Abstract
The salt-sensitive mutant 549 of the cyanobacterium Synechocystis sp. strain PCC 6803 was genetically and physiologically characterized. The mutated site and corresponding wild-type site were cloned and partially sequenced. The genetic analysis revealed that during the mutation about 1.8 kb was deleted from the chromosome of mutant 549. This deletion affected four open reading frames: a gcp gene homolog, the psaFJ genes, and an unknown gene. After construction of mutants with single mutations, only the gcp mutant showed a reduction in salt tolerance comparable to that of the initial mutant, indicating that the deletion of this gene was responsible for the salt sensitivity and that the other genes were of minor importance. Besides the reduced salt tolerance, a remarkable change in pigmentation was observed that became more pronounced in salt-stressed cells. The phycobilipigment content decreased, and that of carotenoids increased. Investigations of changes in the ultrastructure revealed an increase in the amount of characteristic inclusion bodies containing the high-molecular-weight nitrogen storage polymer cyanophycin (polyaspartate and arginine). The salt-induced accumulation of cyanophycin was confirmed by chemical estimations. The putative glycoprotease encoded by the gcp gene might be responsible for the degradation of cyanophycin in Synechocystis. Mutation of this gene leads to nitrogen starvation of the cells, accompanied by characteristic changes in pigmentation, ultrastructure, and salt tolerance level.
The physiological basis for the adaptation to high salinities has been studied intensively in several cyanobacterial species. It includes three main subprocesses: active extrusion of inorganic ions, leading to relatively unchanged internal salt concentrations; accumulation of large internal amounts of organic osmoprotective compounds; and expression of a set of salt stress proteins (17). The ion export is mediated by Na+/H+ antiporters, which are energized by the respiratory chain localized on the cytoplasmic membrane (27), and by H+- ATPases (12). On the basis of the principal osmoprotective compound accumulated, three salt tolerance groups of cyanobacteria could be distinguished (29). Slightly salt-tolerant strains accumulate sucrose or trehalose, moderately halotolerant strains synthesize glucosylglycerol (GG), and halophilic strains contain glycine betaine or glutamate betaine. The synthesis of salt stress proteins was analyzed by amino acid labelling. After comparison of stress protein synthesis in salt-shocked cells with that in cells subjected to other stresses, the occurrence of general and specific stress proteins became obvious. A first general stress protein was identified in Synechocystis sp. strain PCC 6803 as a flavodoxin (11).
The genetic processes involved in cyanobacterial salt adaptation have been investigated for only a short time. With a subtractive hybridization procedure, it was shown that about 10% of the genome of Anabaena torulosa seems to be salt inducible (5). Genes encoding enzymes involved in the ion export process (cytochrome oxidase [3]), in the synthesis of osmoprotective compounds (GG-phosphate phosphatase [18]), and in the transport of osmoprotective compounds (19) have been cloned and functionally characterized. For the identification of additional genes necessary in the process of salt adaptation, the generation of mutants represents a powerful tool, since after the characterization of such mutants processes originally not thought to be essential for salt adaptation can be identified. In 1990, several spontaneous cyanobacterial mutants were obtained that showed a defect in respiration and a salt-sensitive phenotype (21).
We have employed the method of random cartridge mutagenesis (23) to generate salt-sensitive mutants of the cyanobacterium Synechocystis. Synechocystis sp. strain PCC 6803 belongs to the group of moderately halotolerant cyanobacteria (salt resistance limit, about 1.2 M NaCl) and accumulates mainly GG after salt stress (28). Recently, the genome of this strain was completely sequenced (22). Random cartridge mutagenesis offers the advantage that the mutated site is tagged by an antibiotic resistance gene marker, which makes it easier to reclone the affected genes and guarantees a stable mutation in the multicopy genome of this strain. In a first attempt, three salt-sensitive mutants (mutants 143, 406, and 549) that had different remaining salt tolerances were obtained (15). With the antibiotic resistance gene as a probe in Southern hybridization experiments, it was found that different sites of the chromosome were affected in the three mutants. The salt-sensitive phenotype of one mutant (mutant 143) could be correlated with a defect in GG synthesis, while the other two mutants (mutants 406 and 549) were able to accumulate the same amount of osmolytes as the wild type (WT) and the defect leading to reduced salt tolerance remained unknown (15).
In this paper we describe the genetic and physiological characterization of the salt-sensitive Synechocystis mutant 549. The mutated site was cloned and used to screen for the WT genes in a gene library. After sequencing, a deletion affecting four genes was found in mutant 549. A newly generated single mutant showing a defect only in a putative glycoprotease resembled the original mutant 549. After cultivation in high-salt media, a remarkable change in pigmentation accompanied by changes in the ultrastructure was found in salt treated cells of this mutant.
MATERIALS AND METHODS
Strains and culture conditions.
The derivative of Synechocystis sp. strain PCC 6803 with enhanced transforming capacity that was used in all experiments was obtained from S. Shestakov (Moscow State University, Russia). Axenic cells were cultivated on agar plates or in liquid culture at 30°C under constant illumination with a mineral medium (15). Salt-sensitive mutants were generated by random cartridge mutagenesis (23) using the aphII gene (aminoglycoside phosphotransferase II gene, conferring kanamycin resistance) isolated from plasmid pUC4K (35). The whole procedure, including transformation of Synechocystis, was described earlier (15). Transformants were initially selected on media containing 10 μg of kanamycin (Sigma) per ml, while the segregation of clones and the cultivation of mutants were conducted with 50 μg of kanamycin per ml. Escherichia coli JM101 (30) was used for routine DNA manipulations, while strain Q358 (30) served as a host for bacteriophage λ. E. coli was cultivated in Luria broth (LB) medium at 37°C.
DNA manipulations.
Total DNA was isolated from Synechocystis by lysozyme treatment and phenol-chloroform purification (5). All other DNA techniques, such as plasmid isolation, transformation of E. coli, ligations, restriction analysis (restriction enzymes were obtained from Life Technologies), Southern hybridization analysis, plaque hybridization, isolation of λ DNA, and DNA labelling by random priming using [α-32P]dATP (Amersham Buchler), were standard methods (30). The WT genes were cloned from a library of Synechocystis DNA fragments in the λ vector EMBL3, kindly provided by H. D. Osiewacz, Ruhr Universität, Bochum, Germany. DNA fragments for sequencing were generated by subcloning defined restriction fragments into pUC18, pUC19 (37), pUCBM20, pUCBM21 (Boehringer Mannheim), or pGEM7 (Promega). Additionally, partially deleted clones were obtained by double-stranded nested deletion (kit from Pharmacia). DNA sequencing was performed by the dideoxy chain termination method using [α-35S]dATP (Amersham Buchler) and the Sequenase 2.0 kit (USB). For sequencing, double-stranded plasmid DNA was isolated by means of the QIAprep plasmid kit (Qiagen). The site of integration of the aphII gene into the chromosomal DNA was characterized by partial sequencing using the following synthetic primers specific for the aphII gene: kan 5′, CAGGCCTGGTATGAGTCAGC; kan 3′, ATTTTTATCTTGTGCAATGT (custom oligonucleotide synthesis; Pharmacia). Computer analysis of the DNA sequence was done by means of the DNASIS/PROSIS software package (Pharmacia).
Analysis of mutant phenotype.
Salt-shock experiments were performed in CO2-gassed (2%) batch cultures of mutant and WT cells after addition of solid NaCl (usually to give a final concentration of 684 mM NaCl) to the standard medium (containing 2 mM NaCl). Photosynthesis and respiration were measured with a Clark-type oxygen electrode (9). The growth of cells was monitored by estimation of the extinction at 750 nm (A750) at appropriate dilutions employing a regression to calculate cell number and biomass. The cyanophycin (multi-l-arginyl-poly[l-aspartic acid]) content was estimated after isolation of cyanophycin granules, their solubilization by 0.1 N HCl, and chemical estimation of arginine exactly as described previously (4). The protein content was also estimated as described previously (24).
Pigment analyses.
The chlorophyll a (Chl a) and carotenoid concentrations of the samples were determined by high-performance liquid chromatography (HPLC) according to a modified method described previously (32) and with previously published extinction coefficients (25). Due to difficulties encountered in quantitative extraction of phycobiliproteins, the pigment content was routinely estimated from measurements of whole-sample absorption. Absorption was measured with samples placed at the entrance of the integrating sphere accessory (Labsphere; Perkin Elmer) of a spectrophotometer (Lambda 2; Perkin Elmer) to minimize scattering. The pigment-specific absorption coefficient for phycocyanin, A*630, determined for a subsample of WT cells was found to be relevant as well for the mutant cells, indicating that differences in pigment “packaging” were low over the range of absorbances encountered in the different cultures.
Electron microscopy.
Cells used for analyses of ultrastructure were taken directly from cultures, immediately fixed with glutaraldehyde (4% in 0.1 M Na phosphate buffer), and additionally fixed with 1% osmium tetroxide, and water was removed with acetone. These cells were embedded in an epoxy resin (Araldite; Fluka) and cut into ultrathin sections. Sections of cells on the grids were treated with 2% uranyl acetate and lead citrate. The micrographs were obtained with an electron microscope, model EM902 (Zeiss).
RESULTS
Gene cloning.
Integration of the aphII gene into the genome of the salt-sensitive Synechocystis mutant 549 was first characterized in Southern blot experiments, in which an EcoRI fragment of about 5.0 kb hybridized with the aphII gene probe (15). This 5.0-kb EcoRI fragment of chromosomal DNA from mutant 549 was cloned into the plasmid pBR329 (8). Kmr clones of E. coli were selected, and the recombinant plasmids were isolated and characterized by restriction analysis (Fig. 1). The aphII gene cartridge (about 1.3 kb) was obtained together with about 3.7 kb of flanking cyanobacterial DNA. The resulting plasmid, pBRM549, was used to transform the Synechocystis WT. Two hundred Kmr clones were selected and, after complete segregation, tested for salt and chloramphenicol resistance. All transformants tested were sensitive to both high salt concentrations (600 mM NaCl) and chloramphenicol (5 μg/ml; pBR329 harbors a chloramphenicol resistance marker). These results indicate that the salt-sensitive phenotype is coupled to the integration of the aphII gene at the site cloned in pBRM549. The absence of chloramphenicol resistance after transformation with pBRM549 demonstrates that only the EcoRI fragment, not the vector molecule, had been integrated in a double-crossover event because single recombinants should carry all the markers of the plasmid.
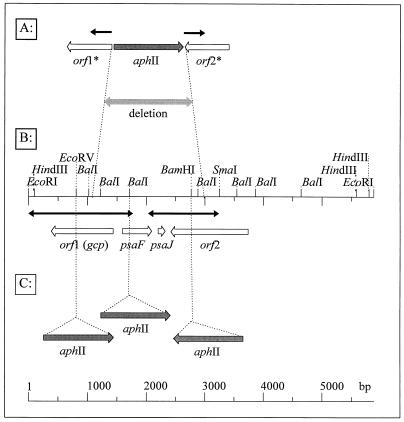
FIG. 1.
Schematic drawing showing the genetic organization, restriction map, and protein-encoding regions of the chromosomal site affected in the Synechocystis mutant 549 (A), the corresponding site of the Synechocystis WT (B), and the insertion sites of the aphII gene in selected sites of the different genes to obtain mutants with single mutations (C). Empty arrows, protein-encoding regions in Synechocystis; darkly shaded arrows, inserted aphII gene cassettes; black arrows, regions sequenced in this work; ∗, partial deleted genes; lightly stippled arrow, deletion that occurred during integration of an aphII gene in mutant 549.
The DNAs flanking the aphII gene in pBRM549 were obtained as HindIII fragments from pBRM549 (the aphII gene contains one HindIII site) and used as probes for screening the Synechocystis gene library to clone the corresponding WT region. Positive plaques hybridizing to both probes were purified to obtain single clones by three screening steps. DNA from one of the lambda clones obtained was isolated and characterized by Southern hybridization analysis using the same probe that was used for screening. With both probes, an EcoRI fragment of about 5.8 kb was specifically recognized. It was cloned into pUC18, leading to the plasmid pM549S (Fig. 1). The plasmid pM549S was transformed into mutant 549 cells, which were then selected on high-salt media (600 mM NaCl). Thousands of clones had been restored to high salt tolerance, indicating that the EcoRI fragment cloned into pM549S contained the complete WT region affected in the mutant 549.
Sequence analysis.
After subcloning of a 3.2-kb SmaI/EcoRI fragment and generation of overlapping deletions by digestion with exonuclease III, the nucleotide sequence of this fragment was determined for both strands starting from the 5′ and 3′ ends (Fig. 1). During sequencing, similarities to previously published sequences from Synechocystis were recognized (7, 36). Therefore, the central part of the 3.2-kb SmaI/EcoRI fragment was not sequenced again. For precise determination of the integration site of the aphII gene during the generation of mutant 549, the cyanobacterial DNA flanking the Kmr cartridge on pBRM549 was partly sequenced with primers specific to the 5′ and 3′ ends of the aphII gene. Sequences identical to the WT fragment (for the 5′ sequence before position 263 and for the 3′ sequence after position 2092) were found (not shown). Therefore, about 1.8 kb of the WT region was deleted during integration of the aphII gene cassette into the genome of mutant 549 (Fig. 1).
The nucleotide sequence was subjected to computer analysis. Four putative protein-coding regions (open reading frames [ORFs]) could be identified on the sequenced SmaI/EcoRI fragment (Fig. 1), all of which were affected by the deletion in mutant 549 (Fig. 1). The deduced amino acid sequences were compared to amino acid sequences from databases. The amino acid sequences of the two completely deleted ORFs were 100% identical to the PsaF and PsaJ proteins of Synechocystis, which were already sequenced (7) and are part of photosystem I. From the gene orf1 upstream of psaF, probably encoding a sialoglycoprotease (slr0807, a gcp gene homolog [22]), the promoter region and the N-terminal part of the protein were deleted in mutant 549. The ORF2 protein, encoded downstream of psaJ, does not show any similarities to known proteins (slr0806 [22]). In mutant 549, about 75 amino acid residues of its C-terminal part are missing (Fig. 1).
Construction of mutants with mutations affecting single genes.
In order to clarify the role of the four ORFs deleted in mutant 549 in salt tolerance in more detail, several insertion mutants with mutations affecting single genes were constructed (Fig. 1) (see Table 1 for nomenclature of the mutants) and physiologically characterized. The psaF and psaJ genes were mutated together by integration of an aphII gene into the BalI site present inside psaF, since both genes are transcribed as an operon (7). A gcp (ORF1) mutant was constructed by cloning the aphII gene cartridge into the internal EcoRV site of the gcp gene homolog. Finally, ORF2 was mutated by insertion of the resistance marker into the BamHI site close to the 3′ end of its coding sequence (Fig. 1). Plasmids showing correct integration of the aphII gene were transformed into Synechocystis, from which Kmr clones originating from homologous recombination with the chromosomal DNA were selected (Table 1).
TABLE 1.
Plasmids and mutants of Synechocystis sp. strain PCC 6803 used and constructed in this study (Fig. 1)
| Designation | Size (kb) | Description |
|---|---|---|
| pBRM549 | 9.2 | pBR329 containing a 5.0-kb EcoRI fragment, the integration site of the aphII gene in mutant 549 |
| pM549S | 8.5 | pUC18 containing a 5.8-kb EcoRI fragment, the wild-type fragment corresponding to the site affected in mutant 549 |
| pM549E/S | 5.9 | pUC18 containing a 3.2-kb EcoRI/SmaI subfragment of pM549S |
| p549ORF1::Km | 7.2 | pM549E/S containing an inactivated ORF1 (gcp gene) (aphII gene inserted at the EcoRV site) |
| p549PSA::Km | 7.2 | pM549E/S containing an inactivated psaF gene (aphII gene inserted at the central BalI site) |
| p549ORF2::Km | 7.2 | pM549E/S containing an inactivated ORF2 (aphII gene inserted at the BamHI site) |
| orf1(gcp) mutant | Synechocystis mutant obtained after transformation of the wild type with p549ORF1::Km | |
| psaF mutant | Synechocystis mutant obtained after transformation of the wild type with p549PSA::Km | |
| orf2 mutant | Synechocystis mutant obtained after transformation of the wild type with p549ORF2::Km |
Levels of salt tolerance of the mutants were compared to those of the WT and the original mutant 549 by growing all clones on solid media and in liquid media in the presence of 2 to 684 mM NaCl. The gcp (ORF1) integration mutant showed a salt-sensitive phenotype similar to that of mutant 549 with a maximal tolerance reduced to less than 550 mM NaCl. In contrast, the mutants impaired in psaF, psaJ, and ORF2 grew as well as the WT in media containing 684 mM NaCl (data not shown). Chromosomal DNA was isolated from all mutants and analyzed by DNA-DNA hybridization with the aphII gene and the genes concerned as probes. In all cases, the aphII gene probe gave signals showing that it was introduced at the expected sites. The hybridizations using the gene probes showed that the mutants were completely segregated, since no signals of the size corresponding to the WT alleles could be observed. Only the results for the salt-sensitive gcp mutant are shown in comparison to the WT in Fig. 2. After restriction by EcoRI and BamHI, a fragment about 1.3 kb larger than the 2.9-kb WT fragment hybridized with DNA of the gcp mutant with the gcp gene as a probe, while after digestion by HindIII and BamHI, in contrast to WT DNA, two fragments were recognized by this probe in the mutant DNA, since the inserted aphII gene contained a HindIII site. With the aphII gene as a probe, the same fragments showed signals with DNA of the gcp mutant, while with WT DNA specific hybridization signals could not be detected (Fig. 2). These hybridization patterns indicated that recombination had occurred via a double-crossover event, with replacement of the WT alleles by the mutated copies.
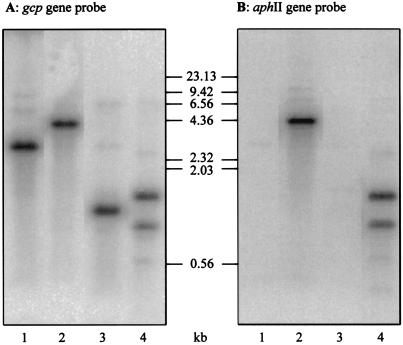
FIG. 2.
Southern blot experiments for characterization of complete segregation of the gcp mutant. The 32P-labelled internal BclI/KpnI fragment of the gcp gene (A) or the 32P-labelled aphII gene (B) was used as a probe for the hybridization to EcoRI-BamHI- (lanes 1 and 2) and to HindIII-BamHI-digested (lanes 3 and 4) chromosomal DNA from the WT (lanes 1 and 3) and the gcp mutant (lanes 2 and 4), respectively. The molecular masses of HindIII-digested λ DNA size standards were drawn by using the positions from the photo of the ethidium bromide-stained gel at the same magnification.
Physiological characterization of the gcp (ORF1) mutant.
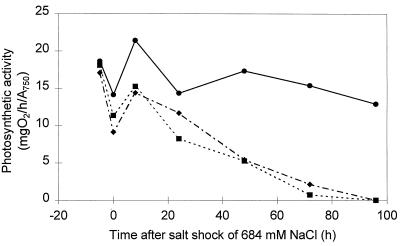
Among the mutants with single mutations constructed in the genes affected in mutant 549, only the gcp mutant showed a significant reduction in its salt tolerance level and it was therefore chosen for further analyses. The growth and photosynthesis of the gcp mutant was compared to those of the original mutant 549 and the WT after applying a salt shock of 684 mM NaCl. In all cases an immediate decrease in photosynthesis was observed after the addition of high salt concentrations (Fig. 3). During the first 8 h after shock, photosynthesis recovered in the WT as well as in the mutants. While the WT cells were able to adapt photosynthesis to the demands of high salt concentrations completely after only 48 h, in cells of the mutant 549 and in the gcp mutant, respectively, it decreased continuously until 96 h, when photosynthesis was completely inhibited (Fig. 3). Growth of the mutants stopped by 48 h after the salt shock, while growth of the WT had already recovered (Table 2).
FIG. 3.
Alterations of photosynthetic oxygen evolution after a salt shock of 684 mM NaCl in WT cells (•), mutant 549 cells (▪), and the gcp mutant (⧫). The diagram represents means from three independent experiments.
TABLE 2.
Alterations in the growth, cyanophycin content (analyzed by electron microscopy), and pigmentation analyzed by HPLC in cells of the gcp mutant and the Synechocystis WT grown in basal medium and after a salt shock of 684 mM NaCl for different times
| Strain | Time (h) | Growth (h−1) | No. of cyanophycin granules/cella | Pigment content (μg/A750)b
|
||||
|---|---|---|---|---|---|---|---|---|
| Chl a | Myxoxa. | Zeaxant. | Echinen. | β-Carotene | ||||
| gcp mutant | Cc | 0.0395 | 4.2 ± 1.0 | 4.03 | 1.82 | 1.14 | 0.82 | 1.28 |
| gcp mutant | 24 | 0.0131 | 3.6 ± 1.7 | 1.04 | 0.45 | 0.37 | 0.67 | 1.21 |
| gcp mutant | 48 | 0.0132 | 6.0 ± 1.6 | 0.56 | 0.35 | 0.24 | 0.51 | 0.98 |
| gcp mutant | 72 | 0 | 6.4 ± 1.2 | 1.55 | 1.19 | 0.56 | 1.03 | 1.49 |
| gcp mutant | 96 | 0 | 7.3 ± 1.3 | 1.1 | 0.90 | 0.41 | 1.05 | 1.33 |
| WT | Cc | 0.0414 | 1.8 ± 1.1 | 7.16 | 1.94 | 1.63 | 0.77 | 1.56 |
| WT | 24 | 0.0179 | 0.9 ± 1.4 | 1.39 | 0.28 | 0.33 | 0.40 | 1.22 |
| WT | 48 | 0.0308 | 0.3 ± 0.4 | 1.2 | 0.19 | 0.25 | 0.31 | 1.10 |
| WT | 72 | 0.0275 | 0.2 ± 0.4 | 2.65 | 0.39 | 0.64 | 0.48 | 1.46 |
| WT | 96 | 0.0268 | 0 | 2.35 | 0.27 | 0.62 | 0.48 | 1.59 |
Mean ± SD (n = 7).
Myxoxa., myxoxanthophyll; Zeaxant., zeaxanthine; Echinen., echinenone.
C, control.
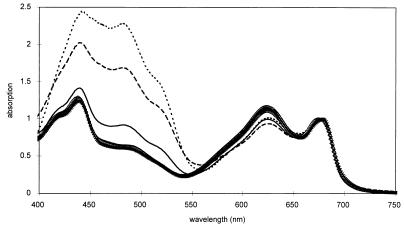
The decrease in photosynthesis was accompanied by a remarkable change in the color of the mutants. Compared with the blue-green color of the WT, cells of the gcp mutant were yellow-green in basal medium and became almost completely yellow after transfer to high-salt medium. With in vivo absorption spectra, which were normalized at the red peak of Chl a, it became obvious that the carotenoids in particular were drastically enhanced compared with Chl a, while the phycobilipigments were slightly reduced (Fig. 4; Table 2). These changes were already visible in cultures in basal medium, but they were increased severalfold after the salt shock. In order to define whether, in addition to the quantitative increase in carotenoids, qualitative changes in their composition also occurred, the pigments were analyzed by HPLC. Quantitative analysis also showed a reduced Chl a content of the gcp mutant cells, leading to an increase in the ratios of all carotenoids per Chl a in the mutant, especially after transfer to salt medium (Table 2). In cells grown in basal medium, the carotenoid contents and ratios were not significantly changed, but in salt-stressed cells of the gcp mutant, the carotenoid content per cell increased and the carotenoid composition was markedly changed. Among the carotenoids, the contents of myxoxanthophyll and echinone were doubled in salt-stressed mutant cells, while the contents of zeaxanthine and β-carotene resembled that of the WT (Table 2).
FIG. 4.
Comparison of in vivo absorption spectra obtained from WT cells and from the gcp mutant grown in basal medium or after a salt shock of 684 mM NaCl for 48 and 96 h. All spectra were normalized at the absorption maximum of Chl a at 680 nm. WT: control cells, ✻; 48 h, ▵; 96 h, ◊. gcp mutant: control cells, ———; 48 h, – – –; 96 h, - - -.
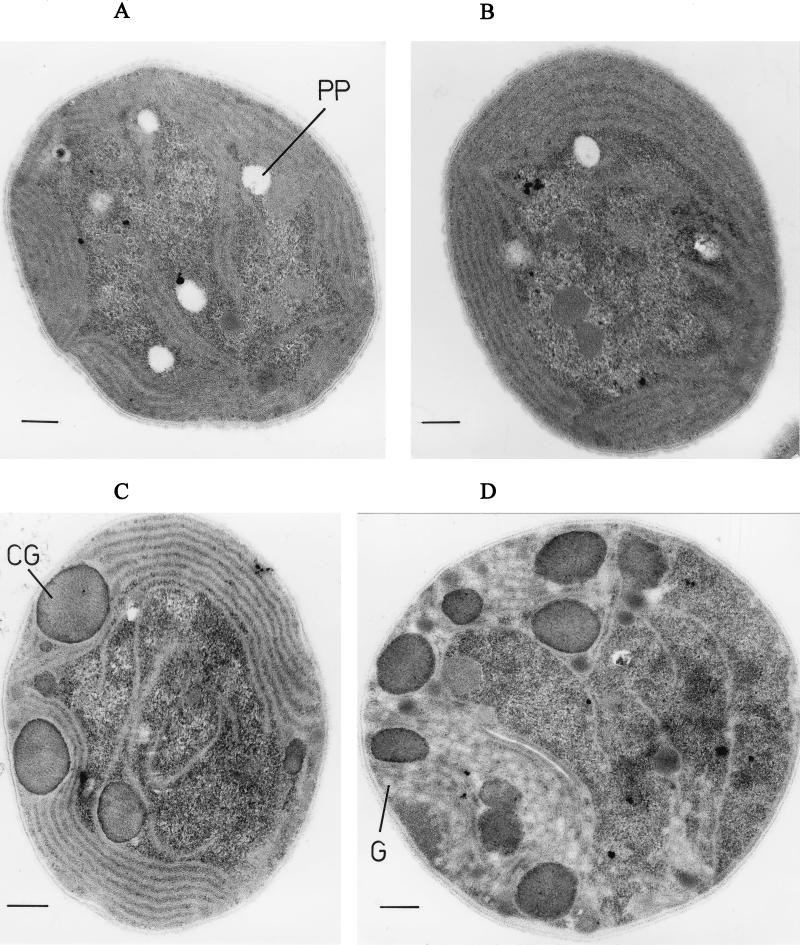
The changes in pigmentation made it very promising to search for alterations in the ultrastructure of the cells. Cells grown in basal medium and salt-stressed cells were compared by transmission electron microscopy, where remarkable structural alterations became obvious. The amount and size of inclusion bodies were particularly enhanced in the gcp mutant (Fig. 5). This increase became even more pronounced in salt-stressed cells. In mutant cells treated for 96 h with high salt concentrations, about 20 to 30% of the cell volume was occupied by these inclusion bodies. On the basis of their characteristic internal structures they could be identified as cyanophycin granules (33, 34). Therefore, a defect in the gcp gene homolog leads to a massive accumulation of cyanophycin in Synechocystis. This accumulation of cyanophycin granules could be confirmed by chemical estimations of the cyanophycin content. In cells of the gcp mutant stressed for 96 h by 684 mM NaCl, about 40 times more cyanophycin was found than in the WT, while in control cells the difference was not significant (WT, control, 0.49 μg of cyanophycin/mg of protein; salt-stressed cells, 0.93 μg/mg; gcp mutant, control, 0.48 μg/mg; salt-stressed cells, 21.48 μg/mg). In addition to the accumulation of cyanophycin granules, the accumulation of large amounts of glycogen, seen as small white granules between thylakoids (34), and a decrease in large white areas outside the thylakoid space were found in comparison to WT cells (Fig. 5). These large white areas in the micrographs of the cells represent holes, most probably caused by the volatilization of polyphosphate granules under the electron beam in thin sections (34). Furthermore, the organization of the thylakoid system disappeared in salt-stressed cells of the gcp mutant. Only some irregularly positioned thylakoid fragments remained visible in cells treated for 72 to 96 h with 684 mM NaCl. In salt-treated cells of the WT, almost no differences were visible compared with differences in cells grown in basal medium (Fig. 5), while in the cyanobacterium Microcystis firma significant salt-induced alterations in the ultrastructure were detected (31).
FIG. 5.
Comparison by electron microscopy of the ultrastructures of WT cells (A and B) and of the gcp mutant (C and D) grown in basal medium (A and C) or after a salt shock of 684 mM NaCl for 96 h (B and D). The bars represent 0.25 μm. CG, cyanophycin granules; G, glycogen; PP, polyphosphate.
DISCUSSION
During the characterization of Synechocystis mutant 549, a deletion of about 1.8 kb was found. The occurrence of deletions with random cartridge mutagenesis was also found in previous studies, in which 1.3 to 50 kb has been lost, leading to very stable mutants (6, 16). These deletions make it difficult to decide which of the affected ORFs is responsible for the observed phenotype. In order to identify the gene responsible for the reduced salt tolerance of mutant 549, mutants with single mutations were generated and characterized regarding salt tolerance. The psaFJ genes, which were completely deleted in mutant 549, seem to be nonessential for salt adaptation in Synechocystis, since an insertion mutant impaired in psaF could grow on 684 mM NaCl. Nevertheless, in the kinetics of adaptation to high salt concentrations, differences compared to the WT were detectable (unpublished data). These subunits of photosystem I are of minor importance for its function, because psaFJ mutants showed no significant alterations in photosynthetic activity (7). Recently, it was found that the PsaF protein might be involved in electron transfer from plastocyanin to P700 in higher plants (20). orfII, encoding a protein of completely unknown function, was partly deleted in mutant 549, but an insertion mutant specific for this gene showed no alterations after growth in high-salt and basal medium compared with the WT.
The reduction in salt tolerance found for mutant 549 could be reproduced in experiments with an insertion mutation in orfI or gcp. Both the mutant 549 and the gcp mutant showed a reduction of remaining salt tolerance to the same extent (limit less than 550 mM NaCl) and the same kinetic behavior after a lethal salt shock of 684 mM NaCl (Fig. 3). Therefore, it could be concluded that the partial deletion of this gene in mutant 549 was responsible for the mutant’s reduced salt tolerance. Compared with mutants of Synechocystis defective in the synthesis of the osmoprotective compound GG, the salt tolerance of the gcp mutant remained relatively high. GG mutants were also not able to restore the immediate decrease in photosynthesis after a salt shock of 684 mM NaCl. Photosynthesis became completely inhibited by about 30 h, and their salt tolerance limit was reduced to less than 350 mM NaCl (15, 16). Therefore, the gcp gene product seems not to be directly involved in basic processes of salt adaptation and its defect might lead only indirectly to a reduction in salt tolerance.
Last year the complete genome sequence of Synechocystis was published (22). The ORF1 protein was identified as a putative sialoglycoprotease, which might be involved in the degradation of proteins. A comparison of the Gcp protein to several other putative glycoproteases from heterotrophic bacteria showed high sequence similarities (about 40% identical amino acid residues) (Fig. 6). Most of the related sequences were obtained during genome sequencing projects. The function of these proteins was derived from sequence comparisons alone. Only for the protein from Pasteurella haemolytica has the function as a protease been biochemically proven. This protease, belonging to the group of metalloproteases, showed specificity for a glycosylated protein, the glycophorin A, with a major cleavage site at Arg-31–Asp-32 (2). A similar protein from E. coli was thought to be involved in the regulation of a macromolecular operon (26).
FIG. 6.
Amino acid sequence alignment of the gcp gene homolog (ORF1) from Synechocystis sp. strain PCC 6803 (SYNE [22]) and other putative gcp genes from several bacteria. HINF, Haemophilus influenzae (10); ECOL, Escherichia coli (26); PHAE, Pasteurella haemolytica (1); MLEP, Mycobacterium leprae (33a). Uppercase letters are used when amino acids are identical to those in the Synechocystis sequence (shaded boxes).
Besides the reduced salt tolerance, the most obvious alterations in the phenotype of the Synechocystis gcp mutant were related to changed pigmentation and a remarkable accumulation of cyanophycin and glycogen. An increase in the carotenoids and especially in echinenone and myxoxanthophyll, as well as in glycogen accumulation, was also observed for salt-adapted cells of the WT (9, 32), but these increases and the decrease in phycocyanin were much more pronounced in the salt-stressed cells of the gcp mutant. Cyanophycin, composed of polyaspartate and arginine, which is synthesized without the participation of ribosomes, has been found only as a high-molecular-weight nitrogen reserve in many cyanobacteria, mainly accumulating in cells during the transition to stationary phase (33). The enhanced content of cyanophycin could be best explained by the assumption that the putative protease encoded by the gcp gene is responsible for cyanophycin degradation in the Synechocystis WT. The enzymes involved in cyanophycin synthesis and degradation are only poorly characterized in cyanobacteria, but they were found to be constitutively expressed (33), except in Anabaena, where the level of both activities increased in heterocysts during their differentiation (14). A defect in the degradative activity would lead to uncontrolled accumulation of cyanophycin. Under our culture conditions, cyanophycin synthesis seems to be especially induced in high-salt media, since in the control cells the cyanophycin level showed no (chemical estimation), or only minor (ultrastructural studies), changes in the gcp mutant, while it is massively induced in salt-treated cells. This massive accumulation of cyanophycin, which could not be remobilized, would starve the cells for nitrogen. The observed changes in pigmentation, as well as the accumulation of large amounts of glycogen, are very characteristic of nitrogen-starved cyanobacteria (13). A reduced supply or availability of nitrogen reduces protein synthesis relative to glycogen and induces the degradation of phycobilisomes, because the phycobiliproteins serve not only as light-harvesting pigments but also as a nitrogen storage mechanism in cyanobacteria. In contrast to phycocyanin, the content of carotenoids as nitrogen-free pigments is enhanced, especially that of xanthophylls, decreasing effects of excess light absorption by the remaining pigments (13). The unfavorable situation of shortened nitrogen supply seems to lead to more dramatic effects, when a second stress factor is introduced, such as salt stress. Under the combined stresses of nitrogen starvation and salt, the cell is unable to adapt to a new steady state and lysis occurs. The gcp mutant also shows interesting possibilities for biotechnological applications, since after a salt shock the cyanophycin accumulation is dramatically enhanced and the content of several carotenoids, especially echinenone and myxoxanthophyll, which could be used as antioxidants, is also increased. In further studies, the effects of the gcp gene defect on the nitrogen metabolism and on general stress tolerance will be investigated with this mutant.
ACKNOWLEDGMENTS
We thank B. Haselkorn, University of Chicago, for critical reading of the manuscript. The excellent technical assistance of B. Brzezinka, I. Dörr, and F. Fischer is greatly appreciated. Many thanks are due to the Centre for Electron Microscopy of the Faculty of Medicine, especially to G. Fulda for performing the ultrastructural studies.
The work at the University of Rostock was supported by a grant from the Deutsche Forschungsgemeinschaft.
REFERENCES
- 1.Abdullah K M, Lo R Y C, Mellors A. Cloning, nucleotide sequence, and expression of the Pasteurella haemolytica A1 glycoprotease gene. J Bacteriol. 1991;173:5597–5603. doi: 10.1128/jb.173.18.5597-5603.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdullah K M, Udoh E A, Shewen P E, Mellors A. A neutral glycoprotease of Pasteurella haemolytica A1 specifically cleaves O-sialoglycoproteins. Infect Immun. 1992;60:56–62. doi: 10.1128/iai.60.1.56-62.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alge D, Peschek G A. Characterization of a ctaCDE operon-like genomic region encoding subunits I–III of the cytochrome c oxidase of the cyanobacterium Synechocystis PCC 6803. Biochem Mol Biol Int. 1993;29:511–525. [PubMed] [Google Scholar]
- 4.Allen M M. Inclusions: cyanophycin. Methods Enzymol. 1988;167:321–325. [Google Scholar]
- 5.Apte S K, Haselkorn R. Cloning of salinity stress-induced genes from the salt-tolerant nitrogen-fixing cyanobacterium Anabaena torulosa. Plant Mol Biol. 1990;15:723–733. doi: 10.1007/BF00016122. [DOI] [PubMed] [Google Scholar]
- 6.Chauvat F, Rouet P, Bottin H, Boussac A. Mutagenesis by random cloning of an Escherichia coli kanamycin resistance gene into the genome of the cyanobacterium Synechocystis sp. PCC 6803: selection of mutants defective in photosynthesis. Mol Gen Genet. 1989;216:51–59. doi: 10.1007/BF00332230. [DOI] [PubMed] [Google Scholar]
- 7.Chitnis P R, Purvis D, Nelson N. Molecular cloning and targeted mutagenesis of the gene psaF encoding subunit III of the photosystem I from the cyanobacterium Synechocystis sp. PCC 6803. J Biol Chem. 1991;266:20146–20151. [PubMed] [Google Scholar]
- 8.Covarrubias L, Bolivar F. Construction and characterization of new cloning vehicles. VI. Plasmid pBR329, a new derivative of pBR328 lacking the 428 base pair inserted duplication. Gene. 1982;17:79–89. doi: 10.1016/0378-1119(82)90103-2. [DOI] [PubMed] [Google Scholar]
- 9.Erdmann N, Fulda S, Hagemann M. Glucosylglycerol accumulation during salt acclimation of two unicellular cyanobacteria. J Gen Microbiol. 1992;138:363–368. [Google Scholar]
- 10.Fleischmann R D, Adams M D, White O, Clayton R A, Kirkness E F, Kerlavage A R, Bult C J, Tomb J-F, Doughtery B A, Merrik J M, McKenney K, Sutton G, Fitzhugh W, Fields C A, Gocayne J D, Scott J D, Shirley R, Liu L-I, Glodek A, Kelley J M, Weidman J F, Phillips C A, Spriggs T, Hedblom E, Cotton M D, Utterback T R, Hanna M C, Nguyen D T, Saudek D M, Brandon R C, Fine L D, Fritchman J L, Fuhrmann J L, Geoghagen N S M, Gnehm C L, McDonald L A, Small K V, Fraser C M, Smith H O, Venter J C. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science. 1995;269:496–512. doi: 10.1126/science.7542800. [DOI] [PubMed] [Google Scholar]
- 11.Fulda S, Hagemann M. Salt treatment induces accumulation of flavodoxin in the cyanobacterium Synechocystis sp. PCC 6803. J Plant Physiol. 1995;146:520–526. [Google Scholar]
- 12.Gabbay-Azaria R, Pick U, Ben-Hayyim G, Tel-Or E. The involvement of a vanadate-sensitive ATPase in plasma membranes of a salt tolerant cyanobacterium. Physiol Plant. 1994;90:692–698. [Google Scholar]
- 13.Grossman A R, Schaefer M R, Chiang G G, Collier J L. The response of cyanobacteria to environmental conditions: light and nutrients. In: Bryant D, editor. Molecular biology of cyanobacteria. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 751–767. [Google Scholar]
- 14.Gupta M, Carr N G. Enzyme activities related to cyanophycin metabolism in heterocysts and vegetative cells of Anabaena spp. J Gen Microbiol. 1981;125:17–23. [Google Scholar]
- 15.Hagemann M, Zuther E. Selection and characterization of mutants of the cyanobacterium Synechocystis sp. PCC 6803 unable to tolerate high salt concentrations. Arch Microbiol. 1992;158:429–434. [Google Scholar]
- 16.Hagemann M, Richter S, Zuther E, Schoor A. Characterization of a glucosylglycerol-phosphate accumulating, salt-sensitive mutant of the cyanobacterium Synechocystis sp. PCC 6803. Arch Microbiol. 1996;166:83–91. doi: 10.1007/s002030050360. [DOI] [PubMed] [Google Scholar]
- 17.Hagemann M, Erdmann N. Environmental stresses. In: Rai A K, editor. Cyanobacterial nitrogen metabolism and environmental biotechnology. Heidelberg, Germany: Springer; 1997. pp. 156–221. [Google Scholar]
- 18.Hagemann M, Schoor A, Jeanjean R, Zuther E, Joset F. The stpA gene from Synechocystis sp. strain PCC 6803 encodes the glucosylglycerol-phosphate phosphatase involved in cyanobacterial osmotic response to salt shock. J Bacteriol. 1997;179:1727–1733. doi: 10.1128/jb.179.5.1727-1733.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hagemann M, Richter S, Mikkat S. The ggtA gene encodes a subunit of the transport system for the osmoprotective compound glucosylglycerol in Synechocystis sp. strain PCC 6803. J Bacteriol. 1997;179:714–720. doi: 10.1128/jb.179.3.714-720.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hippler M, Reichert J, Sutter M, Zak E, Altschmied L, Schröer U, Herrmann R G, Haehnel W. The plastocyanin binding domain of photosystem I. EMBO J. 1996;15:6374–6384. [PMC free article] [PubMed] [Google Scholar]
- 21.Jeanjean R, Onana B, Peschek G A, Joset F. Mutants of the cyanobacterium Synechocystis PCC 6803 impaired in respiration and unable to tolerate high salt concentrations. FEMS Microbiol Lett. 1990;68:125–130. [Google Scholar]
- 22.Kaneko T, Sato S, Kotani H, Tanaka A, Asamizu E, Nakamura Y, Miyajima N, Hirosawa M, Sugiura M, Sasamoto S, Kimura T, Hosouchi T, Matsuno A, Muraki A, Nakazaki N, Nruo K, Okumura S, Shimpo S, Takeuchi C, Wada T, Watanabe A, Yamada M, Yasuda M, Tabata S. Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC 6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Res. 1996;3:109–136. doi: 10.1093/dnares/3.3.109. [DOI] [PubMed] [Google Scholar]
- 23.Labarre J, Chauvat F, Thuriaux P. Insertional mutagenesis by random cloning of antibiotic resistance genes into the genome of the cyanobacterium Synechocystis strain PCC 6803. J Bacteriol. 1989;171:3449–3457. doi: 10.1128/jb.171.6.3449-3457.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 25.Mantoura R F C, Llewellyn C A. The rapid determination of algal chlorophyll and carotenoid pigments and their breakdown products in natural waters by reverse-phase high-performance liquid chromatography. Anal Chim Acta. 1983;15:297–314. [Google Scholar]
- 26.Nesin M, Lupski J R, Svec P, Godson G N. Possible new genes as revealed by molecular analysis of a 5-kb Escherichia coli chromosomal region 5′ to the rpsU-dnaG-rpoD macromolecular-synthesis operon. Gene. 1987;51:149–161. doi: 10.1016/0378-1119(87)90303-9. [DOI] [PubMed] [Google Scholar]
- 27.Peschek G A, Molitor V, Trnka M, Wastyn M, Erber W. Characterization of cytochrome-c oxidase in isolated and purified plasma and thylakoid membranes from cyanobacteria. Methods Enzymol. 1988;167:437–449. [Google Scholar]
- 28.Reed R H, Stewart W D P. Osmotic adjustment and organic solute accumulation in unicellular cyanobacteria from freshwater and marine habitats. Mar Biol. 1985;88:1–9. [Google Scholar]
- 29.Reed R H, Borowitzka L J, Mackay M A, Chudek J A, Foster R, Warr S R C, Moore D J, Stewart W D P. Organic solute accumulation in osmotically stressed cyanobacteria. FEMS Microbiol Rev. 1986;39:51–56. [Google Scholar]
- 30.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 31.Schiewer U, Jonas L. Die Wirkung unterschiedlicher NaCl-Konzentrationen auf die Ultrastruktur von Blaualgen. I. Microcystis firma. Arch Protistenkd. 1977;119:127–145. [Google Scholar]
- 32.Schubert H, Fulda S, Hagemann M. Effects of adaptation to different salt concentrations on photosynthesis and pigmentation of the cyanobacterium Synechocystis sp. PCC 6803. J Plant Physiol. 1993;142:291–295. [Google Scholar]
- 33.Simon R D. Inclusion bodies in the cyanobacteria: cyanophycin, polyphosphate, polyhedral bodies. In: Fay P, VanBaalen C, editors. The cyanobacteria. Amsterdam, The Netherlands: Elsevier Science Publishers B. V.; 1987. pp. 199–226. [Google Scholar]
- 33a.Smith, D. R., and K. Robison. 1994. Unpublished data.
- 34.Stanier G (Cohen-Bazire) Fine structure of cyanobacteria. Methods Enzymol. 1988;167:157–172. [Google Scholar]
- 35.Vieira J, Messing J. The pUC plasmids, an M13mp7-derived system for the insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982;19:259. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- 36.Xu Q, Yu L, Chitnis V P, Chitnis P R. Function and organization of photosystem I in a cyanobacterial mutant strain lacks PsaF and PsaJ subunits. J Biol Chem. 1994;269:3205–3211. [PubMed] [Google Scholar]
- 37.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]