Lipopolysaccharide-Binding Protein, and Other Inflammatory Markers in Obesity and After Bariatric Surgery. Metab. Syndr. Relat. Disord. 2016;14:279–288. doi: 10.1089/met.2015.0170. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are contained within the article and Supplementary Materials.