Abstract
Obesity is one of the main risk factors for progression of chronic kidney disease (CKD). Weight loss interventions have limited efficacy in patients with pre-dialysis CKD. Our objective is to test the efficacy of a cognitive behavioral therapy program for obesity management in patients with CKD. We will conduct a randomized controlled intervention trial to evaluate the effects of cognitive behavioral therapy for obesity on weight loss, change in proteinuria, weight maintenance, quality of life, depression symptoms, and anxiety symptoms in patients with CKD. The duration of the intervention will be 16 weeks. The primary study outcomes will be body mass index (BMI) and proteinuria. CKD patients will be randomized into two groups: an intervention group with cognitive behavioral therapy, sessions with a dietitian and a kinesiologist, and a control group with sessions with a dietitian and a kinesiologist, without cognitive behavioral therapy. Study outcomes will be assessed at baseline, immediately after the 16-week intervention, 3 months after the end of the intervention, and 12 months after the end of the intervention. This study will be the first to evaluate the efficacy of cognitive behavioral therapy for obesity in patients with CKD. We expect that our results will contribute to new ways of non-pharmacological treatment of CKD.
Clinical trial registration
ClinicalTrials.Gov, NCT05927337.
Keywords: Cognitive behavioral therapy, Obesity, Proteinuria, Chronic kidney disease, Non-pharmacological interventions
1. Introduction
Obesity is one of the major risk factors for chronic kidney disease (CKD) along with arterial hypertension, diabetes, cardiovascular disease, age, genetic factors, and other lifestyle factors, such as smoking [1,2]. Studies examining the relationship between obesity and kidney disease show that obesity increases the risk of kidney disease, and leads to more rapid disease progression and kidney failure in individuals already diagnosed with kidney disease [3]. With early diagnosis, appropriate treatment and lifestyle modification, disease progression can be limited and, in some cases, even halted [4].
Therapeutic interventions for weight loss in patients with CKD mostly focus on lifestyle modification (dietary and exercise interventions), pharmacological treatment and surgical treatment [3,5]. Weight loss interventions resulted in greater change in body mass index change in the intervention groups, on average for 2.18 kg/m2 (p = 0.12) more than the control group; on average patients in intervention groups lost 3.7 kg (p = 0.07) more than controls [5]. In one of the systematic reviews [5] the interventions had uncertain effects on proteinuria (mean difference −0.29 g/day, p = 0,22),. In another systematic review, the interventions significantly reduced proteinuria levels by −1.31 g/day (p > 0.01) [3].
Incorporating clinical psychological and psychotherapeutic interventions into the management of overweight and obese patients can contribute significantly to the success of lifestyle changes and increased motivation for weight loss [6]. Indeed, cognitive processes play an important role in the maintenance of dysfunctional eating patterns [7]. Cognitive behavioural therapy and behavioural therapies are widely used and effective in the weight loss process [8,9,23]. The starting point of behavioural and cognitive behavioural therapies is the assumption that behaviours are learned and therefore can be unlearned, changed or replaced through a therapeutic process involving a variety of behavioural and cognitive techniques (Foster et al., 2005; Spahn et al., 2010).
One of the successful cognitive behavioral therapy programs for weight loss and weight maintenance is Individualized Cognitive Behavioural Therapy for Obesity [10]. It has three central goals, i) losing and maintaining an appropriate weight, ii) adopting and maintaining a lifestyle that allows for adequate weight control, and iii) developing a stable mindset about weight control [11]. In the therapeutic process, the therapist develops a collaborative relationship with the patient in which the patient plays an active role in abandoning unhealthy lifestyle habits and developing more appropriate ones that facilitate the achievement and maintenance of a healthy weight [10]. The approach was developed for different levels of obesity, is delivered in an individual or group format, and includes a preparatory phase (one to two sessions) in which the level of obesity, associated health and psychological problems are assessed and the patient is engaged in the treatment, followed by a first phase (16 sessions over 24 weeks) that focuses on weight loss, and a second phase (12 sessions over 48 weeks) in which the work focuses on weight maintenance. Program lasts 18 months [10]. The shortened and adapted program (only 22 sessions) has also been shown to be successful, with participants reducing their body weight by an average of 11.5 % [12].
The impact end efficacy of weight loss interventions in patients with CKD are limited in terms of clinical effectiveness, and further research with different approaches for weight loss are needed. To our knowledge, there are no studies examining the effectiveness of cognitive behavioral therapy for weight loss in patients with pre-dialysis CKD and the impact on renal end points relevant for CKD progression. Considering the lack of knowledge on the success of psychological interventions in weight loss and weight maintenance process in patents with CKD, well-designed interventional studies are needed. We adapted (shortened) the Individualized cognitive behavioural therapy for obesity management [10] and we will test its efficacy in overweight CKD patients.
Therefore, the following research questions were identified:
-
i.
How efficient is a cognitive behavioral intervention for obesity management in terms of weight loss and change in proteinuria in patients with CKD?
-
ii.
What is the long-term efficacy of cognitive behavioral intervention for obesity management in patients with CKD?
-
iii.
Do personality traits and self-efficacy influence weight loss?
-
Iv.
What is the impact of cognitive behavioral intervention for obesity management on quality of life, depression and anxiety symptoms?
The aim of our study will be to test the efficacy of a cognitive behavioral therapy program for obesity management in patients with CKD.
2. Materials and methods
2.1. Participants
Participants will be recruited at the outpatient nephrology clinic of University Medical Center Ljubljana, Slovenia. The study will include 40 participants with chronic kidney disease stages 2–4 (proteinuria form). Decision to focus on earlier stages is primarily driven by the goal of intervening at an earlier stage of the disease and potentially slow the progression of disease. Participants will be randomly assigned to the intervention (20) or control (20) group with gender stratification. Table 1 provides an overview of the inclusion and exclusion criteria. The study will be conducted in accordance with the ethical standards of the 1964 Declaration of Helsinki and was approved by the Slovenian Medical Ethical Committee (number 0120-26/2023/3). Participants will sign an informed consent form before participating in the study. The study has been registered at ClinicalTrials.gov under NCT05927337.
Table 1.
Study criteria.
| Inclusion criteria | Exclusion criteria |
|---|---|
| CKD from 2nd to 4th stage | Dementia |
| BMI index ≥30 kg/m2 or BMI ≥28 kg/m2 and WC ≥ 94 cm (men) or ≥ 80 cm (women) | Acute psychiatric illness or chronic, poorly managed psychiatric illness; |
| Estimated daily proteinuria ≥200 mg of protein per g of creatinine in the urine | Bioimpedance findings of low lean body mass index below that expected for age and sex (or presence of any other sarcopenic obesity criteria) |
| Diabetes type 2 or not | Active chronic inflammatory disease (e.g. active vasculitis, SLE, rheumatoid arthritis) or active cancer |
| Active nephrotic syndrome | |
| NYHA grade 3 or 4 heart failure | |
| Spontaneous weight loss of 5 % or more in the last 6-month period | |
| Receiving induction immunosuppression therapy for autoimmune renal disease Pharmacological treatment of obesity or any other active obesity treatment |
|
| Any other clinical factor that puts the patient at risk with regard to metabolic stability and daily energy expenditure. |
CKD: chronic kidney disease, BMI: body mass index, WC: waist circumference, SLE: Systemic lupus erythematosus, NYHA: New York Heart Association Functional Classification.
2.2. Study design
This is a randomized, controlled, open- This is a randomized, controlled, open-label trial designed to examine the effects of a cognitive behavioral intervention for obesity management on weight loss, proteinuria change, weight maintenance, other measures of metabolic regulation, psychological aspects (depression symptoms, anxiety symptoms, quality of life, eating disorders risk factors) and the relationship between psychological variables and the success of the cognitive behavioral intervention.
After screening, recruitment, and baseline measurements, patients will be randomized in 1:1 ratio into two groups, using sealed envelopes. Randomization will be performed using the Clinical Trial Randomization Tool form The National Cancer Institute's Division of Cancer Prevention.
Intervention group: participants will be enrolled in a 4-month program of cognitive behavioural therapy for obesity management. The program is described in more detail in the next section. The program consists of 12 individual cognitive behavioral therapy sessions with psychologist. Sessions are weekly for the first eight weeks and bi-monthly for the next eight weeks. Participants will have their physical parameters measured and psychological characteristics assessed at baseline, at the end of the intervention, and three months and one year after the end of the program. Participants will also have 3 individual sessions with a dietician and 3 individual sessions with a kinesiologist.
Control group: participants who will have measurements and assessment, 3 individual sessions with a dietician, and 3 individual sessions with a kinesiologist.
This study follows the SPIRIT guidelines and reporting template [13], that is available in supplementary material file.
2.3. Procedures
Participants will be approached in the nephrology outpatient clinic. The study will be described, and the risks and benefits will be explained to each participant prior to enrolment in the study. Refusal to participate in the study will not affect further medical treatment and care. We will explain to participants that they can stop participating at any time, without consequences on their medical treatment and care. Principal investigator will obtain informed consent from potential study participants.
We do not anticipate that participation in the study will involve risks or significant burdens for participants. The burden is mainly time-related, as patients in the control group will have to take time for the assessment and measurements, which will take place four times for 60 min, and for three visits with the dietitian and kinesiologist, which will last 45 min. Patients in the intervention group will need to make time for the assessment, which will take place four times for 60 min, for 12 individual therapeutic sessions with the psychologist, which will last 45 min, and for three visits with the dietitian and kinesiologist, which will last 45 min. We believe that the potential benefits for the patients involved far outweigh the burden of participating in the study. At the same time, we also believe that, based on the data on the safety of lifestyle interventions known so far, the safety of participants is sufficiently high or that the study intervention poses no significant health risks. Subjects who we know are unsuitable for obesity reduction programs based on published data to date (e.g., patients with cancer, inflammatory diseases) will not be eligible for participation in the study based on the above criteria. We will ensure that the sessions with the dietitian and kinesiologist will take place on the same days as the sessions with the psychologist if the patients agree. The schedule will be individualized and adapted to fit the patient's other commitments and activities.
2.3.1. Study interventions and protocol
The intent of the intervention is to help patients with CKD achieve appropriate weight management. The intervention is based on the Individualized cognitive behavioural therapy for obesity management [10]. A team of clinical psychologists, psychologists, medical doctors and researchers collaborated to develop an intervention based on the previously mentioned program. Main six modules are the same as in the original program, we changed the duration of overall program, reduced the number of sessions, and slightly adjusted the content, but topics are the same as in the program by Dalle Grave et al. [10]. Table 2 shows the main content of the program and each session.
Table 2.
Intended treatment plan and key content summary of the sessions.
| Session | Module | Content |
|---|---|---|
| 1 | Monitoring food intake, physical activity, and body weight | Introduction Cognitive behavioral therapy model Real-time monitoring of food intake, physical activity, and weekly weighing |
| 2 | Changing eating | Energy intake, nutrition, and energy deficit Planning when, where and what to eat Mindful eating |
| 3 | Developing an active lifestyle | Encouraging physical activity Creating more active and less sedentary lifestyle Cycle of change Goal setting |
| 4 | Addressing obstacles to weight loss | Education on weight loss obstacles Weigt loss obstacles questionnaire Personal formulation of weight loss obstacles |
| 5 | Addressing obstacles to weight loss | Changing environment Reducing environmental stimuli |
| 6 | Addressing obstacles to weight loss | Using food as reward Barriers for physical activity Problem solving |
| 7 | Addressing obstacles to weight loss | Negative automatic thoughts Thinking errors |
| 8 | Addressing weight loss dissatisfaction | Unrealistic weight loss goals Dysfunctional primary goals for losing weight Negative body image |
| 9 | Addressing obstacles to weight maintenance | Weight maintenance education Processes for weight maintenance |
| 10 | Addressing obstacles to weight maintenance | Relapse prevention Developing a weight maintenance plan |
| 11 | Addressing obstacles to weight maintenance | Developing a weight maintenance plan |
| 12 | Addressing obstacles to weight maintenance | Conclusion |
The session structure is the same as in the original program [10]. The duration of each session is 45 min and includes the following parts: greeting and weighing (5 min), homework review (10 min), setting the agenda for the session (2 min), main part of the session and setting new homework (25 min), closing the session (3 min).
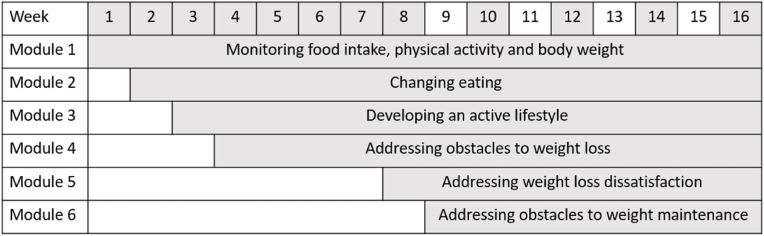
Based on individual characteristics, modules and sessions will be flexible and tailored to the needs of the patient. Therefore, not all patients will have the same session schedule. Fig. 1 shows the planned treatment schedule broken down by weeks and modules. The shaded numbers represent weeks of cognitive behavioral therapy sessions.
Fig. 1.
Program layout.
2.3.1.1. Sessions with the dietician
In the first session, the dietitian assesses the participant's eating habits based on an interview and food diary. He provides advice for shaping eating habits according to the dietary guidelines for patients with CKD. Emphasis is placed on maintaining an appropriate energy deficit; evenly distributing meals throughout the day; adequate protein intake; regular consumption of fruits, vegetables, and legumes; limiting salt intake; and avoiding processed and fast foods. The patient will attend a follow-up appointment with a dietitian in one month, at which time the dietitian will determine the success of the intervention to date and recommend additional interventions. The second follow-up appointment will take place in another 2 months (3 months after the first appointment). The dietitian will work with the participant to analyse the success of the intervention. The sessions will take place in the first week, in week 5 and in week 12.
2.3.1.2. Sessions with the kinesiologist
In the first session, the kinesiologist will assess the participant's physical fitness and ask them about their current physical activity. In the second session, the kinesiologist works with the patient to create an exercise plan. In the third session, the plan is evaluated and adjusted. Sessions will take place in the first week 1, in week 5 and in week 12.
2.3.2. Primary study end points
Change in body mass index: change in body mass index between the start of the program (baseline) and immediately after the end of the program (4 months after the start), three months after the end of the program (7 months after the start) and 1 year after the end of the program (16 months after the start). Body mass index will be calculated using body height and body weight according to the formula BMI = kg/m2.
Change in proteinuria: change in proteinuria levels between the start of the program (baseline) and immediately after the end the of program (4 months after the start), three months after the end of the program (7 months after the start) and 1 year after the end of the program (16 months after the start). Proteinuria will be measured from urine sample.
2.3.3. Secondary study end points
Table 3 summarises when which outcomes will be measured and assessed. Secondary biological outcomes of the study include other measures of metabolic regulation - waist circumference, body fat, LDL cholesterol, blood pressure, fasting blood glucose, glycated haemoglobin (HbA1c), waist circumference and blood pressure will be measured at the outpatient visit by a trained nurse; other parameters will be measured from a blood sample, collected by a trained nurse. Secondary psychological outcomes will include personality traits, depression symptoms, anxiety symptoms, self-efficacy, quality of life and risk factors for eating disorders. Psychological assessment will be performed by a psychologist. We will use the following questionnaires and scales:
-
-
Beck's Depression Inventory BDI-II [14]: The BDI-II is used to assess depressive symptoms in an individual. The questionnaire has a self-report format, it includes 21 statements to which the individual responds on a four-point scale.
-
-
State-Trait Anxiety Inventory STAI [15]: The STAI questionnaire includes the STAI-1 and STAI-2 formats. The STAI-1 assesses anxiety as a state and the STAI-2 assesses anxiety as a trait - a general predisposition of an individual to perceive certain situations as threatening. The questionnaire has a self-report format, with 20 statements in each format, to which the individual responds on a four-point scale.
-
-
SF-36v2® Healty outcome Survey - Generic Multidimensional Short-form Quality-of-Life Survey [16]: The SF-36v2 is a generic quality of life questionnaire used to assess eight domains of health, which can be grouped into two components - physical and mental health. The questionnaire has a self-report format, comprising 36 statements, each of which is answered on a three- or five-point scale.
-
-
Eating Disorder Examination Questionnaire EDE-Q 6 [17]: The questionnaire assesses behaviours and attitudes related to eating disorders in the past 28 days. The questionnaire has a self-report format, comprising 28 statements to which the individual responds on a seven-point scale.
-
-
Big Five Questionnaire BFQ [18]: The questionnaire assesses the five big personality factors - energy, agreeableness, conscientiousness, emotional stability and openness, each of the factors is divided into two sub-factors, and the questionnaire also includes a social desirability scale. The questionnaire has a self-report format, comprising 132 statements to which the individual responds on a five-point scale.
-
-
General Self-Efficacy Scale GSE [19]: The scale is designed to assess optimistic beliefs about oneself to cope with life's challenges. The questionnaire has a self-report format, comprising 10 statements to which the individual responds on a four-point scale.
Table 3.
Study outcomes.
| Body weight | WC | Blood sample | Urin sample | BP | SF-36v2® | BDI-II | STAI | BFQ | EDE-Q 6 | GSI | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline (0 months) | X | X | X | X | X | X | X | X | X | X | X |
| At the end of the program (4 months from baseline) | X | X | X | X | X | X | X | X | X | ||
| 3 months after the end (7 months from baseline) | X | X | X | X | X | X | X | X | X | ||
| 12 months after the end (16 months from baseline) | X | X | X | X | X | X | X | X | X |
WC: waist circumference, BP: blood pressure, SF-36v2®: SF-36v2® Healty outcome Survey - Generic Multidimensional Short-form Quality-of-Life Survey, BDI-II: Beck's Depression Inventory, STAI: State-Trait Anxiety Inventory; BFQ: Big Five Questionnaire; EDE-Q 6: Eating Disorder Examination Questionnaire; GSI: General Self-Efficacy Scale.
2.3.4. Statistical methods including sample size calculation
The sample size was calculated using G*Power software, version 3.1; [20], based on the results of the study by Fernández-Ruiz et al. [21], calculated on the size of the effect of weight reduction and the expected variability of this parameter. The alpha error probability was set to 0.05, the 1-beta error probability to 0.89, while the effect size was taken from the previously mentioned study (0.56). A sample size of 34 participants was calculated. Considering an attrition rate of 20 %, a total number of 40 participants is required, with 20 participants assigned to each group. Analyses will be conducted according to the intention-to-treat principle.
SPSS software, version 29 (SPSS, Inc., Chicago, IL, United States) will be used for all calculations. Data will be presented with mean ± standard deviation and 95 % confidence interval, where applicable. Normality will be tested using the Shapiro–Wilk test and an additional Q– Q-plot visual inspection. To determine group differences in clinical and demographic variables, t tests or Mann-Whitney tests will be used. Correlations and linear regression will also be calculated and analysed. Main effects will be analysed using a mixed general linear model (GLM), with group (intervention and control) and time point (baseline and after 4 months) as factors. After determining the interaction effect, a secondary analysis will be used to determine the time effect in both groups. In addition, the magnitude of the effect for the dependent variables will be determined using partial eta squared (η2). Statistical significance will be set at values of p < 0.05.
As for missing data, we will do our best to avoid missing data. Data will be collected very carefully. Psychological assessments will be done individually, psychologists will review the questionnaires when they are fully completed. Participants will be reminded in advance to collect blood and urine samples, and we will try to balance this with patients' other commitments and activities, and on days with other appointments, so that it is not an additional burden for them.
3. Discussion
The presented study will be the first randomized controlled intervention using cognitive behavioral therapy to manage obesity and proteinuria in patients with CKD. By testing the potential beneficial effect of a non-pharmacological intervention, we will address a significant risk factor in CKD. We hypothesize that after the intervention, the intervention group will lose significantly more weight compared to the control group, significantly reduce proteinuria levels, and achieve a clinically meaningful effect size in the areas of weight loss, reduced proteinuria levels, weight maintenance, quality of life, depression, and anxiety symptoms compared to the control group. Interventions for weight management in CKD have limited and unclear effects on weight loss and proteinuria [5]. Therefore, there is a need for studies exploring new approaches to weight management in CKD, as obesity is a major risk for the onset of CKD and its progression to end-stage disease.
Current obesity management approaches (biomedical approaches, behavioral treatment, bariatric surgery) have some drawbacks, such as high dropout rates and poor long-term weight maintenance, and bariatric surgery is indicated only for a small group of patients. All approaches overlook the role of cognitive processes in the weight loss process [10]. Cognitive processes play an important role in eating behaviors and obesity management [7,10,22]. Cognitive processes are an essential component of cognitive behavioral therapy. Recent meta-analyses show that cognitive behavioral therapy is an effective approach for successful weight loss and maintenance of lost weight [8,9,23].
None of the studies conducted to date examined the efficacy of cognitive behavioral therapy for weight management in patients with pre-dialysis CKD. With this in mind, it is reasonable to investigate the efficacy of cognitive behavioral therapy for obesity management in a group of patients with CKD. In our research protocol we will address the unanswered questions by implementing cognitive behavioral therapy in the management of obesity and proteinuria in patients with CKD. Based on our findings, we could develop recommendations and protocols aimed at improving lifestyle and addressing obesity risk factors in CKD progression. Psychological interventions could become part of a standard treatment approach for patients with early-stage CKD. If our hypotheses are confirmed, we may offer an evidence-based improvement in non-pharmacological treatment of CKD.
Ethics statement
The study was reviewed and approved by Slovenian Medical Ethical Committee (0120-26/2023/3). The participants will provide their written informed consent to participate in this study. All data will be anonymized, data will be stored in password protected files on password protected computer, only accessible by the research team. All paper-based data will be stored in a locked filing cabinet. To ensure data-quality, all collected data will be entered by one and cross-checked by another researcher.
Author contributions
KKM, JP, JK and BLZ conceptualized the study design. KKM drafted the manuscript, JP, JK, BLZ, AMP and ŠB reviewed the manuscript. All authors have read and approved the final version of the manuscript.
Funding
The study is funded by the public Slovenian Research Agency (Grant No. L3-1838 and postdoctoral research project Z3-3212). KKM is funded by Slovenian Research Agency Young researcher program and is PhD student at University of Ljubljana.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.conctc.2023.101236.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Kidney Disease Improving Global Outcomes KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney International Suplements. 2013;3(1) doi: 10.1038/ki.2013.243. www.publicationethics.org [DOI] [PubMed] [Google Scholar]
- 2.Nawaz S., Chinnadurai R., Al-Chalabi S., Evans P., Kalra P.A., Syed A.A., Sinha S. Obesity and chronic kidney disease: a current review. Obesity Science and Practice. 2023;9(2):61–74. doi: 10.1002/osp4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Navaneethan S.D., Yehnert H., Moustarah F., Schreiber M.J., Schauer P.R., Beddhu S. Weight loss interventions in chronic kidney disease: a systematic review and meta-analysis. Clin. J. Am. Soc. Nephrol. 2009;4(10):1565–1574. doi: 10.2215/CJN.02250409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Kidney Foundation . National Kidney Foundation; 2002. Clinical Practice Guidelines for Chronic Kidney Disease : Evaluation, Classification and Stratification. [Google Scholar]
- 5.Conley M.M., McFarlane C.M., Johnson D.W., Kelly J.T., Campbell K.L., MacLaughlin H.L. Interventions for weight loss in people with chronic kidney disease who are overweight or obese. Cochrane Database Syst. Rev. 2021;3(3) doi: 10.1002/14651858.CD013119.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castelnuovo G., Pietrabissa G., Manzoni G.M., Cattivelli R., Rossi A., Novelli M., Varallo G., Molinari E. Cognitive behavioral therapy to aid weight loss in obese patients: current perspectives. Psychol. Res. Behav. Manag. 2017;10:165–173. doi: 10.2147/PRBM.S113278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jansen A., Houben K., Roefs A. A cognitive profile of obesity and its translation into new interventions. Front. Psychol. 2015;6(NOV) doi: 10.3389/fpsyg.2015.01807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacob A., Moullec G., Lavoie K.L., Catherine Laurin, Cowan T., Tisshaw C., Kazazian C., Raddatz C., Bacon S.L. Impact of cognitive-behavioral interventions on weight loss and psychological outcomes: a meta-analysis. Health Psychol. 2018;37(5):417–432. doi: 10.1037/hea0000576.supp. [DOI] [PubMed] [Google Scholar]
- 9.Kurnik Mesarič K., Pajek J., Logar Zakrajšek B., Bogataj Š., Kodrič J. Cognitive behavioral therapy for lifestyle changes in patients with obesity and type 2 diabetes: a systematic review and meta-analysis. Sci. Rep. 2023;13(1) doi: 10.1038/s41598-023-40141-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dalle Grave R., Massimiliano S., El Ghoch M., Calugi S. Springer International Publishing; 2018. Treating Obesity with Personalized Cognitive Behavioral Therapy. [Google Scholar]
- 11.Dalle Grave R., Sartirana M., Calugi S. Personalized cognitive-behavioural therapy for obesity (CBT-OB): theory, strategies and procedures. Biopsychosoc. Med. 2020;14(1) doi: 10.1186/s13030-020-00177-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dalle Grave R., Calugi S., Bosco G., Valerio L., Valenti C., El Ghoch M., Zini D. Personalized group cognitive behavioural therapy for obesity: a longitudinal study in a real-world clinical setting. Eat. Weight Disord. 2020;25(2):337–346. doi: 10.1007/s40519-018-0593-z. [DOI] [PubMed] [Google Scholar]
- 13.Chan A.-W., Tetzlaff J., Altman D., Laupacis A., Gøtzsche P., Krleža-Jerić K., Hróbjartsson A., Mann H., Dickersin K., Berlin J., Doré C., Parulekar W., Summerskill W., Groves T., Schulz K., Sox H., Rockhold F., Rennie D., Moher D. SPIRIT 2013 Statement: defining standard protocol items for clinical trials. Ann. Intern. Med. 2013;158(3):200–207. doi: 10.7326/0003-4819-158-3-201302050-00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beck A.T., Steer R.A., Brown G.K. Psychological Corporation; 1996. Manual for the Beck Depression Inventory-II. [Google Scholar]
- 15.Spielberger C.D., Gorsuch R.L., Lushene R., Vagg P.R., Jacobs G.A. Consulting Psychologists Press; 1983. Manual for the State-Trait Anxiety Inventory. [Google Scholar]
- 16.Ware J., Kosinski M., Bjorner J., Turner-Bowker D., Gandek B., Maruish M. QualityMetric Incorporated; 2007. ser's Manual for the SF-36v2® Health Survey. [Google Scholar]
- 17.Fairburn C.G., Beglin S.J. In: Cognitive Behavior Therapy and Eating Disorders. Fairburn C.G., editor. Guilford Press; 2008. Eating disorder examination questionnaire (EDE-Q 6.0) pp. 309–313. [Google Scholar]
- 18.Caprara G.V., Barbaranelli C., Borcogni L., Perucini M. The “Big Five Questionnaire”: a new questionnaire to assess the five factor model. Pers. Indiv. Differ. 1993;15(3):281–288. [Google Scholar]
- 19.Schwarzer R., Jerusalem M. In: Measures in Health Psychology: A User's Portfolio. Causal and Control Beliefs. Weinman J., Wright S., Johnston M., editors. NEFR-Nelson; 1995. General self-efficacy scale; pp. 35–37. [Google Scholar]
- 20.Faul F., Erdfelder E., Buchner A., Lang G.-A. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav. Res. Methods. 2009;41:1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- 21.Fernández-Ruiz V.E., Armero-Barranco D., Paniagua-Urbano J.A., Sole-Agusti M., Ruiz-Sánchez A., Gómez-Marín J. Short-medium-long-term efficacy of interdisciplinary intervention against overweight and obesity: randomized controlled clinical trial. Int. J. Nurs. Pract. 2018;24(6) doi: 10.1111/ijn.12690. [DOI] [PubMed] [Google Scholar]
- 22.Stahre L., Tärnell B., Håkanson C.-E., Hällström T. A randomized controlled trial of two weight-reducing short-term group treatment programs for obesity with an 18-month follow-up. Int. J. Behav. Med. 2007;14(1):48–55. doi: 10.1007/BF02999227. [DOI] [PubMed] [Google Scholar]
- 23.Comsa L., David O., David D. Outcomes and mechanisms of change in cognitive-behavioral interventions for weight loss: a meta-analysis of randomized clinical trials. Behav. Res. Ther. 2020;132 doi: 10.1016/j.brat.2020.103654. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.