Abstract
Bordetella pertussis, the causative agent of whooping cough, produces a wide array of factors that are associated with its ability to cause disease. The expression and regulation of these virulence factors is dependent upon the bvg locus (originally designated the vir locus), which encodes two proteins: BvgA, a 23-kDa cytoplasmic protein, and BvgS, a 135-kDa transmembrane protein. It is proposed that BvgS responds to environmental signals and interacts with BvgA, a transcriptional regulator which upon modification by BvgS binds to specific promoters and activates transcription. An additional class of genes is repressed by the bvg locus. Expression of this class, the bvg-repressed genes (vrgs [for vir-repressed genes]), is reduced under conditions in which expression of the aforementioned bvg-activated virulence factors is maximal; this repression is dependent upon the presence of an intact bvgAS locus. We have previously identified a locus required for regulation of all of the known bvg-repressed genes in B. pertussis. This locus, designated bvgR, maps to a location immediately downstream of bvgAS. We have undertaken deletion and complementation studies, as well as sequence analysis, in order to identify the bvgR open reading frame and identify the cis-acting sequences required for regulated expression of bvgR. Studies utilizing transcriptional fusions of bvgR to the gene encoding alkaline phosphatase have demonstrated that bvgR is activated at the level of transcription and that this activation is dependent upon an intact bvgAS locus.
Whooping cough is an acute respiratory disease caused by the small gram-negative bacterium Bordetella pertussis. B. pertussis expresses several factors that contribute to its ability to cause disease (15, 16, 33, 43, 45). Several of these factors, including filamentous hemagglutinin, pertactin, fimbriae, and the recently described tracheal colonization factor, contribute to the interaction between the bacterium and host cells. Other virulence factors are exotoxins which impair the function of immune cells and/or are capable of causing damage to host tissues. These include pertussis toxin, adenylate cyclase toxin, dermonecrotic toxin, and tracheal cytotoxin. Expression of these virulence factors, with the exception of tracheal cytotoxin, is activated at the level of transcription by a single locus, referred to as the bvg locus (originally designated the vir locus) (3, 38, 39, 42, 44). The bvg locus encodes a two-component regulatory system consisting of a sensor protein, BvgS, and a transcriptional activator, BvgA. Although the relevant signals for regulation of the bvg locus in vivo are unknown, activity of the bvg locus is repressed when cells are grown in the presence of MgSO4 or nicotinic acid or when they are grown at a reduced temperature in vitro (26). This bvg-mediated change in the patterns of transcription in response to environmental signals is referred to as phenotypic modulation. Under nonmodulating conditions, autophosphorylation of BvgS on a conserved histidine residue is followed by two intramolecular phosphotransfer reactions and the transfer of the phosphate moiety to a conserved aspartate residue on BvgA (41). Upon phosphorylation by BvgS, BvgA binds to cis-acting sequences in the promoter regions of the bvg-activated genes and activates transcription (7, 8, 22).
In addition to the virulence factors which are activated by the bvg locus, a second class of genes, which is repressed by the bvg locus, has been described (24). The function(s) of the bvg-repressed genes is unknown. The fact that these genes are repressed by the locus responsible for regulation of known virulence genes suggests that they play a role in the pathogenesis of the bacterium, perhaps by contributing to late stages in the infectious cycle. Alternatively, their inappropriate expression may interfere with the bacterium’s ability to cause disease. Five bvg-repressed genes have been identified to date (vrg6, vrg18, vrg24, vrg53, and vrg73), and the DNA sequences of the 5′ ends of these genes have been determined (4, 5). Examination of the upstream regions of these genes revealed the presence of a conserved sequence element in four of the five genes (vrg6, vrg18, vrg24, and vrg53). The exception, vrg73, does not appear to contain this element. A 6-bp linker inserted into the conserved sequence element, as well as a single base pair change within the element in one of these genes, vrg6, eliminated responsiveness to modulation and resulted in constitutive expression (4, 5). A construct in which the sequences upstream of the initiating codon in vrg6 were replaced with a constitutive B. pertussis promoter was still regulated normally, demonstrating that the cis-acting sequences required for bvg-dependent repression of this locus are located downstream of the translation start site (5). The conserved sequence element in vrg6 was shown by Southwestern analysis to be bound by a 34-kDa protein which is present in nonmodulated cells but absent in modulated cells (5). These results, taken together, have led to a proposed model of bvg repression in which the bvg-repressed genes are regulated by a repressor protein, the expression or activity of which is activated by the bvg locus. A locus required for expression of repressor activity has been identified and shown to be located immediately downstream of the bvgS gene (30). This locus has been designated bvgR.
In this report, we describe the identification and characterization of the BvgR open reading frame. The presence of several errors in the published sequence of the region downstream of bvgS resulted in a failure to recognize previously the presence of this large open reading frame in the bvg locus (3). Analysis of the corrected sequence, presented herein, and the results of complementation analyses demonstrate that the bvgR gene is located immediately downstream of bvgS and that it is transcribed convergently to bvgAS. Activation of expression of the bvgR gene by BvgA was demonstrated to be at the level of transcription.
MATERIALS AND METHODS
Bacterial strains, plasmids, oligonucleotides, and media.
The bacterial strains and plasmids used in this study are presented in Table 1. Escherichia coli strains were grown on L agar or in L broth supplemented with antibiotics when appropriate (31). B. pertussis strains were grown on Bordet-Gengou agar (Difco) containing 1% proteose peptone (Difco) and 15% defibrinated sheep blood. Concentrations of antibiotics, unless stated otherwise, were as follows: gentamicin sulfate, 10 μg/ml; kanamycin sulfate, 10 μg/ml; nalidixic acid, 50 μg/ml; rifampin, 50 μg/ml; streptomycin sulfate, 100 μg/ml. Plasmids were transformed into E. coli DH5α (Bethesda Research Laboratories, Bethesda, Md.).
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant features | Reference or source |
|---|---|---|
| E. coli K-12 strains | ||
| DH5α | High-efficiency transformation | BRLa |
| SM10 | Tra functions of IncP plasmids integrated into chromosome | 35 |
| S17 | Tra functions of IncP plasmids integrated into chromosome | 35 |
| B. pertussis strains | ||
| Tohama I | Patient isolate | 23 |
| BP907 | Tohama I; Nalr Strr ΔbvgA | 40 |
| BP947 | Tohama I; Nalr StrrfhaB-lacZ | 37 |
| TM1081 | Tohama I; Nalr StrrfhaB-lacZ vrg6-phoA | 30 |
| TM1126 | TM1081-bvgR (in-frame insertional mutant) | 30 |
| TM1311 | ΔbvgA orf2-phoA | This study |
| TM1312 | ΔbvgA orf1-phoA (= bvgR-phoA) | This study |
| TM1315 | fhaB-lacZ orf2-phoA | This study |
| TM1316 | fhaB-lacZ orf1-phoA ( = bvgR-phoA) | This study |
| Plasmids | ||
| pBBRKAN | Replication in Bordetella; mobilizable by IncP Tra functions | 2 |
| pTM193 | pBBRKAN with multiple cloning site inserted | This study |
| pSS2000 | Apr GmrrpsL oriT cos | 30 |
BRL, Bethesda Research Laboratories.
Strain and plasmid construction.
pTM193 derivatives bearing B. pertussis chromosomal fragments derived from the region downstream of the bvgA and bvgS genes were constructed as follows. Oligonucleotides MCS-1 (5′-CGCCG CGGATCCATATGAGCTC TAGATC TAG TAC TAG TCGACCATGG TACCAATTGAATTCGCTAGCATGC-3′) and MCS-2 (5′-CGGCATGCTAGCGAAT TCAAT TGGTACCATGG TCGAC TAG TAC TAGATC TAGAGC TCATATGGATCCGCGG-3′) were annealed to generate a linker bearing the restriction enzyme sites NarI, SacII, BamHI, NdeI, SacI, XbaI, BglII, ScaI, SpeI, SalI, HincII, NcoI, KpnI, MunI, EcoRI, NheI, SphI, and NarI. This linker was cloned into the NarI site of plasmid pBBRKAN to generate plasmid pTM193. The 9.0-kb BglII-BamHI, 6.0-kb SalI, 4.0-kb XhoI, 3.5-kb BglII-XhoI, or 1.3-kb SalI-XhoI restriction fragment derived from the region downstream of the bvg locus was cloned into the multiple cloning site of plasmid pTM193 to generate plasmids pTM193:BgB, pTM193:S, pTM193:X, pTM193:BgX, and pTM193:SX, respectively. Restriction fragments bearing BglII-proximal deletions of the 3.5-kb BglII-XhoI fragment were generated by PCR with primers X1 (5′-CTGACTCGAGAATGGCCTGCCGGTCCGCCACATCGAGCAG-3′), and either Bg2 (5′-GTCAAGATCTGGCTACGAATTGGCGCGCCGCATACGCGCC-3′), Bg3 (5′-GTCAAGATCTGGCCGAAGGTCTCGGACATGGCGCACAGGC-3′), Bg4 (5′-GTCAAGATCTTGGTACGTACGCTTGCGGCGCAGTCCGCCG-3′), Bg5 (5′-GTCAAGATCTCCTTACGGATAATTGGGCGGCATCTCGCGG-3′), or Bg6 (5′-GTCAAGATCTCATGGGCGGGGCCACATGGTCGCCCAGCAG-3′). Restriction fragments bearing XhoI-proximal deletions of the 3.5-kb BglII-XhoI fragment were generated by PCR with primers Bg1 (5′-GACTAGATCTCGAGAATGGCCTGCCGGTCCGCCACATC-3′) and either X2 (5′-GTCACTCGAGCGCTGGTGGTTTCGATGCGGCCGCTGAAGG-3′), X3 (5′-GTCACTCGAGGCGACCCCGCATTGATCGGTATCGCCGACC-3′), X4 (5′-GTCACTCGAGCGGCCACATGCTGGTCACCGATCTGCTCAAC-3′), or X5 (5′-GTCACTCGAGCATGATCCACTGGACCAACAGCGCTCGCAG-3′). The products of these PCRs were double-stranded DNA fragments which contained terminal BglII and XhoI restriction enzyme sites. After digestion with BglII and XhoI, these fragments were inserted into plasmid pTM193, which had been digested with BglII and SalI. Although the entire sequences of the PCR products were not determined, at least four independent PCRs were performed to generate those products and the PCR products from each reaction gave the same result, indicating that differences in activity between the truncated DNA fragments generated by PCR and the full-length wild-type fragment are consequences of the deletion of required sequences at the termini rather than results of misincorporation of nucleotides during the extension reactions.
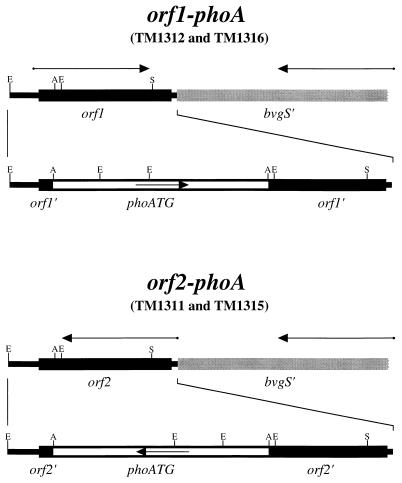
Strains with transcriptional fusions of the E. coli gene for alkaline phosphatase (phoA) to open reading frames downstream of bvgAS were constructed as follows. Oligonucleotide XApX (5′-TCGAGGGCCC-3′) was self-annealed to generate a linker which permits the insertion of an ApaI site into an XhoI site. This linker was inserted into the XhoI site in plasmid pBS KS+, generating plasmid pBS:XApX. The BglII-BamHI fragment from the B. pertussis bvg locus was inserted into plasmid pSS2000 to generate plasmid pTM030. Plasmid pTM030 was digested with BglII, XhoI, and mung bean nuclease and religated to generate plasmid pTM057. A double-stranded DNA fragment bearing the E. coli phoA gene was synthesized by PCR with primers phoA1 (5′-GCGGATCCGTATTTGTACATGGAGAAAATAAAATGAAACAAAGCAC-3′) and phoA2 (5′-GCGGATCCTTATTTCAGCCCCAGAGCGGCTTTCATGG-3′). This PCR resulted in the production of an altered form of the phoA gene in which the native GTG initiating codon was replaced by an ATG initiating codon. Oligonucleotides phoA1 and phoA2 both contain a BamHI restriction enzyme site, allowing insertion of the phoA-bearing PCR product into the BamHI site of plasmid pBS:XApX. This fragment can be inserted in either of two possible orientations, generating plasmids pTM073 and pTM074. The phoA gene was transferred from plasmid pTM073 as an ApaI fragment into the ApaI site of plasmid pTM057. Clones containing the phoA fragment were identified and designated either pTM092 or pTM093, depending on the orientation of the inserted DNA fragment. E. coli SM10 bearing pTM092 or pTM093 was mated with B. pertussis BP907 and BP947, as described below, and exconjugates in which the plasmid sequences had integrated into the chromosome were isolated by selection with gentamicin. Isolates in which plasmid sequences were lost from the chromosome but in which the orf1-phoA or orf2-phoA transcriptional fusion was retained were isolated by selection for streptomycin resistance on Bordet-Gengou agar plates and by screening for alkaline phosphatase activity in the absence of modulators. PhoA+ Strr exconjugates of BP907 bearing the orf1-phoA and orf2-phoA fusions, as well as PhoA+ Strr exconjugates of BP947 bearing the orf1-phoA and orf2-phoA fusions, were isolated and have been designated TM1312, TM1311, TM1316, and TM1315, respectively (Fig. 1).
FIG. 1.
B. pertussis TM1311, TM1312, TM1315, and TM1316. The orf1-phoA transcriptional fusion present in strains TM1312 and TM1316 and the orf2-phoA transcriptional fusion present in strains TM1311 and TM1315 are shown. The putative BvgR (ORF1 and ORF2) coding sequences are represented by black boxes. The 3′ terminus of the bvgS coding sequence is represented by gray boxes. The alkaline phosphatase coding sequence is represented by open boxes. The putative directions of transcription of the native genes and transcriptional fusions are indicated by arrows. Restriction enzyme recognition sequences are indicated as follows: E, EcoRI; B, BamHI; S, ScaI; X, XhoI; N, NcoI; A, ApaI.
Bacterial conjugations.
Matings between E. coli and B. pertussis strains were performed by swabbing bacteria from fresh plate cultures of each strain onto a Bordet-Gengou agar plate supplemented with 10 mM MgCl2 but without any antibiotics. After 3 h of incubation at 37°C, bacteria were swabbed onto Bordet-Gengou agar containing the appropriate antibiotics for selection of exconjugates, and incubation was continued at 37°C. Prior to mating, B. pertussis strains were grown for 3 days and E. coli strains were grown overnight at 37°C.
Quantitative alkaline phosphatase and β-galactosidase assays.
Bacteria to be assayed were recovered by sterile swabs into 3.5 ml of Tris-HCl, pH 8.0, and the absorbance at 600 nm was measured. For measurement of β-galactosidase activity, 0.05 ml of cell suspension was added to 1 ml of Z buffer, cells were permeabilized by the addition of 30 μl of 0.1% sodium dodecyl sulfate and 30 μl of chloroform followed by vortexing, and the assay was completed as described by Miller (31). For measurement of alkaline phosphatase, 0.5 ml of cell suspension was added to 0.5 ml of Tris-HCl (pH 8.0), the cells were permeabilized as above, and the assay was completed as described by Brickman and Beckwith (9). Units in both cases were defined by the following equation: Units = [1,000 × A420 − (1.75 × A550)]/(T × V × A600) where T is the incubation time, in minutes, and V is the volume of permeabilized cells added to the assay mixture, in milliliters.
Sequence analysis.
DNA fragments cloned in plasmid pBS KS+ were sequenced by the dideoxy sequencing method with the Sequenase 7-deaza dGTP DNA sequencing kit (United States Biochemical, Cleveland, Ohio). Computer analysis of DNA and protein sequences was performed with the GCG sequence analysis software package (Genetics Computer Group Inc., Madison, Wis.) and with the MacVector sequence analysis programs (International Biotechnologies Inc., New Haven, Conn.).
RESULTS
Complementation studies of the bvgR locus.
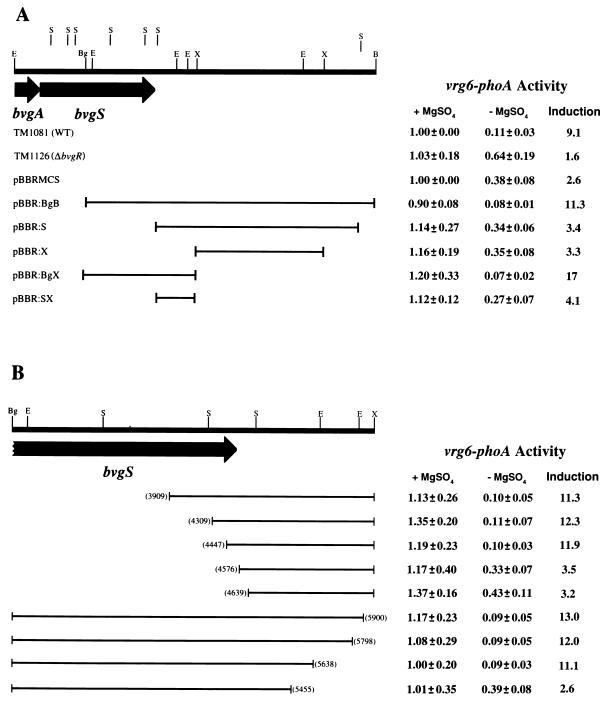
We identified previously a locus in B. pertussis that is required for regulation of the bvg-repressed genes (30). This locus, designated bvgR, was mapped to a position immediately downstream of bvgAS. In order to functionally define the limits of the bvgR locus, we utilized the fact that bvgR-dependent regulation of a vrg6-phoA fusion can be restored in the bvgR-deficient strain TM1126 when the intact bvgR locus is provided in trans (30). A collection of restriction fragments containing different segments of DNA from the region immediately downstream of bvgAS were cloned into plasmid pTM193 (Fig. 2). These constructs were transferred by conjugation into strain TM1126, which contains a 12-bp in-frame insertion within the bvgR locus, a transcriptional fusion of the E. coli lacZ gene to the bvg-activated gene fhaB, and a transcriptional fusion of the E. coli phoA gene to the bvg-repressed gene vrg6. Quantitative enzyme assays for alkaline phosphatase and β-galactosidase activities after growth in either the presence or absence of 50 mM MgSO4 were performed in order to evaluate the ability of the exconjugates to complement the bvgR deficiency in strain TM1126. In all of the strains assayed, expression of the fha-lacZ fusion was induced between 13- and 38-fold, indicating that the functions of BvgA and BvgS were not altered in these constructs relative to the wild-type strain. As seen previously, the activity of the vrg6-phoA fusion in the wild-type strain shows an approximately 10-fold reduction in activity when the cells are grown in the absence of MgSO4, while the activity of the vrg6-phoA fusion in strain TM1126 is reduced less than twofold under the same conditions (Fig. 2A). Strain TM1126 bearing plasmid pTM193 alone or pTM193:S, pTM193:X, or pTM193:SX failed to restore wild-type regulation of the vrg6-phoA fusion in strain TM1126 (Fig. 2A). Introduction of plasmid pTM193:BgB or pTM193:BgX restored wild-type regulation of the reporter fusion in strain TM1126. These results indicated that the sequences required for regulated expression of BvgR were located between the BglII site in bvgS and the XhoI site 1.5 kb downstream of bvgS.
FIG. 2.
Deletion and complementation analysis of the bvgR locus. The transcriptional activities of the vrg6-phoA transcriptional fusions in wild-type (WT) strain TM1081, strain TM1126, and strain TM1126 bearing the indicated pTM193 derivatives are shown. The bvg sequences inserted into the multiple cloning site of each pTM193 derivative are indicated by solid lines. (A) Effects of pTM193 alone and pTM193 bearing large restriction fragments derived from the bvg locus on expression of the vrg6 locus. (B) Effects of pTM193 bearing truncated versions of the bvg BglII-XhoI restriction fragment on expression of the vrg6 locus. The endpoints of deletions are indicated in parentheses. Nucleotide numbers correspond to those in the published sequence of bvgAS (3). Alkaline phosphatase activities are reported relative to strain TM1081 grown in the presence of MgSO4 (36.1 units). All reported values are averages of at least six independent assays. Restriction enzyme recognition sequences are indicated as follows: E, EcoRI; B, BamHI; S, SalI; X, XhoI; Bg, BglII.
In order to more precisely define the limits of the bvgR locus, a nested set of deletions of the BglII-XhoI fragment were generated by PCR and DNA fragments containing these deletions were inserted into plasmid pTM193. These constructs were transferred by conjugation into strain TM1126, and their ability to complement the bvgR deficiency in strain TM1126 was determined. Deletion of the sequences between the BglII site and position 4447 in the published sequence of bvgAS did not effect the ability of the plasmid to complement the bvgR deficiency in strain TM1126 (Fig. 2B). However, a deletion extending to position 4576 eliminated the ability of the plasmid to provide bvgR function in trans. Deletion of the sequences between the XhoI site and position 5638 in the published sequence of bvgAS did not affect the ability of the plasmid to complement the bvgR deficiency in strain TM1126; however, a deletion of sequences up to position 5455 eliminated the ability of the plasmid to provide bvgR function in trans.
None of the bvgR-bearing pTM193 derivatives conferred constitutive repression of the vrg6-phoA fusion in strain TM1126. This result indicates that there is not sufficient transcriptional activity through the multiple cloning site of the plasmid to confer expression of enough BvgR to repress transcription at the vrg6 locus. This conclusion is supported by the fact that insertion of all DNA fragments yielded the same result regardless of the orientation of the insert within the plasmid vector (data not shown). These results indicate that expression of the bvgR locus carried on the pTM193 derivatives is being driven by the native bvgR promoter rather than by promoters provided by the vector. These results indicate that the cis-acting sequences required for the regulated expression of bvgR are located between positions 4447 and 5638 of the published bvgAS sequence.
Sequencing of the bvgR locus.
A previous examination of the published sequence of the region immediately downstream of the bvgAS genes revealed three open reading frames which were disrupted by all of the mutations known to eliminate repressor activity in B. pertussis (30). Although the work of Beattie et al. (5) suggested that the bvg-dependent repressor was a protein of approximately 34 kDa, none of the open reading frames downstream of bvgS were predicted to encode a protein of that size. In addition, the boundaries of the bvgR locus, as determined by deletion and complementation analysis, were mapped to positions nearly 200 bp downstream from the ends of any of the three predicted open reading frames. In order to examine the possibility that the region defined by the deletion and complementation analysis contained an open reading frame large enough to encode a protein of approximately 34 kDa, we determined the DNA sequence between the SalI site at position 4272 in the published sequence and the XhoI site at position 5991. We identified 8 nucleotide residues between the 3′ end of the bvgS gene and the XhoI site downstream of bvgS which were in error in the published sequence (see Fig. 3). Sequencing was performed on both strands of DNA for the entire region specified above. Nucleotides which were not in agreement with the published sequence were sequenced at least two times on each strand. Thus, a total of at least four independent sequencing reactions were performed, and the readings for each corrected nucleotide were in agreement in all of the reactions. Examination of the corrected sequence revealed the presence of two open reading frames predicted to encode proteins of approximately 30 kDa (Fig. 1). Open reading frame 1 (ORF1) extends from position 547 to position 1422 in the corrected sequence (Fig. 3) and is predicted to encode a 32-kDa protein. Open reading frame 2 (ORF2) extends from position 1347 to position 520 in the corrected sequence and is predicted to encode a 29-kDa protein. Transcription of ORF1 and bvgAS is predicted to occur convergently. Sequences which are good matches to the E. coli ribosomal binding site and the E. coli −10 promoter element are found upstream of ORF1 at positions 474 to 479 and positions 510 to 516, respectively. ORF2 and bvgAS are predicted to be transcribed in the same direction. Sequences which are good matches to the E. coli ribosomal binding site and −10 promoter element are also found upstream of ORF2, at positions 1379 to 1373 and 1417 to 1412, respectively.
FIG. 3.
Nucleotide sequence of bvgR. The nucleotide sequence of bvgR and the 3′ end of bvgS and the corresponding amino acids are shown. A potential promoter element (−10) and ribosomal binding site (RBS) are indicated by boxed-in sequences. Corrections of the published sequence are indicated as follows: nucleotides that are deleted in the published sequence are indicated by asterisks, and at those positions where a nucleotide was inserted or substituted in the published sequence, the nucleotide present in the published sequence is shown above the corrected sequence.
Activation of bvgR.
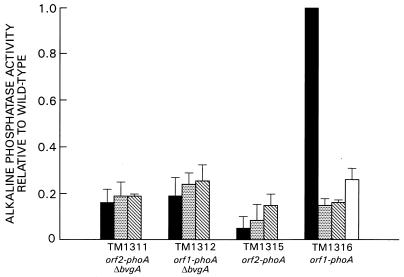
In an effort to determine which of the two open reading frames encodes the bvg-dependent repressor, an ApaI fragment containing a promoterless E. coli phoA gene was inserted in both orientations into the ApaI site located at position 5853 of the published bvgAS sequence (Fig. 1). This allowed construction of transcriptional fusions of the phoA gene to ORF1 and ORF2. These constructs were transferred by conjugation into strain BP947, which contains a transcriptional fusion of the E. coli lacZ gene to fha, and strain BP907, which contains an in-frame deletion of the bvgA locus. The orientation of the phoA gene with respect to the bvg locus was determined by restriction enzyme digestion of plasmid DNA prior to introduction into strains BP947 and BP907 and also by PCR analysis of chromosomal DNA derived from strains TM1311, TM1312, TM1315, and TM1316 (data not shown). The activities of the bvg-phoA and fha-lacZ fusions in each strain were determined by analysis of the exconjugates with quantitative enzyme assays for alkaline phosphatase and β-galactosidase after growth at 37°C. The results of this analysis are shown in Fig. 4. The fha-lacZ fusions in strains TM1315 and TM1316 demonstrated high levels of expression when the cells were grown in the absence of modulators. This activity was reduced approximately 25-fold upon growth of the cells in the presence of 50 mM MgSO4 or 20 mM nicotinic acid and approximately 5-fold upon growth at 25°C (data not shown). Low-level constitutive alkaline phosphatase activity was detected under all of the conditions examined for the transcriptional fusion of the phoA gene to ORF2 (orf2-phoA) (strains TM1311 and TM1315). The transcriptional fusion of the phoA gene to ORF1 demonstrated high levels of expression in the wild-type background (strain TM1316) in the absence of modulators. This expression was reduced approximately 6.5-fold when the bacteria were grown in the presence of 50 mM MgSO4. The orf1-phoATG fusion demonstrated low-level constitutive activity in strain TM1312, which bears an in-frame deletion of the bvgA locus. We investigated the regulation of expression of the orf1-phoA fusion further by determining the level of alkaline phosphatase activity expressed in strain TM1316 after growth in the presence of 20 mM nicotinic acid or after growth at 25°C, two conditions that have also been shown to modulate the activity of bvg-activated genes. The high level of alkaline phosphatase activity seen in strain TM1316 upon growth in the absence of modulators was reduced approximately 6.5-fold when the cells were grown in the presence of 20 mM nicotinic acid and approximately 4-fold when the cells were grown at 25°C.
FIG. 4.
Transcriptional activation of bvgR. Alkaline phosphatase activities of transcriptional fusions of the E. coli gene encoding alkaline phosphatase to ORF1 and ORF2 are shown after growth in the presence and absence of modulators. Black bars, no modulator; dotted bars, 50 mM MgSO4; diagonally striped bars, 20 mM nicotinic acid; white bar, growth at 25°C. Activities are reported relative to strain TM1316 grown in the absence of MgSO4 (153.26 units). All reported values are averages of at least four independent assays.
These results demonstrate that expression of ORF1 is activated at the level of transcription and that this expression is dependent upon the presence of an intact bvgA locus. Moreover, this activity is reduced upon growth under conditions known to down-modulate the expression of bvgA-activated genes. Our observations indicate that ORF1 encodes a bvgA-activated protein. This, along with the result that only a low constitutive level of transcriptional activity was detected through ORF2 under conditions known to promote expression of bvg-activated genes, has led us to assign a bvgR coding function to ORF1.
Analysis of the bvgR sequence.
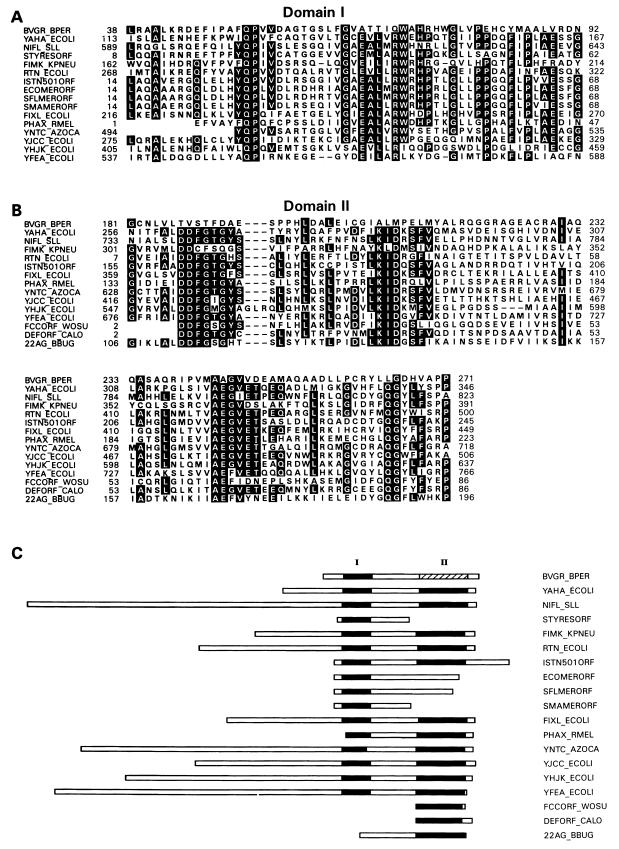
The predicted protein sequence of BvgR was determined and compared to sequences in the Swiss Protein, Protein Information Resource, and GenBank translated sequence databases. A search of the databases with the BLAST program showed that BvgR has significant homology to 18 proteins or predicted proteins found in a variety of eubacterial organisms, although the functions of these proteins are unknown (Fig. 5). The rtn locus of E. coli was identified by a transposon insertion that conferred an increased rate of adaptive mutation (19). The mechanism by which the rtn locus confers this phenotype, or if disruption of the rtn open reading frame is even responsible for the phenotype, has yet to be determined. The 22-kDa antigen of Borrelia burgdorferi has been identified, but its function is unknown (28). The functions of two of these predicted proteins have only been inferred, based upon their sequence homologies to characterized proteins in other organisms. These are the NifL homolog of Synechocystis sp. and the FixL homolog of E. coli (21) (D90789). It should be noted that the homology between these predicted proteins and BvgR does not extend to the characterized examples of these proteins (NifL of Klebsiella pneumoniae and FixL of Rhizobium meliloti). The functions of the remaining 14 of these predicted proteins are unknown, although their open reading frames are within or are tightly linked to operons with defined functions. Sequence alignment by the LFASTA program initially showed that a region of 55 amino acid residues was highly conserved among 16 of the 19 proteins (Fig. 5A). Domain I of BvgR is most closely related to domain I of YAHA_ECOLI, with 31% identity and 47% similarity over the 55-amino-acid region. Alignment of the 15 protein sequences identified as having homology to BvgR within domain I revealed the presence of a second region of high sequence homology (Fig. 5B). Although this family of proteins was originally defined by homology to domain I of BvgR, BvgR itself shows very poor conservation of domain II. Within this family, we have observed that in addition to the high degree of sequence conservation within the two domains, the spacing between the two domains and the distance between domain II and the carboxy-terminal ends of the predicted proteins are also conserved. (Fig. 5C). It should be noted that we have included in this analysis only those proteins and predicted proteins for which some information is available. An additional 12 open reading frames in the Synechocystis genome, 9 open reading frames in the E. coli genome, and 2 open reading frames in the Mycobacterium tuberculosis genome that contain these two conserved sequence elements were identified, bringing the total number of potential members of this protein family to 42.
FIG. 5.
Alignment of the predicted amino acid sequence of BvgR with a new family of proteins. BVGR_BPER, predicted protein BvgR of B. pertussis; YAHA_ECOLI, hypothetical 40.7-kDa protein encoded by the betT 3′ region of E. coli (27); NIFL_SLL, NifL homolog of Synechocystis sp. (21); STYRESORF, open reading frame 4 of the Salmonella typhimurium resolvase operon (25); FIMK_KPNEU, FimK protein of K. pneumoniae (17); RTN_ECOLI, Rtn protein of E. coli (19, 20); ISTN501ORF, unidentified reading frame 2 of the ISTN501-encoded mercuric ion resistance operon of Pseudomonas aeruginosa (10–12, 14, 32); ECOMERORF, unidentified reading frame 2 of the E. coli mercuric ion resistance operon (R100) (1, 11); SFLMERORF, unidentified reading frame 2 of the Shigella flexneri mercuric ion resistance operon (Tn501) (11); SMAMERORF, unidentified reading frame 2 of the Serratia marcesens mercuric ion resistance operon (pDU1358) (18); FIXL_ECOLI, FixL homolog of E. coli (D90789); PHAX_RMEL, unidentified reading frame X of the pha operon of R. meliloti (X93358); YNTC_AZOCA, hypothetical 80.5-kDa protein encoded by the ntrC 5′ region of Azorhizobium caulinodans (34); YJCC_ECOLI, hypothetical 60.8-kDa protein encoded by the ssb-soxS intergenic region (6); YHJK_ECOLI, hypothetical 73.1-kDa protein encoded by the dctA-dppF intergenic region (36); YFEA_ECOLI, hypothetical protein encoded by the gltX 5′ region of E. coli (13, 46). FCCORF_WOSU, unidentified open reading frame in the fcc 5′ region of Wolinella succinogenes (Y10581); DEFORF_CALO, unidentified open reading frame in the def 3′ region of Calothrix sp. (29); 22AG_BBUG, 22-kDa antigen of B. burgdorferi (28). Black boxes indicate positions conserved in at least 51% of the aligned sequences.
Examination of the predicted BvgR sequence with the Motifs program did not reveal the presence of any of the sequence motifs defined in the PROSITE dictionary of protein sites and patterns. No sequences with significant homologies were identified when the predicted protein sequence encoded by ORF2 was compared to sequences in the Swiss Protein, Protein Information Resource, and GenBank translated sequence databases with the BLAST program.
DISCUSSION
The locus required for regulation of the bvg-repressed genes in B. pertussis is located immediately downstream of bvgAS (30). This locus was designated bvgR, for Bordetella virulence gene repression. Examination of the published sequence of the bvgAS locus revealed the presence of three open reading frames in the region immediately downstream of bvgAS that are predicted to be affected by all of the mutations that have been demonstrated to abolish repressor activity (30). The largest of these open reading frames is predicted to encode a protein of approximately 25 kDa. Previous results published by Beattie et al. have suggested that the repressor protein that binds to the conserved sequence element in the vrg6 gene is a protein of approximately 34 kDa (5). It is possible that rather than repressing the bvg-repressed genes directly, the product of the bvgR locus may be required for the expression or activity of the repressor protein. If, however, bvgR does encode the repressor protein, then either the repressor is smaller than 34 kDa in size or the bvgR open reading frame is larger than those open reading frames present in the published sequence. In order to begin to address these possibilities, we sought to functionally define the upstream and downstream limits of the bvgR locus. Various plasmid derivatives of plasmid pTM193 bearing sequences derived from the bvgR locus were introduced into strain TM1126, which bears an insertional mutation in the bvgR locus which abrogates bvgR function. The ability of these derivatives to provide bvgR function in trans was determined. This analysis allowed localization of the cis-acting sequences required for regulated expression of bvgR to between positions 4447 and 5639 in the published bvgAS sequence. It should be noted, however, that although this system provides an effective method for determining the boundaries of the bvgR locus, it provides only an indirect measure of the level of bvgR expression. It is possible, for example, that promoter elements contributing to bvgR expression reside upstream of position 5639, since a requirement for these sequences would not be detected by the assay utilized in this study if less than 100% of bvgR expression was sufficient to fully activate expression at the vrg6 locus. Future studies will focus on the identification and characterization of all cis-acting sequences that contribute to bvgR expression.
Having determined the functional boundaries of the bvgR locus, we next undertook the sequencing of the region between the end of the bvgS open reading frame and the XhoI site 1.5 kb downstream of this site. This analysis resulted in the identification of 8 nucleotides which were in error in the published sequence (Fig. 3). Examination of the corrected sequence revealed the presence of two large open reading frames downstream of bvgS within the boundaries determined by deletion and complementation analysis to be sufficient to provide bvgR activity in trans (Fig. 3). One open reading frame (ORF2) was located almost in its entirety within the other open reading frame (ORF1), although the two open reading frames are encoded on opposite strands of the DNA. Both open reading frames would be expected to be disrupted by all of the mutations known to abolish repressor activity in B. pertussis, and both open reading frames are large enough to encode the protein identified as the repressor protein in the studies of Beattie et al. (5). The presence of two large open reading frames immediately downstream of bvgAS, in opposite orientation to each other, suggests two models for the regulated transcription of bvgR. If bvgR is encoded by ORF2, it lies immediately downstream of bvgS oriented in the same direction as bvgA and bvgS, which would allow all three proteins to be expressed from a single transcript under the regulation of the bvgAS promoter. If, however, bvgR is encoded by ORF1, its transcription is presumably driven from its own bvgA-activated promoter. Analysis of the transcription in the region downstream of bvgS revealed that there is only a low constitutive level of transcription through ORF2. In contrast, there was a high level of transcriptional activity through ORF1 that was reduced upon growth under conditions known to modulate the expression of bvg-activated genes; this transcription was dependent upon an intact bvgA locus. These results demonstrate that ORF1, but not ORF2, encodes a bvg-activated gene and support the conclusion that bvgR is encoded by ORF1. Although it remains to be demonstrated that bvgR encodes the actual repressor of the bvg-repressed genes, the fact that the product of the bvgR locus is predicted to be a protein of 32 kDa is consistent with that conclusion. It is interesting that the bvg-activated transcription of bvgA and bvgS does not extend through the bvgR locus. This is perhaps a little surprising, given that the end of bvgR lies only 43 nucleotides downstream from bvgS. Examination of the sequence between bvgS and bvgR reveals the presence of several large potential stem-loop structures. The presence of one or more of these stem-loop structures may lead to transcriptional termination in this region. Perhaps this is required in order to ensure that overlapping transcription of bvgAS and bvgR does not interfere with expression of the products of these genes.
The predicted BvgR protein encoded by ORF1 contains a domain that is strongly conserved among a large number of predicted proteins from a variety of eubacterial species (Fig. 5). This family of predicted proteins has been identified based on its homology to domain I of BvgR. Most of the members of this family are characterized by two domains of strong sequence homology which are located at the carboxy-terminal ends of the proteins and which reside approximately 130 residues apart. Several members of this family of proteins have either domain I or domain II but not both. The assignment of BvgR to this family of proteins is based on its high degree of conservation within domain I. In this region, BvgR is most closely related to YAHA_ECOLI, with 30% identity and 46% similarity over the 55 residues. BvgR shows weak but discernible conservation in domain II, suggesting that BvgR either has lost the requirement for the function of Domain II or has adapted this domain for an alternative function. The functional role(s), if any, of domain I and domain II is only speculative at this time, since no known function has been demonstrated for any of the members of this protein family. It is interesting to note that members of this family of proteins are encoded by open reading frames which either lie within or are very closely linked to functional operons. The identification and characterization of BvgR allows, for the first time, the assignment of an activity to one of the members of this family of proteins. It will be interesting to evaluate whether the members of this family are involved in regulation of expression of the operons to which they are so closely linked.
In this study, we have identified the bvgR open reading frame. The close correlation between the predicted size of the bvgR product and the protein shown by Beattie et al. (5) to bind the putative repressor binding site in the vrg6 gene suggests that BvgR is the actual repressor protein that binds to the conserved sequence element found within the coding sequences of the bvg-repressed genes. Our results demonstrate that the expression of bvgR is activated at the level of transcription by the products of the bvgAS genes. We propose that this activation is the result of binding of phosphorylated BvgA to the bvgR promoter. Ongoing and future work is and will be focused on characterizing in detail the activation of bvgR expression and determining the mechanism by which BvgR represses the expression of its target genes.
ACKNOWLEDGMENT
We thank Gopa Raychaudhuri for many helpful discussions and for critical reading of the manuscript.
REFERENCES
- 1.Allmeier H, Cresnar B, Greck M, Schmitt R. Complete nucleotide sequence of Tn1721: gene organization and a novel gene product with features of a chemotaxis protein. Gene. 1992;111:11–20. doi: 10.1016/0378-1119(92)90597-i. [DOI] [PubMed] [Google Scholar]
- 2.Antoine R, Locht C. Isolation and molecular characterization of a novel broad-host-range plasmid from Bordetella bronchiseptica with sequence similarities to plasmids from Gram-positive organisms. Mol Microbiol. 1992;6:1785–1799. doi: 10.1111/j.1365-2958.1992.tb01351.x. [DOI] [PubMed] [Google Scholar]
- 3.Arico B, Miller J F, Roy C, Stibitz S, Monack D, Falkow S, Gross R, Rappuoli R. Sequences required for expression of Bordetella pertussis virulence factors share homology with prokaryotic signal transduction proteins. Proc Natl Acad Sci USA. 1989;86:6671–6675. doi: 10.1073/pnas.86.17.6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beattie D T, Knapp S, Mekalanos J J. Evidence that modulation requires sequences downstream of the promoters of two vir-repressed genes of Bordetella pertussis. J Bacteriol. 1990;172:6997–7004. doi: 10.1128/jb.172.12.6997-7004.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beattie D T, Mahan M J, Mekalanos J J. Repressor binding to a regulatory site in the DNA coding sequence is sufficient to confer transcriptional regulation of the vir-repressed genes (vrg genes) in Bordetella pertussis. J Bacteriol. 1993;175:519–527. doi: 10.1128/jb.175.2.519-527.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blattner F R, Burland V, Plunket G, Sofia H J, Danials D L. Analysis of the Escherichia coli genome. V. DNA sequence of the region from 89.2 to 92.8 minute. Nucleic Acids Res. 1993;21:5408–5417. doi: 10.1093/nar/21.23.5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boucher P E, Murakami K, Ishihama A, Stibitz S. Nature of DNA binding and RNA polymerase interaction of the Bordetella pertussis BvgA transcriptional activator at the fha promoter. J Bacteriol. 1997;179:1755–1763. doi: 10.1128/jb.179.5.1755-1763.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boucher P E, Stibitz S. Synergistic binding of RNA polymerase and BvgA phosphate to the pertussis toxin promoter of Bordetella pertussis. J Bacteriol. 1995;177:6486–6491. doi: 10.1128/jb.177.22.6486-6491.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brickman E, Beckwith J. Analysis of the regulation of Escherichia coli alkaline phosphatase synthesis using deletions and f80 transducing phages. J Mol Biol. 1975;96:307–316. doi: 10.1016/0022-2836(75)90350-2. [DOI] [PubMed] [Google Scholar]
- 10.Brown N L, Ford S J, Pridmore R D, Fritzinger D C. Nucleotide sequence of a gene from the Pseudomonas transposon Tn501 encoding mercuric reductase. Biochemistry. 1983;22:4089–4095. doi: 10.1021/bi00286a015. [DOI] [PubMed] [Google Scholar]
- 11.Brown N L, Misra T K, Winnie J N, Schmidt A, Seiff M, Silver S. The nucleotide sequence of the mercuric resistance operons of plasmid R100 and transposon Tn501: further evidence for mer genes which enhance the activity of the mercuric ion detoxification system. Mol Gen Genet. 1986;202:143–151. doi: 10.1007/BF00330531. [DOI] [PubMed] [Google Scholar]
- 12.Brown N L, Winnie J N, Fritzinger D C, Pridmore R D. The nucleotide sequence of the tnpA gene completes the sequence of the Pseudomonas transposon Tn501. Nucleic Acids Res. 1985;13:5657–5669. doi: 10.1093/nar/13.15.5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brun Y V, Breton R, Lanouette P, Lapointe J. Precise mapping and comparison of two evolutionarily related regions of the Escherichia coli K-12 chromosome. Evolution of valU and lyst from an ancestral tRNA operon. J Mol Biol. 1990;214:825–843. doi: 10.1016/0022-2836(90)90339-N. [DOI] [PubMed] [Google Scholar]
- 14.Diver W P, Grinsted J, Fritzinger D C, Brown N L, Altenbuchner J, Rogowsky P, Schmitt R. DNA sequences of and complementation by the tnpR genes of Tn21, Tn501, and Tn1721. Mol Gen Genet. 1983;191:189–193. doi: 10.1007/BF00334812. [DOI] [PubMed] [Google Scholar]
- 15.Finn T M, Shahin R, Mekalanos J J. Characterization of vir-activated TnphoA gene fusions in Bordetella pertussis. Infect Immun. 1991;59:3273–3279. doi: 10.1128/iai.59.9.3273-3279.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finn T M, Stevens L A. Tracheal colonization factor: a Bordetella pertussis secreted virulence determinant. Mol Microbiol. 1995;16:625–634. doi: 10.1111/j.1365-2958.1995.tb02425.x. [DOI] [PubMed] [Google Scholar]
- 17.Gerlach G F, Clegg S, Allen B L. Identification and characterization of the genes encoding the type 3 and type 1 fimbrial adhesins of Klebsiella pneumoniae. J Bacteriol. 1989;171:1262–1270. doi: 10.1128/jb.171.3.1262-1270.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Griffin H G, Foster T J, Silver S, Misra T K. Cloning and DNA sequence of the mercuric and organomercuric-resistance determinants of plasmid pDU1358. Proc Natl Acad Sci USA. 1987;84:3112–3116. doi: 10.1073/pnas.84.10.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hall B G. The rtn gene of Proteus vulgaris is actually from Escherichia coli. J Bacteriol. 1997;179:2433–2434. doi: 10.1128/jb.179.7.2433-2434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hwang U W, Lee D W, Kim C S, Chae K. Structural analysis of an rtn gene isolated from Proteus vulgaris. Mol Cells. 1994;4:387–391. [Google Scholar]
- 21.Kaneko T, Sato S, Kotani H, Tanaka A, Asamizu E, Nakamura Y, Miyajima N, Hirosawa M, Sugiura M, Sasamoto S, Kimura T, Hosouchi T, Matsuno A, Muraki A, Nakazaki N, Naruo K, Okumura S, Shimpo S, Takeuchi C, Wada T, Watanabe A, Yamada M, Yasuda M, Tabata S. Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC6803. II. Sequence determination of the entire genome and assignment of potential protein coding regions. DNA Res. 1996;3:109–136. doi: 10.1093/dnares/3.3.109. [DOI] [PubMed] [Google Scholar]
- 22.Karimova G, Bellalou J, Ullmann A. Phosphorylation-dependent binding of BvgA to the upstream region of the cyaA gene of Bordetella pertussis. Mol Microbiol. 1996;20:489–496. doi: 10.1046/j.1365-2958.1996.5231057.x. [DOI] [PubMed] [Google Scholar]
- 23.Kasuga T, Nakase Y, Ukishima K, Takatsu K. Studies on Haemophilus pertussis. V. Relation between the phase of bacilli and the progress of the whooping-cough. Arch Exp Med. 1954;27:57–62. [PubMed] [Google Scholar]
- 24.Knapp S, Mekalanos J J. Two trans-acting regulatory genes (vir and mod) control antigenic modulation in Bordetella pertussis. J Bacteriol. 1988;170:5059–5066. doi: 10.1128/jb.170.11.5059-5066.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krause M, Guiney D G. Identification of a multimer resolution system involved in stabilization of the Salmonella dublin virulence plasmid pSDL2. J Bacteriol. 1991;173:5754–5762. doi: 10.1128/jb.173.18.5754-5762.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lacey B W. Antigenic modulation of Bordetella pertussis. J Hyg. 1960;31:423–434. doi: 10.1017/s0022172400038134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lamark T, Kaasen I, Eshoo M W, Falkenberg P, McDougall J, Strom A R. DNA sequence and analysis of the bet genes encoding the osmoregulatory choline-glycine betaine pathway of Escherichia coli. Mol Microbiol. 1991;5:1049–1064. doi: 10.1111/j.1365-2958.1991.tb01877.x. [DOI] [PubMed] [Google Scholar]
- 28.LeFabvre R B, Probert W S, Perng G C. Characterization of a chromosomal gene and the antigen it expresses from the lyme disease agent Borrelia burgdorferi. J Clin Microbiol. 1993;31:2146–2151. doi: 10.1128/jcm.31.8.2146-2151.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mazel D, Coic E, Blanchard S, Saurin W, Marliere P. A survey of polypeptide deformylase function throughout the eubacterial lineage. J Mol Biol. 1997;266:939–949. doi: 10.1006/jmbi.1996.0835. [DOI] [PubMed] [Google Scholar]
- 30.Merkel T J, Stibitz S. Identification of a locus required for the regulation of bvg-repressed genes in Bordetella pertussis. J Bacteriol. 1995;177:2727–2736. doi: 10.1128/jb.177.10.2727-2736.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1972. [Google Scholar]
- 32.Misra T K, Brown N L, Fritzinger D C, Pridmore R D, Barnes W M, Haberstroh L, Silver S. The nucleotide sequence of the mercuric resistance operons of plasmidd R100 and transposon Tn501: further evidence for mer genes which enhance the activity of the mercuric ion detoxification system. Proc Natl Acad Sci USA. 1984;81:5975–5979. doi: 10.1073/pnas.81.19.5975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mooi F R. Virulence factors of Bordetella pertussis. Antonie van Leeuwenhoek. 1988;54:465–474. doi: 10.1007/BF00461865. [DOI] [PubMed] [Google Scholar]
- 34.Pawlowski K, Klosse U, de Bruijn F J. Characterization of a novel Azorhizobium caulinodans ORS571 two-componant regulatory system, NtrY/NtrX, involved in nitrogen fixation and metabolism. Mol Gen Genet. 1991;231:124–138. doi: 10.1007/BF00293830. [DOI] [PubMed] [Google Scholar]
- 35.Simon R, Priefer U, Puhler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Bio/Technology. 1983;1:784–789. [Google Scholar]
- 36.Sofia H J, Burland V, Danials D L, Plunket G, Blattner F R. Analysis of the Escherichia coli genome. IV. DNA sequence of the region from 76.0 to 81.5 minutes. Nucleic Acids Res. 1994;22:2576–2586. doi: 10.1093/nar/22.13.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stibitz S. Use of conditionally counterselectable suicide vectors for allelic exchange. Methods Enzymol. 1994;235:458–465. doi: 10.1016/0076-6879(94)35161-9. [DOI] [PubMed] [Google Scholar]
- 38.Stibitz S, Aaronson W, Monack D, Falkow S. Phase-variation in Bordetella pertussis by Frameshift mutation in a Gene for a Novel Two-component System. Nature. 1989;338:226–229. doi: 10.1038/338266a0. [DOI] [PubMed] [Google Scholar]
- 39.Stibitz S, Miller J F. Coordinate regulation of virulence in Bordetella pertussis mediated by the vir (bvg) locus. In: Miller V L, Kaper J B, Portnoy D A, Isberg R R, editors. Molecular genetics of bacterial pathogenesis. Washington, D.C: American Society for Microbiology; 1994. pp. 407–422. [Google Scholar]
- 40.Stibitz S, Yang M S. Subcellular localization and immunological detection of proteins encoded by the vir locus of Bordetella pertussis. J Bacteriol. 1991;173:4288–4296. doi: 10.1128/jb.173.14.4288-4296.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uhl M A, Miller J F. Autophosphorylation and phosphotransfer in the Bordetella pertussis BvgAS signal transduction cascade. Proc Natl Acad Sci USA. 1994;91:1163–1167. doi: 10.1073/pnas.91.3.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Uhl M A, Miller J F. Bordetella pertussis BvgAS virulence control system. In: Hoch J A, Silhavy T J, editors. Two-component signal transduction. Washington, D.C: ASM Press; 1995. pp. 333–349. [Google Scholar]
- 43.Weiss A A, Hewlett E L. Virulence factors of Bordetella pertussis. Annu Rev Microbiol. 1986;40:661–686. doi: 10.1146/annurev.mi.40.100186.003305. [DOI] [PubMed] [Google Scholar]
- 44.Weiss A A, Hewlett E L, Meyers A, Falkow S. Tn5-induced mutations affecting virulence factors of Bordetella pertussis. Infect Immun. 1983;42:33–41. doi: 10.1128/iai.42.1.33-41.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weiss A A, Melton A R, Walker K E, Andraos-Selim C, Meidl J J. Use of the promoter fusion transposon Tn5lac to identify mutations in Bordetella pertussis vir-regulated genes. Infect Immun. 1989;57:2674–2682. doi: 10.1128/iai.57.9.2674-2682.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamamoto Y, Aiba H, Baba T, Hayashi K, Inada T, Isono K, Itoh T, Kimura S, Kitagawa M, Makino K, Miki T, Mitsuhashi N, Mizobuchi K, Mori H, Nakade S, Nakamura Y, Nashimoto H, Oshima T, Oyama S, Saito N, Sampei G, Satoh Y, Sivasundaram S, Tagami H, Takahashi H, Takeda J, Takemoto K, Uehara K, Wada C, Yamagata S, Horiuchi T. Construction of a contiguous 874 kb sequence of the Escherichia coli K-12 genome corresponding to 50.0–68.8 min region on the linkage map and analysis of its sequence features. DNA Res. 1997;4:91–113. doi: 10.1093/dnares/4.2.91. [DOI] [PubMed] [Google Scholar]