Abstract
Syzygium cumini L. is an evergreen tree belonging to family Myrtaceae, employed for different traditional uses like diabetes, inflammation, and fever. The current study aimed to compare the chemical compositions of the essential oils (EOs) isolated from different organs of Syzygium cumini (leaves (Scl), fruits (Scf), seeds (Scs), and bark (Scb)) using a GC/MS analysis. Also, a chemometric analysis was applied to explore the main similarities and differences among different organs using a Principal Component Analysis (PCA) and a hierarchal cluster analysis (HCA). Furthermore, in vitro antiaging activities were investigated via anti-collagenase, anti-elastase, and anti-hyaluronidase assays. The GC-MS analysis revealed 82 compounds representing 92.13%, 99.42%, 100%, and 92.97% in Scl, Scf, Scs, and Scb, respectively. The predominant components were α-pinene, β-pinene, (E)-β-caryophyllene, α-caryophyllene, caryophyllene oxide, and α-humulene epoxide II with variable percentages. All EOs were positioned on positive PC1, except for Scs, which was positioned on the negative side in a separate quadrant. The HCA dendrogram displayed the closeness of Scl and Scb, which was not clearly recognized in the PCA score plot. Moreover, the Scs oils were totally discriminated from other parts. The Scl and Scs oils showed superior anti-collagenase, anti-elastase, and anti-hyaluronidase activities. Thus, S. cumini oils should be considered for cosmetic preparations to retard skin aging manifestations.
Keywords: antiaging, anti-collagenase, anti-elastase, essential oils, GC/MS, HCA, PCA, Syzygium cumini
1. Introduction
The aging process is a biochemical process caused by oxidative stress, which is attributed to endogenous free radicals, which leads to the progression of age-related manifestations [1]. Reactive oxygen species (ROS) or free radicals are reported to cause a change in the skin cell composition as well as damage to the cell membranes, leading to both DNA damage and cell death [2]. Furthermore, ROS play crucial roles in the aging process by destroying the skin’s major proteins like elastin and collagen via the activation of elastase and collagenase enzymes. Moreover, ROS causes the degradation of hyaluronic acid via the activation of hyaluronidases, which prevent proper skin hydration [3]. Antioxidants are the natural protection compounds found in the skin and scavenge the excessive free radicals in the body. When the load of ROS in the body is expressively higher than that of natural antioxidants, this leads to a condition called oxidative stress. Therefore, the use of external antioxidants from sources like diet or pharmaceuticals to neutralize ROS and counter the aging process is highly important [4]. Various studies have reported that natural products have many important biological roles such as antibacterial [5], antifungal [6], and anticancer activities [7], as well as their abilities to significantly contribute to the total antiaging and antioxidant activities in various plants [8]. This led to an increase in the interest to investigate antiaging in terms of the activities from different natural sources [9,10].
Plant essential oils are well known in traditional medicine as well as in aromatherapy for the management of numerous diseases [11,12,13,14]. Essential oils extracted from different plant sources were reported to possess antioxidant and antiaging properties [15,16]. Essential oils have also been reported as promising agents for the treatment of neurodegenerative diseases because they possess strong free radical scavenging activity, and hence, cause the inhibition of oxidative stress in the body [17,18].
Syzygium cumini L. Skeels (common name: Pamposia or Jamun) is an evergreen tree that belongs to the family Myrtaceae [19]. It is widely allocated in India and in Southeast Asian countries [20]. Traditionally, different parts of S. cumini have been employed for the treatment of various ailments. The leaves are utilized as remedies for indigestion, dysentery, stomach pain, and diabetes [21,22]. In addition. The leaves are applied as anti-inflammatory, antipyretic, and antiemetic agents [23,24,25]. However, the fruits have been used for gastric complaints as anti-flatulent and stomachic agents and for the treatment of dysentery [20]. Moreover, the fruits have been employed for the treatment of cough, inflammation, and diabetes [22,26]. Regarding S. cumini seeds, the powdered seeds have been described for dysentery and diabetes [20,27], and they have also been applied externally to cure sores and ulcers [28]. The decoction of the bark has been utilized to cure diabetes [29], dysentery, headache, and to improve appetite [26], in addition to the treatment of recurrent miscarriages [28].
Various studies have reported the effectiveness of the essential oil of S. cumini leaves as an antifungal [19,30,31], antibacterial, and antioxidant agent [31,32,33,34,35]. Moreover, the essential oil was reported to have molluscicidal, lishmanicidal [23,36,37], anti-inflammatory [38,39], larvicidal [40], and cytotoxic activities [37]. Few studies have been concerned with the biological effect of the essential oils of fruits, barks, and seeds, where most of the studies were conducted on the various extracts. Moresco et al. proved that the aqueous extract of S. cumini seeds have anti-inflammatory and antioxidant activities [41]. However, the alcoholic extract exhibited antioxidant and antipyretic activities [20]. Fruit extracts have been reported to exhibit anticancer, antioxidant [20,42,43], and hypoglycemic activities [44]. The aqueous, ethanol, and n-hexane extracts from the leaves, fruit, and bark of Syzygium cumini (L.) displayed antifungal activities [30]. The fruit pulp of Syzygium cumini (L.) Skeels has been reported for its protective effects against anxiety and dementia in aged mice [45], and this may be attributed to the plant’s antioxidant properties [46].
In light of prior research demonstrating the antioxidant properties of Syzygium cumini essential oils, this study not only aims to investigate the potential antiaging effects of essential oils obtained from Syzygium cumini leaves but also to comprehensively analyze the chemical composition and investigate the antiaging properties of oils extracted from other plant organs, including fruits, bark, and seeds. This study represents the first comparative metabolic analysis of essential oil compositions from various Syzygium cumini plant organs, utilizing GC/MS profiling coupled with chemometric analysis, specifically Principal Component Analysis (PCA) and Hierarchical Cluster Analysis (HCA), to identify the key similarities and differences among these plant organs. Furthermore, this study seeks to assess the antiaging potentials of these essential oils through anti-collagenase, anti-elastase, and anti-hyaluronidase inhibition assays.
2. Results and Discussion
2.1. GC/MS Analysis of the Essential Oils
A GC-MS analysis of the essential oils (EOs) isolated from different organs of Syzygium cumini could detect 82 compounds representing 92.13%, 99.42%, 100%, and 92.97% of Syzygium cumini leaves (Scl), fruits (Scf), seeds (Scs), and bark (Scb), respectively, as illustrated in Table 1. α-pinene, β-pinene, (E)-β-caryophyllene, α-caryophyllene, caryophyllene oxide, and α-humulene epoxide II were the main identified components in all of the studied organs with variable percentages. Caryophyllene oxide (17.24%) and α-terpineol (12.31%) are the major identified components in the leaf EOs, with small amounts of α-humulene epoxide II (8.27%) and bornyl acetate (5.85%). Regarding the EOs of fruits, the main detected compounds are caryophyllene oxide (22.63%), (E)-β-caryophyllene (13.6%), α-pinene (13.3%), α-terpineol (12.48%), α-caryophyllene (10.05%), α-humulene epoxide II (7.62%), and β-pinene (6.58%). However, (E)-β-caryophyllene (60.79%) and α-caryophyllene (25.15%) are the key components comprising the seed oil with little amounts of α-pinene (5.47%) and β-pinene (5.20%). For the stem bark, its essential oil is characterized by the presence of caryophyllene oxide (26.92%), α-humulene epoxide II (10.44%), α-pinene (7.29%), and α-terpineol (6.79%).
Table 1.
Chemical compositions of the essential oils based on GC/MS analysis (n = 3).
| No. | KI | Compound | Relative Abundance% | ||||
|---|---|---|---|---|---|---|---|
| Cal. | Rep. | Scl | Scf | Scs | Scb | ||
| 1. | 932 | 939 | α-pinene | 0.69 | 13.13 | 5.47 | 7.92 |
| 2. | 946 | 946 | Camphene | - | 0.55 | - | 0.21 |
| 3. | 972 | 971 | α-thujene | 0.86 | - | - | - |
| 4. | 974 | 974 | β-pinene | 0.45 | 6.58 | 5.20 | 3.34 |
| 5. | 991 | 991 | β-Myrecene | - | 0.42 | - | 0.06 |
| 6. | 1024 | 1028 | o-Cymene | 0.48 | - | - | 0.36 |
| 7. | 1028 | 1029 | D-Limonene | 1.69 | 2.67 | - | 4.08 |
| 8. | 1059 | 1060 | γ-terpinene | 0.21 | - | - | - |
| 9. | 1071 | 1071 | Linalool oxide | - | - | - | 0.05 |
| 10. | 1097 | 1097 | α-Pinene epoxide | - | - | - | 0.34 |
| 11. | 1100 | 1100 | Linalool | 0.48 | - | - | 0.7 |
| 12. | 1106 | 1105 | trans-para-Mentha-2,8-dien-1-ol | 0.44 | - | - | 0.46 |
| 13. | 1113 | 1111 | Fenchol | 1.12 | 0.62 | - | 0.68 |
| 14. | 1122 | 1122 | cis-2-p-Menthen-1-ol | 0.28 | - | - | 0.20 |
| 15. | 1126 | 1125 | α-Campholenal | 0.13 | - | - | 0.17 |
| 16. | 1134 | 1134 | Limonene 1,2-epoxide | - | - | - | 0.12 |
| 17. | 1139 | 1139 | (-)-trans-Pinocarveol | 0.67 | 0.5 | - | 0.78 |
| 18. | 1145 | 1143 | cis-Verbenol | 0.75 | - | - | 0.27 |
| 19. | 1148 | 1148 | Camphene hydrate | 0.77 | - | - | 0.41 |
| 20. | 1163 | 1164 | Pinocarvone | 0.12 | 0.43 | - | 0.31 |
| 21. | 1166 | 1166 | Endo-Borneol | 0.85 | 0.57 | - | 0.59 |
| 22. | 1178 | 1182 | Terpinen-4-ol | 1.60 | - | - | 0.45 |
| 23. | 1187 | 1188 | p-Cymen-8-ol | 1.48 | - | - | 0.22 |
| 24. | 1193 | 1192 | α-Terpineol | 12.31 | 12.48 | - | 6.79 |
| 25. | 1198 | 1198 | Myrtenal | 0.73 | 0.75 | - | 0.70 |
| 26. | 1210 | 1210 | Verbenone | 0.40 | - | - | 0.29 |
| 27. | 1221 | 1220 | α-Fenchyl acetate | 1.45 | - | - | 0.58 |
| 28. | 1224 | 1227 | 2-Hydroxy-1,8-cineole | 0.32 | - | - | 0.06 |
| 29. | 1243 | 1243 | p-Cumic aldehyde | 0.22 | - | - | - |
| 30. | 1247 | 1246 | Carvone | 0.11 | - | - | 0.25 |
| 31. | 1277 | 1274 | p-Menth-1-en-7-al | 1.75 | - | - | 0.12 |
| 32. | 1287 | 1287 | Bornyl acetate | 5.85 | - | - | 1.5 |
| 33. | 1294 | 1293 | p-Cymen-7-ol | 0.23 | - | - | - |
| 34. | 1301 | 1297 | trans-Pinocarvyl acetate | 0.42 | - | - | 0.52 |
| 35. | 1306 | 1305 | Carvacrol | 0.61 | - | - | 0.39 |
| 36. | 1351 | 1351 | α-Terpinyl acetate | 0.14 | - | - | - |
| 37. | 1379 | 1379 | α-Copaene | 0.17 | - | - | 0.1 |
| 38. | 1385 | 1385 | Geranyl acetate | - | - | - | 0.22 |
| 39. | 1423 | 1422 | trans-β-Caryophyllene | 1.35 | 13.6 | 60.79 | 0.34 |
| 40. | 1431 | 1432 | α-Ionone | 0.19 | - | - | 0.16 |
| 41. | 1443 | 1447 | Aromandendrene | 0.1 | - | - | 0.15 |
| 42. | 1458 | 1459 | α-Caryophyllene | 1.33 | 10.05 | 25.15 | 0.38 |
| 43. | 1466 | 1466 | Alloaromadendrene | 0.31 | - | - | - |
| 44. | 1474 | 1479 | Aristolochene | 0.53 | - | - | 0.20 |
| 45. | 1480 | 1480 | γ-Muurolene | 0.33 | 4.04 | - | 0.15 |
| 46. | 1492 | 1492 | β-Selinene | 0.76 | - | 0.51 | 0.15 |
| 47. | 1504 | 1504 | α-Muurolene | 0.16 | - | - | 0.71 |
| 48. | 1528 | 1528 | γ-Cadinene | 1.56 | - | 1.14 | 0.47 |
| 49. | 1559 | 1552 | Isopatchoulane | - | - | - | 0.76 |
| 50. | 1567 | 1567 | trans-(E)-Nerolidol | 1.03 | - | - | 0.23 |
| 51. | 1577 | 1581 | Palustrol | 1.93 | 0.60 | - | 0.58 |
| 52. | 1586 | 1586 | (+)-Spathulenol | 4.49 | - | - | 0.32 |
| 53. | 1592 | 1592 | Caryophyllene oxide | 17.24 | 22.63 | 1.27 | 26.92 |
| 54. | 1599 | 1598 | Viridiflorol | 0.32 | - | - | 0.41 |
| 55. | 1609 | 1608 | Epiglobulol | 2.39 | 1.26 | - | 0.58 |
| 56. | 1618 | 1613 | α-Humulene epoxide II | 8.27 | 7.62 | 0.46 | 10.44 |
| 57. | 1640 | 1641 | Caryophylla-4(12),8(13)-dien-5α-ol | 1.59 | 0.88 | - | 1.55 |
| 58. | 1644 | 1644 | Caryophylla-4(12),8(13)-dien-5β-ol | 0.62 | - | - | 0.87 |
| 59. | 1648 | 1648 | τ-Cadinol | 0.38 | - | - | 0.55 |
| 60. | 1653 | 1652 | δ-Cadinol | 0.1 | - | - | 0.14 |
| 61. | 1659 | 1655 | trans-Guai-11-en-10-ol | 1.49 | - | - | 1.41 |
| 62. | 1678 | 1679 | α-Bisabolene oxide | 0.76 | - | - | 2.1 |
| 63. | 1731 | 1733 | (Z)-α-Bisabolene epoxide | 0.54 | - | - | 1.05 |
| 64. | 1744 | 1746 | Bisabolone | 0.22 | - | - | 0.47 |
| 65. | 1785 | 1789 | β-bisabolen-15-ol | 0.43 | - | - | 0.63 |
| 66. | 1839 | 1838 | Hexahydro farnesyl acetone | - | - | - | 0.31 |
| 67. | 1971 | 1961 | Cembrene A | 0.34 | - | - | - |
| 68. | 2026 | 2033 | Kaur-16-ene | 0.49 | - | - | - |
| 69. | 2084 | 2085 | 1-Octadecanol | - | - | - | 0.07 |
| 70. | 2097 | 2100 | n-Heneicosane | - | - | - | 0.1 |
| 71. | 2114 | 2114 | Phytol | 0.27 | - | - | - |
| 72. | 2128 | 2120 | Phenethyl anthranilate | 0.28 | - | - | 0.34 |
| 73. | 2155 | 2161 | Cembrenol | 0.32 | - | - | - |
| 74. | 2169 | 2158 | incensole | 3.41 | - | - | - |
| 75. | 2197 | 2200 | n-Docosane | - | - | - | 0.13 |
| 76. | 2297 | 2300 | n-Tricosane | 0.13 | - | - | 0.43 |
| 77. | 2303 | 2302 | Methyl cis-11-eicosenoate | 0.12 | - | - | - |
| 78. | 2397 | 2400 | n-Tetracosane | - | - | - | 0.32 |
| 79. | 2496 | 2500 | n-Pentacosane | 0.2 | - | - | 0.87 |
| 80. | 2696 | 2700 | n-Heptacosane | - | - | - | 1.53 |
| 81. | 2895 | 2900 | n-Nonacosane | 0.21 | - | - | 1.77 |
| 82. | 3095 | 3100 | n-Hentriacontane | - | - | - | 1.38 |
| Total identified compounds% | 92.13% | 99.42% | 100% | 92.97% | |||
Compounds were identified based on the compounds’ mass spectral data and retention indices compared with those of the NIST Mass Spectral Library (December 2011), the Wiley Registry of Mass Spectral Data, 8th edition, and many authentic standards. The content (%) was calculated in triplicate using the normalization method based on the GC-MS data. The presented data are shown as the average of three replicas. (-): Unidentified in the sample. Standard deviation did not exceed 3% for any of the values. KI: Kovats index calculated on Rtx-5MS column. (Scl) S. cumini leaves, (Scf) S. cumini fruits, (Scs) S. cumini seeds, (Scb) S. cumini bark.
Our results were slightly different from that reported in the literature; this may be attributed to various factors such as the geographical source, harvesting period, method of oil isolation, environment, and growth conditions that may affect the essential oil composition [11,47]. In Egypt, various authors reported the leaf essential oil composition of S. cumini, where Elansary et al. reported α-pinene (17.53%), α-terpineol (16.67%), and alloocimene (13.55%) as its major components [33]. However, in other studies, the abundant constituents of the oils were α-pinene (32.32%), β-pinene (12.44%), and (E)-caryophyllene (11.19%) [34]. In accordance, Badawy et al. also recognized α-pinene (17.26%) and β-pinene (11.28%) as the main constituents in the leaf oil in addition to α-terpineol (13.88%) [31]. It was noticed that α-pinene, β-pinene, and (E)-caryophyllene were present in higher percentages in the literature when compared to our results; however, α-terpineol was closely related to the results reported in the literature. Also, a recent study carried out by El-Nashar et al. reported α-pinene (21.09%), β-(E)-ocimene (11.80%), D-limonene (8.08%), β-pinene (7.33%), and α-terpineol (5.38%) as predominant components [11].
Several studies reported the leaf essential oil constituents of S. cumini growing in Brazil, where α-pinene (31.85%), (Z)-β-ocimene (28.98%), and (E)-β-ocimene (11.71%) were the major detected ones [23]. However, α-caryophyllene (25.24%), β-caryophyllene (16%), and α-terpineol (9.08%), together with α-pinene (21.20%), globulol (15.30%), eugenol (11.20%), and α-terpineol (8.88%), were the key components [37] in other reported studies [38]. On the contrary, the leaf essential oil composition of S. cumini growing in India exhibited significant variations, where α-humulene (12.30%), in addition to β-caryophyllene (6.34%) and α-terpineol (5.71%), were the major detected constituents [48]. In another study, the principal components were α-pinene (21.5%), α-terpinessential oil (9.5%), δ-cadinene (8.3%), and (E)-ocimene (6.8%), [49]. Saroj et al. reported that α-pinene (17.2%), β-pinene (8.6%), (Z)-β-ocimene (10.9%), (E)-β-ocimene (9.6%), and δ-cadinene (7.5%) were identified as major constituents [19]. However, τ-cadinol (21.44%) and τ-muurolol (12.01%), globulol (7.98%), caryophyllene (6.69%), δ-cadinene (6.56%), and α-pinene (6.32%) were also found [50].
Concerning the fruits, (Z)-ocimene (29.95%), (E)-ocimene (23.03%), β-myrcene (6.99%), and α-terpineol (6.46%) were the major identified constituents in the essential oil isolated from the pulp [51]. Similarly, the major compounds identified in unripe fruit pulp were (E)-β-ocimene (25.59%), β-ocimene (16.83%), caryophyllene (20.31%), and humulene (12.83%) [52]. However, in another study, α-cadinol (25.8%), α-pinene (12.4%), β-pinene (8.0%), and myrcene (8.4%) were the principal components isolated [49].
2.2. Chemometric Analysis of the Essential Oils
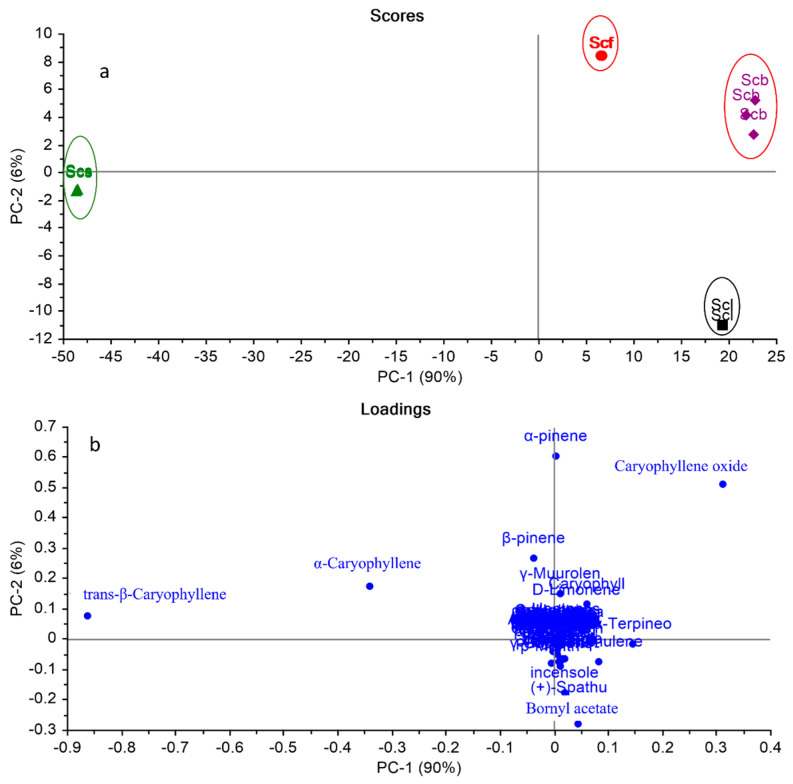
As observed in Table 1, the GC-MS data displayed both qualitative and quantitative variations among different organs that cannot be interpreted by the naked eye. The application of a multivariate analysis using a principal component analysis (PCA) and a hierarchal cluster analysis (HCA) enables the exploration of the similarities and differences between various samples based on the GC/MS data analysis [53,54]. A matrix of the total number of samples and their replicates (12 samples) multiplied by 82 variables (GC/MS peak area%) was constructed in MS Excel® (Microsoft 365), and then exposed to multivariate analyses (PCA and HCA). Figure 1a,b display the PCA score and loading plots based on the GC-MS analysis of the chemical compositions of the essential oils of various organs of S. cumini, respectively.
Figure 1.
PCA score plot (a) and loading plot (b) based on GC-MS identification of the chemical compositions of the essential oils of various organs of Syzygium cumini. Abbreviations: (Scf) S. cumini, (Scl) S. cumini leaves, (Scf) S. cumini fruits, (Scs) S. cumini seeds, (Scb) S. cumini bark.
The PCA score plot (Figure 1a) described about 96% of the data variance by the first two PCs, where PC1 explained 90% and PC2 explained 6%. Various S. cumini organs were grouped into four main clusters on three different quadrants. All S. cumini organs were positioned on positive PC1, except for the seeds that were positioned on the negative side in a separate quadrant. It was noticed that the S. cumini leaves (Scl) were placed on the lower right quadrant, completely discriminated from the other organs. Although the fruits (Scf) and the barks (Scb) were placed on the same quadrant (positive PC1 and PC2), they were clearly separated from each other. The loading plot (Figure 1b) displayed the key discriminating markers responsible for the PCA score plot pattern. (E)-β-Caryophyllene was the main marker responsible for the separation of S. cumini seeds (Scs) in a separate quadrant. Bornyl acetate and (+)-Spathulenol were the major components accountable for the segregation of leaves (Scl) in a single quadrant. Although caryophyllene oxide presents in approximately the same percentage in both Scf and Scb, it was observed that it applies a high load for the segregation of the barks only. However, α-pinene could separate Scf from the bark, although they were in the same quadrant. From the loading plot, it was observed that the key markers responsible for the segregation of various organs are not those that are present in high percentages, which proves the significance of the whole metabolic profile in the discrimination between various organs, and not only the ones that are identified in higher concentrations.
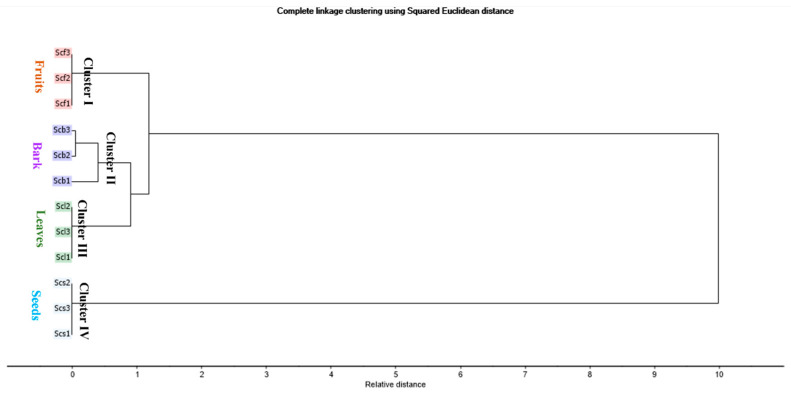
An HCA was applied to explore the closeness of various organs based on the relative measured distance. The dendrogram (Figure 2) revealed the segregation of various organs into four main clusters. Cluster I, II, III, and IV displayed Scf, Scb, Scl, and Scs, respectively. The HCA dendrogram disclosed the closeness of the barks and the leaves, which was not clearly recognized in the PCA score plot. Moreover, the HCA dendrogram confirmed that Scs was totally discriminated from the other parts.
Figure 2.
HCA dendrogram based on GC-MS identification of the chemical compositions of the essential oils of various organs of Syzygium cumini (leaves, fruits, bark stems, and seeds), as displayed in Table 1.
2.3. Assessment of Anti-Collagenase, Anti-Elastase, and Anti-Hyaluronidase Activities
Collagen and elastin are vital protein components of the skin epidermis that play important roles in sustaining the elasticity of the skin [55]. Collagen makes up 70–80% of the skin’s dry weight and provides the dermis with structural and mechanical integrity. Although elastin is a minor component of the skin (2–4% of the extra cellular matrix), it has a significant role in providing the skin with its elasticity [56]. Elastin forms elastic fibers, which provide the stretch and recoil of the skin [57]. Elastin has a low rate of turnover, which causes it to be susceptible to loss over time [58]. Both collagen and elastic fibers show marked alterations with age in their three-dimensional arrangements. The synthesis of collagen reduces gradually, resulting in the decrease and disorganization in collagen in aged skin [59].
Skin aging is also caused by moisture loss in the skin. Hyaluronic acid is the key molecule involved in skin moisture with a unique ability to retain and bind water molecules [60]. The levels of elastin, collagen, as well as hyaluronic acid decrease gradually in the aging process, hence contributing to the loss of skin integrity and elasticity [61].
Many factors such as aging, the excessive exposure to UV light, as well as oxidative stress result in the activation of hydrolyzing enzymes like collagenase, elastase, and hyaluronidase, which will lead to the development of wrinkles and irregularities in the skin tone [62]. Therefore, the inhibition of collagenase, elastase, and hyaluronidase activities is one of the effective approaches to protect the skin from aging manifestations [63].
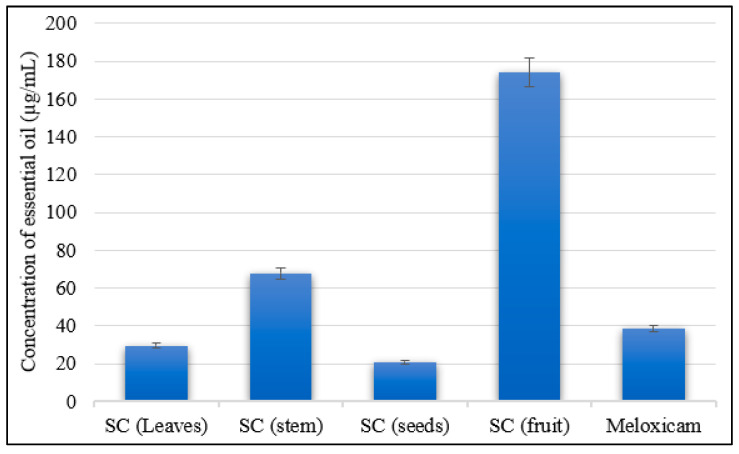
As clarified in Figure 3, S. cumini seeds’ and leaves’ essential oils showed significant and remarkable anti-collagenase activities with IC50 values of 20.80 µg/mL and 29.39 n µg/mL, respectively, showing superior inhibitory effects compared to Meloxicam (IC50 = 38.48 µg/mL) as a standard drug. On the other hand, S. cumini bark essential oil showed moderate anti-collagenase activity with an IC50 value of 67.96 µg/mL, and S. cumini fruit essential oil exhibited weak anti-collagenase activity among all essential oil samples, with an IC50 value of 173.90 µg/mL, compared to the reference standard.
Figure 3.
Collagenase inhibitory activity of the essential oils of various organs of Syzygium cumini (leaves, fruits, bark stems, and seeds).
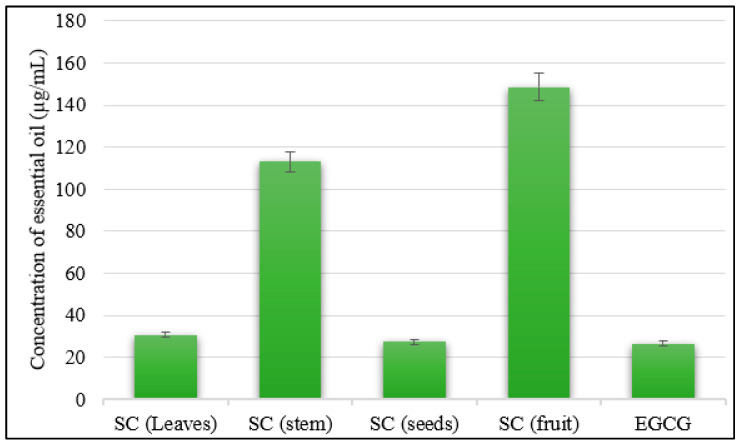
Similarly, the anti-elastase effects of S. cumini seeds’ and leaves’ essential oils showed significant results, with IC50 values of 27.44 µg/mL and 30.82 µg/mL, respectively, compared to epigallocatechin gallate (EGCG) (IC50 = 26.43 µg/mL) as the standard (Figure 4). On the other hand, the S. cumini stem bark and fruit essential oils showed lower inhibitions to the elastase effect, with IC50 values of 113.00 µg/mL and 148.50 µg/mL, respectively, compared to the reference standard.
Figure 4.
Elastase inhibitory activities of the essential oils of various organs of Syzygium cumini (leaves, fruits, bark stems, and seeds).
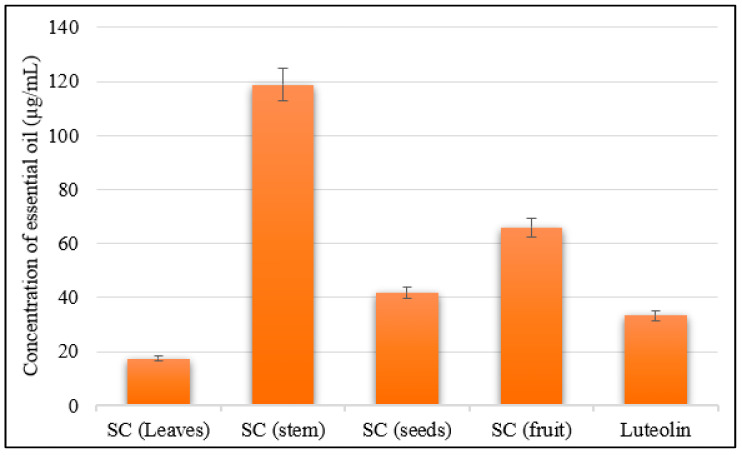
Moreover, among the four S. cumini essential oil samples, the leaf essential oil showed the highest inhibitory activity against the hyaluronidase enzyme, with an IC50 value of 17.35 µg/mL, showing greater inhibition compared to Luteolin (IC50 = 33.25 µg/mL) as a reference standard control. The seed essential oil exhibited a remarkable anti- hyaluronidase effect with an IC50 value of 41.74 µg/mL. The fruit oil showed a moderate anti-hyaluronidase effect with an IC50 value of 65.91 µg/mL, while the stem bark essential oil exhibited the least inhibitory effect (IC50 = 118.70 µg/mL) compared with the reference control (Figure 5).
Figure 5.
Hyaluronidase inhibitory activities of the essential oils of various organs of Syzygium cumini (leaves, fruits, bark stems, and seeds).
In line with these results, a remarkable anti-collagenase activity of the essential oil isolated from another species of Syzygium coriaceum was previously reported with an IC50 value of 0.84 mg/mL [64]. Further prior studies have reported the antioxidant effects of S. cumini leaves’ essential oils (0.47 mg/100 mg) with a ferric reducing effect [34] and 1,1-diphenyl-2-picrylhydrazy (DPPH) radical scavenging activity, with a total antioxidant activity of 11.13% [33]. Further, a previous study reported the antioxidant activities of S. cumini fruits due to their richness in anthocyanins and carotenoids [65]. Moreover, S. cumini seeds were also reported for their antioxidant effects [66]. In addition to that, the antiaging activities of the essential oils derived from the leaves of the plant may be attributed to the high abundance of caryophyllene oxide (17.24%) in the oil; caryophyllene oxide was reported previously for its antiaging and antioxidant effects, where it showed strong cholinesterase inhibitory activity [67], and this was further evidenced by its significant binding interactions as well as docking scores with the acetylcholinesterase enzyme [68]. Moreover, the essential oil derived from the seeds of the plant showed significant anti-elastase as well as anti-collagenase activities, and this also may be attributed to the richness of the oil with β-caryophyllene (60.79%), which was reported for its antioxidant effects [69,70].
3. Materials and Methods
3.1. Plant Material
Different plant organs of Syzygium cumini (leaves, fruits, seeds, and bark stems) were collected in May 2022 from a private garden in the Masken Abo-Zabal region (N 30°17′43.386″, E 31°22′27.9804″), Qualiobya, Egypt. The plant was botanically identified by Therease Labib, the taxonomy specialist at the herbarium of the El-Orman Botanical Garden, Giza, Egypt. Voucher specimens were deposited with a code of PHG-P-SC-348 at the herbarium of the Department of Pharmacognosy, Faculty of Pharmacy, Ain Shams University, Cairo, Egypt. The symbols of Scl, Scf, Scs, and Scb are given to Syzygium cumini leaves, fruits, seeds, and bark stems, respectively.
3.2. Isolation of the Essential Oils
About 1 kg of different plant organs of Syzygium cumini (leaves, fruits, bark stems, and seeds) were subjected to hydrodistillation for 4 h using a Clevenger-type apparatus. The oils were collected, desiccated, and then stored at −4 °C in sealed vials, and protected from light for further analysis.
3.3. GC/MS Analysis of Essential Oils
The essential oil compositions of the different studied organs were analyzed via GC chromatograms and mass spectra using a Shimadzu GC/MS-QP 2010 (Kyoto, Japan) coupled to a mass spectrometer (SSQ 7000 quadrupole: Thermo-Finnigan, Bremen, Germany). A capillary column was applied (Rtx-5MS, 30 m length, 0.25 mm internal diameter, and 0.25 µm film thickness; Restek Co., Bellefonte, PA, USA. An initial temperature was adjusted at 45 °C for 2 min with gradual elevation to 300 °C at 5 °C/min for 5 min. The temperatures of the injector and detector were kept at 250 °C and 280 °C, respectively. Essential oil samples were diluted in n-hexane prior to analysis (1% v/v). Sample injection was applied automatically (1 µL, split ratio of 1:15). Helium carrier gas was utilized (flow rate of 1.41 mL/min). The mass spectrometer was carried out under the following conditions: ionization voltage of 70 eV, ion source temperature of 200 °C, and the scan range was adjusted from 35 to 500 m/z. Essential oil compositions were elucidated from their mass spectra and retention indices (RIs) relative to a mixture of a homologous series of standard n-alkanes (C8–C28) injected under the same conditions. The series of standard saturated n-alkanes (C8–C28) were obtained from Sigma-Aldrich Inc. (St. Louis, MO, USA), with a concentration of 1000 µg/mL for each component in hexane, and stored at 2–8 °C.
3.4. Identification of the Oil Components
The components of the essential oils were characterized via a comparison of their GC/MS spectra, fragmentation patterns, and retention indices with those reported in the literature [11,12]. The retention indices were calculated relative to a homologous series of n-alkanes (C8–C28) injected under the same conditions. These data were evaluated with NIST-11, Wiley Registry of Mass Spectral Database, and the data described in the literature [71,72].
3.5. Chemometric Analysis
The data obtained from GC-MS were subjected to chemometric analysis, where a matrix of the total number of samples (4 organs) and their replicates (4 × 3 = 12 samples) multiplied by 82 variables (GC/MS peak area % of the identified compounds) was constructed in MS Excel®, and then exposed to multivariate analyses (PCA and HCA). PCA was first employed as a primary step to explore the similarities and differences among different studied organs and to distinguish the markers responsible for this pattern. A PCA model was performed using cross validation method. Hierarchal cluster analysis (HCA) was then applied to allow for the clustering of different organs. The clustering pattern was created using the complete linkage method, applying squared Euclidean distance. PCA and HCA were accomplished utilizing Unscrambler® X 10.4 software (Computer Aided Modeling, AS, Norway) [73,74].
3.6. Assessment of In Vitro Antiaging Activities
3.6.1. Anti-Collagenase Activity
The inhibitory ability of Syzygium cumini essential oils against collagenase activity was tested using a fluorometric collagenase inhibitor screening kit (BioVision, Waltham City, MA, USA, catalog no. # K833-100) according to the method described previously [75]. Meloxicam was used as the reference control. The control and the tested oil samples were prepared in concentrations of 1, 10, 100, and 1000 μg/mL for the anti-collagenase analysis. A flat-bottom 96-well plate was used to prepare oil samples. The collagenase substrate was dissolved initially in Collagenase Assay Buffer (CAB). Then, preparation of the samples for analysis was performed by mixing them with collagenase as well as CAB. Inhibitor control samples were prepared by mixing the inhibitor Meloxicam (80 mM) with CAB buffer and the diluted collagenase enzyme. Preparation of the enzyme control was performed by mixing both diluted collagenase and CAB together. The background control used was the CAB buffer. All samples were then incubated at 25 °C for 15 min. During this time, mixing of the collagenase with CAB was performed to prepare the reaction mixture. In the next step, the prepared samples were mixed thoroughly with the reaction mixture. The emitted fluorescence was measured using a microplate reader (FilterMax F5, Thermo Fisher, Waltham, MA, USA) at 490 nm excitation wavelength and 520 nm emission wavelength. The fluorescence was measured in kinetic mode for 60 min at 37 °C. All samples were prepared in this method in duplicates, and the collagenase inhibitory activities of the tested samples were calculated by applying the following equation: % relative inhibition = [(enzyme control−sample)/enzyme control] * 100.
3.6.2. Anti-Elastase Activity
Syzygium cumini oil samples were investigated for their anti-elastase activities using EnzChek® Elastase Assay Kit (E-12056, Thermo Fisher, Waltham, MA, USA) according to the method reported previously [76]. The oil samples were prepared in clear-bottom 96-well plates for fluorometric assay. Elastase substrate, elastase enzyme solutions, in addition to the inhibitor control were prepared as per the described method. Diluted elastase solution was firstly added to the wells. Tested oil samples, enzyme control, as well as inhibitor control (Meloxicam) were added to the subsequent wells. All samples were mixed using a shaker and then incubated at 37 °C for 5 min. The fluorometric reaction mix was prepared by mixing the assay buffer with the substrate, which was added and then mixed with each sample. The fluorescence was measured using a microplate reader (FilterMax F5, Thermo Fisher) at 400 nm excitation wavelength and 505 nm emission wavelength. Measurement of the emitted fluorescence was conducted in kinetic mode for 30 min at 37 °C with protection from light. All measured samples were prepared in duplicates, and the anti-elastase inhibitory of the samples was calculated by applying the following equation: % relative inhibition = [ΔRFU (test inhibitor)/ΔRFU (EC)] * 100.
3.6.3. Anti-Hyaluronidase Activity
The anti-hyaluronidase activity was evaluated spectrophotometrically by assessing the N-acetylglucosamine produced from sodium hyaluronate [77]. A total of 50 µL of bovine hyaluronidase (7900 units mL−1, Sigma, Burlington, MA, USA) prepared in 0.1 M acetate buffer was mixed with 100 µL of a designated concentration of the oil sample dissolved in 5% DMSO. In the following step, the prepared reaction mix was then incubated in a water bath for 20 min at 37 °C. The control group was prepared via treatment with 100 µL of 5% DMSO instead of the sample. Luteolin was used as a reference control. The reaction mixture’s optical density was measured spectrophotometrically at 585 nm.
3.7. Statistical Analysis
All analyses were carried out in triplicate. Values are conveyed as means ± SD. Statistical significance was determined using one-way ANOVA followed by Tukey’s post hoc test (significance level at p < 0.05).
4. Conclusions
As a conclusion, we described a detailed comparative study on the chemical compositions of the essential oils isolated from different organs of S. cumini grown in Egypt. All tested oils were found to be plentiful in α-pinene, β-pinene, (E)-β-caryophyllene, α-caryophyllene, caryophyllene oxide, and α-humulene epoxide II. Further, we explored the main similarities and differences among different organs using multivariate chemometric analyses (PCA and HCA). On the biological side, the leaf and seed essential oils exhibited superior inhibitory activities against the enzymes involved in the aging process such as collagenase, elastase, and hyaluronidase, making these essential oils and their major constituents good candidates in adjuvant therapy for anti-skin-aging cosmetic preparations. Moreover, in vivo studies on anti-wrinkle activity are recommended along with toxicity, pharmacokinetics, and pharmacodynamics studies to assemble a molecular mechanistic profile for the isolated essential oils in the management of skin aging, encouraging the utilization of S. cumini essential oils as herbal pharmaceutical products.
Author Contributions
Formal analysis, software, investigation, and writing—original draft, N.S.A.; formal analysis, software, writing—review and editing, and supervision, H.A.G.; conceptualization, resources, methodology, data curation, and formal analysis, H.A.S.E.-N. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available upon request from the corresponding authors: dr.naglaa@gmu.ac.ae (N.S.A.) and heba_pharma@pharma.asu.edu.eg (H.A.S.E.-N.).
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Guerra-Araiza C., Álvarez-Mejía A.L., Sánchez-Torres S., Farfan-García E., Mondragón-Lozano R., Pinto-Almazán R., Salgado-Ceballos H. Effect of natural exogenous antioxidants on aging and on neurodegenerative diseases. Free Radic. Res. 2013;47:451–462. doi: 10.3109/10715762.2013.795649. [DOI] [PubMed] [Google Scholar]
- 2.Pillai S., Oresajo C., Hayward J. Ultraviolet radiation and skin aging: Roles of reactive oxygen species, inflammation and protease activation, and strategies for prevention of inflammation-induced matrix degradation—A review. Int. J. Cosmet. Sci. 2005;27:17–34. doi: 10.1111/j.1467-2494.2004.00241.x. [DOI] [PubMed] [Google Scholar]
- 3.Wölfle U., Seelinger G., Bauer G., Meinke M.C., Lademann J., Schempp C.M. Reactive molecule species and antioxidative mechanisms in normal skin and skin aging. Ski. Pharmacol. Physiol. 2014;27:316–332. doi: 10.1159/000360092. [DOI] [PubMed] [Google Scholar]
- 4.Sivamani R.K., Jagdeo J.R., Elsner P., Maibach H.I. Cosmeceuticals and Active Cosmetics. CRC Press; Boca Raton, FL, USA: 2015. [Google Scholar]
- 5.Ishaq A.R., El-Nashar H.A., Younis T., Mangat M.A., Shahzadi M., Ul Haq A.S., El-Shazly M. Genus Lupinus (Fabaceae): A review of ethnobotanical, phytochemical and biological studies. J. Pharm. Pharmacol. 2022;74:1700–1717. doi: 10.1093/jpp/rgac058. [DOI] [PubMed] [Google Scholar]
- 6.Ashmawy N.S., El-Labbad E.M., Hamoda A.M., El-Keblawy A.A., El-Shorbagi A.-N.A., Mosa K.A., Soliman S.S. The Anti-Candida Activity of Tephrosia apollinea Is More Superiorly Attributed to a Novel Steroidal Compound with Selective Targeting. Plants. 2022;11:2120. doi: 10.3390/plants11162120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El-Nashar H.A., Mostafa N.M., El-Badry M.A., Eldahshan O.A., Singab A.N.B. Chemical composition, antimicrobial and cytotoxic activities of essential oils from Schinus polygamus (Cav.) cabrera leaf and bark grown in Egypt. Nat. Prod. Res. 2021;35:5369–5372. doi: 10.1080/14786419.2020.1765343. [DOI] [PubMed] [Google Scholar]
- 8.El-Nashar H.A., El-Labbad E.M., Al-Azzawi M.A., Ashmawy N.S. A new xanthone glycoside from Mangifera indica L.: Physicochemical properties and in vitro anti-skin aging activities. Molecules. 2022;27:2609. doi: 10.3390/molecules27092609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corrêa R.C., Peralta R.M., Haminiuk C.W., Maciel G.M., Bracht A., Ferreira I.C. New phytochemicals as potential human anti-aging compounds: Reality, promise, and challenges. Crit. Rev. Food Sci. Nutr. 2018;58:942–957. doi: 10.1080/10408398.2016.1233860. [DOI] [PubMed] [Google Scholar]
- 10.El-Shawi O.E., El-Nashar H.A.S., Abd El-Rahman S.S., Eldahshan O.A., Singab A.N.B. Protective effect of Acrocarpus fraxinifolius extract against hepatic fibrosis induced by Gamma irradiation and carbon tetrachloride in albino rats. Int. J. Radiat. Biol. 2023;99:270–280. doi: 10.1080/09553002.2022.2087926. [DOI] [PubMed] [Google Scholar]
- 11.El-Nashar H.A.S., Eldehna W.M., Al-Rashood S.T., Alharbi A., Eskandrani R.O., Aly S.H. GC/MS Analysis of Essential Oil and Enzyme Inhibitory Activities of Syzygium cumini (Pamposia) Grown in Egypt: Chemical Characterization and Molecular Docking Studies. Molecules. 2021;26:6984. doi: 10.3390/molecules26226984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Panda S., Sahoo S., Tripathy K., Singh Y.D., Sarma M.K., Babu P.J., Singh M.C. Essential oils and their pharmacotherapeutics applications in human diseases. Adv. Tradit. Med. 2020;22:1–15. doi: 10.1007/s13596-020-00477-z. [DOI] [Google Scholar]
- 13.El-Nashar H.A.S., Eldahshan O.A., Elshawi O.E., Singab A.N.B. Phytochemical Investigation, Antitumor Activity, and Hepatoprotective Effects of Acrocarpus fraxinifolius Leaf Extract. Drug Dev. Res. 2017;78:210–226. doi: 10.1002/ddr.21395. [DOI] [PubMed] [Google Scholar]
- 14.El-Nashar H., Eldahshan O., Singab A. The tribe Caesalpinieae (Fabaceae): An updated review on pharmacological aspects. Med. Aromat. Plants. 2015;4:215. [Google Scholar]
- 15.Fahmy N.M., Elhady S.S., Bannan D.F., Malatani R.T., Gad H.A. Citrus reticulata Leaves Essential Oil as an Antiaging Agent: A Comparative Study between Different Cultivars and Correlation with Their Chemical Compositions. Plants. 2022;11:3335. doi: 10.3390/plants11233335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gad H.A., El Hassab M.A., Elhady S.S., Fahmy N.M. Insights on Citrus clementina essential oil as a potential antiaging candidate with a comparative chemometric study on different cultivars. Ind. Crops Prod. 2023;194:116349. doi: 10.1016/j.indcrop.2023.116349. [DOI] [Google Scholar]
- 17.Tomaino A., Cimino F., Zimbalatti V., Venuti V., Sulfaro V., De Pasquale A., Saija A. Influence of heating on antioxidant activity and the chemical composition of some spice essential oils. Food Chem. 2005;89:549–554. doi: 10.1016/j.foodchem.2004.03.011. [DOI] [Google Scholar]
- 18.Gad H.A., Mamadalieva R.Z., Khalil N., Zengin G., Najar B., Khojimatov O.K., Al Musayeib N.M., Ashour M.L., Mamadalieva N.Z. GC-MS Chemical Profiling, Biological Investigation of Three Salvia Species Growing in Uzbekistan. Molecules. 2022;27:5365. doi: 10.3390/molecules27175365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saroj A., Pragadheesh V.S., Palanivelu, Yadav A., Singh S.C., Samad A., Negi A.S., Chanotiya C.S. Anti-phytopathogenic activity of Syzygium cumini essential oil, hydrocarbon fractions and its novel constituents. Ind. Crops Prod. 2015;74:327–335. doi: 10.1016/j.indcrop.2015.04.065. [DOI] [Google Scholar]
- 20.Benherlal P.S., Arumughan C. Chemical composition and in vitro antioxidant studies on Syzygium cumini fruit. J. Sci. Food Agric. 2007;87:2560–2569. doi: 10.1002/jsfa.2957. [DOI] [PubMed] [Google Scholar]
- 21.Ayyanar M., Subash-Babu P. Syzygium cumini (L.) Skeels: A review of its phytochemical constituents and traditional uses. Asian Pac. J. Trop. Biomed. 2012;2:240–246. doi: 10.1016/S2221-1691(12)60050-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dharani N. A Review of Traditional Uses and Phytochemical Constituents of Indigenous Syzygium Species in East Africa. Pharm. J. Kenya. 2016;22:123–127. [Google Scholar]
- 23.Dias C.N., Rodrigues K.A.F., Carvalho F.A.A., Carneiro S.M.P., Maia J.G.S., Andrade E.H.A., Moraes D.F.C. Molluscicidal and Leishmanicidal Activity of the Leaf Essential Oil of Syzygium cumini (L.) Skeels from Brazil. Chem. Biodivers. 2013;10:1133–1141. doi: 10.1002/cbdv.201200292. [DOI] [PubMed] [Google Scholar]
- 24.Dickel M.L., Rates S.M.K., Ritter M.R. Plants popularly used for loosing weight purposes in Porto Alegre, South Brazil. J. Ethnopharmacol. 2007;109:60–71. doi: 10.1016/j.jep.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 25.Khare C.P. Indian Medicinal Plants: An Illustrated Dictionary. Springer Science & Business Media; Berlin/Heidelberg, Germany: 2008. [Google Scholar]
- 26.Jain A., Katewa S.S., Galav P.K., Sharma P. Medicinal plant diversity of Sitamata wildlife sanctuary, Rajasthan, India. J. Ethnopharmacol. 2005;102:143–157. doi: 10.1016/j.jep.2005.05.047. [DOI] [PubMed] [Google Scholar]
- 27.Naga Raju G.J., Sarita P., Ramana Murty G.A.V., Ravi Kumar M., Seetharami Reddy B., John Charles M., Lakshminarayana S., Seshi Reddy T., Reddy S.B., Vijayan V. Estimation of trace elements in some anti-diabetic medicinal plants using PIXE technique. Appl. Radiat. Isot. 2006;64:893–900. doi: 10.1016/j.apradiso.2006.02.085. [DOI] [PubMed] [Google Scholar]
- 28.Sharma H.K., Chhangte L., Dolui A.K. Traditional medicinal plants in Mizoram, India. Fitoterapia. 2001;72:146–161. doi: 10.1016/S0367-326X(00)00278-1. [DOI] [PubMed] [Google Scholar]
- 29.Chhetri D.R., Parajuli P., Subba G.C. Antidiabetic plants used by Sikkim and Darjeeling Himalayan tribes, India. J. Ethnopharmacol. 2005;99:199–202. doi: 10.1016/j.jep.2005.01.058. [DOI] [PubMed] [Google Scholar]
- 30.Jabeen K., Javaid A. Antifungal activity of Syzygium cumini against Ascochyta rabiei—The cause of chickpea blight. Nat. Prod. Res. 2010;24:1158–1167. doi: 10.1080/14786410902941154. [DOI] [PubMed] [Google Scholar]
- 31.Badawy M.E.I., Abdelgaleil S.A.M. Composition and antimicrobial activity of essential oils isolated from Egyptian plants against plant pathogenic bacteria and fungi. Ind. Crops Prod. 2014;52:776–782. doi: 10.1016/j.indcrop.2013.12.003. [DOI] [Google Scholar]
- 32.Shafi P.M., Rosamma M.K., Jamil K., Reddy P.S. Antibacterial activity of Syzygium cumini and Syzygium travancoricum leaf essential oils. Fitoterapia. 2002;73:414–416. doi: 10.1016/S0367-326X(02)00131-4. [DOI] [PubMed] [Google Scholar]
- 33.Elansary H., Salem M.Z.M., Ashmawy N., Yacout M. Chemical Composition, Antibacterial and Antioxidant Activities of Leaves Essential Oils from Syzygium cumini L., Cupressus sempervirens L. and Lantana camara L. from Egypt. J. Agric. Sci. 2012;4:144–152. doi: 10.5539/jas.v4n10p144. [DOI] [Google Scholar]
- 34.Mohamed A.A., Ali S.I., El-Baz F.K. Antioxidant and antibacterial activities of crude extracts and essential oils of Syzygium cumini leaves. PLoS ONE. 2013;8:e60269. doi: 10.1371/journal.pone.0060269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hanif M.U., Hussain A.I., Aslam N., Kamal G.M., Chatha S.A.S., Shahida S., Khalid M., Hussain R. Chemical Composition and Bioactivities of Essential Oil from Leaves of Syzygium cumini (L.) Skeels Native to Punjab, Pakistan. Chem. Biodivers. 2020;17:e1900733. doi: 10.1002/cbdv.201900733. [DOI] [PubMed] [Google Scholar]
- 36.Rodrigues K.A.d.F., Amorim L.V., Dias C.N., Moraes D.F.C., Carneiro S.M.P., Carvalho F.A.d.A. Syzygium cumini (L.) Skeels essential oil and its major constituent α-pinene exhibit anti-Leishmania activity through immunomodulation in vitro. J. Ethnopharmacol. 2015;160:32–40. doi: 10.1016/j.jep.2014.11.024. [DOI] [PubMed] [Google Scholar]
- 37.Da Silva V.P., Alves C.C.F., Miranda M.L.D., Bretanha L.C., Balleste M.P., Micke G.A., Silveira E.V., Martins C.H.G., Ambrosio M.A.L.V., de Souza Silva T., et al. Chemical composition and in vitro leishmanicidal, antibacterial and cytotoxic activities of essential oils of the Myrtaceae family occurring in the Cerrado biome. Ind. Crops Prod. 2018;123:638–645. doi: 10.1016/j.indcrop.2018.07.033. [DOI] [Google Scholar]
- 38.Machado R.R.P., Jardim D.F., Souza A.R., Scio E., Fabri R.L., Carpanez A.G., Grazul R.M., de Mendonça J.P.R.F., Lesche B., Aarestrup F.M. The effect of essential oil of Syzygium cumini on the development of granulomatous inflammation in mice. Rev. Bras. Farmacogn. 2013;23:488–496. doi: 10.1590/S0102-695X2013005000030. [DOI] [Google Scholar]
- 39.Siani A.C., Souza M.C., Henriques M.G., Ramos M.F. Anti-inflammatory activity of essential oils from Syzygium cumini and Psidium guajava. Pharm. Biol. 2013;51:881–887. doi: 10.3109/13880209.2013.768675. [DOI] [PubMed] [Google Scholar]
- 40.Stalin N., Swamy P.S. Screening of phytochemical and pharmacological activities of Syzygium caryophyllatum (L.) Alston. Clin. Phytosci. 2018;4:3. [Google Scholar]
- 41.Moresco R.N., Sperotto R.L., Bernardi A.S., Cardoso R.F., Gomes P. Effect of the aqueous extract of Syzygium cumini on carbon tetrachloride-induced hepatotoxicity in rats. Phytother. Res. 2007;21:793–795. doi: 10.1002/ptr.2158. [DOI] [PubMed] [Google Scholar]
- 42.El-Moneim A., Afify M., Fayed S., Shalaby E., El-Shemy H. Syzygium cumini (pomposia) active principles exhibit potent anticancer and antioxidant activities. Afr. J. Pharm. Pharmacol. 2011;5:948–956. [Google Scholar]
- 43.Li L., Adams L.S., Chen S., Killian C., Ahmed A., Seeram N.P. Eugenia jambolana Lam. berry extract inhibits growth and induces apoptosis of human breast cancer but not non-tumorigenic breast cells. J. Agric. Food Chem. 2009;57:826–831. doi: 10.1021/jf803407q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bopp A., De Bona K.S., Bellé L.P., Moresco R.N., Moretto M.B. Syzygium cumini inhibits adenosine deaminase activity and reduces glucose levels in hyperglycemic patients. Fundam. Clin. Pharmacol. 2009;23:501–507. doi: 10.1111/j.1472-8206.2009.00700.x. [DOI] [PubMed] [Google Scholar]
- 45.Malik N., Javaid S., Ashraf W., Siddique F., Rasool M.F., Alqahtani F., Ahmad T., Abrar M.A., Imran I. Long-Term Supplementation of Syzygium cumini (L.) Skeels Concentrate Alleviates Age-Related Cognitive Deficit and Oxidative Damage: A Comparative Study of Young vs. Old Mice. Nutrients. 2023;15:666. doi: 10.3390/nu15030666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Halim M.A., Kanan K.A., Nahar T., Rahman M.J., Ahmed K.S., Hossain H., Mozumder N.H.M.R., Ahmed M. Metabolic profiling of phenolics of the extracts from the various parts of blackberry plant (Syzygium cumini L.) and their antioxidant activities. LWT. 2022;167:113813. doi: 10.1016/j.lwt.2022.113813. [DOI] [Google Scholar]
- 47.Barra A. Factors affecting chemical variability of essential oils: A review of recent developments. Nat. Prod. Commun. 2009;4:1147–1154. doi: 10.1177/1934578X0900400827. [DOI] [PubMed] [Google Scholar]
- 48.Kumar A., Naqvi A.A., Kahol A.P., Tandon S. Composition of leaf oil of Syzygium cumini L. from north India. Indian Perfum. 2004;48:439–441. [Google Scholar]
- 49.Nishandhini S., Sudha V., Mallavarapu G.R., Murugan R. Chemical Compositions, α-Amylase Inhibitory and Antioxidant Activities of the Essential Oils from Unripe Fruit Pulp and Leaves of Syzygium cumini. Int. J. Pharm. Pharm. Sci. 2015;7:515–519. [Google Scholar]
- 50.Sarma N., Begum T., Pandey S., Gogoi R., Munda S., Lal D. Chemical Composition of Syzygium cumini (L.) Skeels Leaf Essential Oil with Respect to its Uses from North East Region of India. J. Essent. Oil Bear. Plants. 2020;23:601–607. doi: 10.1080/0972060X.2020.1796822. [DOI] [Google Scholar]
- 51.Vijayanand P., Jagan Mohan Rao L., Narasimham P. Volatile flavour components of jamun fruit (Syzygium cumini L) Flavour Fragr. J. 2001;16:47–49. doi: 10.1002/1099-1026(200101/02)16:1<47::AID-FFJ944>3.0.CO;2-L. [DOI] [Google Scholar]
- 52.Mehta P.K., de Sousa Galvão M., Soares A.C., Nogueira J.P., Narain N. Volatile Constituents of Jambolan (Syzygium cumini L.) Fruits at Three Maturation Stages and Optimization of HS-SPME GC-MS Method Using a Central Composite Design. Food Anal. Methods. 2018;11:733–749. doi: 10.1007/s12161-017-1038-4. [DOI] [Google Scholar]
- 53.Demirci B., Kırcı D., Öztürk G., Demirci F. Effect of Extraction Time on Origanum onites L. Infusions and Essential Oils—Biological Evaluation, Statistical Principal Component and Hierarchial Cluster Analyses. Chem. Biodivers. 2022;19:e202200482. doi: 10.1002/cbdv.202200482. [DOI] [PubMed] [Google Scholar]
- 54.Ashmawy N.S., Hamoda A.M., Gad H.A., El-Keblawy A.A., Soliman S.S. Newly-sprouted leaves at the stem base differ anatomically and histochemically from the crown leaves in Ficus johannis. Bot. Lett. 2023;170:591–599. doi: 10.1080/23818107.2023.2192264. [DOI] [Google Scholar]
- 55.Solano F. Amino Acids in Nutrition and Health. Springer; Cham, Switzerland: 2020. Metabolism and functions of amino acids in the skin; pp. 187–199. [DOI] [PubMed] [Google Scholar]
- 56.Tzaphlidou M. The role of collagen and elastin in aged skin: An image processing approach. Micron. 2004;35:173–177. doi: 10.1016/j.micron.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 57.Almine J.F., Wise S.G., Weiss A.S. Elastin signaling in wound repair. Birth Defects Res. Part C Embryo Today Rev. 2012;96:248–257. doi: 10.1002/bdrc.21016. [DOI] [PubMed] [Google Scholar]
- 58.Wong R., Geyer S., Weninger W., Guimberteau J.C., Wong J.K. The dynamic anatomy and patterning of skin. Exp. Dermatol. 2016;25:92–98. doi: 10.1111/exd.12832. [DOI] [PubMed] [Google Scholar]
- 59.Lewis P.N., White T.L., Young R.D., Bell J.S., Winlove C.P., Meek K.M. Three-dimensional arrangement of elastic fibers in the human corneal stroma. Exp. Eye Res. 2016;146:43–53. doi: 10.1016/j.exer.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Papakonstantinou E., Roth M., Karakiulakis G. Hyaluronic acid: A key molecule in skin aging. Derm. Endocrinol. 2012;4:253–258. doi: 10.4161/derm.21923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Langton A.K., Halai P., Griffiths C.E., Sherratt M.J., Watson R.E. The impact of intrinsic ageing on the protein composition of the dermal-epidermal junction. Mech. Ageing Dev. 2016;156:14–16. doi: 10.1016/j.mad.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 62.Callaghan T., Wilhelm K.P. A review of ageing and an examination of clinical methods in the assessment of ageing skin. Part 2: Clinical perspectives and clinical methods in the evaluation of ageing skin. Int. J. Cosmet. Sci. 2008;30:323–332. doi: 10.1111/j.1468-2494.2008.00455.x. [DOI] [PubMed] [Google Scholar]
- 63.Madan K., Nanda S. In-vitro evaluation of antioxidant, anti-elastase, anti-collagenase, anti-hyaluronidase activities of safranal and determination of its sun protection factor in skin photoaging. Bioorg. Chem. 2018;77:159–167. doi: 10.1016/j.bioorg.2017.12.030. [DOI] [PubMed] [Google Scholar]
- 64.Jugreet B.S., Lall N., Anina Lambrechts I., Reid A.-M., Maphutha J., Nel M., Hassan A.H., Khalid A., Abdalla A.N., Van B.L. In Vitro and In Silico Pharmacological and Cosmeceutical Potential of Ten Essential Oils from Aromatic Medicinal Plants from the Mascarene Islands. Molecules. 2022;27:8705. doi: 10.3390/molecules27248705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Faria A.F., Marques M.C., Mercadante A.Z. Identification of bioactive compounds from jambolão (Syzygium cumini) and antioxidant capacity evaluation in different pH conditions. Food Chem. 2011;126:1571–1578. doi: 10.1016/j.foodchem.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 66.Kumar M., Hasan M., Lorenzo J.M., Dhumal S., Nishad J., Rais N., Verma A., Changan S., Barbhai M.D., Chandran D. Jamun (Syzygium cumini (L.) Skeels) seed bioactives and its biological activities: A review. Food Biosci. 2022;50:102109. doi: 10.1016/j.fbio.2022.102109. [DOI] [Google Scholar]
- 67.Karakaya S., Yilmaz S.V., Özdemir Ö., Koca M., Pınar N.M., Demirci B., Yıldırım K., Sytar O., Turkez H., Baser K.H.C. A caryophyllene oxide and other potential anticholinesterase and anticancer agent in Salvia verticillata subsp. amasiaca (Freyn & Bornm.) Bornm. (Lamiaceae) J. Essent. Oil Res. 2020;32:512–525. [Google Scholar]
- 68.Leporini M., Bonesi M., Loizzo M.R., Passalacqua N.G., Tundis R. The essential oil of Salvia rosmarinus Spenn. from Italy as a source of health-promoting compounds: Chemical profile and antioxidant and cholinesterase inhibitory activity. Plants. 2020;9:798. doi: 10.3390/plants9060798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gushiken L.F.S., Beserra F.P., Hussni M.F., Gonzaga M.T., Ribeiro V.P., de Souza P.F., Campos J.C.L., Massaro T.N.C., Hussni C.A., Takahira R.K., et al. Beta-caryophyllene as an antioxidant, anti-inflammatory and re-epithelialization activities in a rat skin wound excision model. Oxid. Med. Cell Longev. 2022;2022:9004014. doi: 10.1155/2022/9004014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Flores-Soto M.E., Corona-Angeles J.A., Tejeda-Martinez A.R., Flores-Guzman P.A., Luna-Mujica I., Chaparro-Huerta V., Viveros-Paredes J.M. β-Caryophyllene exerts protective antioxidant effects through the activation of NQO1 in the MPTP model of Parkinson’s disease. Neurosci. Lett. 2021;742:135534. doi: 10.1016/j.neulet.2020.135534. [DOI] [PubMed] [Google Scholar]
- 71.Adams R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. 4th ed. Allured Publishing Corporation; Carol Stream, IL, USA: 2007. [Google Scholar]
- 72.Gad H.A., Ayoub I.M., Wink M. Phytochemical profiling and seasonal variation of essential oils of three Callistemon species cultivated in Egypt. PLoS ONE. 2019;14:e0219571. doi: 10.1371/journal.pone.0219571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.El Bishbishy M.H., Gad H.A., Aborehab N.M. Chemometric discrimination of three Pistacia species via their metabolic profiling and their possible in vitro effects on memory functions. J. Pharm. Biomed. Anal. 2020;177:112840. doi: 10.1016/j.jpba.2019.112840. [DOI] [PubMed] [Google Scholar]
- 74.Gad H.A., Mukhammadiev E.A., Zengen G., Musayeib N.M.A., Hussain H., Bin Ware I., Ashour M.L., Mamadalieva N.Z. Chemometric Analysis Based on GC-MS Chemical Profiles of Three Stachys Species from Uzbekistan and Their Biological Activity. Plants. 2022;11:1215. doi: 10.3390/plants11091215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Van Wart H.E., Steinbrink D.R. A continuous spectrophotometric assay for Clostridium histolyticum collagenase. Anal. Biochem. 1981;113:356–365. doi: 10.1016/0003-2697(81)90089-0. [DOI] [PubMed] [Google Scholar]
- 76.Stein R.L., Trainor D.A. Mechanism of inactivation of human leukocyte elastase by a chloromethyl ketone: Kinetic and solvent isotope effect studies. Biochemistry. 1986;25:5414–5419. doi: 10.1021/bi00367a011. [DOI] [PubMed] [Google Scholar]
- 77.Kim Y.-S., Noh Y.-K., Lee G.-I., Kim Y.-K., Lee K.-S., Min K.-R. Inhibitory effects of herbal medicines on hyaluronidase activity. Korean J. Pharmacogn. 1995;26:265–272. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon request from the corresponding authors: dr.naglaa@gmu.ac.ae (N.S.A.) and heba_pharma@pharma.asu.edu.eg (H.A.S.E.-N.).