Abstract
Background
Food allergy has considerably increased in recent years and this situation has been aggravated mainly by the consumption of more processed and complex foods, since minor or potentially allergenic foods are not required to be labeled. Manihot esculenta (cassava) is a widely consumed food in South America, Africa, and Asia and can be used in the production of flour and starch, as well as several other products. This root can cause allergic reactions with symptoms ranging from mild to severe.
Methods
Thus, the aim of this study was the characterization of the immunogenic cassava proteins responsible for sensitizing patients allergic to it. Using a 2D-SDS-PAGE based proteomic approach, six proteins were identified, including Fructose Bisphosphate Aldolase (FBA). Recombinant FBA was produced in Expi293 cells and evaluated by immunoblotting with the serum of 10 individual study subjects.
Results
Our results showed six cassava IgE-reactive proteins. From those, recombinant fructose bisphosphate aldolase (FBA) showed a positivity of 80% among tested sera, proving to be a highly sensitizing protein.
Conclusion
The recombinant FBA molecule obtained in this study can be important for in vivo diagnostic assays, by producing more accurate results, and for desensitization protocols, in which the use of the isolated molecule produces more precise results by avoiding secondary sensitization.
Trial registration
All patients signed a consent form approved by the internal ethics committee CAPPesq, Comissão de Ética para Análise de Projetos de Pesquisa do HC FMUSP (CAAE: 10420619.6.0000.0068).
Keywords: Food hypersensitivity, Manihot, Fructose-biphosphate aldolase, Proteome
Background
More than 220 million people worldwide suffer from food allergy, and in recent decades this number assumed an increasing prevalence and severity.1,2 The food allergy situation was worsened mainly by the consumption of more processed and complex foods that may contain minor or potentially allergenic foods, which can be validated by the higher food allergy prevalence in more industrialized and western regions.2,3
Cassava (Manihot esculenta crantz) is a shrub of the Euphorbiaceae family. Its tuberous roots composed of 85% starch and only 2% protein, and it has a high energy value. Cassava is native to South America, but can also be found in Asia and Africa as it is commonly cultivated in tropical and subtropical regions. It is highly tolerant of drought and harsh weather conditions and very productive in poor soils and marginal lands, being available throughout the year due to the flexibility of planting and harvesting the crop. These nutritional and agronomic characteristics establish cassava as a reliable crop for food security in many countries.4,5
Cassava is the third largest food source of carbohydrates and vitamins for about 700 million people, especially in developing countries, being an important raw material for starch extraction.5, 6, 7 In Brazil, cassava is known as “mandioca”, but it can be found with others names in other countries such as: “tapioca” in, Thailand and Vietnam, “cassava” in West India, and “yucca” in other South America regions.8,9 It can be consumed fried or cooked and serves as a basis for the production of several products such as flour, which is part of the daily meals of most Brazilians, animal feed, alcohol, sweets, glue for paper and fabrics, and biodegradable plastics.5,10,11
So far, 3 allergens have been described for cassava. FBA (Fructose-Bisphosphate Aldolase), with a theoretical molecular mass of 39.9 kDa, GAPDH (Glyceraldehyde-3-Phosphate Dehydrogenase) theoretical molecular mass of 36.8 kDa, and Man e 5 (glutamic acid rich protein) theoretical molecular mass of 30 kDa.8 In inhibition assays, Man e 5 partially inhibited IgE-reactivity to Hev b 5 (a described latex allergen), suggesting that the IgE-reactivity to cassava may occur because of prior sensitization by Hev b 5.8
Here we characterized immunogenic cassava proteins using sera from cassava-allergic patients followed by the development and IgE-reactivity testing of a recombinant FBA molecule.
Materials and methods
Patient selection
Based on clinical history, 10 patients with cassava allergy confirmed by prick-to-prick test with raw cassava were selected from the Allergy and Immunology Clinic from Ambulatório de Alergia e Imunologia do Hospital das Clínicas da Universidade de São Paulo. Sera from these patients were used in ImmunoCAP 100 tests (Thermo Fisher) for detection of specific IgE for latex and its available allergens: Hev b 1 (rubber elongation factor), Hev b 3 (small rubber particle protein), Hev b 5 (acidic protein), Hev b 6 (hevein precursor), Hev b 8 (profilin) and Hev b 11 (class I chitinase). All patients signed a consent form approved by the internal ethics committee (CAAE: 10420619.6.0000.0068). Besides, 2 individuals, 1 atopic without manic allergy and 1 non-atopic were included as controls for 1D immunoblot.
Cassava extract preparation
Cassava (Manihot esculenta) was purchased from a local supermarket and properly sanitized in running water. The protein extract was produced from maceration of freeze-dried cassava (Super Modulyo – Thermo Savant) using the phenolic extraction and precipitation protocol with 100 mM ammonium acetate/methanol in the presence of protease inhibitors, adapted from Carpentier et al.12 The protein concentration was measured by Bradford13 and the extract was stored at −20 °C until further use.
1D and 2D gel electrophoresis
For 1D gel electrophoresis, 20 μg of cassava protein extract was solubilized in denaturing/reducing sample buffer and applied to each well of a 12% (m/v) SDS-PAGE. For the isoelectric focusing (IEF) of 2D electrophoresis, the proteins were solubilized in a rehydration solution (DeStreak plus 0.5% (v/v) IPG buffer (GE Healthcare Biosciences AB, Uppsala, Sweden). Immobiline Dry Strips (IPG) with a pI range of 4–7 were rehydrated in this protein solution overnight aiming at a good separation of different proteins with different pIs likely to be found in the whole extract. The strips were subjected to IEF in an IPGphor system (GE Healthcare Biosciences AB, Uppsala, Sweden) at 300V, 1000V and 5000V until reaching 5750 Vh. For reduction and alkylation, the two following steps were performed, an incubation with 1% (w/v) DTT for 15 min followed by 4% (w/v) IAA (iodoacetamide) for 15 min, before strips were loaded onto 12% (w/v) polyacrylamide gels for proteins separation by reducing SDS-PAGE. Proteins were stained with Coomassie Blue Colloidal14 and gel images were captured using ImageQuant LAS 4000 (GE Healthcare Biosciences AB, Uppsala, Sweden).
1D and 2D immunoblot
After separation, proteins from the 1D and 2D gel were electrotransferred onto nitrocellulose membranes and subsequently blocked with TBS added with 0.1% (v/v) Tween 20 and 5% of (w/v) nonfat dry milk (Biorad – Blotting-Grade Blocker) for total extract and at 10% (w/v) for recombinant FBA for 1 h at room temperature. Membranes were incubated overnight at 4 °C with patient sera at 1:5 individually for 1D and (1:10) pooled for 2D. Bound IgE was detected with goat anti-human IgE (ε), HRP conjugate (Invitrogen-California, USA) standardized at 1:5000 as a second antibody. Immunoreactive bands were scanned by ImageQuant LAS 4000 (GE Healthcare Biosciences AB, Uppsala, Sweden) using Enhanced Chemiluminescence (ECL) Kit (GE Healthcare Biosciences, Uppsala, Sweden).
Mass spectrometry and data analysis
For mass spectrometry analyses, IgE-reactive spots from 2D-gels were digested with trypsin (Promega - Madison, WI, EUA) according to the protocol designed by Shevchenko et al,15 and the mass spectrometry analyses of cassava 2D gel excised spots were performed according to Dias et al.16 Tryptic digests of the cassava were solubilized in 0.1% (v/v) formic acid solution (solution A) and submitted to Ultimate 3000 HPLC (Dionex, Germering, Germany) coupled to a Q Exactive Orbitrap™ mass spectrometer (Thermo Fisher Scientific, Bremen, Germany). Peptides were loaded on a Trap Column with nano Viper Fitting (P/N 164,649, C18, 5 mm × 30 μm, Thermo Fisher Scientific, Bremen, Germany) and eluted at a flow rate of 300 nL/min using an isocratic gradient of 4% solution B (100% acetonitrile containing 0.1% (v/v) formic acid) for 3 min. Thereafter, peptides were loaded on a C18 PicoChip column (Reprosil-Pur®, C18-AQ, 3 μm, 120 Å, 105 mm, New Objective, Woburn, MA, USA) using a segmented concentration gradient from 4 to 55% B for 30 min, 55–90% B for 1 min, 90% B over 5 min and then returning to 4% B over 20 min at a flow rate of 300 nL/min. Ion polarity was set to positive ionization mode using data-dependent acquisition (DDA) mode. Mass spectra were acquired with a scan range of m/z 200–2000, resolution of 70 000 and injection time of 100 ms. The fragmentation chamber was conditioned with collision energy between 29 and 35% with a resolution of 17 500, 50 ms of injection time, m/z 4.0 of isolation window, and dynamic exclusion of 10 s. The spectrometric data were acquired using the Thermo Xcalibur™ software (version 4.0.27.19, Thermo Fisher Scientific Bremen, Germany).
Raw data were submitted to the PatternLab for Proteomics software version 4.0.0.8417 and searched against the NCBI database (http://blast.ncbi.nlm.nih.gov) by using the Taxonomy Manihot. The following search parameters were used: semi-tryptic cleavage products (2 tryptic missed cleavage allowed), carbamidomethylation of cysteine as fixed modification, and oxidation of methionine as variable modification. Parent mass tolerance error was set at 40 ppm and fragment mass error at 0.02 ppm. Protein identifications were considered with a minimum of 1 fragment ion per peptide, 5 fragment ions per protein, 2 peptides per protein, and a false discovery rate (FDR) set to lower than 1%, estimated by a simultaneous search against a reversed database.
Construction of FBA expression clones
The gene of fructose bisphosphate aldolase (1164 bp) was optimized with codons preferentially used by mammals, a Kozak sequence included at the 5′ end to favor protein expression, an IgE signal peptide to target proteins for exocytosis, and a histidine tag, for further purification on a nickel column. The designed gene was synthesized (GenOne Biotech©) and cloned into the expression vector pcDNA3.1 (Thermo Fisher), using the restriction enzymes XbaI and ApaI (Invitrogen).
Cell transfection and protein expression
The human cell line Expi293F was cultured in suspension in the recomended Expression medium (Gibco A14351-01) in shake flasks under standard procedures at 125 rpm and 37°C with 8% CO2.
The pCDNA3.1 plasmid containing the optimized FBA gene was transfected into 300×106 Expi293F cells in 100 ml Expi293 Expression Medium using ExpiFectamine 293 Transfection Kit and Opti-MEM Reduced Serum Medium (GIBCO BRL) for 16–18 h, added with 600 μl Enhancer 1 and 6 ml Enhancer 2. Cell cultures were expanded for 5 days to allow for protein expression. Cell viability was determined by Trypan Blue exclusion assay.
Recombinant protein purification
Due to the presence of histidine tag in the protein, purification of the supernatant was performed by metal affinity chromatography (IMAC). The supernatant containing the proteins was diluted 1:1 in phosphate-buffered saline (PBS, pH 7.4) along with resin (Ni Sepharose 6 Fast Flow - Cytivia) and 5 mM imidazole for every 100 mL of diluted supernatant. This mixture was left under gentle agitation for 1 h and 30 min at 4C. For protein purification, the pre-treated crude supernatant was loaded onto a plastic column (20 mL), and this loaded column was washed with PBS containing 5 mM imidazole. The protein was then eluted in 50 mM, 250 mM and 500 mM imidazole. Finally, the elution was buffer exchanged with PBS by dialysis. Protein concentration was determined in Spectrophotometer (Nanodrop ND1000 - Uniscience), concentration (M) = Absorbance at 280 nm/extinction coefficient.
Simulation of gastric digestion with pepsin
To assess protein stability during gastric digestion, an in vitro simulation of rFBA protein degradation in the presence of the enzyme Pepsin (Sigma - P6887) was performed. The enzyme was diluted in HCL 0.1 M pH 2 to a concentration of 3 μg/μL and stored at −20°C until the time of use as a stock solution. For the experiment, in microtubes, 60 μg of rFBA protein was incubated along with 0.4% pepsin and 114 μL HCL 0.45 M pH 2 solution at 37°C under gentle agitation in ThermoMixer C (Eppendorf). The reaction was then stopped at different timepoints: 10 min, 30 min and 1 h, with 2 μL of NaOH 1 M solution and the microtubes were frozen at −20°C until application on a 12% SDS-PAGE acrylamide gel for visualization, in which 3 μg of protein were loaded per well.
Results
The 10 individuals included in this study had clinical symptoms of cassava allergy which were confirmed by prick-to-prick test with raw cassava. Six were also allergic to latex, in addition to other fruits (Table 1). For this reason, prick to prick test for latex (glove) and ImmunoCAP specific IgE for latex and its individual allergens were performed. All individuals were female with a median age range of 55 years. Demographic data and skin tests and ImmunoCAP results are summarized in Table 1.
Table 1.
Demographic, clinical features and sensitivities of patients
| Patient | Age | Health Professional | Symptoms after latex exposure | Symptoms after manioc exposure | Episodes of Anaphylaxis | Prick to Prick Raw Cassava | Prick to Prick Rubber Latex | ImmunoCAP |
Other Allergies | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Latex | Hev b 3 | Hev b 5 | Hev b 6 | Hev b 8 | Hev b 11 | |||||||||
| 1 | 72 | No | S, NS, EI | D, C, S, CO, A, N | 3 | + | + | + | - | + | + | - | - | Latex, Papaya, Peach |
| 2 | 55 | Yes | U, A | D, U | 2 | + | - | + | - | + | + | - | + | – |
| 3 | 61 | Yes | C, S, SI | D, S, CO, A | 1 | + | - | - | - | - | - | - | - | – |
| 4 | 55 | Yes | SI, D, C | D, S, CO, U, A | 1 | + | + | + | - | + | - | - | - | Kiwi, Passion Fruit, Pineapple, Avocado, Grape. Eggplant |
| 5 | 31 | Yes | NS | A | 0 | + | - | - | - | - | - | - | - | – |
| 6 | 37 | Yes | NS | U, A | 0 | + | + | + | - | + | - | - | - | Latex |
| 7 | 55 | No | U | D, C, S, CO, U, A, N, DI | 7 | + | + | + | - | + | - | - | - | Latex, Papaya, Peach, Banana |
| 8 | 52 | Yes | D, U, A | D, C, S, CO, U, A | 4 | + | + | + | + | + | + | - | - | Latex, Papaya |
| 9 | 70 | No | NI | D, C, S, CO, U | 1 | + | + | - | - | + | + | - | + | Latex, Papaya, Pineapple |
| 10 | 62 | Yes | NI | D, A, N, AP | ND | + | + | + | + | + | - | - | - | Latex, Papaya, Avocado |
D: dyspnea; C: cough; S: sneeze; CO: coryza; U: urticaria; A: angioedema; N: nausea; AP: abdominal pain; DI: diarrhea; SI: skin itching; NS: no symptons; NO: nasal obstruction; EI: eye itching; NI: no information; Prick to Prick + positive (≥ 3x3 mm) - negative (≤ 3x3); ND: not done; ImmunoCAP + positive (≥ 0,35 kUA/L); Hev b (Hevea brasiliensis)
We were able to extract 1.5 mg of protein from 2 g of freeze-dried cassava using our precipitation protocol. Cassava proteins were evaluated on 1D SDS-PAGE and showed a wide variety of bands ranging from about 8 to 120 kDa (Fig. 1a). Primary investigation of IgE reactivity by 1D immunoblotting using individual sera from allergic patients revealed different IgE sensitization profiles with various bands being recognized mainly from 25 to 110 kDa. For control, sera from non-allergic individuals were included and no bands were visible. Also, a lane with the secondary antibody alone without any serum was run. In this case it was possible to visualize some very faint bands in the 2nd antibody control lane that should not interfere with the results due to their low density (Fig. 1a). From a 2D immunoblotting approach with pooled sera, seven spots between 39 and 110 kDa and between isoelectric points from 4 to 7 were detected, and therefore excised from the gel for identification (Fig. 1b). Of these, all had positive hits in the database search (Table 2) and those with >2 unique peptides and molecular weight corresponding to that found in the 2D gel were selected. All proteomic data for spots identification are shown in the Supplementary Table 1.
Fig. 1.
SDS-PAGE and Immunoblot analysis of cassava extract. (a) Cassava extract (E) on reducing SDS-PAGE. Immunoblot of individual allergic patients' sera,1, 2, 3, 4, 5, 6, 7, 8, 9, 10 second antibody control (2nd) and patients non-allergic (C1: non-atopic and C2: atopic) (b) 2D-SDS-PAGE of cassava protein extract (I) and Western blotting (II) showing the IgE binding of pooled sera1, 2, 3, 4, 5, 6, 7, 8, 9, 10 to cassava protein. pI, isoelectric point; MW, molecular weight, kDa, kiloDalton
Table 2.
Allergenic proteins identified in cassava extract
| Spota | Access Codeb | Identification | Organism | Functional Category | UPc | Experi. Mw/pld | Theo. Mw/ple |
|---|---|---|---|---|---|---|---|
| 1-2 | A0A2C9WB58 | Alpha-1,4 Glucan Phosphorylase | Manihot esculenta | Carbohydrate metabolism | 2-3 | 100/5-6 | 107/5.94 |
| 3-4 | A0A251IQB4 | Peptidase_S9 | Manihot esculenta | Proteolysis | 19-36 | 72/5-6 | 74/5.04 |
| 5 | A0A2C9VPN2 | Cytosol_AP | Manihot esculenta | Proteolysis | 33 | 60/6,5 | 60/6.69 |
| 6 | A0A2C9VH59 | FBA | Manihot esculenta | Glycolysis | 9 | 36/7 | 38/6.91 |
| 6 | A0A2C9VQ42 | GAPDH | Manihot esculenta | Oxidoreductase | 4 | 36/7 | 36/7.73 |
| 7 | A0A2C9UKP7 | ATP Synthase | Manihot esculenta | ATP biosynthetic process | 10 | 55/4-5 | 60/6.26 |
Spot numbers as indicated in Fig. 1b.
Accession numbers according to Uniprot database.
Unique Peptides.
Experimental molecular mass and pI.
Theorical mass kDa and pI of identified proteins retrieved from Expasy.org.
Six proteins were identified in the seven excised spots, including apha-1,4 glucan phosphorylase, peptidase_S9, cytosol_AP domain-containing protein, fructose biphosphate aldolase (FBA), glyceraldehyde 3 phosphate dehydrogenase (GAPDH), and ATP synthase. These proteins are related to carbohydrate metabolism, proteolysis, glycolysis, oxidoreductase, and ATP biosynthetic process. According to our research on Allergome, only ATP synthase and FBA have been reported as allergens, and only FBA is included in the World Health Organization (WHO) and International Union of Immunological Societies (IUIS) Allergen Nomenclature Subcommittee database, while the other proteins identified in the 2D WB are not yet reported as allergenic.
Since FBA has already been described as an allergen and as our patients’ sera recognized the protein in the cassava extract, a recombinant FBA protein (rFBA) was produced and expressed in an Expi293F cell system to determine whether this protein could be specifically recognized by IgE derived from patient sera. To investigate this, an immunoblot was performed using the rFBA and individual patient sera. The rFBA protein was specifically recognized by serum IgE from 8 out of 10 patients (Fig. 2). Sera from patients 5 and 9 did not react with FBA.
Fig. 2.
FBA (fructose-bisphosphate aldolase) recombinant protein (R) on reducing SDS-PAGE. Immunoblot of individual allergic patients' sera (n = 10). MW, molecular weight, kDa, kiloDalton
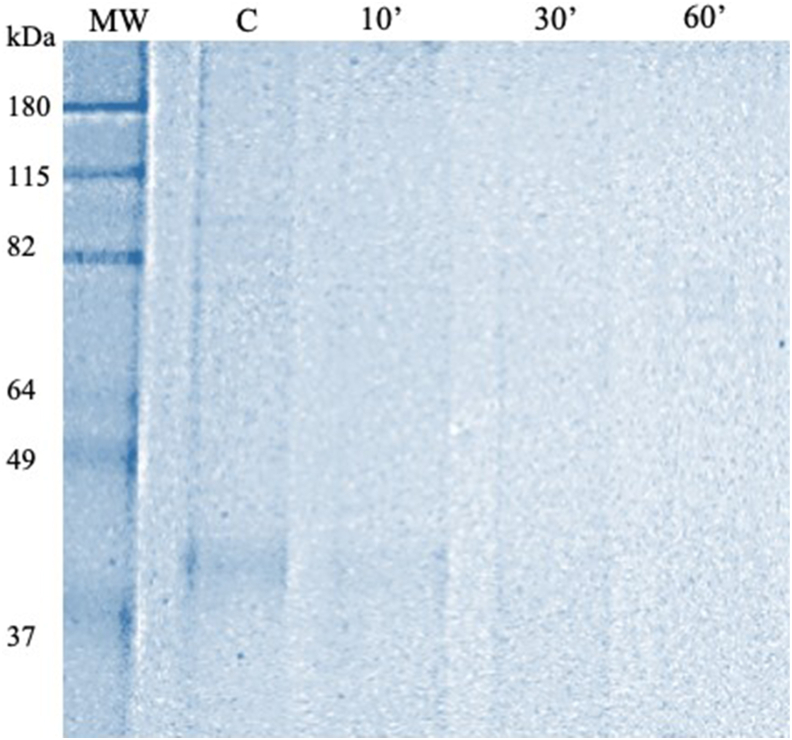
To verify how long rFBA would take to degrade in an environment with a pH similar to that found in the stomach acid. A gastric simulation with the pepsin enzyme was performed. It is possible to observe that after 10 min of incubation, a partial digestion of the rFBA began, at 30 min it was still possible to see some traces of the protein, but after 1 h there was no longer any detection of intact protein (Fig. 3).
Fig. 3.
Gastric simulation with pepsin the protein fructose biphosphate aldolase in SDS gel - PAGE 12 % acrylamide. MM: Molecular marker; C: undigested protein control with pepsin; times: 10 min, 30 min and 60 min - incubation time at 37 °C with pepsin enzyme; kDa: kiloDalton
Discussion
Cassava was elected by the United Nations (UN) as the most relevant food of the 21st century, having been so named it due to its versatility, as it is consumed by people and animals, in addition to being used in the manufacture of various derivatives.18
In order to investigate potential allergens in cassava, we extracted its proteins and performed immunoblotting with sera from ten patients with confirmed cassava allergy. The blots were incubated with an IgE-specific secondary antibody to ensure that only IgE binding was detected. It is possible to identify different IgE recognition patterns among the individuals and the presence of strongly reactive protein bands (Fig. 1a). Some individuals have several IgE-reactive bands, mainly between 30 and 115 kDa, while others have a cleaner profile, that is, with few IgE bindings (Fig. 1a). Mass spectrometry analysis of the excised 2D-SDS-PAGE spots identified two proteins around 36 and 39 kDa as homologous to GAPDH and FBA respectively, corroborating a previous study19 when we also showed IgE reactivity to these molecules in another group of patients. As previously reported the protein band around 30 kDa that appears in all samples, except sample 9 is probably Man e 5 that is shown to not be detected on 2D blot (8).
The closest species to Manihot esculenta, which also belongs to the Euphorbiaceae family, is Hevea brasiliensis (latex), whose proteome includes FBA and GAPDHs proteins.20 In our cohort, 70% (7/10) were health professionals and of these 42.9% (3/7) from them were are also allergic to latex. It is feasible to infer that sensitization to these allergens form cassava may have occurred due to constant contact with powder from latex glove.
We successfully produced and expressed in an Expi293F cell system an rFBA protein, and this protein can be classified as a major allergen in our cohort, considering the criteria for allergen nomenclature, since 8/10 patients recognized the isolated molecule.21 From the 8 individuals who showed IgE reactivity against rFBA protein, 6 are healthcare professionals, showing that FBA can be a cross-reactive allergen. Of note, patient 5 is not allergic to latex and did not react against FBA indicating she is sensitized to another exclusive allergen from manioc, not yet identified, that do not cross react with latex.
FBA is a molecule with native tetrameric conformation, labile and the main routes of exposure described are by inhalation and ingestion.22,23 This protein has been described by the IUIS as an allergen in several sources, being first described in cockroach24 and also found in other sources such as: shrimp22,25 being also related to anaphylaxis after physical activity,26,27 tuna, salmon, and cod.28
Additionally, FBA from wheat flour was identified as IgE-reactive29 in individuals with baker's asthma, one of the most common causes of occupational asthma. However, in our cohort, individuals who presented IgE reactivity to rFBA did not report allergic symptoms to wheat and sensitization to wheat was not tested. Interestingly though, all but one of the rFBA positive individuals have respiratory symptoms. In both cassava and wheat, FBA has a close molecular mass, 36 and 37 kDa respectively,30 with 87% of identity between them considering amino acid sequence (data not shown). As baker's asthma, sensitization occurs in a very specific group of individuals exposed by inhalation, our cohort does not appear to be sensitized by wheat. For cassava, sensitization is more likely have happened by ingestion.
Since FBA proteins from both sources are remarkably similar, differences in allergenicity may be due to cooking process and the presence of different food matrices. It is known that alterations in the molecules such as denaturation, conformational and structural changes due to the loss of secondary and/or tertiary interactions or the formation of new rearrangements of disulfide bonds, can make foods become more or less allergenic.31,32 Studies have shown that food matrix components can not only alter the structure of allergens, but also change the biological processes of allergens sensitization in the human body, thereby affecting their allergenicity. Processing causes physical-chemical interactions between components released from the food matrix and the allergens. These processes can promote the formation of aggregate between allergens and the matrix, limiting the destruction of the allergen epitope, but can also lead to the generation of new allergens.33 Further studies are necessary to elucidate the mechanisms that may be involved in this case.
GAPDH that was also herein identified as IgE-reactive has been already recognized by the IUIS as allergen in other sources such as Pan h 13 in Striped catfish, Per a 13 from the American cockroach and Tri a 34 from wheat. Furthermore, although not considered in the official IUIS nomenclature, it has also been reported as an allergen of latex,20 macadamia nut,34 ribwort plantain35 and also Canabis sativa.36 GAPDH is strongly involved in glycolysis/gluconeogenesis and carbon fixation in photosynthetic plants as part of the pentose phosphate reductive cycle for energy metabolism34
Among the proteins identified in 2D immunoblotting, only ATP synthase (protein responsible for ATP synthesis and known to be involved in the regulating plant cell death)37 has already been described as an allergen in pollen37 and in Cannabis sativa.36 Three proteins not previously identified as IgE-reactive were identified for the first time in this study and are potential novel cassava allergens: Alpha-1,4 Glucan Phosphorylase, an enzyme known to play a role in both starch synthesis and degradation and its association with carbohydrate and energy metabolism, can be found in abundance in green fruits, such as mango and banana, where its presence is related to the ripening process of the fruit;38,39 Peptidase S9, an enzyme involved in proteolysis;40 and finally cytosol_AP, which has a molecular function related to metalloaminopeptidase activity.41
In recent years, the food allergy research focused on the allergenic potential of purified allergens rather than the whole food. The production of recombinant forms has proven to be quite efficient due to their higher purity index when compared to natural extracts, in which protein concentrations are varied.42 The use of recombinant allergens in the clinic can increases the specificity of the skin test by allowing only one specific component to sensitize the skin without the risk of exposing the individual to a set of other sensitization components, thus improving the guidance of the desensitization process. Even so, although their standardization may still be a problem, extracts from natural sources are still the only ones available for allergy diagnosis by skin tests, because. Despite the great advances in the characterization of allergens, its recombinants proteins for in vivo use are still difficult to obtain as they demand rigorous and expensive tests are necessary for their manufacture and commercialization.42,43
Furthermore, our cohort is comprised only of women. It has been described that female sex hormones increase the risk and levels of symptoms in food allergies. In cases of asthma, it has already been proven that estrogen has an aggravating role while testosterone has a protective role.44 In addition, the greater permeability of the female skin and mucosa barrier, combined with a lower metabolic capacity, can be decisive for the onset of symptoms, as more disease-causing foods are absorbed and take longer to leave the body.45,46
Conclusion
Altogether, herein we identified seven IgE-reactive proteins in Manihot esculenta, were described, including two that were previously identified as allergens and included a patient allergic to manioc that do not react to latex indicating the presence of allergens in cassava that are not cross-reacting. We also produced a recombinant allergen that can lead to the development of new diagnostic strategies using the specific molecule, such as more specific in vivo tests and therapies in individuals allergic to Manihot esculenta.
Abbreviations
FBA, Fructose-Biphosphate Aldolase; GAPDH, Glyceraldehyde-3-Phosphate Dehydrogenase; SDS-PAGE, Sodium Dodecyl-Sulfate Polyacrylamide Gel Electrophoresis; IEF, Isoeletric Focusing; pI, Isoeletric Point; 1D, One-Dimensional; 2D, Two-Dimensional; ECL, Enhanced Chemiluminescence; DDA, Data-Dependent Acquisition; FDR, False Discovery Rate; IMAC, Metal Affinity Chromatography; PBS, Phosphate-Buffered Saline; rFBA, Recombinante Protein Fructose-Biphosphate Aldolase; HCL, Hydrochloric Acid; NaOH, Sodium Hydroxide; kDa, Kilodalton; IgE, Immunoglobulin E; WHO, World Health Organization; IUIS, International Union of Immunological Societies.
Funding
This research was funded by Young Research Award of FAPESP (Project 2012/14019-2), Conselho Nacional de Desenvolvimento Científico e Tecnológico (INCT/CNPq-iii grant 465434/2014-2), Ventura, AKRM and Alves, SP were beneficiary of fellowships from CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Finance Code 001).
Availability of data and materials
The data that support the findings of this study, which are captured in the current article, are available from the corresponding author upon reasonable request.
Author contributions
We hereby state that all authors have contributed substantially for this work to be completed, fulfilling the authorship criteria.
Ethics approval
The study was reviewed and approved by an Hospital das Clínicas da Universidade de São Paulo, approval number 10420619.6.0000.0068.
Authors’ consent for publication
All authors have reviewed this manuscript and consent to its publication.
Declaration of competing interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgements
We thank Dr. Ana Moretti for her technological support in protein expression and purification.
Footnotes
Full list of author information is available at the end of the article
Supplementary data to this article can be found online at https://doi.org/10.1016/j.waojou.2023.100845.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.De Martinis M., Sirufo M.M., Suppa M., Ginaldi L. New perspectives in food allergy. Int J Mol Sci. 2020;21(4) doi: 10.3390/ijms21041474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sicherer S.H., Sampson H.A. Food allergy: a review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J Allergy Clin Immunol. 2018;141(1):41–58. doi: 10.1016/j.jaci.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Taylor S.L., Hefle S.L. Food allergen labeling in the USA and Europe. Curr Opin Allergy Clin Immunol. 2006;6(3):186–190. doi: 10.1097/01.all.0000225158.75521.ad. [DOI] [PubMed] [Google Scholar]
- 4.Sheffield J., Taylor N., Fauquet C., Chen S. The cassava (Manihot esculenta Crantz) root proteome: protein identification and differential expression. Proteomics. 2006;6(5):1588–1598. doi: 10.1002/pmic.200500503. [DOI] [PubMed] [Google Scholar]
- 5.Dias, Larissa Tavares e Leonel, Magali Caracterização físico-química de farinhas de mandioca de diferentes localidades do Brasil. Ciência e Agrotecnologia [online] 2006;30(4):692–700. doi: 10.1590/S1413-70542006000400015. Available in: Epub 23 Nov 2007. ISSN 1981-1829. [DOI] [Google Scholar]
- 6.Antolin-Amerigo D., Rodriguez-Rodriguez M., Barbarroja-Escudero J., Postigo Resa I., Uribe-Etxebarría M.C., Alvarez-Mon M. Hypersensitivity to cassava: an allergen-based assessment. J Investig Allergol Clin Immunol. 2012;22(5):385–386. [PubMed] [Google Scholar]
- 7.Li K., Zhu W., Zeng K., et al. Proteome characterization of cassava (Manihot esculenta Crantz) somatic embryos, plantlets and tuberous roots. Proteome Sci. 2010;8:10. doi: 10.1186/1477-5956-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santos K.S., Gadermaier G., Vejvar E., et al. Novel allergens from ancient foods: Man e 5 from manioc (Manihot esculenta Crantz) cross reacts with Hev b 5 from latex. Mol Nutr Food Res. 2013;57(6):1100–1109. doi: 10.1002/mnfr.201200433. [DOI] [PubMed] [Google Scholar]
- 9.Cohen KdO., Oliveira S.S., Chisté R.C. Quantificação de teores de compostos cianogênicos totais em produtos elaborados com raízes de mandioca. 2007. https://ainfo.cnptia.embrapa.br/digital/bitstream/item/19073/1/Doc-290.pdf Available in:
- 10.Zhu F. Composition, structure, physicochemical properties, and modifications of cassava starch. Carbohydr Polym. 2015;122:456–480. doi: 10.1016/j.carbpol.2014.10.063. [DOI] [PubMed] [Google Scholar]
- 11.Effect of Cassava Production on Biodiversity. Food and Agriculture Organization of United Nations (FAO). Available in: http://www.fao.org/3/y2413e/y2413e0c.htm.
- 12.Carpentier S.C., Witters E., Laukens K., Deckers P., Swennen R., Panis B. Preparation of protein extracts from recalcitrant plant tissues: an evaluation of different methods for two-dimensional gel electrophoresis analysis. Proteomics. 2005;5(10):2497–2507. doi: 10.1002/pmic.200401222. [DOI] [PubMed] [Google Scholar]
- 13.Kruger N.J. The Bradford method for protein quantitation. Methods Mol Biol. 1994;32:9–15. doi: 10.1385/0-89603-268-X:9. [DOI] [PubMed] [Google Scholar]
- 14.Candiano G., Bruschi M., Musante L., et al. Blue silver: a very sensitive colloidal Coomassie G-250 staining for proteome analysis. Electrophoresis. 2004;25(9):1327–1333. doi: 10.1002/elps.200305844. [DOI] [PubMed] [Google Scholar]
- 15.Shevchenko Mw An, Vorm 0, Jensen 0. N., et al. 1996. A strategy for identifying gel-separated proteins in sequence databases by MS alone. [DOI] [PubMed] [Google Scholar]
- 16.Dias Ê., de Oliveira L.A., Lauria P.S.S., et al. Bothrops leucurus snake venom protein profile, isolation and biological characterization of its major toxin PLA. Toxicon. 2022;213:27–42. doi: 10.1016/j.toxicon.2022.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Carvalho P.C., Lima D.B., Leprevost F.V., et al. Integrated analysis of shotgun proteomic data with PatternLab for proteomics 4.0. Nat Protoc. 2016;11(1):102–117. doi: 10.1038/nprot.2015.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.ONU. Onu Declara a mandioca como o alimento do séc. XXI. https://cantinho.live/2020/02/05/onu-declara-a-mandioca-como-o-alimento-do-seculo-xxi/ Avaliable: Acess Sep 29, 2022.
- 19.Santos K.S., Galvao C.E., Gadermaier G., et al. Allergic reactions to manioc (Manihot esculenta Crantz): identification of novel allergens with potential involvement in latex-fruit syndrome. J Allergy Clin Immunol. 2011;128(6):1367–1369. doi: 10.1016/j.jaci.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 20.D'Amato A., Bachi A., Fasoli E., et al. In-depth exploration of Hevea brasiliensis latex proteome and "hidden allergens" via combinatorial peptide ligand libraries. J Proteonomics. 2010;73(7):1368–1380. doi: 10.1016/j.jprot.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Caraballo L., Valenta R., Acevedo N., Zakzuk J. Are the terms major and minor allergens useful for precision allergology? Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.651500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamath S.D., Rahman A.M., Voskamp A., et al. Effect of heat processing on antibody reactivity to allergen variants and fragments of black tiger prawn: a comprehensive allergenomic approach. Mol Nutr Food Res. 2014;58(5):1144–1155. doi: 10.1002/mnfr.201300584. [DOI] [PubMed] [Google Scholar]
- 23.Lv G.Y., Guo X.G., Xie L.P., et al. Molecular characterization, gene evolution, and expression analysis of the fructose-1, 6-bisphosphate aldolase (FBA) gene family in wheat. Front Plant Sci. 2017;8:1030. doi: 10.3389/fpls.2017.01030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chuang J.G., Su S.N., Chiang B.L., Lee H.J., Chow L.P. Proteome mining for novel IgE-binding proteins from the German cockroach (Blattella germanica) and allergen profiling of patients. Proteomics. 2010;10(21):3854–3867. doi: 10.1002/pmic.201000348. [DOI] [PubMed] [Google Scholar]
- 25.Gamboa P.M., Bartolomé B., García Lirio E., Cuesta-Herranz J., Pastor-Vargas C. Aldolase: a new Crustacea allergen. Ann Allergy Asthma Immunol. 2018;121(2):246–247. doi: 10.1016/j.anai.2018.04.025. [DOI] [PubMed] [Google Scholar]
- 26.Akimoto S., Yokooji T., Ogino R., et al. Identification of allergens for food-dependent exercise-induced anaphylaxis to shrimp. Sci Rep. 2021;11(1):5400. doi: 10.1038/s41598-021-84752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tonomura K., Fujimoto R., Okuda Y., et al. [A case of food-dependent exercISe-induced anaphylaxIS by shrimp: fructose 1, 6- BISphosphate aldolase IS supposed as causative component despite negative allergen-specific ige test (immunocap. Arerugi. 2019;68(1):48–53. doi: 10.15036/arerugi.68.48. [DOI] [PubMed] [Google Scholar]
- 28.Kuehn A., Hilger C., Lehners-Weber C., et al. Identification of enolases and aldolases as important fish allergens in cod, salmon and tuna: component resolved diagnosis using parvalbumin and the new allergens. Clin Exp Allergy. 2013;43(7):811–822. doi: 10.1111/cea.12117. [DOI] [PubMed] [Google Scholar]
- 29.Weiss W., Huber G., Engel K.H., et al. Identification and characterization of wheat grain albumin/globulin allergens. Electrophoresis. 1997;18(5):826–833. doi: 10.1002/elps.1150180529. [DOI] [PubMed] [Google Scholar]
- 30.Baur X., Posch A. Characterized allergens causing bakers' asthma. Allergy. 1998;53(6):562–566. doi: 10.1111/j.1398-9995.1998.tb03931.x. [DOI] [PubMed] [Google Scholar]
- 31.Davis P.J., Williams S.C. Protein modification by thermal processing. Allergy. 1998;53(46 Suppl):102–105. doi: 10.1111/j.1398-9995.1998.tb04975.x. [DOI] [PubMed] [Google Scholar]
- 32.Zailatul Hani Mohamad Yadzir R.M., Bakhtiar Faizal, Abdullah Noormalin, Murad Shahnaz. BioMed Research International; 2015. Tropomyosin and Actin Identified as Major Allergens of the Carpet Clam (Paphia Textile) and the Effect of Cooking on Their Allergenicity; p. 6. Article ID 254152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Q. In: Trends in Food Science & Technology. Lin S., Sun N., editors. 2022. How does food matrix components affect food allergies, food allergens and the detection of food allergens? A systematic review; pp. 280–290. [Google Scholar]
- 34.Rost J., Muralidharan S., Lee N.A. A label-free shotgun proteomics analysis of macadamia nut. Food Res Int. 2020;129 doi: 10.1016/j.foodres.2019.108838. [DOI] [PubMed] [Google Scholar]
- 35.Gadermaier G., Eichhorn S., Vejvar E., et al. Plantago lanceolata: an important trigger of summer pollinosis with limited IgE cross-reactivity. J Allergy Clin Immunol. 2014;134(2):472–475. doi: 10.1016/j.jaci.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 36.Nayak A.P., Green B.J., Sussman G., et al. Characterization of Cannabis sativa allergens. Ann Allergy Asthma Immunol. 2013;111(1):32–37. doi: 10.1016/j.anai.2013.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huerta-Ocampo J., Valenzuela-Corral A., Robles-Burgueño M.D.R., et al. Proteomic identification of allergenic proteins in red oak. World Allergy Organ J. 2020;13(3) doi: 10.1016/j.waojou.2020.100111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chin C.F., Teoh E.Y., Chee M.J.Y., Al-Obaidi J.R., Rahmad N., Lawson T. Comparative proteomic analysis on fruit ripening processes in two varieties of tropical mango (mangifera indica) Protein J. 2019;38(6):704–715. doi: 10.1007/s10930-019-09868-x. [DOI] [PubMed] [Google Scholar]
- 39.Mainardi J.A., Purgatto E., Vieira A., et al. Effects of ethylene and 1-methylcyclopropene (1-MCP) on gene expression and activity profile of alpha-1,4-glucan-phosphorylase during banana ripening. J Agric Food Chem. 2006;54(19):7294–7299. doi: 10.1021/jf061180k. [DOI] [PubMed] [Google Scholar]
- 40.UniProt - UniProtKB Protein knowledgebase https://www.uniprot.org/uniprotkb/A0A251IQB4/entry - Avaliable in:
- 41.UniProt - UniProtKB Protein knowledgebase https://www.uniprot.org/uniprotkb/A0A2C9VPN2/entry - Avaliable in:
- 42.Niederberger V., Eckl-Dorna J., Pauli G. Recombinant allergen-based provocation testing. Methods. 2014;66(1):96–105. doi: 10.1016/j.ymeth.2013.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Valenta R., Karaulov A., Niederberger V., et al. Allergen extracts for in vivo diagnosis and treatment of allergy: is there a future? J Allergy Clin Immunol Pract. 2018;6(6):1845. doi: 10.1016/j.jaip.2018.08.032. 55.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koper I., Hufnagl K., Ehmann R. Gender aspects and influence of hormones on bronchial asthma - secondary publication and update. World Allergy Organ J. 2017;10(1):46. doi: 10.1186/s40413-017-0177-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Afify S.M., Pali-Schöll I. Adverse reactions to food: the female dominance - a secondary publication and update. World Allergy Organ J. 2017;10(1):43. doi: 10.1186/s40413-017-0174-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jensen-Jarolim E. Gender effects in allergology - secondary publications and update. World Allergy Organ J. 2017;10(1):47. doi: 10.1186/s40413-017-0178-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study, which are captured in the current article, are available from the corresponding author upon reasonable request.