Objectives
Learn the common causes of neutropenia
Recognize the severity of the neutropenia and indications for hospital admission
Understand the diagnostic workup in a patient with neutropenia
Understand the treatment options that are available for severe neutropenia
Normal levels of neutrophils are essential to prevent infections. Neutropenia is a relatively common disorder most often due to chemotherapy treatments, adverse drug reactions, or autoimmune disorders. The differential diagnosis of neutropenia is broad and encompasses congenital, infectious, rheumatologic, and iatrogenic causes.1, 2 Severe neutropenia or agranulocytosis, defined as an absolute neutrophil count of less than 0.5 × 109/L (<500/μL), is uncommon but can cause morbidity and mortality from infections. Epidemiologic studies show a wide variation in the incidence of neutropenia among geographic regions, with an average annual incidence of 56.4 cases of neutropenia and 9.0 cases of agranulocytosis per million people in the United States.1 Data from the International Agranulocytosis and Aplastic Anemia Study in Europe show an annual incidence of 6.2 cases of agranulocytosis per million people.2 Both studies assessed only hospitalized patients with severe neutropenia due primarily to noncytotoxic drugs, infections, or immune disorders and excluded those patients who were treated with cytotoxic drugs. Male and nonwhite patients had an increased risk, and patients between 50 and 59 years of age had the highest risk. No epidemiologic studies adequately address the relative severity, frequency, and mortality of the different causes of neutropenia. In this review, we present the case of a patient with apparently acute severe neutropenia.
Summary points
Patients with a neutrophil count of less than 0.5 × 109/L (500 cells/μL) and signs or symptoms of infection require immediate hospitalization and prophylactic antibiotic therapy
Severe neutropenia is most commonly due to cell destruction that is mediated by the immune system or hypoplasia, rather than sequestration
Drugs are the most common cause of neutropenia, and medicines that can cause neutropenia should be discontinued whenever possible
The appropriate use of cytokines is dependent on the underlying cause of neutropenia
METHODS
Information on the topics discussed in this review was obtained through a literature search using MEDLINE and the following keywords: neutropenia, agranulocytosis, sarcoidosis, and autoimmune. In addition, popular textbooks of hematology and rheumatology were consulted.
The patient, aged 45, had a five-week history of chest heaviness, palpitations, and flulike symptoms that are increasing in severity. In the past, she has had paroxysmal atrial tachycardia for which she intermittently takes quinidine sulfate, but she is not taking any other medications. On physical examination, she has a fever (temperature, 38.7°C), axillary lymphadenopathy, and an enlarged spleen. Her electrocardiogram shows sinus tachycardia. Her leukocyte count is 0.77 × 109/L (770/μL) with an absolute neutrophil count of 0.38 × 109/L (380/μL), hematocrit of 0.39, and a platelet count of 106 × 109/L (106 × 103/μL). Her serum lactate dehydrogenase level is 600 U/L (reference range, 0-250).
Does this patient require hospitalization, and what studies should be considered on initial evaluation?
The physician should focus first on the severity of the neutropenia and on the presence of symptoms suggesting infection. Any patient with an absolute neutrophil count of less than 0.5 × 109/L and with fever or signs of infection and those with evidence of a malignant disease need immediate hospitalization. The cause of the neutropenia should be rapidly evaluated, relevant tissue and fluid specimens obtained for culture, and a regimen of supportive antibiotic therapy started.
In the diagnostic evaluation, it is important to ask about prior infections and to look at previous complete blood cell counts. Physicians should ask about weight loss, fever, or night sweats; medications; recent viral infections; and rheumatologic complaints. During the physical examination, the physician should look for and record the presence or absence of fever, mucositis, gingivitis or abscess, adenopathy, and hepatosplenomegaly. The findings of a microscopic examination of the patient's peripheral blood smear may suggest megaloblastosis, myelodysplasia, or hematologic cancer.
Patients without infectious symptoms and with only mild neutropenia, which could be due to a medicine or a recent viral infection, may be monitored with blood cell counts several times per week for 6 to 8 weeks. The cause of neutropenia in children is likely to be benign and to resolve without any intervention. Patients at risk for acquiring human immunodeficiency virus (HIV) infection should undergo testing for HIV, and patients with symptoms and findings suggestive of collagen vascular disease should have the appropriate serologic tests carried out. A bone marrow evaluation is considered mandatory in patients whose neutropenia does not resolve after discontinuation of medications and in any patient with multiple cytopenias. The bone marrow aspirate may be sent for cytogenetic analysis and flow cytometry to rule out a malignant disorder or myelodysplasia. The biopsy specimen may also be assessed for infectious causes, such as mycobacteria and fungi, that could explain the neutropenia. Hematologic consultation should be considered for all patients except those with mild neutropenia or with an obvious drug-related cause.
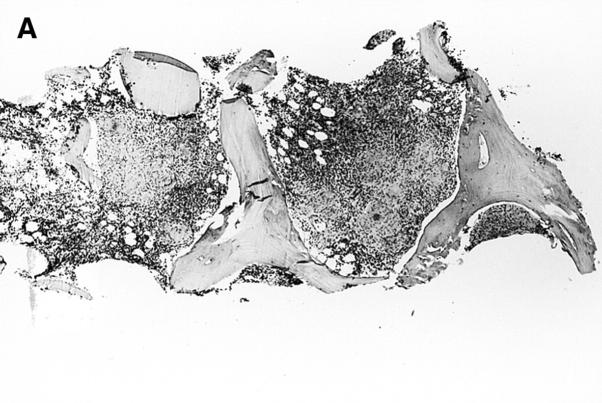
The patient is afebrile and does not appear acutely ill. She has no history of weight loss or night sweats. Mild anemia was noted one year ago, but her other blood cell counts have been normal. Blood and urine specimens are sent to the laboratory for culture. A radiograph of her chest shows bilateral hilar adenopathy and upper zone interstitial markings, which have progressed since a previous chest radiograph in January 1997, 13 months prior to admission. The results of her liver function tests, serum vitamin B12, and folate concentrations are normal. Computed tomographic scans show hilar, retroperitoneal, axillary, and inguinal adenopathy and confirm mild splenomegaly. A bone marrow aspirate shows a mildly left-shifted myeloid lineage but there is no evidence of a clonal proliferation by flow cytometry, ruling out a hematopoietic malignancy. Examination of a bone marrow biopsy specimen shows that the marrow has been replaced with noncaseating granulomas (figure 1,figure 1). Mycobacterial and fungal staining do not show any organisms.
Figure 1.
(Top) Low- and (bottom) high-power fields of a bone marrow biopsy specimen showing noncaseating granulomas.
Figure 1.
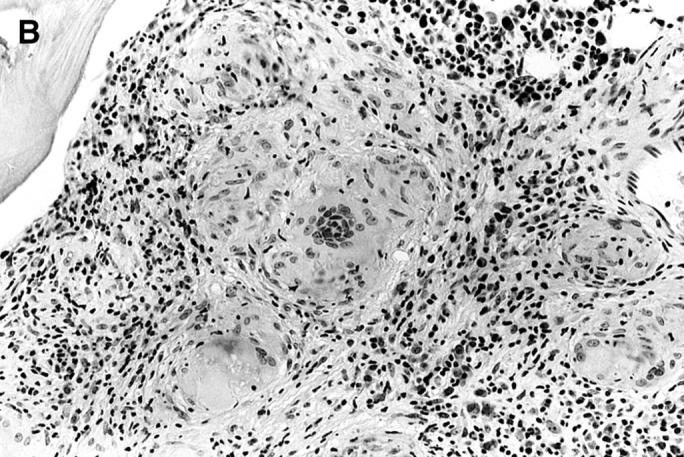
(Top) Low- and (bottom) high-power fields of a bone marrow biopsy specimen showing noncaseating granulomas.
What is the most likely underlying cause of the patient's neutropenia, and what other testing might be considered?
The mechanisms underlying the development of neutropenia mirror those for all of the formed blood elements—namely, defective production, accelerated destruction, or sequestration outside the circulating blood pool (table). A useful initial distinction can be made between congenital or hereditary and acquired causes of neutropenia. The congenital syndromes are rare, present in childhood, and associated with a history of recurrent infections and anomalous neutrophil structure.
Table 1.
Classification of neutropenias
| Neutropenia type | Example |
|---|---|
| Acquired | |
| Production | Cytotoxic chemotherapy, irradiation |
| Drugs—chloramphenicol, phenothiazines | |
| Metabolic disease | |
| Nutritional deficiency—vitamin B12, folate | |
| Infectious | |
| |
| Malignant—leukemia, lymphoma myelodysplasia | |
| Destruction | Collagen vascular disease—systemic lupus erythematosus, rheumatoid arthritis, Sjögren's syndrome |
| Drugs—penicillins, cephalosporins, quinidine sulfate | |
| Infections | |
| Malignant diseases—large granular lymphocyte leukemia | |
| Sequestration | Hypersplenism |
| Congenital | |
| Kostmann syndrome | |
| Cyclic neutropenia | |
| EBV = Epstein-Barr virus, CMV = cytomegalovirus, HIV = human immunodeficiency virus | |
Neutropenia is more likely to be acquired than congenital. The most common cause of acutely acquired neutropenia is cytotoxic chemotherapy or radiation therapy for malignant diseases; it affects up to 40% of patients given treatment. Drug reactions are the most common cause of isolated neutropenia, although the precise incidence is not known because reliable information on the use of drugs is not readily available. The neutropenia may be mediated either by direct marrow suppression, as seen with the use of chloramphenicol and phenothiazines, or by immune destruction of the neutrophil or myeloid precursors, as seen with the use of penicillins, cephalosporins, and quinidine.3 Neutropenia that is drug induced and immunologically mediated is likely to be underdiagnosed, because testing for the antineutrophil antibodies is not widely available, and the detection of drug-dependent antibodies is seldom attempted.4, 5 Because immune agranulocytosis has a 20% mortality, it must be considered an extremely serious condition.6,7,8 There is an inverse correlation between the level of marrow hypoplasia and the time it takes for marrow to recover. Patients who have renal insufficiency or bacteremia at the time of presentation have a significantly higher mortality.8, 9
Viral infections are a common cause of neutropenia, due either to bone marrow suppression or to peripheral destruction. The agents commonly implicated include Epstein-Barr virus, cytomegalovirus, hepatitis A and B viruses, parvovirus, Influenzavirus species, and measles.10, 11 Infection with HIV is also associated with neutropenia and approximately 70% of patients infected with HIV are neutropenic during their illness.12 The HIV virus not only may suppress hematopoiesis but also increases the risk of acquiring other infections. Furthermore, therapy with antiretroviral agents may dramatically decrease neutrophil counts.
Any bacterial infection can cause neutropenia, but it is most commonly seen in salmonellosis, brucellosis, pertussis, and rickettsial infections.12 Disseminated tuberculosis is also known to cause neutropenia. It is, however, unusual in fungal infections unless the bone marrow is extensively involved, as occasionally seen with disseminated histoplasmosis.13
Neutropenia may be a prominent feature of collagen vascular diseases. About 50% of patients with systemic lupus erythematosus have white blood cell counts of less than 4.5 × 109/L (<4,500/μL), but severe neutropenia is unusual and should prompt a search for other causes.14, 15 Splenomegaly and neutropenia (Felty's syndrome) may develop in patients with rheumatoid arthritis.16, 17 These patients may have an absolute neutrophil count of less than 0.1 × 109/L and recurrent major and minor infections. Other collagen vascular diseases such as Sjögren's syndrome, polymyalgia rheumatica, and mixed connective tissue disease have been known to cause autoimmune neutropenia.18, 19 The specific mechanism of the neutropenia associated with collagen vascular diseases is not known; however, circulating immune complexes and antineutrophil antibodies directed against specific neutrophil antigens have been identified in these patients.20
Both benign and malignant hematopoietic diseases may cause neutropenia. For example, vitamin B12 and folate deficiencies cause not only neutropenia but also anemia and thrombocytopenia.21, 22 Megaloblastic features are essentially always present in the circulating cells and in the marrow precursors. Leukemia, multiple myeloma, and myelodysplasia may all cause neutropenia by suppressing normal myelopoiesis.23, 24 Because the patient had combined cytopenias, a marrow aspiration was carried out to rule out malignancy and to evaluate the likelihood of an infectious cause. The finding of hilar adenopathy, hepatosplenomegaly, and a pattern of apical pulmonary infiltrates suggested the diagnosis of sarcoid. These findings are not specific, however, and may be associated with fungal and mycobacterial infections, toxoplasmosis, viral disease, systemic lupus erythematosus, and Wegener's granulomatosis. The diagnosis of sarcoidosis is one of exclusion—ruling out infectious and immune causes for the clinical findings and for the tissue granulomas.25 In this patient, the severity of the neutropenia was unusual for sequestration, and it was necessary to look for other causes.
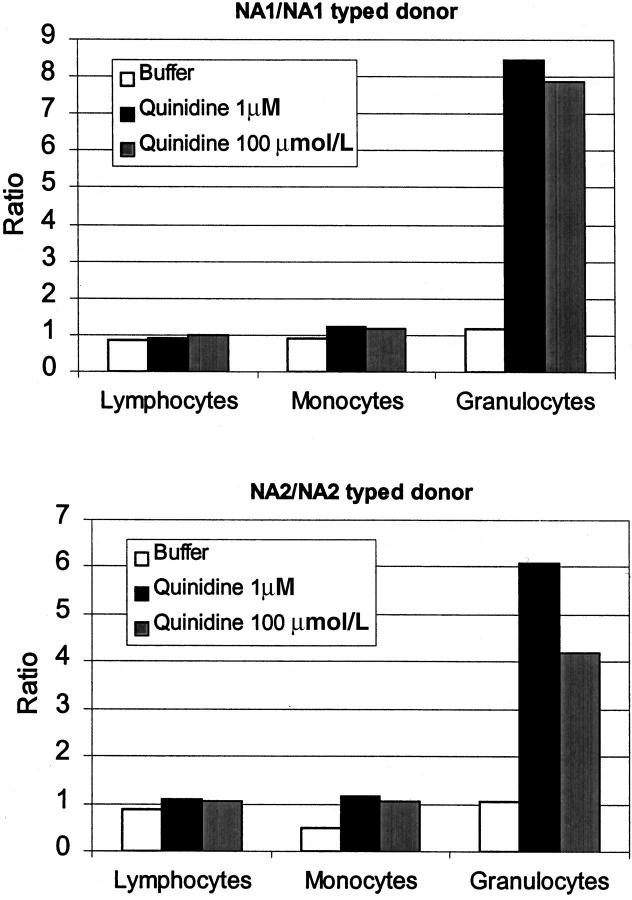
The patient is afebrile during her admission, and her initial cultures are negative for pathogens. Her neutropenia resolves two days after the quinidine is discontinued, and she is discharged to home. Her bone marrow cultures subsequently show no growth of organisms. Brucellosis, Q fever, and other rickettsial diseases are ruled out with acute and convalescent titers. Tests are negative for HIV, cytomegalovirus, and hepatitis A, B, and C. Results of an Epstein-Barr virus serologic test are consistent with previous exposure. Serologic tests are negative for antinuclear and antineutrophil cytoplasmic antibodies. Of note, her rheumatoid factor is 47 IU/mL (reference range, 0-11) and an angiotensin-converting enzyme level is 99 U/L (reference range, 8-52). Antibody test results suggest that the patient has antineutrophil antibodies that are dependent on quinidine (figure 2).
Figure 2.
The patient's serum was incubated with NA1/NA1- (top) or NA2/NA2 (bottom)-typed donor cells in buffer or quinidine solutions. NA1 and NA2 are granulocyte-specific antigens that differ in their amino acid sequence. Antibodies may be specific to one or the other antigen. Leukocyte-bound antibodies were detected with antihuman antibody labeled with fluoresceine. The ratios of the mean peak channel of the patient's serum are shown compared with that of the control serum for lymphocytes, monocytes, and granulocytes.
What other interventions might be considered as treatment of neutropenia?
Neutropenia that is drug-induced often resolves within days to weeks after the medicine is discontinued. Watchful waiting is the first option for patients whose neutropenia is thought to be due to a viral infection or medication. Neutropenia resulting from chemotherapy or radiation may, in specific instances, be treated with cytokines such as granulocyte colony—stimulating factor or granulocyte-macrophage colony—stimulating factor. The use of cytokines after patients have already become neutropenic from treatment has not been shown to substantially decrease the mortality from infections, the duration of fever, or the duration of antibiotic therapy. The American Society of Clinical Oncology recommends the prophylactic use of cytokines for patients at high risk for neutropenia developing from cytotoxic drugs in intensive doses.26, 27 Once neutropenia is established as a result of chemotherapy, current recommendations are to consider cytokine therapy if there is evidence of fungal infection, pneumonia, hypotension, or sepsis syndrome. Granulocyte-macrophage colony—stimulating factor ameliorates the neutropenia in severe infantile agranulocytosis (Kostmann syndrome), cyclic neutropenia, and idiopathic neutropenias.28,29,30 The decision to initiate therapy should be made only in consultation and principally in patients who are at an increased risk for infections. Other more aggressive interventions such as allogeneic marrow transplantation have been effective in patients with diseases of the bone marrow such as Kostmann syndrome, aplastic anemia, and myelodysplastic syndromes and in several malignant hematopoietic diseases.31
At follow-up two months later, the patient is found to have a normal white blood cell count with persistent mild anemia and thrombocytopenia. She is asymptomatic except for painful pretibial erythema nodosum. In the absence of serious pulmonary problems, she is not being treated for sarcoidosis.
This patient had signs and symptoms of multiple disease processes. The extremely low neutrophil count is not typical of sequestration from splenomegaly, which in this case was related to sarcoidosis. The presence of antineutrophil antibodies that were dependent on quinidine suggests that the neutropenia was most likely due to the destruction of white blood cells by the immune system. However, autoimmune disorders related to quinidine are not known to cause splenomegaly or bone marrow granulomas.32 Sarcoidosis, which was considered only after excluding infectious and rheumatologic causes, would explain these findings in this patient. Leukopenia is also seen with sarcoidosis, especially in those who have splenomegaly, but is usually due to decreased lymphocyte counts and not to neutropenia.33 Thus, our patient had both sarcoidosis and neutropenia induced by quinidine. Patients with sarcoidosis have a humoral immune system that is dysregulated and also have circulating autoantibodies, suggesting that they may be at increased risk for neutropenia that is drug-dependent34
Acknowledgments
We thank Barbara Roberts for performing the antineutrophil antibody assays.
Competing interests: None declared
References
- 1.Strom BL, Carson JL, Schinnar R, et al. Descriptive epidemiology of agranulocytosis. Arch Intern Med 1992;152: 1475-1480. [PubMed] [Google Scholar]
- 2.Risks of agranulocytosis and aplastic anemia: a first report of their relation to drug use with special reference to analgesics. The International Agranulocytosis and Aplastic Anemia Study. JAMA 1986;256: 1749-1757. [PubMed] [Google Scholar]
- 3.Price TH, Dale DC. The selective neutropenias. Clin Haematol 1978;7: 501-521. [PubMed] [Google Scholar]
- 4.Salama A, Mueller-Eckhardt C. Immune-mediated blood cell dyscrasias related to drugs. Semin Hematol 1992;29: 54-63. [PubMed] [Google Scholar]
- 5.Stroncek DF, Vercellotti GM, Hammerschmidt DE, et al. Characterization of multiple quinine-dependent antibodies in a patient with episodic hemolytic uremic syndrome and immune agranulocytosis. Blood 1992;80: 241-248. [PubMed] [Google Scholar]
- 6.Hurtado R, Candelaria M, Majluf-Cruz A, et al. Drug-induced agranulocytosis treated with granulocyte-macrophage colony stimulating factor. Rev Invest Clin 1994;46: 59-61. [PubMed] [Google Scholar]
- 7.Roddie P, Dorrance H, Cook MK, et al. Treatment of sulphasalazine-induced agranulocytosis with granulocyte macrophage-colony stimulating factor. Aliment Pharmacol Ther 1995;9: 711-712. [DOI] [PubMed] [Google Scholar]
- 8.Julia A, Olona M, Bueno J, et al. Drug-induced agranulocytosis: prognostic factors in a series of 168 episodes. Br J Haematol 1991;79: 366-371. [DOI] [PubMed] [Google Scholar]
- 9.Vincent PC. Drug-induced aplastic anaemia and agranulocytosis: incidence and mechanisms. Drugs 1986;31: 52-63. [DOI] [PubMed] [Google Scholar]
- 10.Baranski B, Young N. Hematologic consequences of viral infections. Hematol Oncol Clin North Am 1987;1: 167-183. [PubMed] [Google Scholar]
- 11.Kurtzman G, Young N. Viruses and bone marrow failure. Baillieres Clin Haematol 1989;2: 51-67. [DOI] [PubMed] [Google Scholar]
- 12.Zon LI, Groopman JE. Hematologic manifestations of the human immune deficiency virus (HIV). Semin Hematol 1988;25: 208-218. [PubMed] [Google Scholar]
- 13.Strausbaugh LJ. Hematologic manifestations of bacterial and fungal infections. Hematol Oncol Clin North Am 1987;1: 185-206. [PubMed] [Google Scholar]
- 14.Simantov R, Laurance J, Nachman RL. The cellular hematology of systemic lupus erythematosus. In: Lahita RG, ed. Systemic lupus erythematosus 3rd ed. San Diego, CA: Academic Press; 1999: 765-791.
- 15.Shoenfeld Y, Ehrenfeld M. Hematologic manifestations. In: Schur P, ed. The clinical management of systemic lupus erythematosus. 2nd ed. Philadelphia: Lippincott-Raven; 1996: 95-108.
- 16.Sienknecht CW, Urowitz MB, Pruzanski W, Stein HB. Felty's syndrome: clinical and serological analysis of 34 cases. Ann Rheum Dis 1977;36: 500-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Breedveld FC, Fibbe WE, Cats A. Neutropenia and infections in Felty's syndrome. Br J Rheumatol 1988;27: 191-197. [DOI] [PubMed] [Google Scholar]
- 18.Starkebaum G, Dancey JT, Arend WP. Chronic neutropenia: possible association with Sjogren's syndrome. J Rheumatol 1981;8: 679-684. [PubMed] [Google Scholar]
- 19.Bux J, Mueller-Eckhardt C. Autoimmune neutropenia. Semin Hematol 1992;29: 45-53. [PubMed] [Google Scholar]
- 20.Bux J, Kissel K, Nowak K, et al. Autoimmune neutropenia: clinical and laboratory studies in 143 patients. Ann Hematol1991;63: 249-252. [DOI] [PubMed] [Google Scholar]
- 21.Dale DC. Neutropenia. In: Beutler E, Lichtman MA, Coller BS, Kipps TJ, eds. Williams' Hematology. 5th ed. New York: McGraw-Hill; 1995: 815-824.
- 22.Coates BD, Baehner R. Leukocytosis and leukopenia. In: Hoffman R, Benz EJ, Sanford SJ, Furie B, Cohen HJ, Silberstein LE, eds. Hematology: basic principles and practice. 2nd ed. New York: Churchill-Livingstone; 1995: 773-781.
- 23.Rappaport ES, Helbert B, Ladd DJ, et al. Myelodysplastic syndrome: identification in the routine hematology laboratory. South Med J 1987;80: 969-974. [DOI] [PubMed] [Google Scholar]
- 24.Russin SJ, Fillipo BH, Adler AG. Neutropenia in adults: what is its clinical significance? [published erratum appears in Postgrad Med 1990;88(6):30]. Postgrad Med 1990;88(2): 209-216. [DOI] [PubMed] [Google Scholar]
- 25.Belfer MH, Stevens RW. Sarcoidosis: a primary care review. Am Fam Physician 1998;58: 2041-2050. [PubMed] [Google Scholar]
- 26.American Society of Clinical Oncology. 1997 update of recommendations for the use of hematopoietic colony-stimulating factors: evidence-based, clinical practice guidelines. J Clin Oncol 1997;15: 3288. [DOI] [PubMed] [Google Scholar]
- 27.American Society of Clinical Oncology. Recommendations for the use of hematopoietic colony-stimulating factors: evidence-based, clinical practice guidelines. J Clin Oncol 1994;12: 2471-2508. [DOI] [PubMed] [Google Scholar]
- 28.Jakubowski AA, Souza L, Kelly F, et al. Effects of human granulocyte colony-stimulating factor in a patient with idiopathic neutropenia. N Engl J Med 1989;320: 38-42. [DOI] [PubMed] [Google Scholar]
- 29.Hammond WP 4th, Price TH, Souza LM, et al. Treatment of cyclic neutropenia with granulocyte colony-stimulating factor. N Engl J Med 1989;320: 1306-1311. [DOI] [PubMed] [Google Scholar]
- 30.Bonilla MA, Gillio AP, Ruggeiro M, et al. Effects of recombinant human granulocyte colony-stimulating factor on neutropenia in patients with congenital agranulocytosis. N Engl J Med 1989;320: 1574-1580. [DOI] [PubMed] [Google Scholar]
- 31.Thomas ED, Blume KG, Forman SG. Hematopoietic cell transplantation. 2nd ed. Oxford, England: Blackwell Science; 1999.
- 32.O'Reilly RA. Splenomegaly in 2,505 patients at a large university medical center from 1913 to 1995, 1963 to 1995: 449 patients. West J Med 1998;169: 88-97. [PMC free article] [PubMed] [Google Scholar]
- 33.Eid A, Carion W, Nystrom JS. Differential diagnoses of bone marrow granuloma. West J Med 1996;164: 510-515. [PMC free article] [PubMed] [Google Scholar]
- 34.Kataria YP, Holter JF. Immunology of sarcoidosis. Clin Chest Med 1997;18: 719-739. [DOI] [PubMed] [Google Scholar]