Abstract
Adenoid cystic carcinoma is a rare tumor that typically originates from secretory glands, most commonly found in the salivary glands. However, it can also develop as a primary cutaneous adenoid cystic carcinoma, which appears identical under the microscope to adenoid cystic carcinoma originating in other tissues. Distinguishing between primary cutaneous adenoid cystic carcinoma and extracutaneous adenoid cystic carcinoma with cutaneous metastases is crucial for determining the prognosis and appropriate management of the condition. In this case report, we describe a case of primary cutaneous adenoid cystic carcinoma located on the hand with lung metastases. Proper differentiation, treatment planning and regular clinical follow-up to monitor for any signs of recurrence or metastasis are essential to ensure favorable outcomes for patients with this rare neoplasm.
Keywords: Primary cutaneous adenoid cystic carcinoma, Adenoid cystic carcinoma, Lung metastases
Introduction
Adenoid cystic carcinoma (ACC) is commonly known as a malignant neoplasm of salivary glands in the head and neck region [1]. Rarely, ACC arises directly in the skin, this diagnosis can be made only after excluding metastatic deposits from other more common sites. In this report, we present a case of primary cutaneous adenoid cystic carcinoma (PCACC) in a 65-year-old male who also had pulmonary metastasis. This case adds to the limited body of knowledge on PCACC with metastatic spread and underscores the importance of further understanding and managing this rare entity.
Case report
A 79-year-old man, who was in overall good health, reported a gradually enlarging skin lesion on his left hand over the course of 6 months. The patient denied any history of trauma, and his medical history was unrelated to the current condition. During the physical examination, a large mass measuring approximately 8 × 6 cm in size was observed in the medial part of the left hand. No palpable lymph nodes or signs of organ enlargement were detected during the examination.
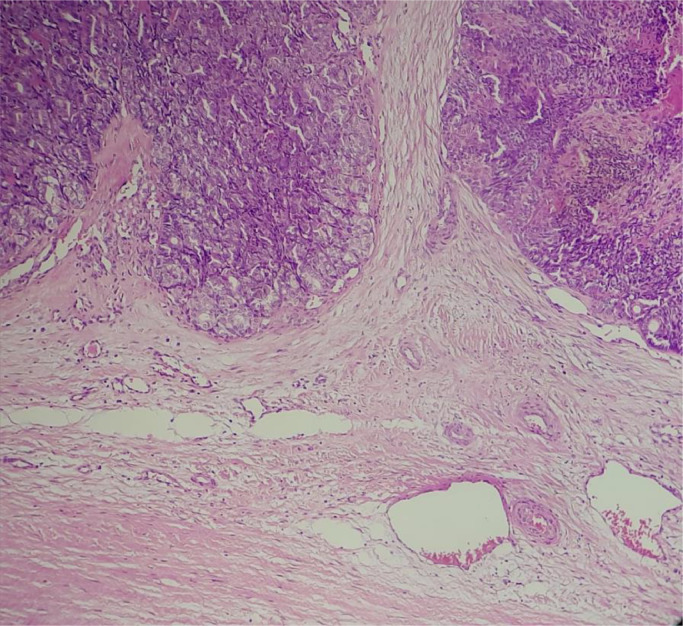
Biopsy showed a dermal tumor arranged predominantly in a sieve-like pattern, composed of cords and strands of basaloid cells with a cribriform, glandular, and cystic arrangement (Fig. 1).
Fig. 1.
Infiltrating intradermal basaloid tumor with cribriform pattern, mucinous secretion, and basement membrane material.
Considering the history, otorhinolaryngology examination, head, and neck imaging, which excluded the possibility of salivary gland origin, a diagnosis of PCACC was considered as the most likely explanation for the observed tumor characteristics.
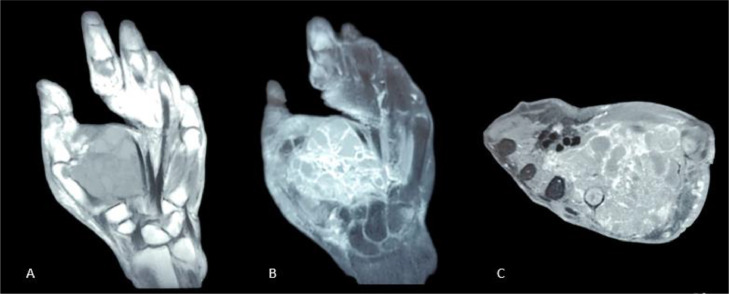
A hand MRI was conducted to assess the extent of the tumor and its extension to the surrounding structures. The MRI revealed a tumor of the thenar fossa which exhibited heterogeneous tissue density and contained cystic pockets separated by thick septa enhanced after gadolinium injection. The tumor infiltrated the metacarpals of the thumb and 2nd finger, along with their respective flexor tendons, and infiltration of the interosseous muscles of the 1st, 2nd, and 3rd interdigital spaces was also observed (Fig. 2). This MRI analysis provides information about the important extent and involvement of the tumor in the hand's anatomical structures, aiding in determining that the appropriate treatment for the patient was wide hand amputation.
Fig. 2.
Coronal T1 (A) with coronal (B) and axial T1 (C) FAT SAT sequences after gadolinium injection showing the tumor of the thenar fossa which exhibited heterogeneous tissue density and contained cystic pockets separated by thick septation.
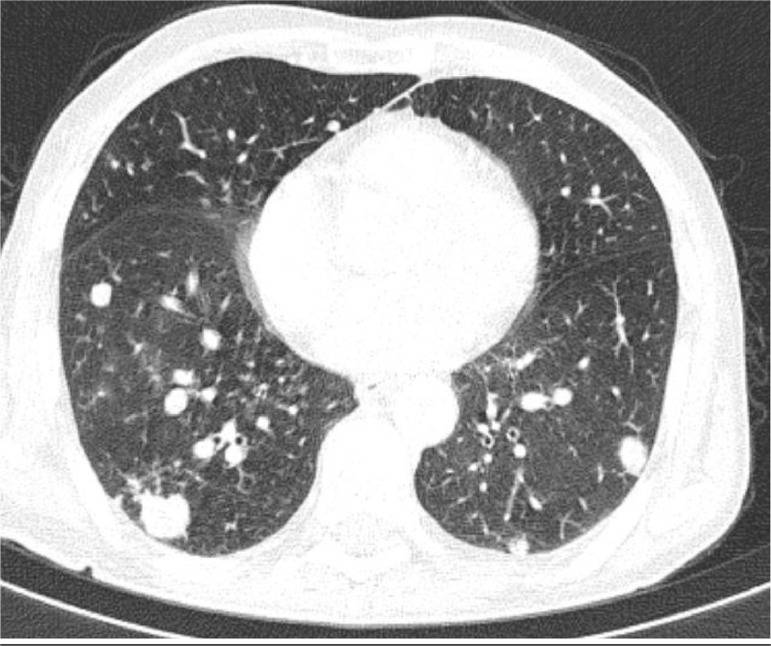
Contrast-enhanced CT chest revealed multiple lobulated mass lesions in both lungs showing contrast enhancement with no evidence of necrosis or calcification (Fig. 3). The findings were suggestive of metastasis.
Fig. 3.
CT chest showing multiple lobulated mass lesions in both lungs which are suggestive of metastasis.
A chemotherapy regimen with vinorelbine and cisplatin was initiated. The patient received 4 cycles over 4 months, and a follow-up CT scan indicated lesion stability, with the target lesions maintaining the same diameter and no new lesions appearing. It was decided to continue with the same treatment.
Discussion
ACC is most frequently observed as a tumor originating from both major and minor salivary glands. However, it can also develop in various other primary locations, such as the external auditory canal, lacrimal gland, respiratory tract, esophagus, breast, thymus, uterus, cervix, vulva, prostate, and skin. When ACC originates directly within the skin, it is termed primary cutaneous ACC. It is essential to distinguish this form from cases where ACC in the skin results from metastasis or direct extension from a salivary gland ACC. For our discussion, only primary cutaneous ACC is of relevance [3,4].
PCACC was first described by Boggio in 1975, and it has been classified by the World Health Organization as a rare skin tumor associated with skin appendages or adnexa. Typically, primary cutaneous ACC manifests as a slowly progressing nodule, which can often remain undiagnosed for an extended period [2,5]. This primary form shares histological similarities with ACC originating from the salivary glands. As a result, distinguishing between cutaneous and extracutaneous ACC is primarily reliant on clinical assessment. The diagnosis of cutaneous ACC can only be confirmed when there is no historical or current evidence of ACC arising from an extracutaneous source [5]. This differentiation is crucial because ACC originating from salivary glands is an aggressive tumor, often leading to death in most patients due to local recurrence and widespread metastases. On the other hand, cutaneous ACC tends to have a relatively slower progression despite a higher likelihood of local recurrence. This distinction holds paramount significance in determining the prognosis and management approach for patients [3,5].
While local recurrence is a common occurrence, distant metastases are infrequent. In cases where metastasis does occur, most reports describe involvement of lymph nodes, with pulmonary, chest wall, pericardium, bone, nose, and liver being less common sites of metastasis. Among these, lymph nodes and lungs are the most frequently affected metastatic sites in primary cutaneous ACC. Due to the potential for metastasis, careful and regular monitoring of patients is essential for timely intervention and appropriate management [2,3,5].
Microscopically, PCACC exhibits distinctive features. It is composed of islands and cords of basaloid cells, organized in solid, cribriform, or tubular patterns, and these structures are embedded within a loosely arranged fibromucinous stroma. The tumor is situated in the mid and deep dermis and lacks any connection with the epidermis. The margins of the tumor appear invasive, and about half of the cases show perineural involvement [5,6].
The tumor cells produce a basal lamina, which undergoes hyalinization and shows positive staining with PAS (Periodic Acid-Schiff) while being resistant to diastase. Additionally, the adenoid spaces or pseudolumina accumulate hyaluronic acid and sulfated mucins. Immunohistochemically, the tumor cells typically exhibit reactivity to markers such as EMA (Epithelial Membrane Antigen), cytokeratin, S-100, and actin [5].
The most critical differential diagnosis in this case is adenoid basal cell carcinoma, which shares similarities with PCACC. It is crucial to differentiate between them, as adenoid basal cell carcinoma does not metastasize, unlike PCACC. To aid in challenging cases, immunohistochemistry can be employed. PCACC typically shows positive staining for EMA, CEA, and S100, which is not observed in adenoid basal cell carcinoma [7,3].
Other primary skin tumors that should be differentiated include mucinous carcinoma of the skin, primary cutaneous cribriform apocrine carcinoma (PCCAC), dermal cylindroma, and spiradenoma. Mucinous carcinoma shows positivity for sialomucins. PCCAC lacks perineural invasion and exhibits a cribriform architecture throughout the tumor with nuclear pleomorphism, whereas PCACC demonstrates uniform cells. Spiradenoma presents small adenoid cystic areas only focally, and dermal cylindroma displays solid nests of tightly fitting tumor cells with hyaline material surrounding them. Proper differentiation of these tumor types is essential for accurate diagnosis and appropriate treatment decisions [5], [6], [7].
The gold standard treatment for PCACC involves performing a wide local surgical resection with a minimum of a 2 cm margin around the tumor. This extensive resection aims to eliminate any recurrences that might arise due to the presence of perineural extensions, which can be difficult to detect. In some cases, patients may also receive adjuvant or therapeutic radiation therapy to further target any remaining cancer cells. However, chemotherapy is not a common therapeutic option for treating this type of neoplasm [6,8].
Due to its propensity for local recurrence, it is essential for patients to undergo regular clinical follow-up to monitor for any signs of recurrence or metastasis. Early detection of any potential recurrence is crucial for prompt intervention and improving the patient's prognosis [4,8].
Patient consent
Written informed consent for the publication of this case report was obtained from the patient.
Footnotes
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Xu YG, Hinshaw M, Longley BJ, Ilyas H, Snow SN. Cutaneous adenoid cystic carcinoma with perineural invasion treated by mohs micrographic surgery-a case report with literature review. J Oncol. 2010;2010 doi: 10.1155/2010/469049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Droussi H, Ettalbi S, Ouahbi S, Soussou M, Boukind S. Carcinome adénoïde kystique du cuir chevelu. Ann Dermatol Venereol. 2011;138(5):418–421. doi: 10.1016/j.annder.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 3.Singh A, Ramesh V. Primary cutaneous adenoid cystic carcinoma with distant metastasis: a case report and brief literature review. Indian J Dermatol Venereol Leprol. 2010;76(2):176–179. doi: 10.4103/0378-6323.60573. [DOI] [PubMed] [Google Scholar]
- 4.Pozzobon LD, Glikstein R, Laurie SA, Hanagandi P, Michaud J, Purgina B, et al. Primary cutaneous adenoid cystic carcinoma with brain metastases: case report and literature review. J Cutan Pathol. 2016;43:137–141. doi: 10.1111/cup.12563. [DOI] [PubMed] [Google Scholar]
- 5.Naylor E, Sarkar P, Perlis CS, Giri D, Gnepp DR, Robinson-Bostom L. Primary cutaneous adenoid cystic carcinoma. J Am Acad Dermatol. 2008;58(4):636–641. doi: 10.1016/j.jaad.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 6.Raychaudhuri S, Santosh KV, Satish Babu HV. Primary cutaneous adenoid cystic carcinoma of the chest wall: Aa rare entity. J Cancer Res Ther. 2012;8(4):633–635. doi: 10.4103/0973-1482.106583. [DOI] [PubMed] [Google Scholar]
- 7.Iro S, Raiteb M, Maadane A, Elmrini S, Slimani F. Cutaneous adenoid cystic carcinoma of lower eyelid: case report of a rare malignant entity. Ann Med Surg. 2021;67 doi: 10.1016/j.amsu.2021.102467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferreira S, Fernandes IC, Coelho A, Selores M. Primary cutaneous adenoid cystic carcinoma of the abdomen: a rare entity. Vol. 26. 2020. [PubMed]