Abstract
The introduction of domestic horses transformed Indigenous societies across the grasslands of Argentina, leading to the emergence of specialized horse cultures across the Southern Cone. However, the dynamics of this introduction are poorly chronicled by historic records. Here, we apply archaeozoological and biomolecular techniques to horse remains from the site of Chorrillo Grande 1 in southern Argentina. Osteological and taphonomic analyses suggest that horses were pastorally managed and used for food by Aónikenk/Tehuelche hunter-gatherers before the onset of permanent European settlement, as early as the mid-17th century. DNA-based sex identifications suggest consumption of both male and female horses, while ceramic residue also shows use of guanaco products. Sequential isotope analyses on horse dentition reveal an origin in southern Patagonia and movement of these animals between the Río Coig and Río Gallegos basins. These results reinforce emerging evidence for rapid Indigenous-mediated dispersal of horses in the Americas and demonstrate that horses catalyzed rapid economic and social transformations.
Archaeological evidence shows early adoption of horses in southern Patagonia.
INTRODUCTION
Perhaps nowhere in the ancient world was the impact of domestic horses on human societies more impactful than in the plains and foothills of the Southern Cone of South America after the onset of European colonization. On horseback, South American Indigenous nations became prolific horse herders and riders and, in many cases, maintained their sovereignty from European colonists deep into the 19th and 20th centuries. Although the spread of horses was partially chronicled in historic records, in some of the areas most deeply affected, European record-keeping began decades or even centuries after the first horses reached South American shores. In recent years, archaeological science has emerged as a powerful tool for understanding human-horse relationships in prehistory. In North America, study of archaeological horse bones has revealed a more rapid integration of domestic horses into Indigenous lifeways than previously inferred from Euroamerican documents (1). Here, we apply archaeozoological and biomolecular methods (DNA sequencing, radiocarbon dating, and isotope analysis) to archaeological horse remains from the site of Chorrillo Grande 1, located in southern Argentina (Fig. 1), to explore the timing and nature of the early spread of domestic horses into southern Patagonia.
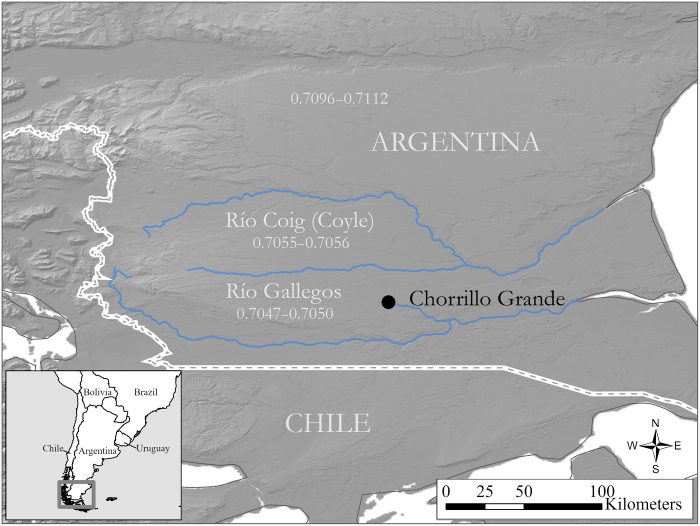
Fig. 1. Study area.
Study site in southern Patagonia, along with reference data for 87Sr/86S ratios from water sources in the adjoining river drainages of Argentina (35). Map by Dr. J. Conver.
Dispersal of horses to South America
Wild equids, including both ancestral members of the genus Equus as well as their donkey-like relatives, Hippidion, flourished in South America during the Pleistocene—the osteological remains of Ice Age horses were encountered by Darwin during his voyages on the HMS Beagle during the 19th century (2). Early migrants to the South American continent encountered and made use of wild equids, as evidenced by their appearance in archaeological sites (3, 4). However, endogenous horses appear to have become extinct in the region sometime during the Late Pleistocene, with perhaps later persistence at higher latitudes and higher altitude zones (5). For most of the Holocene, equids remained absent from the continent until their historical reintroduction by European settlers during the 16th century.
Historical records suggest that Spanish settlers were likely the first Europeans to reintroduce equids to the Southern Cone, first bringing horses to the area around the Río de la Plata delta in ca. 1536 during the colonization of Buenos Aires (6). When the colony failed a few years later because of the impacts of starvation and conflict with Indigenous people in the region, horses and other livestock were abandoned and occupants moved further upriver to the site of Asunción. When permanent resettlement returned to Buenos Aires several decades later in ca. 1580, feral horses were already abundant on the local landscape, in the interim having also spread into the southern Andes through Spanish colonial activity on the Pacific coast.
By the 19th century, the hunter-gatherers of the Andean foothills and the Pampas were widely known by Europeans for their equestrian prowess, particularly the Aonik’enk (Tehuelche) to the south, and the Gününa küna (Puelche) to the north. Among the region’s horse cultures, domestic horses were both herded as domestic livestock as well as used in mounted hunts of wild guanaco (Lama guanicoe) and rhea (Rhea sp.), a large terrestrial bird (6–9). European visitors to Patagonia and the Pampas noted the use of horses in ceremonies, funerary practices, and nearly every aspect of daily life, from making tents to stringed instruments (10–12). Archaeological data suggest that introduction of horses prompted major economic shifts by the 18th and 19th centuries, including a major shift away from marine resources (13–16).
Although documents establish that domestic horse populations were already booming in some areas during the 16th century, their dispersal deeper into higher latitude areas of the Pampas and Patagonia is more difficult to trace via textual evidence. Magellan visited the region ca. 1520, but establishment of a permanent European footprint in Patagonia lagged behind other zones, and historical records lagged alongside. The first documents tracing the local use of domestic horses by Indigenous peoples in the region date to ca. 1741, with permanent colonial settlements not established in most of the region until over a century later (13). As a result, tracing the adoption of early dispersal and integration of domestic horses into Patagonia appears to lie beyond the bounds of the region’s sparse historical record.
Archaeological and biomolecular proxies for horse use
In recent years, archaeozoology has emerged as a valuable toolkit for tracing the dispersal and socioeconomic integration of domestic horses in antiquity, particularly in cases where gaps in the historical and archaeological record make these processes difficult to otherwise study. The archaeological presence of early domestic horses is likely underappreciated. In North America, for example, horse remains from Indigenous contexts are sometimes misclassified as Pleistocene or modern specimens (17). Even in cases where only isolated skeletal elements can be recovered, advances in biomolecular analysis allow information such as species, sex, and parentage to be gleaned from even poorly preserved horse remains (18). In the American Southwest and Rocky Mountains, direct radiocarbon dating of horse remains, paired with osteology, DNA, and isotope analyses, demonstrates a rapid and Indigenous-mediated dispersal of domestic horses long before the direct arrival of European colonists (1). Therefore, a multimethodological study of archaeological horse remains has the potential to provide important insights into the timing and nature of early horse use by Indigenous societies in the Southern Cone.
Although existing archaeozoological data on domestic horses is minimal, previous scholarship has identified horse remains from Indigenous cultural contexts in Argentina from the eastern Pampas (19) and the Andean front (20). In Patagonia, some horse remains from previously identified archaeological contexts show the presence of horse in both colonial and Aonik’enk assemblages (21–24). At Laguna Cóndor, an Aonik’enk midden, three horse bones showed evidence for modification by humans, suggesting a minor role for horses in the diet (25). However, so far, none of the horse remains from these assemblages has been directly dated, and available dates only constrain these assemblages to the historic era.
The site: Chorrillo Grande 1
To assess the potential of archaeological horse remains to shed light on the early dispersal of domestic horses into the Southern Cone, we analyzed equine skeletal material from the site of Chorrillo Grande 1, located on the bottom of the Mack Aike canyon, on the north bank of the Gallegos River in southern Patagonia, Argentina. Today, the canyon provides shelter from the prevailing winds and a valuable source of water for fauna, mainly guanacos. Chorrillo Grande 1 itself is an Aonik’enk-affiliated site discovered during road construction, a particularly artifact-dense locality along the many artifactual and archaeofaunal concentrations distributed along the canyon, which span more than three millennia. During survey of surface materials exposed during road construction, researchers documented Guanaco remains, glass beads dating to the 18th and 19th centuries, glass scrapers, fragments of metal artifacts, lithic projectile points, sidescrapers, scrapers, cores, and lithic debris along with domestic horse remains (26). The assemblage likely represents an accretional accumulation of material spanning several centuries.
We recovered a total of nine horse specimens from this site, including two right tibia fragments, a right upper fourth premolar (P4), a left partial upper cheektooth row (P2, P3, M1, and M3), a right lower fourth premolar (P4), and a right radius fragment. For each specimen, we conducted osteological, taphonomic, and paleopathological analyses, assessing evidence of weathering, cultural modification, and skeletal indicators potentially linked with transport or human activity. We also conducted direct radiocarbon dating and DNA sequencing, revealing species, parentage, and sex for four of the specimens examined. For the tooth row, we supplemented these analyses with a detailed sequential stable and strontium isotope study to assess dietary inputs and mobility.
RESULTS
Radiocarbon dating
All four horse specimens from Chorrillo Grande 1 submitted for radiocarbon dating (two tibia bone fragments, a radius bone fragment, and a tooth fragment, see Supplementary Text) produced successful accelerator mass spectrometry (AMS) results following collagen extraction and measurement at the University of Arizona AMS facility in Tucson, Arizona (Fig. 2). Two of these horse specimens produced dates from the early 18th to mid-20th centuries. More sensitive radiocarbon discrimination between samples from this period is typically impossible due to a plateau in the calibration curve. However, one specimen, a right tibia fragment, confidently predates 1800 CE (ca. 1645–1808 cal. CE, 2 sigma calibrated range, AA114998), while a second, from the tooth row, appears to likely predate 1700 CE (ca. 1515–1800 cal. CE, AA115001). A guanaco bone shaft fragment was also successfully dated to ca. 1522–1800 cal. CE (2 sigma calibrated range, AA115000), and carbonized food residue from a single ceramic sherd was dated to a similar range (ca. 1645–1800 cal. CE, 2 sigma, UCIAMS-280680). When analyzing horse occupation at the site as a Bayesian uniform phase model in OxCal (https://c14.arch.ox.ac.uk/oxcal/OxCal.html) (27) and assuming the date of 1536 (start of the Mendoza expedition) as the earliest possibility date of horse introduction, the posterior modeled probability distribution for the adoption of horses at Chorrillo Grande 1 has a median modeled start date of ca. 1617 cal. CE, with the most likely date for this adoption falling between 1540 and 1650 cal. CE (1 sigma). When all dates from the site, including guanaco and foodcrust dates, are also included, Bayesian uniform phase modeling produces an even narrower posterior distribution for the onset of site activity, with a median modeled start date of ca. 1627 cal. CE and the most likely boundary falling between 1599 and 1653 cal. CE (1 sigma).
Fig. 2. Radiocarbon dates and modeled boundaries from Chorrillo Grande 1.
Radiocarbon dates and Bayesian model estimation (assuming a single, uniform phase model bounded by the historical introduction of horses to Buenos Aires in 1536) for site activity at Chorrillo Grande 1, produced in OxCal (27) using the INTCAL 20 calibration curve (57).
Osteology and taphonomy
Analysis of the horse skeletal remains from Chorrillo Grande reveals evidence of processing and consumption of both adult and juvenile horses. While all specimens were weathered [score of “2” or greater following criteria outlined by Behrensmeyer (28) from surficial exposure, perhaps removing cut marks if they were initially present], all three limb bone specimens identified in the assemblage showed modifications associated with marrow/grease extraction (Fig. 3). Two specimens showed evidence of spiral fracturing, while a third appeared to have been struck with a blunt object midshaft, producing a greenstick fracture. Of these, the radius fragment was also burned and partially calcined.
Fig. 3. Culturally modified horse bones.
Limb bone remains (tibia and radius) from Chorrillo Grande 1, showing evidence of spiral fracturing.
Although no specimens were recovered that could be used in morphological sex estimation, both epiphyseal fusion (29) and crown height (30) enabled estimation of the age of each specimen (Table 1). These estimates show the presence of both adult horses (6 to 10 years) and juvenile animals (1.75 to 3.5 years). Although a typical minimum number of individuals estimate based only on the skeletal elements present would suggest that at least two individuals were present, when also considering the age information, it appears that at least three individuals are represented in the assemblage (at least two juvenile specimens and one adult).
Table 1. Skeletal elements and associated age estimates.
Age estimates for horse remains recovered from Chorrillo Grande 1.
| Specimen | Age estimate | Basis |
|---|---|---|
| Upper left P2, P3, M1, M3, upper right P4 | 6–10 years | Crown height |
| Lower right cheektooth (P4, M1, or M2) | 5–7 years | Crown height |
| Right radius (proximal) | ~3.5 years | Epiphyseal fusion |
| Right tibia (distal) | ~2 years | Epiphyseal fusion |
| Right tibia (distal) | 1.75–2 years | Epiphyseal fusion |
Ancient DNA
DNA was successfully extracted from three of four specimens and analyzed at the Centre for Anthropobiology and Genomics of Toulouse, University of Toulouse, France. Species estimation of all specimens using the Zonkey pipeline (18) confirmed that all likely represent caballine domestic horses and indicated that two of the three submitted specimens originated from female horses/mares (Table 2). These results were subsequently confirmed with full genomic sequencing reported elsewhere (1).
Table 2. Genomic results.
Genomic (Zonkey) estimates of sex and species from Chorrillo Grande 1.
| Specimen | Age estimate | DNA sequences* | EquCab2 high-quality alignments | mtDNA high-quality alignments | Species estimate | Sex estimate |
|---|---|---|---|---|---|---|
| SAH2 (right tibia) | 2+ years | 102,693,340 | 693,968 | 87 | Domestic horse (Equus caballus) | Female |
| SAH4 (lower R P4) | 7–8 years | 22,997,682 | 165,467 | 67 | Domestic horse (E. caballus) | Male |
| SAH5 (right tibia) | ~2 years | 44,673,568 | 88,405 | 26 | Domestic horse (E. caballus) | Female |
| SAH6 (right radius, distal) | <3.5 years | 969 | 1 | – | – | – |
*Collapsed + uncollapsed pairs.
Isotope analysis
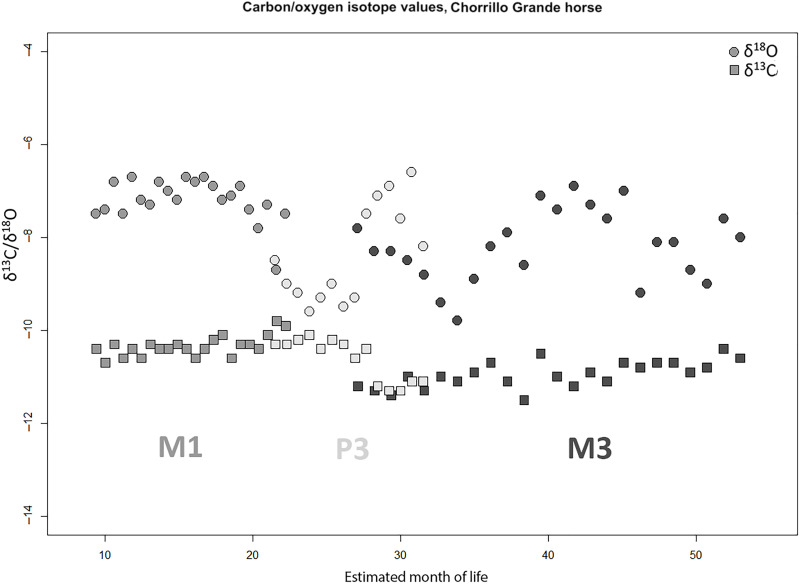
Stable carbon isotope data from the adult horse specimen (table S3) indicate that the animal spent its time foraging on plants using the C3 photosynthetic pathway, most likely the cold-adapted grasses that predominate in the region today (31), classified as an arid-cold steppe (32). We conducted sequential sampling of tooth enamel that mineralized at different stages of the animal’s life, including the upper left first molar (M1, 8 to 24 months), upper left third premolar (P3, 26 to 36 months), and upper left third molar (M3, 25 to 55 months) (33), producing a three-dimensional model of each tooth using an EinScanSE structured light scanner and measuring crown height after Levine (30). For each tooth, we produced an estimated age at mineralization, assuming a maximum crown height based on reference values from Levine (30) and assuming incremental mineralization from the crown downward over intervals specified by Hoppe et al. (33).
When plotted against estimated age at mineralization, measured stable carbon isotope values (δ13C) show very minimal variation across this period (Fig. 4). Bulk strontium values sampled from the middle of each enamel column (Table 3) show an increase in 87Sr/86Sr values from the early stage of the animal’s life (~0.7052, M1) toward the end of the mineralization sequence (~0.7058, M3). There is a latitudinal gradient in strontium values recorded in the waters of the main fluvial basins in the region, showing very low values in the south (87Sr/86Sr = 0.704863 ± 0.000129, n = 4), due to the young basaltic geology of the Pali Aike volcanic field (34), gradually increasing northward toward the genetically mixed and more radiogenic deposits underlying the Coig basin (87Sr/86Sr = 0.70559 ± 0.00001, n = 4) (Fig. 1) (35). Hence, these values may suggest that the animal was originally born in southern latitudes near the Rio Gallegos (with contributions from the mother’s milk before weaning unlikely given the section of the tooth that was sampled) but moved northward during his lifetime toward the Rio Coig drainage during the animal’s third/fourth years of life, eventually meeting its end in the Rio Gallegos region (probably between the ages of 6 and 10, as estimated by crown height). Because of latitudinal gradients in 87Sr/86Sr across Argentina, measured values would exclude habitation meaningfully further north or in marine coastal ecosystems, either of the Atlantic Ocean or Strait of Magellan during this period of the animal’s life. Together, these isotope data suggest that the Chorrillo Grande 1 horse was born and lived primarily in southern Patagonia during the time periods represented by tooth mineralization.
Fig. 4. Sequential isotope data from Chorrillo Grande 1 horse.
Serially sampled table carbon and oxygen isotopes for horse SAH3 from Chorrillo Grande 1, by estimated month of life [calculated from tooth wear curves in Levine (30)].
Table 3. Strontium isotope data.
Strontium isotope values for Chorrillo Grande 1, SAH3.
| Specimen | Mineralization range | Bulk 87Sr/86Sr |
|---|---|---|
| ULM1 | 8–24 months | 0.705205 |
| ULP3 | 26–36 months | 0.705417 |
| ULM3 | 25–55 months | 0.705829 |
Stable oxygen isotope values (δ18O) show variation that we interpret as corresponding primarily to seasonal changes in temperature and moisture in an environment with a highly seasonal pattern of temperature and precipitation (36). Alternative sources of variation, such as the consumption of isotopically enriched standing water, cannot be ruled out but are difficult to quantify. Consistent highly positive δ18O isotope values characterize southern Patagonian latitudes east of the Andes relative to windward, high-elevation Andean water sources (37, 38). Unexpectedly, positive δ18O values, given the extreme southern latitude of Chorrillo Grande 1, are produced by reprecipitation of transpired Amazonian moisture (38), which is the main source of precipitation near the Patagonian Atlantic coast in settings comparable to Chorrillo Grande 1 (37). While incipient efforts to build δ18O isoscapes in southern Patagonia (39) will allow more resolved reconstructions, we consider that consistent 87Sr/86Sr and δ13C values combined with cyclical variation in δ18O favors the influence of seasonality in imbibed water sources. Therefore, although occasional consumption of isotopically enriched, standing water cannot be excluded, we consider it unlikely to result in similarly patterned equid M1, P3, and M3 enamel δ18O values.
Ceramic residues
Sixteen ceramic sherds, which are rare in southern Patagonia (40), were recovered at one corner of the site. These were apparently all fired in a reduced environment, and were recovered within an area of 0.5 m2, likely belonging to a single original vessel. Carbonized surface residue from one of these pottery sherds from Chorrillo Grande 1, which showed visible foodcrust, was extracted following existing protocols (41) and analyzed by gas chromatography–mass spectrometry (GC-MS) and GC-combustion-isotope ratio MS (GC-C-IRMS).
Lipids were well preserved and present in high concentrations (ceramic = 1.3 mg g−1, foodcrust = 10.2 mg g−1). The presence of mid-chain ketones (hentriacontan-16-one, tritriacontan-16-one, and pentatriacontan-18-one) with a reconstituted P:S fatty acid ratio of 1.57 (42) is consistent with terrestrial animal fats and provides evidence that the pottery was heated. The presence of ω-(o-alkylphenyl) alkanoic acids (APAAs) with carbon length 18 and 20 (APAA C18/20 ratio = 0.03) (43) further collaborates the use of this pottery for cooking. The APAA C18 isomeric distributions in the ceramic and foodcrust sample (fig. S2) both have a distribution maximizing at E and F comparable to terrestrial animals (43, 44). Phytanic acid diastereomer SRR% (3S,7R,11R,15-phytanic acid) values are within the range of modern ruminant adipose tissues (ceramic = 73%, foodcrust = 58%) (45). Stable carbon isotope values of palmitic (C16:0) and stearic acid (C18:0) show Δ13C offsets of −1.5‰ and −2‰ that are comparable to values of modern authentic (C3) ruminant fats (table S4). Conversely, the reference range for authentic horse adipose fats is 2.1 to 1.2‰ (mean = −0.05‰) (46, 47). This interpretation is supported by the triacylglycerol distribution of the ceramic sample (fig. S3), comparable to degraded ruminant fats (48), as opposed to horse fats (46).
Minimal offsets between the bulk and fatty acid carbon measurements (−0.3) indicate that both methods essentially measure the same component (i.e., lipids). High lipid concentrations as well as a high C:N atomic ratio (34.3) of the bulk foodcrust sample further support an oily sample constitution (tables S4 and S5 and figs. S1 to S3). Deuterium isotope values of C16:0 (−247.09 ± 3) and C18:0 (−216.36 ± 3; ΔD = 30.73) fatty acids from the ceramic sample (table S5) are comparable to reference data of terrestrial animals published elsewhere (47, 49).
DISCUSSION
Although historic records provide no direct insights into the early spread of domestic horses across the high latitudes of Patagonia, analysis of domestic horse remains from the site of Chorrillo Grande 1 provides evidence of an early and rapid integration of horses into Indigenous (Aonik’enk/Tehuelche) societies before the arrival of formal European settlement in the region. Radiocarbon dating of horse bones from this locality reveals several specimens dated with high confidence to ca. 1800 or earlier, with at least one specimen confidently predating ca. 1700 CE, decades before the first historic observation of horses in the region. Bayesian model estimates for the integration of horses into lifeways at Chorrillo Grande point toward the early 17th century.
Osteological and biomolecular analysis of these horse remains indicates that while horses were apparently raised locally, they were integrated into lifeways in unique and important ways. Ceramic and foodcrust residues from one sherd provided evidence of the processing of ruminant carcass fats, likely in this context from guanaco, potentially for the extraction of bone marrow as reflected in the oily sample constitution. DNA sequencing indicates that both juvenile horses were female, with limb bone specimens showing direct indication of human consumption (spiral fracturing, burning). This archaeological pattern fits a well-known cultural tradition appearing in later historical documents. European observers in later decades noted the Tehuelche preference for mare’s meat and mare’s blood in culinary traditions and ritual, in part because of the superior tenderness, taste, and fat of animals in this demographic (6, 7, 50). Although genetic analyses of a larger number of remains are necessary to validate statistical significance of these findings, identification of similar patterns in 17th and 18th century archaeological materials could suggest that regular mare consumption dates to the earliest stages of the horse’s introduction.
Consumption of breeding-age female horses is uncommon in typical Eurasian patterns of management, which tend to maximize herd reproduction. In most Eurasian pastoral contexts, modern or ancient, domestic horses of this demographic are rarely harvested because of the impact of the loss of breeding-age female horses on group fertility (51). Identification of young female horse consumption among Indigenous groups of the Southern Cone as early as the 17th century could indicate that domestic horses were slaughtered only occasionally (such as in rituals and feasts) or that by this time Aonik’enk groups had such a wealth in domestic horses so as to exclude major concerns over herd proliferation.
Multimethodological analysis of horse remains from Chorrillo Grande, located in the Rio Gallegos region of Patagonia in southern Argentina, points toward a likely rapid dispersal of domestic horses into the region following their initial introduction in northern Argentina in the 16th century. Osteological data, paired with isotope analysis and DNA, point to the hunting or herding of locally sourced young and breeding-age horses as early as the 17th century by Indigenous hunters or herders. These results reinforce other recent analyses suggesting that secondary dispersal of domestic horses took place faster and at a much larger scale across much of Indigenous America than previously chronicled in European historical records.
MATERIALS AND METHODS
Osteology
For each horse specimen, we confirmed identification as Equus using comparative faunal reference collections in the Archaeozoology Laboratory at the University of Colorado Museum of Natural History in Boulder, CO, USA. Each specimen was assessed for evidence of human modification, including spiral fractures, cut marks, burning, or other modification, and weathering was classified using the categorical system of Behrensmeyer (28). Age at death for postcranial elements was assessed on the basis of epiphyseal fusion data from Silver (29), while age at death for teeth was estimated using eruption and crown height wear curves from Levine (30). Each specimen was also assessed carefully for the presence of pathology or other modifications indicative of human activity (52–54).
Radiocarbon dating
Approximately 500 mg of crushed bone and tooth dentin sample was subjected to acid-base-acid pretreatment followed by ultrafiltration. Surface-cleaned samples were crushed to a coarse powder. The powder was extracted in continuous flow cells with the sequence 0.1 M HCl, 0.5 M NaOH, 0.1 M HCl, and 0.001 M HCl, with water washes in between. The resulting crude collagen was gelatinized by heating to 65°C for 16 hours and then filtered through a 0.45-μm glass fiber filter. The pass-through fraction was ultrafiltered, and the retained, greater than 30,000 molecular weight fraction was recovered and lyophilized. Ultrafiltered collagen was combusted in an Elementar varioISOTOPE Select elemental analyzer to determine the quality control parameters carbon yield and C/N atomic ratios. An Elementar precisION isotope ratio mass spectrometer was used to determine carbon δ13C versus vPDB at ±0.1‰ precision. Nitrogen δ15N was measured versus AIR with a precision of ±0.3‰. Quality control results are presented in table S1. Combustion and graphitization were accomplished using an Ionplus AGE system. Radiocarbon measurement was by accelerator MS using standard methods, and the NBS oxalic acid standards SRM-4990B and SRM-4990C. Measurements were background-subtracted and isotope fractionation–corrected. Uncalibrated radiocarbon dates are quoted at ±1 sigma uncertainties.
Foodcrust residue was prepared at the Keck Laboratory at the University of California-Irvine. The sample was treated with acid-base-acid (1 N HCl, 1 N NaOH, 75°C, the base step repeated until the solutions remained clear), washed repeatedly with ultrapure MQ water to pH >4, and vacuum-dried. A sample of 1.5 to 2 mg of pretreated organics was combusted with CuO and Ag wire getter at 900°C in quartz tubes sealed under vacuum, graphitized by hydrogen reduction at 525°C with an iron powder catalyst (55), and the Fe-graphite mixture was pressed into an Al sample holder. Radiocarbon was measured on a National Electrostatics 1.5SDH Compact Accelerator Mass Spectrometer system (56). Radiocarbon age is shown as conventional 14C age corrected for isotopic fractionation, with 1 sigma uncertainty that reflects scatter in repeated runs and uncertainties in the measurement of blanks and normalizing standards (NIST Oxalic Acid 1, SRM4990B) as well as counting statistics.
Calibration was carried out using the IntCal20 dataset (57) and OxCal v4.4 (27). Calibrated results, quoted at the 95% confidence interval, are listed in table S2. Calibration plots are shown in Fig. 2. OxCal v4.4 was also used to construct a simple uniform phase model with boundary estimates for activity at the site Chorrillo Grande 1 following Bronk Ramsey (27), and assuming that the Mendoza expedition of 1536 was an initial bounding event, and the recovery of the specimens in 2020 field survey was a second bounding event, using only horse remains (model 1) and using all measured dates (model 2). All code used in the analysis is provided in Supplementary Text.
Isotope analysis
For each tooth, we estimated the age at mineralization for each sequential measurement by calculating the ratio of measured crown height (30) to maximum crown height reference values for each tooth as measured by Levine (30). This ratio was then compared against the measured intervals of tooth mineralization measured by Hoppe et al. (33) to estimate the life interval represented by the remaining sequence of crown for each tooth, assuming a similar maximum height for the Chorrillo Grande 1 specimens (table S3).
For δ13C and δ18O analysis, approximately 8 mg of enamel powder was drilled from the sampled teeth at regular intervals with the distance being recorded from the enamel dentine junction. One percent NaClO was then added to these samples before three rinses in purified H2O, followed by the addition of 0.1 M acetic acid followed by a further three rinses. The samples were then lyophilized for 4 hours. Roughly 3 mg of sample was then weighed into glass tubes before reaction with 100% phosphoric acid. The evolved gasses were analyzed for δ13C and δ18O by Thermo Gasbench II connected to a Thermo Delta V Advantage mass spectrometer at the Max Planck Institute of Geoanthropology. δ13C and δ18O values were corrected using a three-point calibration compared against international standards [IAEA-603 (δ13C = 2.5; δ18O = −2.4), IAEA-CO-8 (δ13C = −5.8; δ18O = −22.7), IAEA-NBS 18 (δ13C = −5.014‰, δ18O = −23.2‰) and an in-house standard USGS44 (δ13C = −42.2)] Replicate analysis of USGS44 standards indicates that machine measurement error is ca. ±0.1‰ for δ13C and ±0.2‰ for δ18O. Overall measurement precision was determined by analysis of repeat extracts from a bovid tooth enamel standard (n = 20, ±0.2‰ for δ13C and ±0.2‰ for δ18O).
For the bulk strontium samples from each tooth, we took a sample along the midpoint of the buccal edge amounting to 20 mg in total. Pretreatment for strontium isotope analysis was undertaken in the clean chemistry laboratory of the MC-ICP-MS facility, Department of Geological Sciences at the University of Cape Town. The sampled tooth enamel powder was dissolved in 2 ml of concentrated HNO3 in a closed Savillex paraformaldehyde beaker. The beaker was then heated on a hotplate at 140°C for 1 hour. The sample was then dried down and redissolved in 1.5 ml of 2 M HNO3 for strontium separation chemistry following Pin et al. (58). The separated strontium fraction was subsequently dried down, dissolved in 2 ml of 0.2% HNO3, and diluted to 200-ppb (parts per billion) strontium concentration. A Nu Instruments NuPlasma HR MC-ICP-MS at the same facility was used for strontium isotope analysis (59). Analyses of NIST SRM987 acted as a bracketing reference standard using a 87Sr/86Sr value of 0.710255 (60). The data were corrected for isobaric rubidium interference at 87 amu using the measurement of 85Rb and the natural 85Rb/87Rb ratio. Instrumental mass fractionation was corrected using the measured 86Sr/88Sr ratio, the exponential law, and a true 86Sr/88Sr ratio of 0.1194. Analysis of an in-house carbonate reference material processed and measured with samples from this study (87Sr/86Sr = 0.708908; 2σ = 0.000018) agreed well with long-term results (87Sr/86Sr = 0.708911; 2σ = 0.000040, n = 414).
Organic residue analysis
One ceramic sherd was sampled for organic residue analysis. After removing a small layer (~1 mm) to avoid contamination from handling, the ceramic powder sample (ca. 1 g) was collected by drilling into the ceramic fabric. The foodcrust was collected with a sterile scalpel (ca. 20 mg). The samples were extracted using acidified methanol following established protocols (41). In short, 4 ml (1 ml for foodcrusts) of methanol was added to the homogenized sample, which was then sonicated for 15 min. Subsequently, 800 μl (200 μl for foodcrusts) of sulfuric acid was added to the mixture. The samples were placed in a heating block (4 hours) at 70°C. Lipid extraction followed using n-hexane (3 × 2 ml). The ceramic sample was also subjected to solvent extraction following established procedures (61). The ceramic powder (ca. 1 g) was extracted three times with dichloromethane/methanol 2:1 (v/v) wash (3 × 2 ml). All acid- and solvent-extracted samples were derivatized using N,O-bis(trimethylsilyl)trifluoroacetamide. All samples were analyzed by GC–flame ionization detection, GC-MS, and GC-C-IRMS.
Ancient DNA
Genetic analyses were carried out in the ancient DNA facilities of the Centre for Anthropobiology and Genomics of Toulouse (University Paul Sabatier, France). Ancient DNA was first extracted from bone and/or dental powder and incubated with USER enzymes to eliminate the impact of postmortem DNA damage on downstream sequence analyses. USER-treated extracts were immortalized into Illumina triple-indexed DNA libraries, which were polymerase chain reaction–amplified to recover enough material for DNA sequencing on the Illumina MiniSeq DNA sequencing instrument (PE 81 × 2 cycles). DNA sequences were demultiplexed according to the presence of unique DNA indexes, end-trimmed, and further collapsed if showing sufficient pair overlap, before being aligned against the EquCab2 horse reference genome (62) and the horse mitochondrial reference genome (63). The full experimental procedures are provided in full detail in the studies of Librado et al. (64) and Schubert et al. (18).
Acknowledgments
We thank B. Halvorsen (Bella Vista ranch), G. Povaszan (Bon Accord ranch), and C. Ruibal (Alquinta ranch) for their hospitality and help during fieldwork and J. Southon for assistance with radiocarbon dating.
Funding: This research received funding from the NSF (Collaborative Research: Horses and Human Societies in the American West, #1949305), the University of Colorado Center for the Humanities and the Arts (CHA), the Max Planck Society, the CNRS, University Paul Sabatier (AnimalFarm IRP), and the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (grant agreement 681605-PEGASUS), as well the Universidad Nacional de la Patagonia Austral (Project 29/A476-1) and the Agencia Nacional de Promoción Científica y Tecnológica (Project 2021 I A 01013). Publication of this article was funded by the University of Colorado Boulder Libraries Open Access Fund.
Author contributions: W.T.T.T. and J.B.B. designed the research, conducted the research, and wrote the manuscript. R.B., J.B.C., F.C.M., L.A.B., J.L.C., G.H., M.A., O.E.C., A.L., H.M.T., J.L., X.L., L.C., S.S., A.S.-O., P.L.R., M.L., L.O., P.R., and E.L.J. conducted the research, analyzed data, and assisted in writing the manuscript.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
Supplementary Materials
This PDF file includes:
Supplementary Text
Tables S1 to S5
Figs. S1 to S3
REFERENCES AND NOTES
- 1.Taylor W. T. T., Librado P., Horse C. J. A., Gover C. S. C., Arterberry J.; A. L. Afraid of Bear-Cook, Heron H. L., Hair R. M. Y., Gonzalez M., Means B., Crane S. H., Bull W. W. Y., Knife B. D.; A. Afraid of Bear, Collin C. T., Ward C., Pasqual T. A., Chauvey L., Tonasso-Calviere L., Schiavinato S., Seguin-Orlando A., Fages A., Khan N., Der Sarkissian C., Liu X., Wagner S., Leonard B. G., Manzano B. L., O’Malley N., Leonard J. A., Bernáldez-Sánchez E., Barrey E., Charliquart L., Robbe E., Denoblet T., Gregersen K., Vershinina A. O., Weinstock J., Šikanjić P. R., Mashkour M., Shingiray I., Aury J.-M., Perdereau A., Alquraishi S., Alfarhan A. H., Al-Rasheid K. A. S., Vukǐević T. T., Buric M., Sauer E., Lucas M., Brenner-Coltrain J., Bozell J. R., Thornhill C. A., Monagle V., Perri A., Newton C., Hall W. E., Conver J. L., Le Roux P., Buckser S. G., Gabe C., Belardi J. B., Barrón-Ortiz C. I., Hart I. A., Ryder C., Sponheimer M., Shapiro B., Southon J., Hibbs J., Faulkner C., Outram A., Rosa L. P., Palermo K., Solé M., William A., McCrory W., Lindgren G., Brooks S., Eché C., Donnadieu C., Bouchez O., Wincker P., Hodgins G., Trabert S., Bethke B., Roberts P., Jones E. L., Collin Y. R. H., Orlando L., Early dispersal of domestic horses into the Great Plains and northern Rockies. Science 379, 1316–1323 (2023). [DOI] [PubMed] [Google Scholar]
- 2.C. Darwin, L. Jenyns, T. C. Eyton, G. R. Waterhouse, R. Owen, J. Gould, T. Bell, The Zoology of the Voyage of HMS Beagle, Under the Command of Captain Fitzroy, RN, During the Years 1832 to 1836: Published with the Approval of the Lords Commissioners of Her Majesty’s Treasury (Smith, Elder and Company, 1839). [Google Scholar]
- 3.Martin F. M., Fell cave reinterpreted. Chungará 54, 535–556 (2022). [Google Scholar]
- 4.L. Miotti, M. Salemme, D. Hermo, Archaeology of Piedra Museo Locality: An Open Window to the Early Population of Patagonia (Springer Nature, 2022). [Google Scholar]
- 5.Villavicencio N. A., Corcoran D., Marquet P. A., Assessing the causes behind the Late Quaternary extinction of horses in South America using species distribution models. Front. Ecol. Evol. 7, (2019).. [Google Scholar]
- 6.P. Mitchell, Horse Nations: The Worldwide Impact of the Horse on Indigenous Societies Post-1492 (OUP Oxford, 2015). [Google Scholar]
- 7.G. C. Musters, At Home with the Patagonians: A Year’s Wanderings Over Untrodden Ground from the Straits of Magellan to the Rio Negro (J. Murray, 1873). [Google Scholar]
- 8.L. F. Dixie, A Través de la Patagonia (Compañía de Tierras Sud Argentino, 1880). [Google Scholar]
- 9.J. Beerbohm, Vagando por la Patagonia (Heliasta, 1881). [Google Scholar]
- 10.Palermo M. Á., Reflexiones sobre el llamado“ complejo ecuestre” en la Argentina. RUNA, archivo para las ciencias del hombre 16, (1986). [Google Scholar]
- 11.A. Guinnard, Three Years’ Slavery Among the Patagonians: An Account of His Captivity (R. Bentley, 1871). [Google Scholar]
- 12.M. T. Boschín, L. R. Nacuzzi, Ensayo Metodológico Para la Reconstrucción Etnohistórica: Su Aplicación a la Comprensión del Modelo Tehuelche Meridional (Colegio de Graduados en Antropología, 1979). [Google Scholar]
- 13.M. Martinic Beros, Los Aónikenk, Historia y Cultura (Universidad de Magallanes, 1995). [Google Scholar]
- 14.Moreno E. J., Videla B. A., Rastreando ausencias: La hipótesis Ddel abandono del uso de los recursos marinos en el momento ecuestre en la Patagonia continental. Magallania 36, 91–104 (2008). [Google Scholar]
- 15.R. Goñi, Reacomodamientos poblacionales de momentos históricos en el noroeste de Santa Cruz. Proyecciones arqueológicas, Tendencias teórico-metodológicas y casos de estudio en la Arqueología de Patagonia, 389–396 (2013). [Google Scholar]
- 16.L. A. Borrero, F. M. Martin, Fragmented records. Fuego-Patagonian hunter-gatherers and archaeological change, in Archaeology on the Threshold. J. Wardle, R. Hitchcock, M. Schmader, P.-L. Yu., Eds. (University Press of Florida, 2023), pp. 68–88. [Google Scholar]
- 17.Taylor W. T. T., Hart I., Jones E. L., Brenner-Coltrain J., Jobe J. T., Britt B. B., Gregory McDonald H., Li Y., Zhang C., Le Roux P., Gover C. Q. S., Schiavinato S., Orlando L., Roberts P., Interdisciplinary analysis of the Lehi horse: Implications for early historic horse cultures of the north american west. American Antiquity 86, 465–485 (2021). [Google Scholar]
- 18.Schubert M., Mashkour M., Gaunitz C., Fages A., Seguin-Orlando A., Sheikhi S., Alfarhan A. H., Alquraishi S. A., Al-Rasheid K. A. S., Chuang R., Ermini L., Gamba C., Weinstock J., Vedat O., Orlando L., Zonkey: A simple, accurate and sensitive pipeline to genetically identify equine F1-hybrids in archaeological assemblages. J. Archaeol. Sci. 78, 147–157 (2017). [Google Scholar]
- 19.D. Mazzanti, C. Quintana, Fauna y ambiente en la subsistencia indigena durante el siglo XVIII en Tandilia oriental. Anuario IEHS 27, 209–221 (2012). [Google Scholar]
- 20.T. Navarro, Análisis arqueofaunistico del sitio El Panteon 1 (Las Ovejas, Neuquén). La Zaranda de Ideas. Revista de Jóvenes Investigadores en Arqueología 14, 41–54 (2016). [Google Scholar]
- 21.A. Guillermo, Zooarqueología de la transición prehispánica y posthispánica del sitio arqueológico Casa de Piedra de Ortega (Río Negro, Argentina). Arqueología 24, 251–253 (2018). [Google Scholar]
- 22.A. Guillermo, F. J. Fernández, J. A. Cordero, Impacto de la fauna exótica doméstica en la subsistencia humana en la cuenca superior del río Limay: la evidencia de Casa de Piedra de Ortega (Río Negro, Argentina). Arqueología 26, 171–195 (2020). [Google Scholar]
- 23.M. J. Silveira, Análisis de los restos faunísticos de la Cueva Grande del Arroyo Feo (Santa Cruz). Relaciones de la Sociedad Argentina de Antropología 13, 229–247 (1979). [Google Scholar]
- 24.M. J. Silveira, J. A. Cordero, Zooarqueología del sitio La Marcelina 1. Provincia de Río Negro, Argentina. AtekNa 4, 67–141 (2014). [Google Scholar]
- 25.Lorena L’Heureux G., Borrero L., El uso de la fauna en laguna Cóndor, provincia de Santa Cruz, Argentina. Magallania. 44, 249–257 (2016). [Google Scholar]
- 26.E. L. Jones, W. T. T. Taylor, J. B. Belardi, G. Neme, A. Gil, P. Roberts, C. Thornhill, G. W. L. Hodgins, L. Orlando, Caballos y humanos en el Nuevo mundo: Investigaciones arqueológicas en América del Norte y perspectivas para Argentina. Anales de Arqueología y Etnología 74, 247–268 (2019). [Google Scholar]
- 27.C. B. Ramsey, Bayesian analysis of radiocarbon dates. Radiocarbon 51, 337–360 (2009). [Google Scholar]
- 28.A. K. Behrensmeyer, Taphonomic and ecologic information from bone weathering. Paleobiology 4, 150–162 (1978) [Google Scholar]
- 29.I. A. Silver, The ageing of domestic animals. Sci. Archaeol., 283–302 (1969). [Google Scholar]
- 30.M. A. Levine, The use of crown height measurements and eruption-wear sequences to age horse teeth, in Ageing and Sexing Animal Bones from Archaeological Sites, B. Wilson, C. Grigson, S. Payne, Eds. (BAR, 1982), pp. 223–250. [Google Scholar]
- 31.Tessone A., Srur A., Aranibar J. N., δ13C and δ15N of plants in a longitudinal transect from the Andes to the Atlantic coast: Terrestrial baseline for paleodietary and paleocological studies. J. Archaeol. Sci. Rep. 47, 103787 (2023). [Google Scholar]
- 32.Kottek M., Grieser J., Beck C., Rudolf B., Rubel F., World Map of the Köppen-Geiger climate classification updated. Meteorol. Z. 15, 259–263 (2006). [Google Scholar]
- 33.Hoppe K. A., Stover S. M., Pascoe J. R., Amundson R., Tooth enamel biomineralization in extant horses: Implications for isotopic microsampling. Palaeogeogr. Palaeoclimatol. Palaeoecol. 206, 355–365 (2004). [Google Scholar]
- 34.D’Orazio M., Agostini S., Mazzarini F., Innocenti F., Manetti P., Haller M. J., Lahsen A., The Pali Aike Volcanic Field, Patagonia: Slab-window magmatism near the tip of South America. Tectonophysics. 321, 407–427 (2000). [Google Scholar]
- 35.Brunet F., Gaiero D., Probst J. L., Depetris P. J., Gauthier Lafaye F., P., Stille δ13C tracing of dissolved inorganic carbon sources in Patagonian rivers (Argentina). Hydrol. Process. 19, 3321–3344 (2005). [Google Scholar]
- 36.G. Oliva, L. González, P. Rial, E. Livraghi, El ambiente en la Patagonia Austral. Ganadería ovina sustentable en la Patagonia Austral, 17–80 (2001). [Google Scholar]
- 37.C. Mayr, L. Langhamer, H. Wissel, W. Meier, T. Sauter, C. Laprida, J. Massaferro, G. Försterra, A. Lücke, Atmospheric controls on hydrogen and oxygen isotope composition of meteoric and surface waters in Patagonia. Hydrol. Earth Syst. Sci. Discuss., 1–22 (2018). [Google Scholar]
- 38.Stern L. A., Blisniuk P. M., Stable isotope composition of precipitation across the southern Patagonian Andes. J. Geophys. Res. 107, 3–14 (2002). [Google Scholar]
- 39.J. Merler Carbajo, A. Tessone, Estudios paleodietarios y de movilidad en grupos cazadores-recolectores de la cuenca del Río Pinturas, Santa Cruz, Argentina. Magallania 51, 1–16 (2023). [Google Scholar]
- 40.N. A. Cirigliano, Hallazgo de fragmentos cerámicos en la meseta Bella Vista (campo volcánico Pali Aike, provincia de Santa Cruz, Argentina). Magallania 49 (2021). [Google Scholar]
- 41.Craig O. E., Saul H., Lucquin A., Nishida Y., Taché K., Clarke L., Thompson A., Altoft D. T., Uchiyama J., Ajimoto M., Gibbs K., Isaksson S., Heron C. P., Jordan P., Earliest evidence for the use of pottery. Nature 496, 351–354 (2013). [DOI] [PubMed] [Google Scholar]
- 42.Baeten J., Jervis B., De Vos D., Waelkens M., Molecular evidence for the mixing of meat, fish and vegetables in Anglo‐Saxon coarseware from Hamwic,UK. Archaeometry 55, 1150–1174 (2013). [Google Scholar]
- 43.Bondetti M., Scott E., Courel B., Lucquin A., Shoda S., Lundy J., Labra-Odde C., Drieu L., Craig O. E., Investigating the formation and diagnostic value of ω-(o-alkylphenyl)alkanoic acids in ancient pottery. Archaeometry 63, 594–608 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Evershed R. P., Copley M. S., Dickson L., Hansel F. A., Experimental evidence for the processing of marine animal products and other commodities containing polyunsaturated fatty acids in pottery vessels. Archaeometry 50, 101–113 (2008). [Google Scholar]
- 45.Lucquin A., Gibbs K., Uchiyama J., Saul H., Ajimoto M., Eley Y., Radini A., Heron C. P., Shoda S., Nishida Y., Lundy J., Jordan P., Isaksson S., Craig O. E., Ancient lipids document continuity in the use of early hunter–gatherer pottery through 9,000 years of Japanese prehistory. Proc. Natl. Acad. Sci.U.S.A. 113, 3991–3996 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.S. N. Dudd, Molecular and Isotopic Characterisation of Animal Fats in Archaeological Pottery (University of Bristol, 1999). [Google Scholar]
- 47.A. K. Outram, N. A. Stear, R. Bendrey, S. Olsen, A. Kasparov, V. Zaibert, N. Thorpe, R. P. Evershed, The earliest horse harnessing and milking. Science 323, 1332–1335 (2009). [DOI] [PubMed] [Google Scholar]
- 48.M. Regert, Analytical strategies for discriminating archeological fatty substances from animal origin. Mass Spectrom. Rev. 30, 177–220 (2011). [DOI] [PubMed] [Google Scholar]
- 49.L. Cramp, R. P. Evershed, Reconstructing aquatic resource exploitation in human prehistory using lipid biomarkers and stable isotopes, in Treatise on Geochemistry (Elsevier, 2014), pp. 319–339. [Google Scholar]
- 50.G. Claraz, Viaje al río Chubut: Aspectos Naturalísticos y Etnológicos (1865–1866) (Ediciones Continente, 2008). [Google Scholar]
- 51.M. A. Levine, The origins of horse husbandry on the Eurasian steppe, in Late Prehistoric Exploitation of the Eurasian Steppe, M. Levine, Y. Rassamakin, A. Kislenko, N. Tatarintseva, Eds. (McDonald Institute, 1999), pp. 5–58. [Google Scholar]
- 52.L. Bartosiewicz, E. Gal, Shuffling Nags, Lame Ducks: The Archaeology of Animal Disease (Oxbow Books, 2013). [Google Scholar]
- 53.Bendrey R., New methods for the identification of evidence for bitting on horse remains from archaeological sites. J. Archaeol. Sci. 34, 1036–1050 (2007). [Google Scholar]
- 54.Li Y., Zhang C., Taylor W. T. T., Chen L., Flad R. K., Boivin N., Liu H., You Y., Wang J., Ren M., Xi T., Han Y., Wen R., Ma J., Early evidence for mounted horseback riding in northwest China. Proc. Natl. Acad. Sci. U.S.A. 117, 29569–29576 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Santos G. M., Moore R. B., Southon J. R., Griffin S., Hinger E., Zhang D., AMS14C sample preparation at the KCCAMS/UCI facility: status report and performance of small samples. Radiocarbon 49, 255–269 (2007). [Google Scholar]
- 56.Southon J., Santos G., Druffel-Rodriguez K., Druffel E., Trumbore S., Xu X., Griffin S., Ali S., Mazon M., The keck carbon cycle AMS laboratory, University of California, Irvine: Initial operation and a background surprise. Radiocarbon 46, 41–49 (2004). [Google Scholar]
- 57.Reimer P. J., Austin W. E. N., Bard E., Bayliss A., Blackwell P. G., Ramsey C. B., Butzin M., Cheng H., Lawrence Edwards R., Friedrich M., Grootes P. M., Guilderson T. P., Hajdas I., Heaton T. J., Hogg A. G., Hughen K. A., Kromer B., Manning S. W., Muscheler R., Palmer J. G., Pearson C., van der Plicht J., Reimer R. W., Richards D. A., Marian Scott E., Southon J. R., Turney C. S. M., Wacker L., Adolphi F., Büntgen U., Capano M., Fahrni S. M., Fogtmann-Schulz A., Friedrich R., Köhler P., Kudsk S., Miyake F., Olsen J., Reinig F., Sakamoto M., Sookdeo A., Talamo S., The IntCal20 northern hemisphere radiocarbon age calibration curve (0–55 cal kBP). Radiocarbon 62, 725–757 (2020). [Google Scholar]
- 58.Pin C., Briot D., Bassin C., Poitrasson F., Concomitant separation of strontium and samarium-neodymium for isotopic analysis in silicate samples, based on specific extraction chromatography. Anal. Chim. Acta 298, 209–217 (1994). [Google Scholar]
- 59.Copeland S. R., Sponheimer M., le Roux P. J., Grimes V., Lee-Thorp J. A., de Ruiter D. J., Richards M. P., Strontium isotope ratios (87Sr/86Sr) of tooth enamel: a comparison of solution and laser ablation multicollector inductively coupled plasma mass spectrometry methods. Rapid Commun. Mass Spectrom. 22, 3187–3194 (2008). [DOI] [PubMed] [Google Scholar]
- 60.Waight T., Baker J., Peate D., Sr isotope ratio measurements by double-focusing MC-ICPMS: techniques, observations and pitfalls. Int. J. Mass Spectrom. 221, 229–244 (2002). [Google Scholar]
- 61.Evershed R. P., Heron C., John Goad L., Analysis of organic residues of archaeological origin by high-temperature gas chromatography and gas chromatography-mass spectrometry. Analyst 115, 1339–1342 (1990). [Google Scholar]
- 62.Wade C. M., Giulotto E., Sigurdsson S., Zoli M., Gnerre S., Imsland F., Lear T. L., Adelson D. L., Bailey E., Bellone R. R., Blöcker H., Distl O., Edgar R. C., Garber M., Leeb T., Mauceli E., MacLeod J. N., Penedo M. C. T., Raison J. M., Sharpe T., Vogel J., Andersson L., Antczak D. F., Biagi T., Binns M. M., Chowdhary B. P., Coleman S. J., Valle G. D., Fryc S., Guérin G., Hasegawa T., Hill E. W., Jurka J., Kiialainen A., Lindgren G., Liu J., Magnani E., Mickelson J. R., Murray J., Nergadze S. G., Onofrio R., Pedroni S., Piras M. F., Raudsepp T., Rocchi M., Røed K. H., Ryder O. A., Searle S., Skow L., Swinburne J. E., Syvänen A. C., Tozaki T., Valberg S. J., Vaudin M., White J. R., Zody M. C.; Broad Institute Genome Sequencing Platform, Broad Institute Whole Genome Assembly Team, Lander E. S., Lindblad-Toh K., Genome sequence, comparative analysis, and population genetics of the domestic horse. Science 326, 865–867 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xu X., Arnason U., The complete mitochondrial DNA sequence of the horse, Equus caballus: Extensive heteroplasmy of the control region. Gene 148, 357–362 (1994). [DOI] [PubMed] [Google Scholar]
- 64.Librado P., Khan N., Fages A., Kusliy M. A., Suchan T., Tonasso-Calvière L., Schiavinato S., Alioglu D., Fromentier A., Perdereau A., Aury J.-M., Gaunitz C., Chauvey L., Seguin-Orlando A., Der Sarkissian C., Southon J., Shapiro B., Tishkin A. A., Kovalev A. A., Alquraishi S., Alfarhan A. H., Al-Rasheid K. A. S., Seregély T., Klassen L., Iversen R., Bignon-Lau O., Bodu P., Olive M., Castel J.-C., Boudadi-Maligne M., Alvarez N., Germonpré M., Hoyo M. M.-D., Wilczyński J., Pospuła S., Lasota-Kuś A., Tunia K., Nowak M., Rannamäe E., Saarma U., Boeskorov G., Lōugas L., Kyselý R., Peške L., Bălășescu A., Dumitrașcu V., Dobrescu R., Gerber D., Kiss V., Szécsényi-Nagy A., Mende B. G., Gallina Z., Somogyi K., Kulcsár G., Gál E., Bendrey R., Allentoft M. E., Sirbu G., Dergachev V., Shephard H., Tomadini N., Grouard S., Kasparov A., Basilyan A. E., Anisimov M. A., Nikolskiy P. A., Pavlova E. Y., Pitulko V., Brem G., Wallner B., Schwall C., Keller M., Kitagawa K., Bessudnov A. N., Bessudnov A., Taylor W., Magail J., Gantulga J.-O., Bayarsaikhan J., Erdenebaatar D., Tabaldiev K., Mijiddorj E., Boldgiv B., Tsagaan T., Pruvost M., Olsen S., Makarewicz C. A., Lamas S. V., Canadell S. A., Espinet A. N., Iborra M. P., Garrido J. L., González E. R., Celestino S., Olària C., Arsuaga J. L., Kotova N., Pryor A., Crabtree P., Zhumatayev R., Toleubaev A., Morgunova N. L., Kuznetsova T., Lordkipanize D., Marzullo M., Prato O., Gianni G. B., Tecchiati U., Clavel B., Lepetz S., Davoudi H., Mashkour M., Berezina N. Y., Stockhammer P. W., Krause J., Haak W., Morales-Muñiz A., Benecke N., Hofreiter M., Ludwig A., Graphodatsky A. S., Peters J., Kiryushin K. Y., Iderkhangai T.-O., Bokovenko N. A., Vasiliev S. K., Seregin N. N., Chugunov K. V., Plasteeva N. A., Baryshnikov G. F., Petrova E., Sablin M., Ananyevskaya E., Logvin A., Shevnina I., Logvin V., Kalieva S., Loman V., Kukushkin I., Merz I., Merz V., Sakenov S., Varfolomeyev V., Usmanova E., Zaibert V., Arbuckle B., Belinskiy A. B., Kalmykov A., Reinhold S., Hansen S., Yudin A. I., Vybornov A. A., Epimakhov A., Berezina N. S., Roslyakova N., Kosintsev P. A., Kuznetsov P. F., Anthony D., Kroonen G. J., Kristiansen K., Wincker P., Outram A., Orlando L., The origins and spread of domestic horses from the Western Eurasian steppes. Nature 598, 634–640 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Text
Tables S1 to S5
Figs. S1 to S3