Abstract
In this study, we analyzed the components of Mentha spp. essential oils (EOs) and evaluated their major constituents and binary combinations against Reticulitermes dabieshanensis. We also determined the activities of esterases (ESTs), glutathione S-transferases (GSTs), and acetylcholinesterase activity (AChE) in treated insects. According to our findings, the most effective oils were those obtained from M. citrata (with the major constituent linalool constituting 45.1%), M. piperita (menthol, 49.1%), and M. spicata (carvone, 69.0%), with LC50 values of 0.176, 0.366, and 0.146 μL/L, respectively. The LC50 values were recorded for linalool (0.303 μL/L), followed by menthol (0.272 μL/L), and carvone (0.147 μL/L). The insecticidal potency increased with binary mixtures of major active constituents, with carvone strongly synergizing the toxicity of linalool and menthol against R. dabieshanensis. Compared to the control, except for M. citrata treated with no difference in α-NA or GST activity, the activities of ESTs and GST in other treatment groups were significantly increased. Additionally, our results found that Mentha spp. EOs and their major constituents inhibited the activity of AChE in vivo and in vitro. Finally, we performed a structure-based virtual screening of linalool, menthol, and carvone to identify that linalool had the greatest potential to bind to the active site of AChE. The present study suggests that Mentha spp. EOs could provide an additional approach for the management of termites over synthetic insecticides.
Keywords: Mentha, fumigation activity, Reticulitermes dabieshanensis, binominal mixture, detoxifying enzyme, acetylcholinesterase
1. Introduction
Termites comprise a group of social wood-feeding cockroaches with over 3000 species [1]. These pests are a significant threat to agriculture and forestry in tropical and subtropical regions and can cause extensive economic and social harm [2]. Reticulitermes flaviceps is responsible for causing serious damage to agriculture and forestry in China [3]. However, traditional methods of chemically controlling termites often have negative impacts on the environment and human health. Therefore, plant essential oils (EOs) are gaining more attention as a safe and effective insecticide to control termites.
Mentha, an aromatic herb, is a species widely cultivated and used in the Labiatae family. It has approximately 19 varieties and 13 natural hybrids [4]. Most Mentha plants are annual and widely distributed across Europe, Asia, Africa, and America [5]. It can be used as herbal medicine to treat colds, flu, fever, headaches, and other diseases [6], as well as as a tea drink [7]. Mentha plants are rich in essential oils, and their primary chemical components include linalool acetate, menthol, and carvone [8,9]. Previous studies have reported that the essential oils and extracts from Mentha plants exhibit robust antioxidant and insecticidal properties [7]. For instance, Mentha spicata (EO) demonstrates strong fumigation efficacy against Acanthoscelides obtectus [10], Helicoverpa armigera [11], Plutella xylostella [12], Culex quinquefasciatus, Aedes aegypti, and Anopheles stephensi [13]. Furthermore, M. viridis EO exhibits potent oviposition inhibitory activity against female adults of Tetranychus turkestani [14] and Acanthoscelides obtectus [10]. M. piperita has been found to have preferable contact activity against T. urticae [15], as well as Sitophilus oryzae, Tribolium castaneum, and Rhizopertha dominica [16,17]. M. piperita has also been found to affect the parasitism of Amyloodinium ocellatum and Piaractus brachypomus [18] and to have a certain oviposition inhibition effect on Acanthoscelides obtectus [19]. Additionally, the essential oil of M. citrata has a good contact-killing effect on Lipaphis erysimi [20]. However, few reports exist regarding the research of Mentha EO in controlling termites. Therefore, this study aimed to examine the biological activity and mechanism of Mentha EO on termites to explore additional plant resources for termite control and provide a scientific foundation for the development of safe and green new termite control agents.
The lipophilicity of EOs can interfere with the basic metabolism, physiology, biochemistry, and behavioral selection of insects. Additionally, certain plant EOs can affect the development, reproduction, and survival of insects, as supported by research conducted by Suwansirisilp et al. [21], Kulkarni et al. [22], and Kumrungsee et al. [23]. A study found that the essential oil of green mint can inhibit the activity of detoxification enzymes in termites, resulting in termite death. Furthermore, its main components also display potent insecticidal activity against termites, as demonstrated by Yang et al. [3].
The objective of this study was to assess the insecticidal properties of three Mentha EOs and their major constituents and mixed compounds against Reticulitermes dabieshanensis and to determine the mechanism of toxicity through in vivo and in vitro enzyme activity determination and molecular docking simulation in order to establish a theoretical foundation for the development of new termite control agents. Additionally, chromatography-mass spectrometry (GC–MS) was used to analyze the components of these essential oils.
2. Results
2.1. Chemical Composition of Mentha spp. EOs
The chemical composition of Mentha spp. EOs was determined by GC–MS. The percentage fractions of each constituent in the total composition are listed in Table 1 and Figure S1. In M. citrata EO, a total of 10 constituents were found, accounting for 97.5% of the EO mass. The primary constituent was linalool (45.1%), while other constituents present at a noteworthy level were linalyl acetate (42.9%), caryophyllene (2.5%), and α-terpineol (2.1%). M. piperita EO contained 10 compounds, constituting 99.9%, and menthol (49.1%), menthone (27.5%), and isomenthol (5.6%) were the major constituents. M. spicata EO had a total of 5 identified constituents, accounting for 98.1%. The major constituent was carvone (69.0%), and other significant constituents were limonene (17.9%), menthol (8.5%), and menthone (2.2%).
Table 1.
Major chemical constituents of Mentha spp. essential oils.
| No. | Constituents | RIExp. | RILit. | Relative Area (%) | ||
|---|---|---|---|---|---|---|
| M. citrata | M. piperita | M. spicata | ||||
| 1 | α-Pinene | 939 | 932 | - | 0.7 | - |
| 2 | β-Pinene | 979 | 979 | 0.9 | 0.9 | - |
| 3 | β-Myrcene | 991 | 990 | 0.6 | - | - |
| 4 | Carane | 1010 | 1009 | - | 5.5 | 0.5 |
| 5 | Limonene | 1027 | 1027 | 1.6 | 5.2 | 17.9 |
| 6 | Linalool | 1097 | 1098 | 45.1 | - | - |
| 7 | Isopulegol | 1141 | 1145 | - | 0.7 | - |
| 8 | Menthone | 1149 | 1151 | - | 27.5 | 2.2 |
| 9 | Isomenthol | 1155 | 1158 | - | 5.6 | - |
| 10 | Menthol | 1170 | 1167 | - | 49.1 | 8.5 |
| 11 | α-Terpineol | 1191 | 1191 | 2.1 | - | - |
| 12 | Pulegone | 1235 | 1235 | - | 1.5 | - |
| 13 | Carvone | 1243 | 1240 | - | - | 69.0 |
| 14 | Linalyl acetate | 1253 | 1252 | 42.9 | - | - |
| 15 | Neryl acetate | 1356 | 1358 | 0.5 | - | - |
| 16 | Geranyl acetate | 1380 | 1379 | 0.8 | - | - |
| 17 | Caryophyllene | 1419 | 1417 | 2.5 | 3.2 | - |
| 18 | Caryophyllene oxide | 1587 | 1589 | 0.5 | - | - |
| Total | 97.5 | 99.9 | 98.1 | |||
2.2. Vapor Activity of Mentha spp. EO and the Major Constituent
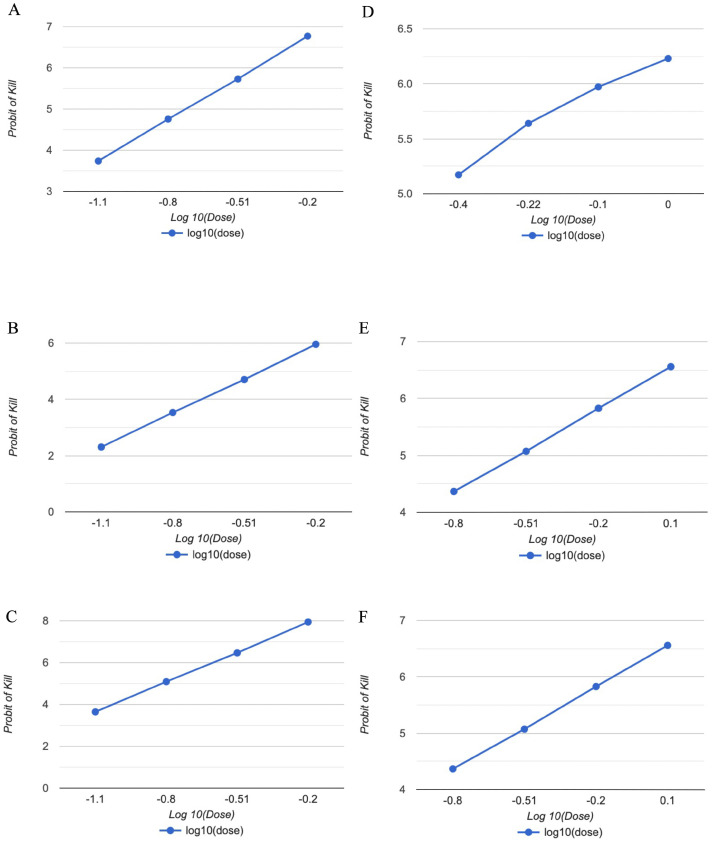
The results achieved revealed that three essential oils and major constituents had dose-dependent insecticidal effects against R. dabieshanensis (Figure 1) compared to the control, which induced no mortality at the concentration tested after 24 h of exposure. According to Table 2, the LC50 values of M. citrata, M. piperita, M. spicata EOs, linalool, menthol, and carvone against R. dabieshanensis were 0.176, 0.366, 0.146, 0.303, 0.272, and 0.147 μL/L, respectively.
Figure 1.
Variation in R. dabieshanensis mortality as a function of essential oils [(A): M. citrata; (B): M. piperita; (C): M. spicata; (D): Linalool; (E): Menthol; (F): Carvone] doses by contact as per the probit transformation model.
Table 2.
LC50 values (μL/L) of Mentha spp. EOs and their major constituents against R. dabieshanensis.
| EOs | LC30 (95% CI) |
LC50 a (95% CI b) |
LC90 (95% CI) |
Slope ± SD | (χ2) c | p Value |
|---|---|---|---|---|---|---|
| M. citrata | 0.119 (0.088–0.149) |
0.176 (0.139–0.225) |
0.460 (0.336–0.770) |
0.25 ± 0.2867 | 29.156 | 0.006 |
| M. piperita | 0.272 (0.219–0.325) |
0.366 (0.306–0.449) |
0.756 (0.585–1.181) |
0.205 ± 0.3583 | 22.459 | 0.049 |
| M. spicata | 0.116 (0.089–0.139) |
0.146 (0.119–0.178) |
0.256 (0.205–0.385) |
0.2883 ± 0.3416 | 34.256 | 0.001 |
| Linalool | 0.176 (0.114–0.230) |
0.303 (0.233–0.362) |
1.130 (0.889–1.674) |
0.1317 ± 0.285 | 9.893 | 0.703 |
| Menthol | 0.164 (0.132–0.196) |
0.272 (0.230–0.320) |
0.936 (0.731–1.318) |
0.2183 ± 0.1183 | 10.952 | 0.615 |
| Carvone | 0.111 (0.083–0.133) |
0.147 (0.121–0.178) |
0.290 (0.226–0.483) |
0.2367 ± 0.2533 | 42.123 | 0.001 |
a LC50, or Median lethal concentration, is the concentration at which half of the insects died. b CI95, Confidence intervals the activity of two compounds was considered significantly different when the 95% CIs failed to overlap. c Pearson’s chi-square, suggesting the goodness of fit test, is not significant (ns) at p > 0.05.
2.3. Fumigation Activity of Binary Mixtures
The binary mixtures of carvone (LC30) + linalool (LC30), carvone (LC30) + linalool (LC50), carvone (LC30) + menthol (LC30), and carvone (LC30) + menthol (LC30) in all the ratios showed a synergistic effect against R. dabieshanensis (Table 3).
Table 3.
Relative toxicity of binominal mixtures of the major constituents of Mentha spp. EOs against R. dabieshanensis after 24 h of post-treatment.
| Binary Mixtures | Con. | N | Mortality (%) | χ2 | Effect | |||
|---|---|---|---|---|---|---|---|---|
| Pure Compound | Binary Mixtures | |||||||
| Oa | Ob | Em | Om | |||||
| Carvone + Linalool | LC30 + LC30 | 60 | 35.00 | 6.67 | 39.33 | 53.33 | 4.99 | Synergy |
| Carvone + Linalool | LC30 + LC50 | 60 | 35.00 | 25.00 | 51.25 | 41.67 | 1.79 | Addtive |
| Carvone + Linalool | LC50 + LC30 | 60 | 51.67 | 6.67 | 54.89 | 83.33 | 14.74 | Synergy |
| Carvone + Menthol | LC30 + LC30 | 60 | 35.00 | 25.00 | 51.25 | 33.33 | 6.26 | Synergy |
| Carvone + Menthol | LC30 + LC50 | 60 | 35.00 | 45.00 | 64.25 | 51.67 | 2.46 | Addtive |
| Carvone + Menthol | LC50 + LC30 | 60 | 51.67 | 25.00 | 63.75 | 81.67 | 5.04 | Synergy |
| Linalool + Menthol | LC30 + LC30 | 60 | 6.67 | 25.00 | 30.00 | 33.33 | 0.37 | Addtive |
| Linalool + Menthol | LC30 + LC50 | 60 | 6.67 | 45.00 | 48.67 | 48.33 | 0.00 | Antagonist |
| Linalool + Menthol | LC50 + LC30 | 60 | 25.00 | 25.00 | 43.75 | 33.33 | 2.48 | Addtive |
2.4. ESTs, GST, and AChE Enzyme Activities
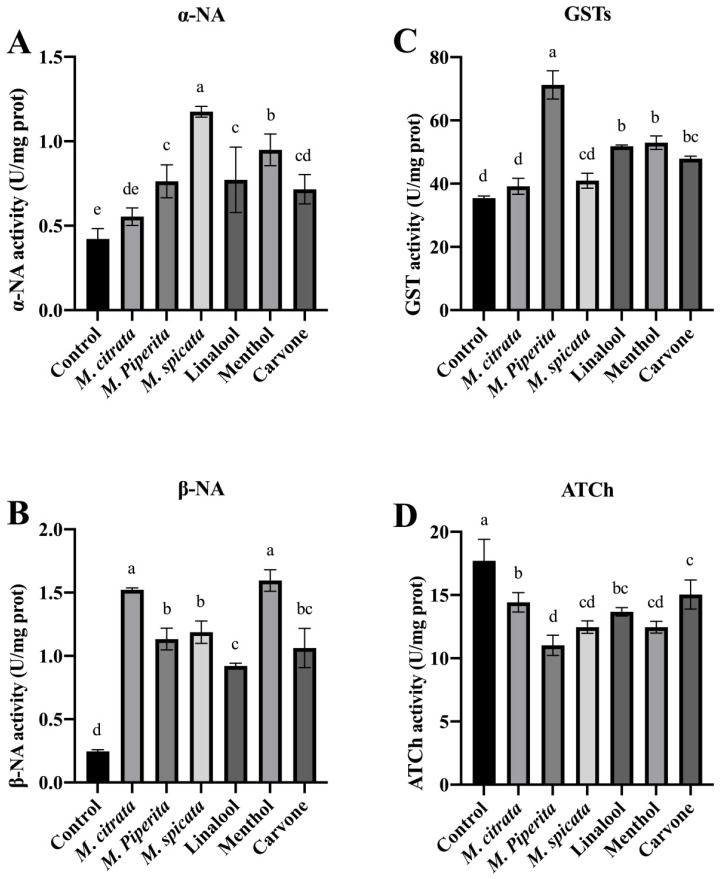
The R. dabieshanensis treated by M. piperita and M. spicata EOs, along with linalool, menthol, and carvone, demonstrated increased activity of esterases (for α-NA, F = 18.080, d.f. = 6, 14, p = 0.0001; for β-NA, F = 89.120, d.f. = 6, 14, p < 0.0001). However, the highest esterase activity was recorded in the larvae treated with M. spicata (α-NA) and M. citrata (β-NA) (Figure 2). The activity of GSTs significantly increased in R. dabieshanensis exposed to M. piperita and M. spicata EOs, linalool, menthol, and carvone in comparison to the control (F = 29.821, d.f. = 6, 14, p < 0.0001) (Figure 2). However, there were no differences in the activity of α-NA or GSTs between the M. citrata and control groups. Conversely, the activity of acetylcholinesterase significantly decreased in all treatments (F = 19.872, d.f. = 6, 14, p < 0.0001), with M. piperita EO exhibiting the highest inhibition activity among the oils and compounds tested.
Figure 2.
The effects of Mentha spp. EOs and their major constituents on the activities of esterases, glutathione S-transferase, and acethylcholine esterase (U/mg protein, respectively), of R. dabieshanensis. (A): activity of esterases (α-NA) for 24 h of LC30 treatment; (B): activity of esterases (β-NA) for 24 h of LC30 treatment; (C): activity of GSTs for 24 h of LC30 treatment; (D): activity of AChE for 24 h of LC30 treatment. Means (±SD) values with different letters (a–e) are significantly different at a level of p < 0.05, according to Duncan’s test.
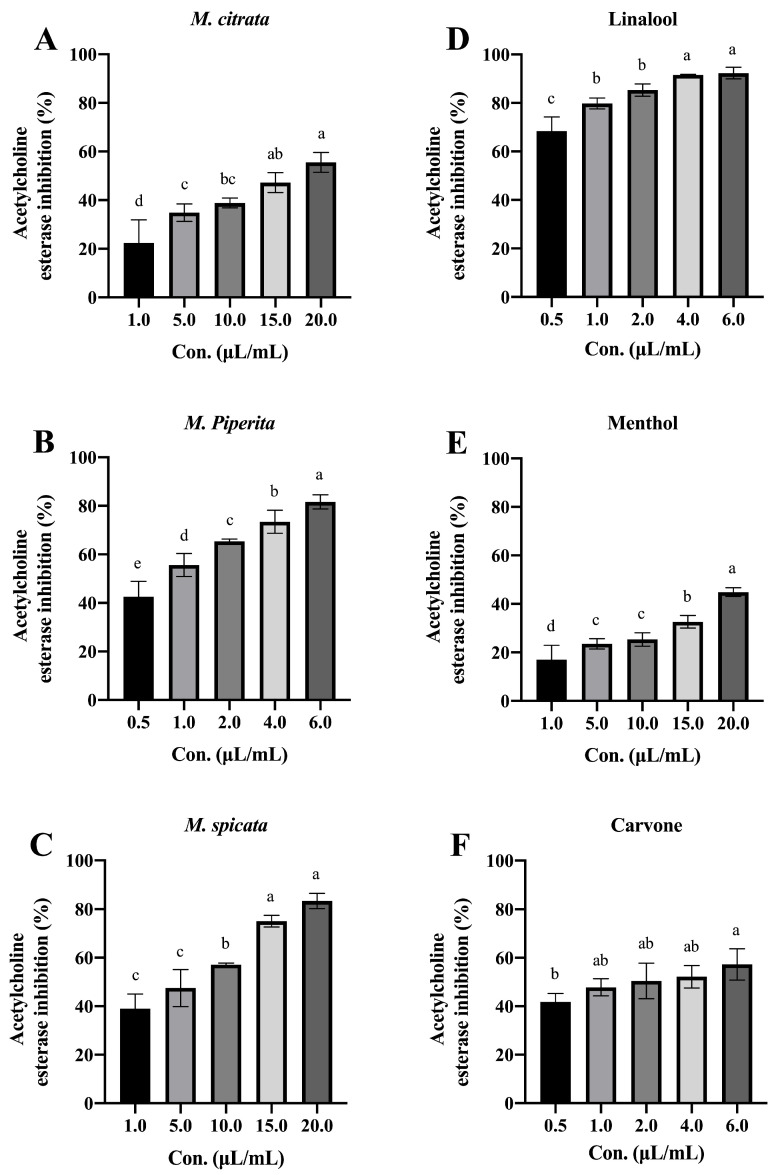
The in vitro inhibitory AChE effects of Mentha spp. EO against R. dabieshanensis increased significantly as the concentration increased (for M. citrata EO, F = 16.806; df = 4, 10; p < 0.001; for M. piperita EO, F = 36.839; df = 4, 10; p < 0.001; for M. spicata EO, F = 46.694; df = 4, 10; p < 0.001; Figure 3A–C). According to Table 4, the IC50 values of M. citrata, M. piperita, and M. spicata EOs against R. dabieshanensis were 18.295, 0.765, and 3.228 μL/mL, respectively. Additionally, each tested compound (linalool and menthol) also showed a significant variation in AChE inhibitory activity at different concentrations (for linalool, F = 28.551; df = 4, 10; p < 0.001; for menthol, F = 32.160; df = 4, 10; p < 0.001; Figure 3D,E), but carvone has no significant difference (F = 3.433; df = 4, 10; p = 0.052; Figure 3F), with an IC50 of 0.136, 76.790, and 1.922 μL/mL, respectively (Table 4).
Figure 3.
In vitro assay for estimation of acetylcholine esterase inhibition activity of Mentha spp. EOs and their major constituents against R. dabieshanensis. (A): M. citrata; (B): M. piperita; (C): M. spicata; (D): Linalool; (E). Menthol; (F). Carvone. Means (±SD) values with different letters (a–e) are significantly different at a level of p < 0.05, according to Duncan’s test.
Table 4.
In vitro assay for estimation of inhibition concentration rate (IC50, μL/mL) for Mentha spp. EOs and their major constituents.
| EOs | IC50 a (95% CI b) | Slope ± SD | (χ2) c | p Value |
|---|---|---|---|---|
| M. citrata | 18.295 (12.681–32.359) | 0.0787 ± 0.1615 | 20.597 | 0.810 |
| M. piperita | 0.765 (0.596–0.931) | 0.0961 ± 0.3489 | 9.795 | 0.711 |
| M. spicata | 3.228 (1.780–4.782) | 0.1163 ± 0.2551 | 48.124 | 0.001 |
| Linalool | 0.136 (0.066–0.218) | 0.0596 ± 0.656 | 7.738 | 0.860 |
| Menthol | 76.790 (36.58–166.367) | 0.0647 ± 0.093 | 21.700 | 0.060 |
| Carvone | 1.922 (1.131–3.308) | 0.0353 ± 0.3933 | 12.262 | 0.506 |
a LC50, or Median lethal concentration, is the concentration at which half of the insects died. b CI95, Confidence intervals the activity of two compounds was considered significantly different when the 95% CIs failed to overlap. c Pearson’s chi-square, suggesting the goodness of fit test, is not significant (ns) at p > 0.05.
2.5. Molecular Docking
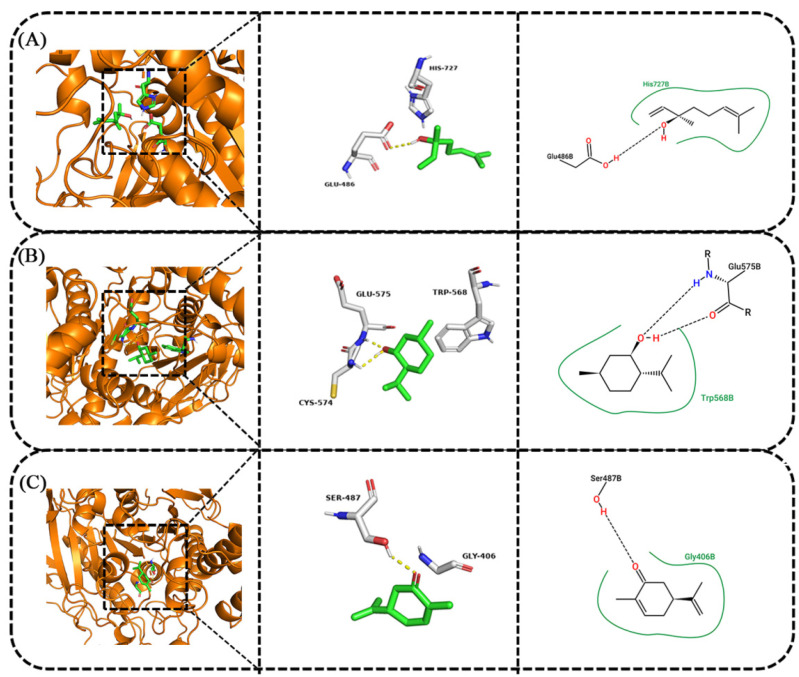
Molecular docking of compounds linalool, menthol, and carvone with the acetylcholinesterase protein model (R. dabieshanensis) was carried out using the Discovery Studio 2019 software packages. The molecular docking results of linalool, menthol, and carvone are shown in Figure 4. From the results, compounds containing a hydroxyl group exhibited similar binding modes with acetylcholinesterase (Figure 4A), and the hydroxyl group played a vital role in binding, which formed a hydrogen bond and an attractive change interaction with His727B and Glu486B. For compound menthol (Figure 4B), with its great fumigant toxicity against R. dabieshanensis, the hydroxyl group interacts with Glu575B and forms a hydrogen bond. The H atom and O atom formed alkyl interactions with Glu575B, respectively. For compound carvone (Figure 4C), which has the best fumigant toxicity against R. dabieshanensis, the carbonyl group of carvone interacts with the hydroxyl group of Ser487B to form hydrogen bonds. These docking results indicated that compounds like linalool, menthol, and carvone could readily dock into the different binding sites of acetylcholinesterase. The docking results further revealed that the three compounds could be promising acetylcholinesterase inhibitors.
Figure 4.
Molecular docking diagrams of compounds. (A): Linalool; (B): Menthol; (C): Carvone.
3. Discussion
Essential oils are characterized by being easily volatile, having low residuality, and having multiple modes of action, making them often considered substitutes for chemical pesticides. They can effectively control pests in small-scale agricultural systems [2,24]. Menthas are famous spices widely used in food, beverages, pharmaceuticals, and cosmetics for their valuable EOs. With their low cost, low environmental impact, and high safety for humans, mint plants are widely cultivated in Europe, Asia, Africa, and the Americas. Mentha EOs can be utilized in sustainable pest control.
Our results demonstrate that M. citrata, M. piperita, and M. spicata EOs had strong vapor activity in R. dabieshanensis. In a similar investigation carried out by Kumar et al. [25], they reported that M. piperita and M. citrata essential oils showed high toxicity against Musca domestica. L. Kedia et al. [26] demonstrated the insecticidal efficacy of M. spicata EO, showing 98.46% oviposition deterrency, 100% ovicidal activity, 88.84% larvicidal activity, 72.91% pupaecidal activity, and 100% antifeedant activity against Callosobruchus chinensis at 0.1 μL/mL. Govindarajan et al. [13] showed M. spicata EO to have toxic effects on C. quinquefasciatus (LC50 = 62.62 ppm), Aedes aegypti (LC50 = 56.08 ppm) and Anopheles stephensi (LC50 = 49.71 ppm). Studies also reported the effectiveness of M. piperita EO against Liposcelis bostrychophila [27], Culex pipiens [28], Tetranychus urticae [29], Phthorimaea absoluta [30], Tribolium castaneum, and Sitophilus oryzae [16,17]. The results of the present study and the previous ones by several authors showed similar trends in the toxicity of the essential oils used, even in different orders of insects.
Here, M. citrata and M. spicata EOs demonstrated the highest insecticidal activity in comparison to their constituents against R. dabieshanensis. The latest report shows that the main component, eugenol, of Ocimum basilicum EO has the highest insecticidal activity against R. dabieshanensis [31]. Similarly, Piri et al. [32] found that Ajwain EO exhibited the highest insecticidal activity against Tuta absoluta larvae compared to its components. Shahriari et al. [33] found that the Ajwain EO was more toxic to Ephestia kuehniella larvae than thymol. In the present study, M. piperita EO was less toxic to R. dabieshanensis than their main constituent, menthol, as has also been reported in some previous studies [12,34]. Strong toxicity of linalool (LC50 = 2.11 μL/L) and carvone (10.87 μL/L) against Callosobrunchus maculatus has been reported by Oyedeji et al. [35]. Hussein et al. [36] showed high toxicity against Aphis nerii. The observed insecticidal activity of Mentha EOs in the current study could be related to the synergistic/antagonist effects of the individual components within the EO. Previous studies have shown that a mixture of several active compounds in essential oils enhances their respective insecticidal activity [3,32].
The mixture of EOs/extracts increases the spectrum of action of insecticides, as different species have different reactions to individual EOs/extracts. The study by Kim et al. [37] demonstrated that the binary mixtures of basil and mandarin EOs against Spodoptera litura resulted in a synergistic interaction. According to Aungtikun et al. [38], combinations of Cymbopogon citratus, Illicium verum, and Myristica fragrans resulted in synergistic effects on Musca domestica, respectively. By contrast, Haouel-Hamdi et al. [39] demonstrated that the mixtures of Mentha rotundifolia and M. longifolia EOs showed antagonistic effects on Sitophilus oryzae. Additionally, binart mixtures of Moringa olerifera, Azadirachta indica, and Eucalyptus globulus leaf extract were synergistically effective against Diurophous noxia [40]. The major constituents of EOs are blended to form a new formulation (binary mixtures), which has additive or synergistic properties upon toxicity. In our study, carvone strongly synergized the toxicity of linalool and menthol against R. dabieshanensis. Similarly, Pavela [41,42] demonstrated that the binary mixtures of carvone and linalool resulted in synergistic effects on the adult mortality of Spodoptera littoralis and C. quinquefasciatus, respectively. Additionally, ocimene strongly synergized the toxicity of β-myrcene, L-limonene, geraniol, and L-menthol against Planococcus lilacinus [43]. In addition, the study of Prasannakumar et al. [30] revealed that the combined use of 4-carene and α-pinene was very effective against Phthorimaea absoluta. In terms of individual compounds, the binary mixture activity of pure compounds may be more pronounced because they have many mechanisms of action and may delay the emergence of resistance in pests.
Furthermore, earlier studies by Pavela et al. [44,45] and Sánchez-Gómez et al. [46], indicating exposure to effective insecticidal essential oils LC30 or LD30 has an impact on the development of C. quinquefasciatus and S. littoralis larvae, as well as on the longevity of adult Musca domestica, have been reported. Considering that there is little information on the physiological effects of essential oils at low lethal concentrations/doses (i.e., LC30 and LD30) on termites. Therefore, the impact of exposure to effective insecticidal essential oils and their main components, LC30 or LD30, on the EST and GST detoxifying enzymes of termites was evaluated.
ESTs and GST detoxifying enzymes are involved in the metabolism of botanical insecticides and are typically enhanced by exogenous compounds [31]. The present study found that exposing R. dabieshanensis to LC30 of Mentha spp. EOs and their major constituents significantly increased the levels of ESTs and GSTs in vivo. Our study is similar to earlier studies by Shahriari et al. [33], Piri et al. [32], Wang et al. [47], Kumrungsee et al. [22], Wei et al. [48], and Yang et al. [3], indicating enhancements in detoxifying enzymes from insect treatments with EOs and their compounds. Earlier studies suggested the toxicity of EOs constituents may be related to cell damage in insect tissues [48,49,50]. Given the importance of these enzymes in cell protection from plant allelochemicals, the induction of their activity can be considered a defense mechanism in R. dabieshanensis against EO.
AChE plays an important role in the mechanism of action of certain EOs or their constituents to produce insecticidal effects [45]. The present study demonstrates that EO and its major compounds significantly inhibit acetylcholinesterase of R. dabieshanensis in vivo and in vitro, which has also been reported by Piri et al. [32], Wang et al. [44], and Yang et al. [31]. In this study, we performed structure-based virtual screening of linalool, menthol, and carvone to identify compounds with the potential to produce binding profiles. Our results indicate that linalool has the greatest potential to bind to the active site of AChE. This finding is consistent with that of [51], where docking simulations showed that linalool (approximately −40 kcal/mol) exhibited the most favorable energy grid scores when compared to the other 24 compounds.
4. Materials and Methods
4.1. Insects
R. dabieshanensis was collected at Linglong Mountain (30.2251°N, 119.6843°E) in Lin’an District, Hangzhou. They were fed with water and newspaper and were maintained in an incubator (27 ± 1 °C, 80 ± 5% relative humidity, L:D = 0:24 h) in the laboratory. We selected healthy and uniform-sized workers for the experiments.
4.2. Mentha spp. EOs and the Constituents
Mentha citrata, M. piperita, and M. spicata EOs were obtained from Poli Co., Ltd. (Shanghai, China); The major constituents, carvone (97%), menthol (98%), and linalool (95%), were purchased from Sigma-Aldrich (Shanghai, China) and stored at 4 °C until the experiments.
4.3. GC-MS Analysis
The chemical composition of Mentha spp. EOs was analyzed using GC-MS. The capillary pillar used was HP-5MS with a dimension of 30 m × 0.25 mm i.d. and 0.25 μm film thickness, and a sample volume of 1.0 μL was injected. The carrier gas flow rate of helium was set at 1.0 mL/min, with a split ratio of 1:50 and an initial column temperature of 50 °C for 10 min. The temperature was raised, then increased at a rate of 10 °C/min until it reached 250 °C. The inlet temperature was set at 250 °C. Mass spectrometry conditions were set using an EI ion source with a voltage of 70 eV, an ion source temperature of 220 °C, an interface temperature of 250 °C, and a scanning range of 15–500 m/z. Chemical compositions were identified using the NIST11.LIB database and literature [52] by comparing their retention index.
4.4. Fumigant Toxicity
The fumigation activity of R. dabieshanensis was tested using the method described by Yang et al. [3]. A qualitative filter paper measuring 1.5 × 6 cm was affixed to the lid of a glass tank with a capacity of 1 L (10 cm diameter × 12.5 cm), and then three kinds of EOs and three main components were added to the filter paper, respectively. The acetone treatment was used as the control. Five concentrations of each substance were selected, and each concentration had three replicates, and 20 workers with similar body vitality were selected for each replicate. The experiment was conducted in an insect incubator set at 25 ± 1 °C and 75 ± 5% RH under dark conditions. After 24 h, the termites were observed, and the number of deaths was recorded.
4.5. Fumigation Activity of Binary Mixtures
The binary mixtures of the major constituents of Mentha EO (carvone, menthol, and linalool) were performed using the method of Yang et al. [3]. The three compounds were combined in a 1:1 ratio at doses of LC30:LC30, LC30:LC50 and LC50:LC30. Each dose was tested on 20 workers three times.
Observed mortalities were compared to expected mortalities using the formula:
| E = Oa + Ob(1 − Oa) |
where E is expected mortality, Oa and Ob are the observed mortality of the first and second compounds used in the binary mixtures, respectively.
The effects of binary mixtures were determined as antagonistic, additive, or synergistic using χ2 comparative analysis.
| χ2 = (Om − E)2/E |
where E is the expected mortality from the binary mixture and Om is observed mortality, when χ2 > 3.84 and χ2 < 3.84 were considered synergistic or additive effects [32,42].
4.6. Determination of Enzyme Activity
4.6.1. Enzyme Assays
The worker adults of termites were exposed to the LC30 concentrations of Mentha spp. EOs and their major constituents to determine their effects on the esterases, glutathione S-transferases, and acetylcholine esterase. Samples were prepared by homogenizing 10 termites in 1 mL phosphate buffer (0.1 M, pH 7.0), centrifuging 12,000× g for 15 min at 4 °C, and storing the supernatants at −80 °C for late use. All biochemical tests were repeated three times.
4.6.2. Esterases (ESTs)
The ESTs test was determined by using the method of Piri et al. [32]. To a 96-well microplate, 20 μL of 10 mM α-NA and β-NA and 50 μL of 1 mM Fast Blue RR Salt were added. Next, 10 μL of enzyme solution was added and incubated at 27 °C for 5 min. The absorbance (OD value) was then measured at 450 nm and recorded every 1 min for 10 min. A control group was set up with 10 μL of enzyme solution added to 10 μL of distilled water. Each experiment was repeated more than three times.
4.6.3. Glutathione S-Transferases (GSTs)
GSTs activity was determined using the method described by Piri et al. [32]. In 96-well microtiter plates, 20 μL of 20 mM CDNB and 510 μL of enzyme solution were added. The mixture was incubated at 27 °C for 5 min, the OD value at 340 nm was measured, and the measurement was recorded every 1 min for a total of 10 min. Each experiment was repeated more than three times.
4.6.4. In Vivo Acetylcholinesterase (AChE) Activity Assay
The activity of acetylcholinesterase was determined according to the method of Ellman et al. [53]. The reaction solution was incubated at 25°C for 5 min and consisted of 80 μL of 0.1 M phosphate buffer with a pH of 7.0, 50 μL 10 mM acetylcholine iodide, and 50 μL of 10 mM 5,5-dithiobis-2-nitrobenzoic acid (DTNB). The OD value was measured at 405 nm using a 96-well microplate reader.
4.6.5. In Vitro Acetylcholinesterase Activity Assay
In an in vitro AChE activity assay, ten termiters were ground using a porcelain mortar in 0.1 M Tris-HCl buffer (pH 7.8), which contained NaCl (20 mM) and 0.5% Triton X-100. The resulting mixture was then centrifuged at 15,000× g for 15 min at 4 °C. M. piperita EO and menthol (diluted to 1.0, 5.0, 10.0, 15.0, and 20.0 μL/mL in 99.5% alcohol), as well as M. citrata, M. spicata EOs, linalool, and carvone (0.5, 1.0, 2.0, 4.0, and 6.0 μL/mL, v/v in alcohol), were prepared. Afterwards, 20 μL of each constituent was mixed separately with 40 μL of enzyme solution, 50 μL acetylthiocholine iodide (10 mM), 10 μL DTNB (4 mM), and 100 μL of Tris-HCl buffer in a 96-well microplate. The mixture was incubated for 30 min before being read at absorbance at 405 nm, with alcohol being used as a control.
4.7. Homology Modeling and Molecular Docking
The Uniprot database (https://www.uniprot.org/, accessed on 10 September 2023) was used to search the amino acid sequence of the acetylcholinesterase from R. dabieshanensis. The protein sequence A0A6L2PI50_COPFO was modeled using the acetylcholinesterase template from Coptotermes formosanus, and the 3D structure was established with SWISS-MODEL (http://swissmodel.expasy.org/, accessed on 10 September 2023). The model was evaluated using Ramachandran. The energy-minimizing structures of linalool, menthol, and carvone were obtained with Discovery Studio 2019. The molecular docking of these compounds with acetylcholinesterase was performed by Auto Dock Tools. The bling box for Auto Dock Tools was defined as x, y, and z with specified coordinates (e.g., x = −1.911, y = −17.384, z = 24.253), and other parameters were set to their default values. Subsequently, the optimal pose with the best docking score was selected and visualized.
4.8. Statistical Analysis
The results were expressed as means ± standard errors. The doses causing 50% and 90% (LC50 and LC90, respectively) mortality were determined by probit analysis using the Online Tool (OPSTAT) (http://14.139.232.166/Probit/probitanalysis.html, accessed on 30 October 2023). The inhibition rate was tested through the analysis of variance (ANOVA), followed by Duncan’s new multiple range method Significant Difference test at the p = 0.05 level of significance using SPSS (version 19.0; SPSS Inc., Chicago, IL, USA).
5. Conclusions
Our results indicate that M. citrata, M. piperita, and M. spicata EOs and their major compounds, linalool, menthol, and carvone, are highly toxic to termites. This toxicity is achieved through the inhibition of AChE activity, which suggests that these compounds could be developed as control agents for termites. However, before future application in the field, it is necessary to determine the effects of these active substances on non-target organisms, as well as to design sustained-release formulations to improve the durability of essential oils. This may lead to effective management of underground termites.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/plants12234034/s1, Figure S1. Gas chromatogram of the essential oils from Mentha citrata (A), Mentha piperita (B) and Mentha spicata (C).
Author Contributions
Conceptualization, Y.X. and Z.W.; methodology, Z.W., C.J. and D.Z.; software, C.J.; validation, Z.W., Y.C. and S.Y.; formal analysis, Y.C.; investigation, S.Y. and X.Y.; resources, S.Y. and X.Y.; data curation, Y.C.; writing—original draft preparation, Y.X., C.J. and Z.W.; writing—review and editing, Y.X.; visualization, X.Y. and D.Z.; supervision, Y.X. and D.Z.; project administration, Y.X.; funding acquisition, Y.X. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
The data set used and analyzed during the present study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the General Project of Zhejiang Provincial Department of Education (Professional Degree Graduate special project) (Y202352474).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Chouvenc T., Šobotník J., Engel M.S., Bourguignon T. Termite evolution: Mutualistic associations, key innovations, and the rise of Termitidae. Cell. Mol. Life Sci. 2021;78:2749–2769. doi: 10.1007/s00018-020-03728-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jin C., Han H., Xie Y., Li B., Zhang Z., Zhang D. Toxicity, behavioral effects, and chitin structural chemistry of Reticulitermes flaviceps exposed to Cymbopogon citratus EO and its major constituent citral. Insects. 2022;13:812. doi: 10.3390/insects13090812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang X., Han H., Li B., Zhang D., Zhang Z., Xie Y. Fumigant toxicity and physiological effects of spearmint (Mentha spicata, Lamiaceae) essential oil and its major constituents against Reticulitermes dabieshanensis. Ind. Crops Prod. 2021;171:113894. doi: 10.1016/j.indcrop.2021.113894. [DOI] [Google Scholar]
- 4.Salehi B., Stojanović-Radić Z., Matejić J., Sharopov F., Antolak H., Kręgiel D., Sen S., Sharifi-Rad M., Acharya K., Sharifi-Rad R., et al. Plants of Genus Mentha: From Farm to Food Factory. Plants. 2018;7:70. doi: 10.3390/plants7030070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mamadalieva N.Z., Akramov D.K., Ovidi E., Tiezzi A., Nahar L., Azimova S.S., Sarker S.D. Aromatic medicinal plants of the Lamiaceae Family from Uzbekistan: Ethnopharmacology, essential oils composition, and biological activities. Medicines. 2017;4:8. doi: 10.3390/medicines4010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahendran G., Rahman L.U. Ethnomedicinal, phytochemical and pharmacological updates on Peppermint (Mentha × piperita L.)—A review. Phytother. Res. 2020;34:2088–2139. doi: 10.1002/ptr.6664. [DOI] [PubMed] [Google Scholar]
- 7.Zhao H., Ren S., Yang H., Tang S., Guo C., Liu M., Tao Q., Ming T., Xu H. Peppermint essential oil: Its phytochemistry, biological activity, pharmacological effect and application. Biomed. Pharmacother. 2022;154:113559. doi: 10.1016/j.biopha.2022.113559. [DOI] [PubMed] [Google Scholar]
- 8.Singh P., Pandey A.K. Prospective of essential oils of the genus Mentha as biopesticides: A review. Front. Plant Sci. 2018;9:1295. doi: 10.3389/fpls.2018.01295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bouyahya A., Lagrouh F., El Omari N., Bourais I., El Jemli M., Marmouzi I., Salhi N., Faouzi M.E.A., Belmehdi O., Dakka N. Essential oils of Mentha viridis rich phenolic compounds show important antioxidant, antidiabetic, dermatoprotective, antidermatophyte and antibacterial properties. Biocatal. Agric. Biotechnol. 2020;23:101471. doi: 10.1016/j.bcab.2019.101471. [DOI] [Google Scholar]
- 10.Papachristos D.P., Stamopoulos D.C. Repellent, toxic and reproduction inhibitory effects of essential oil vapours on Acanthoscelides obtectus (Say) (Coleoptera: Bruchidae) J. Stored Prod. Res. 2002;38:117–128. doi: 10.1016/S0022-474X(01)00007-8. [DOI] [Google Scholar]
- 11.Boulamtat R., Mesfioui A., El-Fakhouri K., Oubayoucef A., Sabraoui A., Aasfar A., El-Bouhssini M. Chemical composition, and insecticidal activities of four plant essential oils from Morocco against larvae of Helicoverpa armigera (Hub.) under field and laboratory conditions. Crop. Prot. 2021;144:105607. doi: 10.1016/j.cropro.2021.105607. [DOI] [Google Scholar]
- 12.Koundal R., Dolma S.K., Chand G., Agnihotri V.K., Reddy S.G.E. Chemical composition and insecticidal properties of essential oils against diamondback moth (Plutella xylostella L.) Toxin Rev. 2020;39:371–381. doi: 10.1080/15569543.2018.1536668. [DOI] [Google Scholar]
- 13.Govindarajan M., Sivakumar R., Rajeswari M., Yogalakshmi K. Chemical composition and larvicidal activity of essential oil from Mentha spicata (Linn.) against three mosquito species. Parasitol. Res. 2012;110:2023–2032. doi: 10.1007/s00436-011-2731-7. [DOI] [PubMed] [Google Scholar]
- 14.Zandi-Sohani N., Ramezani L. Evaluation of five essential oils as botanical acaricides against the strawberry spider mite Tetranychus turkestani Ugarov and Nikolskii. Int. Biodeterior. Biodegrad. 2015;98:101–106. doi: 10.1016/j.ibiod.2014.12.007. [DOI] [Google Scholar]
- 15.Reddy S.G.E., Dolma S.K. Acaricidal activities of essential oils against two spotted spider mite, Tetranychus urticae Koch. Toxin Rev. 2018;37:62–66. doi: 10.1080/15569543.2017.1320805. [DOI] [Google Scholar]
- 16.Mackled M.I., El-Hefny M., Bin-Jumah M., Wahba T.F., Allam A. Assessment of the toxicity of natural oils from Mentha piperita, Pinus roxburghii, and Rosa spp. against three stored product insects. Processes. 2019;7:861. doi: 10.3390/pr7110861. [DOI] [Google Scholar]
- 17.Rajkumar V., Gunasekaran C., Christy I.K., Dharmaraj J., Chinnaraj P., Paul C.A. Toxicity, antifeedant and biochemical efficacy of Mentha piperita L. essential oil and their major constituents against stored grain pest. Pestic. Biochem. Phys. 2019;156:138–144. doi: 10.1016/j.pestbp.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 18.Ferreira L.C., Cruz M.G.D., Lima T.B.C., Serra B.N.V., Jerônimo G.T. Antiparasitic activity of Mentha piperita (Lamiaceae) essential oil against Piscinoodinium pillulare and its physiological effects on Colossoma macropomum (Cuvier, 1818) Aquaculture. 2019;512:734343. doi: 10.1016/j.aquaculture.2019.734343. [DOI] [Google Scholar]
- 19.Hategekimana A., Erler F. Fecundity and fertility inhibition effects of some plant essential oils and their major components against Acanthoscelides obtectus Say (Coleoptera: Bruchidae) J. Plant Dis. Prot. 2020;127:615–623. doi: 10.1007/s41348-020-00311-3. [DOI] [Google Scholar]
- 20.Sampson B.J., Tabanca N., Kirimer N., Demirci B., Baser K.H.C., Khan I.A., Spiers J.M., Wedge D.E. Insecticidal activity of 23 essential oils and their major compounds against adult Lipaphis pseudobrassicae (Davis) (Aphididae: Homoptera) Pest Manag. Sci. 2005;61:1122–1128. doi: 10.1002/ps.1100. [DOI] [PubMed] [Google Scholar]
- 21.Suwansirisilp K., Visetson S., Prabaripai A., Tanasinchayakul S., Grieco J.P., Bangs M.J., Chareonviriyaphap T. Behavioral responses of Aedes aegypti and Culex quinquefasciatus (Diptera: Culicidae) to four essential oils in Thailand. J. Pest Sci. 2013;86:309–320. doi: 10.1007/s10340-012-0464-8. [DOI] [Google Scholar]
- 22.Kulkarni R.R., Pawar P.V., Joseph M.P., Akulwad A.K., Sen A., Joshi S.P. Lavandula gibsoni and Plectranthus mollis essential oils: Chemical analysis and insect control activities against Aedes aegypti, Anopheles sfttephensi and Culex quinquefasciatus. J. Pest Sci. 2013;86:713–718. doi: 10.1007/s10340-013-0502-1. [DOI] [Google Scholar]
- 23.Kumrungsee N., Pluempanupat W., Koul O., Bullangpoti V. Toxicity of essential oil compounds against diamondback moth, Plutella xylostella, and their impact on detoxification enzyme activities. J. Pest Sci. 2014;87:721–729. doi: 10.1007/s10340-014-0602-6. [DOI] [Google Scholar]
- 24.Isman M.B. Botanical insecticides in the twenty-first century-fulfilling their promise? Annu. Rev. Entomol. 2020;65:233–249. doi: 10.1146/annurev-ento-011019-025010. [DOI] [PubMed] [Google Scholar]
- 25.Kumar P., Mishra S., Malik A., Satya S. Efficacy of Mentha × piperita and Mentha citrata essential oils against housefly, Musca domestica L. Ind. Crops Prod. 2012;39:106–112. doi: 10.1016/j.indcrop.2012.02.021. [DOI] [Google Scholar]
- 26.Kedia A., Prakash B., Mishra P.K., Chanotiya C.S., Dubey N.K. Antifungal, antiaflatoxigenic, and insecticidal efficacy of spearmint (Mentha spicata L.) essential oil. Int. Biodeter. Biodeg. 2014;89:29–36. doi: 10.1016/j.ibiod.2013.10.027. [DOI] [Google Scholar]
- 27.Pang X., Feng Y.X., Qi X.J., Wang Y., Almaz B., Xi C., Du S. Toxicity and repellent activity of essential oil from Mentha piperita Linn. leaves and its major monoterpenoids against three stored product insects. Environ. Sci. Pollut. Res. 2020;27:7618–7627. doi: 10.1007/s11356-019-07081-y. [DOI] [PubMed] [Google Scholar]
- 28.Kharoubi R., Rehimi N., Khaldi R., Haouari-Abderrahim J., Soltani N. Phytochemical screening and insecticidal activities of essential oil of Mentha × piperita L. (Lamiales: Lamiaceae) and their enzymatic properties against mosquito Culex pipiens L. (Diptera: Culicidae) J. Essent. Oil Bear. Plants. 2021;24:134–146. doi: 10.1080/0972060X.2021.1888158. [DOI] [Google Scholar]
- 29.De Souza L.P., Zuim V., Stinguel P., Pinheiro P.F., Zago H.B. Toxicity of essential oil of Mentha piperita (Lamiaceae) and its monoterpenoid menthol against Tetranychus urticae Kogan 1836 (Acari: Tetranychidae) An. Acad. Bras. Ciênc. 2022;94:e20200427. doi: 10.1590/0001-3765202220200427. [DOI] [PubMed] [Google Scholar]
- 30.Prasannakumar N.R., Jyothi N., Saroja S., Lokesha A.N. Insecticidal properties of Ocimum basilicum and Mentha piperita essential oils against South American Tomato moth, Phthorimaea absoluta (Meyrick) (Lepidoptera: Gelichiidae) Pestic. Biochem. Phys. 2023;190:105329. doi: 10.1016/j.pestbp.2022.105329. [DOI] [PubMed] [Google Scholar]
- 31.Yang X., Jin C., Wu Z., Han H., Zhang Z., Xie Y., Zhang D. Toxicity and physiological effects of nine Lamiaceae essential oils and their major compounds on Reticulitermes dabieshanensis. Molecules. 2023;28:2007. doi: 10.3390/molecules28052007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piri A., Sahebzadeh N., Zibaee A., Sendi J.J., Shamakhi L., Shahriari M. Toxicity and physiological effects of ajwain (Carum copticum, Apiaceae) essential oil and its major constituents against Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) Chemosphere. 2020;256:127103. doi: 10.1016/j.chemosphere.2020.127103. [DOI] [PubMed] [Google Scholar]
- 33.Shahriari M., Sahbzadeh N., Zibaee A., Khani A., Senthil-Nathan S. Metabolic response of Ephestia kuehniella zeller (Lepidoptera: Pyralidae) to essential oil of ajwain and thymol. Toxin Rev. 2017;36:204–209. doi: 10.1080/15569543.2017.1294605. [DOI] [Google Scholar]
- 34.Xie Y., Huang Q., Rao Y., Hong L., Zhang D. Efficacy of Origanum vulgare essential oil and carvacrol against the housefly, Musca domestica L. (Diptera: Muscidae) Environ. Sci. Pollut. Res. 2019;26:23824–23831. doi: 10.1007/s11356-019-05671-4. [DOI] [PubMed] [Google Scholar]
- 35.Oyedeji A.O., Okunowo W.O., Osuntoki A.A., Olabode T.B., Ayo-Folorunso F. Insecticidal and biochemical activity of essential oil from Citrus sinensis peel and constituents on Callosobrunchus maculatus and Sitophilus zeamais. Pestic. Biochem. Phys. 2020;168:104643. doi: 10.1016/j.pestbp.2020.104643. [DOI] [PubMed] [Google Scholar]
- 36.Hussein H.S., El-Deeb D.A., Tawfeek M.E., Abdelgaleil S.A.M. Contact and fumigant toxicities of monoterpenes and phenylpropenes, and their possible mode of action to oleander aphid. Int. J. Trop. Insect Sci. 2022;42:2195–2201. doi: 10.1007/s42690-022-00740-7. [DOI] [Google Scholar]
- 37.Kim S., Yoon J., Tak J.H. Synergistic mechanism of insecticidal activity in basil and mandarin essential oils against the tobacco cutworm. J. Pest Sci. 2021;94:1119–1131. doi: 10.1007/s10340-021-01345-8. [DOI] [Google Scholar]
- 38.Aungtikun J., Soonwera M., Sittichok S. Insecticidal synergy of essential oils from Cymbopogon citratus (Stapf.), Myristica fragrans (Houtt.), and Illicium verum Hook. f. and their major active constituents. Ind. Crops Prod. 2021;164:113386. doi: 10.1016/j.indcrop.2021.113386. [DOI] [Google Scholar]
- 39.Haouel-Hamdi S., Soltani A., Jmal R., Messaoud C., Zaouali Y., Boushih E., Jemâa J.M.B. Use of binary mixtures of three Mentha essential oils for the control of rice weevil Sitophilus oryzae (Curculionidae) Int. J. Trop. Insect Sci. 2021;41:1333–1342. doi: 10.1007/s42690-020-00326-1. [DOI] [Google Scholar]
- 40.Ali H., Qasim M., Saqib H.S.A., Arif M., Islam S. Synergetic effects of various plant extracts as bio-pesticide against wheat aphid (Diurophous noxia L.) (Hemiptera: Aphididae) Afr. J. Agric. Sci. Technol. 2015;3:310–315. [Google Scholar]
- 41.Pavela R. Acute, synergistic and antagonistic effects of some aromatic compounds on the Spodoptera littoralis Boisd. (Lep., Noctuidae) larvae. Ind. Crops Prod. 2014;60:247–258. doi: 10.1016/j.indcrop.2014.06.030. [DOI] [Google Scholar]
- 42.Pavela R. Acute toxicity and synergistic and antagonistic effects of the aromatic compounds of some essential oils against Culex quinquefasciatus Say larvae. Parasitol. Res. 2015;114:3835–3853. doi: 10.1007/s00436-015-4614-9. [DOI] [PubMed] [Google Scholar]
- 43.Arokiyaraj C., Bhattacharyya K., Reddy S.G.E. Toxicity and synergistic activity of compounds from essential oils and their effect on detoxification enzymes against Planococcus lilacinus. Front. Plant Sci. 2022;13:1016737. doi: 10.3389/fpls.2022.1016737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pavela R., Maggi F., Petrelli R., Cappellacci L., Buccioni M., Palmieri A., Canale A., Benelli G. Outstanding insecticidal activity and sublethal effects of Carlina acaulis root essential oil on the housefly, Musca domestica, with insights on its toxicity on human cells. Food Chem. Toxicol. 2022;136:111037. doi: 10.1016/j.fct.2019.111037. [DOI] [PubMed] [Google Scholar]
- 45.Pavela R., Maggi F., Mazzara E., Torresi J., Cianfaglione K., Benelli G., Canale A. Prolonged sublethal effects of essential oils from non-wood parts of nine conifers on key insect pests and vectors. Ind. Crops Prod. 2011;168:113590. doi: 10.1016/j.indcrop.2021.113590. [DOI] [Google Scholar]
- 46.Sánchez-Gómez S., Pagán R., Pavela R., Mazzara E., Spinozzi E., Marinelli O., Zeppa L., Morshedloo M.R. Lethal and sublethal effects of essential oil-loaded zein nanocapsules on a zoonotic disease vector mosquito, and their non-target impact. Ind. Crops Prod. 2022;176:114413. doi: 10.1016/j.indcrop.2021.114413. [DOI] [Google Scholar]
- 47.Wang Z., Xie Y., Sabier M., Zhang T., Deng J., Song X., Liao Z., Li Q., Yang S., Cao Y., et al. Trans-anethole is a potent toxic fumigant that partially inhibits rusty grain beetle (Cryptolestes ferrugineus) acetylcholinesterase activity. Ind. Crops Prod. 2021;161:113207. doi: 10.1016/j.indcrop.2020.113207. [DOI] [Google Scholar]
- 48.Wei H., Liu J., Li B., Zhan Z., Chen Y., Tian H., Lin S., Gu X. The toxicity and physiological effect of essential oil from Chenopodium ambrosioides against the diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae) Crop. Protect. 2015;76:68–74. doi: 10.1016/j.cropro.2015.06.013. [DOI] [Google Scholar]
- 49.Kiran S., Prakash B. Toxicity and biochemical efficacy of chemically characterized Rosmarinus officinalis essential oil against Sitophilus oryzae and Oryzaephilus surinamensis. Ind. Crops Prod. 2015;74:817–823. doi: 10.1016/j.indcrop.2015.05.073. [DOI] [Google Scholar]
- 50.Shahriari M., Zibaee A., Sahebzadeh N., Shamakhi L. Effects of a-pinene, trans-anethole, and thymol as the essential oil constituents on antioxidant system and acetylcholine esterase of Ephestia kuehniella Zeller (Lepidoptera: Pyralidae) Pestic. Biochem. Physiol. 2018;150:40–47. doi: 10.1016/j.pestbp.2018.06.015. [DOI] [PubMed] [Google Scholar]
- 51.de Sena Filho J.G., de Almeida A.S., Pinto-Zevallos D., Barreto I.C., de Holanda Cavalcanti S.C., Nunes R., Teodoro A.V., Xavier H.S., Filho J.M.B., Guan L., et al. From plant scent defense to biopesticide discovery: Evaluation of toxicity and acetylcholinesterase docking properties for Lamiaceae monoterpenes. Crops Prot. 2023;164:106126. doi: 10.1016/j.cropro.2022.106126. [DOI] [Google Scholar]
- 52.Adams R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. 4th ed. Allured Publishing Corporation; Carol Stream, IL, USA: 2007. [Google Scholar]
- 53.Ellman G.L., Courtney K.D., Andres V., Featherstone R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data set used and analyzed during the present study are available from the corresponding author upon reasonable request.