Abstract
The prsDE genes encode a type I protein secretion system required for the secretion of the nodulation protein NodO and at least three other proteins from Rhizobium leguminosarum bv. viciae. At least one of these proteins was predicted to be a glycanase involved in processing of bacterial exopolysaccharide (EPS). Two strongly homologous genes (plyA and plyB) were identified as encoding secreted proteins with polysaccharide degradation activity. Both PlyA and PlyB degrade EPS and carboxymethyl cellulose (CMC), and these extracellular activities are absent in a prsD (protein secretion) mutant. The plyA gene is upstream of prsD but appears to be expressed at a very low level (if at all) in cultured bacteria. A plyB::Tn5 mutant has a very large reduction in degradation of EPS and CMC. Cultures of plyB mutants contained an increased ratio of EPS repeat units to reducing ends, indicating that the EPS was present in a longer-chain form, and this correlated with a significant increase in culture viscosity. Thus, PlyB may play a role in processing of EPS. Analysis of the symbiotic properties of a plyA plyB double mutant revealed that these genes are not required for symbiotic nitrogen fixation and that nodulation was not significantly affected. PlyA and PlyB are similar to bacterial and fungal polysaccharide lyases; they contain 10 copies of what we propose as a novel heptapeptide repeat motif that may constitute a fold similar to that found in the family of extracellular pectate lyases. PlyA and PlyB lack the Ca2+-binding RTX nonapeptide repeat motifs usually found in proteins secreted via type I systems. We propose that PlyA and PlyB are members of a new family of proteins secreted via type I secretion systems and that they are involved in processing of EPS.
The Rhizobium-legume symbiosis involves an exchange of signalling molecules between plant and bacterium. The main signals produced by the bacterium are lipochitooligosaccharides (Nod factors), which are synthesized by the products of the nod genes and are essential for nodulation. The structure and variety of different Nod factors determine the types of legumes that can be nodulated. Some rhizobial strains also secrete proteins that may be involved in signalling during nodulation (13, 14, 23, 44), providing an extension of the Nod factor-based signalling pathway.
The prsDE genes encode two components of a type I protein secretion system that is required for the secretion of the nodulation signalling protein NodO and at least three other proteins from Rhizobium leguminosarum bv. viciae (16). One of the other proteins secreted via the Prs system is thought to be a glycanase that cleaves the bacterial expolysaccharide (EPS), since a prsD mutant lacks the ability to degrade EPS in a plate assay and produces EPS with an increased degree of polymerization (16). EPS is required at an early stage in nodule invasion, and a low-molecular-weight form has been implicated in the infection of roots by Rhizobium meliloti (2, 43). However, the mutant of R. leguminosarum bv. viciae defective in protein secretion and cleavage of EPS formed fully infected nodules containing bacteroids (16).
Protein secretion via a type I pathway involves a C-terminal, noncleaved secretion signal (46), and, indeed, the C-terminal 24 amino acids of NodO is essential for its secretion (39). No clear consensus sequence exists for these C-terminal secretion signals; their specificity seems to reside in conserved properties of secondary structure (48, 53). Most proteins that are secreted via type I secretion systems (including NodO) contain a characteristic nonapeptide tandem called RTX (repeat in toxin), so called because these repeats are present in many secreted protein toxins. Members of this family of toxins include Escherichia coli alpha-hemolysin, Erwinia chrysanthemi proteases, Pseudomonas aeruginosa alkaline protease, and Serratia marcescens protease and lipase. The structures of some proteins carrying this motif have been determined (3, 4). The RTX repeats form a β-roll structure stabilized by Ca2+ ions coordinated between adjacent coils of the β-roll. In some cases, the RTX repeats are required for efficient secretion, especially of large, heterologous proteins (12, 25, 39, 46).
The genes encoding type I secretion systems are usually adjacent to the genes encoding the secreted proteins (11, 15, 26, 27, 47), whereas nodO is unlinked to the genes required for NodO secretion (37). Genes encoding other proteins secreted by the PrsDE system are unlinked to nodO, since they are not present on the symbiotic plasmid pRL1JI (16). Therefore the R. leguminosarum bv. viciae Prs system is apparently different from most other characterized type I systems, which usually secrete only a single protein or several very similar proteins that are encoded by genes adjacent to one another.
MATERIALS AND METHODS
Microbiological methods.
Rhizobia were grown at 28°C in TY medium (6) with appropriate antibiotics at the following concentrations (micrograms per milliliter): streptomycin, 400; kanamycin, 20; gentamicin, 200; spectinomycin, 20; tetracycline, 10; lividomycin, 5. Where required for the induction of nod genes, hesperetin was added to a final concentration of 1 μM. Culture optical densities were measured at 600 nm with an MSE Spectro-Plus spectrophotometer. The bacterial strains and plasmids used are described in Table 1 or the text.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristics | Reference |
|---|---|---|
| Strains | ||
| 8401 | R. leguminosarum lacking pSym; Strr | 24 |
| 8401(pRL1JI) | Derivative of 8401 carrying the symbiotic plasmid pRL1JI | 9 |
| A408 | 8401 prsD1::Tn5 | 16 |
| A412 | 8401(pRL1JI) prsD1::Tn5 | 16 |
| A501 | 8401(pRL1JI) plyA2::Tn5 | This work |
| A503 | 8401(pRL1JI) plyA1::Tn5 | This work |
| A575 | 8401(pRL1JI) prs6::Tn5 | This work |
| A590 | 8401(pRL1JI) prsD2::Tn5 | This work |
| A591 | 8401(pRL1JI) prsD3::Tn5 | This work |
| A599 | 8401(pRL1JI) prsD4::Tn5 | This work |
| A600 | 8401(pRL1JI) plyB1::Tn5 | This work |
| A616 | Transductant of 8401 with plyB1::Tn5 | This work |
| A617 | Transductant of 8401(pRL1JI) with plyB1::Tn5 | This work |
| A632 | 8401 plyA2::Tn5 | This work |
| A633 | 8401 plyA1::Tn5 | This work |
| A636 | 8401 plyA::SpcrplyB1::Tn5 | This work |
| A638 | 8401(pRL1JI) plyA3::Spcr | This work |
| A640 | 8401(pRL1JI) plyA3::SpcrplyB1::Tn5 | This work |
| Plasmids | ||
| pGV910-C1 | pGV910 derivative carrying truncated egl from A. caulinodans on a 4.3-kb EcoRI fragment from pRGC1 | 17 |
| pIJ7298 | pLAFR1 cosmid carrying prsDE and plyA | 16 |
| pIJ7349 | 9.6-kb EcoRI fragment from pIJ7298 carrying prsDE, in pIJ1891 | 16 |
| pIJ7419 | pIJ7298 derivative with plyA2::Tn5 | This work |
| pIJ7421 | pIJ7298 derivative with plyA1::Tn5 | This work |
| pIJ7474 | 16-kb EcoRI fragment from pIJ7419 carrying plyA2::Tn5 in pBluescript (SK+) | This work |
| pIJ7647 | pLAFR1 cosmid carrying plyB | This work |
| pIJ7653 | pIJ7647 derivative with plyB1::Tn5 | This work |
| pIJ7708 | 2-kb EcoRI-BamHI fragment with plyB from pIJ7647 in pUC18 | This work |
| pIJ7709 | 2-kb EcoRI-BamHI fragment with plyB from pIJ7647 in pIJ1891 | This work |
| pIJ7754 | 12-kb EcoRI fragment with plyA::Spcr in pBluescript(SK+) | This work |
| pIJ7765 | Derivative of pJQ200(KS) carrying 12-kb EcoRI fragment with plyA::Spcr from pIJ7754 | This work |
| pIJ7871 | plyA cloned behind a vector promoter in pKT230 | This work |
| pRGC1 | pLAFR1 cosmid carrying the egl endoglycanase gene from A. caulinodans | 17 |
For polysaccharide analysis, bacteria were grown in Y medium (38) containing mannitol (0.2%, wt/vol) as the carbon source. E. coli strains were grown in L broth (31), at 37°C. Plasmids were transferred to E. coli by transformation and to Rhizobium by triparental mating with a helper plasmid. Strains A616 and A617 were made by transduction (8) of 8401 and 8401(pRL1JI) with phage RL38 propagated on the glycanase mutant A600.
Mutagenesis of pIJ7298 was done as follows. An E. coli strain carrying pIJ7298 and Tn5 on the chromosome was grown overnight in an inhibitory concentration of kanamycin (1 mg/ml) to enrich for cells in which Tn5 had transposed onto pIJ7298. The cells were subcultured in fresh medium containing no kanamycin, and plasmid DNA was isolated. Transformants of E. coli carrying Tn5 in pIJ7298 were selected on tetracycline and kanamycin. Recombination of the Tn5 mutations into R. leguminosarum bv. viciae was done by marker exchange (35).
Nodulation tests were conducted on peas (Pisum sativum var. Wisconsin Perfection) with a minimum of 12 plants per test or on vetch (Vicia hirsuta), as described by Knight et al. (22), with at least 20 plants per test.
Construction of plyA plyB double mutants.
To construct strains carrying mutations in both plyA and plyB, a plyA3::Spcr allele was constructed. The 16-kb EcoRI fragment carrying plyA2::Tn5 was cloned from pIJ7419 into pBluescript(SK+) (Stratagene) to form pIJ7474. Digestion of pIJ7474 with HpaI resulted in the loss of an internal Tn5 fragment that included the kanamycin resistance gene. The 2-kb Spcr cassette from pHP45Ω (32) was cloned as a SmaI fragment into the HpaI-digested pIJ7474 to make pIJ7754. The resulting plyA3::Spcr allele was cloned as an EcoRI fragment into the sacB suicide vector pJQ200(KS) (33) to make pIJ7765. The double mutants A636 and A640 were made by marker exchange of the plyA3::Spcr allele from pIJ7765 into the plyB mutants A616 and A617, respectively. Double mutants were identified as spectinomycin-, kanamycin-, and sucrose-resistant, gentamicin-sensitive colonies.
Protein analysis.
For rapid and sensitive detection of secreted NodO, 250 μl of culture supernatant was loaded onto a nitrocellulose membrane (Sartorius) with a slot blot apparatus. The membrane was then immunostained with a mixture of NodO-specific monoclonal antibodies (39) and goat anti-rat immunoglobulin conjugated to horseradish peroxidase (Sigma). The bound complex was visualized with the enhanced chemiluminescence (ECL) kit (Amersham) as specified by the manufacturer.
For analysis of secreted proteins, rhizobia were grown for 24 h at 28°C in TY medium to an optical density of 0.6. Culture-supernatant proteins were concentrated by precipitation with 10% trichloroacetic acid, as described previously (13), except that after precipitation, the trichloroacetic acid was extracted by washing the precipitate with acetone. Proteins from an equivalent of 5 to 10 ml of culture supernatant were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (7) with 8% acrylamide and visualized by staining with Coomassie brilliant blue R-250.
PlyB was labelled with [35S]methionine by using an E. coli T7 RNA polymerase expression system. The EcoRI-BamHI fragment carrying plyB (see Fig. 1b) was cloned behind the T7 promoter in pT7-5 (40). Expression and labelling with [35S]methionine were carried out in E. coli K38(pGP1-2) in the presence of rifampin as described previously (40). Labelled proteins were detected by autoradiography of SDS-PAGE gels. The molecular weight standards used were the broad-range prestained markers from New England Biolabs.
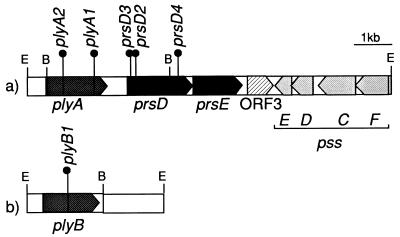
FIG. 1.
(a) Map of the 9.6-kb EcoRI fragment carring the prsDE genes. Downstream from prsDE and ORF3 and in the opposite orientation are four genes (pssFCDE) homologous to glycosyl transferases in R. leguminosarum bv. trifolii (45) and R. meliloti (19). Upstream from prsDE is plyA, encoding a putative polysaccharide lyase. (b) The plyB gene is located in a 3.6-kb EcoRI fragment. Tn5 insertions identified in prsD, plyA, and plyB are indicated by solid circles. E, EcoRI; B, BamHI.
Plate assays for enzyme activities.
For detection of glycanase activity, EPS was precipitated from 5-day cultures of strain A408 with 3 volumes of ethanol and redissolved in sterile water. EPS was incorporated into Y agar plates at about 2 mg ml−1. Colonies were grown for 3 days on this medium and then washed off with water. The plates were flooded with 0.1% Congo Red (42) for 15 min and washed with 5% acetic acid. Degradation of EPS was observed as clearings (reduction of staining) under the colony. Carboxymethyl cellulose (CMC) or polygalacturonic acid degradation was detected similarly, except that colonies were grown on Y agar containing 0.1% CMC or polygalacturonic acid instead of EPS. CMC plates were washed for 10 min with 1 M NaCl before being washed with 5% acetic acid.
Isolation of glycanase mutants and complementation by pIJ7647.
For the isolation of mutants lacking polysaccharide-degrading activity, Tn5-induced mutants were picked onto CMC or EPS agar plates and analyzed for the ability to produce clearings. Colonies which gave reduced degradation were rescreened by the same procedure. For the isolation of a cosmid (pIJ7647) which complemented the plyB mutant for activity on CMC agar, an R. leguminosarum bv. viciae cosmid library (9) was introduced into A617 (plyB). Transconjugants were selected for streptomycin, kanamycin, and tetracycline resistance and were tested for CMC degradation on plates. The Tn5 mutation from A617 was recombined onto pIJ7647, to make pIJ7653, by conjugating A617(pIJ7647) with an E. coli strain carrying a helper plasmid. E. coli transconjugants which contained recombinant derivatives of pIJ7647 carrying the Tn5 insertion from A617 were selected on L medium containing kanamycin and tetracycline, and the location of Tn5 was mapped with several restriction endonucleases. The precise location of Tn5 was obtained by cloning EcoRI-BamHI fragments containing each end of the Tn5 into pUC18 and sequencing from the ends of Tn5 with an oligonucleotide primer.
Polysaccharide analysis.
Culture supernatants were analyzed by the anthrone method (29) to determine the concentration of sugars and by the Lever (28) method to determine the concentration of reducing sugars. Measurements of total sugars were made with reference to a standard mixture of sugars corresponding to the molar ratio of sugar residues present in the bacterial EPS (glucose/glucuronic acid/galactose ratio, 5:2:1) (10). The ratio of EPS repeat units to reducing ends was calculated on the assumption that EPS accounted for all the carbohydrate present in the culture supernatants. An estimate of the molar concentration of the EPS repeat unit was calculated on the assumption that the concentration of the repeat unit is one-eighth of the total estimated sugar concentration. This was then divided by the measured molar concentration of reducing sugars to obtain the ratio of EPS repeat units to reducing ends. The results are presented as the mean values from duplicate experiments (see Table 2). Variation between duplicate experiments was less than 20%. Qualitatively similar results were obtained in several different experiments, although the actual values were variable (probably due to changes occurring during growth), making it difficult to meaningfully average all the results. The viscosities of the cultures were compared by measuring the time taken to flow between marks on a 0.1-ml glass pipette. The readings were normalized against the results for the wild-type strain 8401(pRL1JI). The results are given as the mean values from duplicate experiments and are representative of several experiments (see Table 2). Variation between duplicate experiments was less than 10%. Sugars in the EPS were converted to alditol acetate derivatives and analyzed by gas chromatography (21).
TABLE 2.
Analysis of EPSs
| Strain | Relative culture viscosity | Amt of carbohydrate in culture supernatant (μg/ml) | Amt of reducing sugars in culture supernatant (μg/ml) | Ratio of EPS repeat units to reducing ends |
|---|---|---|---|---|
| 8401(pRL1JI) | 1.0 | 1,800 | 2.7 | 82 |
| A412 (prsD) | 2.7 | 2,400 | <0.2 | >1,200 |
| A632 (plyA) | 0.65 | 2,100 | 2.5 | 100 |
| A616 (plyB) | 2.0 | 2,900 | <0.2 | >1,425 |
DNA manipulation and sequence analysis.
Standard DNA manipulations were carried out by the method of Sambrook et al. (36). A 9.6-kb EcoRI fragment, cloned in pBluescript (SK+) (Stratagene) and carrying plyA, prsD, and prsE, was digested with NarI and SmaI and religated, resulting in the deletion of 6.2 kb. The remaining 3.4-kb carrying plyA and part (809 bp) of prsD was cloned into pKT230 as an EcoRI-SacI fragment to form pIJ7871. Double-stranded DNA sequencing was carried out on pIJ7708 and on subcloned fragments of pIJ7349 by using exonuclease III deletions to generate templates. Cycle sequencing was done on a Perkin-Elmer PCR 9600 instrument with M13 forward and reverse primers, using ABI “Prism” Dye Primer cycle-sequencing ready-reaction kits. Reactions were run on ABI 377 and 373A DNA sequencers.
Sequence analysis was carried out with the GCG package (version 8; Genetics Computer Group, Madison, Wis.). Homologous proteins in the data library at the National Center for Biotechnology Information were identified with the blastp program (1). Predictions of secondary structure for protein sequences were obtained with the PredictProtein server (34).
RESULTS
Isolation of mutants defective in polysaccharide degradation.
Previously we observed that mutation of prsD in R. leguminosarum bv. viciae abolished the secretion of several proteins and that this correlated with an inability of the prsD mutant to cleave EPS and CMC (16). On the assumption that a gene(s) encoding such one secreted protein(s) may be close to prsD, we extended the DNA sequence upstream of prsD (there was no evidence for such genes downstream of prsD). This sequencing revealed an open reading frame (ORF) upstream of and in the same orientation as prsD (Fig. 1a). Since the ORF ends 541 bp upstream from prsD, it is unlikely to be transcribed as part of the same operon; indeed, a Tn5 insertion in this ORF (see below) had no polar effect on the secretion of NodO (data not shown), indicating that the mutation is not polar on prsD, which is required for NodO secretion. The 450 bp upstream of the ORF is predicted to be noncoding and therefore may include a promoter.
Since the predicted protein sequence encoded by this ORF has significant similarity to that of bacterial and fungal polysaccharide lyases (see below), we thought that it might be responsible for the CMC and/or EPS degradation by R. leguminosarum bv. viciae, and we called the gene plyA. Two Tn5 insertions within plyA were identified by restriction mapping of mutated derivatives of pIJ7298 (which carries plyA and prsDE); the Tn5 insertion sites were confirmed by DNA sequencing from the Tn5 ends (Fig. 1a). The mutations (plyA1::Tn5 and plyA2::Tn5) were recombined into R. leguminosarum bv. viciae by homologous recombination to make the mutants A632 (plyA1::Tn5) and A633 (plyA2::Tn5). Neither mutant was affected in its ability to degrade CMC or EPS in agar plates containing these substrates. This is illustrated for A632 grown on CMC-agar plates (Fig. 2a); as shown, the degradation seen below the A632 colony is not significantly different from that seen with the isogenic control strain (strain 8401). In contrast, degradation of CMC is essentially absent from the prsD (protein secretion) mutant A408. These observations showed that the degradation of CMC and EPS observed in the plate assays does not require plyA and must be due to a secreted enzyme encoded by another gene.
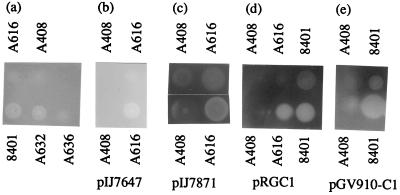
FIG. 2.
Degradation of CMC by ply mutants. CMC degradation is estimated by the lack of staining (seen as cleared regions) by Congo Red in the agar directly below the colonies. (a) Comparison of the CMC degradation by 8401 (wild-type control), A632 (plyA), A616 (plyB), A408 (prsD), and A636 (plyA plyB) on CMC agar. (b) Introduction of pIJ7647 (carrying plyB) results in increased degradation of CMC by A616 (plyB) but not by A408 (prsD). (c) Introduction of pIJ7871 (carrying plyA) causes strong degradation by A616 (plyB) but not by A408 (prsD). (d) Egl from A. caulinodans, expressed from pRGC1, causes increased CMC degradation by 8401 and A616 (plyB) but not by A408 (prsD). (e) Similar results were obtained with Egl expressed from pGV910-C1, except that the CMC degradation extended beyond the diameter of the colony.
To identify this other gene, plate assays were used to screen for mutants unable to degrade EPS or CMC. A population of Tn5-induced mutants was screened on plates containing either CMC or EPS, and colonies that gave a reduced clearing in comparison with the control strain were identified. This screen was expected to result in the isolation of two classes of mutants: those with mutations in the prs genes, which would prevent secretion of the endoglycanase, and those with mutations in a gene encoding a secreted enzyme that degrades CMC and/or EPS. From a total of 12,000 mutant colonies screened, 5 were identified which produced reduced clearing on both EPS and CMC plates. No colonies that degraded only one of the substrates were identified. Genetic complementation studies and mapping of the sites of Tn5 insertions revealed that four of the five mutations were in prsD, and biochemical studies (data not shown) confirmed that these mutants were identical to the prsD protein secretion mutant described previously (16). The insertion sites of three of the prsD Tn5 mutations are shown in Fig. 1a.
One mutant was not complemented by the prsDE plyA region cloned on pIJ7298. The Tn5 from this mutant cotransduced (100%) with the defect in CMC and EPS degradation, confirming that a single event caused both phenotypes. The test of CMC degradation by one of the transductants (A616) is illustrated in Fig. 2a. This reveals that there is very little CMC degradation, although a low residual level appears to be present, suggesting that another CMC-degrading enzyme may be present. A similar observation was made with EPS degradation (data not shown). The residual level of CMC degradation by the mutant A616 is highlighted in Fig. 2c, in which the level of staining is enhanced. This shows the presence of a zone of degradation below the A616 colony. There also appears to be a low level of CMC degradation below the prsD secretion mutant A408; this may be due to a low level of lysis of some cells in the colony. To determine if the mutation in A616 caused a general effect on protein secretion, the Tn5 mutation was transduced into a strain carrying the nodO gene on the symbiotic plasmid. This mutant (A617) was confirmed to be defective in CMC degradation but retained the ability to secrete NodO (data not shown). This indicates that the Tn5 mutation affects a gene involved in CMC and EPS degradation rather than a component of the type I protein secretion system.
Identification of plyB, which encodes a polysaccharide-degrading enzyme.
A cosmid library of Rhizobium DNA was crossed into the mutant (A616) defective for CMC and EPS degradation to identify complementing cosmids. The transconjugant colonies were screened for restoration of degradation by using CMC agar plates, and the cosmid pIJ7647 was isolated from one such complemented colony. When pIJ7647 was transferred back into A616, it again restored CMC degradation, demonstrating that pIJ7647 complements the mutation in A616 (Fig. 2b). A 2-kb EcoRI-HindIII fragment subcloned from pIJ7647 could also complement the mutant, and DNA sequencing of this 2-kb fragment revealed a long ORF (1,494 bp). DNA sequencing from the ends of the Tn5 mutation (cloned from A616) revealed that the Tn5 is inserted 666 nucleotides downstream of the proposed translation start of the ORF (Fig. 1b). The ORF encoded a predicted protein of 51.5 kDa that is 76% similar (71% identical) to PlyA, and the gene was called plyB. In comparison with PlyB, PlyA contains an extra 50 amino acids located 120 residues from the C terminus. Apart from this insert, the similarity extends along the full length of two proteins.
Both PlyA and PlyB show significant similarity to bacterial and fungal polysaccharide lyases, including about 20% identity to PlyD from Aspergillus niger (20), PelC from E. chrysanthemi (41), and SpsR from Sphingomonas strain 888. SpsR may be involved in processing of the sphingan EPS produced by Sphingomonas (49). These similarities strongly suggest that plyB and plyA encode enzymes with polysaccharide-degrading activity; the observation that mutation of plyB strongly reduces the degradation of EPS suggests that the primary role of PlyB is the processing of EPS. Since the secretion of CMC and EPS degradation activity is blocked by mutation of prsD, we conclude that PlyB is secreted via the prsDE-encoded type I secretion system. It is clear that there is little or no degradation of CMC by the prsD mutant A408, even when the plyB gene is cloned on the multicopy plasmid pIJ7647 (Fig. 2b).
In view of the level of identity between PlyA and PlyB, it seemed likely that plyA also encodes an enzyme with polysaccharide-degrading activity. To test this, plyA was cloned behind a vector promoter to generate pIJ7871, which was then introduced into the plyB mutant (A616), in which the EPS and CMC degradation is greatly reduced. As shown in Fig. 2c, pIJ7871 strongly increases the degradation of CMC, and similar results were seen on EPS-agar plates (not shown). There was no significant CMC (or EPS) degradation when plyA (on pIJ7871) was introduced into the protein secretion mutant A408 (prsD). On the basis of these results, we conclude that PlyA, like PlyB, is secreted via the prsDE-encoded type I secretion system. Such a type I-dependent secretion of PlyA and PlyB is consistent with the absence of potential N-terminal secretion signals in PlyA or PlyB and suggests that they probably have C-terminal secretion signals.
Secretion of an A. caulinodans endoglycanase.
An endoglycanase gene (egl) was previously cloned from Azorhizobium caulinodans (17). The egl gene product shows no similarity to PlyA and PlyB or to any other proteins shown to be secreted by a type I secretion system. The cloned egl gene (on pRGC1) was transferred to A616 (plyB) and shown to induce strong degradation of CMC (Fig. 2d). However, there was no degradation of EPS by this strain (data not shown), demonstrating that although the egl gene product can degrade CMC, it cannot degrade the EPS from R. leguminosarum bv. viciae.
The observation (Fig. 2d) that the cloned egl gene does not cause CMC degradation in the protein secretion mutant A408 (prsD) demonstrates that Egl secretion is prsD dependent. Geelen et al. (17) described a derivative of the egl gene that retained CMC degradation activity even though a large part of the gene encoding the N-terminal domain of the Egl protein was deleted. This deleted form of the Egl protein could be also secreted by R. leguminosarum bv. viciae, as observed by the strongly enhanced degradation of CMC by R. leguminosarum bv. viciae 8401 carrying the deleted form of egl (on pGV910-C1). This demonstrates that the deleted N-terminal region of Egl is not required for its secretion. The observed secretion is PrsD dependent as judged by the lack of CMC degradation by the prsD mutant A408 carrying the deleted form of egl (Fig. 2e).
The pattern of CMC degradation by the strain carrying the deleted egl gene was different from that observed in all of the other assays. In all the other cases, the zone of CMC degradation was observed only directly below the colony; i.e., there was no observed diffusion of CMC degradation activity beyond the edge of the colony. However, with the deleted derivative of egl (on pGV910-C1), the zone of CMC degradation extended well beyond the edge of the colony (Fig. 2e). The wild-type form of Egl was shown to be cell associated in A. caulinodans (17), and this also seems to be the case in R. leguminosarum bv. viciae with wild-type Egl, which does not diffuse beyond the edge of the colony (Fig. 2d). It may be that this cell association is mediated via the N-terminal half of the protein and that deletion of this region allows a more freely diffusible activity.
Analysis of culture-supernatant proteins of ply mutants.
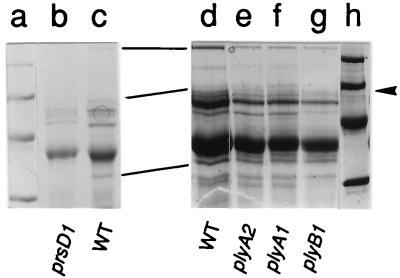
Previously we identified three proteins (other than NodO), with Mrs of 33,000, 65,000, and 110,000, which were not secreted by a prsD mutant. To determine which (if any) of these proteins was absent from plyA or plyB mutants, culture supernatant proteins from 8401 (wild type), A616 (plyB1::Tn5), A632 (plyA2::Tn5), and A633 (plyA1::Tn5) were concentrated, separated by SDS-PAGE, and stained with Coomassie blue (Fig. 3). No bands were absent from the culture supernatants of the plyA mutants; the bands previously identified as being secreted via PrsDE were all present, indicating that none of these is encoded by plyA. A band running at about 65 kDa was absent from (or significantly reduced in amount in) the culture supernatant of the plyB mutant A616 (Fig. 3, lane g). This band corresponds to one of the proteins previously observed to be missing from the culture supernatant of a prsD mutant (Fig. 3). However, the predicted molecular mass of PlyB (51.5 kDa) is significantly lower than the apparent molecular mass of the missing protein band (65 kDa). The only other difference compared with the control concerns a protein of 30 kDa, whose was reduced in the supernatant of the plyB mutant (Fig. 3, lane g), but this decrease was more variable between different preparations. We considered it possible that the PlyB protein migrated aberrantly on SDS-PAGE. plyB was expressed in E. coli by using a T7 expression system in which expressed proteins were labelled with [35S]methionine (40). A plyB-specific band of 52 kDa was detected following SDS-PAGE and autoradiography (data not shown). Therefore, we conclude that PlyB migrates normally on SDS-PAGE and that we are unable to detect its absence in the culture supernatant of the plyB mutant. The absence of the 65-kDa protein from the Coomassie blue-stained gel of the plyB mutant may be due to a secondary effect (such as lack of expression of a downstream gene) in the plyB mutant.
FIG. 3.
Two Coomassie blue-stained SDS-PAGE gels showing secreted proteins from R. leguminosarum bv. viciae mutants. Culture supernatant proteins were precipitated from the prsD mutant A412 (lane b), wild-type (WT) 8401(pRL1JI) (lanes c and d), the plyA mutants A501 (lane e) and A503 (lane f), and the plyB mutant A617 (lane g). Molecular size markers (94, 67, 43, and 30 kDa) are shown in lanes a and h. The strains were grown in the absence of the nod gene inducer hesperetin. Proteins present in 8401(pRL1JI) but absent from the secretion mutant A412 are indicated in lanes c and d. One of these proteins (arrowhead) is apparently also absent from A617 (lane g). The gel containing lanes a, b, and c was run slightly differently from the other gel; the lines highlight the positions of the proteins that were absent from the supernatant of prsD mutants.
Analysis of EPSs of ply mutants.
The prsD mutant was found to have an increased ratio of total to reducing sugars in culture supernatants and an increase in culture viscosity over the wild-type strain (16). We analyzed the effects of the plyA and plyB mutations on the EPS by using the same methods that we previously used to characterize the EPS of the prsD mutant. Parallel experiments were done with the mutants containing or lacking the symbiotic plasmid pRL1JI, which was found to have no effect on the results. Therefore, for simplicity and to enable a direct comparison with our previous data (which was obtained with pRL1JI-containing strains), we present only the results of the analysis of EPS in the plyA and plyB mutants carrying pRL1JI.
The amount of EPS (determined by measuring the total sugar content by the anthrone method) made by the plyA mutant (A501) was similar to that observed with the control strain [8401(pRL1JI)], and there was also no observed difference in the concentration of reducing sugars (an estimate of the numbers of free ends of EPS) (Table 2). Therefore, the calculated ratio of EPS repeats to reducing sugars is similar to that in the control, 8401(pRL1JI). The viscosity of cultures of A501 (plyA) was not increased. These observations indicate that the plyA mutation does not greatly affect EPS processing, at least under the growth conditions used. The plyB mutant had a slightly higher level of EPS, since the total sugar content was slightly higher than that of the control [8401(pRL1JI)] (Table 2), but the concentration of reducing sugars was below the limits of sensitivity of the assay. Under the conditions used, the assay could detect reducing sugars at concentrations above 0.2 μg ml−1. Therefore, the level of reducing sugars found with the plyB mutant (A617) was less than 1/10 of the level seen with the control [8401(pRL1JI)]. On this basis, the ratio of EPS repeats to reducing ends in the plyB mutant was calculated to be more than 100 times that observed with the wild-type. The measurements of viscosity supported these results; A617 (plyB) cultures were twice as viscous as control [8401(pRL1JI)] cultures after 5 days of growth (Table 2), indicating that the EPS of the mutant is longer than normal. Even when the culture supernatants were adjusted to have similar carbohydrate concentrations, the viscosity of the plyB mutant was much higher (about 1.8- to 2-fold) than that of the wild type. There is a theoretical possibility that these altered characteristics of the plyB mutant are due to the formation of a new EPS. However, gas chromatography analysis of alditol acetate derivatives of culture supernatant polysaccharides from the plyB mutant (and the plyA mutant) demonstrated that the ratio and amount of neutral monosaccharides were the same as in the wild-type control (data not shown). Therefore, the increase in culture viscosity and estimated polysaccharide length observed in the plyB mutant is unlikely to be due to the formation of a novel high-molecular-weight polysaccharide.
Analysis of the role of plyA and plyB in the symbiosis.
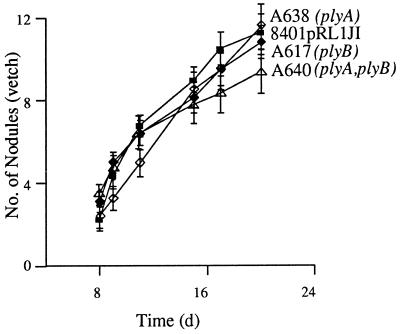
The protein secretion mutant A412 (prsD), which cannot secrete NodO, PlyB, PlyA, or other proteins, induces Fix− nodules on peas. As is typical of Fix− mutants, this was accompanied by an increase in nodule number from an average of about 80 to about 120 nodules/plant (16). This phenotype cannot be explained by the absence of secreted NodO, since nodules induced by a strain lacking nodO are Fix+ (14). One or more proteins that are secreted via the PrsDE system are presumably required for the development of an effective symbiosis. The number of nodules induced on peas by A638 (plyA) and A617 (plyB) was normal (an average of about 80 nodules per plant after 24 days of growth), and this correlated with normal levels of nitrogen fixation (based on measurements of acetylene reduction). The possibility remained that plyA and plyB were fulfilling a similar function and that either one of them was sufficient for nitrogen fixation; therefore, a double mutant was constructed. The plyA plyB double mutant (A640) was also normal with respect to the number of nodules formed and levels of symbiotic nitrogen fixation, demonstrating that PlyA and PlyB are not required to establish a nitrogen-fixing symbiosis. The rate of nodulation of the various mutants on vetch was measured (Fig. 4). A640 (plyA plyB) induced slightly fewer nodules than did the control [8401(pRL1JI)], A638 (plyA), and A617 (plyB). This indicates that plyA and plyB have little influence on the efficiency of nodulation.
FIG. 4.
Nodulation of vetch by 8401 pRLIJI (▪), the plyA mutant A638 (◊), the plyB mutant A617 (⧫), and the double mutant A640 (plyA plyB) (▵). Standard errors are shown.
Since plyB mutants retain a small residual activity on CMC plates and EPS plates, another enzyme which is able to degrade CMC and EPS may be present. Mutant strains lacking both plyA and plyB retained the same level of residual activity seen with the plyB mutant on CMC agar and EPS agar. The residual activity in the plyB mutant is therefore not dependent on PlyA and may be due to an additional polysaccharide-degrading enzyme(s) secreted by R. leguminosarum bv. viciae. The plyA plyB double mutant was also indistinguishable from the plyB mutant in terms of culture viscosity and supernatant polysaccharides (data not shown).
Structural characteristics of PlyA and PlyB.
Our data suggest that PlyA and PlyB cleave EPS and depend on the prsDE genes for their secretion. The C-terminal 70 amino acids of PlyA and PlyB are very similar and are expected to contain a secretion signal. NodO, PlyA, PlyB, and Egl, all proposed to be secreted by PrsDE, have a characteristic motif at the extreme C terminus. This is made up of a negatively charged residue followed by several hydrophobic residues and is often present in proteins secreted via type I secretion systems (18).
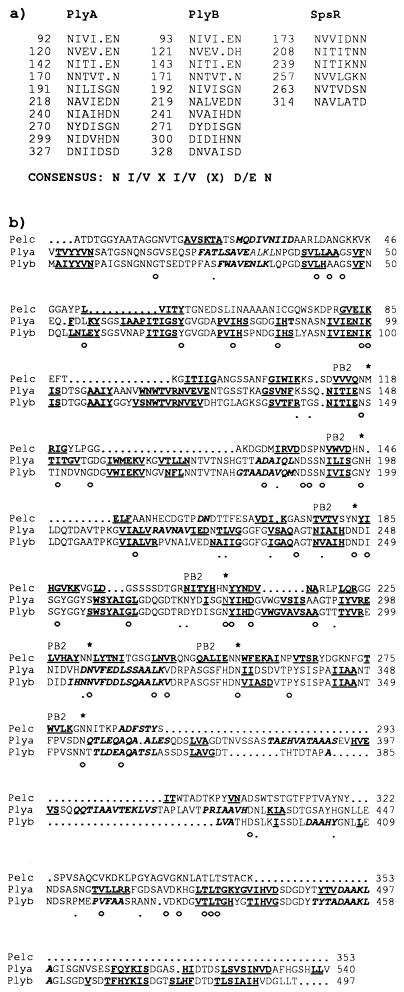
Another characteristic shared by most of the proteins secreted via type I secretion systems is the presence of glycine- and aspartate-rich nonapeptide RTX repeats (46), which form a Ca2+-binding β-roll structure (3, 4). NodO (30 kDa) contains 12 such RTX repeats (13). PlyA and PlyB do not contain an RTX domain, and there is no sequence similarity between NodO and PlyA or PlyB, which are secreted via the same (PrsDE) secretion system. However, we noted that PlyA and PlyB each contain 10 repeats of a novel motif with the approximate consensus N(I/V)X(I/V)X(D/E)N (Fig. 5a). Predictions of secondary structure for PlyA and PlyB suggest that they are composed predominantly of β-strand, particularly in the regions containing the proposed novel repeats (Fig. 5b). Six similar repeats are present in SpsR (Fig. 5a), which has about 20% identity to PlyA and PlyB.
FIG. 5.
(a) Repeat motifs found in PlyA, PlyB, and SpsR. The position in the sequence of the first amino acid in each repeat is indicated. A rough consensus sequence is shown. PlyA and PlyB each contain 10 copies of the motif; SpsR contains 6 copies. (b) Sequence alignment of PelC from E. chrysanthemi with PlyA and PlyB from R. leguminosarum bv. viciae, showing conservation of secondary structure. The mature form of PelC (lacking the N-terminal signal peptide) was used for the alignment. Residues that are conserved in all three proteins (○) and between PelC and either PlyA or PlyB (·) are indicated beneath the alignment. Structural elements are indicated in boldface type, with α-helices in italics and β-strands underlined. The secondary structure of PelC is known from the crystal structure (49). The β-strands that form the PB2 β-sheet in PelC are indicated. The residues which form stacks stabilizing the parallel β-helix of PelC are indicated by asterisks above the sequence. The stacks are composed mainly of Asn residues, which occur as the second residue after the end of the PB2 β-strands. The secondary structure of PlyA and PlyB was predicted by PHDsec (34) and aligns well with the known structure of PelC, suggesting that these proteins may have a similar fold.
The crystal structures of PelC and PelE from E. chrysanthemi (which are similar to PlyA and PlyB) have been solved (50, 51). Both form a parallel β-helix, with three β-sheets, PB1, PB2, and PB3, stabilized by specific stacking interactions between amino acid side chains in the core of the helix. The prediction of secondary structure for PlyA and PlyB was obtained with PHDsec (34) and was superimposed onto a sequence alignment of PlyA and PlyB with PelC. The predicted secondary structure aligned well with the known PelC structure (Fig. 5b). The repeat motifs tended to align with the β-strands forming the PB2 β-sheet. In particular, the Asn residues involved in the formation of a distinctive “asparagine ladder” between PB2 and PB3 in pectate lyases are conserved in PlyA and PlyB (Fig. 5b). These conserved asparagines often correspond to the last residue of the repeat motif described above. Another feature of the PelC structure is the presence of an N-terminal α-helix that caps the β-helix. PlyA and PlyB are predicted to form an α-helix in a similar position (around residue 30). Therefore, rather than forming a β-roll structure based on RTX repeats like other proteins previously shown to be secreted by type I systems, PlyA and PlyB may share a similar fold with the superfamily of extracellular pectate lyases.
In view of the apparent structural similarities between PlyA or PlyB and pectate lyases, we checked for hydrolysis of polygalacturonic acid, which has been seen previously in R. leguminosarum bv. trifolii (30). R. leguminosarum bv. viciae produced clearings on agar plates that contained polygalacturonic acid, but this activity was not dependent on plyA or plyB, since isogenic strains carrying mutations in either or both genes had similar clearings to the control strain, 8401 (data not shown). Therefore, although PlyA and PlyB are suggested to form a similar structure to the family of pectate lyases, they apparently do not have the same substrate specificity as this family.
DISCUSSION
Two genes, plyA and plyB, which are very similar to each other and encode products that are similar to polysaccharide lyases have been identified. PlyA and PlyB are secreted by the Prs protein secretion system encoded by prsDE and cleave EPS and CMC. The lack of secreted PlyB can account for the increase in the length of EPS chains observed in the prsD mutant.
Some of the proteins secreted via the prsDE system may remain cell associated. Cellulase and endoglycanase activities in R. leguminosarum bv. trifolii and A. caulinodans were found to be cell associated (17, 30). Our results also suggest that Egl is secreted via the PrsDE type I secretion system but may remain associated with the cell surface. Such an association could account for the lack of diffusion of CMC-degrading activity from colonies and could be somewhat different from the secretion of soluble proteins such as NodO, proteases, and alpha-hemolysin secreted via type I exporters. R. leguminosarum bv. trifolii, which had a cell-associated extracellular cellulase activity, had no cellulase activity in culture supernatants (30). Similarly, the Egl endoglycanase from A. caulinodans was also concluded to be a cell-associated extracellular enzyme (17). The diffusion of enzyme activity from colonies carrying the truncated form of Egl may indicate that this protein does not remain cell associated and might suggest a role for the N-terminal part of the protein in surface association. It is possible that the activities encoded by plyA and plyB are also predominantly cell associated, since the areas of clearing formed by R. leguminosarum bv. viciae on CMC and EPS agar are visible only directly underneath the colonies. If a freely diffusible activity were present, more diffuse clearing around the colonies would be expected. The small amount of 65-kDa protein in culture supernatants that is thought to correspond to PlyB may be due to the release of a relatively low proportion of the PlyB protein from the cell surface.
It was previously proposed that at least one of the proteins secreted via the Prs system was required for symbiotic nitrogen fixation. The evidence presented here demonstrates that PlyA and PlyB are not required for nitrogen fixation and that they do not have a strong effect on nodulation. Work with R. meliloti has demonstrated that a particular size range of EPS produced by the bacterium is required for activity in nodulation (2, 43). So far, two enzymes, encoded by exoK and exsH, that cleave the bacterial EPS have been identified in R. meliloti (5, 52). ExoK is thought to be secreted in a sec-dependent manner since it has a potential N-terminal transit peptide, and ExsH (which contains four RTX repeats) is thought to be a glycosyl hydrolase that is secreted via the R. meliloti PrsDE type I secretion system (52). These glycosyl hydrolases are not homologous to PlyA and PlyB.
The residual low level of degradation of CMC and EPS by the plyA plyB double mutant suggests that an additional polysaccharidase may be produced by R. leguminosarum bv. viciae. To demonstrate a requirement for these enzymes in the symbiosis, it may therefore be necessary to mutate additional genes. Although plyA mutants are apparently unaffected in the ability to degrade CMC and EPS, we have demonstrated, by placing plyA under the control of a constitutive promoter, that PlyA has glycanase activity. Our results suggest that under free-living conditions, plyA is not highly expressed, and they imply that plyA and plyB may be under different regulatory controls. An in situ analysis (with appropriate gene fusions) of plyA and plyB gene expression during legume nodulation would reveal if either gene is expressed during legume infection.
Four gene products have been identified so far that require prs genes for secretion from R. leguminosarum bv. viciae: NodO, PlyA, PlyB, and Egl (from A. caulinodans). Analysis of extracellular proteins indicates that other proteins are also secreted via the same system. SpsR from Sphingomonas may also be secreted via a type I secretion system. Like PlyA, it is encoded in a cluster of EPS-biosynthetic genes and is expected to be a secreted protein involved in EPS processing. SpsR has no N-terminal signal sequence (49) but does have a negatively charged residue followed by several hydrophobic residues at its extreme C terminus, a motif often present in proteins secreted via type I secretion systems (18). Two genes, atrB and atrD, encoding a type I secretion system are also present within the sps gene cluster (49), and these genes may be required for the secretion of SpsR.
PlyA, PlyB, and SpsR share a novel repeat motif. These repeats may be involved in the formation of a similar structure to the parallel β-helix formed by the pectate lyase superfamily, on the basis of conservation of significant structural features. The repeat sequences align with the PB2 β-strands of PelC (50), and the terminal asparagines in the repeats align with the residues in PelC that form the asparagine ladder stabilizing a tight turn between PB2 and PB3. The conservation of these asparagines is particularly significant in view of the otherwise low overall sequence similarity. These features suggest the existence of a family of bacterial proteins involved in polysaccharide processing, including PlyA, PlyB, and SpsR, that are secreted by type I secretion systems.
The Egl protein shows no similarity to PlyA, PlyB, or any of the RTX family of proteins. Therefore, Egl lacks the RTX repeats, and we found no evidence for the PlyA-type heptapeptide repeat. However, Egl does contain several copies of a large (150-amino-acid) repeat that is similar to Ca2+-binding domains of some proteins (17). Thus, Egl may be a member of a different class of polysaccharide degrading enzymes that are secreted via a type I system. It remains to be determined if the repeat domains in PlyA and PlyB or Egl play any role in their secretion. However, the observation that there are three classes of secreted proteins, each with a different type of internal-repeat structure, suggests that repeated domains may be important for the secretion or folding of these proteins in the extracellular environment.
ACKNOWLEDGMENTS
We thank M. Sutton and J. Peart for the gift of NodO-specific monoclonal antibodies, M. Holsters and D. Geelen for pRGC1 and pGU910-C1, P. Bovill and A. Wilkinson for help with DNA sequencing, A. Marry and M. McCann for help with analysis of sugar derivatives, E. Brown for help with screening mutants, O. Mayans and L. Lo Leggio for informed and helpful discussions about the parallel β-helix protein structure analysis, M. Dow for critical comments on the manuscript, and G. Sawyers and M. Wexler for help with 35S labelling of proteins.
This work was supported by the BBSRC. A.Z. is supported by CONICET Argentina.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Meyers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Battisti L, Lara J C, Leigh J A. Specific oligosaccharide form of the Rhizobium meliloti exopolysaccharide promotes nodule invasion in alfalfa. Proc Natl Acad Sci USA. 1992;89:5625–5629. doi: 10.1073/pnas.89.12.5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baumann U. Crystal structure of the 50kDa metallo protease from Serratia marcescens. J Mol Biol. 1994;242:244–251. doi: 10.1006/jmbi.1994.1576. [DOI] [PubMed] [Google Scholar]
- 4.Baumann U, Wu S, Flaherty K M, McKay D B. Three-dimensional structure of the alkaline protease of Pseudomonas aeruginosa: a two-domain protein with a calcium binding parallel beta roll motif. EMBO J. 1993;12:3357–3364. doi: 10.1002/j.1460-2075.1993.tb06009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Becker A, Kleickmann A, Arnold W, Pühler A. Analysis of the Rhizobium meliloti exoH/exoK/exoL fragment: ExoK shows homology to excreted endo-β-1,3-1,4-glucanases and ExoH resembles membrane proteins. Mol Gen Genet. 1993;238:145–154. doi: 10.1007/BF00279541. [DOI] [PubMed] [Google Scholar]
- 6.Beringer J E. R factor transfer in Rhizobium leguminosarum. J Gen Microbiol. 1974;84:188–198. doi: 10.1099/00221287-84-1-188. [DOI] [PubMed] [Google Scholar]
- 7.Bradley D J, Wood E A, Larkins A P, Galfrè G, Butcher G W, Brewin N J. Isolation of monoclonal antibodies reacting with peribacteroid membranes and other components of pea root nodules containing Rhizobium leguminosarum. Planta. 1988;173:149–160. doi: 10.1007/BF00403006. [DOI] [PubMed] [Google Scholar]
- 8.Buchanan-Wollaston A V. Generalized transduction in Rhizobium leguminosarum. J Gen Microbiol. 1979;112:135–142. [Google Scholar]
- 9.Downie J A, Ma Q-S, Knight C D, Hombrecher G, Johnston A W B. Cloning of the symbiotic region of Rhizobium leguminosarum: the nodulation genes are between the nitrogenase genes and the nifA-like gene. EMBO J. 1983;2:947–952. doi: 10.1002/j.1460-2075.1983.tb01526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dudman W F, Franzen L E, Darvill J E, McNeil M, Darvill A G, Albersheim P. The structure of the acidic polysaccharide secreted by Rhizobium phaseoli strain-127k36. Carbohydr Res. 1983;117:141–156. [Google Scholar]
- 11.Duong F, Lazdunski A, Cami B, Murgier M. Sequence of a cluster of genes controlling synthesis and secretion of alkaline protease in Pseudomonas aeruginosa: relationships to other secretory pathways. Gene. 1992;121:47–54. doi: 10.1016/0378-1119(92)90160-q. [DOI] [PubMed] [Google Scholar]
- 12.Duong F, Lazdunski A, Murgier M. Protein secretion by heterologous bacterial ABC-transporters: the C-terminus secretion signal of the secreted protein confers high recognition specificity. Mol Microbiol. 1996;21:459–470. doi: 10.1111/j.1365-2958.1996.tb02555.x. [DOI] [PubMed] [Google Scholar]
- 13.Economou A, Hamilton W D O, Johnston A W B, Downie J A. The Rhizobium nodulation gene nodO encodes a Ca2+-binding protein that is exported without N-terminal cleavage and is homologous to haemolysin and related proteins. EMBO J. 1990;9:349–354. doi: 10.1002/j.1460-2075.1990.tb08117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Economou A, Davies A E, Johnson A W B, Downie J A. The Rhizobium leguminosarum biovar viciae nodO gene can enable a nodE mutant of Rhizobium leguminosarum biovar trifolii to nodulate vetch. Microbiology. 1994;140:2341–2347. [Google Scholar]
- 15.Felmlee T, Pellett S, Welch R A. Nucleotide sequence of an Escherichia coli chromosomal hemolysin. J Bacteriol. 1985;163:94–105. doi: 10.1128/jb.163.1.94-105.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finnie C, Hartley N M, Findlay K C, Downie J A. The Rhizobium leguminosarum prsDE genes are required for secretion of several proteins, some of which influence nodulation, symbiotic nitrogen fixation and exopolysaccharide modification. Mol Microbiol. 1997;25:135–146. doi: 10.1046/j.1365-2958.1997.4471803.x. [DOI] [PubMed] [Google Scholar]
- 17.Geelen D, Van Montagu M, Holsters M. Cloning of an Azorhizobium caulinodans endoglucanase gene and analysis of its role in symbiosis. Appl Environ Microbiol. 1995;61:3304–3310. doi: 10.1128/aem.61.9.3304-3310.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghigo J M, Wandersman C. A carboxyl-terminal four amino motif is required for secretion of the metalloprotease PrtG through the Erwinia chrysanthemi protease secretion pathway. J Biol Chem. 1994;269:8979–8985. [PubMed] [Google Scholar]
- 19.Glucksmann M A, Reuber T L, Walker G C. Genes needed for the modification, polymerization, export, and processing of succinoglycan by Rhizobium meliloti: a model for succinoglycan biosynthesis. J Bacteriol. 1993;175:7045–7055. doi: 10.1128/jb.175.21.7045-7055.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gysler C, Harmsen J A M, Kester H C M, Visser J, Heim J. Isolation and structure of the pectin lyase D-encoding gene from Aspergillus niger. Gene. 1990;89:101–108. doi: 10.1016/0378-1119(90)90211-9. [DOI] [PubMed] [Google Scholar]
- 21.Kim J-B, Carpita N C. Changes in esterification of the uronic acid groups of cell wall polysaccharides during elongation of maize coleoptiles. Plant Physiol. 1992;98:646–653. doi: 10.1104/pp.98.2.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knight C D, Rossen L, Robertson J G, Wells B, Downie J A. Nodulation inhibition by Rhizobium leguminosarum multicopy nodABC genes and analysis of early stages of plant infection. J Bacteriol. 1986;166:552–558. doi: 10.1128/jb.166.2.552-558.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krishnan H B, Kuo C-L, Pueppke S G. Elaboration of flavonoid-induced proteins by the nitrogen-fixing soybean symbiont Rhizobium fredii is regulated by both NodD1 and NodD2, and is dependent on the cultivar specificity locus, nolXWBTUV. Microbiology. 1995;141:2245–2251. [Google Scholar]
- 24.Lamb J W, Hombrecher G, Johnston A W B. Plasmid-determined nodulation and nitrogen-fixation abilities in Rhizobium phaseoli. Mol Gen Genet. 1982;186:449–452. [Google Scholar]
- 25.Létoffé S, Wandersman C. Secretion of CyaA-PrtB and HlyA-PrtB fusion proteins in Escherichia coli: involvement of the glycine-rich repeat domain of Erwinia chrysanthemi protease B. J Bacteriol. 1992;174:4920–4927. doi: 10.1128/jb.174.15.4920-4927.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Létoffé S, Delepelaire P, Wandersman C. Protease secretion by Erwinia chrysanthemi: the specific secretion functions are analogous to those of Escherichia coli haemolysin. EMBO J. 1990;9:1375–1382. doi: 10.1002/j.1460-2075.1990.tb08252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Létoffé S, Ghigo J M, Wandersman C. Secretion of the Serratia marcescens HasA protein by an ABC transporter. J Bacteriol. 1994;176:5372–5377. doi: 10.1128/jb.176.17.5372-5377.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lever M. A new reaction for colorimetric determination of carbohydrates. Anal Biochem. 1972;47:273–279. doi: 10.1016/0003-2697(72)90301-6. [DOI] [PubMed] [Google Scholar]
- 29.Loewus F A. Improvement in the anthrone method for determination of carbohydrates. Anal Chem. 1952;24:219. [Google Scholar]
- 30.Mateos P F, Jimenez-Zurdo J I, Chen J, Squartini A S, Haack S K, Martinez-Molina E, Hubbell D H, Dazzo F B. Cell-associated pectinolytic and cellulolytic enzymes in Rhizobium leguminosarum biovar trifolii. Appl Environ Microbiol. 1992;58:1816–1822. doi: 10.1128/aem.58.6.1816-1822.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 32.Prentki P, Krisch H M. In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984;29:303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- 33.Quandt J, Hynes M F. Versatile suicide vectors which allow direct selection for gene replacement in Gram-negative bacteria. Gene. 1993;127:15–21. doi: 10.1016/0378-1119(93)90611-6. [DOI] [PubMed] [Google Scholar]
- 34.Rost B. Predicting one-dimensional protein structure by profile based neural networks. Methods Enzymol. 1996;266:525–539. doi: 10.1016/s0076-6879(96)66033-9. [DOI] [PubMed] [Google Scholar]
- 35.Ruvkun G B, Ausubel F M. A general method for site-directed mutagenesis in prokaryotes. Nature. 1981;289:85–88. doi: 10.1038/289085a0. [DOI] [PubMed] [Google Scholar]
- 36.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 37.Scheu A K, Economou A, Hong G-F, Ghelani S, Johnston A W B, Downie J A. Secretion of the Rhizobium leguminosarum nodulation protein NodO by haemolysin-type systems. Mol Microbiol. 1992;6:231–238. doi: 10.1111/j.1365-2958.1992.tb02004.x. [DOI] [PubMed] [Google Scholar]
- 38.Sherwood M T. Improved synthetic medium for the growth of Rhizobium. J Appl Bacteriol. 1970;33:708–713. doi: 10.1111/j.1365-2672.1970.tb02253.x. [DOI] [PubMed] [Google Scholar]
- 39.Sutton J M, Peart J, Dean G, Downie J A. Analysis of the C-terminal secretion signal of the Rhizobium leguminosarum nodulation protein NodO: a potential system for the secretion of heterologous proteins. Mol Plant-Microbe Interact. 1996;9:671–680. doi: 10.1094/mpmi-9-0671. [DOI] [PubMed] [Google Scholar]
- 40.Tabor S. Expression using the T7 RNA polymerase/promoter system. In: Ausubel F A, Brent R, Kingston R E, Moore D D, Seidman J A, Smith J A, Struhl K, editors. : Current protocols in molecular biology. New York, N.Y: Greene Publishing and Wiley Interscience; 1990. pp. 16.2.1–16.2.11. [Google Scholar]
- 41.Tamaki S J, Gold S, Robeson M, Manulis S, Keen N T. Structure and organization of the pel genes from Erwinia chrysanthemi EC16. J Bacteriol. 1988;170:3468–3478. doi: 10.1128/jb.170.8.3468-3478.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Teather R M, Wood P J. Use of Congo red-polysaccharide interactions in enumeration and characterization of cellulolytic bacteria from the bovine rumen. Appl Environ Microbiol. 1982;43:777–780. doi: 10.1128/aem.43.4.777-780.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Urzainqui A, Walker G C. Exogenous suppression of the symbiotic deficiencies of Rhizobium meliloti exo mutants. J Bacteriol. 1992;174:3403–3406. doi: 10.1128/jb.174.10.3403-3406.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Rhijn P, Luyten E, Vlassak K, Vanderleyden J. Isolation and characterization of a pSym locus of Rhizobium sp. BR816 that extends nodulation ability of narrow host range Phaseolus vulgaris symbionts to Leucaena leucocephala. Mol Plant-Microbe Interact. 1996;9:74–77. doi: 10.1094/mpmi-9-0074. [DOI] [PubMed] [Google Scholar]
- 45.van Workum W A T, Canter-Cremers H C J, Wijfjes A H M, van der Kolk C, Wijffelman C A, Kijne J W. Cloning and characterisation of four genes of Rhizobium leguminosarum bv. trifolii involved in exopolysaccharide production and nodulation. Mol Plant-Microbe Interact. 1997;10:290–301. doi: 10.1094/MPMI.1997.10.2.290. [DOI] [PubMed] [Google Scholar]
- 46.Wandersman C. Secretion across the bacterial membrane. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Rezikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C: ASM Press; 1996. pp. 955–966. [Google Scholar]
- 47.Wassif C, Cheek D, Belas R. Molecular analysis of a metalloprotease from Proteus mirabilis. J Bacteriol. 1995;177:5790–5798. doi: 10.1128/jb.177.20.5790-5798.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wolff N, Ghigo J M, Delepelaire P, Wandersman C, Delepelaire M. C-terminal secretion signal of Erwinia chrysanthemi protease secreted by a signal peptide-independent pathway: proton NMR and circular dicroism conformational studies in membrane-mimetic environments. Biochemistry. 1994;33:6792–6801. doi: 10.1021/bi00188a007. [DOI] [PubMed] [Google Scholar]
- 49.Yamazaki M, Thorne L, Mikolajczak M, Armentrout R W, Pollock T J. Linkage of genes essential for synthesis of a polysaccharide capsule in Sphingomonas strain S88. J Bacteriol. 1996;178:2676–2687. doi: 10.1128/jb.178.9.2676-2687.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yoder M D, Keen N T, Jurnak F. New domain motif: the structure of pectate lyase C, a secreted plant virulence factor. Science. 1993;260:1503–1507. doi: 10.1126/science.8502994. [DOI] [PubMed] [Google Scholar]
- 51.Yoder M D, Lietzke S E, Jurnak F. Unusual structural features in the parallel β-helix in pectate lyases. Structure. 1993;1:241–251. doi: 10.1016/0969-2126(93)90013-7. [DOI] [PubMed] [Google Scholar]
- 52.York G M, Walker G C. The Rhizobium meliloti exoK gene and prsD/prsE/exsH genes are components of independent degradative pathways which contribute to production of low molecular weight succinoglycan. Mol Microbiol. 1997;25:117–134. doi: 10.1046/j.1365-2958.1997.4481804.x. [DOI] [PubMed] [Google Scholar]
- 53.Zhang F, Yin Y, Arrowsmith C H, Ling V. Secretion and circular dicroism analysis of the C-terminal signal peptides of HlyA and LktA. Biochemistry. 1995;34:4193–4201. doi: 10.1021/bi00013a007. [DOI] [PubMed] [Google Scholar]