Abstract
Rhodobacter capsulatus ORF1696 mutant strains were created by insertion of antibiotic resistance cartridges at different sites within the ORF1696 gene in a strain that lacks the light-harvesting II (LHII) complex. Steady-state absorption spectroscopy profiles and the kinetics of the light-harvesting I (LHI) complex assembly and decay were used to evaluate the function of the ORF1696 protein in various strains. All of the mutant strains were found to be deficient in the LHI complex, including one (ΔNae) with a disruption located 13 codons before the 3′ end of the gene. A 5′-proximal disruption after the 31st codon of ORF1696 resulted in a mutant strain (ΔMun) with a novel absorption spectrum. The two strains with more 3′ disruptions (ΔStu and ΔNae) were restored nearly to the parental strain phenotype when trans complemented with a plasmid expressing the ORF1696 gene, but ΔMun was not. The absorption spectrum of ΔMun resembled that of a strain which had a polar mutation in ORF1696. We suggest that a rho-dependent transcription termination site exists between the MunI and proximal StuI sites of ORF1696. A comparison of LHI complex assembly kinetics showed that assembly occurred 2.6-fold faster in the parental strain than in strain ΔStu. In contrast, LHI complex decay occurred 1.7-fold faster in the ORF1696 parental strain than in ΔStu. These results indicate that the ORF1696 protein has a major effect on LHI complex assembly, and models of ORF1696 function are proposed.
Purple nonsulfur bacteria such as Rhodobacter capsulatus are able to grow chemotrophically or phototrophically. Photosynthesis is anaerobic, and the components of the photosynthetic apparatus are formed gratuitously in the dark in response to oxygen limitation (5). An intracytoplasmic membrane system (ICM), contiguous with and derived from the cytoplasmic membrane, contains the apparatus necessary to sustain photosynthetic growth. In R. capsulatus the photosynthetic machinery includes the light-harvesting I (LHI) and II (LHII) complexes B875 and B800-B850, respectively, and the reaction center (RC) complex, each of which contains bacteriochlorophyll a (Bchl) and carotenoid pigments (15).
The LHI antenna complex contains two polypeptide subunits, α and β, each spanning the ICM once due to a hydrophobic central region, which are encoded by the pufA and pufB genes. Conserved His residues bind Bchl within the transmembrane segments of α and β. The currently understood mechanisms of in vivo and in vitro assembly of the LHI complex were reviewed recently (14, 23). Strains of R. capsulatus defective in Bchl synthesis exhibit enhanced turnover of LH peptides, and binding of Bchl is believed to be essential for the proper membrane insertion of photosynthetic pigment-binding proteins (15). In one report on site-directed LHI peptide mutants, several (especially N-terminal) residues were implicated in appropriate pigment binding or complex assembly, and some mutations seemed to affect the structure of the RC (3). In other studies, site-directed mutations resulted in increased degradation rates of LHI peptides, and a model has been proposed in which electrostatic interaction between the negatively charged N terminus of the β polypeptide and the positively charged N terminus of the α polypeptide is a prerequisite for assembly of the LHI complex (14).
Following or concurrent with in vivo assembly in the R. capsulatus ICM, the LHI complex closely associates with the RC complex and efficiently transfers light energy to the RC. Chemical cross-linking experiments indicated a close association between these two complexes in the ICM of R. capsulatus (26), and it is likely that the LHI complex forms a ring around the RC (12, 33).
Little is known about proteins that might interact with LH complex components to facilitate membrane insertion of protein subunits, delivery of Bchl, complex assembly, or stabilization in the ICM. It is known that mutations of two homologous genes, pucC and ORF1696, result in analogous reductions in the LHII and LHI complexes, respectively and specifically. Although pucC mutants completely lack the LHII complex (15), secondary mutations in pucC deletion strains result in partial restoration of the LHII spectrum (21, 22). It was found that disruption of the open reading frame ORF1696 (which is located immediately 5′ of and cotranscribed with the puhA gene; see Fig. 1) reduced the LHI complex steady-state level in the ICM of R. capsulatus (6, 41). However, it was not clear to what extent the consequences of ORF1696 disruption were due to cis or trans effects. Furthermore, if the ORF1696 gene product really is required to obtain maximal levels of the LHI complex, an important question is whether the ORF1696 protein acts to inhibit LHI complex turnover or to enhance LHI assembly.
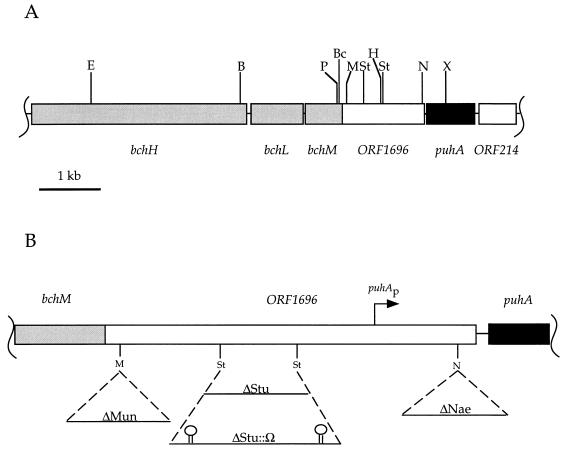
FIG. 1.
(A) Restriction map of ORF1696 and flanking genes found in an 8-kb region of the photosynthesis gene cluster of the R. capsulatus genome (2). Abbreviations: B, BamHI; Bc, BclI; E, EcoRI; H, HindIII; M, MunI; N, NaeI; P, PstI; St, StuI; X, XhoI. (B) Schematic illustration of ORF1696 and flanking genes with sites of antibiotic resistance cartridge insertion. Solid lines below the genes represent antibiotic resistance cartridge DNA molecules. Stem-loop symbols represent transcriptional terminators. Dashed lines indicate the site of cartridge insertion in ORF1696. The direction of transcription is from left to right, and the puhA promoter (puhAp) is indicated by the bent arrow.
In this paper we report the results of disruption-complementation analyses of several ORF1696 mutants generated by insertion of antibiotic resistance cartridges at different sites within ORF1696. We address the question of the function (assembly versus stabilization) provided by the ORF1696 protein in kinetic analyses of LHI assembly and decay rates in an ORF1696 mutant compared to the parental ORF1696+ strain.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The wild-type R. capsulatus strain SB1003 (39), the ORF1696 mutant ZY1 (6), and the expression plasmid pJAJ9 (19) were described previously. The Escherichia coli strain C600 r− m+ (9) was used for the routine cloning of plasmids. Strains SM10 (30) and HB101(pRK2013) (11) were used for the diparental and triparental conjugational transfer of plasmids, respectively, into R. capsulatus. Other bacterial strains and plasmids used in this study are described below.
Growth conditions and media.
E. coli cultures were grown in Luria-Bertani medium (28) at 37°C. Antibiotics were added as required at the following concentrations (in micrograms per milliliter): ampicillin, 200; kanamycin sulfate, 50; tetracycline-HCl, 10; and trimethoprim, 40. R. capsulatus cultures were grown in RCV minimal medium as described previously (22) with antibiotics added as required in the following concentrations (in micrograms per milliliter): kanamycin sulfate, 10; tetracycline-HCl, 0.5; and spectinomycin-2HCl, 10.
In vitro DNA techniques and plasmid constructions.
Restriction endonuclease digestion, DNA ligation, agarose gel electrophoresis, transformation of E. coli, and other recombinant DNA procedures were carried out essentially as described previously (28).
The relative positions of genes and restriction sites in DNA fragments were as shown in Fig. 1A. Plasmid pCY42 was constructed by subcloning the 4.2-kb ORF1696::Ω fragment from pΔPUHA::Ω2 (37) as a BclI-to-PvuII fragment into the BamHI and PstI (made blunt with T4 DNA polymerase) sites of pJAJ9. This resulted in the forced cloning of the ORF1696::Ω fragment in an orientation which placed ORF1696 under the transcriptional control of the puf operon promoter present on pJAJ9. The Ω cartridge, which replaces the puhA gene sequences downstream of ORF1696, provided a rho-independent transcriptional terminator that should protect the 3′ end of the ORF1696 message from exonucleolytic degradation.
Plasmid pCY34 was constructed by subcloning the 3.4-kb BamHI-to-XhoI DNA fragment, which includes sequences encoding the bch′HLMORF1696puhA′ genes, from pUC13::EcoF (36) into pUC13 (25) previously digested with BamHI and SalI. Plasmid pCY1800 was constructed by subcloning the 1.8-kb PstI DNA fragment, which includes sequences encoding the bch′MORF1696puhA′ genes, from pCY34 into pUC13 previously digested with PstI, such that the orientation of the bch′MORF1696puhA′ DNA insert was in the direction of transcription of the lac promoter.
Plasmids pCYNAE and pCYMUN were constructed by digesting plasmid pCY34 separately with the restriction endonuclease NaeI or MunI. Following digestion with MunI, the 5′-overhanging end of the plasmid DNA was made blunt with T4 DNA polymerase. The pUC4KIXX plasmid (Pharmacia Biotech, Inc., Baie d’Urfe, Quebec, Canada) was digested with SmaI to generate a 1.2-kb blunt-ended DNA fragment encoding kanamycin resistance (Kmr) and a 5′ segment of the bleomych resistance (Bler) gene. The Kmr cassette was separated from plasmid DNA by agarose gel electrophoresis, purified, and separately ligated into either the NaeI or MunI (end made blunt)-linearized pCY34 plasmid.
Plasmid pRKPUHA2 was made by digesting pRKPUHA1 (37) with HindIII to remove a 1.0-kb DNA fragment encoding the translational start codon and the 5′ sequences of the ORF1696 gene but leaving the puhAp promoter sequences intact on the remaining vector DNA. The 11.9-kb vector DNA was gel purified and self-ligated to yield pRKPUHA2.
Plasmid pRR3 was created by ligation of the Ω cartridge (27) SmaI fragment into the pCY34 StuI site.
Plasmid pRR5 was constructed by subcloning the 2.2-kb bch′HLMORF1696′KIXX′ DNA fragment, obtained from pCYMUN digested with EcoRI and BglII, into the 9.9-kb expression plasmid pPUFP1 (10) cut with the same enzymes (separated from a 1.2-kb DNA fragment and purified). The gentamicin resistance (Gmr) cartridge from plasmid pWKR440 (a gift from W. Klipp) was subcloned as a 2.6-kb HindIII DNA fragment into plasmid pRR5, which had been digested with HindIII, to allow selection of this plasmid with gentamicin. Plasmid pRR5C was constructed by digesting pRR5 with SmaI to release a 3.2-kb DNA fragment encoding the bchH′LMORF1696puhA′ sequences and an 11.5-kb linear vector fragment. The 11.5-kb vector DNA was purified by agarose gel electrophoresis and recircularized in a dilute ligation reaction to yield pRR5C. Plasmid pRRMun+ was constructed by digesting pCY1800 with MunI, generating blunt ends with Klenow fragment, and then subcloning the 1.2-kb SmaI KIXX cartridge DNA fragment from pUC4KIXX into the blunt-ended pCY1800 DNA.
Plasmid pRRMun+ was digested with EcoRI and StuI to give a 1.9-kb linear DNA segment encoding a 3′ segment of bchM, including the 5′ 90 nucleotides of ORF1696 followed by the KIXX fragment sequences. This 1.9-kb fragment was purified and ligated into pRR5C DNA linearized by digestion with EcoRI and SmaI. The resulting 13.5-kb plasmid, pRR6, places the ORF1696 sequences under the transcriptional control of the puf promoter and specifies Kmr and Gmr.
Strain construction.
Plasmids were mobilized by conjugation from E. coli into the R. capsulatus gene transfer agent (GTA) overproducer strain DE442 (38) by use of the mobilizing vector pDPT51, present in E. coli Tec5, as described previously (32). The ORF1696::Kmr insertions in plasmids pCYNAE and pCYMUN were transduced into the R. capsulatus puc operon deletion strain, ΔLHII (22), by GTA-mediated interposon mutagenesis as described previously (37). GTA filtrate containing the ORF1696::Kmr insertion between the StuI sites was a gift from C. Bauer’s lab. The ORF1696::Ω insertion in plasmid pRR3 was transduced into the R. capsulatus LHII− mutant strain, MW442 (29). The ORF1696 mutants of ΔLHII were named according to the site(s) at which the Kmr (or Ω) cassette was inserted in the ORF1696 gene, i.e., ΔNae, ΔStu, ΔStu::Ω, and ΔMun. Thus, the Kmr cartridge was inserted 1,392, 411, and 87 nucleotides downstream of the putative ATG start codon for ORF1696 in strains ΔNae, ΔStu, and ΔMun, respectively. The ΔMun and ΔStu insertions are upstream of the reported site of the puhA promoter, puhAp (6), and the Bler gene segment is translationally out of frame with the 3′ segments of ORF1696. The ΔNae insertion is downstream of puhAp and also creates a translationally in-frame fusion between the remaining 60 nucleotides of ORF1696 and a 5′ segment of the Bler gene on the SmaI fragment of the KIXX Kmr cartridge (4).
LHI decay kinetics experiments.
Strains ΔLHII and ΔStu were grown to early stationary phase (absorbance at 650 nm [A650] = 1.5 to 2.0) in RCV medium under anaerobic, photosynthetic conditions in 900-ml Roux bottles. Zero hour samples were removed from these cultures, and the cells were pelleted by centrifugation and stored at −80°C for later analysis. Portions (500 ml) of the photosynthetic cultures were used to inoculate 9.5 liters of RCV medium in a 20-liter fermenter with aeration maintained at or near 20% partial O2 pressure by sparging with air at a constant rate of 3 liters/min as well as by automatic adjustment of the impeller revolutions-per-minute value. Triplicate samples were removed every 2 h, and the cells were pelleted by centrifugation and stored at −80°C prior to analysis.
LHI assembly kinetics.
Strains ΔLHII and ΔStu were grown under highly aerated conditions in 100 ml of RCV medium in a 1-liter Erlenmeyer flask incubated in a gyratory shaker at 300 rpm to an optical density of 2.0 to 2.5 A650 units. A zero hour sample (5.0 ml) was removed from these highly aerated cultures, the remaining portion (95 ml) of the cultures was used to inoculate 700 ml of fresh RCV medium in separate 1-liter flasks, and the flasks were incubated at 150 rpm in a gyratory shaker. Samples were removed from these semiaerobic cultures every 30 min, and the cells were pelleted and stored at −80°C.
Analytical methods.
All measurements were done on a minimum of two independent cultures and were highly reproducible (10 to 15% variation). For spectroscopy, cell samples were resuspended in 24% bovine serum albumin in RCV medium and analyzed for pigment-protein complex levels as previously described (22). LHI complex levels were determined as the integrated area under the absorbance peak at 875 nm, normalized to cell numbers by multiplying spectra by a factor to give an A650 (due to light scattering) of 0.2, with SpectraCalc and GRAMS 386 software packages (Galactic Industries Corp.). The contribution of the RC to the 875-nm peaks was assumed to be negligible. In the kinetics experiments, the values of the A875/A650 ratios obtained were plotted graphically as a function of time, and the slopes of the resultant lines were calculated. Total cellular Bchl content was determined by acetone extraction as described previously (31). The β-galactosidase activities were determined as described previously (22).
RESULTS
ORF1696 gene disruption-complementation experiments.
Disruption of ORF1696 with a Kmr cartridge resulted in a decrease in LHI antenna complex levels in strains ZY1 and ZY3, but it was not clear if this phenotype was due solely to the loss of an ORF1696 gene product since trans-complementation experiments were not done (6). Therefore, the plasmid pCY42 (which contains the ORF1696 gene transcribed from the puf promoter) was introduced into ZY1, and the absorption spectroscopy profile of intact cells of this strain, grown under semiaerobic conditions, was compared with those generated from strain ZY1(pJAJ9) and the wild-type strain SB1003(pJAJ9) grown under the same conditions. The LHI complex level in ZY1(pCY42), manifested as a shoulder on the long-wavelength slope of the 850-nm LHII absorbance peak, was restored nearly to that of the wild-type strain (40). This trans-complementation experiment indicates that the ORF1696 gene product is required to obtain the wild-type level of the LHI complex in an LHII+ background.
An attempt was made, by gene disruption analysis, to identify regions of the ORF1696 protein that might be important for this activity, by using the well-characterized LHII− strain ΔLHII (22) to allow quantitative measurements of the relative amounts of the LHI complex. Gene disruptions were initially made at three sites in ORF1696 using a Kmr cartridge, which rarely results in a polar effect when the Kmr gene is inserted in the same orientation as the disrupted gene (10). Figure 1B gives a schematic representation of the ORF1696 gene and flanking regions and the sites of insertion of antibiotic resistance cartridges at the MunI site (yielding strain ΔMun), between the StuI sites (yielding strains ΔStu and ΔStu::Ω), and at the NaeI site (yielding strain ΔNae).
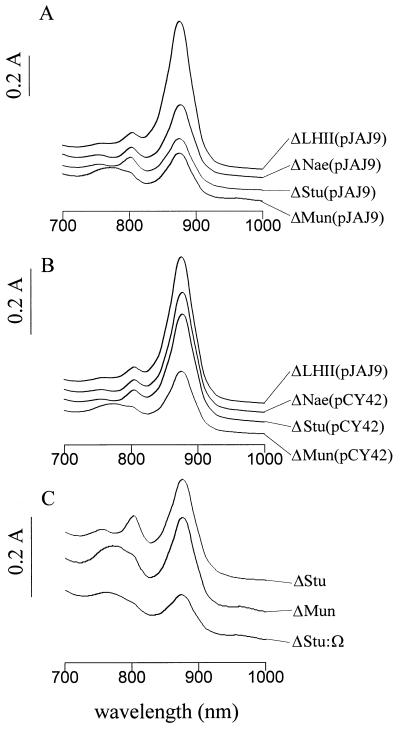
The effect that each of the above Kmr disruptions had on LHI complex levels is shown in Fig. 2A. Each strain gave rise to a spectrum containing a peak at 875 nm corresponding to LHI absorption and an RC peak at 800 nm. The areas of the A875 peaks were determined for each of the mutant strains and compared to that of the ΔLHII (ORF1696+) parental strain containing the expression plasmid pJAJ9. It was found that the LHI peak area was reduced to 20% in ΔStu, to 30% in ΔMun, and to 50% in ΔNae of the level in ΔLHII(pJAJ9). These results imply that deletion of as few as 13 of the C-terminal amino acid residues of the ORF1696 protein (in strain ΔNae) impairs its function in maintaining the wild-type steady-state level of LHI.
FIG. 2.
Absorption spectra obtained with intact cells grown under semiaerobic conditions. (A) Parental strain ΔLHII(pJAJ9) and the Kmr disruption mutants ΔNae(pJAJ9), ΔStu(pJAJ9), and ΔMun(pJAJ9). (B) Parental strain ΔLHII(pJAJ9) and pCY42-complemented ΔNae, ΔStu, and ΔMun strains. (C) Strains ΔMun and ΔStu compared with ΔStu::Ω.
Plasmid pCY42 (which contains ORF1696 expressed from the puf promoter of pJAJ9) was introduced into strains ΔStu and ΔNae, and when these ORF1696 complemented strains were grown under semiaerobic conditions the area of the LHI complex peak was increased to 73 and 83% of the ΔLHII(pJAJ9) level, respectively, as shown in Fig. 2B. Thus, there was nearly complete restoration of the level of LHI by trans complementation of ΔStu and ΔNae with the ORF1696 gene.
In the ΔMun mutant the reduction in LHI was accompanied by a reduction in the size of the RC peak at 800 nm and the appearance of a broad, heterogeneous area of absorbance spanning from 750 to 790 nm (Fig. 2A). Several hypotheses were considered to explain the novel absorption spectrum of the ΔMun strain, including the following: (i) the loss of amino acids encoded by ORF1696 sequences between the MunI and StuI sites altered the activity of the ORF1696 N-terminal peptide; (ii) the 45-amino-acid fusion peptide translated as a result of the ΔMun mutation had an altered activity; and (iii) a polar effect was exerted by the Kmr cassette on the expression of the puhA and 3′ sequences (37), which was manifested in ΔMun but absent from ΔStu and ΔNae.
Hypotheses 1 and 2 were tested by trans complementation of the ΔMun mutation with plasmid pCY42 or by expression of the 45-amino-acid ΔMun ORF1696 fusion peptide in strains ΔLHII and ΔStu. These experiments did not change the 750- to 790-nm absorbance or the 800-nm RC peak of any of the strains tested (40), thus ruling out the first two hypotheses.
Hypothesis 3 was first tested by introducing plasmid pRKPUHA2, which carries the intact puhA gene and the puhA promoter region, into the ΔMun strain. The broad area of absorbance from 750 to 790 nm in strain ΔMun (pRKPUHA2) was not reduced, although there was a slight increase in the 800-nm RC peak (40). This result indicates that a polar effect on puhA gene expression exists in the ΔMun strain and that this polar effect extends to ORF214 and perhaps open reading frames located 3′ of ORF214 (37). Because of the uncertainty of how many genes located 3′ of puhA might be affected by a polar mutation in ORF1696, we next adopted a different approach.
To determine the phenotype of a genuinely polar mutation in ORF1696, which would reduce expression of all transcriptionally coupled genes located 3′ of ORF1696, the Ω cartridge was inserted between the StuI sites of the cloned ORF1696 gene and recombined into the chromosome of the LHII− strain MW442. Although this Ω disruption is at the same position as the Kmr disruption of the ΔStu mutant, the Ω cartridge contains translational and transcriptional stop signals (27) and has been shown to have a strong polar effect in R. capsulatus (34), whereas the KIXX Kmr cartridge rarely has a polar effect (10). As shown in Fig. 2C, the ΔStu::Ω strain was found to have a broad region of absorbance from 750 to 790 nm and the 800-nm RC peak was reduced. This ΔMun-like absorption spectrum of ΔStu::Ω, in contrast to the ΔStu spectrum, indicates that insertion of the Kmr cartridge at the MunI site in ORF1696 exerts a polar effect on the expression of puhA and other downstream genes, whereas the absence of the 750- to 790-nm absorbance in the ΔStu and ΔNae strains indicates a lack of polarity (see the Discussion section).
Complementation of the ΔMun strain with ORF1696 (in pCY42) resulted in an increase of the LHI peak area from 30 to 41% of that of the ΔLHII parental strain (Fig. 2B). This amount of LHI restoration was much less than that seen with ΔStu(pCY42) (73%) and ΔNae(pCY42) (83%) (compare Fig. 2A and B). We interpret this relatively slight increase in ΔMun(pCY42) LHI levels as being due to a partial polar effect of the ΔMun ORF1696 disruption on the expression of puhA and ORF214 genes, the expression of which is required to obtain the normal level of LHI (37). Complementation of the ΔMun strain with the puhA gene in pRKPUHA2 did not result in a significant increase in the LHI peak (40), which we attribute to the absence of a full-length ORF1696 protein.
Effect of a Kmr cartridge disruption of ORF1696 on the transcription and translation of a pufB::lacZ fusion.
Northern blot analysis demonstrated that a ORF1696 Kmr disruption mutation had no effect on the levels of pufBA mRNA, and so the ORF1696 protein should not modulate transcription of the puf operon or mRNA decay (6). We tested the possibility that the ORF1696 protein acts to regulate pufB gene expression at the level of transcription and/or translation by introducing plasmid pXCA::935 (1), which encodes a pufB′::lac′Z translational fusion driven by the puf promoter, into strains ΔLHII and ΔStu. After growth under semiaerobic conditions, the β-galactosidase activities obtained with ΔLHII(pXCA::935) were 754 (±44) U and with ΔStu(pXCA::935) were 677 (±2) U. This experiment confirms that the ORF1696 protein does not significantly affect transcription of puf genes and indicates that it does not modulate translation of pufB mRNA. Thus, the ORF1696 protein regulates LHI complex levels at a posttranslational level.
Kinetic analyses of LHI formation and decay.
In principle, the ORF1696 protein could function to stabilize (e.g., protect from proteolysis) the otherwise-assembled LHI complex in the ICM or it could be a catalyst to assemble the LHI complex from the α- and β-polypeptide subunits and pigment molecules. We differentiated between these two possibilities by kinetic analyses of the LHI complex formation and the decay in the ΔLHII and ΔStu strains.
To evaluate the role of the ORF1696 protein in LHI complex assembly, cultures of strains ΔStu and ΔLHII were grown with high aeration to repress expression of the photosynthetic apparatus. These cultures were used as inocula for semiaerobic cultures in which cells were induced to express photosynthesis genes and assemble the LHI complex de novo. Growth rates of the two strains were monitored, and no significant differences were detected. Thus, any differences between these strains in LHI levels would be attributable to an effect of the ORF1696 disruption in ΔStu on LHI accumulation, as opposed to a difference in their growth rates.
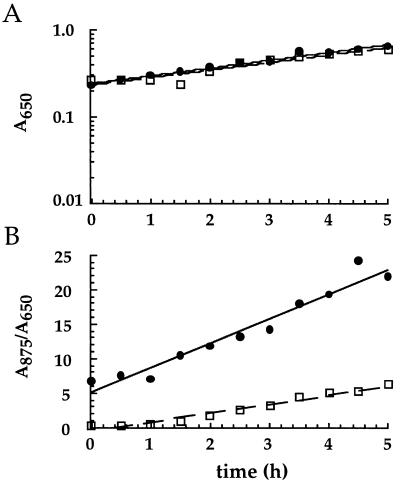
Figure 3 illustrates the time course of a representative experiment comparing the rates of growth and of LHI accumulation in cells of ΔStu and ΔLHII over a 5-h period after a shift to semiaerobic conditions. As summarized in Table 1, LHI accumulated in both strains, although more slowly in the ΔStu strain (average slope of 1.4) than in the ΔLHII strain (average slope of 3.7). Comparison of the LHI accumulation slopes obtained for these two strains in three independent experiments revealed a range of 2.2- to 3.4-fold differences in the slopes and an average difference of 2.6-fold.
FIG. 3.
Representative graphs of growth (A) and of LHI assembly (B) over time for ΔStu (dashed line) and ΔLHII (solid line) strains. Inocula were grown under highly aerated conditions and then transferred to growth conditions with reduced aeration to induce LHI synthesis.
TABLE 1.
Average growth, LHI assembly, and decay rates
| Expta | Strain | Culture doubling time (h)b | Slope of line from LHI assembly/decay graphb | Ratioc |
|---|---|---|---|---|
| Assembly | ΔLHII | 2.9 (0.7) | 3.7 (0.1) | |
| ΔStu | 3.1 (0.5) | 1.4 (0.3) | 2.6 | |
| Decay | ΔLHII | 3.2 (0.2) | −2.7 (0.3) | |
| ΔStu | 3.4 (0.2) | −1.6 (0.2) | 1.7 |
Three independent cultures of each strain were grown for each experiment.
Values in parentheses are the significant differences for the average values listed.
Ratios of mean ΔLHII to ΔStu decay or assembly slopes.
The differences in the rates of accumulation of the LHI complex in the experiments described above could be due to differences in efficiencies of assembly, differences in stability, or a combination of these two processes. To differentiate between these alternatives, we compared the rates of decay of the LHI complex in ΔLHII and ΔStu cultures that underwent a shift from anaerobic-photosynthetic to aerobic-respiratory conditions of growth. The logic underlying these experiments was that LHI would be formed maximally during anaerobic-photosynthetic growth but would be synthesized at a reduced rate under aerobic-respiratory growth and that the rates of decay of 875-nm peak areas would approximate the relative stabilities of the LHI complex in these two strains.
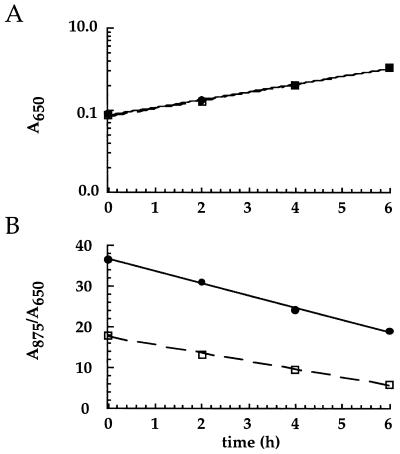
Figure 4 shows the time course of a representative experiment comparing the rates of growth and LHI decay in ΔStu and ΔLHII. Cells of both strains contained smaller amounts of the LHI complex as the fermenter cultures grew aerobically, but, surprisingly, the LHI complex was lost slightly more rapidly from the ΔLHII cells than from the ΔStu cells. Since the growth rates of these strains were very similar (average generation times of 3.2 to 3.4 h), the difference in rates of LHI decay is due to a difference in LHI stability rather than to a difference resulting from different rates of cell growth and division.
FIG. 4.
Representative graphs of culture growth (A) and of LHI decay (B) over time for ΔStu (dashed line) and ΔLHII (solid line) strains. Inocula were grown under anaerobic-photosynthetic growth conditions and then transferred to highly aerated growth conditions to repress LHI synthesis.
Table 1 summarizes the results of six independent experiments and shows that the LHI complex is slightly less stable in ΔLHII than in ΔStu (the average ratio of the ΔLHII to the ΔStu slopes for LHI decay was 1.7). The assembly rate of LHI was greater in ΔLHII than in ΔStu (ΔLHII/ΔStu ratio of 2.6). We conclude that the ORF1696 protein functions to enhance assembly of the LHI antenna complex.
DISCUSSION
ORF1696 is an open reading frame encoding 477 amino acids that is located 3′ of the bchFNBHLM genes and immediately 5′ of the puhA gene (Fig. 1) within the photosynthesis gene cluster of R. capsulatus (2). Transcription of these genes initiates at two promoters, one located upstream of the bchF gene and another located within ORF1696 (6). Hydropathy analyses of the primary amino acid sequence of ORF1696 show it to be very hydrophobic, and gene fusion experiments indicate that it is an integral membrane protein with 12 transmembrane segments (40). Therefore, the ORF1696 protein is likely to be located in the ICM, which contains the LHI antenna complex.
Our data demonstrate that Kmr interposon mutations of the ORF1696 gene in R. capsulatus ΔNae and ΔStu reduced the amount of the LHI complex and were complemented in trans with a plasmid-borne copy of ORF1696 (Fig. 2). The β-galactosidase activities of ΔStu and ΔLHII mutants harboring plasmid pXCA::935, in which the lac′Z gene is fused translationally in frame with pufB and transcribed from the puf promoter, were similar, indicating that pufB mRNA transcription and translation are not significantly affected by the mutation in ORF1696. Therefore, restoration of the LHI complex in the ΔStu(pCY42) and ΔNae(pCY42) strains to near-normal levels shows that a ORF1696 gene product enhances the steady-state level of the LHI complex in the ICM.
Several reasons might account for the incomplete restoration of the LHI complex observed in the complemented ΔNae and ΔStu strains. For example, the ORF1696 gene may not be expressed as strongly from the pCY42 plasmid as it is expressed from its natural chromosomal location. Although the Kmr cassette could have a marginally polar effect in ΔStu and ΔNae (see below), it is clear that the absence of the ORF1696 gene product is the primary reason for the reduced LHI content of these strains.
The 50% reduction of the LHI complex level observed in the ΔNae mutant suggests that the loss of as few as the 13 C-terminal amino acid residues of ORF1696 impairs its function. Since the 5′ remnant of the Bler gene on the Kmr cartridge was fused translationally in frame to the 3′ codons of ORF1696 in ΔNae, no ORF1696 amino acid residues were deleted per se. That is, 97 heterologous amino acids were added to Ala-464 of the N-terminal segment of ORF1696 at the disruption site, and the C-terminal 13-amino-acid segment of ORF1696 (starting with Gly-465) was fused to the truncated Bler protein. Nevertheless, this disruption greatly interfered with the function of the transected ORF1696 protein (Fig. 2).
Since all of the ORF1696 mutants contained small amounts of LHI, the N-terminal segments remaining in the three mutants described here, as short as 31 amino acid residues in the ΔMun mutant, conceivably could contribute to LHI assembly, or else LHI is assembled inefficiently in the complete absence of ORF1696 activity. We suggest that ORF1696 is a major factor in LHI complex assembly but that either there are additional assembly factors or LHI forms spontaneously to a limited degree in vivo, as has been observed in vitro (23).
The low level of the LHI complex in ΔStu::Ω, compared to the levels in ΔStu and ΔMun (Fig. 2C), is attributed to a combination of the direct effect of the disruption of ORF1696 and the indirect polar effect of the Ω disruption. The RC and LHI complexes are closely associated in the ICM, which could provide mutual stabilization as a result of protein-protein interactions (26, 33, 37). In fact, the amounts of the RC complex 800-nm peak in these three mutants correspond to the following order: ΔStu > ΔMun > ΔStu::Ω (Fig. 2C). Thus, the Ω cartridge insertion in ΔStu::Ω and the ΔMun Kmr cartridge insertion seem to have polar effects to different degrees on transcription of puhA and genes located 3′ of puhA, such as ORF214, which have been observed to reduce RC and LHI levels when mutated (37). This conclusion is supported by the results of the trans-complementation experiments on the ΔMun strain (Fig. 2B). We suggest that a rho-dependent transcription termination site exists between the MunI site and the nearest of the two StuI restriction sites within the ORF1696 sequence and gives rise to the polar effect observed in ΔMun but not in ΔStu or ΔNae. Our results indicate that transcription initiated at the bchF promoter is required for normal expression of puhA, ORF214, and perhaps other genes located 3′ of ORF214. This interpretation supports the proposal that the bch-puhA superoperon, previously thought to end immediately after puhA (6), extends beyond the puhA gene (7, 37).
It is difficult to account for the appearance of the broad, heterogeneous region of absorbance extending from approximately 750 to 790 nm in the ΔMun and the ΔStu::Ω spectra that accompanied the reduction in the 800-nm RC peak (Fig. 2). This absorbance could in principle arise from Bchl degradation products due to pigment-protein complex turnover or to biosynthetic intermediates, but absorption spectra of pigments in acetone extracts of ΔMun and ΔLHII cells were superimposable (40). Thus, this spectrum seems to be due to Bchl molecules abnormally associated with proteins.
Our comparisons of the kinetics of LHI assembly and decay in strains ΔLHII and ΔStu show that ORF1696 functions to enhance LHI assembly (Fig. 3 and 4; Table 1). It is conceivable that ORF1696 can act reversibly in an equilibrium-driven process to promote loss of Bchl from or disassembly of the LHI complex in the absence of Bchl synthesis, as indicated by the LHI decay kinetics (Fig. 4B; Table 1).
Although the differences between the ΔLHII and the ΔStu slopes for LHI accumulation are significant (Fig. 3B and Table 1), the chromosomal disruptions of ORF1696 did not result in the complete loss of LHI under steady-state conditions (Fig. 2). This partial phenotype resulting from the mutation of ORF1696 raises questions as to how many factors are involved in maintaining the LHI complex at normal steady-state levels. Although the exact step at which ORF1696 exerts its role in the assembly of the LHI complex, whether by interacting with LHI polypeptides or with pigments, is unknown, we propose below two nonexclusive models.
Firstly, in vivo translocation of the LHI α and β polypeptides into the ICM has been hypothesized to involve an integral membrane protein, such as ORF1696, as well as DnaK and GroEL homologs (14, 24). These cytoplasmic chaperonins may bind to and escort the LHI polypeptides to the ICM, where a membrane-bound translocation apparatus (which may consist of ORF1696 alone or include additional factors) enhances the stable insertion of LHI α and β polypeptides into the membrane. This process would be followed by, or coincide with, ORF1696-independent binding of Bchl and carotenoid molecules to the membrane-spanning portions of the LHI α and β polypeptides to yield the mature LHI holocomplex.
Secondly, ORF1696 might act as an intermediary, transferring Bchl molecules from the terminus of the Bchl biosynthetic pathway to the LHI apoproteins, to enhance formation of the mature LHI holocomplex. Amino acid sequence comparisons between the R. capsulatus ORF1696 protein, homologs, and PucC sequences found in other purple nonsulfur bacteria reveal the presence of several histidines that are conserved to different degrees, including an invariant histidine residue at position 152 of the R. capsulatus ORF1696, which could participate in transient binding of Bchl (40). However, site-directed mutagenesis of the ORF1696 His-152 residue to Asn or Phe did not significantly affect LHI complex levels in trans-complementation experiments (40).
Homologs of ORF1696 have been found in the purple nonsulfur bacteria Rhodopseudomonas viridis (35), Rhodospirillum rubrum (8), and Rhodobacter sphaeroides (6, 13) and are similarly located 5′ to the puhA gene of these species. Furthermore, the homologous pucC gene is required for LHII formation and is present in bacteria that contain the LHII complex (15–17), and ORF1696 homologs have been discovered in the cyanobacterium Synechocystis sp. strain PCC6803 (20) and in Prochlorococcus marinus (18). It will be interesting to see if proteins encoded by these open reading frames play a role in LH complex assembly in these species as does the ORF1696 protein in R. capsulatus.
It is clear that ORF1696 is a gene that encodes a protein involved in LHI complex assembly in R. capsulatus, and so we propose that the name be changed to the genetic designation lhaA, for light-harvesting complex assembly.
ACKNOWLEDGMENTS
We thank Gary Lesnicki for technical assistance, Victor Yih for the construction of plasmids, I.-P. Chen and H. Michel for provision of unpublished Rhodopseudomonas viridis sequence data, W. R. Hess for communicating unpublished information, and W. Klipp and C. Bauer for generous provision of materials.
This research was supported by a grant from the Canadian NSERC to J.T.B.
REFERENCES
- 1.Adams C W, Forrest M E, Cohen S N, Beatty J T. Structural and functional analysis of transcriptional control of the Rhodobacter capsulatus puf operon. J Bacteriol. 1989;171:473–482. doi: 10.1128/jb.171.1.473-482.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alberti M, Burke D E, Hearst J E. Structure and sequence of the photosynthetic gene cluster. In: Blankenship R E, Madigan M T, Bauer C E, editors. Anoxygenic photosynthetic bacteria. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 1083–1106. [Google Scholar]
- 3.Babst M, Albrecht H, Wegmann I, Brunisholz R, Zuber H. Single amino acid substitutions in the B870 α and β light-harvesting polypeptides of Rhodobacter capsulatus: structural and spectral effects. Eur J Biochem. 1991;202:277–284. doi: 10.1111/j.1432-1033.1991.tb16373.x. [DOI] [PubMed] [Google Scholar]
- 4.Barany F. Single-stranded hexameric linkers: a system for in-phase insertion mutagenesis and protein engineering. Gene. 1985;37:111–123. doi: 10.1016/0378-1119(85)90263-x. [DOI] [PubMed] [Google Scholar]
- 5.Bauer C E, Bird T H. Regulatory circuits controlling photosynthesis gene expression. Cell. 1996;85:5–8. doi: 10.1016/s0092-8674(00)81074-0. [DOI] [PubMed] [Google Scholar]
- 6.Bauer C E, Buggy J, Yang Z, Marrs B L. The superoperonal organization of genes for pigment biosynthesis and reaction center proteins is a conserved feature in R. capsulatus: analysis of overlapping bchB and puhA transcripts. Mol Gen Genet. 1991;228:433–444. doi: 10.1007/BF00260637. [DOI] [PubMed] [Google Scholar]
- 7.Beatty J T. Organization of photosynthesis gene transcripts. In: Blankenship R E, Madigan M T, Bauer C E, editors. Anoxygenic photosynthetic bacteria. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 1209–1219. [Google Scholar]
- 8.Bérard J, Gingras G. The puh structural gene coding for the H subunit of the Rhodospirillum rubrum photoreaction center. Biochem Cell Biol. 1991;69:122–131. doi: 10.1139/o91-019. [DOI] [PubMed] [Google Scholar]
- 9.Bibb M J, Cohen S N. Gene expression in Streptomyces: construction and application of promoter-probe plasmid vectors in Streptomyces lividans. Mol Gen Genet. 1982;187:265–277. doi: 10.1007/BF00331128. [DOI] [PubMed] [Google Scholar]
- 10.Bollivar D W, Suzuki J Y, Beatty J T, Dobrowski J M, Bauer C E. Directed mutational analysis of bacteriochlorophyll a biosynthesis in Rhodobacter capsulatus. J Mol Biol. 1994;237:622–640. doi: 10.1006/jmbi.1994.1260. [DOI] [PubMed] [Google Scholar]
- 11.Boyer H W, Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969;41:459. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- 12.Cogdell R J, Fyfe P K, Barrett S J, Prince S M, Freer A A, Isaacs N W, McGlynn P, Hunter C N. The purple bacterial photosynthetic unit. Photosynth Res. 1996;48:55–63. doi: 10.1007/BF00040996. [DOI] [PubMed] [Google Scholar]
- 13.Donohue T J, Hoger J H, Kaplan S. Cloning and expression of the Rhodobacter sphaeroides reaction center H gene. J Bacteriol. 1986;168:953–961. doi: 10.1128/jb.168.2.953-961.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drews G. Formation of the light-harvesting I (B870) of anoxygenic phototrophic purple bacteria. Arch Microbiol. 1996;166:151–159. doi: 10.1007/s002030050370. [DOI] [PubMed] [Google Scholar]
- 15.Drews G, Golecki J R. Structure, molecular organization, and biosynthesis of membranes of purple bacteria. In: Blankenship R E, Madigan M T, Bauer C E, editors. Anoxygenic photosynthetic bacteria. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 231–257. [Google Scholar]
- 16.Gibson L C D, McGlynn P, Chaudhri M, Hunter C N. A putative coproporphyrinogen III oxidase in Rhodobacter sphaeroides. II. Analysis of a region of the genome encoding hemF and the puc operon. Mol Microbiol. 1992;6:3171–3186. doi: 10.1111/j.1365-2958.1992.tb01773.x. [DOI] [PubMed] [Google Scholar]
- 17.Hagemann G E, Katsiou E, Forkl H, Steindorf A C J, Tadros M H. Gene cloning and regulation of gene expression of the puc operon from Rhodovulum sulfidophilum. Biochim Biophys Acta. 1997;1351:341–358. doi: 10.1016/s0167-4781(96)00228-x. [DOI] [PubMed] [Google Scholar]
- 18.Hess, W. R. Personal communication.
- 19.Johnson J A, Wong W K R, Beatty J T. Expression of cellulase genes in Rhodobacter capsulatus by use of plasmid expression vectors. J Bacteriol. 1986;167:604–610. doi: 10.1128/jb.167.2.604-610.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaneko, T., S. Sato, H. Kotani, A. Tanaka, E. Asamizu, Y. Nakamura, N. Miyajima, M. Hirosawa, M. Sugiura, S. Sasamoto, T. Kimura, T. Hosouchi, A. Matsuno, A. Muraki, N. Nakazaki, K. Naruo, S. Okumura, S. Shimpo, C. Takeuchi, T. Wada, A. Watanabe, M. Yamada, M. Yasuda, and S. Tabata. 1996. Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Res. 3(Suppl.):185–209. [DOI] [PubMed]
- 21.LeBlanc H. Ph.D. thesis. Vancouver, Canada: University of British Columbia; 1995. [Google Scholar]
- 22.LeBlanc H N, Beatty J T. Rhodobacter capsulatus puc operon: promoter location, transcript sizes and effects of deletions on photosynthetic growth. J Gen Microbiol. 1993;139:101–109. doi: 10.1099/00221287-139-1-101. [DOI] [PubMed] [Google Scholar]
- 23.Loach P A, Parkes-Loach P S. Structure-function relationships in core light-harvesting complexes (LHI) as determined by characterization of the structural subunit and by reconstitution experiments. In: Blankenship R E, Madigan M T, Bauer C E, editors. Anoxygenic photosynthetic bacteria. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 437–471. [Google Scholar]
- 24.Meryandini A, Drews G. Import and assembly of the a and b-polypeptides of the light-harvesting complex I (B870) in the membrane system of Rhodobacter capsulatus investigated in an in vitro translation system. Photosynth Res. 1996;47:21–31. doi: 10.1007/BF00017750. [DOI] [PubMed] [Google Scholar]
- 25.Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- 26.Peters J, Drews G. Chemical cross-linking studies of the light-harvesting pigment-protein complex B800-850 of Rhodopseudomonas capsulata. Eur J Cell Biol. 1983;29:115–120. [PubMed] [Google Scholar]
- 27.Prentki P, Krisch H M. In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984;29:303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- 28.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 29.Scolnik P A, Zannoni D, Marrs B L. Spectral and functional comparisons between the carotenoids of the two antenna complexes of Rhodopseudomonas capsulata. Biochim Biophys Acta. 1980;593:230–240. doi: 10.1016/0005-2728(80)90061-4. [DOI] [PubMed] [Google Scholar]
- 30.Simon R, Priefer U, Pühler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram-negative bacteria. Bio/Technology. 1983;1:37–45. [Google Scholar]
- 31.Steenburgen C L M, Korthals H J. Distribution of phototrophic microorganisms in the anaerobic and microaerophilic strata of Lake Vechten (The Netherlands). Pigment analysis and role in primary production. Limnol Oceanogr. 1982;27:883–895. [Google Scholar]
- 32.Taylor D P, Cohen S N, Clark W G, Marrs B L. Alignment of genetic and restriction maps of the photosynthesis region of the Rhodopseudomonas capsulata chromosome by a conjugation-mediated marker rescue technique. J Bacteriol. 1983;154:580–590. doi: 10.1128/jb.154.2.580-590.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walz T, Ghosh R. Two-dimensional crystallization of the light-harvesting I-reaction centre photounit from Rhodospirillum rubrum. J Mol Biol. 1997;265:107–111. doi: 10.1006/jmbi.1996.0714. [DOI] [PubMed] [Google Scholar]
- 34.Wellington C L, Bauer C E, Beatty J T. Photosynthesis gene superoperons in purple nonsulfur bacteria: the tip of the iceberg? Can J Microbiol. 1992;38:20–27. [Google Scholar]
- 35.Wiessner C. Ph.D. dissertation. Frankfurt, Germany: Johann Wolfgang Goethe-Universität; 1990. [Google Scholar]
- 36.Wong D H-K. M.Sc. thesis. Vancouver, Canada: The University of British Columbia; 1994. [Google Scholar]
- 37.Wong D K-H, Collins W J, Harmer A, Lilburn T G, Beatty J T. Directed mutagenesis of the Rhodobacter capsulatus puhA gene and orf214: pleiotropic effects on photosynthesis reaction center and light-harvesting I complexes. J Bacteriol. 1996;178:2334–2342. doi: 10.1128/jb.178.8.2334-2342.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yen H C, Hu N T, Marrs B L. Characterization of the gene transfer agent made by an overproducer mutant of Rhodopseudomonas capsulata. J Mol Biol. 1979;131:157–168. doi: 10.1016/0022-2836(79)90071-8. [DOI] [PubMed] [Google Scholar]
- 39.Yen H C, Marrs B. Map of genes for carotenoid and bacteriochlorophyll biosynthesis in Rhodopseudomonas capsulata. J Bacteriol. 1976;126:619–629. doi: 10.1128/jb.126.2.619-629.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Young C S. Ph.D. thesis. Vancouver, Canada: University of British Columbia; 1997. [Google Scholar]
- 41.Zsebo K M, Hearst J E. Genetic-physical mapping of a photosynthetic gene cluster from R. capsulata. Cell. 1984;37:937–947. doi: 10.1016/0092-8674(84)90428-8. [DOI] [PubMed] [Google Scholar]