Abstract
Multiple studies have indicated that distinct metabolites are involved in the occurrence and development of osteopenia (ON) and osteoporosis (OP); however, these metabolites in OP and ON have not yet been classified and standardized. This systematic review and meta-analysis included 21 articles aiming to investigate the distinct metabolites in patients with ON and OP. The quality of the included articles was generally high; seventeen studies had >7 stars, and the remaining four received 6 stars. This systematic review showed that three metabolites (phosphatidylcholine (PC) (lipid metabolites), galactose (carbohydrate metabolites), and succinic acid (other metabolites)) increased, four (glycylglycine (gly-gly), cystine (amino acids), sphingomyelin (SM) (lipid metabolites) and glucose (carbohydrate metabolites)) decreased, and five (glutamine, hydroxyproline, taurine (amino acids), lysophosphatidylcholine (LPC) (lipid metabolites), and lactate (other metabolites)) had conflicting directions in OP/ON. The results of the meta-analysis show that gly-gly (MD = −0.77, 95%CI −1.43 to −0.11, p = 0.02) and cystine (MD = −5.52, 95%CI −7.35 to −3.68, p < 0.00001) decreased in the OP group compared with the healthy control group. Moreover, LPC (MD = 1.48, 95%CI 0.11 to 2.86, p = 0.03) increased in the OP group compared with the healthy control group. These results indicate that distinct metabolites were associated with ON and OP, which could be considered a predictor for OP.
Keywords: osteoporosis, osteopenia, distinct metabolites, metabolomics
1. Introduction
Osteoporosis (OP) is a systemic metabolic bone disease characterized by low bone mineral density (BMD) and the destruction of bone microstructure, which can increase the risk of fractures [1]. Osteopenia (ON) is a stage between the normal BMD and OP, where bone density is suboptimal but still not as low as OP [2]. Without adequate treatment, ON tends to develop into OP. There are some 200 million OP patients in the world, which is expected to reach 400 million in the next decade [3]. In addition, 20% of people aged > 50 experience OP-related fractures, among which hip fracture is the most devastating [4]. By 2020, the number of ON cases has reached 47 million [5]. It has been estimated that the medical expenses for OP fractures in China will reach CNY 132 billion by 2035, while this portion of healthcare expenditure may climb to CNY 163 billion yuan by 2050 [6].
Currently, OP and ON are mainly diagnosed according to BMD value by dual-energy X-ray absorptiometry (DXA) [7,8], and these diseases are considered preventable and treatable. The mainstream therapies include estrogen treatment, alendronate sodium, calcium supplementation, and Chinese medicine [9]. Proper treatment is believed to effectively decrease the risk of fracture even for those who have already suffered from brittle fracture [10], and the key point for effective treatment is early diagnosis. For example, as one of the mainstream therapies, calcium supplementation can hardly restore BMD to normal levels and has weak effects on fractures [11]; however, it is effective as an early preventive measure [12]. Should the diagnosis be made as early as possible, the treatment effectiveness and cost would both be satisfying. Therefore, an effective predictor, or a biomarker, which can warn the potential of the diseases, is warranted.
Generally, biomarkers are often discovered based on the mechanisms of disease [13]. OP is considered to be the disorder of bone homeostasis, which means the imbalance between the bone formation mediated by osteoblast (OB) and the bone resorption mediated by osteoclast (OC) [14]. Nonetheless, the specific mechanism of bone homeostasis disorder remains unclear. Multiple factors have been proven to contribute to OP, such as age, gender, hormone level, use of oral glucocorticoids, etc. [15]. Older individuals, especially postmenopausal women, are the most affected by ON or OP [16]. Respectable efforts have been made to construct predictive models using these factors; however, there are no large-scale clinical applications for these models [17].
Great expectations have been placed on bone turnover markers (BTMs), including procollagen type I amino-terminal peptide (PINP) and C-terminal telopeptide of type I collagen (CTX-I), in the monitoring and treatment of OP and ON [18]. Nevertheless, BTMs are proteins or matrix-degradation products produced and released by OB and OC, so they cannot exert an early predictive role before the functional abnormalities of OB and OC occur [19]. Due to this natural variability, their independent prognostic value for OP is uncertain [20]. Therefore, the proper candidates for the predictive biomarkers of OP and ON are still an issue worth considering.
In recent years, the role of metabolites in OP or ON has been considered [21], as the disordered body metabolism can affect bone metabolism, leading to the decrease in BMD and causing OP [22]. Since OP and ON are metabolic diseases, and given the rapid progress in metabolomic technique, it is possible and reasonable to elucidate the characteristics of the disease status by using some or a pattern of metabolites in the body.
Metabolomics, a popular method for systematically profiling small molecules, is widely used in various metabolic diseases [23]. This approach can dynamically reflect the small molecule metabolites and their changes in organisms [24]. Increasing evidence has indicated that the altered metabolites relate to the ON/OP [25], which provided novel insights into a prediction of OP and highlighted the potential of metabolites as markers for OP. Recently, preclinical and clinical studies have proved that distinct metabolites can be used to help predict ON/OP and for monitoring during the treatment of OP [26,27,28]. Moreover, it may provide a feasible solution to perform the differential metabolite between the OP or ON and healthy participants. However, the distinct metabolites in OP and ON have not yet been classified and standardized.
The present systematic review and meta-analysis aimed to summarize the differential metabolites in OP and ON patients compared with healthy people, to establish the metabolic pathways associated with OP/ON, and further discuss their potential in predicting OP so as to facilitate a new perspective for the early identification and prevention of OP in clinical practice.
2. Materials and Methods
Our systematic review and meta-analysis adhered to the standard criteria PRISMA [29]. This research protocol has been registered in the PROSPERO registry (ID = CRD42022365040).
2.1. Search Strategy
PubMed, Web of Science, Embase, the Cochrane Library, the Chinese databases of China National Knowledge Internet (CNKI), and the Wan-fang database were searched for relevant articles published from their inception up to 5 August 2022 without restrictions on countries or article type. Our search terms combined interventions (Metabolomics) with diseases (ON, OP) in humans. The detailed search strategy is shown in Table S1.
2.2. Eligibility Criteria
Inclusion criteria were the following: (1) Human study; (2) OP and ON were defined by measuring BMD; (3) Randomized controlled trial, case-control study, cross-sectional study; (4) Metabolites were identified by metabolomics technique in blood or urine samples; (5) published in English or Chinese language. Reviews, studies on children, adolescents, and pregnant women, secondary OP, subjects with other basic diseases aside from OP, patients receiving treatment that affected the metabolite level within three months, and non-original articles were excluded.
2.3. Study Selection
Firstly, we removed duplicates, after which the titles and abstracts of the remaining articles were screened separately by two researchers, and the studies that did not meet the predefined criteria were excluded. Moreover, the full text of the eligible articles was subjected to further screening. Any disagreements were discussed with the third researcher to minimize selection bias.
2.4. Data Extraction
Two independent researchers extracted information from eligible articles, including the first name of the author, year of publication, study design, number of OP or ON and control, age, body mass index (BMI), metabolomics technique, biological sample, the name of different metabolites, the variation trend, and the concentration of metabolites and associated metabolic pathways. The data were shown in graphs, and WebPlotDigitizer-4.5 was used to extract data from graphs. Disagreements were resolved through discussion and consensus was reached with a third investigator. Classify different metabolites according to amino acid, lipid, carbohydrate, and other metabolites.
2.5. Quality Assessment
We evaluated and scored the quality of the case-control studies by the Newcastle–Ottawa Scale (NOS), including whether the sample selection was random, comparability of cases and controls, and exposure [30]. Among these categories, each item in selection and exposure could obtain at most one point, while in comparability, it could obtain up to two points. The quality assessment of the cross-sectional study was conducted by using a modified Newcastle–Ottawa Quality Assessment Scale [31], which includes the following items: (1) Selection (composed of three items): representativeness of the sample, sample size, and non-response rate; (2) Comparability (one item): between respondents and non-respondents; (3) Outcome and Analysis (four items): assessment of outcome of hypersensitivity, reporting of point estimate (prevalence), reporting of the measure of variability for the point estimate and accounting for correlation between multilevel units. Two independent researchers implemented the quality assessment, and arbitration was conducted by the third researcher in case of disagreement.
2.6. Statistical Analysis
A qualitative analysis was performed for the different metabolites by counting the frequency across the included studies. For the concentrations of different metabolites, a meta-analysis was conducted across studies using the MD with the confidence intervals 95% (95%CI) when 2 different studies reported the same metabolites. Finally, a random effect model was applied when the heterogeneity was high (I2 > 50%); conversely, the fixed effect model was applied. Sensitivity analyses were performed to investigate the influence of possible bias by removing studies one by one.
3. Results
3.1. Literature Search Results
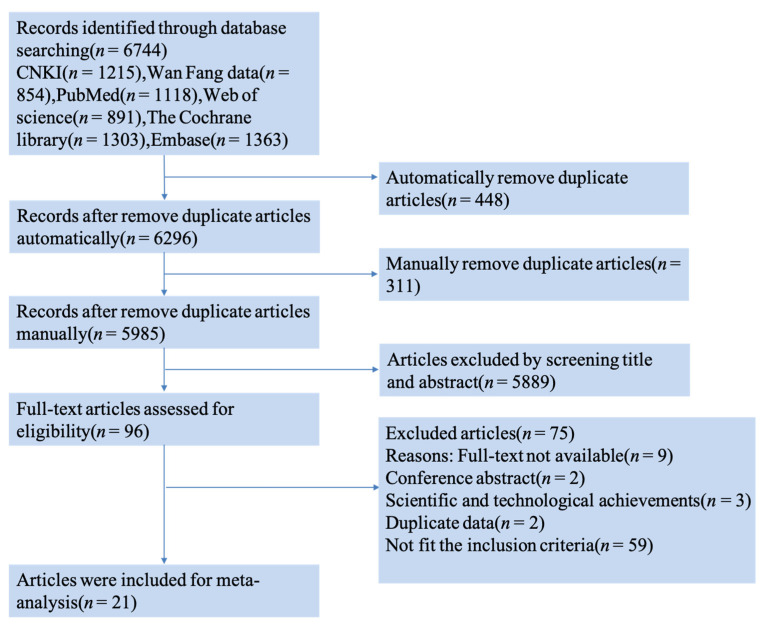
The literature search and selection of articles are shown in Figure 1. A total of 6744 articles were found in Chinese and English databases, 759 of which were deleted due to the repetitive content. Among the 5985 titles and abstracts that were reviewed independently by two researchers, 5889 were excluded. After reading the complete text, 75 articles were excluded for various reasons (Figure 1). Finally, 21 articles, 4 in Chinese and 17 in English, were included.
Figure 1.
Flowchart of literature screening.
3.2. Characteristics of Included Studies
All the studies were published between 1997 and 2022, including 20 case-control studies and one cross-sectional study. Among these, one study [32] focused on ON as the target disease, six studies [27,33,34,35,36,37] focused on both ON and OP, while the remaining fourteen studies [38,39,40,41,42,43,44,45,46,47,48,49,50,51] concentrated on OP. Notably, there were five studies [38,48,49,50,51] on OP that primarily focused on the differentiation of traditional Chinese medical (TCM) syndromes in their study design. A total of 3721 participants were recruited from China, Hungary, Japan, Jordan, Brazil, Korea, and the USA. In addition, 15 studies reported on age and BMI, and six studies [39,41,42,44,47,51] failed to record BMI. The included studies analyzed the metabolites from blood and urine samples via GC-MS, LC-MS, and HNMR. The characteristic of each study is shown in Table 1.
Table 1.
The characteristics and NOS of the included studies.
| References | Country | Study Design | No. of OP or ON/Control | Age of OP or ON/Control (years) | BMI of OP or ON/Control (m/kg2) | Technique | Biological Sample | Key Findings | NOS | |
|---|---|---|---|---|---|---|---|---|---|---|
|
Yin et al., 2021 [48] |
China | case-control | 30 OP vs. 30 control | 66.1 ± 7.5/64.3 ± 6.3 | 24.34 ± 2.99/24.11 ± 2.87 | UPLC/MS | blood | 15 different metabolites in OP with Yin deficiency syndrome: Glycocholic Acid, Bilirubin, Diloxanide, etc. |
7 | |
| Zhu 2020 [49] | China | case-control | 30 OP(A) vs. 30 OP(I) 30 control | 65.47 ± 7.54/66.1 ± 7.47 /55.97 ± 9.47 |
22.93 ± 3.42/24.34 ± 2.99 /24.11 ± 2.87 |
UPLC/MS | serum | 15 different metabolites | 10 (↑) *: Inosine, Lucidenic acid G, etc. | 7 |
| 5 (↓) *: Dodecanoic acid, Cohibin B, etc. | ||||||||||
|
Li 2020 [50] |
China | case-control | 120 OP vs. 18 control | 46–87 | 14.69–33.33 | HNMR | serum | 20 different metabolites: Glutamine, Leucine, etc. | 6 | |
|
Guo et al., 2022 [51] |
China | case-control | 20 OP vs. 12 control | 62.7 ± 2.2/47.5 ± 5.4 | NA/NA * | UPLC/MS/MS | serum | 157 different metabolites | 93 (↑): L-isoleucine, γ-Aminobutyric acid, etc. 64 (↓): Alanine, Glutamate, etc. |
7 |
|
Yin et al., 2022 [38] |
China | case-control | 30 OP vs. 30 control | 65.47 ± 7.54/55.97 ± 9.47 | 22.93 ± 3.42/24.11 ± 2.87 | UPLC/MS | serum | 11 potential metabolite biomarkers of KYADS: Indole, Lotusine, etc. | 6 | |
|
Poor et al., 2003 [39] |
Hungary | case-control | 11 OP vs. 13 control | 53.8 ± 4.9/56.6 ± 5.7 | NA/NA | capillary gas chromatography | urine | 8 Urinary steroid different metabolites: Tetrahydro-corticosterone, 11-O-androsterone, etc. | 6 | |
|
Wang et al., 2019 [33] |
China | case-control | Male: 40 OP vs. 46 ON vs. 46 control Female: 60 OP vs. 61 ON vs. 61 control |
Male: 66.9 ± 2.9/67.2 ± 1.3/67.4 ± 1.4 Female: 60.7 ± 3.9/60.8 ± 4.0/60.1 ± 4.2 |
Male: 23.3 ± 2.5/23.4 ± 2.5/23.4 ± 2.4 Female: 26.8 ± 3.5/26.7 ± 3.5/26.7 ± 3.5 |
LC-MS/MS | blood | Male: 8 metabolites in males showed significant differences between the three groups Female: 12 metabolites showed significant differences between the three groups |
8 | |
|
Miyamoto et al., 2017 [40] |
Japan | case-control | 5 OP vs. 42 control | 55.83 ± 3.6/56.34 ± 3.5 | 23.09 ± 1.8/22.25 ± 2.53 | LC/MS | serum | protein metabolism | (↓) Gly-Gly, cystine (↑) hydroxyproline |
6 |
|
Aleidi et al., 2021 [34] |
Jordan | case-control | 25 OP vs. 22 ON vs. 22 control | 66.16 ± 1.78/64.64 ± 1.72 /54.82 ± 1.03 |
30.70 ± 1.4/30.38 ± 1.84 /32.21 ± 1.1 |
UPLC/MS | serum | 94 dysregulated metabolites: | 52 (↑) 42 (↓) |
8 |
|
Deng et al., 2021 [41] |
China | case-control | 32 OP vs. 32 control | 60.47 ± 12.39/60.59 ± 14.14 | NA/NA | UHPLC-HRMS | serum | The differential metabolites | (↑) PE, TG(18:0/18:0/18:0), cyclic Melatonin, etc. (↓): LPC, 4-Hydroxyproline, etc. |
9 |
|
Cao et al., 2021 [42] |
China | case-control | 36 OP vs. 55 control | 57.51 ± 4.59 | NA/NA | LC-MS | blood | 10 different lipid metabolites: | 6 (↑): PC (18:0/20:4), TG (16:0/10:0/20:4), CL (19:0/18:2/20:0/22:6), CL (75:4), PC (36:5), Tand G (54:4) 4 (↓): PC (36:2), CL (22:3/18:0/18:0/20:4), LPC (18:1), SM (d16:0/18:1) |
7 |
|
Kou et al., 2022 [43] |
China | case-control | 50 OP vs. 50 control | 69.3 ± 9.3/66.3 ± 10 | 23.8 ± 3.2/23.5 ± 4.4 | GC/LC-MS | serum | 18 different metabolites | 8 | |
|
Pontes et al., 2019 [35] |
Brazil | case-control | 24 OP vs. 26 ON vs. 28 control | 60. 8 ± 6.0/61.88 ± 7.9/ 60.38 ± 6.2 |
25.58 ± 4.8/27.20 ± 5.2/ 25.35 ± 3.4 |
H NMR | serum | 9 different metabolites OP | 6 (↑): Cholesterol, Leucine, isoleucine, Lactate, Unsaturated lipids, Allantoin 3 (↓): Tyrosine, Choline, Taurine |
7 |
|
Zhang et al., 2022 [44] |
China | case-control | 120 OP vs. 80 control | 71/70 | NA/NA | LC-MS/MS | serum | (↑) NEOs and their metabolites |
7 | |
|
LIM et al., 1997 [32] |
Korea | case-control | 34 ON vs. 25 control | 56.8 ± 0.4/57.2 ± 0.4 | 23.15 ± 0.36/24.38 ± 0.36 | GC-MS | urinary | 18 estrogen metabolites: | 7 | |
|
Qi et al., 2016 [36] |
China | case-control | 67 OP vs. 114 ON vs. 79 control | 58.37 ± 4.78/57.03 ± 4.53/ 54.43 ± 4.9 |
23.52 ± 3.39/23.56 ± 3.05/ 24.75 ± 3.21 |
GC-MS | serum | 12 different metabolites between low BMD and control 5 free fatty acids (LA, Oleic acid, AA and 11, 14-Eicosadienoic acid) correlations with BMD |
8 | |
|
Zhao et al., 2018 [45] |
USA | case-control | 65 OP vs. 71 control | 31.2 ± 4.9/31.8 ± 55.3 | 21.9 ± 2.5/29.7 ± 8.6 | LC-MS | serum | 14 metabolites, 7 amino acids and amino acid derivatives, 5 lipids (including three bile acids), and 2 organic acids were significantly associated with the risk for low BMD |
7 | |
|
Yu et al., 2018 [37] |
China | case-control | 77 OP vs. 92 ON vs. 71 control | 57.97 ± 4.07/56.72 ± 4.79/ 54.71 ± 4.81 |
23.12 ± 3.08/23.01 ± 2.98/ 24.73 ± 3.14 |
GC–MS | Urine | 17 different metabolites | 8 | |
|
You et al., 2014 [46] |
China | cross-sectional study | Premenopausal: 134 OP vs. 349 control Postmenopausal: 77 OP vs. 41 control |
Premenopausal: 44.7 ± 0.29/44.9 ± 0.19 Postmenopausal: 52.5 ± 0.29/50.7 ± 0.47 |
Premenopausal: 21.2 ± 0.27/22.5 ± 0.17 Postmenopausal: 21.8 ± 0.56/24.3 ± 0.60 |
GC–MS | blood | 7 different metabolites | 2 (↑): Acetate, Glutamine 5 (↓): Lactate, Acetone, Lipids, VLDLs, Glucose |
9 |
|
Mei et al., 2020 [27] |
China | case-control | Discovery set: 83 OP vs. 205 ON vs. 413 control Replication set: 107 OP vs. 68 ON vs. 103 control |
Discovery set: 63.0 ± 9.1/59.0 ± 10.8/ 52.9 ± 12 Replication set: 70.3 ± 9.5/66.5 ± 13.9/ 62.6 ± 12.7 |
Discovery set: 22.8 ± 2.9/24.2 ± 3.3/ 24.7 ± 3.2 Replication set: 22.4 ± 3.7/23.2 ± 3.2/ 24.3 ± 3.7 |
LC-MS | blood | 47 different metabolites (13 amino acids, 2 carboxylic acids, 14 glycerophospholipids, 3 purines and purine derivatives, 7 sphingolipids, and 8 others) |
9 | |
|
Miyamoto et al., 2018 [47] |
Japan | case-control | 33 OP vs. 46 control | 39–61 | NA/NA | LC/MS | serum | 24 different metabolites | 8 | |
* (↑): Increased expression; (↓): Decreased expression; NA/NA: Not available.
3.3. Risk of Bias of Included Studies
The risk of bias in case-control studies was assessed by NOS, and the cross-sectional study was conducted using a modified Newcastle–Ottawa Quality Assessment Scale. The quality of seventeen studies was >7 stars, and that of the four other studies equaled 6 stars. The maximum score for NOS is 9 stars and the minimum score for NOS is 6 stars. The risk of bias evaluation of the included studies is shown in Table 1. The assessment details for every item are listed in Table S2.
3.4. Qualitative Synthesis
In this systematic review and meta-analysis, we collected many different metabolites, including amino acid metabolites, lipid metabolites, carbohydrate metabolites, and other metabolites in ON/OP. Distinct metabolites identified in ON and OP are shown in Table 2. We have provided a list of abbreviations in Table S3.
Table 2.
Metabolites altered in ON and OP.
| Category | Metabolites | Variation Trend | Reference |
|---|---|---|---|
| Amino Acids | glutamine | ↓ * | Wang et al. [33] |
| ↑ * | Zhao et al. [45] | ||
| ↑ | You et al. [46] | ||
| ↑ | Miyamoto et al. [40,47] | ||
| hydroxyproline | ↑ | Wang et al. [33] | |
| ↑ | Miyamoto et al. (2017) [40] | ||
| ↓ | Deng et al. [41] | ||
| ↑ | Miyamoto et al. (2018) [47] | ||
| gly-gly | ↓ | Miyamoto et al. (2017) [40] | |
| ↓ | Kou et al. [43] | ||
| ↓ | Miyamoto et al. (2018) [47] | ||
| cystine | ↓ | Miyamoto et al. (2017) [40] | |
| ↓ | Zhao et al. [45] | ||
| ↓ | Miyamoto et al. (2018) [47] | ||
| taurine | ↓ | Pontes et al. [35] | |
| ↑ | Zhao et al. [45] | ||
| ↓ | Yu et al. [37] | ||
| Lipid Metabolites | PC | ↑ | Aleidi et al. [34] |
| ↑ | Cao et al. [42] | ||
| ↑ | Kou et al. [43] | ||
| LPC | ↑ | Wang et al. [33] | |
| ↓ | Deng et al. [41] | ||
| ↓ | Cao et al. [42] | ||
| ↑ | Kou et al. [43] | ||
| ↑ | Miyamoto et al. (2018) [47] | ||
| SM | ↓ | Cao et al. [42] | |
| ↓ | Kou et al. [43] | ||
| Carbohydrate Metabolites | glucose | ↓ | Kou et al. [43] |
| ↓ | You et al. [46] | ||
| Other Metabolites | lactate | ↑ | Kou et al. [43] |
| ↑ | Pontes et al. [35] | ||
| ↓ | You et al. [46] | ||
| succinic | ↑ | Deng et al. [41] | |
| ↑ | Zhao et al. [45] | ||
| ↑ | Yu et al. [37] |
* ↑: Increased expression; ↓: Decreased expression.
3.5. Amino Acids
More than 10 studies compared the differences in amino acid levels between ON/OP and healthy subjects. In particular, a study carried out by Zhao et al. [45] evaluated the association between BMD and amino acids, reporting that seven amino acids and their derivatives were significantly associated with low BMD. Moreover, a study conducted in China that included 701 participants [27] found that 13 amino acids were the risk factors for ON/OP through the LC-MS metabolomics method. At least five studies detected the level of glutamine (which can be converted to glutamic acid in the body) and glutamic acid and analyzed the relationship between glutamine or glutamic acid and BMD. Three studies [45,46,47] reported a negative correlation between glutamine and BMD; however, these results contradict those of another study [33] in which the glutamine level declined in the participants with ON or OP compared with the healthy controls. Interestingly, Wang et al. [33] also found that the concentration of glutamine was elevated in the OP group compared to the ON group. Among four studies [33,40,41,47] that analyzed the relationship between hydroxyproline and BMD, three found significant negative associations, unlike the study of Deng et al. [41]. Miyamoto and Kou [40,43,47] observed that gly-gly and glycine levels were significantly lower in the low BMD group than in the high BMD group. Furthermore, the concentrations of cystine and cysteine were decreased in the individuals with OP [40,45,47], which suggested that cysteine may also be a protective factor for BMD. Two studies [35,37] revealed that the taurine level declined in patients with low BMD, contrary to another study [45].
3.6. Lipid Metabolites
The correlations between lipid metabolites and ON/OP were also analyzed.
Eleven studies focused on lipid metabolites, seven of which evaluated the levels of phosphatidylcholine (PC) and its metabolite lysophosphatidylcholine (LPC) in different participants. Three case-control studies [34,42,43] revealed a negative correlation between serum PC and BMD; two other studies [41,42] discovered a significantly lower level of LPC in the OP group than in the normal BMD group. However, three studies [33,43,47] reported opposite results, where the LPC level was higher in the low BMD group than in the normal group.
More than 10 differentially expressed phospholipids, including sphingomyelin (SM), cardiolipin (CL), phosphatidic acid (PA), glycerophosphate, etc., identified by high-throughput techniques, were found in the ON/OP group. Among four studies that determined the level of SM, two [42,43] suggested a positive correlation between SM and BMD.
In addition, two studies [34,43] reported a close correlation between low carbon number-saturated lipids (palmitic acid, stearic acid) and OP.
3.7. Carbohydrate Metabolites
Sugar metabolites, including galactose, glucose, and fructose, were closely correlated with the prevalence of ON/OP. Two Chinese studies [43,46] reported lower glucose levels and one study [37] indicated a higher galactose concentration in the OP group than in the control group.
3.8. Other Metabolites
Organic acids: at least nine analyses recruited organic acids. The determination in 74 individuals with OP and 78 control subjects [35,43] showed that the lactate level was significantly higher in OP than in the control group. However, an opposite result was also reported [46]. Succinic acid was indicated as an increased risk for low BMD [37,41,45].
Acylcarnitines: C5-DC, C3-DC-M, acetylcarnitine, and 3-hydroxy-11-octadecenoylcarnitine were associated with ON/OP [33,34,45]; however, there was little overlap among these studies.
Urea cycle metabolites, ornithine, citrulline, and arginine, were considered OP risk factors [27,33,43,47].
Bile acids: two studies [41,45] identified more than eight bile acids (BAs) and the detected BAs were associated with OP.
Other compounds, steroids [32,39], purines [27], neonicotinoids, and their characteristic metabolites [44] were associated with ON/OP in isolated reports.
3.9. Metabolites and Traditional Chinese Medicine Syndrome
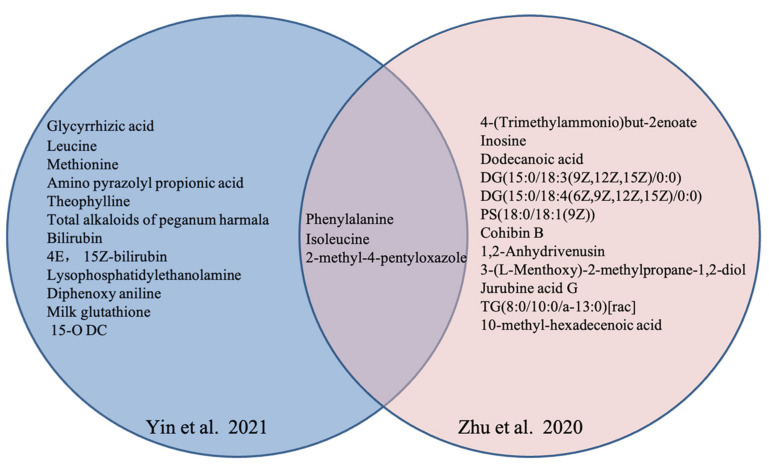
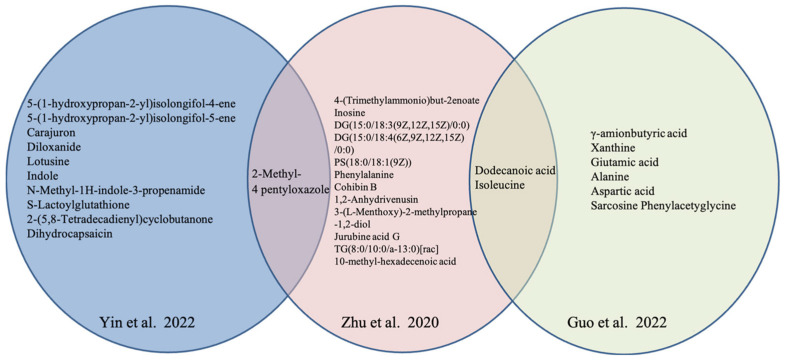
Notably, four studies [48,49,50,51] in Chinese and one in English language [38] explored the potential distinct metabolites in OP with TCM syndrome. Multiple differential metabolites, including amino acids (phenylalanine, isoleucine), oxazole (2-methyl-4-pentyloxazole), and organic acids (dodecanoic acid) were anchored to characterize the various TCM syndrome of OP from healthy subjects. The distinct metabolites in Yin deficiency of liver and kidney syndrome are shown in Figure 2, and those in Kidney-Yang deficiency syndrome are listed in Figure 3.
Figure 2.
Distinct metabolites in OP with Yin deficiency of liver and kidney in different studies [48,49].
Figure 3.
Distinct metabolites in OP with Kidney-Yang deficiency in different studies [38,49,51].
3.10. Pathways Analysis
Some of the included studies described metabolic pathways with ON/OP. As depicted in Table 3, 10 studies [34,37,41,42,43,45,47,48,50,51] analyzed differential pathways related to ON/OP, including amino acids metabolism pathways (e.g., valine, leucine, and isoleucine biosynthesis and degradation, tryptophan metabolism, histidine metabolism, taurine metabolism), and lipid metabolism pathways (e.g., glycerophospholipid metabolism, biosynthesis of unsaturated fatty acids). In addition, Li et al. [50], Aleidi et al. [34], and Zhao et al. [45] certified that the pathways of the aminoacyl-tRNA biosynthesis were differently expressed in the group of ON/OP. The TCA cycle was the most important metabolic pathway related to ON/OP found by Yu et al. [37] and Miyamoto et al. [47]. Finally, several studies found that choline metabolism, acetaldehyde, dicarboxylate, and glucose metabolism were expressed differently in the ON/OP.
Table 3.
Associated metabolic pathways involved in ON/OP.
| Study | Pathways | Analysis Methods |
|---|---|---|
| Yin et al., 2021 [48] |
Bile secretion | Enrichment analysis of KEGG signaling pathway |
| Secondary bile acid biosynthesis | ||
| Cholesterol metabolism | ||
| Caffeine metabolism | ||
| Pyruvate metabolism | ||
| Primary bile acid biosynthesis | ||
| Li et al., 2020 [50] |
Valine, leucine, and isoleucine biosynthesis and degradation | Enrichment analysis and topology analysis |
| Aminoacyl-tRNA biosynthesis | ||
| Glycolysis or Gluconeogenesis | ||
| Glycerophospholipid metabolism | ||
| Glyoxylate and dicarboxylate metabolism | ||
| TCA cycle | ||
| Taurine and hypotaurine metabolism | ||
| Guo et al., 2022 [51] |
Tryptophan metabolism | Enrichment analysis of KEGG signaling pathway |
| Glutathione metabolism | ||
| Phospholipase D signaling pathway | ||
| Arginine, proline with alanine metabolism | ||
| Aleidi et al. 2021 [34] |
Histidine metabolism | The pathway analysis module |
| Aminoacyl-tRNA biosynthesis | ||
| Glyoxylate and dicarboxylate metabolism | ||
| Biosynthesis of unsaturated fatty acids | ||
| Deng et al., 2021 [41] |
Lipids pathways | NA |
| Cao et al., 2021 [42] |
Choline metabolism | The bubble diagram of pathway enrichment analysis |
| Glycerophospholipid metabolism | ||
| Retrograde endocannabinoid signaling | ||
| Linoleic acid metabolism | ||
| Alpha-linolenic acid metabolism | ||
| Arachidonic acid metabolism | ||
| Kou et al., 2022 [43] |
Glucose metabolism | Database searching (KEGG) and consulting relevant literature |
| Amino acids metabolism | ||
| Choline metabolism | ||
| Inflammatory response | ||
| Zhao et al., 2018 [45] |
Alanine, aspartate, and glutamate metabolism | MetaboAnalyst 3.0 software |
| Butanoate metabolism | ||
| Taurine and hypotaurine metabolism | ||
| Aminoacyl-tRNA biosynthesis | ||
| Glutathione metabolism | ||
| Primary bile acid biosynthesis | ||
| Glycine, serine, and threonine metabolism | ||
| Yu et al., 2018 [37] |
Taurine metabolism | MetaboAnalyst 3.0 software |
| β-alanine metabolism | ||
| Galactose metabolism | ||
| TCA cycle | ||
| Proparoate metabolism | ||
| Nitrogen metabolism | ||
| Butanoate metabolism | ||
| Miyamoto et al., 2018 [47] |
TCA cycle | NA |
| Urea cycle | ||
| Pentose phosphate pathway |
3.11. Meta-Analysis for Metabolites
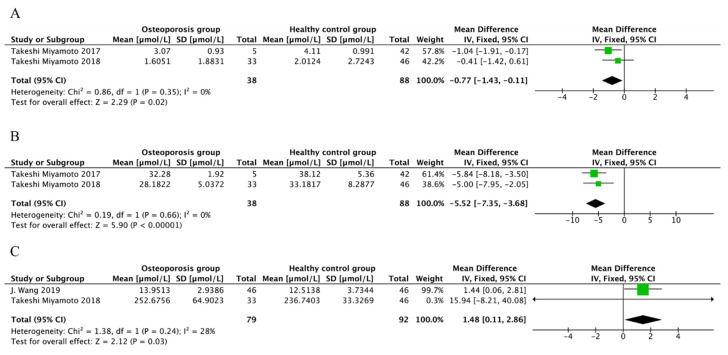
A metabolite was included in the meta-analysis if at least two studies reported its concentration with the variables of mean and SD. Due to the limited consistency of the included studies, three metabolites, including gly-gly, cystine, and LPC, were included in the meta-analysis. The results reveal that the level of gly-gly (MD = −0.77, 95%CI −1.43 to −0.11, p = 0.02) and cystine (MD = −5.52, 95%CI −7.35 to −3.68, p < 0.00001) decreased in the OP group compared to the control group, without heterogeneity among the included studies (I2 = 0%, p > 0.1) (Figure 4A,B). The level of LPC was higher in the OP group than in the control group (MD = 1.48, 95%CI 0.11 to 2.86, p = 0.03) (Figure 4C). Due to the low degree of heterogeneity, a fixed effect model was used for meta-analysis.
Figure 4.
Forest plot for different metabolites (μmol/L). (A) Forest plot of the concentration of gly-gly in OP patients and controls. Data from references [40,47]. The level of gly-gly decreased in OP group compared to the control group. (B) Forest plot of the concentration of cystine in OP patients and controls. Data from references [40,47]. The results reveal that the level of cystine decreased in OP group compared to the control group. (C) Forest plot of the concentration of LPC in OP patients and control. Data from references [33,47]. The level of LPC was higher in OP group than the control group. Note: The green boxes represent the point estimates for each study, and the black boxes represent the combined values of the study results.
4. Discussion
This systematic review and meta-analysis identified a number of blood or urine metabolites related to ON/OP in humans. In addition, this systematic review detected several metabolic pathways associated with OP/ON, such as amino acids metabolism pathways, lipids metabolism pathways, and the TCA cycle. Moreover, this systematic review also analyzed potential distinct metabolites in OP with different TCM syndromes.
Studies found that amino acid metabolism can regulate bone metabolism and is involved in the BMD [52,53,54]. Amino acids may directly regulate the proliferation, differentiation, and apoptosis of OB and OC [55,56]. Certainly, amino acids also affect OB and OC through their actions on the secretion of insulin-like growth factor 1 (IGF-1) [57]. In addition, amino acids influence BMD by affecting the intestinal absorption of calcium [58]. As one of the amino acids, proline has a positive association with BMD, a derivative that can increase serum estradiol and alkaline phosphatase [59]. Some studies have reported on the role of hydroxyproline in the stability of collagen [60]. However, the present meta-analysis did not determine a definitive association between hydroxyproline and BMD. Notably, in the study of Wang et al. [33], the level of hydroxyproline in ON was elevated while it declined in OP compared to the normal controls, which may indicate that hydroxyproline is associated with the severity of ON. Therefore, more studies are needed to clarify this association. Moreover, tyrosine, which is present in thyroid hormones, has been proven to exert a protective effect against OP and can stimulate the expression of OB for the production of collagen [61]. Unfortunately, in the present work, only one study reported the correlation between BMD and the level of tyrosine. Furthermore, cystine and cysteine could accelerate bone regeneration by activating OB differentiation [62]. In this work, we demonstrated a positive association between cystine and BMD. Some previous studies reported on the potential role of glutamine and glutamic acid in bone metabolism. Glutamate receptors can be expressed in bone cells, and their signaling can regulate bone remodeling [63]. In addition, glutamine can provide the needed energy for bone resorption of OC through the TCA [64]. However, this meta-analysis has not found a definitive association between glutamic acid and BMD due to the lack of reports on the concentration values of included studies. In our meta-analysis, we detected a significant difference in gly-gly levels between individuals with OP and healthy individuals. Compared with the healthy control group, the gly-gly level was decreased in the OP group, which indicated that gly-gly could predict low BMD in OP.
Lipidomics has suggested that a number of lipids may be predictive of ON/OP. Moreover, many studies have found that lipid metabolism disorders may have a certain relationship with the incidence of OP [65,66]. Phospholipids are the main component of bio- membrane. The articles included in this systematic review reported that more than 10 phospholipids (e.g., SM, CL, PA, glycerophosphate), identified by high-throughput techniques, were distinct in those with and without ON/OP. SM can generate ceramides under hydrolyzed, which produces reactive oxygen species, thereby inhibiting bone formation and leading to the occurrence of OP [67]. In this study, at least two articles suggested a positive association between SM and BMD, where the level of SM was decreased under hydrolyzed, thus leading to OP, closely related to inflammatory reactions [68]. Triglycerides (TGs) and diacylglycerols (DGs) have been reported to regulate inflammation reactions, promoting the expression of inflammatory factors such as IL-6, IL-1β, and IL-8 and inducing oxidative stress and OP [69]. However, included studies reported inconsistent results, thus suggesting that further research is still needed. As a metabolite of PC, LPC plays a crucial role in the occurrence of OP. On the one hand, LPC is the component of oxidized low-density lipoprotein (ox-LDL), which can facilitate the differentiation from bone marrow mesenchymal stem cells (BMSCs) to adipocytes and inhibit BMSCs in the differentiation from BMSCs to OB, thus accelerating the occurrence of OP [42]. On the other hand, LPC has also been identified as a potential OC differentiation factor [70]. More importantly, LPC can increase the concentration of free calcium in intracellular [71] and reduce the concentration of calcium in bone, leading to OP. Our meta-analysis also demonstrated a significantly negative association between the concentration of LPC and BMD, which is consistent with the previous findings. BAs were reported to be associated with ON and OP. A recent study suggested that sodium deoxycholate reduces intestinal calcium absorption, whereas lithocholic acid stimulates calcium absorption [45]. UCDA, a secondary bile acid, can promote OB differentiation and bone mineralization. However, different studies reported inconsistent results, suggesting that the type of BAs should be considered when the relationship between BAs and BMD has already been analyzed.
In our study, the sugars (e.g., galactose, glucose, and fructose) were associated with BMD, which may be partly explained by abnormal energy metabolism affecting OP [72]. Moreover, our research found that organic acids like succinic acid had an increased risk for low BMD. Succinic acid is important in mitochondrial function, and mitochondrial dysfunction is related to age-related diseases such as OP. Moreover, succinic acid receptor activation can enhance the function of OC, leading to a decrease in bone mass.
This research found that amino acid metabolism had an extreme difference in OP with Yin liver and kidney deficiency. The metabolic state of different syndrome types of TCM requires different energy, which leads to changes in amino acid metabolism. Organic acids and their metabolites showed apparent differences in the OP group with Kidney-Yang deficiency compared with the healthy group. According to up-to-date research, Kidney-Yang deficiency is closely related to the neuroendocrine system [73], often showing the clinical manifestations of sympathetic insufficiency, such as a pale complexion and lukewarm limbs. This may be related to the metabolic disorder of organic and amino acids, affecting the nervous system.
The strengths of the present study include that we perform an extensive literature search. We searched the main databases in English and Chinese, thus minimizing the possibility of missing any major published report. Next, to the best of our knowledge, this is the first study that performed qualitative synthesis to evaluate the relationship between distinct metabolites and the occurrence of OP. This is also the first study that investigated the relationship between distinct metabolites and OP with different TCM syndrome types, thus providing a theoretical basis for the biological nature of TCM syndrome types. Moreover, we described associated metabolic pathways in this review. Finally, the included studies were of high quality, although most results show significant heterogeneity.
Despite the novelty of our research, several limitations should be pointed out. First, most included studies reported inconsistent results, so only a few meta-analyses could be conducted. Second, menstruation and BMI are important influencing factors of OP, but we did not perform subgroup analysis to exclude the influence of both due to the limited number of articles in the meta-analysis. Third, not all trials clearly distinguished the two groups of ON and OP according to BMD, and the severity of the disease needed to be standardized. Finally, due to the limitation of the research type, our results could only provide clues for finding predicted metabolites.
5. Conclusions
Distinct metabolites are associated with ON and OP. Our results suggest that gly-gly and cystine negatively affect BMD. However, LPC is positively associated with the occurrence of OP, which provides evidence that metabolomics can be used as predictive biomarkers for ON and OP. In addition, this systematic review found that amino acids metabolism pathways, lipids metabolism pathways, and TCA cycle are associated with OP/ON. Moreover, this systematic review also demonstrated that phenylalanine, isoleucine, and 2-methyl-4-pentyloxazole were closely linked to OP with Yin deficiency of the liver and kidney, while dodecanoic acid, isoleucine, and 2-methyl-4-pentyloxazole had a relationship with OP with Kidney-Yang deficiency. However, we found only possible candidates for diagnostic purposes in this work. The associations between metabolites and BMD should be further investigated and verified by future high-quality and large prospective cohort studies.
Acknowledgments
Thanks to all authors for their contributions to this study and the support of the fund for this study.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu15234895/s1, Table S1: Search strategy. Table S2: The NOS assessment scale for every study. Table S3: The list of abbreviations.
Author Contributions
Y.W. designed this meta-analysis. X.H., J.S., Z.L., Y.Z. and M.J. searched aimed articles. Y.W., Y.L. and M.L. extracted data. Y.W. wrote the manuscript and submitted the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable for studies not involving humans or animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no competing interest.
Funding Statement
The National Natural Science Foundation of China (Grant No. 82074290) and the National Training Program for Innovative Backbone Talents of Traditional Chinese Medicine (Grant No. ZX2020002).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Ensrud K.E., Crandall C.J. Osteoporosis. Ann. Intern. Med. 2017;167:Itc17–Itc32. doi: 10.7326/AITC201708010. [DOI] [PubMed] [Google Scholar]
- 2.Johnston C.B., Dagar M. Osteoporosis in Older Adults. Med. Clin. N. Am. 2020;104:873–884. doi: 10.1016/j.mcna.2020.06.004. [DOI] [PubMed] [Google Scholar]
- 3.Coughlan T., Dockery F. Osteoporosis and fracture risk in older people. Clin. Med. 2014;14:187–191. doi: 10.7861/clinmedicine.14-2-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yong E.L., Logan S. Menopausal osteoporosis: Screening, prevention and treatment. Singap. Med. J. 2021;62:159–166. doi: 10.11622/smedj.2021036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karaguzel G., Holick M.F. Diagnosis and treatment of osteopenia. Rev. Endocr. Metab. Disord. 2010;11:237–251. doi: 10.1007/s11154-010-9154-0. [DOI] [PubMed] [Google Scholar]
- 6.Zhenlin Z. Guidelines for the Diagnosis and Treatment of Primary Osteoporosiss (2022) Chin. Gen. Med. 2023;26:1671–1691. [Google Scholar]
- 7.Bandaru S., Hare K., Krueger D., Binkley N. Do patients that fracture with normal DXA-measured BMD have normal bone? Arch. Osteoporos. 2020;15:70. doi: 10.1007/s11657-020-00745-0. [DOI] [PubMed] [Google Scholar]
- 8.Ma J., Lin X., Chen C., Li S., Zhang S., Chen Z., Li D., Zhao F., Yang C., Yin C., et al. Circulating miR-181c-5p and miR-497-5p Are Potential Biomarkers for Prognosis and Diagnosis of Osteoporosis. J. Clin. Endocrinol. Metab. 2020:105. doi: 10.1210/clinem/dgz300. [DOI] [PubMed] [Google Scholar]
- 9.Tella S.H., Gallagher J.C. Prevention and treatment of postmenopausal osteoporosis. J. Steroid Biochem. Mol. Biol. 2014;142:155–170. doi: 10.1016/j.jsbmb.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delaney M.F. Strategies for the prevention and treatment of osteoporosis during early postmenopause. Am. J. Obstet. Gynecol. 2006;194:S12–S23. doi: 10.1016/j.ajog.2005.08.049. [DOI] [PubMed] [Google Scholar]
- 11.Bolland M.J., Grey A., Reid I.R. Should we prescribe calcium or vitamin D supplements to treat or prevent osteoporosis? Climacteric. 2015;18((Suppl. S2)):22–31. doi: 10.3109/13697137.2015.1098266. [DOI] [PubMed] [Google Scholar]
- 12.Chiodini I., Bolland M.J. Calcium supplementation in osteoporosis: Useful or harmful? Eur. J. Endocrinol. 2018;178:D13–D25. doi: 10.1530/EJE-18-0113. [DOI] [PubMed] [Google Scholar]
- 13.Thudium C.S., Löfvall H., Karsdal M.A., Bay-Jensen A.C., Bihlet A.R. Protein biomarkers associated with pain mechanisms in osteoarthritis. J. Proteom. 2019;190:55–66. doi: 10.1016/j.jprot.2018.04.030. [DOI] [PubMed] [Google Scholar]
- 14.Kim J.M., Lin C., Stavre Z., Greenblatt M.B., Shim J.H. Osteoblast-Osteoclast Communication and Bone Homeostasis. Cells. 2020;9:2073. doi: 10.3390/cells9092073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelsey J.L. Risk factors for osteoporosis and associated fractures. Public Health Rep. 1989;104:14–20. [PMC free article] [PubMed] [Google Scholar]
- 16.Muñoz M., Robinson K., Shibli-Rahhal A. Bone Health and Osteoporosis Prevention and Treatment. Clin. Obstet. Gynecol. 2020;63:770–787. doi: 10.1097/GRF.0000000000000572. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y., Wang L., Sun Y., Wu M., Ma Y., Yang L., Meng C., Zhong L., Hossain M.A., Peng B. Prediction model for the risk of osteoporosis incorporating factors of disease history and living habits in physical examination of population in Chongqing, Southwest China: Based on artificial neural network. BMC Public Health. 2021;21:991. doi: 10.1186/s12889-021-11002-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eastell R., Szulc P. Use of bone turnover markers in postmenopausal osteoporosis. Lancet Diabetes Endocrinol. 2017;5:908–923. doi: 10.1016/S2213-8587(17)30184-5. [DOI] [PubMed] [Google Scholar]
- 19.Brown J.P., Don-Wauchope A., Douville P., Albert C., Vasikaran S.D. Current use of bone turnover markers in the management of osteoporosis. Clin. Biochem. 2022;109–110:1–10. doi: 10.1016/j.clinbiochem.2022.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Jain S., Camacho P. Use of bone turnover markers in the management of osteoporosis. Curr. Opin. Endocrinol. Diabetes Obes. 2018;25:366–372. doi: 10.1097/MED.0000000000000446. [DOI] [PubMed] [Google Scholar]
- 21.Yu X.H., Cao R.R., Yang Y.Q., Zhang L., Lei S.F., Deng F.Y. Systematic evaluation for the causal effects of blood metabolites on osteoporosis: Genetic risk score and Mendelian randomization. Front. Public Health. 2022;10:905178. doi: 10.3389/fpubh.2022.905178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Polyzos S.A., Anastasilakis A.D., Efstathiadou Z.A., Yavropoulou M.P., Makras P. Postmenopausal osteoporosis coexisting with other metabolic diseases: Treatment considerations. Maturitas. 2021;147:19–25. doi: 10.1016/j.maturitas.2021.02.007. [DOI] [PubMed] [Google Scholar]
- 23.Chen X., Yu D. Metabolomics study of oral cancers. Metabolomics. 2019;15:22. doi: 10.1007/s11306-019-1483-8. [DOI] [PubMed] [Google Scholar]
- 24.Guijas C., Montenegro-Burke J.R., Warth B., Spilker M.E., Siuzdak G. Metabolomics activity screening for identifying metabolites that modulate phenotype. Nat. Biotechnol. 2018;36:316–320. doi: 10.1038/nbt.4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang X., Xu H., Li G.H., Long M.T., Cheung C.L., Vasan R.S., Hsu Y.H., Kiel D.P., Liu C.T. Metabolomics Insights into Osteoporosis Through Association with Bone Mineral Density. J. Bone Miner. Res. 2021;36:729–738. doi: 10.1002/jbmr.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lv H., Jiang F., Guan D., Lu C., Guo B., Chan C., Peng S., Liu B., Guo W., Zhu H., et al. Metabolomics and Its Application in the Development of Discovering Biomarkers for Osteoporosis Research. Int. J. Mol. Sci. 2016;17:2018. doi: 10.3390/ijms17122018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mei Z., Dong X., Qian Y., Hong D., Xie Z., Yao G., Qin A., Gao S., Hu J., Liang L., et al. Association between the metabolome and bone mineral density in a Chinese population. EBioMedicine. 2020;62:103111. doi: 10.1016/j.ebiom.2020.103111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen X., Wang Z., Duan N., Zhu G., Schwarz E.M., Xie C. Osteoblast-osteoclast interactions. Connect. Tissue Res. 2018;59:99–107. doi: 10.1080/03008207.2017.1290085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vrabel M. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Rev. Espaola Nutr. Humana Dietética. 2009;18:e123. [PMC free article] [PubMed] [Google Scholar]
- 30.Wells G.A., Shea B.J., O’Connell D., Peterson J., Tugwell P. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Non-Randomized Studies in Meta-Analysis. University of Liverpool; Liverpool, UK: 2000. [Google Scholar]
- 31.Favaro Zeola L., Soares P.V., Cunha-Cruz J. Prevalence of dentin hypersensitivity: Systematic review and meta-analysis. J. Dent. 2019;81:1–6. doi: 10.1016/j.jdent.2018.12.015. [DOI] [PubMed] [Google Scholar]
- 32.Lim S.K. Altered Hydroxylation of Estrogen in Patients with Postmenopausal Osteopenia. J. Clin. Endocrinol. Metab. 1997;82:1001–1006. doi: 10.1210/jcem.82.4.3875. [DOI] [PubMed] [Google Scholar]
- 33.Wang J., Yan D., Zhao A., Hou X., Zheng X., Chen P., Bao Y., Jia W., Hu C., Zhang Z.L. Discovery of potential biomarkers for osteoporosis using LC-MS/MS metabolomic methods. Osteoporos. Int. 2019;30:1491–1499. doi: 10.1007/s00198-019-04892-0. [DOI] [PubMed] [Google Scholar]
- 34.Aleidi S.M., Alnehmi E.A., Alshaker M., Masood A., Benabdelkamel H., Al-Ansari M.M., Abdel Rahman A.M. A Distinctive Human Metabolomics Alteration Associated with Osteopenic and Osteoporotic Patients. Metabolites. 2021;11:628. doi: 10.3390/metabo11090628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pontes T.A., Barbosa A.D., Silva R.D., Melo-Junior M.R., Silva R.O. Osteopenia-osteoporosis discrimination in postmenopausal women by 1H NMR-based metabonomics. PLoS ONE. 2019;14:e0217348. doi: 10.1371/journal.pone.0217348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qi H., Bao J., An G., Ouyang G., Zhang P., Wang C., Ying H., Ouyang P., Ma B., Zhang Q. Association between the metabolome and bone mineral density in pre- and post-menopausal Chinese women using GC-MS. Mol. Biosyst. 2016;12:2265–2275. doi: 10.1039/C6MB00181E. [DOI] [PubMed] [Google Scholar]
- 37.Yu L., Qi H., An G., Bao J., Ma B., Zhu J., Ouyang G., Zhang P., Fan H., Zhang Q. Association between metabolic profiles in urine and bone mineral density of pre- and postmenopausal Chinese women. Menopause. 2019;26:94–102. doi: 10.1097/GME.0000000000001158. [DOI] [PubMed] [Google Scholar]
- 38.Yin H., Zhu T.C., Zhang Y.F., Wang J.W. Metabolomics-based screening for potential serum biomarkers of kidney yang defi ciency syndrome in osteoporosis patients: A pilot study. Asian J. Surg. 2022;45:2494–2495. doi: 10.1016/j.asjsur.2022.05.118. [DOI] [PubMed] [Google Scholar]
- 39.Poór V., Bufa A., Bíró I., Wilhelm F., Juricskay S. Examination of sex steroids in the urines of postmenopausal women with osteoporosis. Chromatographia. 2004;60:S165–S168. doi: 10.1365/s10337-004-0205-0. [DOI] [Google Scholar]
- 40.Miyamoto T., Hirayama A., Sato Y., Koboyashi T., Katsuyama E., Kanagawa H., Miyamoto H., Mori T., Yoshida S., Fujie A., et al. A serum metabolomics-based profile in low bone mineral density postmenopausal women. Bone. 2017;95:1–4. doi: 10.1016/j.bone.2016.10.027. [DOI] [PubMed] [Google Scholar]
- 41.Deng D., Pan C., Wu Z., Sun Y., Liu C., Xiang H., Yin P., Shang D. An Integrated Metabolomic Study of Osteoporosis: Discovery and Quantification of Hyocholic Acids as Candidate Markers. Front. Pharmacol. 2021;12:725341. doi: 10.3389/fphar.2021.725341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cao X., Deng L., Zhou G., Wang L., Han Y., Li G. Disorder of serum lipid metabolism in patients with postmenopausal osteoporosis based on untargeted lipidomics. Int. J. Clin. Exp. Med. 2021;14:789–799. [Google Scholar]
- 43.Kou J., He C., Cui L., Zhang Z., Wang W., Tan L., Liu D., Zheng W., Gu W., Xia N. Discovery of Potential Biomarkers for Postmenopausal Osteoporosis Based on Untargeted GC/LC-MS. Front. Endocrinol. 2022;13:849076. doi: 10.3389/fendo.2022.849076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang H., Zhu K., Du J., Ou M., Hou J., Wang D., Wang J., Zhang W., Sun G. Serum concentrations of neonicotinoids and their characteristic metabolites in elderly population from South China: Association with osteoporosis. Environ. Res. 2022;203:111772. doi: 10.1016/j.envres.2021.111772. [DOI] [PubMed] [Google Scholar]
- 45.Zhao Q., Shen H., Su K.J., Zhang J.G., Tian Q., Zhao L.J., Qiu C., Zhang Q., Garrett T.J., Liu J., et al. Metabolomic profiles associated with bone mineral density in US Caucasian women. Nutr. Metab. 2018;15:57. doi: 10.1186/s12986-018-0296-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.You Y.S., Lin C.Y., Liang H.J., Lee S.H., Tsai K.S., Chiou J.M., Chen Y.C., Tsao C.K., Chen J.H. Association between the metabolome and low bone mineral density in Taiwanese women determined by (1)H NMR spectroscopy. J. Bone Miner. Res. 2014;29:212–222. doi: 10.1002/jbmr.2018. [DOI] [PubMed] [Google Scholar]
- 47.Miyamoto T., Hirayama A., Sato Y., Koboyashi T., Katsuyama E., Kanagawa H., Fujie A., Morita M., Watanabe R., Tando T., et al. Metabolomics-based profiles predictive of low bone mass in menopausal women. Bone Rep. 2018;9:11–18. doi: 10.1016/j.bonr.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yin S., Wang J.W., Zhang Y.F., Ma Y., Zhu T.C., Chen H., Wang G.X., Dou W.W., Su Q.J. Screening study of the serum metabolite biomarkers in osteoporosis patients with liver-kidney Yin deficiency syndrome by UPLC-MS. Chin. J. Osteoporos. 2021;27:1316–1322. [Google Scholar]
- 49.Zhu T.C. Master’s Thesis. Nanjing University of Traditional Chinese Medicine; Nanjing, China: 2020. Clinical Metabolomics of Primary Osteoporosis Based on Yin/Yang Deficiency Syndrome. [Google Scholar]
- 50.Li X.F. Master’s Thesis. Shandong University of Traditional Chinese Medicine; Nanjing, China: 2019. Research of Common Cyndrome Factors for Postmenopausal Osteoporosis Based on Metabolomics. [Google Scholar]
- 51.Guo S.N., Qi X.N., Yao X.S., Ren L., Chen W.N. Metabolomics analysis based on UPLC-MS /MS in primary osteoporosis with Kidney-Yang deficiency syndrome. Chin. J. Osteoporos. 2022;28:1410–1415. [Google Scholar]
- 52.Suzuki A., Iwata J. Amino acid metabolism and autophagy in skeletal development and homeostasis. Bone. 2021;146:115881. doi: 10.1016/j.bone.2021.115881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cui Z., Feng H., He B., He J., Tian Y. Relationship Between Serum Amino Acid Levels and Bone Mineral Density: A Mendelian Randomization Study. Front. Endocrinol. 2021;12:763538. doi: 10.3389/fendo.2021.763538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Panahi N., Fahimfar N., Roshani S., Arjmand B., Gharibzadeh S., Shafiee G., Migliavacca E., Breuille D., Feige J.N., Grzywinski Y., et al. Association of amino acid metabolites with osteoporosis, a metabolomic approach: Bushehr elderly health program. Metabolomics. 2022;18:63. doi: 10.1007/s11306-022-01919-2. [DOI] [PubMed] [Google Scholar]
- 55.Shen L., Hu G., Karner C.M. Bioenergetic Metabolism In Osteoblast Differentiation. Curr. Osteoporos. Rep. 2022;20:53–64. doi: 10.1007/s11914-022-00721-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Da W., Tao L., Zhu Y. The Role of Osteoclast Energy Metabolism in the Occurrence and Development of Osteoporosis. Front. Endocrinol. 2021;12:675385. doi: 10.3389/fendo.2021.675385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bihuniak J.D., Insogna K.L. The effects of dietary protein and amino acids on skeletal metabolism. Mol. Cell Endocrinol. 2015;410:78–86. doi: 10.1016/j.mce.2015.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tanimoto H., Fox T., Eagles J., Satoh H., Nozawa H., Okiyama A., Morinaga Y., Fairweather-Tait S.J. Acute effect of poly-gamma-glutamic acid on calcium absorption in post-menopausal women. J. Am. Coll. Nutr. 2007;26:645–649. doi: 10.1080/07315724.2007.10719642. [DOI] [PubMed] [Google Scholar]
- 59.Nam S.Y., Yoou M.S., Kim H.M., Jeong H.J. Efficacy of proline in the treatment of menopause. Exp. Biol. Med. 2016;241:611–619. doi: 10.1177/1535370216629011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Srivastava A.K., Khare P., Nagar H.K., Raghuwanshi N., Srivastava R. Hydroxyproline: A Potential Biochemical Marker and Its Role in the Pathogenesis of Different Diseases. Curr. Protein Pept. Sci. 2016;17:596–602. doi: 10.2174/1389203717666151201192247. [DOI] [PubMed] [Google Scholar]
- 61.da Silva R.A., de Camargo Andrade A.F., da Silva Feltran G., Fernandes C., de Assis R.I.F., Ferreira M.R., Andia D.C., Zambuzzi W.F. The role of triiodothyronine hormone and mechanically-stressed endothelial cell paracrine signalling synergism in gene reprogramming during hBMSC-stimulated osteogenic phenotype in vitro. Mol. Cell Endocrinol. 2018;478:151–167. doi: 10.1016/j.mce.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 62.Yamada M., Tsukimura N., Ikeda T., Sugita Y., Att W., Kojima N., Kubo K., Ueno T., Sakurai K., Ogawa T. N-acetyl cysteine as an osteogenesis-enhancing molecule for bone regeneration. Biomaterials. 2013;34:6147–6156. doi: 10.1016/j.biomaterials.2013.04.064. [DOI] [PubMed] [Google Scholar]
- 63.Brakspear K.S., Mason D.J. Glutamate signaling in bone. Front. Endocrinol. 2012;3:97. doi: 10.3389/fendo.2012.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee W.C., Guntur A.R., Long F., Rosen C.J. Energy Metabolism of the Osteoblast: Implications for Osteoporosis. Endocr. Rev. 2017;38:255–266. doi: 10.1210/er.2017-00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sun J., Pan Y., Li X., Wang L., Liu M., Tu P., Wu C., Xiao J., Han Q., Da W., et al. Quercetin Attenuates Osteoporosis in Orchiectomy Mice by Regulating Glucose and Lipid Metabolism via the GPRC6A/AMPK/mTOR Signaling Pathway. Front. Endocrinol. 2022;13:849544. doi: 10.3389/fendo.2022.849544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tian L., Yu X. Lipid metabolism disorders and bone dysfunction-interrelated and mutually regulated (review) Mol. Med. Rep. 2015;12:783–794. doi: 10.3892/mmr.2015.3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.During A. Osteoporosis: A role for lipids. Biochimie. 2020;178:49–55. doi: 10.1016/j.biochi.2020.08.004. [DOI] [PubMed] [Google Scholar]
- 68.Wang T., He C. TNF-α and IL-6: The Link between Immune and Bone System. Curr. Drug Targets. 2020;21:213–227. doi: 10.2174/1389450120666190821161259. [DOI] [PubMed] [Google Scholar]
- 69.Yang S., Feskanich D., Willett W.C., Eliassen A.H., Wu T. Association between global biomarkers of oxidative stress and hip fracture in postmenopausal women: A prospective study. J. Bone Miner. Res. 2014;29:2577–2583. doi: 10.1002/jbmr.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kwak H.B., Lee S.W., Li Y.J., Kim Y.A., Han S.Y., Jhon G.J., Kim H.H., Lee Z.H. Inhibition of osteoclast differentiation and bone resorption by a novel lysophosphatidylcholine derivative, SCOH. Biochem. Pharmacol. 2004;67:1239–1248. doi: 10.1016/j.bcp.2003.10.032. [DOI] [PubMed] [Google Scholar]
- 71.Jung H.J., Im S.S., Song D.K., Bae J.H. Effects of chlorogenic acid on intracellular calcium regulation in lysophosphatidylcholine-treated endothelial cells. BMB Rep. 2017;50:323–328. doi: 10.5483/BMBRep.2017.50.6.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shi Y. The investigation of energy metabolism in osteoblasts and osteoclasts. Hua Xi Kou Qiang Yi Xue Za Zhi. 2021;39:501–509. doi: 10.7518/hxkq.2021.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu B., Shi J., Li Z., Zhang C., Liu P., Yao W., Jia T. Study on Neuroendocrine-Immune Function of Cistanche deserticola and Its Rice Wine Steaming Products in Glucocorti-coid-Induced Rat Model. Evid. Based Complement. Altern. Med. 2020;2020:5321976. doi: 10.1155/2020/5321976. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.