Abstract
Menopausal hormone therapy (HT) was widely used in the past, but with the publication of seminal primary and secondary prevention trials which reported an excess cardiovascular (CV) risk with combined estrogen-progestin, HT use declined significantly. However, over the past 20 years, much has been learned about the relationship between timing of HT use with respect to age and time since menopause, HT route of administration, and cardiovascular disease risk. Four leading medical societies recommend HT for treatment of menopausal women with bothersome menopausal symptoms. In this context, this review, led by the ACC CVD in Women Committee along with leading gynecologists, women’s health internists and endocrinologists, aims to provide guidance on HT use, including selection of patients and HT formulation with a focus on caring for symptomatic women with CVD risk.
Keywords: Menopause, Hormone therapy, Cardiovascular disease, Atherosclerotic heart disease, Venous thromboembolism, Hyperlipidemia, Hypertension
Introduction
Menopause, the permanent cessation of menstruation caused by loss of ovarian function, occurs at a mean age of 52 years (1). Based on the latest United States (U.S.) Census Bureau data, as of 2020, more than 63 million women in the US are age 50 years or older and approximately 6000 women enter menopause each day (2). Vasomotor symptoms (VMS), which include hot flashes and night sweats, represent the most lifestyle limiting symptoms of menopause and are the most common reason women present for care at the time of the menopause transition (3). VMS often include a sudden sensation of heat in the face and chest, persist for up to several minutes and are associated with anxiety, sleep disruption, and reduced quality of life (4). VMS occur in about 75% of women during the menopause transition, and are more prevalent among Black/African- American women, women who smoke, those with mood disorders and those with low income and/or low educational attainment (4).
Menopausal HT was at one time almost universally recommended but with the publication of Heart and Estrogen/Progestin Replacement Study (HERS)(5) and Women’s Health Initiative (WHI) randomized trials, (6, 7) which reported excess cardiovascular risk, HT use has declined substantially (8). Appropriately, no medical societies currently recommend HT for the primary or secondary prevention of CVD (9–14) (Table 1).
Table 1:
Recommendations for HT from 4 different medical societies
| Aspect of treatment | ACOG (10) | NAMS (13) | AACE & ACE (11) | Endocrine Society(12) |
|---|---|---|---|---|
| Principal Indication | Menopause symptoms | Menopause symptoms | Menopause symptoms | Menopause symptoms |
| Prevention of CHD | Not recommended | Not recommended | Not recommended | Not recommended |
| Special Considerations | None | Consideration of age and time from menopause onset | Consideration of age, time from menopause onset and risk of CVD, with lipid profile, smoking history | Consideration of age, time from menopause, and baseline risks of CVD and breast cancer |
| Dose & Route of Administration | Lowest effective dose | Appropriate dose to manage symptoms with consideration of route | Lowest effective dose | Shared decision making to determine formulation, dose, and route |
| Duration of use | Shortest period based on risk- benefit analysis, with recommendation against routine discontinuation in patient ≥ 65 yr of age | May be extended for persistent vasomotor symptoms, prevention of bone loss, or quality of life after attempt at stopping; Reassess benefits and risks regularly | Recommended for ≤ 5 yrs with reduction of dose if continuing | Shortest total duration consistent with the treatment goals and evolving risk assessment of the individual woman |
However, over the past 20 years, the relationship of CVD risk with timing of menopause, initiation of HT, and route of HT delivery has been better understood (15–18). As such, four major North American medical societies, the American College of Obstetricians and Gynecologists (ACOG), American Association of Clinical Endocrinology (AACE), The Endocrine Society (ENDO), and The North American Menopause Society (NAMS), now recommend HT in appropriate patients for the management of menopausal symptoms (10–13) (Table 1). Likewise, in Europe, societies and organizations have recommended HT in low risk patients for the management of menopausal symptoms (19–22). Despite these evidence-based recommendations, physicians, including cardiologists, are reluctant to use HT due to confusion and lack of education regarding who is an appropriate patient for HT use (23, 24).
In this context, the aim of this review, led by the ACC CVD in Women committee along with leading gynecologists, women’s health internists, and endocrinologists who specialize in menopause management, is to provide guidance regarding current understanding of risk and benefits of HT, which women are appropriate candidates for HT and which routes and doses of HT minimize CVD risks in women.
History of Hormone Therapy & Cardiovascular Disease
The intersection between cardiology and reproductive endocrinology dates back to 1905 when Ernest Starling, a physician whose foundational work in cardiovascular physiology is well known, first introduced the concept of the hormone (25). The history of modern-day HT in the U.S. began during the Great Depression when the first commercially available menopausal estrogen product was produced from the urine of pregnant women. For cost-savings, this was later replaced in the early 1940’s with conjugated equine estrogen (CEE), derived from the urine of pregnant mares, and aggressively marketed for the treatment of vasomotor symptoms in postmenopausal women.
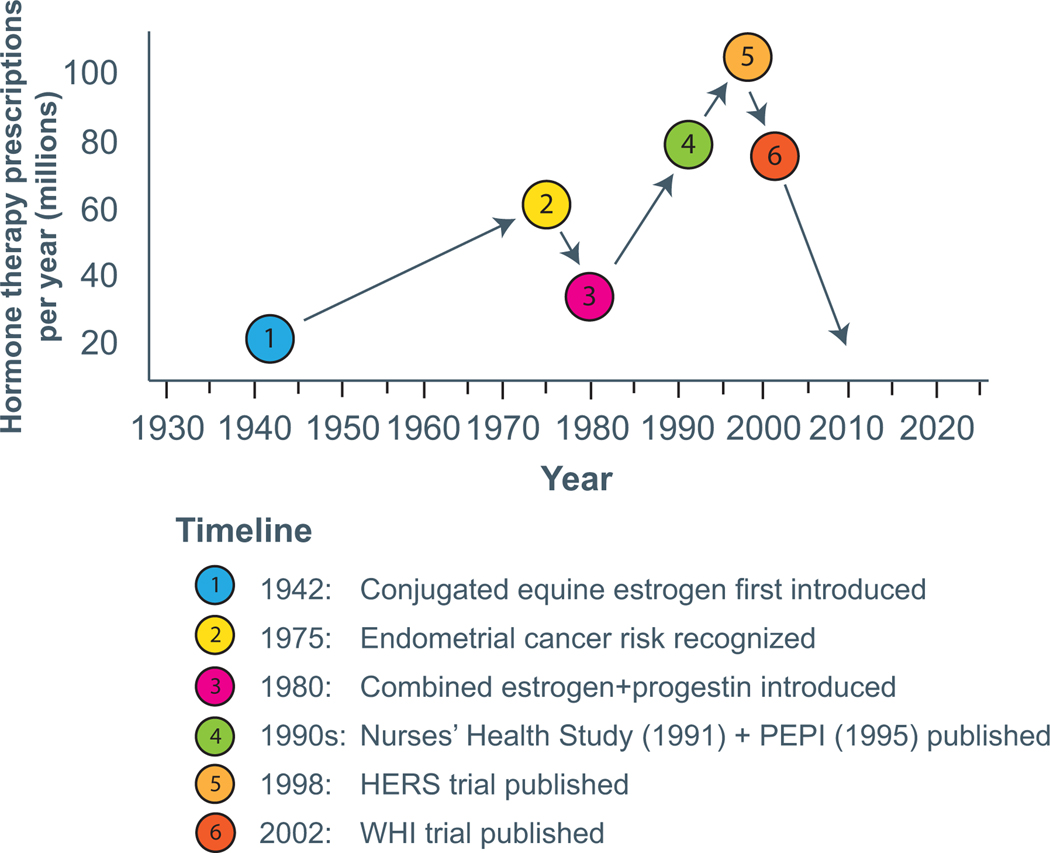
Fueled by a popular book called “Feminine Forever” published in 1966 which proposed that menopause was a hormone deficiency state that led not only to painful intercourse but also to the loss of sex appeal and youth, coupled with the changing status of women and the feminist movement, HT was increasingly prescribed with annual prescriptions exceeding 50 million in the U.S. alone by the 1970’s (26, 27). However, in 1975, several studies demonstrated increased risk of endometrial cancer with unopposed estrogen therapy (ET), prompting a significant reduction in HT use (28, 29). By the early 1980s, recognition that the addition of a progestogen to ET mitigated endometrial (uterine lining) cancer risk and subsequent development of combination estrogen-progestogen therapy (EPT) formulations, prompted a revival and firmly established HT as part of women’s health therapies. The next two decades saw a dramatic increase in HT use, propelled in large part by observational data supporting benefits of estrogen with respect to CVD (25). The most notable of these was the Nurses’ Health Study, a prospective cohort study that demonstrated marked reduction in incident coronary disease and CV death in estrogen users (30). By the late 1990s, HT use reached an all-time peak, 90 million HT prescriptions per year, representing approximately 15 million women (27, 31) (Figure 1).
Figure 1:
Timeline of HT use in US
HT hormone therapy; HERS Heart and Estrogen/progestin Replacement Study; PEPI Postmenopausal Estrogen/Progestins Interventions; WHI Women’s Health Initiative;
Ironically, the first secondary prevention clinical trial assessing CVD effects of estrogen was conducted exclusively in men (32). The Coronary Drug Project randomized over 8000 men after myocardial infarction (MI) to estrogen, niacin, thyroid, clofibrate or placebo. The Estrogen (both 5mg and later 2.5 mg a day arm of the trial) was terminated early due to increased thrombosis and myocardial infarction (32). Two decades later in 1998, the HERS trial, the first randomized trial of EPT vs placebo for the secondary prevention of coronary heart disease (CHD) events among postmenopausal women with established CHD, found no overall CV benefit and a pattern of an early increase in CHD events with HT use, arguing against the initiation of HT for secondary prevention of CHD (5). The HERS data led to a slight reduction in HT prescribing rates following its publication, but the early termination of the landmark Women’s Health Initiative (WHI) EPT trial in 2002, a primary prevention trial (6, 7), led to a dramatic decline in the use of HT worldwide (Figure 1).
The WHI randomized trial enrolled women without CVD between the ages of 50–79 years and represents the largest randomized placebo-controlled trial of systemic HT designed to evaluate the risks and benefit for the primary prevention of chronic diseases, including cardiovascular disease (6, 7). Women with a uterus were randomized to continuous combined oral conjugated equine estrogen (CEE) with medroxyprogesterone acetate (MPA) (CEE+MPA) or placebo and women without a uterus were randomized to CEE-alone or placebo. The initial publications which detailed WHI findings in 2002 (CEE+MPA) and 2004 (CEE-alone), with median age of 63.2 and 63.6 years of age at the time of enrollment, respectively, aggregated participants of all ages, and reported that compared to placebo, risks of coronary heart disease (CHD), stroke and venous thromboembolism (VTE) including pulmonary embolism were increased with HT (6, 7).
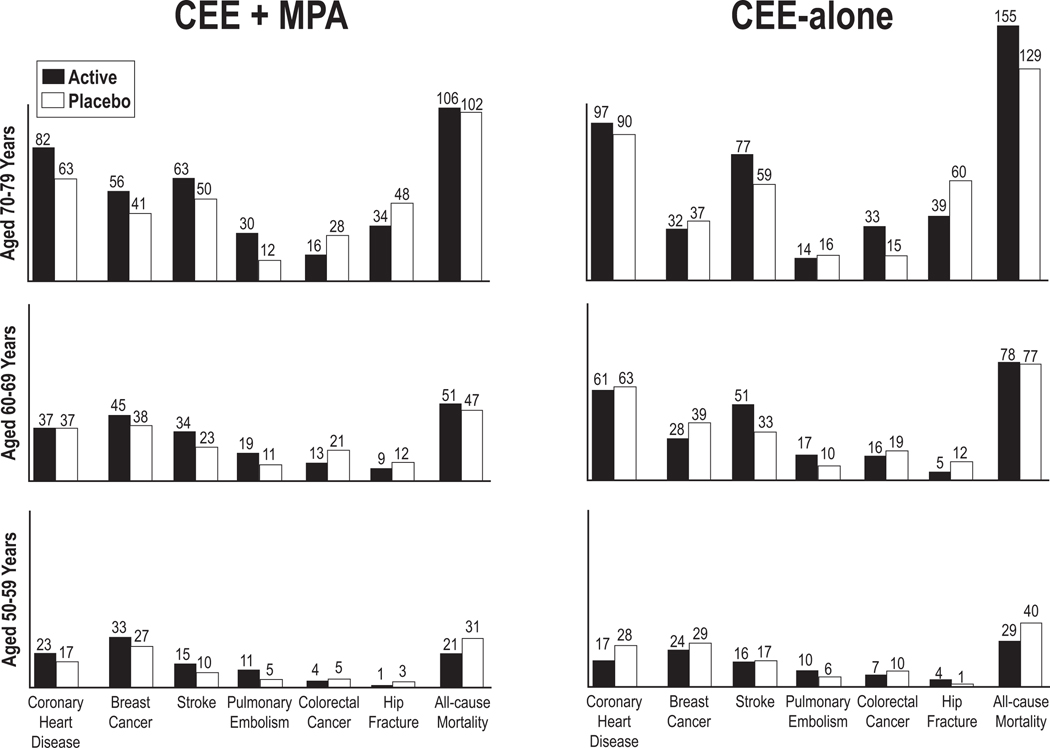
However, subsequent to the initial publication of the primary WHI results, age-stratified analyses with longer cumulative follow up (median duration 13 years) supported a more nuanced approach to HT (15, 16). These analyses demonstrated that the absolute risks of adverse events following HT initiations were much lower for younger women (aged 50–59 years) than for older women and for those who initiated HT within a decade of menopause (Figure 2).
Figure 2:
CEE+MPA and CEE-alone by age
From the Women’s Health Initiative hormone therapy trials: absolute risks (cases per 10,000 person-years) for outcomes in the CEE+MPA and CEE-alone by age group. CEE, conjugated equine estrogens; MPA, medroxyprogesterone acetate. Modified from Manson JE JAMA 2013; 310:1535–68 (15)
These findings support the “timing hypothesis”, which postulates that CV risk associated with HT appears to depend on the timing of initiation in relation to menopause onset (33). This hypothesis arose from a primate model, in which CEE prevented atherosclerosis only in animals treated early after surgically induced menopause and before the onset of atherosclerosis (34). In support of this hypothesis, a reanalysis of the Nurses’s Health Study observed a benefit to starting HT less than four years compared to more than 10 years after menopause (35). In women who started HT earlier, there was a reduced risk of CHD (RR = 0.66, 95% CI 0.54–0.80 for estrogen alone; RR = 0.72, 95% CI 0.56–0.92 for estrogen with progestin). Furthermore, in 2012, the Danish Osteoporosis Prevention study, designed to assess the long term impact of HT (open-label with no placebo) on bone mineral density in 1006 recently menopausal and perimenopausal women, reported on over 10 years of randomized follow up and another 5.7 of post intervention follow up (36). Women receiving HT had a reduced risk of composite cardiovascular safety outcomes, death or hospitalization for myocardial infarction or heart failure (HR 0.48, 95% CI 0.26 to 0.87; P=0.015)(36).
A more recent meta-analysis of 19 randomized controlled trials with a total of 40, 410 postmenopausal women on HT (the majority of which was oral) found no significant increase in all-cause mortality, death from CVD, or MI with HT in both primary and secondary prevention populations. A subgroup analysis based on HT timing found that those who initiated HT within 10 years after menopause had lower mortality (RR 0.70, 95% CI 0.52 to 0.95) and fewer cardiac events (composite of CV death and non-fatal myocardial infarction) (RR 0.52, 95% CI 0.29 to 0.96)(18). In contrast, women who started HT more than 10 years from the onset of menopause were found to have an increased risk of stroke without any effect on mortality or other CVD outcomes (18). Of note, in both groups regardless of the HT timing, investigators observed an increase in venous thromboembolism events (18).
Along with these studies, the ELITE study (Early vs Late Intervention trial with Estradiol) published in 2016, randomized 643 healthy menopausal women to oral estradiol and vaginal progesterone (if a uterus was present) or placebo. HT attenuated progression of subclinical atherosclerosis as measured by carotid intima media thickness for women who were less than 6 years from last menses while those women greater than 10 years from last menses derived no benefit (37). The KEEPS (Kronos Early Estrogen Prevention Study) randomized 727 women within 36 months of their last menses to lower dose oral CEE or transdermal estradiol (E2) plus progesterone in women with a uterus did not identify any difference in the progression of subclinical atherosclerosis between HT vs. placebo. The lack of benefit in the latter study was thought to be due to the lower dose of HT used as well as to the younger age of the women enrolled in the trial and shorter duration of follow up (38). Reassuringly, these studies did not show harm with early initiation of HT.
The studies summarized above have given credibility to the hypothesis that CV risk associated with HT appears to depend on the timing of initiation in relation to menopause onset. Estrogen may have plaque-destabilizing and other adverse effects in the setting of advanced atherosclerosis, but provides an appropriate and safe option for treatment of menopausal symptoms when initiated in healthy women under age 60 or within 10 years of menopause onset.
Types of HT
It is important for cardiologists to understand the various types of HT. We will discuss differences with regard to systemic vs. local, oral vs. transdermal as well as US Food and Drug Administration (FDA) approved HT vs. compounded bioidentical HT.
Systemic Hormone Therapy
Systemic estrogen therapy represents an effective treatment for VMS and other menopause symptoms, including genitourinary syndrome of menopause (GSM). Oral and transdermal estrogen formulations have similar efficacy, with the lowest effective dose generally recommended (4). Most systemic estrogen formulations are also approved for the prevention of osteoporosis. Because the use of estrogen therapy (ET) alone in women with a uterus increases the risk of endometrial hyperplasia and cancer, a progestogen should also be prescribed. The most commonly prescribed systemic oral estrogens and progestogens are detailed in Table 2. A variety of combination oral estrogen and progestogens therapy (EPT) formulations, all of which have demonstrated endometrial safety, are also detailed in Table 2. Of note, oral CEE combined with the selective estrogen receptor modulator, bazedoxifene, is also approved to treat VMS and prevent osteoporosis in women with a uterus. This formulation may be useful for women who prefer not to use a progestogen, including those intolerant of progestogen-related side effects, including adverse effects on mood, weight, headaches and fluid retention (13).
Table 2:
Oral Hormone Therapy
| Oral Estrogen Formulations for Menopausal Hormone Therapy commonly prescribed in the U.S. | |
| Estradiol | 0.5, 1.0, 2.0 mg Standard: 1.0 mg Low: 0.5 mg |
| Conjugated equine estrogen | 0.3, 0.45, 0.625, 0.9, 1.25 mg Standard: 0.625 mg Low: 0.3 mg, 0.45 mg |
| Combination oral estrogen- progestogen formulations available | |
| Estradiol (0.5 mg, 1.0 mg) | Drospirenone (0.25 mg, 0.5 mg) |
| Estradiol (0.5 mg, 1.0 mg) | Norethindrone acetate (0.1 mg, 0.5 mg) |
| Estradiol (1.0 mg) | Norgestimate (0.09 mg) |
| Estradiol (1.0 mg)* | Progesterone (100 mg)* |
| Ethinyl Estradiol (2.5 μg, 5 μg) | Norethindrone acetate (0.5 mg, 1.0 mg) |
| Conjugated equine estrogen (0.3 mg, 0.45 mg, 0.625 mg) |
Medroxyprogesterone acetate (1.5 mg, 2.5 mg, 5 mg) |
| Oral Progestogen Formulations for Menopausal Hormone Therapy commonly prescribed in the U.S. | |
| Medications | Available doses |
| Medroxyprogesterone acetate | 2.5 mg, 5 mg, 10 mg |
| Progesterone* | 100 mg, 200 mg |
formulation contains peanut oil; hypnotic effect, so should be taken at bedtime
Transdermal Estrogens and Progestogens
Transdermal HT formulations, including estrogen alone and estrogen plus progestogen are detailed in Table 3. No randomized controlled trials have compared VTE risk with oral vs. transdermal vs. placebo therapy. However, observational studies have consistently observed lower rates of venous thromboembolism with transdermal compared to oral HT (39–41)(Table 3). Moreover, in 11 randomized controlled studies of lipid metabolism, transdermal HT has shown neutral effects on triglycerides, in contrast with oral HT, which increases triglyceride levels (41). In a randomized trial of 196 women, oral HT significantly increased CRP level compared to placebo (42). However, at 6 months, transdermal estrogen had no significant effect on CRP levels compared to placebo (42). This is likely related to first-pass hepatic metabolism of oral estrogens, which increases triglycerides, coagulation factors, C-reactive protein, and sex hormone-binding globulins (43, 44).
Table 3:
Transdermal Hormone Therapy
| Transdermal Estrogen Formulations for Menopausal Hormone Therapy commonly prescribed in the U.S. | |
|---|---|
| Medications | Available doses* |
| Weekly Estradiol patch | 0.014, 0.025, 0.0375, 0.05, 0.06, 0.075, 0.1 mg Standard: 0.0375–0.05 mg Low: 0.025 mg Ultra-Low: 0.014 mg |
| Twice weekly estradiol patch | 0.025, 0.0375, 0.05, 0.075, 0.1 mg Standard: 0.0375–0.05 mg |
| Combination transdermal estrogen- progestin formulations available * | |
| Estrogen | Progestin |
| Estradiol 0.05 mg | Norethindrone 0.14 mg, 0.25 mg |
| Estradiol 0.045 mg | Levonorgestrel 0.015 mg |
Daily release note
Compounded Hormone Therapy
Among U.S. women using HT, approximately one-third use compounded HT, often marketed as ‘bioidentical’ or ‘natural.’ (45). Most users are unaware that these formulations are not monitored for safety or approved by the FDA. Concerns with compounded HT include risk of contamination, variability in dosing and absorption, limited data on safety and efficacy, lack of a package insert describing risks and significant out-of-pocket cost. FDA-approved ‘bioidentical’ HT, which includes oral, transdermal and vaginal estradiol formulations as well as oral and vaginal progesterone, is biochemically identical to the sex steroids produced by the ovary. Current guidelines and position statements indicate that the FDA approved HT is preferable to compounded HT(46). No studies have compared FDA approved bioidentical HT to standard synthetic HT with regard to cardiovascular outcomes.
Vaginal Estrogen Therapy
In contrast with VMS, which diminish over time, GSM, which includes symptomatic vulvovaginal atrophy, painful intercourse, and recurrent urinary tract infections, increases in prevalence as women age. Low dose vaginal ET is currently the most effective treatment for GSM when symptoms persist after use of nonhormonal, over-the-counter options (47). Low dose vaginal ET formulations are available by prescription, including tablets, inserts, and creams used several times weekly, and a vaginal ring changed every 3 months (Table 4). Low dose vaginal ET is minimally absorbed at current recommended dosing, with circulating estrogen levels typically maintained within the normal postmenopausal range (48). Of note, Estring (estradiol) vaginal ring should not be confused with Femring (estradiol acetate) since Femring delivers systemic doses of estrogen. Use of a progestogen is not recommended with low dose vaginal ET, although any vaginal bleeding in a postmenopausal woman should be thoroughly evaluated irrespective of HT use. Given minimal systemic absorption, low dose vaginal ET is an option for women in whom systemic HT may be contraindicated, including those with a history of estrogen-responsive cancers, CVD, stroke, or VTE (47).
Table 4:
Low Dose Vaginal Estrogen Therapy
| Vaginal Tablets/Inserts | Formulation |
|---|---|
| Estradiol Tablet | E2 10 mcg |
| Estradiol Insert | E2 10 mcg, E2 4 mcg |
| Vaginal Creams | |
| Estradiol Cream | E2 (variable)* |
| Conjugated Estrogen Cream | CE (variable)* |
| Vaginal Ring | |
| Estradiol Ring | E2 7.5 mcg (vaginal therapy) |
Abbreviations: E2 estradiol, CE conjugated estrogens, mcg microgram, g gram
US Food and Drug Administration approved doses of estrogen creams are higher than those proven effective and recommended for clinical practice
Due to ‘class labeling’, the package insert for low dose vaginal ET includes the same boxed warning regarding risks of CVD, endometrial and breast cancer, and probable dementia that accompanies all menopausal HT products. Due to minimal systemic estrogen absorption, this warning is not evidence-based and adversely affects women’s quality of life by discouraging use of these highly effective therapies (49). Several large observational studies confirmed no increased risk of adverse health outcomes, including CVD, VTE or cancer in vaginal ET users (50,51). Women prescribed low dose vaginal ET should be prepared for the black box warning and informed that it is based on the use of higher doses of estrogen to treat VMS while very low doses of estrogen placed directly in the vagina do not appear to be associated with these risks.
Recommendations for HT
Low risk with HT
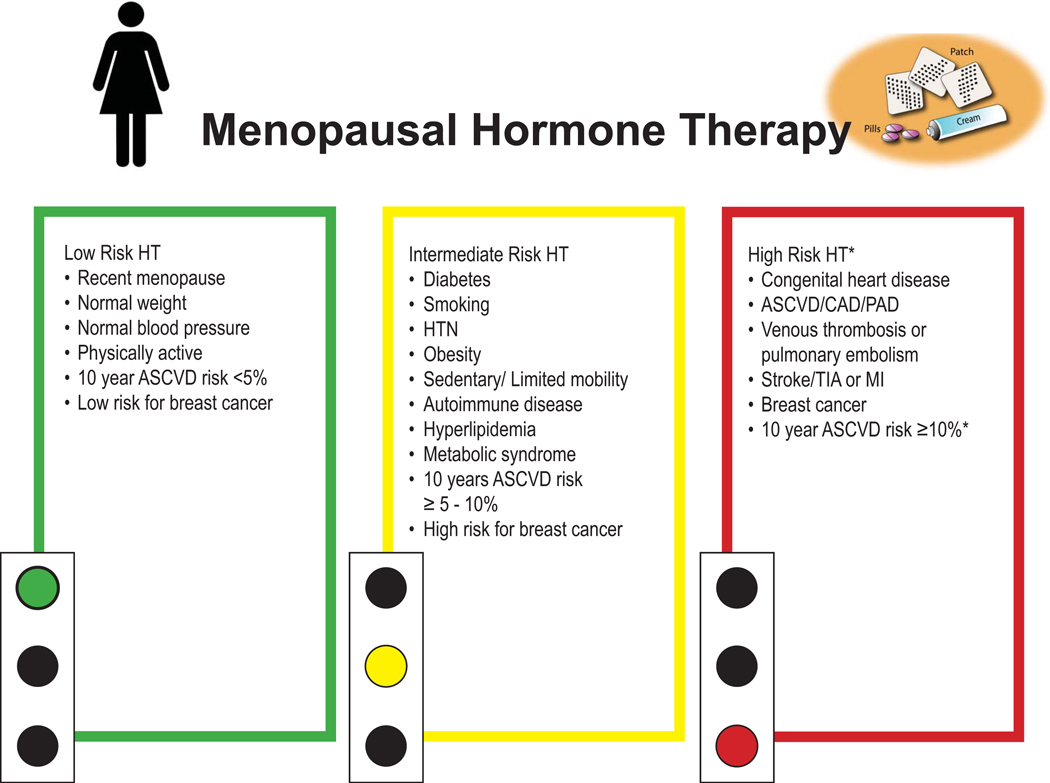
Women less than 60 years, or within 10 years of menopause onset, with 10-year estimated ASCVD risk <5% and do not have an increased risk of breast cancer or history of VTE are considered low risk for major adverse cardiovascular events with initiation of HT for the treatment of menopausal symptoms (Figure 3).
Figure 3:
Menopausal hormone therapy recommendation by patient risk
*Generally advised to avoid systemic HT. Consider alternative therapy and if severe VSM persists, individualized, shared decision making recommended. All women are candidates for low dose vaginal estrogen therapy for GSM.
ASCVD atherosclerotic cardiovascular disease, CAD coronary artery disease, PAD peripheral arterial disease, TIA transient ischemic attack, MI myocardial infarction, HTN hypertension
GSM genitourinary symptoms of menopause
Moderate Risk with HT
Decision making is more difficult when a woman has one or more chronic medical conditions potentially impacting the risk-to-benefit balance of HT use. However, this is the most common scenario faced by clinicians as 80% of women over age 55 have at least one chronic medical condition (52). The presence of CVD risk factors alone does not preclude the use of HT, but a patient’s worsening CV risk profile around the menopause transition emphasizes the need to optimize primary prevention efforts, including lifestyle and pharmacologic management (24).
We review existing evidence for HT use in symptomatic women with prevalent medical conditions including obesity, dyslipidemia, hypertension and diabetes, and provide guidance as to when transdermal ET preparations may be preferred over oral ET (Figure 3).
Obesity
Almost half of women aged 40–59 years in the U.S. are affected by obesity, and the prevalence of obesity in women continues to rise (53). While HT use has shown favorable effects on body composition with preservation of lean body mass and reduction in visceral adiposity, it has not demonstrated a consistent impact on weight (54,55). Moreover, obesity is a risk factor for VTE, and oral HT appears to have a significant additive effect on the increased risk of VTE in overweight women (56). In the WHI, randomized trial of oral systemic HT, there was a threefold increased risk of VTE in overweight women (BMI 25–30) randomized to EPT vs lean (BMI <25) postmenopausal women on placebo (HR 3.80, 95% CI 2.08–6.94). In obese women (BMI >30), there was almost a six-fold increased risk in the EPT group compared to the placebo lean group (HR 5.61, 95% CI 3.12–10.11) (56). In the WHI Observational study (WHI-OS) of over 45,000 women, oral EPT in obese women (BMI>30) was associated with higher cardiovascular event rates (HR 1.21, 95% CI 1.03–1.42) whereas transdermal HT was not associated with greater risk (HR 1.61, 95% CI 0.83–3.12) (57).
In the absence of RCT data, and given that observational studies have consistently found a lower risk of VTE with transdermal vs. oral HT, transdermal ET is preferred.
Dyslipidemia
Despite the favorable impact of oral ET on low-density lipoprotein cholesterol (LDL-C), lipoprotein(a) levels and high density lipoprotein cholesterol (HDL-C), these subclinical benefits have not translated into reduction in CVD events or death (24). In a pooled analysis, the WHI clinical trials found an excess CHD risk among women with a baseline LDL-C ≥130 mg/dL or an LDL-C/HDL-C ratio of >2.5. (HR 1.46, 95% CI 1.02–2.10 and HR 1.73, 95% CI 1.18–2.53, respectively) taking CEE with or without MPA (58). In the HERS trial, during the first year, women not using statins at baseline and assigned to HT appeared to have higher cardiac event rates than women assigned to HT who were taking statins at baseline. However, at 4.1 years of follow up, the event rates were similar between the two groups (59). Moreover, in the WHI trials, statin use did not lower the event rate in women on EPT (6, 7).
Notably, as a deleterious effect on lipids, oral HT use increases triglycerides. Eleven randomized trials have compared lipid effects of oral vs transdermal HT (41). Consistently, these trials have found that, in contrast with oral HT, which increases triglycerides by 5–15%, transdermal HT decreases triglyceride levels by 5–30% (41, 60, 61)(Table 5). In this context, we recommend the transdermal route of ET for symptomatic menopausal women with dyslipidemia, particularly those with a tendency toward hypertriglyceridemia.
Table 5:
Studies comparing Systemic vs. Transdermal HT
| Study Type | Study Quality | Findings | |
|---|---|---|---|
| Lipid | 11 Randomized studies 1 Cohort study |
Reasonable | Overall, greater LDL lowering with oral systemic HT. Consistently higher TG levels with oral systemic HT. Lower TG levels with transdermal HT. |
| VTE | 7 case controlled studies 3 Cohort studies |
Low | Oral Systemic HT increases VTE risk. Transdermal has neutral effect |
| MI | 4 case controlled studies 2 cohort studies |
Poor | None designed to compared the difference between systemic vs. transdermal |
Hypertension
While some studies examining the impact of HT on blood pressure in women with pre-existing hypertension show no clinically meaningful change in blood pressure in oral ET users, WHI showed that CEE either alone or in combination with MPA increased blood pressure by 1–1.5mmHg (62–64). Given that meta-analysis of clinical trials showed that 1 mmHg reduction in systolic blood pressure translated into 2% relative risk reduction in major CVD and 3% reduction in heart failure events, we recommend caution starting HT in women with HTN (65). Of note, uncontrolled blood pressure ≥180/110 is a relative contraindication to starting HT due to the possibly increased risk for stroke. HT can be reconsidered once hypertension is controlled. In the WHI-OS, transdermal ET was associated with lower risk of development of HTN and with neutral effect on blood pressure compared to oral HT (66, 67).
Diabetes
Diabetes increases the risk of CVD by about 4-fold in women but only about two 2-fold in men, and diabetic women have worse outcomes after MI and more CHF compared with diabetic men (68). Women are also at higher risk of other diabetes-related end organ damage. In the WHI trials, regardless of HT treatment, women with diabetes had a 2–3 fold higher risk of all-cause, CVD, and cancer mortality than women without diabetes (69,70). While there is robust evidence supporting favorable impact of HT on glycemic control and insulin resistance in postmenopausal women with and without T2DM, this has not translated into fewer cardiovascular events nor into clinical recommendations for use of HT to prevent diabetes (71–73). In WHI, neither EPT nor ET use in diabetic women increased mortality or cardiovascular events. However, given that diabetic women are at increased risk of cardiac events with concomitant comorbidities such as obesity, hypertension, and hyperlipidemia (specifically hypertriglyceridemia), the transdermal route of ET is preferred for diabetic women with menopause symptoms.
Metabolic Syndrome
The WHI clinical trials found that women with metabolic syndrome randomized to CEE+MPT and CEE alone compared with placebo, had a 2 fold increased CVD risk (74, 75). Metabolic syndrome is often characterized by hypertriglyceridemia and obesity, which portends greater risk of VTE. Therefore, although there are no studies comparing systemic vs. transdermal HT and CV outcomes in women with metabolic syndrome, we recommend transdermal route of ET for menopause symptom relief in this setting.
Monitoring, Discontinuation and Consideration of Extended Use
In the clinical scenarios above, shared-decision making is important, and the need for ongoing HT should be reassessed annually or when new clinical concerns arise, taking into consideration any changes in the patient’s medical history, family history, symptom burden, personal preferences, and treatment goals. HT use should be individualized with dose adjustments based on symptom response. Expert opinion suggests it is reasonable to lower the dose of HT or discontinue HT after several years of use, particularly given that VMS tend to improve for most women over time (76) (Table 1). However, age alone should not dictate when HT is discontinued (10, 77). Among women who initiated systemic HT within 10 years of the onset of menopause, extended use of systemic HT may be appropriate for the treatment of persistent VMS and prevention of osteoporosis in select patients (Table 6). Extended use of systemic HT should not be confused with the initiation of systemic HT by women older than age 60 or more than 10 years following menopause onset—a practice that is known to be associated with an elevated risk of CVD. Women considering extended use of systemic HT should be aware that risk of breast cancer increases with longer duration of combined EPT. Since older age is an independent risk factor for VTE, the use of transdermal ET should be considered, and lower than standard doses can often be used in this setting. When women discontinue systemic HT, the likelihood of recurrent VMS appears to be similar whether HT is abruptly stopped or gradually tapered. Stopping systemic HT often results in an accelerated loss of bone mass and progression of GSM. If GSM represents the only indication for continuing (or starting) HT, low dose vaginal ET should be used (78).
Table 6:
ASCVD risk score and years since menopause onset for initiating HT
| CVD Risk over 10 years ACC/AHA ASCVD Risk Score |
Years since menopause onset | |||
| ≤ 5 | 6–10 | ≥ 10 | ||
| Low Risk (<5%) | HT Acceptable | HT Acceptable | Consider alternatives; HT acceptable with individualized, shared-decision making | |
| Intermediate Risk (≥ 5.0% - < 10%) | HT Acceptable Consider transdermal HT depending on risk factors | HT Acceptable Consider transdermal HT depending on risk factors | Generally advised to avoid systemic HT. Consider alternative therapy and if severe VMS persist, individualized, shared decision making | |
| High Risk (≥ 10%) | Generally advised to avoid systemic HT. Consider alternative therapy and if severe VMS persist, individualized, shared decision making | Generally advised to avoid systemic HT. Consider alternative therapy and if severe VMS persist, individualized, shared decision making | Avoid HT Consider alternative therapy and if severe VMS persist, individualized, shared decision making | |
Who Should Generally Avoid Hormone Therapy
While many women with menopause-related symptoms can safely use HT, certain cardiovascular and non-cardiovascular conditions constitute relative or absolute contraindications for use. For patients with these conditions, shared decision making is advised employing an individualized approach incorporating an assessment of symptom severity, evidence for safety vs. harm relative to the woman’s underlying condition(s)/medical history, and collaboration with other members of her healthcare team (Figure 3).
Coronary Heart Disease and Cardiovascular Risk Factors
HT is generally contraindicated in women with known CHD, including history of myocardial infarction or peripheral artery disease (13). Although discontinuation of HT after acute myocardial infarction is advised, a meta-analysis of 10 trials of HT in 5,766 secondary prevention patients suggest that in this setting the absolute risk of death, MI, angina or revascularization is low (79)(Table 7).
Table 7:
Risk with HT in primary and secondary prevention from meta-analysis of 19 RCT trials (18)
| Primary prevention | Secondary prevention | |
|---|---|---|
| Death All Cause | RR 1.00 (95% CI 0.89–1.12) | RR 1.04 (95% CI 0.87–1.24) |
| Death from CVD | RR 0.81 (95% CI 0.47–1.40) | RR 1.00 (95% CI 0.78–1.29) |
| MI | RR 1.02 (95% CI 0.80–1.31) | RR 0.98 (95% CI 0.81–1.18) |
| Angina | RR 0.90 (95% CI 0.74–1.08) | RR 0.91 (95% CI 0.74–1.12) |
| Revascularization | RR 0.96 (95% CI 0.85–1.09) | RR 0.98 (95% CI 0.63–1.53) |
| VTE | RR 1.92 (95% CI 1.24–2.99) Absolute risk increase 0.008 with NNTH 118 |
RR2.02 (95% CI 1.13–3.62) Absolute risk increase 0.014 with NNTH 71 |
| Stroke | RR 1.32 (95% CI 1.12–1.56) Absolute risk increase 0.006 with NNTH of 165 | RR 1.09 (95% CI 0.89–1.33) |
| PE | RR 1.89 (95% CI 1.17–3.04) Absolute risk increase 0.004 with NNTH 242 | RR 2.48 (95% CI 0.92–6.70) |
CVD Cardiovascular disease; MI myocardial infarction; VTE venous thromboembolism; PE pulmonary embolism; RCT randomized control trials; NNTH number needed to harm (18)
Non-atherosclerotic/non-thrombotic CHD is especially prevalent in women, but current guidelines do not stratify risk of HT use by subtype of disease. For women aged 50–59 years with a history of myocardial infarction with no obstructive coronary artery disease, spontaneous coronary artery dissection, coronary microvascular dysfunction, or coronary vasospasm, an individualized approach to HT is required. Due to the presumed pathophysiological association with female sex hormones in spontaneous coronary artery dissection, we recommend that in general, oral ET be avoided in this group. This recommendation stems from the fact that over 90% of patients with spontaneous coronary artery dissection are female and the observed increase in coronary artery dissection incidence around pregnancy and during the postpartum period, both times characterized by high systemic estrogen levels.
HT is generally contraindicated in women at high risk for CHD with comorbid conditions that remain uncontrolled, including blood pressure ≥ 180/110 mmHg, total cholesterol > 310 mg/dL, and triglycerides > 400 mg/dL (12, 80). Once these risk factors are better controlled, initiation of systemic HT can be considered. Women with high 10-year ASCVD risk (>10%) are generally advised to avoid systemic HT, regardless of years since menopause onset; however, if severe symptoms persist despite use of alternative therapies, individualized risk assessment and shared-decision-making is warranted. The use of a menopause decision-support algorithm, which incorporates the American College of Cardiology (ACC)/American Heart Association (AHA) 10-year risk of cardiovascular disease and years since menopause may aid in risk stratification and determine the appropriateness and continuation of HT (12). Transdermal HT is typically preferred in the setting of significant cardiovascular risk factors, particularly diabetes, hypertension and hypertriglyceridemia.
Venous Thromboembolism and Pulmonary Embolism
Given that oral HT increases the risk of VTE, we recommend that, in general, a history of VTE including deep venous thrombosis and pulmonary embolism should be considered a contraindication to use of systemic oral HT. A large meta-analysis of RCT HT trials showed an increased risk of VTE with both primary (RR 1,92 95% CI 1.24 to 2.99 in 33, 477 participants in 6 studies compared to placebo) and secondary prevention (RR 2.02, 95% CI 1.13 to 3.62; 4399 in 6 studies compared to placebo) trials (18)(Table 7–9). Pulmonary embolism risk was also increased with HT in primary prevention trials (RR 1.89, 95% CI 1.17 to 3.04; 31,732 participants in 3 trials) and in secondary prevention, there was a trend toward increase (RR 2.48, 95% CI 0.92 to 6.70; 3920 participants in 3 studies) (18).
Table 9:
HT initiated >10 years after menopause from Cochrane Review (18)
| Relative Effect (95% CI) | # of participants (# of studies) | Quality of the evidence | |
|---|---|---|---|
| Death All Cause | RR 1.06 (95% CI 0.95–1.18) | 27,750 (12) | High |
| Death from CHD | RR 1.07 (95% CI 0.96 – 1.20) | 23,491 (12) | High |
| VTE | RR 1.96 (95% CI 1.37–2.80) Absolute risk increase 0.01 with NNTH 101 | 27,475 (9) | High |
| Stroke |
RR 1.21 (95% CI 1.06–1.38) Absolute risk increase 0.01 with NNTH 102 |
22,722 (8) | High |
CHD Coronary Heart; NNTH number needed to harm (18)
An underlying thrombophilia without a history of VTE represents a relative contraindication to use of HT (12, 81). Women considering HT who have a personal or family history of VTE or pulmonary embolism, particularly if idiopathic/unprovoked, should undergo an evaluation for potential underlying and/or modifiable thrombophilia. Low dose transdermal HT is not associated with thrombotic risk in observational studies and off-label use may be considered in women with significant VMS and a history of VTE if appropriately anticoagulated (11,40, 42, 82–84). In women with systemic lupus erythematosus (SLE) and high disease activity, positive anticardiolipin antibody, and/or positive lupus anticoagulant, oral HT should generally be avoided. Conversely, HT may be prescribed to women with SLE who have mild to moderate disease activity who do not have additional risk factors for VTE (85).
Stroke
HT is generally contraindicated in patients with history of ischemic stroke. In the WHI trials, an increased risk of ischemic stroke was noted in both the EPT and ET groups regardless of the baseline risk of the patient (86,87). In a meta-analysis of primary prevention trials (719 participants, 4 studies), stroke risk was increased (RR 1.32, 95% CI 1.12 to 1.56) compared to placebo (18)(Table 7–9). In a meta-analysis of secondary prevention trials (5172 participants, 5 studies), there was a trend toward increase in risk (RR 1.09, 95% CI 0.89 to 1.33)(18).
Congenital Heart Disease
Many women with congenital heart disease are now surviving long enough to experience menopause, but there are no data on the safety of HT in this population (88). The treatment strategy should be individualized, with a focus on the underlying cardiopulmonary and postoperative physiology and associated risks. For instance, women with Fontan circulation are predisposed to VTE and therefore should avoid oral HT (89).
Cardiac Transplant
There are little data regarding HT for women after cardiac transplant (90). Given the absence of data, caution and shared decision making should be exercised.
Non-cardiovascular contraindications to HT
Non-cardiovascular contraindications to HT include unexplained vaginal bleeding, history of breast cancer, estrogen-sensitive and/or intermediate-to high-risk stage endometrial cancer, porphyria cutanea tarda, dementia, and active liver or gallbladder disease (13). A detailed analysis of these conditions is beyond the scope of this review.
Premature and Early Menopause
Premature menopause is defined as menopause occurring before age 40 years, and early menopause is defined as menopause occurring before age 45 years. Premature or early menopause may be spontaneous or induced by surgery (bilateral oophorectomy with or without hysterectomy), chemotherapy or radiation therapy. Compared with women who experience menopause at the average age, VMS in women with premature or early menopause are often more severe (4). Furthermore, observational data have indicated that untreated premature menopause regardless of the cause is associated with an elevated risk of CHD, Parkinsonism, cognitive decline, dementia, osteoporosis, and mortality (91). In this population, systemic ET should be initiated unless clear contraindications are present and continued at least until the average age of menopause at age 52(13, 92).
Although clinical trial data are not available, clinical experience suggests that doses higher than the standard doses detailed in Tables 1 and 2 are often needed to adequately treat VMS in patients with premature or early menopause.
Multidisciplinary Co-management of Patient and Shared Decision making
The value of a multidisciplinary approach to care for complex patients has been increasingly recognized throughout the cardiovascular community (93, 94). Successful models for multidisciplinary menopausal medicine clinics have been reported (95, 96). Given the high prevalence of medical comorbidities among perimenopausal women, this approach can be particularly beneficial for safe and effective management of menopausal symptoms (95, 96). A recent study reported significant menopausal symptom improvement in women with a history of malignancy receiving care through a multidisciplinary menopause clinic (95). Comprehensive multidisciplinary care may allow for more streamlined risk assessment, initiation of appropriate therapies for menopause symptoms and longitudinal CV risk reduction through a patient-centered, holistic approach.
Conclusion
Since the publication of landmark HERS and WHI trials, we have learned much regarding benefits and risks of systemic HT that is highly relevant to cardiologists who care for women at risk for or with established CVD. These last two decades have brought nuanced insights into HT use regarding timing and route of administration and have resulted in our recommendation that initiating systemic HT is appropriate for younger, healthy menopausal women with lifestyle limiting bothersome VMS. This paper provides guidance for management of symptomatic women, including those with risk factors for CVD as well as those with stable CVD. Going forward, we need additional data to better understand the risk to benefit balance of initiating HT early and continuing long term and to more fully delineate the differences between various formulations and routes of HT with respect to CVD risk.
Supplementary Material
Table 8:
HT initiated <10 years after menopause from Cochrane Review (18)
| Relative Effect (95% CI) | # of participants (# of studies) | Quality of the evidence | |
|---|---|---|---|
| Death All Cause | RR 0.70 (95% CI 0.52–0.95) | 9088 (5) | Moderate |
| Death from CHD | RR 0.52 (95% CI 0.29–0.96) | 8311 (4) | Moderate |
| VTE | RR 1.74 (95% CI 1.11–2.73) Absolute risk increase 0.008 with NNTH 214 | 9838 (3) | High |
| Stroke | RR 1.37 (95% CI 0.80–2.34) | 8143 (3) | High |
CHD Coronary Heart; NNTH number needed to harm (18)
Disclosure
Andrew Kaunitz – University of Florida receives clinical trial funding from Bayer
Cynthia A Stuenkel- Data and Safety Monitoring Board, managed by ICON Clinical Research, on behalf of Mithra Pharmaceuticals
All other authors (LC, SSF, SNH, ESL, NP, NS, JIS, CLS, KJL) have nothing to disclose
Non-standarrd abbreviations and acronyms
- CEE
conjugated equine estrogen
- CVD
cardiovascular disease
- CHD
coronary heart disease
- DVT
deep vein thrombosis
- EPT
estrogen progesten therapy
- ET
estrogen therapy
- GSM
genitourinary syndrome of menopause
- HT
hormone therapy
- MI
myocardial infarction
- MPA
medroxyprogesterone acetate
- VSM
vasomotor symptoms
- VTE
venous thromboembolic event
- WHI
Women’s Health Initiative
- WHI-OS
Women’s Health Initiative Observational Study
References
- 1.Gold EB, Crawford SL, Avis NE, Crandall CJ, Matthews KA, Waetjen LE, Lee JS, Thurston R, Vuga M, Harlow S, et al. Factors related to age at natural menopause: longitudinal analyses from SWAN 2013 Am J Epidemiol. 2013;178:70–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.US Census 2020 data [Internet] 2020[accessed 2022 July 19]; Available https://www.census.gov/programs-surveys/decennial-census/decade/2020/2020-census-main.html [Google Scholar]
- 3.Nelson HD Menopause Lancet. 2008;371:760–770. [DOI] [PubMed] [Google Scholar]
- 4.Kaunitz AM, Manson JE Management of menopausal symptoms Obstet Gynecol. 2015;126:859–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hulley S, Grady D, Bush T, Furburg C, Herrington D, Riggs B, Vittinghoff E,. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998;280:605–613. [DOI] [PubMed] [Google Scholar]
- 6.Manson JE, Hsia J, Johnson KC, Rossouw JE, Assaf AR, Lasser NL, Trevisan M, Black HR, Heckbert SR, Detrano R et al. ,. Estrogen plus Progestin and the Risk of Coronary Heart Disease. N Engl J Med. 2003;349:523–534. [DOI] [PubMed] [Google Scholar]
- 7.Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SAA, Black H, Bonds D, Brunner R, Brzyski R, Caan B et al. , Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701–1712. [DOI] [PubMed] [Google Scholar]
- 8.Steinkellner AR, Dennison SE, Eldridge SL, Lenzi LL, Chen W, Bowlin SJ A decade of postmenopausal hormone therapy prescribing in the US: long term effect of the Women’s Health Initiative Menopause. 2012;19:616–624. [DOI] [PubMed] [Google Scholar]
- 9.Mosca L, Benjamin EJ, Berra K, Bezanson JL, Dolor RJ, Lloyd –Jones DM, Newby LK, Piña IL, Roger VL, Shaw LJ et al. ,, Effectiveness-based guidelines for the prevention of cardiovascular disease in women −2011 update: a guideline from the American Heart Association Circulation. 2011;123:1243–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.ACOG practice bulletin no. 141: management of menopausal symptoms. Obstet Gynecol. 2014;123:202–216 [DOI] [PubMed] [Google Scholar]
- 11.Cobin RH, Goodman NF. American Association of Clinical Endocrinologists and American College of Endocrinology position statement on menopause - 2017 update. Endocr Pract. 2017;23:869–880 [DOI] [PubMed] [Google Scholar]
- 12.Stuenkel CA, Davis SR, Gompel A, Lumsden MA, Murad MH, Pinkerton JV, Santen RJ Treatment of symptoms of the menopause: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:3975–4011 [DOI] [PubMed] [Google Scholar]
- 13.The NAMS 2022 Hormone Therapy Position Statement Advisory Panel The 2022 hormone therapy position statement of the North American Menopause Society. Menopause. 2022;29:767–794. [DOI] [PubMed] [Google Scholar]
- 14.Grossman DC, Curry SJ, Owens DK, Barry MJ, Davidson KW, Doubeni CA, Epling JW Jr, Kemper AR, Krist AH, Kurth AE et al. , Hormone therapy for the primary prevention of chronic conditions in postmenopause women: US preventive services Task Force recommendation statement JAMA. 2017;318:2224–2233. [DOI] [PubMed] [Google Scholar]
- 15.Manson JE, Chlebowski RT, Stefanick ML, Aragaki AK, Rossouw JE, Prentice RL, Anderson G, Howard BV, Thomson CA, LaCroix AZ et al. , Menopausal hormone therapy and health outcomes during the interventions and extended poststopping phases of the Women’s Health Initiative randomized trials JAMA. 2013;310:1353–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manson JE, Aragaki AK, Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Chlebowski RT, Howard BV, Thomson CA, Margolis KL et al. , Menopausal Hormone therapy and long-term all-cause and cause-specific mortality: the Women’s health Initiative randomized trials. JAMA. 2017;318:927–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salpeter SR, Cheng J, Thabane L, Buckley NS, Salpeter EE, Bayesian meta-analysis of hormone therapy and mortality in younger postmenopausal women Am J Med. 2009;122:1016–1022. [DOI] [PubMed] [Google Scholar]
- 18.Boardman HM, Hartley L, Eisinga A, Main C, Roqué i Figuls M, Bonfill Cosp X, Sanchez RG, Knight B Hormone therapy for preventing cardiovascular disease in post-menopausal women Cochrane Database Syst Rev. 2015:CD002229.doi: 10.1002/14651858.CD002229.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Villiers TJ, Pines A, Panay N, Gambacciani M, Archer DF, Baber RJ, Davis SR, Gompel AA, Hnerderson VW, Langer R et al. , on behalf of the International Menopause Society. Updated 2013 Internal Menopause society recommendations on menopausal hormone therapy and preventive strategies for mildlife health. Climacteric. 2013;16:316–337. [DOI] [PubMed] [Google Scholar]
- 20.Armeni E, Lambrinoudaki I, Ceausu I, Depypere H, Mueck A, Perez-Lopex FR, va der Schouw YT, Senturk LM, Simoncini T, Stevenson JC et al. , Maintaining postreproductive health: a care pathway from the European Menopause and Andropause Society (EMAS) Maturitas. 2016;89:63–72. [DOI] [PubMed] [Google Scholar]
- 21.Lumsden MA, Davies M, Sarri G Guideline development group for menopause: Diagnosis and management (NICE Clinical Guideline No. 23). Diagnosis and management of menopause: the National Institute of Helath and Care Excellence (NICE) guideline. JAMA Intern Med. 2016;176:1205–1206. [DOI] [PubMed] [Google Scholar]
- 22.Maas AHEM, Rosano G, Cifkova R, Chieffo A, van Dijken D, Hamoda H, Kunadian V, Laan E, Lambrinoudaki I, Maclaran K et al. , Cardiovascular Health after menopause transition, pregnancy disorders, and other gynaecologic conditions: a condsensus document from European cardiologists, gynaecologists, and endocrinologist. Eur Heart J. 2021;42:967–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manson JE, Kaunitz AM. Menopause management-- Getting Clinical care back on track N Engl J Med. 2016;374:803–806. [DOI] [PubMed] [Google Scholar]
- 24.El Khoudary SR, Aggarwal B, Beckie TM, Hodis HN, Johnson AE, Langer RD, Limacher MC, Manson JE, Stefanick ML, Allison MA, et al. , Menopause transition and cardiovasuclar disease risk: Implications for timing of early prevention A scientific statement from the American Heart Association Circulation. 2020;142(25):e506–e532. [DOI] [PubMed] [Google Scholar]
- 25.Tata JR., One hundred years of hormones. EMBO Rep. 2005;6(6):490–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson R. Feminine Forever New York J Evans co Inc 1966 [Google Scholar]
- 27.Barrett-Connor E Clinical review 162: cardiovascular endocrinology 3: an epidemiologist looks at hormones and heart disease in women. J Clin Endocrinol Metab. 2003;33:4031–4042. [DOI] [PubMed] [Google Scholar]
- 28.Ziel HK, Finkle WD. Increased Risk of Endometrial Carcinoma among Users of Conjugated Estrogens. New Engl J Med. 1975;293:1167–1170. [DOI] [PubMed] [Google Scholar]
- 29.Smith DC, Prentice R, Thompson DJ, Herrmann WL. Association of exogenous estrogen and endometrial carcinoma. N Engl J Med. 1975;293:1164–1167. [DOI] [PubMed] [Google Scholar]
- 30.Stampfer MJ, Colditz GA, Willett WC, Manson JE, Rosner B, Speizer FE, Hennekens CH Postmenopausal estrogen therapy and cardiovascular disease. Ten-year follow-up from the nurses’ health study. N Engl J Med. 1991;325:756–762. [DOI] [PubMed] [Google Scholar]
- 31.Cagnacci A, Venier M. The Controversial History of Hormone Replacement Therapy. Medicina (Kaunas). 2019;55(9):602. Published 2019 Sep 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.The Coronary Drug Project Findings leading to discontinuation of the 2.5mg a day estrogen group. The coronary drug project research group. JAMA. 1973;226:652–657 [PubMed] [Google Scholar]
- 33.Rossouw JE, Prentice RL, Manson JE, Wu L, Barad D, Barnabei VM, Ko M, LaCroix AZ, Margolis KL, Stefanick ML Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA.2007;297:1465–1477. [DOI] [PubMed] [Google Scholar]
- 34.Mikkola TS, Clarkson TB Estrogen replacement therapy, atherosclerosis, and vascular function Cardiovasc Res. 2002;53:605–619 [DOI] [PubMed] [Google Scholar]
- 35.Grodstein F, Manson JE, Stamper MJ Hormone therapy and coronary heart disease: the role of time since menopause and age at hormone initiation J. Womens Health. 2006;15:35–44 [DOI] [PubMed] [Google Scholar]
- 36.Schierbeck LL, Rejnmark L, Landbo Tofteng C, Stilgren L, Eiken P, Mosekilde L, Køber L, Beck Jensen JE Effect of hormone replacement therapy on cardiovascular events in recently postmenopausal women: randomized trial BMJ. 2012;345:e6409. [DOI] [PubMed] [Google Scholar]
- 37.Hodis HN, Mack WJ, Henderson VW, Shoupe D, Budoff MJ, Selzer RH, Azen SP Vascular Effects of Early versus Late Postmenopausal Treatment with Estradiol. New Engl. JMedicine. 2016;374:1221–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harman SM, Black DM, Naftolin F, Brinton EA, Budoff MJ, Cedars MI, Hopkins PN, Lobo RL, Manson JE, Merriam GR et al. , Arterial imaging outcomes and cardiovascular risk factors in recently menopausal women: a randomized trial. Ann Intern Med. 2014;161:249–260. [DOI] [PubMed] [Google Scholar]
- 39.Bergendal A, Kieler H, Sundström A, Lindén Hirschberg A, Kocoska- Maras L Risk of venous thromboembolism associated with local and systemic use of hormone therapy in peri- and postmenopausal women and in relation to type and route of administration. Menopause. 2016;23(6):593–599. [DOI] [PubMed] [Google Scholar]
- 40.Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases. BMJ. 2019;364:k4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goldstajn SM, Mikus M, Ferrari FA, Bosco M, Uccella S, Noventa M, Torok P, Terzic S, Lagana AS, Garzon S Effects of transdermal versus oral hormone replacement therapy in postmenopause: a systemic review Arch Gynecol Obstet. 2022. Jun 17. doi: 10.1007/s00404-022-06647-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lacut K, Oger E, Le Gal G, Blouch MT, Abgrall JF, Kerlan V, Scarabin PY, Mottier D; SARAH Investigators. Differential effects of oral and transdermal postmenopausal estrogen replacement therapies on C-reactive protein. Thromb Haemost. 2003. Jul;90(1):124–131. PMID: . [PubMed] [Google Scholar]
- 43.Pinkerton JV Hormone therapy for postmenopausal Women N Engl J Med. 2020;382:446–455 [DOI] [PubMed] [Google Scholar]
- 44.Kaunitz AM. Transdermal and vaginal estradiol for the treatment of menopausal symptoms: the nuts and bolts. Menopause. 2012;19(6):602–603 [DOI] [PubMed] [Google Scholar]
- 45.Gass ML, Stuenkel CA, Utian WH, LaCroix A, Liu JH, Shifren JL North American Menopause Society (NAMS) Advisory Panel consisting of representatives of NAMS Board of Trustees and other experts in women’s health. Use of compounded hormone therapy in the United States: report of The North American Menopause Society Survey. Menopause. 2015. Dec;22(12):1276–1284. [DOI] [PubMed] [Google Scholar]
- 46.National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Health Sciences Policy; Committee on the Clinical Utility of Treating Patients with Compounded Bioidentical Hormone Replacement Therapy. The Clinical Utility of Compounded Bioidentical Hormone Therapy: A Review of Safety, Effectiveness, and Use. Jackson LM, Parker RM, Mattison DR, editors. Washington (DC): National Academies Press (US); 2020. Jul 1. PMID: 33048485. [PubMed] [Google Scholar]
- 47.NAMS. The 2020 genitourinary syndrome of menopause position statement of The North American Menopause Society. Menopause. 2020;27:976–992. [DOI] [PubMed] [Google Scholar]
- 48.Santen RJ, Mirkin S, Bernick B and Constantine GD. Systemic estradiol levels with low-dose vaginal estrogens. Menopause. 2020;27:361–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Manson JE, Goldstein SR, Kagan R, Kaunitz AM, Liu JH, Pinkerton JV, Rebar RW, Schnatz PF, Shifren JL, Stenkel CA et al. , Working Group on Women’s H and Well-Being in M. Why the product labeling for low-dose vaginal estrogen should be changed. Menopause. 2014;21:911–916. [DOI] [PubMed] [Google Scholar]
- 50.Bhupathiraju SN, Grodstein F, Stampfer MJ, Willet WC, Crandall CJ, Shifren JL, Manson JE Vaginal estrogen use and chronic disease risk in the Nurses’ Health Study. Menopause. 2018;26:603–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Crandall CJ, Hovey KM, Andrews CA, Chlebowski RT, Stefanick ML, Lane DS, Shifren J, Chen C, Kaunitz AM, Cauley JA et al. , Breast Cancer, Endometrial Cancer, and Cardiovascular Events in Participants who used Vaginal Estrogen in the Women’s Health Initiative Observational Study Menopause. 2018;25:11–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Percent of U.S. Adults 55 and Over with Chronic Conditions. National Center for Health Statistics CDC; https://www.cdc.gov/nchs/health_policy/adult_chronic_conditions.htm. Published 2008. Accessed 12/29/21, 2021. [Google Scholar]
- 53.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL Trends in Obesity Among Adults in the United States, 2005 to 2014. JAMA. 2016;315(21):2284–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen Z, Bassford T, Green SB, Cauley JA, Jackson RD, LaCroix AZ, Leboff M, Stefanick ML, Margolis KL Postmenopausal hormone therapy and body composition--a substudy of the estrogen plus progestin trial of the Women’s Health Initiative. Am J Clin Nutr. 2005;82(3):651–656. [DOI] [PubMed] [Google Scholar]
- 55.Espeland MA, Stefanick ML, Kritz-Silverstein D, Fineburg SE, Waclawiw MA, James MK, Greendale GA Effect of postmenopausal hormone therapy on body weight and waist and hip girths. Postmenopausal Estrogen-Progestin Interventions Study Investigators. J Clin Endocrinol Metab. 1997;82(5):1549–1556. [DOI] [PubMed] [Google Scholar]
- 56.Cushman M, Kuller LH, Prentice R, Rodabough RJ, Psaty BM, Stafford RS, Sodney S, Rosendaal FR Estrogen plus progestin and risk of venous thrombosis JAMA. 2004; 292: 1573–1580. [DOI] [PubMed] [Google Scholar]
- 57.Crandall CJ, Hovey KM, Andrews CA, Cauley JA, Stefanick M, Shufelt C, Prentice RL, Kaunitz AM, Eaton C, Wactawski- Wende J et al. , Comparison of clinical outcomes among users of oral and transdermal estrogen therapy in the Women’s Health Initiative Observational Study Menopause. 2017;24:1145–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bray PF, Larson JC, Lacroix AZ, Manson J, Limacher MC, Rossouw JE, Lasser NL, Lawson WE, Stefanick ML, Langer RD et al. Usefulness of baseline lipids and C - reactive protein in women receiving menopausal hormone therapy as predictors of treatment-related coronary events. Am J Cardiol. 2008;101(11):1599–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Herrington DM, Vittinghoff E, Lin F, Fong J, Harris F, Hunninghake D, Bittner V, Schrott HG, Blumenthal RS, Levy R Statin treatment, cardiovascular events and total mortality in the Heart and Estrogen/Progestin Replacement study Circulation. 2002;105:2962–2967. [DOI] [PubMed] [Google Scholar]
- 60.Ambikairajah A, Walsh E, Cherbuin N Lipid profile differences during menopause; a review with meta-analysis Menopause. 2019;26:1327–1333 [DOI] [PubMed] [Google Scholar]
- 61.Kopper NW, Gudeman J, Thompson DJ. Transdermal hormone therapy in postmenopausal women: a review of metabolic effects and drug delivery technologies. Drug Des Devel Ther. 2009;2:193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mehta J, Manson JE. Menopausal hormone therapy and hypertension: minimizing risk. Menopause. 2021;28:1201–1202. [DOI] [PubMed] [Google Scholar]
- 63.Nair GV, Chaput LA, Vittinghoff E, Herrington DM Heart, Estrogen/Progestin Replacement Study I. Pulse pressure and cardiovascular events in postmenopausal women with coronary heart disease. Chest. 2005;127(5):1498–1506. [DOI] [PubMed] [Google Scholar]
- 64.Shimbo D, Wang L, Lamonte MJ, Allison M, Wellenius GA, Bavry AA, Martin LW, Aragaki A, Newman JD, Swica Y et al. , The effect of hormone therapy on mean blood pressure and visit-to-visit blood pressure variability in postmenopausal women: results from the Women’s Health Initiative randomized controlled trials. J Hypertens. 2014;32(10):2071–2081; discussion 2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, Chalmers J, Rodgers A, Rahimi K Blood pressure lowering for prevention of cardiovascular disease and death: a systemic review and meta-analysis Lancet. 2016;387:957–967. [DOI] [PubMed] [Google Scholar]
- 66.Swica Y, Warren MP, Manson JE, Aragaki AK, Bassuk SS, Shimbo D, Kaunitz, Rossouw J, Stefanick ML, Womack CR Effects of oral conjugated equine estrogens with or without medroxyprogesterone acetate on incident hypertension in the Women’s Health Initiative hormone therapy trials. Menopause. 2018:25:753–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wild RA, Larson JC, Crandall CJ, Shadyab AH, Allison M, Gass M, Shufelt C, Manson JE Hormone therapy formulation, dose, route of delivery and risk of hypertension: findings from the Women’s Health Initiative Observational Study (WHI-OS) Menopause. 2021;28:1108–1116 [DOI] [PubMed] [Google Scholar]
- 68.Cho L, Davis M, Elgendy I, Epps K, Lindley KJ, Mehta PK, Michos ED, Minissian M, Pepine C, Vaccarino V et al. , Summary of updated recommendations for primary prevention of cardiovascular disease in women J Am Coll Cardiol. 2020;75:2602–2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ma Y, Hebert JR, Balasubramanian R, Wedick NM, Howard BV, Rosal MC, Liu S, Bird CE, Olendzki BC, Ockene JK et al. , All-cause, cardiovascular, and cancer mortality rates in postmenopausal white, black, hispanic, and asian women with and without diabetes in the United States: The Women’s health initiative, 1993–2009 Am J epidemiol. 2013; 178:1533–1541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stuenkel CA Menopause, hormone therapy and diabetes Climacteric. 2017;20:11–21. [DOI] [PubMed] [Google Scholar]
- 71.Wild RA, Wu C, Curb JD, Martin LW, Phillips L, Stefanick M, Trevisan M, Manson JE Coronary heart disease events in the Women’s Health Initiative randomized clinical trials. Menopause. 2013;20:254–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Slopien R, Wender-Ozegowska E, Rogowicz-Frontczak A,Meczekalski B, Zozulinska- Ziolekiewicz D, Jaremek JD, Cano A, Chedraui P, Simoncini T et al. , Menopause and diabetes: EMAS clinical guide. Maturitas. 2018;117:6–10. [DOI] [PubMed] [Google Scholar]
- 73.de Lauzon-Guillain B, Fournier A, Fabre A, Simon N, Mesrine S, Boutron-Ruault M-C, Balkau B, Clavel- Chapelon F Menopausal hormone therapy and new-onset diabetes in the French Etude Epidemiologique de Femmes de la Mutuelle Generale de l’Education Nationale (E3N) cohort. Diabetologia. 2009;52(10):2092–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Salpeter SR, Walsh JM, Ormiston TM, Greyber E, Buckley NS, Salpeter EE Meta-analysis: effect of hormone-replacement therapy on components of the metabolic syndrome in postmenopausal women. Diabetes Obes Metab. 2006;8(5):538–554. [DOI] [PubMed] [Google Scholar]
- 75.Nonhormonal management of menopause-associated vasomotor symptoms: 2015 position statement of The North American Menopause Society. Menopause. 2015;22(11):1155–1172; quiz 1173–1154. [DOI] [PubMed] [Google Scholar]
- 76.Faubion SS, Kaunitz AM. Stopping systemic menopausal hormone therapy: Why, when and how. Maturitas. 2016;89:3–4. [DOI] [PubMed] [Google Scholar]
- 77.Gass ML, Maki PM, Shifren JL, Schnatz PF, Kaunitz AM, Shapiro M, Leidy Sievert L NAMS supports judicious use of systemic hormone therapy for women aged 65 years and older. Menopause. 2015;22(7):685–686. [DOI] [PubMed] [Google Scholar]
- 78.Kaunitz AM. Extended Use of Systemic Menopausal Hormone Therapy. Clin Obstet Gynecol. 2018;61(3):517–522. [DOI] [PubMed] [Google Scholar]
- 79.Bretler DM, Hansen PR, Sørensen R,Lindhardsen J, Ahlehoff O, Andersson C, Zabell Abildstrøm S, Torp- Pedersen C, Hilmar Gislason G Discontinuation of hormone replacement therapy after myocardial infarction and short term risk of adverse cardiovascular events: nationwide cohort study. BMJ. 2012. Mar 27;344:e1802. doi: 10.1136/bmj.e1802. PMID: 22453184; PMCID: PMC3313837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Maas AHEM. Hormone therapy and cardiovascular disease: Benefits and harms. Best Pract Res Clin Endocrinol Metab. 2021 Dec;35(6):101576. doi: 10.1016/j.beem.2021.101576. Epub 2021 Sep 10. PMID: 34556415. [DOI] [PubMed] [Google Scholar]
- 81.Peverill RE. Hormone therapy and venous thromboembolism. Best Pract Res Clin Endocrinol Metab. 2003. Mar;17(1):149–164. doi: 10.1016/s1521-690x(02)00079-9. PMID: 12763518. [DOI] [PubMed] [Google Scholar]
- 82.Taylor HS, Pal L, Emre S. Reproductive aging, menopause transition, and menopause hormone therapy. In: Speroff’s Clinical Gynecologic Endocrinology and Infertility. 9th ed. Lippincott Williams & Wilkins; 2019. [Google Scholar]
- 83.Kapoor E, Kling JM, Lobo AS, Faubion SS, Menopausal hormone therapy in women with medical conditions, Best Practice & Research Clinical Endocrinology & Metabolism, Volume 35, Issue 6, 2021, 101578,ISSN 1521–690X, 10.1016/j.beem.2021.101578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Martinelli I, Lensing AWA, Middeldorp S, Levi M, Beyer-Westendorf J, van Bellen Bonno, Bouameauz H, Brighton TA, Cohen AT, Trajanovic M et al. , Recurrent venous thromboembolism and abnormal uterine bleeding with anticoagulant and hormone therapy use Blood. 2016. 17: 1417–1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sánchez-Guerrero J, González-Pérez M, Durand-Carbajal M, Lara- Reyes L, Jiménez- Santana L, Romero- Díaz J, Cravioto MDC Menopause hormonal therapy in women with systemic lupus erythematosus. Arthritis Rheum. 2007. Sep;56(9):3070–3079. doi: 10.1002/art.22855. PMID: 17763408. [DOI] [PubMed] [Google Scholar]
- 86.Wassertheil-Smoller S, Hendrix SL, Limacher M, Heiss Ge, Kooperberg C, Baird A, Kotchen T, Curb D, Black H, Rossouw JE et al. , Effect of estrogen plus progestin on stroke in postmenopausal women: the Women’s Health Initiative: a randomized trial JAMA. 2003;289:2673–2684 [DOI] [PubMed] [Google Scholar]
- 87.Hendrix SL, Wassertheil-Smoller S, Johnson KC, Howard BV, Kooperberg C, Rossouw JE, Trevisan M, Aragaki A, Baird AE, Bray PF et al. , Effects of conjugated equine estrogen on stroke in the Women’s Health Initiative Circulation 2006; 113:2425–2434. [DOI] [PubMed] [Google Scholar]
- 88.Haberer K, Silversides CK. Congenital Heart Disease and Women’s Health Across the Life Span: Focus on Reproductive Issues. Can J Cardiol. 2019. Dec;35(12):1652–1663. doi: 10.1016/j.cjca.2019.10.009. Epub 2019 Oct 16. PMID: 31813502. [DOI] [PubMed] [Google Scholar]
- 89.Bhatt AB, Foster E, Kuehl K, Alpert J, Brabeck S, Crumb S, Davidson WR Jr, Earing MG, Ghoshhajra BB, Karamlou T et al. , American Heart Association Council on Clinical Cardiology. Congenital heart disease in the older adult: a scientific statement from the American Heart Association. Circulation. 2015. May 26;131(21):1884–1931. doi: 10.1161/CIR.0000000000000204. Epub 2015 Apr 20. Erratum in: Circulation. 2015 May 26;131(21):e510. PMID: 25896865. [DOI] [PubMed] [Google Scholar]
- 90.Kobashigawa LC, Hamilton M, Rafiei M, Stern L, Bairey Merz CN Hormone therapy in women after cardiac transplant Transplant Proc. 2013;45:3386–3388. [DOI] [PubMed] [Google Scholar]
- 91.Honigberg Zekavat SM, Aragam K, Finneran P, Klarin D, Bhatt DL, Januzzi JL Jr, Scott NS, Natarajan P Association of premature natural and surgical menopause with incident cardiovascular disease JAMA. 2019;322: 2411–2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kaunitz AM, Kapoor E, Faubion S. Treatment of Women After Bilateral Salpingo-oophorectomy Performed Prior to Natural Menopause. JAMA. 2021;326(14):1429–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jones DR, Chew DP, Horsfall MJ, Ming-Yu Chuang A, Sinhal AR, Joseph MX, Baker RA, Bennets JS, Selvanayagam Jb et al. ,. Multidisciplinary transcatheter aortic valve replacement heart team programme improves mortality in aortic stenosis. Open heart. 2019;6:e000983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Davis MB, Arendt K, Bello NA, Brown H, Briller J, Epps K, Hollier L, Langen E, Park K, Walsh MN et al. , Team-Based Care of Women With Cardiovascular Disease From Pre-Conception Through Pregnancy and Postpartum. J Am Coll Cardiol. . 2021;77:1763–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hollingworth J, Walsh L, Tran S, Ramage L, Patel- Brown S, Ambekar M, Weeks J, Williams L, Cohen PA Does a multidisciplinary menopausal symptoms after cancer clinic reduce symptoms? Support Care Cancer. 2022;30:2245–2252. [DOI] [PubMed] [Google Scholar]
- 96.Sydora BC, Yuksel N, Veltri NL, Marillier J, Sydora CP, Yaskina M, Battochio L, Shandro TML, Ross S Patient characteristics, menopause symptoms, and care provided at an interdisciplinary menopause clinic: retrospective chart review. Menopause. 2018;25:102–105 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.