Abstract
The prevention and treatment of fear-related disorders in offspring affected by pregnancy stress remains challenging at clinic. Here, we examined the effects of gut microbiota of stressed pregnant rats on the fear extinction of their offsprings, and the potential mechanisms. We found that gut microbiota transplantation from rats with pregnancy stress to normal pregnant rats impaired fear extinction, induced microglial activation and synaptic phagocytosis, increased synapse loss in offsprings. Probiotics supplement during pregnancy stress partly normalized pregnancy stress-induced gut microbiota dysbiosis of pregnant rats, and promoted fear memory extinction, inhibited fear memory reappearance, and limited microglial activation and synaptic phagocytosis in offsprings. These data revealed that gut microbiota of stressed pregnant mother improved the development of fear-related disorders of offspring, which may be associated with microglial synaptic pruning.
Keywords: Pregnancy stress, Probiotics, Gut microbiota, Microglia, Fear extinction
1. Introduction
Pregnancy is a critical and sensitive period for shaping offspring and a deleterious prenatal environment (e.g., psychological stress, sudden major life events, natural disasters) can adversely affect the growth and development in utero (Glynn et al., 2018; Han et al., 2021; Jones et al., 2014). Studies have shown that 8–12% of pregnant person meet the clinical diagnostic criteria for anxiety or mental disorders during pregnancy; and a large-scale community study using standardized self-report scales shows that approximately 30% of pregnant person experience pregnancy stress (Van den Bergh et al., 2020; Howard et al., 2014). Pregnancy stress inevitably causes abnormal brain function and behavior in offspring (Vuong et al., 2020), while fear-related disorders exist in an offspring whose mother experiences pregnancy stress (Lee et al., 2011; Bingham et al., 2013; Salm et al., 2015). Patients exposed to a severe traumatic event can re-live the traumatic event for several weeks afterward, and the fear can easily reappear even after successful extinction (First et al., 2021). Previous studies have focused on the effects of pregnancy stress on offspring and its possible mechanism, and have noted that glucocorticoids and inflammatory factors are important for mediating alterations in offspring (Vuong et al., 2020; Moisiadis and Matthews, 2014; Gumusoglu et al., 2017). However, it is very difficult to target the reported mechanisms in treating and preventing the effects of prenatal stress on offsprings. The prevention and treatment of neurological and psychiatric disorders in offspring affected by pregnancy stress remain challenging clinically because of poor compliance and less effectiveness of pharmacologic interventions (YI et al., 2018). There is a tremendous need for more studies focused on treatment of pregnancy stress.
Prenatal stress disrupts the gut microbiome, according to measurements of both α- and β-diversity (Chen et al., 2022; Dawson et al., 2021; Gao et al., 2021). Zijlmans et al. used a phylogenetic microarray to determine that prenatal stress was strongly and persistently associated with fetal microbiota composition (Zijlmans et al., 2015). In the study, especially the gut microbiota of infants of highly stressed mothers was colonized with pathogenic proteobacterial groups (Zijlmans et al., 2015). Microbiome changes affected by prenatal stress were manifested in the mother, and also found in female offspring in adulthood (Gur et al., 2017). Another study showed that pregnancy stress experience may impact offspring development by altering the temporal and spatial dynamics of the microbiome in mothers during pregnancy (Jašarević et al., 2017). The above studies indicate (Gur et al., 2017; Jašarević et al., 2017; Pronovost and Hsiao, 2019) that intestinal microbiota changes in mothers induced by pregnancy stress may significantly affect alterations in offspring.
The alterations in the gut microbiota of mothers influence the development of microglia, the primary resident immune cells, in the offspring's neuroimmune system (Vuong et al., 2020; Hayes et al., 2022). Microglia are the predominant immune response cells and professional phagocytes of the central nervous system and play vital physiological roles in regulating normal brain development and function (Tay et al., 2018). Microglia are essential for fear memory (Wang et al., 2020a) and are likely to be potential targets for treating impaired fear memory extinction (Cui et al., 2021). Previous reports proved that probiotics administration before or during the formation of fear memory promoted the formation (Bifidobacterium longum 1714 and Bifidobacterium breve 1205) or the retention (Lactobacillus rhamnosus) of fear memory (Bravo et al., 2011; Savignac et al., 2015). In our previous study, we found that probiotics (Bifidobacterium longum, Lactobacillus acidophilus, and Enterococcus faecalis) treatment after fear conditioning for 27 days promoted fear extinction in mice, and microbiome changes affected by prenatal stress were manifested in the mother (Cui et al., 2021). We also found that the specific probiotics worked well for antidiarrheal of children. Thus we used the probiotics in the study (Cui et al., 2021). The aim of our study was to investigate whether the effect of probiotics could positively impact the fear memory extinction in offspring caused by pregnancy stress.
Fear extinction is achieved by repeated exposure to fear-eliciting stimuli in the absence of any adverse events during extinction training (Colori, 2018; Buchholz and Abramowitz, 2020). This method is believed to represent a new learning process that causes the extinction of memory formation, for which synaptic plasticity of the hippocampus is regarded essential (Orsini and Maren, 2012; Kalisch et al., 2019). In addition, microglia are vital for synaptic pruning (Wang et al., 2023). In the study, we examined that the effects of probiotics treatment during pregnancy stress on the fear extinction and hippocampal synapse plasticity of offspring in rats. We found that dysbiosis of gut microbiota caused by stress during pregnancy could lead to microglial activation, synapse loss, and stronger synaptic phagocytosis of microglial cells in the hippocampal DG during fear memory extinction in offspring. Probiotics supplementation during pregnancy stress was shown to inhibit these changes. These findings suggest a simple, safe intervention may be applicable as an auxiliary strategy to tackle fear-related disorders in offspring affected by pregnancy stress.
2. Materials and methods
2.1. Animals
Adult female pregnant Sprague-Dawley rats (8 weeks, 250–300 g) were used in this study. They were previously nulliparous, and this was consistent across groups. Rats were purchased from the Laboratory Animal Center (Central South University, Changsha, China). The rats were housed under standard conditions (humidity 50 ± 5%, temperature 22 ± 1 °C) with a 12h light/dark cycle (lights on at 07:00 and off at 19:00) with ad libitum access to food and water. All animals were given one week to acclimate to the colony conditions before the experiments began. The study protocols were approved by the Animal Ethics Committee of Basic Medical School of Central South University (approval number: 2021–XMSB–0052, date of approval: 7 March 2021), and all animal experiments were carried out in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health, Bethesda, MD, USA).
2.2. Experimental procedures
Pregnant rats were paired housed and were randomly assigned to a control, gestational stress, gut microbiome transplantation, sham, and pregnancy stress + probiotics groups. Prenatal stress protocol used what we have previously reported (Liao et al., 2019). Restraint stress was applied from gestational days 16–20, whereby the pregnant female rats were placed into transparent plastic cylinders (length = 21.6 cm; diameter = 8.6 cm) and exposed to three 45-min stress sessions per day (09:00, 13:00, and 17:00). For gut microbiome transplantation, the dams experienced restraint stress on their gestational days 16–20 in advance, and the gut microbiomes for transplantion were obtained from these dams on the 21st gestation day. The fresh cecal contents of stressed pregnant rats could be diluted in the same volume of sterilized phosphate-buffered saline (PBS) to get an estimated suspension solution. The suspension solution was transplanted into normal pregnant rats on their 16th gestation day. A plastic tube (diameter 5.33 mm, length 30 cm) was applied paraffin oil onto its surface. The tube was gently inserted into the cecum through the anus of the normal pregnant rats up to 25 cm from the top of the tube to the anus, and the cecal bacteria solution (2 ml) was slowly injected (Zhou et al., 2019). In addition, the dams of sham group underwent 2 ml of PBS transplantation on the 16th day of gestation. For the treatment paradigm, the probiotics capsules were dissolved in drinking water (≥14 × 105 cfu/ml) and given to rats subjected to restraint stress from gestational days 16–20. After birth, the pups were raised with their mothers and weaned on postnatal day (PND) 21, group-housed with littermates of the same sex. To eliminate the effects of sex, only male offspring were included in our study. C group: The dams of control group were housed normally; S group: The dams were subjected to restraint stress from gestational days 16–20; ST group: Gut microbiomes of stressed dams were transplanted into the normal pregnant rats on the 16th gestation day; SH group: The dams of sham group underwent 2 ml of PBS transplantation on the 16th day of gestation; SP group: Probiotics were given to the stressed dams from gestational days 16–20.
2.3. Auditory fear memory
The experimental behavioral assessment was conducted during offspring adulthood from postnatal day 61–103. For auditory fear conditioning, day 0 consisted of fear acquisition in Context A, which consisted of a shock chamber ((76 × 86 × 79.5) cm, Huaibei Zhenghua, China)) composed of translucent perspex walls with purple patterns, a floor made of 23 stainless steel rods that delivered the shock, and a 70% ethanol scent. Animals were able to freely explore the context freely for 3 min for three consecutive days and 5 min on the day before the auditory fear conditioning to familiarize themselves with the environment and setup. After a 2 min habituation, the rats received five stress trials consisting of a 20-s tone (2800 Hz, 80 dB), serving as the conditioned stimulus (CS), paired with foot shock stress (0.8 mA), serving as the unconditioned stimulus (US), during the last 2 s of the tone (CS and US co-terminated). Each trial was separated by a 90-s inter-trial interval (ITI). After the final CS-US pairing, all rats remained in the conditioning environment for 1 min before being transported back to their home cages. 70% ethanol was used to clean the chambers between groups.
Fear memory extinction training was performed each day between the 27th and 31st days after fear conditioning. Context B consisted of a different operant conditioning chamber from the one used in Context A and was composed of black opaque Perspex walls, a flat plastic floor, and a 0.01% acetic acid scent. After 120 s of habituation, the rats received five presentations of the CS (tone) only (i.e., with no US). Each tone lasted for 20 s and, again, was separated by a 90-s ITI. On the 38th day after fear conditioning, the long-term fear extinction memory was measured in Context B with five trials made up of the CS without the US, again with 90-s ITIs. As freezing is a rodent response to threats, freezing behavior was defined as the absence of any movement except breathing and was scored if there was no movement for at least 1 s through video recorded during each session (Bouchet et al., 2017). Observers were blind to the rats of the different groups. The percentage of time (%time) freezing per epoch was equal to the freezing time for each epoch divided by the total time of each epoch.
2.4. Reagents and antibodies
The probiotics capsule (BIFICO, Sine Pharmaceuticals, Shanghai, China) contained Bifidobacterium longum (≥1.0 × 107 cfu/g), Lactobacillus acidophilus (≥1.0 × 107 cfu/g), and Enterococcus faecalis (≥1.0 × 107 cfu/g). Primary antibody: Iba1 (cat: 019–19741, Wako Chemical, 1:1000); PSD95 (cat: MAB1596, Millipore, 1:100); Synaptophysin (cat: ab16659, Abcam, 1:100). Secondary antibody: cat: 111-545-144, cat: 115-025-166, Jackson immunoresearch, 1:200.
2.5. Brain tissue collection
Rats were deeply anesthetized with inhaled sevoflurane and were perfused transcardially with 0.9% sodium chloride solution, followed by 4% paraformaldehyde (PFA) in PBS. The brains were quickly removed from the skull on an ice-chilled plate and immediately transferred into an ice-cold tube pre-filled with 4% PFA for fixation. Tubes were stored at 4 °C, and after 24 h, the solution was changed to 15% (24 h) and 30% (48–72 h) sucrose-containing PBS buffer sequentially for cryopreservation until the brains sank to the bottom of the tube. The brains were then removed from the 30% sucrose solution and divided into two hemispheres using a scalpel. After the surface water of the sample was blotted by filter paper, the brains were transferred to a disposable mold of tin foil made in advance, carefully embedded at optimal cutting temperature (OCT) to avoid the formation of bubbles and snap-frozen in a box containing liquid nitrogen. The fresh frozen brains were sectioned serially into 20 μm thick coronal slices using a freezing sliding microtome (Leica CM1950, Wetzlar, Germany). The sections we used for immunofluorescent staining were between bregma −3.30 mm and −3.14 mm. We utilized three slices per animal.
2.6. Immunofluorescent staining
The immunofluorescence staining process followed the routine protocol. First, brain slices were transferred to 0.01 M PBS and were washed for 10 min three times. Next, the slices were blocked for 1 h at room temperature (RT) with goat serum (10%) in PBS and were then incubated with the primary antibody at 4 °C overnight. The following day, after three 10-min washes with PBS, the samples were incubated with the appropriate secondary antibody for 2 h at RT in the dark. The samples were then washed three times with PBS and were cover-slipped with Vectamount mounting medium with DAPI (Vector labs H-1000). The slides were then viewed using an LSM800 confocal microscope and Zen 2009 image acquisition software (Carl Zeiss, Jane, Germany). The 3D images of Iba1, the Synaptophysin and PSD95 and PSD95 and Iba1 were overlaid by ten images (1 μm per image) (Hong et al., 2016).
The DG and basolateral amygdala (BLA) were outlined according to the DAPI staining of brain slices. Based on the Iba1 staining, the positive area of Iba1 was calculated by a blinded experimenter using ImageJ software (Cui et al., 2021)(National Institutes of Health, Bethesda, USA). Iba1+ cell numbers in the DG and the BLA was also counted using the reported method (Zheng et al., 2021). Our classification of microglial morphological phenotypes and individual class percentage of microglia were determined using their reported method (Reddaway et al., 2023; Leyh et al., 2021). In manual classification, a trained “scorer” classed each cell as being either ramified, rod-like, reactive or amoeboid whilst being blinded to the experimental group/animal they were from (Reddaway et al., 2023).The number of colocalized spots and the positive area of Synaptophysin and PSD95 within 50 μm* 50 μm area around the midline of granular cell layer of dentate gyrus were quantitatively analyzed. When microglia and PSD95 were colocalized, the 3D images of Iba-1 and PSD95 were overlaid by ten images (1um per image). The number of colocalized spots and the positive area of PSD95 and Iba-1 in granular cell layer of dentate gyrus were quantitatively analyzed. The 3D images were analyzed by ZEN to determine the number of colocalized spots. The number of colocalized spots was then divided by total area of the image stack (Wang et al., 2020a; Hong et al., 2016).
2.7. Cecal matter collection and microbial analysis
One part of dams were utilized for microbial analysis and fetal brain immunofluorescence, and the other part of dams raised their pups normally. The dams were euthanized by CO2 asphyxiation on the 21st day of pregnancy. Samples of cecal matter were quickly collected, instantly frozen in liquid nitrogen, and stored at −80 °C until 16S microbiome analysis. The total microbial genomic DNA was extracted from the cecal content samples using the PF Mag-Bind Stool DNA Kit (Omega Bio-tek, Georgia, U.S.) per the manufacturer's instructions. The quality of extracted DNA was determined by 1% agarose gel electrophoresis, and the concentration and purity of DNA were using a NanoDrop® ND-2000 spectrophotometer (Thermo Scientific, USA). The V3∼V4 hypervariable region sequence of the bacterial 16S rRNA gene was the target area, and 338F-806R with barcode sequence was amplified as the primer to obtain polymerase chain reaction (PCR) products (468bp) by an ABI GeneAmp® 9700 PCR thermocycler (ABI, CA, USA). Forward primer 338F: ACTCCTACGGGAGGCAGCAGCAG; Reverse primer 806R: GGACTACHVGGGTWTCTAAT. Purified amplicons were pooled in equimolar amounts and paired-end sequenced on an Illumina PE300 platform (Illumina, San Diego, USA) using the standard protocols of Majorbio Bio-Pharm Technology Co. Ltd. (Shanghai, China). Raw FASTQ files were de-multiplexed using an in-house Perl script, quality-filtered by fastp (version 0.19.6), and merged by FLASH (version 1.2.11). Next, the optimized sequences were clustered into operational taxonomic units (OTUs) using UPARSE 11 at 97% similarity. The OTU species taxonomic annotation was performed against the Silva 16S rRNA gene database (v138) and RDP classifier (http://rdp.cme.msu.edu/, version 2.13) with a confidence threshold of 70%, and the diversity of gut microbiota in each sample was determined. PICRUSt2 (version 2.2.0) software was used for 16S functional predictive analysis.
2.8. Statistical analysis
Data analyses were performed with GraphPad Prism 8. All data are expressed as the mean ± standard error of the mean (SEM). For comparison among three or more groups, a one-way ANOVA was used to analyze the data from the long-term memory of extinction and immunostaining. Two-way ANOVA was used to analyze the conditioning and extinction training data. A significance level was set at a p-value of <0.05. Bioinformatic gut microbiota analysis was carried out using the Majorbio Cloud platform (https://cloud.majorbio.com). A sample α-diversity was analyzed based on the OTUs information, while the similarity among the microbial communities in different samples was determined by principal coordinate analysis (PCoA) based on the Bray-Curtis dissimilarity using the R (version 3.3.1) package. A Mann-Whitney U test and a Kruskal-Wallis rank-sum test were used to compare the gut microbial communities.
3. Results
3.1. Dysbiosis of gut microbiota caused by pregnancy stress impaired fear extinction in the rats’ offspring
Rats were trained in auditory fear conditioning paradigm to evaluate whether dysbiosis of gut microbiota caused by pregnancy stress impairs fear extinction in rats' offspring. After the training in an auditory fear conditioning paradigm, all rat groups acquired equivalent conditioned auditory fear memory (Fig. 1b). The freezing levels of rats' offspring were examined from the 27th to the 31st days after fear conditioning. As illustrated, all groups of rats were reactivated with the equivalent conditioned fear memory they acquired before (Fig. 1b). In the fear memory extinction training, the freezing levels of the groups showed significant inter-group differences (F(72,683) = 2.406, p < 0.0001, two-way repeated measures). Analysis showed that the freezing levels of the pregnancy stress group (S group) were significantly higher than those of the control group (C group) (p < 0.0001), while the gut microbiome transplantation (ST group) significantly increased the freezing levels compared with the sham group (SH group) (p < 0.0001). For long-term fear extinction memory, similar trends were observed in all groups of rats with significant differences between groups (F(3,28) = 86.90, p < 0.0001, one-way ANOVA). The freezing levels of the S group and ST group remained significantly higher than the C group and SH group (C vs. S: p < 0.0001; SH vs. ST: p < 0.001) (Fig. 1b). No significant difference of freezing levels was detected between C and SH groups and between S and ST groups. Taken together, these preliminary results supported the evidence that dysbiosis of gut microbiota caused by pregnancy stress impaired short-term and long-term fear extinction in the rats’ offspring.
Fig. 1.
Dysbiosis of gut microbiota caused by pregnancy stress impaired fear extinction in rats' offspring. (a) The schedule of pregnancy restraint stress, gut microbiome transplantation, fear conditioning, and behavior test. Pregnancy restraint stress: from gestational day 16–20, three stress sessions were performed daily (45 min per session, starting at 09:00, 13:00, and 17:00), whereby the rats were placed into transparent plastic cylinders (length = 21.6 cm; diameter = 8.6 cm). Gut microbiome transplantation: transplanting gut microbiome from gestational restraint-stressed rats to the control pregnant rats. Fear conditioning: five pairings of 20-s, 2800 Hz, 80 dB auditory conditioned stimulus (CS) co-terminating with 2-s, 0.8 mA foot shock unconditioned stimulus (US). Fear extinction: five CS presentations (90-sec inter-trial interval) for five days in a novel extinction context without the US. Long-term memory: five CS presentations (90-sec inter-trial interval) for one day in the same context of extinction without the US. GD: gestational day. P: postnatal day. (b) The freezing levels of adult offspring of rats in fear conditioning, fear extinction, and long-term memory test. &: p < 0.0001 for C vs. S in fear extinction (two-way repeated measures ANOVA with post hoc analysis). #: p < 0.0001 for ST vs. SH in fear extinction (two-way repeated measures ANOVA with post hoc analysis). C, control; S, pregnancy restraint stress; SH, sham; ST, gut microbiome transplantation; ****p < 0.0001, N = 8/group. All data were presented as means ± SEM.
In addition, we detected the gut microbiota of rats in each group on the 21st day of pregnancy, and compared their difference in gut microbiota (N = 5/group). Pregnancy stress significantly increased α-diversity (Chao index of OUT level, C vs. S: p = 0.008639, SH vs. ST: p = 0.004911, Student's t-test) and there were significant differences among the groups in the β-diversity of pregnant rats (PCoA on OTU level, Adonis: R2 = 0.2250, p = 0.0270) (Fig. 2a). We found that pregnancy stress did not make significant difference on gut microbiota composition on Phylum level (Fig. 2b). For the Genus level, the relative abundance of unclassified_f__Lachnospiraceae (p = 0.01219, Treponema (p = 0.02001), Intestinimonas (p = 0.02157), Lachnospiraceae_UCG-001 (p = 0.01219) and unclassified_k__norank_d__Bacteria (p = 0.01219) in the S group were significantly higher than that in the C group. In parallel, compared with the SH group, the relative abundance of Treponema (p = 0.03445), Oscillibacter (p = 0.02118), and unclassified_k__norank_d__Bacteria (p = 0.03671) were increased in ST group. Furthermore, there was no significant difference between C and SH groups and between S and ST groups in the relative abundance of unclassified_f__Lachnospiraceae (C vs. SH: P = 1; S vs. ST: P = 0.8345), Treponema (C vs. SH: P = 1; S vs. ST: P = 0.5309), Intestinimonas (C vs. SH: P = 1; S vs. ST: P = 0.09369), Oscillibacter (C vs. SH: P = 0.3886; S vs. ST: P = 0.2963), Lachnospiraceae_UCG-001 (C vs. SH: P = 1; S vs. ST: P = 0.09469), unclassified_k__norank_d__Bacteria (C vs. SH: P = 0.8315; S vs. ST: P = 0.07491) (Fig. 2c). These data indicated that transplanting the gut microbiome from gestational restraint-stressed rats to the normal pregnant rats could reproduce the dysbiosis of gut microbiota caused by pregnancy stress in mother rats.
Fig. 2.
Transplanting gut microbiome from gestational restraint-stressed rats to the normal pregnant rats could reproduce the dysbiosis of gut microbiota caused by pregnancy stress in mother rats. (a) α-diversity indicated that pregnancy stress increased gut microbial diversity (Chao index of OUT level, C vs. S: p = 0.008639, SH vs. ST: p = 0.004911, Student's t-test). β-diversity (PCoA on OTU level, Adonis: R2 = 0.2250, P = 0.0270) demonstrated significant differences in bacterial communities among the four groups. (b) Pregnancy stress did not make any significant difference on Phylum level (C vs. S: p > 0.05, SH vs. ST: p > 0.05, Mann-Whitney U test). (c) The relative abundance of unclassified_f__Lachnospiraceae, Treponema, Intestinimonas, Oscillibacter, Lachnospiraceae_UCG-001 and unclassified_k__norank_d__Bacteria on the Genus level showed a significant difference among groups (Kruskal-Wallis rank-sum test). N = 5/group, *p < 0.05, **p < 0.01. Data were represented as means ± SEM.
3.2. Dysbiosis of gut microbiota caused by pregnancy stress affected rats’ microglia development
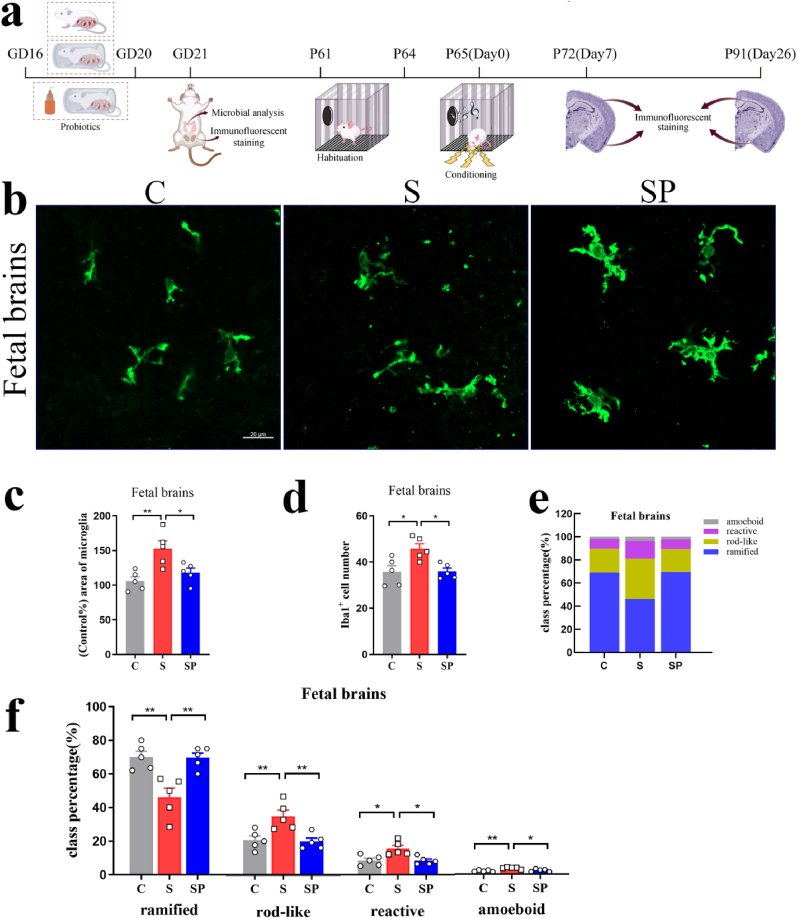
Microglial cells, which are the primary resident immune cells in the central nervous system, play a pivotal role in fear memory extinction (Ten-Blanco et al., 2022; Nguyen et al., 2020). The level of microglia activation in the brain of offspring was evaluated by microglia positive-area percentage, Iba1+ cell numbers, and class percentage of ramified, rod-like, reactive, and amoeboid microglia. Immunostaining was performed using an antibody of Iba1, a common microglial biomarker. In the rats' fetal brains, inter-group differences were observed in the positive area percentage of microglial cells (F(3,16) = 12.13, p = 0.0002, one-way ANOVA). Positive area percentage of microglial cells in the S and ST groups increased significantly in the fetal brains (C vs. S: p = 0.0069; SH vs. ST: p = 0.0014) (Fig. 3b and c). Iba1+ cell numbers in the fetal brains showed a statistical difference between groups (F(3,16) = 8.981, p = 0.0010, one-way ANOVA). Pregnancy stress increased Iba1+ cell numbers in the fetal brains (C vs. S: p = 0.0103), while the transplanted gut microbiome was able to roughly reproduce the effects in offspring microglia (SH vs. ST: p = 0.0104) (Fig. 3d). The microglia of the S and ST groups showed larger somas, spindle-shaped, rod-shaped, and incomplete branches compared with the C and SH groups. Microglia morphological phenotypes were classified by morphological assessment after staining with Iba1. Individual class percentages in the S and ST groups revealed significant increases of amoeboid, reactive, and rod-like microglial cells and a simultaneous decrease of ramified microglia in the fetal brains compared to C and SH groups (amoeboid: C vs. S: p = 0.0122; SH vs. ST: p = 0.0232; reactive: C vs. S: p = 0.0073; SH vs. ST: p = 0.0113; rod-like: C vs. S: p = 0.0046; SH vs. ST: p = 0.0073; ramified: C vs. S: p = 0.0011; SH vs. ST: p = 0.0032) (Fig. 3e and f). The level of microglia activation in the rats’ fetal brains did not differ between C and SH groups and between S and ST groups.
Fig. 3.
Dysbiosis of gut microbiota caused by pregnancy stress affected rats' microglia development. (a) Schematic diagram of the experimental procedure. (b) Confocal images of offspring Iba1(green) staining of C, S, SH, and ST groups on the 21st gestational day. Scale bar = 20 μm. (c) Positive-area percentage of microglial cells in the fetal brains of C, S, SH, and ST groups (d) Iba1+ cell numbers in the rats fetal brains of C, S, SH, and ST groups. (e) Class percentage of ramified, rod-like, reactive, and amoeboid microglia in the rats' fetal brains of C, S, SH, and ST groups. (f) Individual class percentage of the four microglial morphological phenotype classes in the rats' fetal brains of C, S, SH, and ST groups. N = 5 rats in each group. One-way ANOVA, *p < 0.05, **p < 0.01. The data were represented as means ± SEM. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
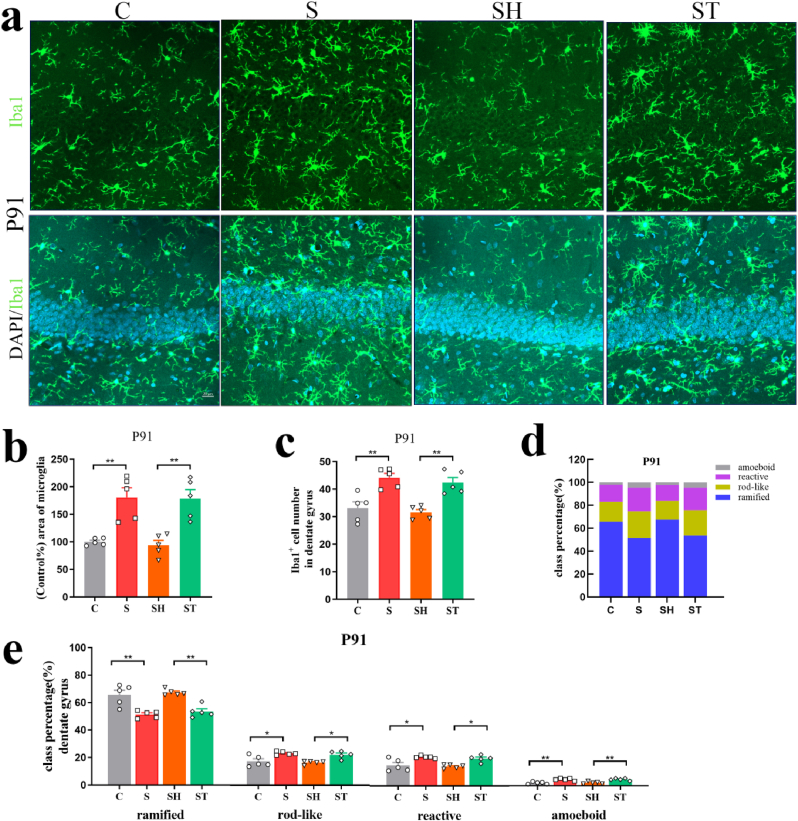
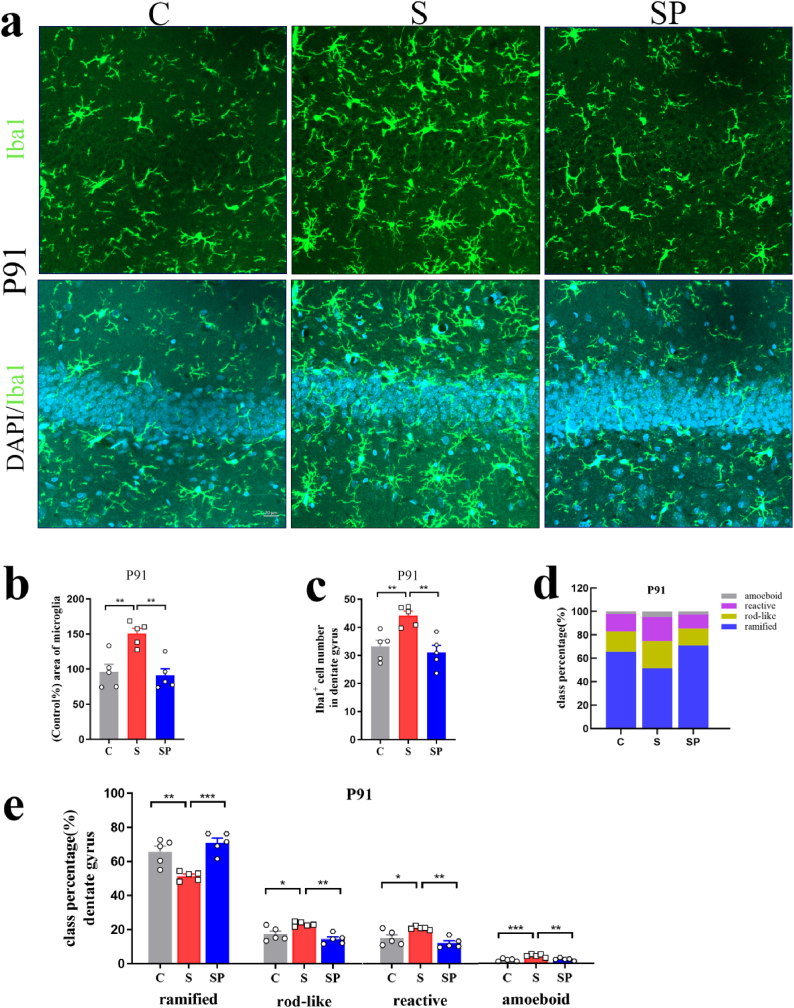
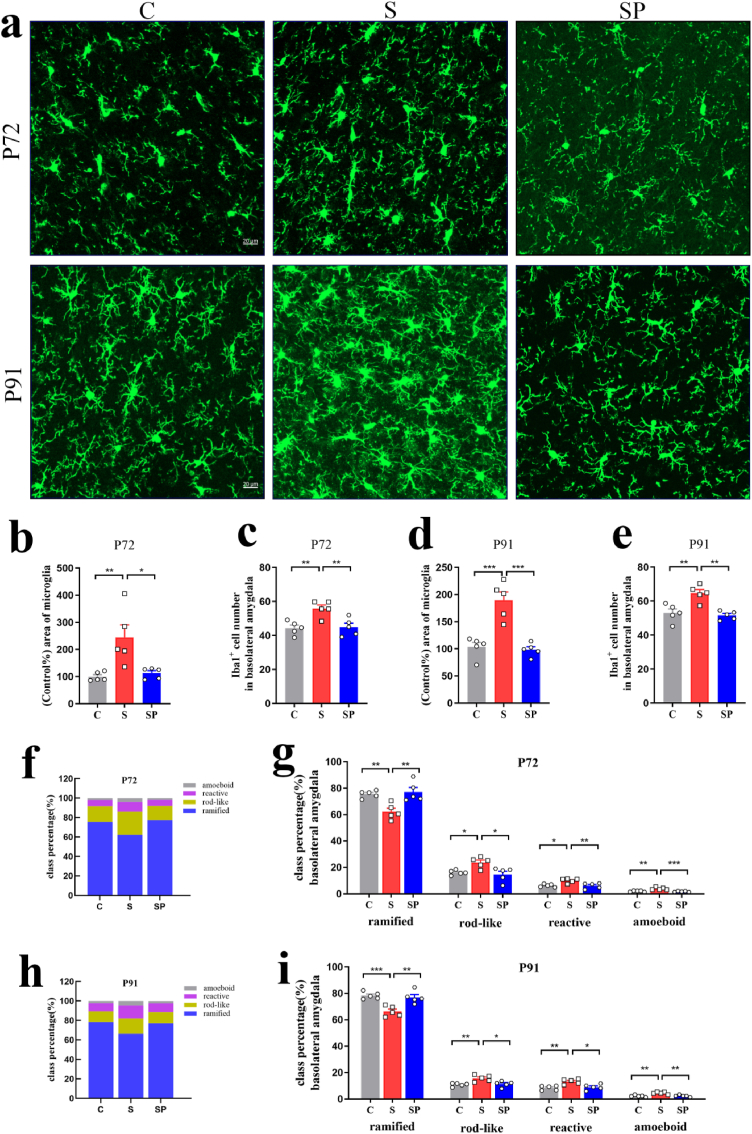
In the rats' adult brains of offspring, inter-group differences were observed in the positive area percentage of microglial cells in the DG of the hippocampus and the BLA (DG: P72: F(3,16) = 10.66, p = 0.0004; P91: F(3,16) = 14.15, p < 0.0001, BLA: P72: F(3,16) = 9.289, p = 0.0009; P91: F(3,16) = 17.79, p < 0.0001, one-way ANOVA). Positive area percentage of microglial cells in the S and ST groups increased significantly on P72 and P91 (DG: P72: C vs. S: p = 0.0015; SH vs. ST: p = 0.0227; P91: C vs. S: p = 0.0019; SH vs. ST: p = 0.0012, BLA: P72: C vs. S: p = 0.0094; SH vs. ST: p = 0.0083; P91: C vs. S: p = 0.0002; SH vs. ST: p = 0.0013) (Fig. 4, Fig. 5, Fig. 6). Iba1+ cell numbers in the DG of the hippocampus and the BLA showed a statistical difference between groups (DG: P72: F(3,16) = 13.16, p = 0.0001; P91: F(3,16) = 13.49, p = 0.0001, BLA: P72: F(3,16) = 11.77, p = 0.0003; P91: F(3,16) = 10.07, p = 0.0006, one-way ANOVA). Pregnancy stress increased Iba1+ cell numbers on P72 and P91 (DG: P72: C vs. S: p = 0.0023; P91: C vs. S: p = 0.0020, BLA: P72: C vs. S: p = 0.0066; P91: C vs. S: p = 0.0043), while the transplanted gut microbiome was able to roughly reproduce the effects in offspring microglia (DG: P72: SH vs. ST: p = 0.0021; P91: SH vs. ST: p = 0.0022, BLA: P72: SH vs. ST: p = 0.0019; P91: SH vs. ST: p = 0.0111) (Fig. 4, Fig. 5, Fig. 6e). Individual class percentages in the S and ST groups revealed significant increases of amoeboid, reactive, and rod-like microglial cells and a simultaneous decrease of ramified microglia on P72 and P91 compared to C and SH groups (DG: P72: reactive: C vs. S: p = 0.0017; SH vs. ST: p = 0.0010; rod-like: C vs. S: p = 0.0037; SH vs. ST: p = 0.0023; ramified: C vs. S: p = 0.0020; SH vs. ST: p = 0.0011; P91: amoeboid: C vs. S: p = 0.0012; SH vs. ST: p = 0.0016; reactive: C vs. S: p = 0.0141; SH vs. ST: p = 0.0162; rod-like: C vs. S: p = 0.0115; SH vs. ST: p = 0.0133; ramified: C vs. S: p = 0.0011; SH vs. ST: p = 0.0013, BLA: P72: amoeboid: C vs. S: p = 0.0027; SH vs. ST: p = 0.0198; reactive: C vs. S: p = 0.0284; SH vs. ST: p = 0.0460; rod-like: C vs. S: p = 0.0379; SH vs. ST: p = 0.0235; ramified: C vs. S: p = 0.0110; SH vs. ST: p = 0.0117; P91: amoeboid: C vs. S: p = 0.0015; SH vs. ST: p = 0.0036; reactive: C vs. S: p = 0.0100; SH vs. ST: p = 0.0099; rod-like: C vs. S: p = 0.0274; SH vs. ST: p = 0.0453; ramified: C vs. S: p = 0.0015; SH vs. ST: p = 0.0024) (Fig. 4, Fig. 5, Fig. 6i). The level of microglia activation in the rats’ adult brains did not differ between C and SH groups and between S and ST groups.The above results indicated that dysbiosis of gut microbiota caused by stress during pregnancy resulted in noticeable activation of microglia in offspring.
Fig. 4.
Dysbiosis of gut microbiota caused by pregnancy stress affected rats' microglia development. (a) Confocal images of offspring Iba1(green) staining in the DG of C, S, SH, and ST groups on P72. Scale bar = 20 μm. (b) Positive-area percentage of microglial cells in the DG of offspring hippocampus of C, S, SH, and ST groups on P72. (c) Iba1+ cell numbers in the DG of offspring hippocampus of C, S, SH, and ST groups on P72. (d) Class percentage of ramified, rod-like, reactive, and amoeboid microglia in the DG of offspring hippocampus of C, S, SH, and ST groups on P72. (e) Individual class percentage of the four microglial morphological phenotype classes in the DG of offspring hippocampus of C, S, SH, and ST groups on P72. N = 5 rats in each group. One-way ANOVA, *p < 0.05, **p < 0.01, ns: no significance. The data were represented as means ± SEM. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 5.
Dysbiosis of gut microbiota caused by pregnancy stress affected rats' microglia development. (a) Confocal images of offspring Iba1(green) staining in the DG of C, S, SH, and ST groups on P91. Scale bar = 20 μm. (b) Positive-area percentage of microglial cells in the DG of offspring hippocampus of C, S, SH, and ST groups on P91. (c) Iba1+ cell numbers in the DG of offspring hippocampus of C, S, SH, and ST groups on P91. (d) Class percentage of ramified, rod-like, reactive, and amoeboid microglia in the DG of offspring hippocampus of C, S, SH, and ST groups on P91. (e) Individual class percentage of the four microglial morphological phenotype classes in the DG of offspring hippocampus of C, S, SH, and ST groups on P91. N = 5 rats in each group. One-way ANOVA, *p < 0.05, **p < 0.01. The data were represented as means ± SEM. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 6.
Dysbiosis of gut microbiota caused by pregnancy stress affected rats' microglia development. (a) Confocal images of offspring Iba1(green) staining in the BLA of C, S, SH, and ST groups on P72 and P91. Scale bar = 20 μm. (b) Positive-area percentage of microglial cells in the offspring BLA of C, S, SH, and ST groups on P72. (c) Iba1+ cell numbers in the offspring BLA of C, S, SH, and ST groups on P72. (d) Positive-area percentage of microglial cells in the offspring BLA of C, S, SH, and ST groups on P91. (e) Iba1+ cell numbers in the offspring BLA of C, S, SH, and ST groups on P91. (f) Class percentage of ramified, rod-like, reactive, and amoeboid microglia in the offspring BLA of C, S, SH, and ST groups on P72. (e) Individual class percentage of the four microglial morphological phenotype classes in the offspring BLA of C, S, SH, and ST groups on P72. (h) Class percentage of ramified, rod-like, reactive, and amoeboid microglia in the offspring BLA of C, S, SH, and ST groups on P91. (i) Individual class percentage of the four microglial morphological phenotype classes in the offspring BLA of C, S, SH, and ST groups on P91. N = 5 rats in each group. One-way ANOVA, *p < 0.05, **p < 0.01, ***p < 0.001. The data were represented as means ± SEM. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
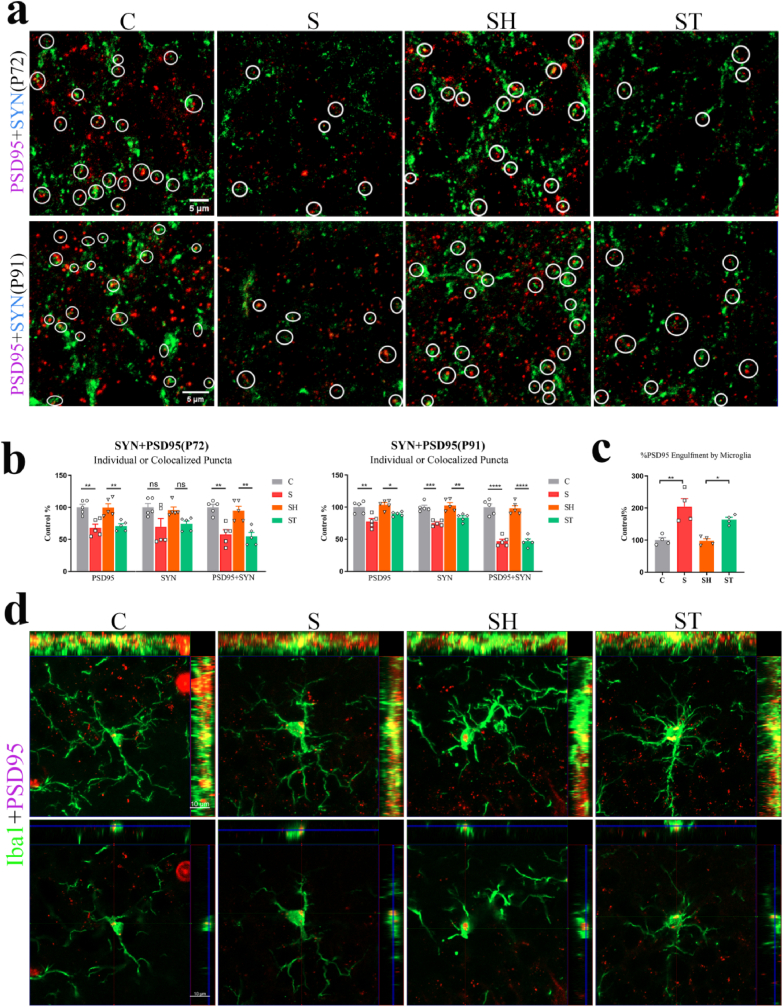
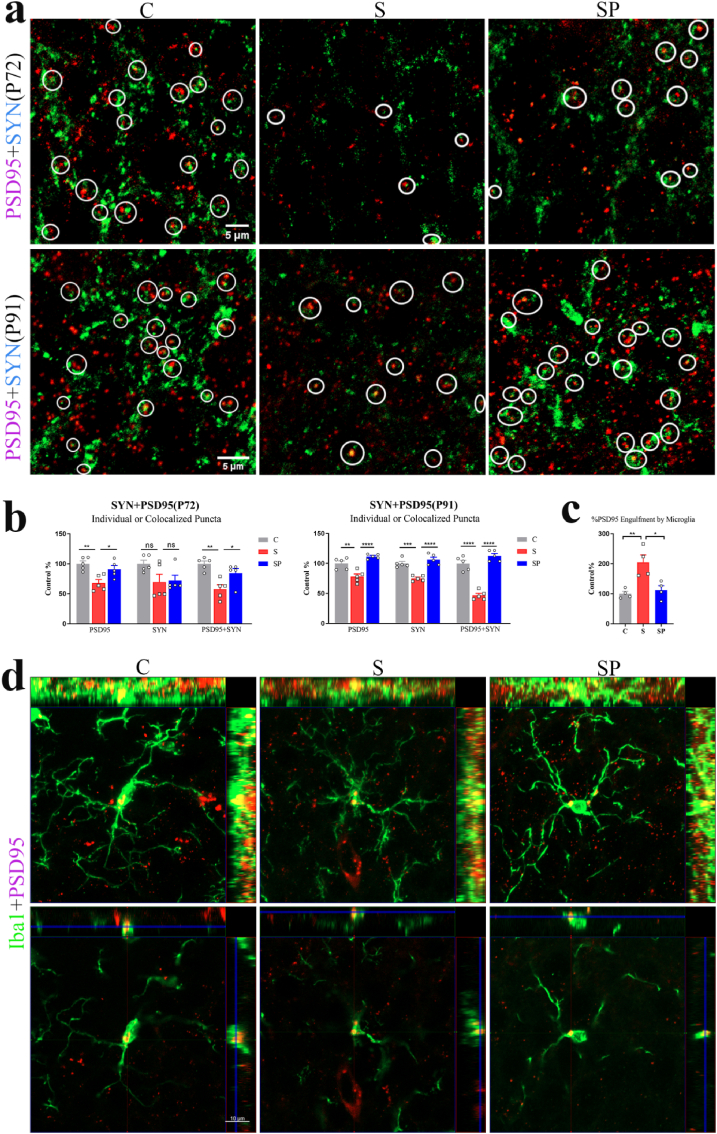
When microglia were dysfunctional, the synaptic components of hippocampal neurons would be engulfed, leading to synaptic loss (Smith et al., 2019; Chen et al., 2020). Therefore, immunofluorescence was used to measure the synaptic integrity of neurons in each group, while synaptophysin and PSD95 were used as the presynaptic and postsynaptic markers, respectively. The synaptic density in the cell layer of the hippocampal DG granule of offspring was also observed in the groups on P72 and P91. Quantification of synaptic puncta displayed a significant difference between the groups (P72: PSD95: F(3,16) = 11.49, p = 0.0003; SYN: F(3,16) = 3.693, p = 0.0341; PSD95+SYN: F(3,16) = 14.00, p < 0.0001. P91: PSD95: F(3,16) = 10.96, p = 0.0004; SYN: F(3,16) = 17.96, p < 0.0001; PSD95+SYN:F(3,16) = 51.29, p < 0.0001; one-way ANOVA).
Results indicated the loss of PSD95 in S group as compared to C group in offspring, as shown in Fig. 7a and b (P72: PSD95: p = 0.0023; PSD95+SYN: p = 0.0013. P91: PSD95: p = 0.0022; PSD95+SYN: p < 0.0001). Additionally, the analyses indicated the loss of PSD95 in the ST group compared with the SH group (P72: PSD95: p = 0.0065; PSD95+SYN: p = 0.0021. P91: PSD95: p = 0.0428; PSD95+SYN: p < 0.0001). The synaptophysin density in the DG of each group remained unaltered on P72. Nevertheless, significant differences in the density of SYN were observed between the groups on P91 (C vs. S: p = 0.0002; SH vs. ST: p = 0.0025; C vs. SH: p > 0.05; S vs. ST: p > 0.05). In the orthographic view (1 μm) of the confocal image, colocalization of PSD95 and Iba1 was found, indicating the phagocytosis of synapses by microglia (Fig. 7d). The areas of PSD95 puncta inside microglia of DG differed significantly between the groups on P72 (F(3,12) = 13.67, p = 0.0004, one-way ANOVA) (Fig. 7c). Statistical analysis revealed a bigger areas of PSD95 puncta inside microglia of S and ST groups compared with the C and SH groups (C vs. S: p = 0.0010, SH vs. ST: p = 0.0267). No significant difference of the density of SYN and PSD95, PSD95 puncta, and the PSD95 puncta area in microglia were detected between C and SH groups and between S and ST groups.
Fig. 7.
Dysbiosis of gut microbiota caused by pregnancy stress caused hippocampal synapse loss in offspring. (a) Confocal images of synaptophysin (SYN, green)- and PSD95 (red)- immunoreactive puncta in granular cell layer of the hippocampal DG of C, S, SH, and ST groups on P72 and P91 in offspring (scale bar, 5 μm). (b) Quantification of synaptic puncta or their apposition using ZEN and Image J indicated a selective loss of PSD95 in the S group compared with the C group (P72: PSD95: p = 0.0023; PSD95+SYN: p = 0.0013. P91: PSD95: p = 0.0022; PSD95+SYN: p < 0.0001; one-way ANOVA) and in the ST group compared with the SH group (P72: PSD95: p = 0.0065; PSD95+SYN: p = 0.0021. P91: PSD95: p = 0.0428; PSD95+SYN: p < 0.0001; one-way ANOVA). N = 5 rats in each group. (c) Using Image J demonstrated that bigger areas of PSD95 puncta inside microglia of S and ST groups compared with the C and SH groups (N = 4/group; S vs. C: p = 0.0010; ST vs. SH: p = 0.0267; one-way ANOVA) on P72 in the offspring. (d) Orthogonal view of confocal images showed colocalization of PSD95 (red) and Iba1(green), suggesting the phagocytosis of microglia on synapses (scale bar, 10 μm). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. ns: no significance. The data were represented as means ± SEM. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.3. Probiotics treatment during pregnancy stress promoted fear extinction in offspring
The above data suggest that fear extinction in offspring was mainly impaired by the dysbiosis of gut microbiota in pregnant rats caused by stress during pregnancy. To explore the effect of probiotics on impaired fear extinction memory in offspring caused by stress during pregnancy, we simultaneously gave the probiotics capsules to rats that were subjected to restraint gestation stress, and then evaluated the behavior of their offspring during adulthood (Fig. 8a). During extinction training, the previously acquired equivalent conditioned fear memories were reactivated in rat groups, and there was a significant between-group difference in freezing levels in the three groups (F(48,372) = 3.935, p < 0.0001, two-way repeated measures) (Fig. 8b). Statistical analysis showed that the freezing levels of rats in the pregnancy restraint stress + probiotics group (SP group) were significantly lower than in the S and C group (S vs. SP: p < 0.0001; SP vs. C: p < 0.0001). Interestingly, the same tendency existed in long-term memory (S vs. SP: p < 0.0001; SP vs. C: p = 0.0003). These data demonstrated that probiotics supplementation improved the impairment of fear extinction because of stress during pregnancy.
Fig. 8.
Probiotics treatment during pregnancy stress promoted fear extinction in offspring. (a) The schedule of pregnancy restraint stress, drug administration, fear conditioning and behavior test. Probiotics: one capsule/day/rat for five days. (b) The freezing levels of adult offspring of rats in fear conditioning, fear extinction, and long-term memory test. $: p < 0.0001 for C vs. S in fear extinction (two-way repeated measures ANOVA with post hoc analysis). +: p < 0.0001 for S vs. SP in fear extinction (two-way repeated measures ANOVA with post hoc analysis). C, control; S, pregnancy restraint stress; SP, pregnancy restraint stress + probiotics, ***p < 0.001, ****p < 0.0001, ns: no significance. n = 5–8. All data were presented as means ± SEM.
To confirm the effects of probiotics supplements on the gut microbiota of pregnant rats, we determined the microbial composition of the three groups and compared the different gut microbiota. Significant differences in α- and β-diversity were found among the rat groups on the 21st day of pregnancy (α-diversity: Chao index of OUT level, p = 0.00911, Kruskal-Wallis rank-sum test; β-diversity: PCoA on OTU level, R2 = 0.2630, P = 0.0020, Adonis) (Fig. 9a). The gut microbial community in the three groups was similar on the Phylum level (S vs. SP: p > 0.05, Mann-Whitney U test) (Fig. 9b). On the Genus level, probiotics treatment lowered the relative abundance of Prevotella (S vs. SP: p = 0.03671), Treponema (C vs. S: p = 0.02001, S vs. SP: p = 0.007495), Lachnospiraceae_UCG-001 (C vs. S: p = 0.01219, S vs. SP: p = 0.01219), Peptococcus (C vs. S: p = 0.02001, S vs. SP: p = 0.0601) unclassified_k__norank_d__Bacteria (C vs. S: p = 0.01219, S vs. SP: p = 0.01219) and Tuzzerella (C vs. S: p = 0.02157, C vs. SP: p = 0.01219), which were increased by pregnancy stress. Moreover, the relative abundance of Lachnospiraceae_NK4A136_group (p = 0.01219), Ruminococcus (p = 0.01219) and Enterococcus (p = 0.009701) in the SP group were significantly higher than in the C group(Fig. 9c). These results showed that probiotics treatment during pregnancy stress normalized gut microbiota dysbiosis induced by pregnancy stress in mother rats.
Fig. 9.
Probiotics treatment during pregnancy stress normalized dysbiosis of gut microbiota induced by pregnancy stress in mother rats. (a) There was a significant difference among the groups in α-diversity of pregnant rats (Chao index of OUT level, p = 0.00911, Kruskal-Wallis rank-sum test). β-diversity (PCoA on OTU level, Adonis: R2 = 0.2630, p = 0.0020) revealed significant differences in bacterial communities among the three groups. (b) Probiotics treatment did not make any significant difference on Phylum level (S vs. SP: p > 0.05, Mann-Whitney U test). (c) The relative abundance of Lachnospiraceae_NK4A136_group, Prevotella, Ruminococcus, Treponema, Lachnospiraceae_UCG-001, Peptococcus, unclassified_k__norank_d__Bacteria, Tuzzerella and Enterococcus on the Genus level showed a significant difference among groups (Kruskal-Wallis rank-sum test). N = 5/group, *p < 0.05, **p < 0.01. Data were represented as means ± SEM.
3.4. Probiotics supplementation during pregnancy could improve impaired fear extinction due to stress by regulating microglial development
The above studies showed that probiotics treatment during pregnancy stress promoted fear extinction in offspring. Subsequent experiments were then conducted to verify whether probiotics can improve the development of microglia in offspring, thereby promoting fear extinction in offspring. The methods for detecting microglia were the same as those outlined in Section 3.2.
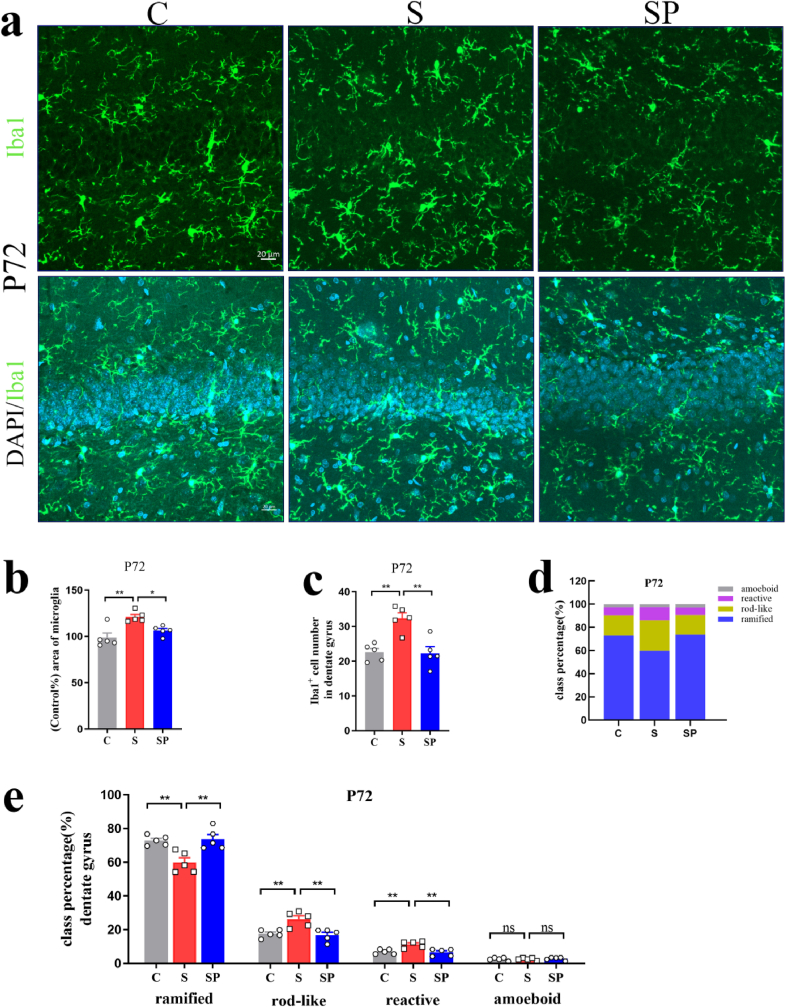
Probiotics treatment normalized microglial branches that were affected by pregnancy stress in the fetal brains (Fig. 10b). There were significant differences in positive-area and Iba1+ cell numbers between groups (Positive-area: F(2,12) = 8.487, p = 0.0050, Iba1+ cell numbers: F(2,12) = 7.157, p = 0.0090; one-way ANOVA). Probiotics treatment significantly reduced the positive area of microglia, Iba1+ cell numbers, and amoeboid, reactive, and rod-like microglial cells in the fetal brains, when the mother experienced pregnancy stress (Positive-area: S vs. SP: p = 0.0306; Iba1+ cell numbers: S vs. SP: p = 0.0179; amoeboid: S vs. SP: p = 0.0176; reactive: S vs. SP: p = 0.0119; rod-like: S vs. SP: p = 0.0068) (Fig. 10c, 10d, 10e, 10f). Probiotics treatment significantly increased the ramified microglia in the fetal brains (S vs. SP: p = 0.0037) (Fig. 10c, 10d, 10e, 10f).
Fig. 10.
Probiotics treatment during pregnancy limited microglial activation of offspring induced by pregnancy stress. (a) Schematic diagram of the experimental procedure. (b) Confocal images of offspring Iba1(green) staining of C, S, and SP groups on the 21st gestational day. Scale bar = 20 μm. (c) Positive-area percentage of microglial cells in the rats' fetal brains of offspring of C, S and SP groups. (d) Iba1+ cell numbers in the rats' fetal brains of C, S and SP groups. (e) Class percentage of ramified, rod-like, reactive, and amoeboid microglia in the rats' fetal brains of C, S and SP groups. (f) Individual class percentage of the four microglial morphological phenotype classes in the rats' fetal brains of C, S and SP groups. N = 5 rats in each group. One-way ANOVA, *p < 0.05, **p < 0.01. The data were represented as means ± SEM. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
In the rats’ adult brains of offspring, there were significant differences in positive-area and Iba1+ cell numbers between groups (Positive-area: DG: P72: F(2,12) = 10.32, p = 0.0025; P91: F(2,12) = 12.53, p = 0.0012, BLA: P72: F(2,12) = 8.233, p = 0.0020; P91: F(2,12) = 23.47, p < 0.0001. Iba1+ cell numbers: DG: P72: F(2,12) = 13.58, p = 0.0008; P91: F(2,12) = 10.74, p = 0.0021, BLA: P72: F(2,12) = 9.703, p = 0.0031; P91: F(2,12) = 13.78, p = 0.0008, one-way ANOVA). Probiotics treatment significantly reduced the positive area of microglia, Iba1+ cell numbers, and amoeboid, reactive, and rod-like microglial cells in the DG of hippocampus and the BLA, when the mother experienced pregnancy stress (Positive-area: DG: P72: S vs. SP: p = 0.0297; P91: S vs. SP: p = 0.0019; BLA: P72: S vs. SP: p = 0.0151; P91: S vs. SP: p = 0.0001. Iba1+ cell numbers: DG: P72: S vs. SP: p = 0.0018; P91: S vs. SP: p = 0.0027; BLA: P72: S vs. SP: p = 0.0076; P91: S vs. SP: p = 0.0012. amoeboid: DG: P91: S vs. SP: p = 0.0043; BLA: P72: S vs. SP: p = 0.0007; P91: S vs. SP: p = 0.0026; reactive: DG: P72: S vs. SP: p = 0.0041; P91: S vs. SP: p = 0.0022; BLA: P72: S vs. SP: p = 0.0090; P91: S vs. SP: p = 0.0115; rod-like: DG: P72: S vs. SP: p = 0.0035; P91: S vs. SP: p = 0.0014; BLA: P72: S vs. SP: p = 0.0103; P91: S vs. SP: p = 0.0148). Probiotics treatment significantly increased the ramified microglia in the adult brains (DG: P72: S vs. SP: p = 0.0039; P91: S vs. SP: p = 0.0005; BLA: P72: S vs. SP: p = 0.0035; P91: S vs. SP: p = 0.0024) (Fig. 11, Fig. 12, Fig. 13). These data suggested that probiotics treatment during pregnancy limited microglial activation of offspring induced by pregnancy stress.
Fig. 11.
Probiotics treatment during pregnancy limited hippocampal microglial activation of offspring induced by pregnancy stress. (a) Confocal images of offspring Iba1(green) staining in the DG of C, S and SP groups on P72. Scale bar = 20 μm. (b) Positive-area percentage of microglial cells in the DG of offspring hippocampus of C, S and SP groups on P72. (c) Iba1+ cell numbers in the DG of offspring hippocampus of C, S and SP groups on P72. (d) Class percentage of ramified, rod-like, reactive, and amoeboid microglia in the DG of offspring hippocampus of C, S and SP groups on P72. (e) Individual class percentage of the four microglial morphological phenotype classes in the DG of offspring hippocampus of C, S and SP groups on P72. N = 5 rats in each group. One-way ANOVA, *p < 0.05, **p < 0.01, ns: no significance. The data were represented as means ± SEM. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 12.
Probiotics treatment during pregnancy limited hippocampal microglial activation of offspring induced by pregnancy stress. (a) Confocal images of offspring Iba1(green) staining in the DG of C, S and SP groups on P91. Scale bar = 20 μm. (b) Positive-area percentage of microglial cells in the DG of offspring hippocampus of C, S and SP groups on P91. (c) Iba1+ cell numbers in the DG of offspring hippocampus of C, S and SP groups on P91. (d) Class percentage of ramified, rod-like, reactive, and amoeboid microglia in the DG of offspring hippocampus of C, S and SP groups on P91. (e) Individual class percentage of the four microglial morphological phenotype classes in the DG of offspring hippocampus of C, S and SP groups on P91. N = 5 rats in each group. One-way ANOVA, *p < 0.05, **p < 0.01, ***p < 0.001. The data were represented as means ± SEM. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 13.
Probiotics treatment during pregnancy limited microglial activation in the BLA of offspring induced by pregnancy stress. (a) Confocal images of offspring Iba1(green) staining in the BLA of C, S and SP groups on P72 and P91. Scale bar = 20 μm. (b) Positive-area percentage of microglial cells in the offspring BLA of C, S and SP groups on P72. (c) Iba1+ cell numbers in the offspring BLA of C, S and SP groups on P72. (d) Positive-area percentage of microglial cells in the offspring BLA of C, S and SP groups on P91. (e) Iba1+ cell numbers in the offspring BLA of C, S and SP groups on P91. (f) Class percentage of ramified, rod-like, reactive, and amoeboid microglia in the offspring BLA of C, S and SP groups on P72. (e) Individual class percentage of the four microglial morphological phenotype classes in the offspring BLA of C, S and SP groups on P72. (h) Class percentage of ramified, rod-like, reactive, and amoeboid microglia in the offspring BLA of C, S and SP groups on P91. (i) Individual class percentage of the four microglial morphological phenotype classes in the offspring BLA of C, S and SP groups on P91. N = 5 rats in each group. One-way ANOVA, *p < 0.05, **p < 0.01, ***p < 0.001. The data were represented as means ± SEM. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Synapse plasticity is the basis of fear extinction (Izquierdo et al., 2016; Krystal et al., 2017). We then explored the effect of probiotics on the synapse density in the granular cell layer of the hippocampal DG of the offspring that experienced stress during pregnancy. There were significant differences in the quantification of synaptic puncta between groups (P72: PSD95: F(2,12) = 8.647, p = 0.0047; SYN: F(2,12) = 2.988, p = 0.0885; PSD95+SYN: F(2,12) = 9.800, p = 0.0030. P91: PSD95: F(2,12) = 21.67, p = 0.0001; SYN: F(2,12) = 26.46, p < 0.0001; PSD95+SYN:F(2,12) = 66.71, p < 0.0001; one-way ANOVA). The loss of PSD95 in the S group was significantly alleviated by probiotics treatment (P72: PSD95: p = 0.0347; PSD95+SYN: p = 0.0441. P91: PSD95: p < 0.0001; PSD95+SYN: p < 0.0001). Likewise, on P91 (but not the P72), the data showed significant differences in SYN density (P72:S vs. SP: p = 0.9843, P91:S vs. SP: p < 0.0001) (Fig. 14a and b). In the orthogonal view (1 μm) of the confocal image, the areas of PSD95 puncta inside microglia of DG showed a significant differences between groups on P72 (F(2,9) = 10.83, p = 0.0040, one-way ANOVA). Post hoc analyses indicated a bigger areas of PSD95 puncta inside microglia of S group compared with the C (p = 0.0054) and SP (p = 0.0113) groups (Fig. 14c and d).
Fig. 14.
Probiotics treatment during pregnancy alleviated hippocampal synapse loss of offspring induced by pregnancy stress. (a) Confocal images of synaptophysin (SYN, green)- and PSD95 (red)- immunoreactive puncta in granular cell layer of the hippocampal DG of C, S and SP groups on P72 and P91 in offspring (scale bar, 5 μm). (b) Quantification of synaptic puncta or their apposition using ZEN and Image J indicated that there was a selective loss of PSD95 in the S group compared with the C group, which was significantly alleviated by probiotics treatment during pregnancy stress (P72: PSD95: S vs. SP: p = 0.0347; PSD95+SYN: S vs. SP: p = 0.0441. P91: PSD95: S vs. SP: p < 0.0001; PSD95+SYN: S vs. SP: <0.0001; one-way ANOVA). N = 5 rats in each group. (c) Image J demonstrated that bigger areas of PSD95 puncta inside the microglia of the S group compared with the C and SP groups (N = 4/group; C vs. S: p = 0.0054; S vs. SP: p = 0.0113; one-way ANOVA) on P72 in offspring. (d) Orthogonal view of confocal images showed colocalization of PSD95 (red) and Iba1(green). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. ns: no significance. The data were represented as means ± SEM. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
Stress during pregnancy causes neurobiological changes in offspring, increasing their susceptibility to neurological and psychiatric disorders (Wei et al., 2021; Lautarescu et al., 2020). For example, fear-related disorders in offspring caused by pregnancy stress could adversely affect life of offspring, although there are only limited studies in this area (Lee et al., 2011; Bingham et al., 2013). However, the prevention and treatment of neurological and psychiatric disorders in offspring affected by pregnancy stress remain challenging clinically because of poor compliance and less effectiveness of pharmacologic interventions (YI et al., 2018). In this study, we found that dysbiosis of gut microbiota caused by pregnancy stress impaired short-term and long-term fear extinction in rats’ offspring. Probiotics supplementation improved the impairment of fear extinction caused by stress during pregnancy. Bifidobacteria are amongst the earliest colonizers of the infant gut (Stark and Lee, 1982). Treatment with probiotics (Bifidobacterium breve CCFM1025) during pregnancy protected the offspring from maternal separation-induced neurobiological and gastrointestinal disorders such as depression-like behaviour and delayed defecation (Zhu et al., 2022). Both Bifidobacterium longum and Bifidobacterium breve treatment were capable of affecting brain or cognitive function (Wang et al., 2019; Asaoka et al., 2022). The administration of Bifidobacterium bifidum NCDO 2203 combined with Lactobacillus acidophilus NCDO 1748 could positively impact the neurodevelopment of the preterm neonates (Baucells et al., 2023). The probiotics capsule (Bifidobacterium longum, Lactobacillus acidophilus, and Enterococcus faecalis) we used in the study may be associated with the neurodevelopment of offspring. However, whether there is a difference in the effect of using the three microbes or one alone need to be further explored.
Studies have reported that prenatal stress can alter the microbiome in pregnant person during pregnancy (Gur et al., 2017; Wang et al., 2020b; Chen and Gur, 2019; Song et al., 2022a). In previous study, metagenome-assembled-genomes that were increased in stressed dams were a member of the UBA9502 genus and the Lachnospiraceae family and a member of the Oscillibacter genus (Antonson et al., 2020). In our study, pregnancy stress increased the relative abundance of unclassified_f__Lachnospiraceae, Treponema, Intestinimonas, Lachnospiraceae_UCG-001 and unclassified_k__norank_d__Bacteria on the Genus level. The variation in genera between the two studies may be related to animal strain, stress patterns, or sample source. In parallel, compared with the SH group, the relative abundance of Treponema, Oscillibacter, and unclassified_k__norank_d__Bacteria were increased in ST group. These data indicated that transplanting gut microbiomes from gestational restraint-stressed rats to normal pregnant rats could reproduce the dysbiosis of gut microbiota and the effects of gestational stress on the behaviors of the offspring in adulthood. The findings of our study provide evidence that dysbiosis of gut microbiota plays a pivotal role in the impairment of fear memory extinction in offspring caused by pregnancy stress. And we found that probiotics treatment lowered the relative abundance of Prevotella, Treponema, Lachnospiraceae_UCG-001, Peptococcus, unclassified_k__norank_d__Bacteria, and Tuzzerella. The probiotics were used to normalize the dysbiosis of gut microbiota caused by stress, corresponding to faster fear extinction in offspring. Furthermore, in this study, Enterococcus only appeared when probiotics were given. The probiotics capsule used in our study contained Enterococcus faecalis, which belongs to the Enterococcus genus. These suggest a close relationship between prenatal stress and gut microbiota. Unfortunately, it is still unclear what the most related gut microbiome and how gut microbiota affects the brains and behavior of offspring. The current knowledge regarding four main microbiota-gut-brain axis routes in rodents: through the vagus nerve; via the hypothalamus-pituitary-adrenal-axis; by metabolism of neuroactive substances; and through modulation of host inflammation (Agirman et al., 2021; Kuijer and Steenbergen, 2023). The gut microbiota is essential for the metabolism of neuroactive substances, such as short-chain fatty acids (SCFAs) (Flint et al., 2015). The three main SCFAs are acetate, butyrate, and propionate (Scheppach, 1994). Acetate and propionate are produced by e.g. Bacteriodetes, whereas e.g. Firmicutes, Bifidobacterium, Lactobacillus, and Clostridium produce butyrate (LeBlanc et al., 2017; Stoeva et al., 2021). In our study, which SCFAs are associated with the faster fear extinction in offspring after probiotics (Bifidobacterium longum, Lactobacillus acidophilus, and Enterococcus faecalis) supplementation remains unclear. More attention should be paid to this current research hotspot in the further studies.
The prenatal period is a time of rapid development, during which the brain is particularly plastic and also sensitive to environmental influences (Davis and Narayan, 2020; Gyllenhammer et al., 2022). Prenatal stress may influence the offspring's brain structure, function, and behavior (Van den Bergh et al., 2020; Fleming et al., 2018; Hoffman et al., 2021; Britto et al., 2017; Franke et al., 2020). Our study showed that pregnancy stress led to microglia activation in the fetal brains, and significant microglial activation in the DG of the hippocampus and the BLA during fear extinction in adult rats. The probiotics could normalize the dysbiosis of gut microbiota in pregnant rats, inhibit microglial activation and promote fear memory extinction in offspring. Studies have shown that alteration of gut microbiota composition in pregnant person during pregnancy affects the neurodevelopment and behavior of offspring (Dawson et al., 2021; Jašarević et al., 2017; Wang et al., 2020b; Kim et al., 2017; Buffington et al., 2016). The gut microbiome of pregnant person promotes fetal thalamocortical axonogenesis and regulates the maturation of microglia, which are the resident macrophages in the brain (Vuong et al., 2020; Gomez de Agüero et al., 2016; Thion et al., 2018; Erny et al., 2015). For the limbic system, the fetal hippocampus and amygdala are known to be sensitive to prenatal because they contain large numbers of glucocorticoid receptors (Graham et al., 2022). It has been showed that microglial cells play a vital role in fear memory (Ten-Blanco et al., 2022; Nguyen et al., 2020) and fear-related neuronal dendritic spine loss (Smith et al., 2019). Thus, microglia are possibly associated with the key targets of pregnancy stress effects passed from mother to offspring. Further study is needed to answer this.
Rather than forgetting, fear extinction creates a new inhibitory memory competing with the original conditoned fear response and inhibiting fear-promoting circuitry via plasticity at the synapses (Krabbe et al., 2018; Bouton et al., 2021; Milad and Quirk, 2002). In our study, pregnancy stress led to the apparent loss of synapses and the synaptic phagocytosis of microglial cells in hippocampal DG of offspring during fear extinction, which reduced the stabilities of new learning-related synapses after fear extinction, thereby easily maintaining the old fear memory. And probiotics treatment during pregnancy inhibited these changes in offspring, rescued synaptic loss and promoted fear memory extinction. During critical neurodevelopment, aberrant activation of microglia cells may lead to harmful synaptic pruning (Notter and Meyer, 2017). The importance of dysregulated synaptic phagocytosis of microglia is also manifested in neurodevelopmental and neuropsychiatric disorders (He et al., 2022; De Schepper et al., 2023; Druart and Le Magueresse, 2019; Madore et al., 2020). And probiotics treatment can inhibit the activation of microglial cells and improve the pathological damage in the depression (Roseburia hominis) (Song et al., 2022b), obesity-induced social deficits and anxiety-like behaviors (Lactobacillus reuteri) (Duan et al., 2021), Alzheimer's disease (Bifidobacterium breve MCC1274) (Abdelhamid et al., 2022) and Parkinson's disease (Clostridium butyricum) (Sun et al., 2021). Taken together, our findings indicate that probiotics treatment during pregnancy stress promotes fear extinction in offspring may be associated with improving microglia development and inhibiting microglial synaptic pruning. However, further research is necessary to determine the specific mechanism.
5. Conclusion
Treatment with probiotics (Bifidobacterium longum, Lactobacillus acidophilus, and Enterococcus faecalis) during pregnancy has protective effects in impairment of fear extinction in the offspring. These may be associated with improving microglia development and inhibiting microglial synaptic pruning. The present findings provided evidence that probiotics may function as an auxiliary and novel strategy to treat fear-related disorders in offspring caused by pregnancy stress.
Ethical statement
All experimental procedures were done based on the standards and protocols approved by the Animal Research Committee of the Xiangya School of Medicine per international conventions.
Funding
This study was supported by grants from the National Natural Science Foundation of China (No. 81871089, 82171466), the Key Research and Development Program of Hunan Province (No. 2022SK2079), Science and Technology Program of Hunan Province (No. 2020TP3003), Fundamental Research Funds for Central Universities of the Central South University (No. 2023XQLH031, 2023XQLH043, 2023XQLH018).
CrediT authorship contribution statement
Ru Zeng: Writing – original draft, Article drafting, Article revising, Acquisition of data. Jie Chen: The design of the study, Acquisition of data. Yihan Peng and Weiye Xu: Validation. Yuanyuan Tao and Min Li: Supervision. Ruqi Zhang and Jingzhuo Meng: Funding acquisition. Zhiyuan Li: Project administration. Leping Zeng, Jufang Huang: Conceptualization, Resources.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors thank Pual M for his contribution in revising the manuscript.
Handling Editor: Dr. John Cryan
Contributor Information
Leping Zeng, Email: zenglp0205@csu.edu.cn.
Jufang Huang, Email: huangjufang@csu.edu.cn.
Data availability
Data will be made available on request.
References
- Abdelhamid M., Zhou C., Jung C.G., Michikawa M. Probiotic Bifidobacterium breve MCC1274 mitigates Alzheimer's disease-related pathologies in wild-type mice. Nutrients. 2022;14(12):2543. doi: 10.3390/nu14122543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agirman G., Yu K.B., Hsiao E.Y. Signaling inflammation across the gut-brain axis. Science. 2021;374(6571):1087–1092. doi: 10.1126/science.abi6087. [DOI] [PubMed] [Google Scholar]
- Antonson A.M., Evans M.V., Galley J.D., Chen H.J., Rajasekera T.A., Lammers S.M., Hale V.L., Bailey M.T., Gur T.L. Unique maternal immune and functional microbial profiles during prenatal stress. Sci. Rep. 2020;10(1) doi: 10.1038/s41598-020-77265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asaoka D., Xiao J., Takeda T., Yanagisawa N., Yamazaki T., Matsubara Y., Sugiyama H., Endo N., Higa M., Kasanuki K., Ichimiya Y., Koido S., Ohno K., Bernier F., Katsumata N., Nagahara A., Arai H., Ohkusa T., Sato N. Effect of probiotic Bifidobacterium breve in improving cognitive function and preventing brain atrophy in older patients with suspected mild cognitive impairment: results of a 24-week randomized, double-blind, placebo-controlled trial. J Alzheimers Dis. 2022;88(1):75–95. doi: 10.3233/JAD-220148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baucells B.J., Sebastiani G., Herrero-Aizpurua L., Andreu-Fernández V., Navarro-Tapia E., García-Algar O., Figueras-Aloy J. Effectiveness of a probiotic combination on the neurodevelopment of the very premature infant. Sci. Rep. 2023;13(1) doi: 10.1038/s41598-023-37393-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham B.C., Sheela Rani C.S., Frazer A., Strong R., Morilak D.A. Exogenous prenatal corticosterone exposure mimics the effects of prenatal stress on adult brain stress response systems and fear extinction behavior. Psychoneuroendocrinology. 2013;38(11):2746–2757. doi: 10.1016/j.psyneuen.2013.07.003. [DOI] [PubMed] [Google Scholar]
- Bouchet C.A., Lloyd B.A., Loetz E.C., Farmer C.E., Ostrovskyy M., Haddad N., Foright R.M., Greenwood B.N. Acute exercise enhances the consolidation of fear extinction memory and reduces conditioned fear relapse in a sex-dependent manner. Learn. Mem. 2017;24(8):358–368. doi: 10.1101/lm.045195.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton M.E., Maren S., McNally G.P. Behavioral and neurobiological mechanisms of pavlovian and instrumental extinction learning. Physiol. Rev. 2021;101(2):611–681. doi: 10.1152/physrev.00016.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo J.A., Forsythe P., Chew M.V., Escaravage E., Savignac H.M., Dinan T.G., Bienenstock J., Cryan J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. U. S. A. 2011;108(38):16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britto P.R., Lye S.J., Proulx K., Yousafzai A.K., Matthews S.G., Vaivada T., Perez-Escamilla R., Rao N., Ip P., Fernald L.C.H., MacMillan H., Hanson M., Wachs T.D., Yao H., Yoshikawa H., Cerezo A., Leckman J.F., Bhutta Z.A. Early childhood development interventions review group, for the lancet early childhood development series steering committee. Nurturing care: promoting early childhood development. Lancet. 2017;389(10064):91–102. doi: 10.1016/S0140-6736(16)31390-3. [DOI] [PubMed] [Google Scholar]
- Buchholz J.L., Abramowitz J.S. The therapeutic alliance in exposure therapy for anxiety-related disorders: a critical review. J. Anxiety Disord. 2020;70 doi: 10.1016/j.janxdis.2020.102194. [DOI] [PubMed] [Google Scholar]
- Buffington S.A., Di Prisco G.V., Auchtung T.A., Ajami N.J., Petrosino J.F., Costa-Mattioli M. Microbial reconstitution reverses maternal diet-induced social and synaptic deficits in offspring. Cell. 2016;165(7):1762–1775. doi: 10.1016/j.cell.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H.J., Gur T.L. Intrauterine microbiota: missing, or the missing link? Trends Neurosci. 2019;42(6):402–413. doi: 10.1016/j.tins.2019.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M.B., Jiang X., Quake S.R., Südhof T.C. Persistent transcriptional programmes are associated with remote memory. Nature. 2020;587(7834):437–442. doi: 10.1038/s41586-020-2905-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H.J., Bischoff A., Galley J.D., Peck L., Bailey M.T., Gur T.L. Discrete role for maternal stress and gut microbes in shaping maternal and offspring immunity. Neurobiol Stress. 2022;21 doi: 10.1016/j.ynstr.2022.100480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colori S. Exposure therapy. Schizophr. Bull. 2018;44(2):229–230. doi: 10.1093/schbul/sbv049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X., Zhou S., Xia G., Chen J., Jiang L., Huang J., Tong J. A multispecies probiotic accelerates fear extinction and inhibits relapse in mice: role of microglia. Neuropharmacology. 2021;193 doi: 10.1016/j.neuropharm.2021.108613. [DOI] [PubMed] [Google Scholar]
- Davis E.P., Narayan A.J. Pregnancy as a period of risk, adaptation, and resilience for mothers and infants. Dev. Psychopathol. 2020;32(5):1625–1639. doi: 10.1017/S0954579420001121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson S.L., O'Hely M., Jacka F.N., Ponsonby A.L., Symeonides C., Loughman A., Collier F., Moreno-Betancur M., Sly P., Burgner D., Tang M.L.K., Saffery R., Ranganathan S., Conlon M.A., Harrison L.C., Brix S., Kristiansen K., Vuillermin P. BIS Investigator Group. Maternal prenatal gut microbiota composition predicts child behaviour. EBioMedicine. 2021;68 doi: 10.1016/j.ebiom.2021.103400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Schepper S., Ge J.Z., Crowley G., Ferreira L.S.S., Garceau D., Toomey C.E., Sokolova D., Rueda-Carrasco J., Shin S.H., Kim J.S., Childs T., Lashley T., Burden J.J., Sasner M., Sala Frigerio C., Jung S., Hong S. Perivascular cells induce microglial phagocytic states and synaptic engulfment via SPP1 in mouse models of Alzheimer's disease. Nat. Neurosci. 2023;26(3):406–415. doi: 10.1038/s41593-023-01257-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druart M., Le Magueresse C. Emerging roles of complement in psychiatric disorders. Front. Psychiatr. 2019;10:573. doi: 10.3389/fpsyt.2019.00573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan C., Huang L., Zhang C., Zhang L., Xia X., Zhong Z., Wang B., Wang Y., Man Hoi M.P., Ding W., Yang Y. Gut commensal-derived butyrate reverses obesity-induced social deficits and anxiety-like behaviors via regulation of microglial homeostasis. Eur. J. Pharmacol. 2021;908 doi: 10.1016/j.ejphar.2021.174338. [DOI] [PubMed] [Google Scholar]
- Erny D., Hrabě de Angelis A.L., Jaitin D., Wieghofer P., Staszewski O., David E., Keren-Shaul H., Mahlakoiv T., Jakobshagen K., Buch T., Schwierzeck V., Utermöhlen O., Chun E., Garrett W.S., McCoy K.D., Diefenbach A., Staeheli P., Stecher B., Amit I., Prinz M. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015;18(7):965–977. doi: 10.1038/nn.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M.B., Gaebel W., Maj M., Stein D.J., Kogan C.S., Saunders J.B., Poznyak V.B., Gureje O., Lewis-Fernández R., Maercker A., Brewin C.R., Cloitre M., Claudino A., Pike K.M., Baird G., Skuse D., Krueger R.B., Briken P., Burke J.D., Lochman J.E., Evans S.C., Woods D.W., Reed G.M. An organization- and category-level comparison of diagnostic requirements for mental disorders in ICD-11 and DSM-5. World Psychiatr. 2021;20(1):34–51. doi: 10.1002/wps.20825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming T.P., Watkins A.J., Velazquez M.A., Mathers J.C., Prentice A.M., Stephenson J., Barker M., Saffery R., Yajnik C.S., Eckert J.J., Hanson M.A., Forrester T., Gluckman P.D., Godfrey K.M. Origins of lifetime health around the time of conception: causes and consequences. Lancet. 2018;391(10132):1842–1852. doi: 10.1016/S0140-6736(18)30312-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint H.J., Duncan S.H., Scott K.P., Louis P. Links between diet, gut microbiota composition and gut metabolism. Proc. Nutr. Soc. 2015;74(1):13–22. doi: 10.1017/S0029665114001463. [DOI] [PubMed] [Google Scholar]
- Franke K., Van den Bergh B.R.H., de Rooij S.R., Kroegel N., Nathanielsz P.W., Rakers F., Roseboom T.J., Witte O.W., Schwab M. Effects of maternal stress and nutrient restriction during gestation on offspring neuroanatomy in humans. Neurosci. Biobehav. Rev. 2020;117:5–25. doi: 10.1016/j.neubiorev.2020.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y., Nanan R., Macia L., Tan J., Sominsky L., Quinn T.P., O'Hely M., Ponsonby A.L., Tang M.L.K., Collier F., Strickland D.H., Dhar P., Brix S., Phipps S., Sly P.D., Ranganathan S., Stokholm J., Kristiansen K., Gray L.E.K., Vuillermin P. The maternal gut microbiome during pregnancy and offspring allergy and asthma. J. Allergy Clin. Immunol. 2021;148(3):669–678. doi: 10.1016/j.jaci.2021.07.011. [DOI] [PubMed] [Google Scholar]
- Glynn L.M., Howland M.A., Sandman C.A., Davis E.P., Phelan M., Baram T.Z., Stern H.S. Prenatal maternal mood patterns predict child temperament and adolescent mental health. J. Affect. Disord. 2018;228:83–90. doi: 10.1016/j.jad.2017.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez de Agüero M., Ganal-Vonarburg S.C., Fuhrer T., Rupp S., Uchimura Y., Li H., Steinert A., Heikenwalder M., Hapfelmeier S., Sauer U., McCoy K.D., Macpherson A.J. The maternal microbiota drives early postnatal innate immune development. Science. 2016;351(6279):1296–1302. doi: 10.1126/science.aad2571. [DOI] [PubMed] [Google Scholar]
- Graham A.M., Doyle O., Tilden E.L., Sullivan E.L., Gustafsson H.C., Marr M., Allen M., Mackiewicz Seghete K.L. Effects of maternal psychological stress during pregnancy on offspring brain development: considering the role of inflammation and potential for preventive intervention. Biol. Psychia.Cogn. Neurosci. Neuroimag. 2022;7(5):461–470. doi: 10.1016/j.bpsc.2021.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumusoglu S.B., Fine R.S., Murray S.J., Bittle J.L., Stevens H.E. The role of IL-6 in neurodevelopment after prenatal stress. Brain Behav. Immun. 2017;65:274–283. doi: 10.1016/j.bbi.2017.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur T.L., Shay L., Palkar A.V., Fisher S., Varaljay V.A., Dowd S., Bailey M.T. Prenatal stress affects placental cytokines and neurotrophins, commensal microbes, and anxiety-like behavior in adult female offspring. Brain Behav. Immun. 2017;64:50–58. doi: 10.1016/j.bbi.2016.12.021. [DOI] [PubMed] [Google Scholar]
- Gyllenhammer L.E., Rasmussen J.M., Bertele N., Halbing A., Entringer S., Wadhwa P.D., Buss C. Maternal inflammation during pregnancy and offspring brain development: the role of mitochondria. Biol. Psychia.Cogn. Neurosci. Neuroimag. 2022;7(5):498–509. doi: 10.1016/j.bpsc.2021.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han V.X., Patel S., Jones H.F., Dale R.C. Maternal immune activation and neuroinflammation in human neurodevelopmental disorders. Nat. Rev. Neurol. 2021;17(9):564–579. doi: 10.1038/s41582-021-00530-8. [DOI] [PubMed] [Google Scholar]
- Hayes L.N., An K., Carloni E., Li F., Vincent E., Trippaers C., Paranjpe M., Dölen G., Goff L.A., Ramos A., Kano S.I., Sawa A. Prenatal immune stress blunts microglia reactivity, impairing neurocircuitry. Nature. 2022;610(7931):327–334. doi: 10.1038/s41586-022-05274-z. [DOI] [PubMed] [Google Scholar]
- He D., Xu H., Zhang H., Tang R., Lan Y., Xing R., Li S., Christian E., Hou Y., Lorello P., Caldarone B., Ding J., Nguyen L., Dionne D., Thakore P., Schnell A., Huh J.R., Rozenblatt-Rosen O., Regev A., Kuchroo V.K. Disruption of the IL-33-ST2-AKT signaling axis impairs neurodevelopment by inhibiting microglial metabolic adaptation and phagocytic function. Immunity. 2022;55(1):159–173.e9. doi: 10.1016/j.immuni.2021.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman D.J., Powell T.L., Barrett E.S., Hardy D.B. Developmental origins of metabolic diseases. Physiol. Rev. 2021;101(3):739–795. doi: 10.1152/physrev.00002.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S., Beja-Glasser V.F., Nfonoyim B.M., Frouin A., Li S., Ramakrishnan S., Merry K.M., Shi Q., Rosenthal A., Barres B.A., Lemere C.A., Selkoe D.J., Stevens B. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science. 2016;352(6286):712–716. doi: 10.1126/science.aad8373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard L.M., Molyneaux E., Dennis C.L., Rochat T., Stein A., Milgrom J. Non-psychotic mental disorders in the perinatal period. Lancet. 2014;384(9956):1775–1788. doi: 10.1016/S0140-6736(14)61276-9. [DOI] [PubMed] [Google Scholar]
- Izquierdo I., Furini C.R., Myskiw J.C. Fear memory. Physiol. Rev. 2016;96(2):695–750. doi: 10.1152/physrev.00018.2015. [DOI] [PubMed] [Google Scholar]
- Jašarević E., Howard C.D., Misic A.M., Beiting D.P., Bale T.L. Stress during pregnancy alters temporal and spatial dynamics of the maternal and offspring microbiome in a sex-specific manner. Sci. Rep. 2017;7 doi: 10.1038/srep44182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones I., Chandra P.S., Dazzan P., Howard L.M. Bipolar disorder, affective psychosis, and schizophrenia in pregnancy and the post-partum period. Lancet. 2014;384(9956):1789–1799. doi: 10.1016/S0140-6736(14)61278-2. [DOI] [PubMed] [Google Scholar]
- Kalisch R., Gerlicher A.M.V., Duvarci S. A dopaminergic basis for fear extinction. Trends Cognit. Sci. 2019;23(4):274–277. doi: 10.1016/j.tics.2019.01.013. [DOI] [PubMed] [Google Scholar]
- Kim S., Kim H., Yim Y.S., Ha S., Atarashi K., Tan T.G., Longman R.S., Honda K., Littman D.R., Choi G.B., Huh J.R. Maternal gut bacteria promote neurodevelopmental abnormalities in mouse offspring. Nature. 2017;549(7673):528–532. doi: 10.1038/nature23910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krabbe S., Gründemann J., Lüthi A. Amygdala inhibitory circuits regulate associative fear conditioning. Biol. Psychiatr. 2018;83(10):800–809. doi: 10.1016/j.biopsych.2017.10.006. [DOI] [PubMed] [Google Scholar]
- Krystal J.H., Abdallah C.G., Averill L.A., Kelmendi B., Harpaz-Rotem I., Sanacora G., Southwick S.M., Duman R.S. Synaptic loss and the pathophysiology of PTSD: implications for ketamine as a prototype novel therapeutic. Curr. Psychiatr. Rep. 2017;19(10):74. doi: 10.1007/s11920-017-0829-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijer E.J., Steenbergen L. The microbiota-gut-brain axis in hippocampus-dependent learning and memory: current state and future challenges. Neurosci. Biobehav. Rev. 2023;152 doi: 10.1016/j.neubiorev.2023.105296. [DOI] [PubMed] [Google Scholar]
- Lautarescu A., Craig M.C., Glover V. Prenatal stress: effects on fetal and child brain development. Int. Rev. Neurobiol. 2020;150:17–40. doi: 10.1016/bs.irn.2019.11.002. [DOI] [PubMed] [Google Scholar]
- LeBlanc J.G., Chain F., Martín R., Bermúdez-Humarán L.G., Courau S., Langella P. Beneficial effects on host energy metabolism of short-chain fatty acids and vitamins produced by commensal and probiotic bacteria. Microb. Cell Factories. 2017;16(1):79. doi: 10.1186/s12934-017-0691-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E.J., Son G.H., Chung S., Lee S., Kim J., Choi S., Kim K. Impairment of fear memory consolidation in maternally stressed male mouse offspring: evidence for nongenomic glucocorticoid action on the amygdala. J. Neurosci. 2011;31(19):7131–7140. doi: 10.1523/JNEUROSCI.4692-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyh J., Paeschke S., Mages B., Michalski D., Nowicki M., Bechmann I., Winter K. Classification of microglial morphological phenotypes using machine learning. Front. Cell. Neurosci. 2021;15 doi: 10.3389/fncel.2021.701673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao L., Wang X., Yao X., Zhang B., Zhou L., Huang J. Gestational stress induced differential expression of HDAC2 in male rat offspring hippocampus during development. Neurosci. Res. 2019;147:9–16. doi: 10.1016/j.neures.2018.11.006. [DOI] [PubMed] [Google Scholar]
- Madore C., Leyrolle Q., Morel L., Rossitto M., Greenhalgh A.D., Delpech J.C., Martinat M., Bosch-Bouju C., Bourel J., Rani B., Lacabanne C., Thomazeau A., Hopperton K.E., Beccari S., Sere A., Aubert A., De Smedt-Peyrusse V., Lecours C., Bisht K., Fourgeaud L., Gregoire S., Bretillon L., Acar N., Grant N.J., Badaut J., Gressens P., Sierra A., Butovsky O., Tremblay M.E., Bazinet R.P., Joffre C., Nadjar A., Layé S. Essential omega-3 fatty acids tune microglial phagocytosis of synaptic elements in the mouse developing brain. Nat. Commun. 2020;11(1):6133. doi: 10.1038/s41467-020-19861-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad M.R., Quirk G.J. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420(6911):70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Moisiadis V.G., Matthews S.G. Glucocorticoids and fetal programming part 2: mechanisms. Nat. Rev. Endocrinol. 2014;10(7):403–411. doi: 10.1038/nrendo.2014.74. [DOI] [PubMed] [Google Scholar]
- Nguyen P.T., Dorman L.C., Pan S., Vainchtein I.D., Han R.T., Nakao-Inoue H., Taloma S.E., Barron J.J., Molofsky A.B., Kheirbek M.A., Molofsky A.V. Microglial remodeling of the extracellular matrix promotes synapse plasticity. Cell. 2020;182(2):388–403.e15. doi: 10.1016/j.cell.2020.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notter T., Meyer U. Microglia and schizophrenia: where next? Mol. Psychiatr. 2017;22(6):788–789. doi: 10.1038/mp.2017.67. [DOI] [PubMed] [Google Scholar]
- Orsini C.A., Maren S. Neural and cellular mechanisms of fear and extinction memory formation. Neurosci. Biobehav. Rev. 2012;36(7):1773–1802. doi: 10.1016/j.neubiorev.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pronovost G.N., Hsiao E.Y. Perinatal interactions between the microbiome, immunity, and neurodevelopment. Immunity. 2019;50(1):18–36. doi: 10.1016/j.immuni.2018.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddaway J., Richardson P.E., Bevan R.J., Stoneman J., Palombo M. Microglial morphometric analysis: so many options, so little consistency. Front. Neuroinf. 2023;17 doi: 10.3389/fninf.2023.1211188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salm A.K., Lally B.E., Borysiewicz E., Fil D., Konat G. Analysis of extinction acquisition to attenuated tones in prenatally stressed and non-stressed offspring following auditory fear conditioning. Physiol. Behav. 2015;139:157–166. doi: 10.1016/j.physbeh.2014.11.027. [DOI] [PubMed] [Google Scholar]
- Savignac H.M., Tramullas M., Kiely B., Dinan T.G., Cryan J.F. Bifidobacteria modulate cognitive processes in an anxious mouse strain. Behav. Brain Res. 2015;287:59–72. doi: 10.1016/j.bbr.2015.02.044. [DOI] [PubMed] [Google Scholar]
- Scheppach W. Effects of short chain fatty acids on gut morphology and function. Gut. 1994;35(1 Suppl. l):S35–S38. doi: 10.1136/gut.35.1_suppl.s35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K.L., Kassem M.S., Clarke D.J., Kuligowski M.P., Bedoya-Pérez M.A., Todd S.M., Lagopoulos J., Bennett M.R., Arnold J.C. Microglial cell hyper-ramification and neuronal dendritic spine loss in the hippocampus and medial prefrontal cortex in a mouse model of PTSD. Brain Behav. Immun. 2019;80:889–899. doi: 10.1016/j.bbi.2019.05.04. [DOI] [PubMed] [Google Scholar]
- Song J., Zhou B., Kan J., Liu G., Zhang S., Si L., Zhang X., Yang X., Ma J., Cheng J., Yang Y., Liu X. Gut microbiota: linking nutrition and perinatal depression. Front. Cell. Infect. Microbiol. 2022;12 doi: 10.3389/fcimb.2022.932309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L., Sun Q., Zheng H., Zhang Y., Wang Y., Liu S., Duan L. Roseburia hominis alleviates neuroinflammation via short-chain fatty acids through histone deacetylase inhibition. Mol. Nutr. Food Res. 2022;66(18) doi: 10.1002/mnfr.202200164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark P.L., Lee A. The microbial ecology of the large bowel of breast-fed and formula-fed infants during the first year of life. J. Med. Microbiol. 1982;15(2):189–203. doi: 10.1099/00222615-15-2-189. [DOI] [PubMed] [Google Scholar]
- Stoeva M.K., Garcia-So J., Justice N., Myers J., Tyagi S., Nemchek M., McMurdie P.J., Kolterman O., Eid J. Butyrate-producing human gut symbiont, Clostridium butyricum, and its role in health and disease. Gut Microb. 2021;13(1):1–28. doi: 10.1080/19490976.2021.1907272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Li H., Jin Y., Yu J., Mao S., Su K.P., Ling Z., Liu J. Probiotic Clostridium butyricum ameliorated motor deficits in a mouse model of Parkinson's disease via gut microbiota-GLP-1 pathway. Brain Behav. Immun. 2021;91:703–715. doi: 10.1016/j.bbi.2020.10.014. [DOI] [PubMed] [Google Scholar]
- Tay T.L., Béchade C., D'Andrea I., St-Pierre M.K., Henry M.S., Roumier A., Tremblay M.E. Microglia gone rogue: impacts on psychiatric disorders across the lifespan. Front. Mol. Neurosci. 2018;10:421. doi: 10.3389/fnmol.2017.00421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ten-Blanco M., Flores Á., Pereda-Pérez I., Piscitelli F., Izquierdo-Luengo C., Cristino L., Romero J., Hillard C.J., Maldonado R., Di Marzo V., Berrendero F. Amygdalar CB2 cannabinoid receptor mediates fear extinction deficits promoted by orexin-A/hypocretin-1. Biomed. Pharmacother. 2022;149 doi: 10.1016/j.biopha.2022.112925. [DOI] [PubMed] [Google Scholar]
- Thion M.S., Ginhoux F., Garel S. Microglia and early brain development: an intimate journey. Science. 2018;362(6411):185–189. doi: 10.1126/science.aat0474. [DOI] [PubMed] [Google Scholar]
- Van den Bergh B.R.H., van den Heuvel M.I., Lahti M., Braeken M., de Rooij S.R., Entringer S., Hoyer D., Roseboom T., Räikkönen K., King S., Schwab M. Prenatal developmental origins of behavior and mental health: the influence of maternal stress in pregnancy. Neurosci. Biobehav. Rev. 2020;117:26–64. doi: 10.1016/j.neubiorev.2017.07.003. [DOI] [PubMed] [Google Scholar]
- Vuong H.E., Pronovost G.N., Williams D.W., Coley E.J.L., Siegler E.L., Qiu A., Kazantsev M., Wilson C.J., Rendon T., Hsiao E.Y. The maternal microbiome modulates fetal neurodevelopment in mice. Nature. 2020;586(7828):281–286. doi: 10.1038/s41586-020-2745-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Braun C., Murphy E.F., Enck P. Bifidobacterium longum 1714™ strain modulates brain activity of healthy volunteers during social stress. Am. J. Gastroenterol. 2019;114(7):1152–1162. doi: 10.14309/ajg.0000000000000203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Yue H., Hu Z., Shen Y., Ma J., Li J., Wang X.D., Wang L., Sun B., Shi P., Wang L., Gu Y. Microglia mediate forgetting via complement-dependent synaptic elimination. Science. 2020;367(6478):688–694. doi: 10.1126/science.aaz2288. [DOI] [PubMed] [Google Scholar]
- Wang S., Egan M., Ryan C.A., Boyaval P., Dempsey E.M., Ross R.P., Stanton C. A good start in life is important-perinatal factors dictate early microbiota development and longer term maturation. FEMS Microbiol. Rev. 2020;44(6):763–781. doi: 10.1093/femsre/fuaa030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Chen H.S., Li H.H., Wang H.J., Zou R.S., Lu X.J., Wang J., Nie B.B., Wu J.F., Li S., Shan B.C., Wu P.F., Long L.H., Hu Z.L., Chen J.G., Wang F. Microglia-dependent excessive synaptic pruning leads to cortical underconnectivity and behavioral abnormality following chronic social defeat stress in mice. Brain Behav. Immun. 2023;109:23–36. doi: 10.1016/j.bbi.2022.12.019. [DOI] [PubMed] [Google Scholar]
- Wei Q., Shi H., Ma X., Shi Y., Zhang Y., Wang L. The impact of maternal stress on offspring birth weight and the mediating effect of dietary patterns: the Shanghai Maternal-Child Pairs Cohort study. J. Affect. Disord. 2021;278:643–649. doi: 10.1016/j.jad.2020.09.077. [DOI] [PubMed] [Google Scholar]
- Yi Nillni, Mehralizade A., Mayer L., Milanovic S. Treatment of depression, anxiety, and trauma-related disorders during the perinatal period: a systematic review. Clin. Psychol. Rev. 2018;66:136–148. doi: 10.1016/j.cpr.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z.H., Tu J.L., Li X.H., Hua Q., Liu W.Z., Liu Y., Pan B.X., Hu P., Zhang W.H. Neuroinflammation induces anxiety- and depressive-like behavior by modulating neuronal plasticity in the basolateral amygdala. Brain Behav. Immun. 2021;91:505–518. doi: 10.1016/j.bbi.2020.11.007. [DOI] [PubMed] [Google Scholar]
- Zhou J., Zhou Z., Ji P., Ma M., Guo J., Jiang S. Effect of fecal microbiota transplantation on experimental colitis in mice. Exp. Ther. Med. 2019;17(4):2581–2586. doi: 10.3892/etm.2019.7263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H., Tian P., Qian X., Gu L., Zhao J., Wang G., Chen W. Perinatal transmission of a probiotic Bifidobacterium strain protects against early life stress-induced mood and gastrointestinal motility disorders. Food Funct. 2022;13(14):7520–7528. doi: 10.1039/d2fo01164f. [DOI] [PubMed] [Google Scholar]
- Zijlmans M.A., Korpela K., Riksen-Walraven J.M., de Vos W.M., de Weerth C. Maternal prenatal stress is associated with the infant intestinal microbiota. Psychoneuroendocrinology. 2015;53:233–245. doi: 10.1016/j.psyneuen.2015.01.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.