Abstract
This comprehensive scientific review provides an in-depth analysis of both the natural compounds, pyrethrins, and their synthetic derivatives, pyrethroids, focusing on their classification, biosynthesis, mechanism of action, general and pharmaceutical uses, as well as their toxicity and environmental impact. Pyrethrins, derived from certain plant species, have long been recognized for their potent insecticidal properties. The review begins by examining the classification of pyrethrins and pyrethroids, elucidating their structural characteristics and unique features within the field of natural and synthetic compounds. The biosynthetic pathways responsible for producing pyrethrins in plants are discussed, highlighting the enzymatic reactions and genetic regulation involved. In addition, the synthesis of pyrethroid derivatives is explored, including both natural and synthetic sources and potential optimization strategies. Understanding the mechanisms of action by which pyrethrins and pyrethroids exert their insecticidal effects is a crucial aspect of this review. Complex interactions with the nervous systems of target organisms are examined, providing insights into their selective toxicity and modes of action. In addition, the various applications of these compounds are explored, from their use in agriculture for pest control to their incorporation into household insecticides and potential pharmaceutical applications. The review also critically evaluates the potential toxicity of pyrethrins and pyrethroids to human health. By consolidating current knowledge and research findings, this review provides a comprehensive understanding of the properties and applications of pyrethrins and pyrethroids, highlighting their benefits and risks, and the importance of responsible and sustainable use in various areas.
Keywords: pyrethrins, pyrethroids, chemical structure, biosynthesis, biological properties, toxicity
1. Introduction
Botanical derivatives have served as fundamental pesticides for centuries and are renowned for their ecological properties [1,2]. Throughout history, various compounds such as sulfur, arsenic, and nicotine sulfate have been used to control pest infestations. After the Second World War, organochlorines and organophosphates emerged, marking a milestone in the development of pesticides. However, due to growing concerns about their environmental impact, alternative approaches such as integrated pest management (IPM) gained ground [3].
The complete ban on persistent organic pollutants, including DDT (dichlorodiphenyltrichloroethane), highlighted the need to re-evaluate pesticide use [4]. Subsequently, the advent of pyrethroids in the 1970s offered a promising alternative, offering efficacy and reduced environmental damage compared to their predecessors [5,6]. Despite their indisputable benefits in limiting agricultural losses and ensuring food security, the widespread use of pyrethroids and other pesticides has raised concerns about their negative effects on human health and the environment [7,8].
In recent years, new aspects of pyrethrins and pyrethroids have been revealed through research projects. For example, the field evaluation of novel spatial repellent controlled release devices against mosquitoes was studied in an outdoor setting in the northern Peruvian Amazonia [9]. Besides, a potential adjunctive agent for treating scabies was discovered: in vitro killing activity of permethrin and tea tree oil on Sarcoptes scabiei collected from patients. Parasites that were not damaged during sampling, and showed full motion were included [10]. Moreover, it was demonstrated for the first time the effect of pyrethroid exposure on the internal microbiota in Aedes albopictus [11].
In the environmental impact area, adverse effects of permethrin and tetramethrin in vitro models of freshwater mussels exposed to 1 mg/L, 10 μg/L, 100 ng/L, and 1 ng/L concentrations of chemicals for 24 h were also determined [12]. For this purpose, reduced glutathione activities were evaluated as biomarkers of the primary gill and digestive gland cell cultures [12]. To elucidate the interactive actions of pesticides on crop pollinators it was determined the individual and joint toxicities of thiamethoxam and other seven pesticides (dimethoate, methomyl, zeta-cypermethrin, cyfluthrin, permethrin, esfenvalerate, and tetraconazole) to honeybees (Apis mellifera) with feeding toxicity test [13]. In another study, the phenotypic and genotypic insecticide-resistance profiles among wild Anopheles were collected over 3 years to assess the longitudinal effects of dual-active-ingredient LLINs (long-lasting insecticidal nets) on insecticide resistance [14].
The application of pyrethroids and carbamates represents an environmental risk and may exert adverse effects on beneficial microorganisms such as Trichoderma, which contribute to the biocontrol of several fungal phytopathogens [15]. It was determined the influence of this insecticide on the release of enzymes such as chitinases, peroxidases, and endoglucanases by a consortium of selected Trichoderma strains grown in liquid culture medium) [15].
This review provides a comprehensive analysis of the origins, development, and implications of pyrethrins and pyrethroids. By examining the historical context and contemporary challenges associated with their use, we aim to provide a comprehensive understanding of the role and impact of these compounds in modern agriculture and public health. In addition, the review sheds light on the intricate mechanisms and comparative toxicological profiles of pyrethrins and pyrethroids in both mammals and insects, highlighting their variable sensitivity and implications for human and environmental health.
2. Natural Occurring Compounds—Pyrethrins
Plant materials containing pyrethrins have been traded in Europe since the middle of the 18th century. The species Chrysanthemum cinerariaefolium first appeared in the Dalmatia region of the Balkans and replaced a species originating from Persia, which proves that the true origin for the use of this plant is the Middle East, and dates to the 17th century. The first studies that set the foundation for the discovery of pyrethrins were elaborated in the 1920s, with the discovery of pyrethrins I and II [16]. These breakthroughs acted as a catalyst for the creation of a new market to sell the herb. The First World War also greatly influenced the countries that had a monopoly on the market, with Kenya becoming the leading exporter of plant products due to its higher concentration of active ingredients. Due to the pressures of World War II and other factors such as insect-borne diseases, these valuable pyrethrins were formulated as aerosols for their use against mosquitoes to decrease the transmission rate of malaria or yellow fever [16].
Another use of this class of plant compounds has been in agriculture as insecticides that are not highly toxic to mammals and are biodegradable. This led to their use on an industrial scale and the need to manufacture large quantities. In the production of pyrethrin-based insecticides, the starting point is generally the plant material, namely the mature inflorescences, which are dried and ground to a fine powder [2]. The powder can be used as such but can also be subjected to extraction with organic solvents.
Subsequently, different synthetic methods were developed for the 6 natural compounds, and new compounds structurally like the natural pyrethrins were created, thus generating a new class of compounds known as pyrethroids. These pyrethroids are superior to the natural compound due to their photostability, pyrethrins have a reduced photostability and are more biodegradable [17].
The term pyrethrum refers to the powdered and dried plant product extracted with organic solvents. In general, the term is used for the name of insecticide, either plant product or extract, which contains all the pyrethrins of this class of compounds. Several species are documented of which, after processing, the final product is called pyrethrum. The most famous species is C. cinerariaefolium (Dalmatian pyrethrum) due to its highest concentration of pyrethrins among all known species that produce this class of compounds, followed by C. coccineum (Persian pyrethrum) whose concentration in active principles is not as renowned [18], but their presence has been documented. Another species of the genus containing this class of compounds is C. balsamita also known as costmary [19]. Two other species from different genera that have been documented to contain pyrethrins are Anacyclus pyrethrum [20] and Tagetes erecta [21], all of which are members of the Asteraceae family.
2.1. Chrysanthemum cinerariaefolium
Chrysanthemum cinerariaefolium alias Tanacetum cinerariaefolium (Dalmatian pyrethrum) is the most industrially cultivated plant for pyrethrum production. The active ingredients are present in all organs of the plant but are concentrated in the inflorescences where, on the surface of the ovaries, there are secretory glandular hairs responsible for the abundant presence of pyrethrins in the plant tissues. Ref. [22] observed that the concentration of pyrethrins is higher in the epidermis of young leaves, and as the leaves mature the highest concentration of pyrethrins is found in the mesophyll of the leaf. This perennial species, since it is the most widely cultivated, has become the subject of most research papers based on the study of pyrethrins. From a morpho-anatomical point of view, the plant presents a capitulum-type inflorescence with central (tubular) yellow flowers and white radiating (ligulate) flowers, slender erect stems, and its leaves are alternate and pinnately lobed.
The existence of five new chemotypes within the species C. cinerariaefolium is documented from a sample of 25 populations in Croatia and distinguishes that the chemotype labeled “A” has the highest concentration of pyrethrin I. The percentage varies between these chemotypes from 0.36% of dry flower weight to 1.30% of dry flower weight. This finding opens the possibility of using this chemotype in the development of a new variety with higher concentrations of active compounds [23].
C. cinerariaefolium plantations usually are established in regions where the well-drained soil is rich in phosphorus, magnesium, and calcium, the average pH of the soil needs to be around 5.6, and, from a climatic point of view, a higher altitude where the precipitation needs to be minimum of 750 mm of rainfall distributed equally across the four seasons [24]. Large plantations and exports of C. cinerariaefolium are not present in Europe whereas one of the largest exporters of this insecticide is in Africa, namely Kenya [25], and also in Australia on the Tasmanian Island [26]. A newer agricultural industry is being developed in the highlands of Papua New Guinea, where the redeveloping of this plant culture offers a helping hand to improve the livelihoods of the local people [27].
2.2. Chemical Structures and Classification
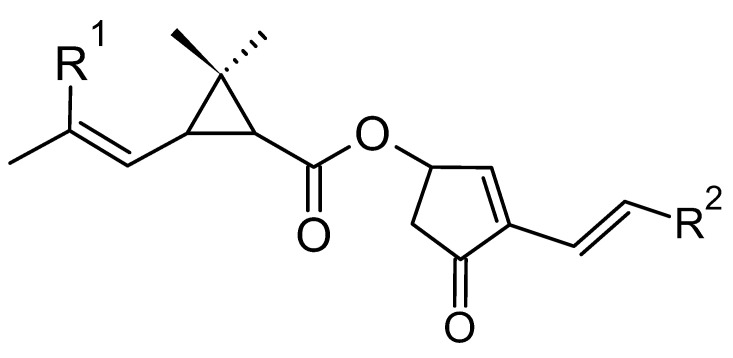
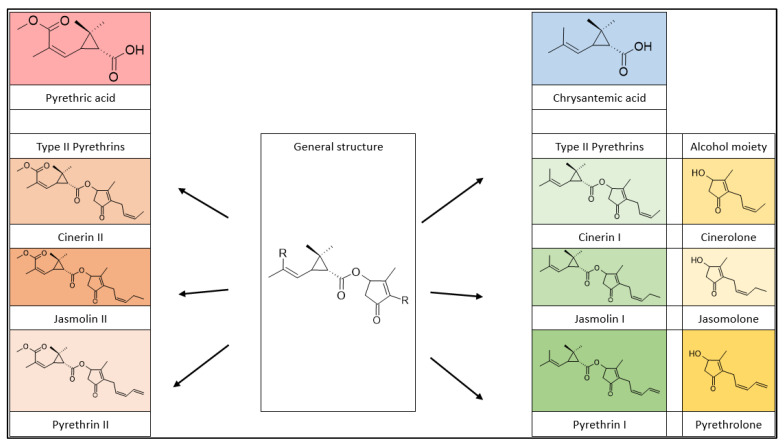
Natural pyrethrins are the six ester compounds that result from the condensation of one alcohol and acid, the general structure is shown below (Figure 1). The acid half may be pyrethric acid or chrysanthemic acid, and the alcohol half may be one of the unsaturated hydroxy-cycloketones (rethrolones): cinerolone, jasmolone, and pyrethrolone. If the compound has pyrethric acid in its structure, then the three acid-alcohol combinations belong to category I of pyrethrins, and if chrysanthemic acid is replaced by pyrethric acid then the three remaining compounds belong to category II of pyrethrins (Figure 2).
Figure 1.
The general structure of pyrethrins.
Figure 2.
Pyrethrin classification.
2.3. Biosynthesis
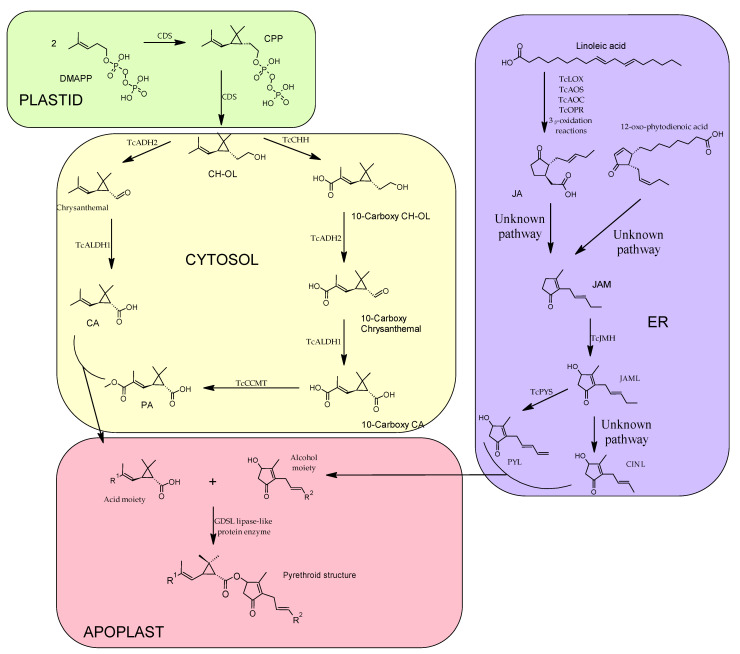
Pyrethrins are the final product of two major pathways merging, as shown below (Figure 3), one pathway is responsible for the synthesis of the acid moiety (crysanthemic acid—CA and pyrethric acid—PA) and another pathway is responsible for the synthesis of the alcohol moiety or rethrolones (jasmolone, cinerolone and pyrethrolone). It has been revealed that most of the reactions involved in the biosynthesis of pyrethrins, in seedlings of C. cinerariaefolium take place in plastids [28]; however, it is also stated that part of these reactions (the alcohol moieties biosynthesis) also take place in the ER (endoplasmic reticulum) and in the peroxisome [29].
Figure 3.
General biosynthetic pathway of pyrethrins depicting the cellular domains where the different reactions of the biosynthesis take place. DMAPP—dimethylallyl diphosphate; CDS—chrysanthemyl diphosphate synthase; CPP—chrysanthemyl diphosphate; CH-OL—chrysanthemol; TcADH2—C. cinerariaefolium alcohol dehydrogenase 2; TcALDH1—C. cinerariaefolium aldehyde dehydrogenase 1; CA—crysanthemic acid; PA—pyrethric acid; TcCHH—chrysanthemol 10-hydroxylase; TcCCMT—10-carboxychrysanthemic acid 10-methyltransferase; TcLOX—T. cinerariaefolium lipoxygenase; TcAOS—allene oxidase synthase; TcAOC—allene oxide cyclase; TcOPR—cis (1)-12-oxo-phytodienenic acid reductase; JA—jasmonic acid; JAM—jasmone; TcJMH—jasmone hydroxylase; JAML—jasmolone; TcPYS—Pyrethrolone synthase; PYL—pyrethrolone; CINL—cinerolone.
In the synthesis of the acid moiety, the starting compound is dimethylallyl diphosphate (DMAPP), which is the universal terpene precursor. Two molecules of the DMAPP are condensed resulting in chrysanthemyl diphosphate (CPP) and after eliminating the phosphate group by hydrolysis the final product of this step is chrysanthemol (CH-OL). This two-step reaction is catalyzed by the enzyme chrysanthemyl diphosphate synthase (CDS) also known as chrysanthemol synthase (CHS) [30], CPP is, because it is an irregular terpene due to the head-to-middle dimerization of DMAPP. CH-OL is converted into CA by two-step oxidation, firstly the alcohol is transformed into an aldehyde (chrysanthemal), catalyzed by C. cinerariaefolium alcohol dehydrogenase 2 (TcADH2), followed by an oxidation to CA, a reaction catalyzed by and aldehyde dehydrogenase 1 (TcALDH1) [31].
An alternative pathway that CH-OL can take is the production of the second acid moiety: PA. The previous two enzymes, TcADH2 and TcALDH1, which are responsible for the oxidation of the C1 hydroxyl group in CH-OL, “team up” with two other enzymes: chrysanthemol 10-hydroxylase (TcCHH), which is a cytochrome P450 oxidoreductase responsible for adding a carboxylic group to C10 after three oxidative processes; and 10-carboxychrysanthemic acid 10-methyltransferase (TcCCMT), a SABATH-class methyltransferase that adds a methyl group to the newly formed C10 carboxylic group [31].
The starting molecule from which all three alcohol moieties are synthesized is linoleic acid, which is converted to jasmonic acid (JA) by a series of consecutive reactions catalyzed by the following enzymes: T. cinerariaefolium lipoxygenase (TcLOX), allene oxidase synthase (TcAOS), allene oxide cyclase (TcAOC), cis (1)-12-oxo-phytodienenic acid reductase (TcOPR), succeeded by three β-oxidation reactions. The next step of converting JA into jasmone (JAM), which needs to pass more chemical reactions to become the alcohol moiety, has yet to be elucidated, although the recent conclusion of [32], states the fact that JA is not the only intermediate that produces the alcohol moiety, instead the precursor might also be 12-oxo-phytodienoic acid. The JAM is further hydroxylated by jasmone hydroxylase (TcJMH) into one of the final rethrolones, jasmolone (JAML) [33]. It has been shown that from JAML, the other two rethrolones can be converted, for example, the reaction of converting JAML into pyrethrolone (PYL) is catalyzed by C. cinerariaefolium cytochrome P450 family member, CYP82Q3 named Pyrethrolone synthase (TcPYS) [29]. Little to nothing is known about the third and last alcohol moiety, cinerolone (CINL), studies are yet to be conducted.
Finally, the end of biosynthesis of pyrethrins is the condensation of the two moieties, a reaction catalyzed by GDSL lipase-like protein enzyme, that takes place in apoplast [34], resulting in a new ester bond between the alcohol and the acid.
The major site of pyrethrin biosynthesis is the glandular trichomes located mainly on the surface of disc floret ovaries of C. cinerariaefolium, although these glandular trichomes, rarely may be present on other aerial parts of the plant. It is considered that these pyrethrin-producing species do not metabolize these kinds of compounds for their insecticidal proprieties nevertheless for self-protecting from herbivores [35]. Different research studies have exhibited that in field conditions, mechanical trauma put on plant individuals did not influence the total concentration of pyrethrins [36], whereas research studies done under specialized parameters (plants grown in growth chambers) have documented increased levels of pyrethrins in vegetative tissue of C. cinerariaefolium, hinting the fact that pyrethrin production may be also mediated by volatile organic compound (VOC) emitted by the plant from the wound location [37]. It is indicated that there is a difference between the levels of pyrethrin I and II in plant tissue from individuals who have been mechanically stressed compared to other individuals who have also been mechanically stressed but have been protected from VOCs secreted by the wound. This finding implies that the biosynthetic pathway of some pyrethrins may be triggered by external factors, such as VOCs, whereas some may be regulated by an internal systemic response [38].
2.4. Mechanism of Action
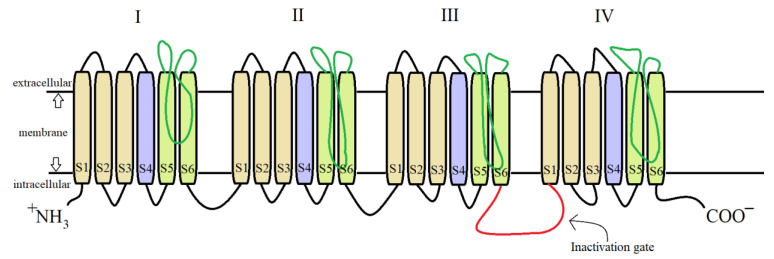
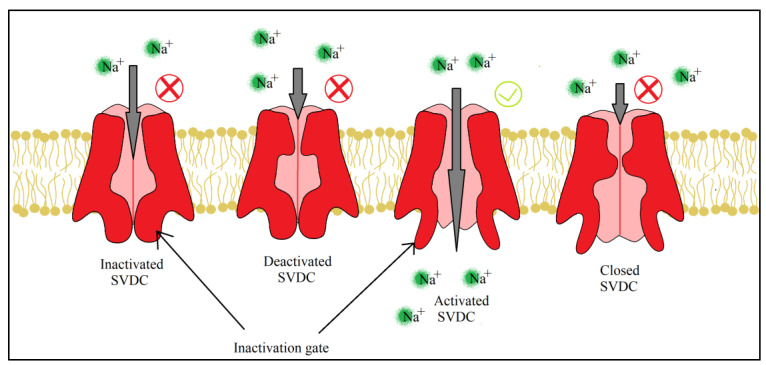
For both pyrethrins and pyrethroids the mechanism of action follows the same pattern, yet because of the biochemical engineering that led to the creation of pyrethroids, the synthetic class has a longer effect and a bigger potency. It has been determined, by molecular genetic studies done on houseflies centered on the resistance of pyrethroids [39], that the central site of insecticidal action revolves around the sodium voltage-dependent channels (SVDC). SVDC consists of a long transmembrane amino acid chain (Figure 4), with the amino and carboxy termini situated intracellular, forming two major subunits: α subunit (which is the main center of action) and aβ auxiliary subunit (responsible for the modulation of sodium channel gating and/or protein expression) [40]. The α subunit is composed of four main domains (I–IV), each domain has six transmembrane segments (S1–S2). The S4 contains positively charged amino acids and it acts as the voltage sensor when the membrane is depolarized receiving a confirmation charge that opens the channel. The channel is represented by the S5, S6, and the linkage segment between them, this segment also confers specificity to Na+ ions. There is an amino acid linkage chain between the domains III and IV which represents the inactivation gate. The channel can exist in four states due to the inactivation gate and one more site named the “activation gate”: the first state when the membrane potential is resting, the channel is closed but the inactivation gate is opened (closed state) when the depolarization is detected, the channel fully opens and the Na+ ions can freely pass thru (activated state). The inactivation state is initiated by the inactivation gate closing, clogging up the channel, after this step the channel finial closes and enters its deactivated state.
Figure 4.
Detailed structure of the α subunit of the SVDC, the transmembrane amino acid chain with its four domains (I–IV), each of them having six transmembrane segments (S1–S6).
SVDC and potassium voltage-dependent channels (PVDC) are responsible for the movement of Na+ and K+ across the membrane which create action potentials, electrical impulses that travel along the neurons [41]. When a neurotransmitter binds to its receptor, in the synapse, a depolarization of the neuron membrane takes place and S4 segments of the SVDC detect the difference in charge, activating the channel and letting Na+ enter the neuron which leads to a further depolarizing of the membrane. This increasing concentration of Na+ oversees the rising phase of the action potential, leading to the membrane polarity switch. The following phase, the falling of the action potential, is produced by the inactivation and closing of the SVDC and the activation and opening of the PVDC, letting the K+ leave the neuron which leads to the hyperpolarization of the membrane. Finally, the PVDC closes and the membrane potential is back to its resting phase. All four stages of the ion channel can be viewed below (Figure 5).
Figure 5.
Pyrethroids and their effects on ion channels, the four states of SVDC, are dependent on the inactivation and activation gate. Readaptation of the representation from [41].
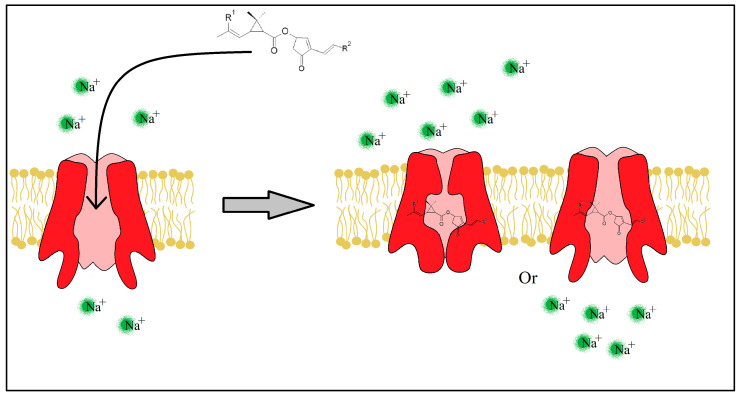
The pyrethrins and pyrethroids lessen the peak of Na+ ions (the rising phase) by slowing the SVDC activation state [42,43], but also prolong the SVDC opening time by slowing the activated and inactivated state of the channel (Figure 6). Immediately after the fast absorption of the insecticide molecule through the insect’s cuticle, the neurotransmission fails causing a paralyzing state and possibly death in seconds or minutes.
Figure 6.
Insecticide molecule mechanism of action, by slowing the Inactivated or Activated state of the SVDC.
The molecular structure and specific geometry enable pyrethrins and pyrethroids to bond to specific sites of the SVDC, mainly the S4–S5 linking segment of the II domain but other binding sites may be included too such as S6 of the III domain and S5 of the II domain, depending on the molecular structure of the insecticide. Regarding the specific geometry of the molecule, these insecticides must have a distinct stereospecificity, for example, all-natural pyrethrins have a 1R, trans configuration which grants the insecticidal action [44]. Moreover, the addition of an α-cyano substituent on the 3-phenoxybenzyl moiety (in different types of pyrethroids furthermore explained in the following chapters) greatly increases the insecticide action. Due to the diversity of the chemical structure of pyrethrins and pyrethroids, it is noted that a special type of chemical substituent or a specific reactive site cannot precisely justify insecticidal activity. For example, between Pyrethrin I and Etofenprox, there are little to nonchemical similarities; however, both molecules exude insecticidal activity. Yet, more details have been outlined about each segment of the pyrethrin molecule and comparisons of the different structures found in synthetic compounds [45,46].
Different mutations on the SVDC-expressing gene are responsible for substituting amino acids from distinct parts of the chain, this replacement leads to fewer binding sites for the insecticidal molecule causing insecticide resistance [47,48].
2.5. General and Pharmaceutical Uses
Since the newly developed pyrethroids have been on the market, the usage of pyrethrins in different forms for repelling insects has greatly decreased. Although the insecticidal power of pyrethrins and pyrethroids is recognized, they are generally associated with different insecticides such as seasmin, sulfoxide, and dicarboximide, for the synergetic potentiation of the effect [49]. Their primary use as a household insecticide is still noted today, different types of sprays, aerosols, solutions, and incense are used to prevent unwanted insects in the household.
A multitude of veterinary products containing this class of compounds are used for their properties to protect pets against insect pests, amongst these formulations can be found collars, powders, shampoos, baths, aerosols, sprays, etc. Among all these formulations for veterinary use, two categories of products can be distinguished, namely products using an organic solvent (alcohol or petroleum), which are more prone to toxic and/or adverse reactions, and products using a water-based solvent, whose bioavailability is relatively low.
The use of these compounds for human purposes has also been documented, namely permethrin for the treatment of various diseases caused by human ectoparasites, such as body lice, head lice, crab lice, and scabies [50]. Pyrethrins and pyrethroids have also been a great weapon against malaria, as mentioned before since the Second World War, these compounds have been used to repel mosquitoes carrying malaria. Nowadays impregnated be nets with different types of insecticides [51,52] are used in parts of the world where malaria Is common. These types of bed nets have been the focus of many debatesIse the long exposIre to insecticides may create different health issues due to the bioaccumulation in the human body [53,54]. Furthermore, it has been thoroughly investigated the resistance of mosquitoes to pyrethrin and pyrethroid-type insecticide [44,51,55], because of the rapid development of this type of phenomenon, especially on the African continent. In addition, it was pointed out the fact that a mixture of natural pyrethrins may be promising for malaria vector control, hinting at the fact that, in the long run maybe a constant juggling between different types of natural and synthetic compounds may be the key to tackle insecticide resistance to this class of compounds [56].
3. Pyrethrin Derivatives—Pyrethroids
3.1. General Considerations about Pyrethroids
Pyrethrin derivatives, known as pyrethroids, have a significant historical trajectory dating back to the mid-20th century, characterized by a series of crucial advancements in the realm of synthetic insecticide development. The milestone achievements include the synthesis of allethrin and bioallethrin in 1949, setting the stage for the subsequent introduction of the first-generation synthetic pyrethroid, resmethrin, in 1962, achieved through structural modifications of natural pyrethrins to enhance stability and elevate insecticidal efficacy [57,58].
The progression in this field expanded with the development of bioresmethrin in 1967, marking the commercial application of pyrethroids in the late 1960s, coinciding with the emergence of potent insecticides like cypermethrin and deltamethrin and solidifying the role of pyrethroids in the domain of pest management [59,60]. By 1983, the widespread utilization of pyrethroids encompassed over 33 million hectares annually, representing a significant share of the global insecticide market at 25.1% [3].
The recognition by the World Health Organization (WHO) of pyrethroids, particularly deltamethrin, and permethrin, for various applications, including the control of disease-transmitting mosquitoes through long-lasting insecticidal nets (LLINs), underscored their environmental advantages and reduced toxicity to humans and mammals [61]. This pivotal acknowledgment emphasized the increasing significance of pyrethroids in the context of global pest management strategies.
Categorized into type I and type II pyrethroids based on distinct chemical attributes influencing their insecticidal properties, these compounds have found extensive use across various sectors. Additionally, the incorporation of piperonyl butoxide as a synergist in commercial pyrethroid formulations has significantly contributed to their sustained efficacy in pest control [62,63].
Despite their pivotal role in pest control, concerns have surfaced regarding the impact of pyrethroids on non-target species and potential health implications for humans. Research efforts have focused on understanding specific toxicity biomarkers associated with pyrethroid exposure, shedding light on their adverse effects on various ecosystems [64,65,66,67,68].
Moreover, the escalating contamination of soil and water bodies with pyrethroids has spurred the exploration of eco-friendly remediation strategies to mitigate their environmental consequences [69,70]. Despite challenges and concerns, the versatility and effectiveness of pyrethroids have firmly established their position as integral components in the global insecticide market, finding applications in diverse sectors ranging from agriculture and forestry to household pest management [71,72]. This chapter aims to comprehensively examine the historical evolution, structural variations, applications, environmental implications, and health considerations associated with the use of pyrethrin derivatives, providing a holistic understanding of their significance and impact on contemporary pest management strategies.
3.2. Classification of Pyrethroids
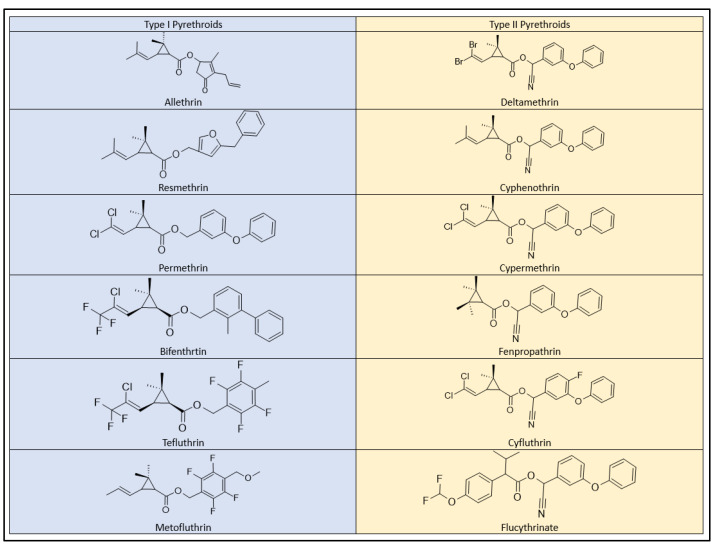
Pyrethroid pesticides exhibit a clear classification into two distinctive categories, known as Type I and Type II, based on their behavioral toxicity and the presence or absence of an α-cyano group within their molecular structures [73]. This division is also reflected in the acute toxicity assessments, where most pyrethroids are grouped into classes I and II (Figure 7), indicating their respective levels of toxicity in rodent models.
Figure 7.
Type I and Type II pyrethroids structures.
Type I pyrethroids, which include allethrin, permethrin, resmethrin, bifenthrin, d-phenothrin, and tetramethrin, do not contain the α-cyano group in their chemical structure. As a result, they exhibit comparatively lower toxicity. In contrast, Type II pyrethroids, such as cypermethrin, deltamethrin, cyhalothrin (lambda), cyfluthrin, and fenvalerate (esfenvalerate), incorporate the α-cyano group, making them notably more toxic [58]. Type II pyrethroids have been associated with salivation, the choreoathetosis-salivation syndrome (CS), and motor dysfunction in mammals [71,72,73,74,75]. Additionally, various other effects, including oxidative stress, impacts on male fertility, and prenatal development, have been documented [66,75,76].
Human biomonitoring (HBM) studies typically monitor pyrethroid exposure through the detection of five specific metabolites in urine: 3-phenoxybenzoic acid (3-PBA), cis- and trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane-1-carboxylic acid (cis-DCCA and trans-DCCA), 4-fluoro-3-phenoxybenzoic acid (F-PBA), and 3-(2,2-dibromovinyl)-2,2-dimethylcyclopropane carboxylic acid (DBCA). Among these, DBCA is specific to deltamethrin, and F-PBA is linked to cyfluthrin but can originate from both isomers. The metabolites are cis- and trans-DCCA are produced from the cis and trans isomers of permethrin, cypermethrin, and cyfluthrin, while 3-PBA is a common metabolite observed with various pyrethroids, including permethrin, cypermethrin, and deltamethrin [77].
However, the intrinsic susceptibility of these pyrethroids to sunlight-induced degradation prompted the development of next-generation compounds to address this issue. These newer compounds are categorized based on their photostability into two generations: first-generation and second-generation synthetic pyrethroids [72].
First-generation synthetic pyrethroids, including resmethrin, tetramethrin, and phenothrin, are derivatives of chrysanthemic acid esters, with susceptibility to photolysis, making them less stable [78]. In contrast, second-generation synthetic pyrethroids, such as permethrin, cypermethrin, and deltamethrin, exhibit both high potency and enhanced photostability, achieved through systemic modifications to their chemical structure in both the acid and alcohol moieties [78].
These pyrethroids share several common physical properties, such as low vapor pressure, low Henry’s law constants, higher octanol-water ratio coefficients (Kow), limited water solubility, enantiomers with identical physical properties, and diastereomers with differing physical attributes [78].
Synthetic pyrethroids have replaced natural pyrethrums due to their improved effectiveness and stability against insects, making them valuable tools for pest control. Various commercial synthetic pyrethroids are widely available, including bifenthrin, permethrin, deltamethrin, resmethrin, sumithrin, fenpropathrin, cyhalothrin, esfenvalerate, beta-cypermethrin, lambda-cyhalothrin, D-phenothrin, D-cyphenothrin, tetramethrin, and allethrin [79].
3.3. Synthesis Strategies of Pyrethroids: Approaches and Mechanistic Considerations
The synthesis of pesticides encompasses various strategies, each tailored to facilitate the creation of novel compounds with specific biological activities. Among the prominent methods, biomimetic synthesis simulates natural reactions within living organisms [80], while substructure splicing amalgamates active pesticide fragments to generate compounds with enhanced biological efficacy [81]. For the efficient synthesis of pyrethroids, the primary approach involves esterification, necessitating the initial production of chrysanthemic acid derivatives and alcohols (or aldehydes) [82,83,84,85].
Pyrethroids, renowned for their potent insecticidal properties and low mammalian toxicity, have evolved significantly, leveraging structural modifications to enhance stability and effectiveness [86]. The integration of aromatic groups into the alcoholic moiety has notably contributed to the improved stability of pyrethroids, allowing for their widespread application in crop protection [82,83,84,85]. This stability feature has propelled the exploration of various modifications, particularly in the alcohol and acid segments, underscoring the versatility of the synthesis approach [82,83,84,85].
3.4. Toxicological Insights into Pyrethroids: Human and Environmental Implications
Pyrethroids, a class of pesticides, have gained recognition for their relatively low toxicity in humans compared to other pesticide groups, including organochlorines, organophosphates, and carbamates [5]. However, type II pyrethroids have demonstrated greater acute oral toxicity than their counterparts, warranting a detailed assessment of their effects [5]. The toxicity profile of pyrethroids is diverse, with type I pyrethroids causing reversible skin and eye irritation upon exposure, while type II pyrethroids pose more severe risks due to their neurotoxic nature, often leading to fatal outcomes [5].
The human metabolism of pyrethroids involves various enzymes, including cytochrome P450s and carboxylesterases, contributing to the degradation of these compounds [87]. Despite their lower impact on humans relative to insects, pyrethroids can still induce alterations in various physiological functions, emphasizing the need for a comprehensive understanding of their toxicological effects [61].
3.4.1. Toxicity in Humans
Pyrethroid poisoning primarily results from the disruption of sodium and chloride channels. Type I pyrethroids cause distinct symptoms known as type I syndrome, while type II pyrethroids, characterized by an additional cyan group in their chemical structure, elicit type II syndrome [88]. Instances of pyrethroid-induced cardiotoxicity have been reported, particularly associated with prallethrin, a common household pesticide used against mosquitoes, cockroaches, and houseflies [89].
Exposure to prallethrin has been linked to alterations in plasma biochemical profiles, with significant changes observed in glucose, phospholipids, nitrite, nitrate, and lipid peroxidase levels [90]. Additionally, allethrin and prallethrin exposure have been associated with increased MUC5AC expression in human airway cells and heightened reactive oxygen species production, underlining their potential impact on respiratory health [91].
Moreover, prallethrin poisoning has been implicated in gastrointestinal, respiratory, and nervous system disturbances, leading to metabolic acidosis and cardiac conduction disturbances [92]. Accidental and suicidal ingestions are the primary causes of pyrethroid poisoning in humans [93], with dermal exposure also being a common entry route [94,95,96].
In recent years, studies on pyrethroid toxicity in humans have increased considerably. As can be seen in Table 1, pathologies associated with pyrethrin exposure affect numerous systems and organs.
Table 1.
Health hazards of pyrethroids based on studies in recent years.
| Subjects | Sampling | Exposure Assessment | Health Effects Related to Pyrethroid Exposure | Reference |
|---|---|---|---|---|
| 512 pregnant woman | urine samples | urinary levels of cis-dibromodimethylvinylcyclopropane carboxylic acid (DBCA), 3-phenoxybenzoic acid (3-PBA), and total pyrethroid pesticides (PYR) metabolites | Exposure to PYRs during pregnancy was linked to higher birth weight, increased length at birth, and longer gestational duration, as well as a reduced likelihood of small for gestational age (SGA) or premature birth. | [97] |
| 537 mother-child pairs | urine samples | dimethyl phosphate (DMP), dimethyl thiophosphate (DMTP), dimethyl dithiophosphate (DMDTP), diethyl phosphate (DEP), diethyl thiophosphate (DETP), diethyl dithiophosphate (DEDTP), 3,5,6-trichloro-2-pyridinol (TCPy; a metabolite of chlorpyrifos, chlorpyrifos-methyl and triclopyr), 3-PBA in urine | The findings of this research suggest that exposure to organophosphate and pyrethroid insecticides during pregnancy could potentially impact the normal growth of the fetus and lead to changes in birth anthropometric measures and gestational age, potentially signaling early disruptions in childhood developmental processes. Such effects might have long-term implications for individual health. Furthermore, our study emphasizes the differing susceptibility between genders to prenatal stressors. Given the limited evidence available at the time of this analysis and the discrepancies in the existing data, further investigation is strongly recommended to validate the reported outcomes and provide additional insights into the observed distinctions. | [98] |
| 524 mother–child pairs | maternal urine samples | 3-PBA in urine | Exposure to 3-PBA both during pregnancy and at age 2 was linked to heightened ADHD (attention deficit hyperactivity disorder) symptoms at age 6, whereas exposure during ages 4 and 6 was connected to ADHD symptoms at age 8. These results suggest the presence of various sensitive phases in early life that could influence the development of ADHD symptoms during the school-age period. | [99] |
| 720 adolescents | urine samples | 3-PBA in urine | Exposure to pyrethroid pesticides was found to be correlated with hearing loss in American teenagers. This research offers new insights into the connection between pyrethroid exposure and the sense of hearing. | [100] |
| 1305 subjects | urine samples | 2,2,3,3-tetramethylcyclopropanecarboxylic acid (TMCA), TFBA, 3-(2-chloro-3,3,3-trifluoroprop-1-enyl)-2,2-dimethylcyclopropanecarboxylic acid (CTFCA), 2,2-dimethyl-3-(2-methylprop-1-enyl)cyclopropanecarboxylic acid (MPCA), DCCA, FPBA, 3-PBA, MTFBL, and MMTFBL. | The findings of this study indicated that pyrethroid exposure was linked to either heightened levels of total prostate-specific antigen (PSA) or changes in the PSA ratio. Our findings propose that long-term exposure to pyrethroids could potentially harm male reproductive organs, particularly the prostate gland. Additionally, the effects of pyrethroid exposure on the body may vary depending on renal function status. The unstable statistical outcomes and the uncertainty surrounding the carcinogenicity of pyrethroids could be attributed to the limited sample size. Further comprehensive studies are required to establish the reproductive toxicity associated with pyrethroids. | [101] |
| 2012 participants (1006 diabetic cases and 1006 matched controls) | serum samples | Serum pyrethroids determined by gas chromatography-mass spectrometry according to a standardized protocol—deltamethrin and fenvalerate | The comprehensive analysis of metabolites revealed that serum pyrethroid insecticides, especially deltamethrin, were linked to various plasma lipid metabolites, many of which participated in the glycerophospholipid metabolism pathway, predominantly characterized by PCs and LPCs. Additionally, the study indicated that four plasma metabolites (namely, PC 32:0, PC 34:4, CE 20:0, and TAG 52:5 [18:2]) and the pathway involving glycerophosphoethanolamine might represent the potential mechanism underlying the relationship between serum pyrethroids and the onset of type 2 diabetes (T2D). | [102] |
| 177 children | urine samples | glyphosate (GLY); aminomethylphosphonic acid (AMPA); 3,5,6-trichloro-2-pyridinol (TCPy), the main chlorpyrifos pesticide metabolite; cis-(2,2-dibromovinyl)-2,2-dime thylcyclopropanecarboxylic acid (cis-DBCA); cis-3-(2,2-dichlorovinyl)- 2,2-dimethylcyclopropane-1-carboxylic acid (cis-DCCA); trans-3-(2,2- dichlorovinyl)-2,2-dimethylcyclopropane-1-carboxylic acid (transDCCA); 3-phenoxybenzoic acid (3-PBA); 4-fluoro-3-phenoxybenzoic acid (4-F-3-PBA); and cis-3-(2-chloro-3,3,3-trifluoroprop-1-enyl)-2,2- dimethylcyclopropanecarboxylic acid (CIF3CA or CFMP) | This study focused on monitoring children’s exposure to pesticides in Cyprus, following the methodology and tools used in the HBM4EU project. While a notable link was found between aminomethylphosphonic acid (AMPA) and the DNA oxidative stress marker in this children’s population, it is essential to replicate these findings in a more extensive study. The absence of significant associations between AMPA/GLY and lipid damage may suggest a biological DNA damage mechanism for AMPA. | [103] |
| 683 mothers | maternal urine samples | cis-DBCA, cis-DCCA, trans-DCCA, and 3-PBA | Exposure during pregnancy to DDT and pyrethroid insecticides might be linked to concerning behaviors in children residing in a malaria-endemic rural area of South Africa. | [104] |
| 726 adults | urine samples | 3-PBA in urine | A direct correlation was noted between 3-PBA and shifts in both low-frequency and high-frequency hearing thresholds in individuals aged 20 to 39 in the United States, suggesting the susceptibility of young adults to the harmful effects of pyrethroid insecticides. | [105] |
| 48 farmworkers | urine samples | five insecticide metabolites [3,5,6-trichloro-2- pyridinol (TCPy; a metabolite of the OP chlorpyrifos) and four metabolites of pyrethroid insecticides: 3-phenoxybenzoic acid (3PBA), 4-fluoro-3-phenoxybenzoic acid (4F3PBA), the sum of cis/trans 3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylic acid (DCCA), and chloro-3,3,3-trifluoro-1-propene-1-yl (CFCA)] | The study showed a connection between exposure to OP and pyrethroid insecticides and a decrease in cortical brain activity in the prefrontal cortex, which might explain previously reported links to cognitive and behavioral function. | [106] |
| 2116 adults | urine samples | 3-PBA in urine | The results of this forward-looking group study revealed that environmental exposure to pyrethroid insecticides was notably linked to a heightened risk of mortality from all causes within the general adult population in the United States. The observed correlation is probably connected to the detrimental impact of pyrethroids on the cardiovascular system. | [107] |
| 384 pregnant women | urine samples | PYRs metabolites: 3-phenoxybenzoic acid (3PBA), 4-fluoro-3-phenoxybenzoic acid (4F3PBA), and cis-2,2dibromovinyl-2,2-dimethylcyclopropane-1-carboxylic acid (DBCA) in urine samples | The results suggested that women in the final trimester of pregnancy and their infants, aged 6–8 months, were widely exposed to PYRs at low doses. The total concentration of PYRs metabolites in infancy exceeded that during pregnancy. While daily PYRs exposure during the third trimester of pregnancy did not impact toddlers’ language development, exposure to PYRs containing 4F3PBA and DBCA during the 6–8 months age range might delay toddlers’ language development. This demonstrates that the phase between 6–8 months old could be a sensitive window for PYRs exposure that affects toddlers’ language development. The likelihood of language development delay in 2-year-old toddlers could be anticipated by PYRs metabolites with 4F3PBA and DBCA during infancy, at 6–8 months. | [108] |
| 1235 adults | urine samples | 3-PBA in urine | The outcomes of our study revealed that exposure to pyrethroids was linked positively with testosterone (TT) and sex hormone-binding globulin (SHBG) in adults and negatively with circulating free testosterone in males. The current study provides backing to the idea that pyrethroids might have disrupted endocrine function on sex hormones at the observed exposure levels of pyrethroids in U.S. adults. Prolonged and chronic exposure to pesticides could potentially lead to changes in serum sex hormones. | [109] |
3.4.2. Biological Mechanisms and Environmental Impact
In both humans and animals, the nervous system serves as the primary target of pyrethroids, leading to acute neurobehavioral effects. The classification of pyrethroids into type I and type II groups is based on their specific neurotoxic manifestations in rodents and other species [109,110,111]. Environmental exposure to pyrethroids can be particularly harmful to aquatic life, emphasizing the need for stringent precautionary measures [112,113,114]. Similarly, pyrethrin and pyrethroid products can pose risks to avian species, particularly in the presence of certain carriers or propellants in spray formulations [115].
4. Conclusions
Pyrethrins and pyrethroids are a dominating group of insecticidal compounds that have been used for a long time and are still being used today, due to their potency and their variability. From natural pyrethrins, which can be utilized especially for their biodegradable properties, to the synthetic derivatives, pyrethroids, which may be used for their potency, this class of organic insecticides displays a lot of variability. It must be acknowledged that without plants, and plant metabolites, a great area of the insecticide compound class would be missing.
From the natural compound’s standpoint, the fully biosynthetic pathway of pyrethrins has yet to be elucidated, nonetheless clarifying the full path may be a key insight into genetically ingenerating subspecies of plants that may yield more pyrethrins, helping the pyrethrum industry flourish.
However, from the pyrethroid’s point of view, the constant demand for a new molecule that is less toxic, and more biodegradable, yet its potency does not lessen, may be an important catalyst to chemical engineering a compound that may satisfy all the necessities. The impetus of constantly developing and innovating the field of pyrethrins and pyrethroids also urges the research of the environmental impact of these compounds and the toxic effects on humans and animals, which in some cases may be fatal or threatening.
To conclude, this promising potential of pyrethrins and pyrethroids seems likely to persist in the future and needs constant innovation since all areas in which these insecticides are used, from agriculture, household insecticides, veterinary industry to the pharmaceutical and medical industry, require the development of new molecules or methods to analyze these compounds for different purposes.
Acknowledgments
This scientific paper was financially supported by the “Carol Davila” University of Medicine and Pharmacy Bucharest through Contract No. CNFIS-FDI-2023-F-0708.
Author Contributions
Conceptualization, C.H., L.N. and I.S.B.; methodology, L.P. and M.V.G.; software., Ș.-C.M. and A.M.; formal analysis, C.H., C.E.G., M.V.G., C.-E.D.-P., L.N., I.S.B., Ș.-C.M., A.M. and L.P.; writing—original draft preparation Ș.-C.M. and A.M.; writing—review and editing, L.P., M.V.G. and C.E.G.; visualization C.H., L.N., Ș.-C.M. and A.M.; supervision, L.P., M.V.G. and C.-E.D.-P.; funding acquisition, C.-E.D.-P. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the Institutional Development Fund, CNFIS-FDI-2023-F-0708.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Thacker J.R. An Introduction to Arthropod Pest Control. Cambridge University Press; Cambridge, UK: 2002. Chapter 1—A brief history of arthropod control; pp. 1–26. [DOI] [Google Scholar]
- 2.Isman M.B. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol. 2006;51:45–66. doi: 10.1146/annurev.ento.51.110104.151146. [DOI] [PubMed] [Google Scholar]
- 3.Carson R., Quistad G.B. Golden age of insecticide research: Past, present, or future? Annu. Rev. Entomol. 1998;43:1–16. doi: 10.1146/annurev.ento.43.1.1. [DOI] [PubMed] [Google Scholar]
- 4.Stockholm Convention on Persistent Organic Pollutants. 2001. [(accessed on 12 September 2023)]. Available online: http://chm.pops.int/portals/0/repository/convention_text/unep-pops-cop-convtext-full.english.pdf.
- 5.Thatheyus A.J., Selvam A.D.G. Synthetic pyrethroids: Toxicity and biodegradation. Appl. Ecol. Environ. Sci. 2013;1:33–36. doi: 10.12691/aees-1-3-2. [DOI] [Google Scholar]
- 6.Chrustek A., Hołyńska-Iwan I., Dziembowska I., Bogusiewicz J., Wróblewski M., Cwynar A., Olszewska-Słonina D. Current research on the safety of pyrethroids used as insecticides. Medicina. 2018;54:61. doi: 10.3390/medicina54040061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peshin R., Bandral R.S., Zhang W., Wilson L., Dhawan A.K. Integrated Pest Management: Innovation-Development Process. Volume 1. Springer; Dordrecht, The Netherlands: 2009. Integrated pest management: A global overview of history, programs and adoption; pp. 1–49. [DOI] [Google Scholar]
- 8.Mahmood I., Imadi S.R., Shazadi K., Gul A., Hakeem K.R. Plant, Soil and Microbes. Springer International Publishing; Cham, Switzerland: 2016. Chapter 13, Effects of Pesticides on Environment; pp. 253–269. [DOI] [Google Scholar]
- 9.Flores-Mendoza C., López-Sifuentes V.M., Vásquez G.M., Stoops C.A., Fisher M.L., Bernier U.R., Perry M., Mollica J., Coltzau D.A., Gurman P., et al. Field Evaluation of Novel Spatial Repellent Controlled Release Devices (CRDs) against Mosquitoes in an Outdoor Setting in the Northern Peruvian Amazon. Trop. Med. Infect. Dis. 2022;7:372. doi: 10.3390/tropicalmed7110372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yürekli A. Adjunctive Agent for Treating Scabies: In vitro Killing Activity of Permethrin and Tea Tree Oil on Sarcoptes scabiei Collected from Patients. Turkiye Parazitol. Derg. 2022;46:334–338. doi: 10.4274/tpd.galenos.2022.29494. [DOI] [PubMed] [Google Scholar]
- 11.Xiao W., Hong P., Yan L., Biao M., Shichao W., Shanzheng B., Xiangna Z. Pyrethroids exposure alters the community and function of the internal microbiota in Aedes albopictus. Ecotoxicol. Environ. Saf. 2023;252:114579. doi: 10.1016/j.ecoenv.2023.114579. [DOI] [PubMed] [Google Scholar]
- 12.Arslan P. Pyrethroid-induced oxidative stress and biochemical changes in the primary mussel cell cultures. Environ. Sci. Pollut. Res. 2023;30:48484–48490. doi: 10.1007/s11356-023-25845-5. [DOI] [PubMed] [Google Scholar]
- 13.Li W., Lv L., Wang Y., Zhu Y.C. Mixture effects of thiamethoxam and seven pesticides with different modes of action on honey bees (Apis mellifera) Sci. Rep. 2023;13:2679. doi: 10.1038/s41598-023-29837-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Messenger L.A., Matowo N.S., Cross C.L., Jumanne M., Portwood N.M., Martin J., Lukole E., Mallya E., Mosha J.F., Kaaya R., et al. Effects of next-generation, dual-active-ingredient, long-lasting insecticidal net deployment on insecticide resistance in malaria vectors in Tanzania: An analysis of a 3-year, cluster-randomised controlled trial. Lancet Planet. Health. 2023;7:e673–e683. doi: 10.1016/S2542-5196(23)00137-7. [DOI] [PubMed] [Google Scholar]
- 15.Mendarte-Alquisira C., Alarcón A., Ferrera-Cerrato R. Growth, Tolerance, and Enzyme Activities of Trichoderma Strains in Culture Media Added with A Pyrethroids-based Insecticide. Rev. Argent. Microbiol. 2023 doi: 10.1016/j.ram.2023.06.004. [DOI] [PubMed] [Google Scholar]
- 16.McLaughlin G.A. Pyrethrum. Elsevier; Amsterdam, The Netherlands: 1973. History of Pyrethrum; pp. 3–15. [DOI] [Google Scholar]
- 17.Demoute J.P. A brief review of the environmental fate and metabolism of pyrethroids. Pestic. Sci. 1989;27:375–385. doi: 10.1002/ps.2780270406. [DOI] [Google Scholar]
- 18.Zeng T., Li J.-W., Zhou L., Xu Z.-Z., Li J.-J., Hu H., Luo J., Zheng R.-R., Wang Y.-Y., Wang C.-Y. Transcriptional Responses and GCMS Analysis for the Biosynthesis of Pyrethrins and Volatile Terpenes in Tanacetum coccineum. Int. J. Mol. Sci. 2021;22:13005. doi: 10.3390/ijms222313005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bestmann H.J., Classen B., Kobold U., Vostrowsky O., Klingaup F. Pflanzliche Insektizide IV*. Die insektizide Wirkung des ätherischen Öls aus dem Balsamkraut, Chrysanthemum balsamita L. Anz. Schadlingskde Pflanzenschutz Umweltschutz. 1987;60:31–34. doi: 10.1007/BF01904708. [DOI] [Google Scholar]
- 20.Elazzouzi H., Fadili K., Cherrat A., Amalich S., Zekri N., Zerkani H., Tagnaout I., Hano C., Lorenzo J.M., Zair T. Phytochemistry, Biological and Pharmacological Activities of the Anacyclus pyrethrum (L.) Lag: A Systematic Review. Plants. 2022;11:2578. doi: 10.3390/plants11192578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta V., Khan S., Verma R.K., Shanker K., Singh S.V., Rahman L.U. Overexpression of chrysanthemyl diphosphate synthase (CDS) gene in Tagetes erecta leads to the overproduction of pyrethrin. Transgenic Res. 2022;31:625–635. doi: 10.1007/s11248-022-00323-9. [DOI] [PubMed] [Google Scholar]
- 22.Zito S.W., Zieg R.G., Staba E.J. Distribution of pyrethrins in oil glands and leaf tissue of Chrysanthemum cinerariaefolium. Planta Med. 1983;47:205–207. doi: 10.1055/s-2007-969986. [DOI] [PubMed] [Google Scholar]
- 23.Grdiša M., Babić S., Periša M., Carović-Stanko K., Kolak I., Liber Z., Jug-Dujaković M., Satovic Z. Chemical diversity of the natural populations of Dalmatian pyrethrum (Tanacetum cinerariifolium) in Croatia. Chem. Biodivers. 2013;10:460–472. doi: 10.1002/cbdv.201200015. [DOI] [PubMed] [Google Scholar]
- 24.Kamau J.K., Kiiya W., Ajanga S., Wanyonyi N., Gathungu G., Mahasi M., Mwangi J., Pertet E. Pyrethrum Propagation. Organization. 2019;3:19. [Google Scholar]
- 25.Wandahwa P., Van Ranst E., Van Damme P. Pyrethrum (Chrysanthemum cinerariaefolium Vis.) cultivation in West Kenya: Origin, ecological conditions and management. Ind. Crops Prod. 1996;5:307–322. doi: 10.1016/S0926-6690(96)00032-5. [DOI] [Google Scholar]
- 26.Greenhill M. Pyrethrum production: Tasmanian success story. Chron. Hortic. 2007;47:5–8. [Google Scholar]
- 27.Yaso M., Yando J., Meckseane W.A., Sitango K., Lindsay E., Corbett P., Wright J., Flowers E., Casey B., Chung B. Pyrethrum industry development and extension activities in the highlands of Papua New Guinea. Int. Symp. Pyrethrum Nat. Insectic. Sci. Ind. Dev. Renew. Tradit. Ind. 2011;1073:43–48. doi: 10.17660/ActaHortic.2015.1073.4. [DOI] [Google Scholar]
- 28.Matsuda K., Kikuta Y., Haba A., Nakayama K., Katsuda Y., Hatanaka A., Komai K. Biosynthesis of pyrethrin I in seedlings of Chrysanthemum cinerariaefolium. Phytochemistry. 2005;66:1529–1535. doi: 10.1016/j.phytochem.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 29.Li W., Lybrand D.B., Zhou F., Last R.L., Pichersky E. Pyrethrin Biosynthesis: The Cytochrome P450 Oxidoreductase CYP82Q3 Converts Jasmolone to Pyrethrolone. Plant Physiol. 2019;181:934–944. doi: 10.1104/pp.19.00499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu H., Li W., Schilmiller A.L., Van Eekelen H., De Vos R.C.H., Jongsma M.A., Pichersky E. Pyrethric acid of natural pyrethrin insecticide: Complete pathway elucidation and reconstitution in Nicotiana benthamiana. New Phytol. 2019;223:751–765. doi: 10.1111/nph.15821. [DOI] [PubMed] [Google Scholar]
- 31.Xu H., Moghe G.D., Wiegert-Rininger K., Schilmiller A.L., Barry C.S., Last R.L., Pichersky E. Coexpression Analysis Identifies Two Oxidoreductases Involved in the Biosynthesis of the Monoterpene Acid Moiety of Natural Pyrethrin Insecticides in Tanacetum cinerariifolium. Plant Physiol. 2018;176:524–537. doi: 10.1104/pp.17.01330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsui R., Takiguchi K., Kuwata N., Oki K., Takahashi K., Matsuda K., Matsuura H. Jasmonic acid is not a biosynthetic intermediate to produce the pyrethrolone moiety in pyrethrin II. Sci Rep. 2020;10:6366. doi: 10.1038/s41598-020-63026-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li W., Zhou F., Pichersky E. Jasmone Hydroxylase, a Key Enzyme in the Synthesis of the Alcohol Moiety of Pyrethrin Insecticides. Plant Physiol. 2018;177:1498–1509. doi: 10.1104/pp.18.00748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kikuta Y., Ueda H., Takahashi M., Mitsumori T., Yamada G., Sakamori K., Takeda K., Furutani S., Nakayama K., Katsuda Y., et al. Identification and characterization of a GDSL lipase-like protein that catalyzes the ester-forming reaction for pyrethrin biosynthesis in Tanacetum cinerariifolium—A new target for plant protection. Plant J. 2012;71:183–193. doi: 10.1111/j.1365-313X.2012.04980.x. [DOI] [PubMed] [Google Scholar]
- 35.Matsuda K. Pyrethrin biosynthesis and its regulation in Chrysanthemum cinerariaefolium. Top. Curr. Chem. 2012;314:73–81. doi: 10.1007/128_2011_271. [DOI] [PubMed] [Google Scholar]
- 36.Baldwin I.T., Karb M.J., Callahan P. Foliar and floral pyrethrins of Chrysanthemum cinerariaefolium are not induced by leaf damage. J. Chem. Ecol. 1993;19:2081–2087. doi: 10.1007/BF00983810. [DOI] [PubMed] [Google Scholar]
- 37.Kikuta Y., Ueda H., Nakayama K., Katsuda Y., Ozawa R., Takabayashi J., Hatanaka A., Matsuda K. Specific regulation of pyrethrin biosynthesis in Chrysanthemum cinerariaefolium by a blend of volatiles emitted from artificially damaged conspecific plants. Plant Cell Physiol. 2011;52:588–596. doi: 10.1093/pcp/pcr017. [DOI] [PubMed] [Google Scholar]
- 38.Hirokazu U., Kazuhiko M. VOC-mediated within-plant communications and nonvolatile systemic signals upregulate pyrethrin biosynthesis in wounded seedlings of Chrysanthemum cinerariaefolium. J. Plant Interact. 2011;6:89–91. doi: 10.1080/17429145.2011.555566. [DOI] [Google Scholar]
- 39.Soderlund D.M., Knipple D.C. Knockdown Resistance to DDT and Pyrethroids in the House Fly (Diptera: Muscidae): From Genetic Trait to Molecular Mechanism. Ann. Entomol. Soc. Am. 1999;92:909–915. doi: 10.1093/aesa/92.6.909. [DOI] [Google Scholar]
- 40.Dong K. Insect sodium channels and insecticide resistance. Invertebr. Neurosci. 2007;7:17–30. doi: 10.1007/s10158-006-0036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wakeling E.N., Neal A.P., Atchison W.D. Pesticides—Advances in Chemical and Botanical Pesticides. Arcler Education Incorporated; Burlington, ON, Canada: 2012. Pyrethroids and Their Effects on Ion Channels. [DOI] [Google Scholar]
- 42.Murayama K., Abbott N.J., Narahashi T., Shapiro B.I. Effects of allethrin and Condylactis toxin on the kinetics of sodium conductance of crayfish axon membranes. Comp. Gen. Pharmacol. 1972;3:391–400. doi: 10.1016/0010-4035(72)90053-5. [DOI] [PubMed] [Google Scholar]
- 43.Narahashi T. Mode of action of pyrethroids. Bull. World Health Organ. 1971;44:337–345. [PMC free article] [PubMed] [Google Scholar]
- 44.Soderlund D.M. Hayes’ Handbook of Pesticide Toxicology. Elsevier; Amsterdam, The Netherlands: 2010. Toxicology and Mode of Action of Pyrethroid Insecticides; pp. 1665–1686. [DOI] [Google Scholar]
- 45.Soderlund D.M. Neurotoxicology of pyrethroid insecticides. Adv. Neurotoxicol. 2020;4:113–165. doi: 10.1016/bs.ant.2019.11.002. [DOI] [Google Scholar]
- 46.Khambay B.P.S., Jewess P.J. Comprehensive Molecular Insect Science. Elsevier; Amsterdam, The Netherlands: 2005. Pyrethroids; pp. 1–29. [DOI] [Google Scholar]
- 47.Du Y., Lee J.-E., Nomura Y., Zhang T., Zhorov B.S., Dong K. Identification of a cluster of residues in transmembrane segment 6 of domain III of the cockroach sodium channel essential for the action of pyrethroid insecticides. Biochem. J. 2009;419:377–385. doi: 10.1042/BJ20082082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O’Reilly A.O., Khambay B.P.S., Williamson M.S., Field L.M., Wallace B.A., Davies T.G.E. Modelling insecticide-binding sites in the voltage-gated sodium channel. Biochem. J. 2006;396:255–263. doi: 10.1042/BJ20051925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anadón A., Martínez-Larrañaga M.R., Martínez M.A. Use and abuse of pyrethrins and synthetic pyrethroids in veterinary medicine. Vet. J. 2009;182:7–20. doi: 10.1016/j.tvjl.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 50.Taplin D. Pyrethrins and Pyrethroids in Dermatology. Arch. Dermatol. 1990;126:213. doi: 10.1001/archderm.1990.01670260083017. [DOI] [PubMed] [Google Scholar]
- 51.Churcher T.S., Lissenden N., Griffin J.T., Worrall E., Ranson H. The impact of pyrethroid resistance on the efficacy and effectiveness of bednets for malaria control in Africa. Elife. 2016;5:e16090. doi: 10.7554/eLife.16090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gleave K., Lissenden N., Chaplin M., Choi L., Ranson H. Piperonyl butoxide (PBO) combined with pyrethroids in insecticide-treated nets to prevent malaria in Africa. Cochrane Database Syst. Rev. 2021;2021:CD012776. doi: 10.1002/14651858.CD012776.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Corcellas C., Feo M.L., Torres J.P., Malm O., Ocampo-Duque W., Eljarrat E., Barceló D. Pyrethroids in human breast milk: Occurrence and nursing daily intake estimation. Environ. Int. 2012;47:17–22. doi: 10.1016/j.envint.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 54.Feo M.L., Eljarrat E., Manaca M.N., Dobaño C., Barcelo D., Sunyer J., Alonso P.L., Menendez C., Grimalt J.O. Pyrethroid use-malaria control and individual applications by households for other pests and home garden use. Environ. Int. 2012;38:67–72. doi: 10.1016/j.envint.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 55.Ranson H., N’Guessan R., Lines J., Moiroux N., Nkuni Z., Corbel V. Pyrethroid resistance in African anopheline mosquitoes: What are the implications for malaria control? Trends Parasitol. 2011;27:91–98. doi: 10.1016/j.pt.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 56.Duchon S., Bonnet J., Marcombe S., Zaim M., Corbel V. Pyrethrum: A Mixture of Natural Pyrethrins Has Potential for Malaria Vector Control. J. Med. Entomol. 2009;46:516–522. doi: 10.1603/033.046.0316. [DOI] [PubMed] [Google Scholar]
- 57.LaForge F.B., Barthel W.F. Constituents of pyrethrum flowers. XVIII. The structure and isomerism of pyrethrolone and cinerolone. J. Org. Chem. 1945;10:114–120. doi: 10.1021/jo01178a004. [DOI] [Google Scholar]
- 58.Kazuya U. The history of extensive structural modifications of pyrethroids. J. Pestic. Sci. 2019;44:215–224. doi: 10.1584/jpestics.D19-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Khambay B.P.S. Pyrethroid Insecticides. Pest Outlook. 2002;13:49–54. doi: 10.1039/b202996k. [DOI] [Google Scholar]
- 60.Kim K.B., Anand S.S., Kim H.J., White C.A., Bruckner J.V. Toxicokinetics and tissue distribution of deltamethrin in adult Sprague-Dawley rats. Toxicol. Sci. 2008;101:197–205. doi: 10.1093/toxsci/kfm277. [DOI] [PubMed] [Google Scholar]
- 61.Bradberry S.M., Cage S.A., Proudfoot A.T., Vale J.A. Poisoning due to pyrethroids. Toxicol. Rev. 2005;24:93–106. doi: 10.2165/00139709-200524020-00003. [DOI] [PubMed] [Google Scholar]
- 62.Fai P.B.A., Kinfack J.S.T., Towa Y.J.T. Acute effects of binary mixtures of Type II pyrethroids and organophosphate insecticides on Oreochromis niloticus. Ecotoxicology. 2017;26:889–901. doi: 10.1007/s10646-017-1819-y. [DOI] [PubMed] [Google Scholar]
- 63.Kuivila K.M., Hladik M.L., Ingersoll C.G., Kemble N.E., Moran P.W., Calhoun D.L., Nowell L.H., Gilliom R.J. Occurrence and potential sources of pyrethroid insecticides in stream sediments from seven U.S. metropolitan areas. Environ. Sci. Technol. 2012;46:4297–4303. doi: 10.1021/es2044882. [DOI] [PubMed] [Google Scholar]
- 64.Burns C.J., Pastoor T.P. Pyrethroid epidemiology: A quality-based review. Crit. Rev. Toxicol. 2018;48:297–311. doi: 10.1080/10408444.2017.1423463. [DOI] [PubMed] [Google Scholar]
- 65.Bordoni L., Nasuti C., Fedeli D., Galeazzi R., Laudadio E., Massaccesi L., López-Rodas G., Gabbianelli R.E. Early impairment of epigenetic pattern in neurodegeneration: Additional mechanisms behind pyrethroid toxicity. Exp. Gerontol. 2019;124:110629. doi: 10.1016/j.exger.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 66.Lu Z., Gan J., Cui X., Moreno L.D., Lin K. Understanding the bioavailability of pyrethroids in the aquatic environment using chemical approaches. Environ. Int. 2019;129:194–207. doi: 10.1016/j.envint.2019.05.035. [DOI] [PubMed] [Google Scholar]
- 67.Ullah S., Li Z., Zuberi A., Zain M.A.U., Mirza M.F.A.B. Biomarkers of pyrethroid toxicity in fish. Environ. Chem. Lett. 2019;17:945–973. doi: 10.1007/s10311-018-00852-y. [DOI] [Google Scholar]
- 68.Deng F., Sun J., Dou R., Yu X., Wei Z., Yang C., Zeng X., Zhu L. Contamination of pyrethroids in agricultural soils from the Yangtze River Delta, China. Sci. Total Environ. 2020;731:139181. doi: 10.1016/j.scitotenv.2020.139181. [DOI] [PubMed] [Google Scholar]
- 69.Cryder Z., Wolf D., Carlan C., Gan J. Removal of urban-use insecticides in a large-scale constructed wetland. Pt AEnviron. Pollut. 2021;268:115586. doi: 10.1016/j.envpol.2020.115586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tang W., Wang D., Wang J., Wu Z., Li L., Huang M., Xu S., Yan D. Pyrethroid pesticide residues in the global environment: An overview. Chemosphere. 2018;191:990–1007. doi: 10.1016/j.chemosphere.2017.10.115. [DOI] [PubMed] [Google Scholar]
- 71.Matsuo N. Discovery and development of pyrethroid insecticides. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2019;95:378–400. doi: 10.2183/pjab.95.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ravula A.R., Yenugu S. Pyrethroid based pesticides—Chemical and biological aspects. Crit. Rev. Toxicol. 2021;51:117–140. doi: 10.1080/10408444.2021.1879007. [DOI] [PubMed] [Google Scholar]
- 73.Ray D.E., Forshaw P.J. Pyrethroid insecticides: Poisoning syndromes, synergies, and therapy. J. Toxicol. Clin. Toxicol. 2000;38:95–101. doi: 10.1081/CLT-100100922. [DOI] [PubMed] [Google Scholar]
- 74.Soderlund D.M. Molecular mechanisms of pyrethroid insecticide neurotoxicity: Recent advances. Arch. Toxicol. 2012;86:165–181. doi: 10.1007/s00204-011-0726-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Saillenfait A.M., Ndiaye D., Sabaté J.P. Pyrethroids: Exposure and health effects—An update. Int. J. Hyg. Environ. Health. 2015;218:281–292. doi: 10.1016/j.ijheh.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 76.Wang N., Huang M., Guo X., Lin P. Urinary Metabolites of Organophosphate and Pyrethroid Pesticides and Neurobehavioral Effects in Chinese Children. Environ. Sci. Technol. 2016;50:9627–9635. doi: 10.1021/acs.est.6b01219. [DOI] [PubMed] [Google Scholar]
- 77.Andersen H.R., David A., Freire C., Fernández M.F., D’Cruz S.C., Reina-Pérez I., Fini J.B., Blaha L. Pyrethroids and developmental neurotoxicity—A critical review of epidemiological studies and supporting mechanistic evidence. Pt 2Environ. Res. 2022;214:113935. doi: 10.1016/j.envres.2022.113935. [DOI] [PubMed] [Google Scholar]
- 78.Laskowski D.A. Physical and chemical properties of pyrethroids. Rev. Environ. Contam. Toxicol. 2002;174:49–170. doi: 10.1007/978-1-4757-4260-2_3. [DOI] [PubMed] [Google Scholar]
- 79.Stout D.M., 2nd, Bradham K.D., Egeghy P.P., Jones P.A., Croghan C.W., Ashley P.A., Pinzer E., Friedman W., Brinkman M.C., Nishioka M.G., et al. American Healthy Homes Survey: A national study of residential pesticides measured from floor wipes. Environ. Sci. Technol. 2009;43:4294–4300. doi: 10.1021/es8030243. [DOI] [PubMed] [Google Scholar]
- 80.Chapuis H., Strazewski P. Shorter puromycin analog synthesis by means of an efficient Staudingere–Vilarrasa coupling. Tetrahedron. 2006;62:12108–12115. doi: 10.1016/j.tet.2006.09.045. [DOI] [Google Scholar]
- 81.Menn J.J. Contemporary frontiers in chemical pesticide research. J. Agric. Food Chem. 1980;28:2–8. doi: 10.1021/jf60227a012. [DOI] [Google Scholar]
- 82.Zheng Z., Wang J., Zhang D., Guan X., Gao S., Chen Z., Zou X. Design, synthesis, and insecticidal activities of novel monohalovinylated pyrethroids. J. Agric. Food Chem. 2011;59:1171–1177. doi: 10.1021/jf103908d. [DOI] [PubMed] [Google Scholar]
- 83.Wang H., Wang Q., Zhao X.F., Liu P., Meng X.H., Yu T., Ji Y.L., Zhang H., Zhang C., Zhang Y., et al. Cypermethrin exposure during puberty disrupts testosterone synthesis via downregulating StAR in mouse testes. Arch. Toxicol. 2010;84:53–61. doi: 10.1007/s00204-009-0479-y. [DOI] [PubMed] [Google Scholar]
- 84.Han Z.-Y., Wu W.-Y., Chen F.-L., Guan X.-L., Fu X.-H., Jiang P., Wan R. Design, Synthesis, Crystal Structure and Insecticidal Evaluation of Novel Arylpyrazole Derivatives Containing Cyhalothroyl Thiourea Moiety. Taylor & Francis; Oxford, UK: 2017. [DOI] [Google Scholar]
- 85.Chang F., Dutta S., Becnel J.J., Estep A.S., Mascal M. Synthesis of the insecticide prothrin and its analogues from biomass-derived 5-(chloromethyl)furfural. J. Agric. Food Chem. 2014;62:476–480. doi: 10.1021/jf4045843. [DOI] [PubMed] [Google Scholar]
- 86.Kiessling L.L., Gestwicki J.E., Strong L.E. Synthetic multivalent ligands in the exploration of cell-surface interactions. Curr. Opin. Chem. Biol. 2000;4:696–703. doi: 10.1016/S1367-5931(00)00153-8. [DOI] [PubMed] [Google Scholar]
- 87.Mikata K., Isobe N., Kaneko H. Biotransformation, and enzymatic reactions of synthetic pyrethroids in mammals. Top. Curr. Chem. 2012;314:113–135. doi: 10.1007/128_2011_254. [DOI] [PubMed] [Google Scholar]
- 88.Ramchandra A.M., Chacko B., Victor P.J. Pyrethroid Poisoning. Indian J. Crit. Care Med. 2019;23((Suppl. 4)):S267–S271. doi: 10.5005/jp-journals-10071-23304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Matsunaga T., Makita M., Higo A., Nishibe I., Dohara K., Shinjo G. Studies on prallethrin, a new synthetic pyrethroid, for indoor applications: I. The insecticidal activities of prallethrin isomers. Med. Entomol. Zool. 1987;38:219–223. doi: 10.7601/mez.38.219. [DOI] [Google Scholar]
- 90.Narendra M., Kavitha G., Helah Kiranmai A., Raghava Rao N., Varadacharyulu N.C. Chronic exposure to pyrethroid-based allethrin and prallethrin mosquito repellents alters plasma biochemical profile. Chemosphere. 2008;73:360–364. doi: 10.1016/j.chemosphere.2008.05.070. [DOI] [PubMed] [Google Scholar]
- 91.Na H.G., Kim Y.D., Choi Y.S., Bae C.H., Song S.Y. Allethrin and prallethrin stimulate MUC5AC expression through oxidative stress in human airway epithelial cells. Biochem. Biophys. Res. Commun. 2018;503:316–322. doi: 10.1016/j.bbrc.2018.06.022. [DOI] [PubMed] [Google Scholar]
- 92.Bhaskar E.M., Moorthy S., Ganeshwala G., Abraham G. Cardiac conduction disturbance due to prallethrin (pyrethroid) poisoning. J. Med. Toxicol. 2010;6:27–30. doi: 10.1007/s13181-010-0032-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Parlato F., Buendia Palacios D., Adão-Serrano M., Gonçalves F., Carreiro C., Gouveia J.L. A Suicide Attempt: Deltamethrin Intoxication. Eur. J. Case Rep. Intern. Med. 2022;9:003573. doi: 10.12890/2022_003573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Osweiler G.D. Toxicology. Williams and Wilkins; Philadelphia, PA, USA: 1996. [Google Scholar]
- 95.Gammon D.W., Chandrasekaran A., AlNaggar S.F. Comparative metabolism and toxicology of pyrethroids in mammals. In: Marrs T.C., editor. Mammalian Toxicology of Insecticides. RSC Publishing; Cambridge, UK: 2012. pp. 137–183. [DOI] [Google Scholar]
- 96.Anadon A., Arés I., Martíinez M.A. Pyrethrins and synthetic pyrethroids: Use in veterinary medicine. In: Ramawat K.G., Mérillon J.M., editors. Natural Products. Springer; Berlin/Heidelberg, Germany: 2013. pp. 4061–4086. [Google Scholar]
- 97.Xu Q., Zhu B., Dong X., Li S., Song X., Xiao X., Zhang C., Lv Y., Zhang X., Li Y. Pyrethroid pesticide exposure during early pregnancy and birth outcomes in southwest China: A birth cohort study. J. Toxicol. Sci. 2020;45:281–291. doi: 10.2131/jts.45.281. [DOI] [PubMed] [Google Scholar]
- 98.Gimenez-Asensio M.J., Hernandez A.F., Romero-Molina D., Gonzalez-Alzaga B., Luzardo O.P., Henríquez-Hernández L.A., Boada L.D., García-Cortés H., Lopez-Flores I., Sanchez-Piedra M.D., et al. Effect of prenatal exposure to organophosphates and pyrethroid pesticides on neonatal anthropometric measures and gestational age. Environ. Res. 2023;232:116410. doi: 10.1016/j.envres.2023.116410. [DOI] [PubMed] [Google Scholar]
- 99.Lee K.S., Lim Y.H., Lee Y.A., Shin C.H., Kim B.N., Hong Y.C., Kim J.I. The association of prenatal and childhood pyrethroid pesticide exposure with school-age ADHD traits. Environ. Int. 2022;161:107124. doi: 10.1016/j.envint.2022.107124. [DOI] [PubMed] [Google Scholar]
- 100.Xu H., Mao Y., Xu B. Association between pyrethroid pesticide exposure and hearing loss in adolescents. Environ. Res. 2020;187:109640. doi: 10.1016/j.envres.2020.109640. [DOI] [PubMed] [Google Scholar]
- 101.Hong D., Min J.Y., Min K.B. Association between pyrethroids and prostate endpoints; stratified according to renal function. Environ. Int. 2021;153:106489. doi: 10.1016/j.envint.2021.106489. [DOI] [PubMed] [Google Scholar]
- 102.Jia C., Qiu G., Wang H., Zhang S., An J., Cheng X., Li P., Li W., Zhang X., Yang H., et al. Lipid metabolic links between serum pyrethroid levels and the risk of incident type 2 diabetes: A mediation study in the prospective design. J. Hazard. Mater. 2023;459:132082. doi: 10.1016/j.jhazmat.2023.132082. [DOI] [PubMed] [Google Scholar]
- 103.Makris K.C., Efthymiou N., Konstantinou C., Anastasi E., Schoeters G., Kolossa-Gehring M., Katsonouri A. Oxidative stress of glyphosate, AMPA and metabolites of pyrethroids and chlorpyrifos pesticides among primary school children in Cyprus. Pt BEnviron. Res. 2022;212:113316. doi: 10.1016/j.envres.2022.113316. [DOI] [PubMed] [Google Scholar]
- 104.An S., Rauch S.A., Maphula A., Obida M., Kogut K., Bornman R., Chevrier J., Eskenazi B. In-utero exposure to DDT and pyrethroids and child behavioral and emotional problems at 2 years of age in the VHEMBE cohort, South Africa. Chemosphere. 2022;306:135569. doi: 10.1016/j.chemosphere.2022.135569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Long L., Tang Y. Urinary pyrethroid metabolite and hearing threshold shifts of adults in the United States: A cross-sectional study. PLoS ONE. 2022;17:e0275775. doi: 10.1371/journal.pone.0275775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mora A.M., Baker J.M., Hyland C., Rodríguez-Zamora M.G., Rojas-Valverde D., Winkler M.S., Staudacher P., Palzes V.A., Gutiérrez-Vargas R., Lindh C., et al. Pesticide exposure and cortical brain activation among farmworkers in Costa Rica. Neurotoxicology. 2022;93:200–210. doi: 10.1016/j.neuro.2022.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bao W., Liu B., Simonsen D.W., Lehmler H.J. Association Between Exposure to Pyrethroid Insecticides and Risk of All-Cause and Cause-Specific Mortality in the General US Adult Population. JAMA Intern. Med. 2020;180:367–374. doi: 10.1001/jamainternmed.2019.6019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen S., Xiao X., Qi Z., Chen L., Chen Y., Xu L., Zhang L., Song X., Li Y. Effects of prenatal and infant daily exposure to pyrethroid pesticides on the language development of 2-year-old toddlers: A prospective cohort study in rural Yunnan, China. Neurotoxicology. 2022;92:180–190. doi: 10.1016/j.neuro.2022.08.002. [DOI] [PubMed] [Google Scholar]
- 109.Xu H., Bo Y. Associations between pyrethroid exposure and serum sex steroid hormones in adults: Findings from a nationally representative sample. Chemosphere. 2022;300:134591. doi: 10.1016/j.chemosphere.2022.134591. [DOI] [PubMed] [Google Scholar]
- 110.Weiner M.L., Nemec M., Sheets L., Sargent D., Breckenridge C. Comparative functional observational battery study of twelve commercial pyrethroid insecticides in male rats following acute oral exposure. Neurotoxicology. 2009;30((Suppl. 1)):S1–S16. doi: 10.1016/j.neuro.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 111.Wolansky M.J., Harrill J.A. Neurobehavioral toxicology of pyrethroid insecticides in adult animals: A critical review. Neurotoxicol. Teratol. 2008;30:55–78. doi: 10.1016/j.ntt.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 112.Bradbury S.P., Coats J.R. Toxicokinetics of fenvalerate in rain-bow trout (Salmo gairdneri) Environ. Toxicol. Chem. 1986;5:567–576. doi: 10.1002/etc.5620050609. [DOI] [Google Scholar]
- 113.Bradbury S.P., Coats J.R. Comparative toxicology of pyrethroid insecticides. Reviews of Environmental Contamination and Toxicology. Volume 108. Springer; New York, NY, USA: 1989. pp. 133–177. US Environmental Res. Lab. [DOI] [PubMed] [Google Scholar]
- 114.Ansari B.A., Kumar K. Cypermethrin toxicity: Effect on the carbohydrate metabolism of the Indian catfish, Heteropneustes fossilis. Sci. Total Environ. 1988;72:161–166. doi: 10.1016/0048-9697(88)90014-9. [DOI] [PubMed] [Google Scholar]
- 115.Bradbury S.P., Coats J.R. Toxicity of fenvalerate to bobwhite quail (Colinus virginianus) including brain and liver residues associated with mortality. J. Toxicol. Environ. Health. 1982;10:307–319. doi: 10.1080/15287398209530253. [DOI] [PubMed] [Google Scholar]