Abstract
Purpose
Papilledema is a very rare complication of leukemia therapies, and particularly tyrosine kinase inhibitor (TKI) therapy. Targeted oncologic therapies are becoming increasingly popular, so it is increasingly important to report rare adverse effects. We present a case of probable papilledema in the setting of ponatinib therapy for acute lymphoblastic leukemia.
Observations
Our patient is a 48-year-old male who was diagnosed with acute lymphocytic leukemia. He underwent stem cell transplantation and shortly after was placed on ponatinib therapy. After initiation of ponatinib, he began to note decreased clarity in the inferonasal visual field of his right eye, corroborated on Humphrey visual field (HVF) testing. Neuroimaging was only notable for a partially empty sella. Lumbar puncture demonstrated opening pressures at the upper limit of normal (23 cm H2O) but with normal cellular constituents and chemistry. Slit lamp exam did not reveal any signs of ocular inflammation. Dilated funduscopic examination (DFE) revealed 360-degree blurring of the right optic disc margin as well as 270-degree blurring of the left optic disc (sparing the temporal border). Optical coherence tomography of the retinal nerve fiber layer (OCT-RNFL) showed increased RNFL thickness of 272 μm in the right eye and 113 μm in the left eye. In the absence of evidence for other possible etiologies of optic disc edema, ponatinib-induced papilledema was suspected. No changes to the ponatinib regimen were made; however, the patient was started on acetazolamide 500 mg twice a day. At three-month follow up, the patient reported resolution of his right eye blurriness and his repeat HVF, OCT-RNFL, and DFE showed resolution of optic disc edema, supporting that his initial bilateral optic disc swelling was likely ponatinib-induced papilledema.
Conclusions and importance
This is the first report of probable ponatinib-induced papilledema. This case expands on the literature of TKI induced papilledema and demonstrates successful treatment with an oral acetazolamide regimen.
Keywords: Papilledema, Ponatinib, Intracranial hypertension, Tyrosine kinase inhibitor, Acute lymphocytic leukemia
Highlights
-
•
As reported with other tyrosine kinase inhibitors, ponatinib can induce papilledema.
-
•
Ponatinib induced papilledema can be treated with acetazolamide.
-
•
Visual disturbances during leukemia treatment should receive ophthalmic work up.
-
•
Patients taking ponatinib should consider periodic eye exams during treatment.
1. Introduction
Papilledema, disc edema due to raised intracranial pressure (ICP), is a very rare complication of leukemia therapy and particularly tyrosine kinase inhibitor (TKI) therapy.1, 2, 3, 4 This condition has variable presentations and can present asymptomatically. Targeted oncologic therapies are becoming increasingly popular, and more uncommon adverse events may not appear during the initial drug studies that comprise a drug monograph.3 Thus, it is important to report rare adverse effects seen in clinical practice. We present a case of probable papilledema in the setting of ponatinib therapy for acute lymphoblastic leukemia.
2. Case report
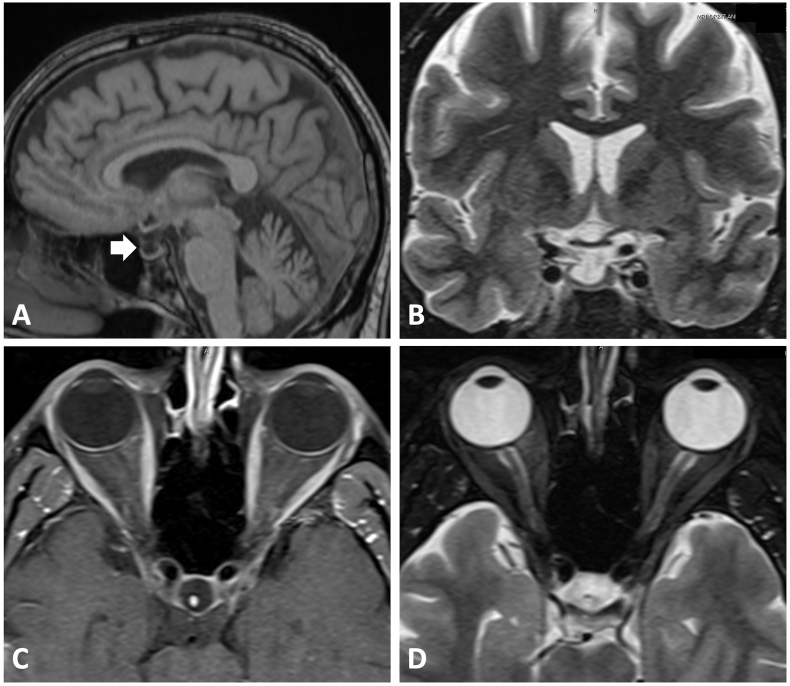
Our patient is a 48-year-old man who was first diagnosed with acute lymphocytic leukemia in 2020 after presenting with unexplained ecchymosis on his limbs. He underwent stem cell transplant in July 2021 and was placed on ponatinib afterward. Shortly after initiation of ponatinib, he began to note decreased clarity in the inferonasal visual field of his right eye. Vision in his left eye was unaffected. Given the visual symptom, his oncologist referred him to an ophthalmologist in January 2022 who noted optic nerve edema in the right eye on fundus exam. Subsequently, MRI neuroimaging and a lumbar puncture (LP) with cerebrospinal-fluid (CSF) analysis were ordered. The MRI was negative for any signs of metastases or leptomeningeal enhancement. There was no evidence of optic nerve enhancement as well. However, the imaging was notable for a partially empty sella (Fig. 1). This finding is nonspecific but can be seen in setting of raised ICP. The lumbar puncture was notable for normal cellular constituent and chemistry as well as cytology free of malignant cells. The opening pressure from lumbar pressure was at the upper limit of normal of 23 cm H2O. Given his visual symptoms and optic disc edema, the patient was referred to the Mitchel and Shannon Wong Eye Institute (MSWEI) for further evaluation of his optic disc swelling. Of note, he was placed on two separate short courses of prednisone for potential concerns of graft-vs-host disease.
Fig. 1.
Brain MRI images. (A) Sagittal T1 image showing empty sella (indicated by the white arrow). (B) Coronal T2-weighted image displaying no concerning compressive lesion onto the optic chiasm. Axial (C) T1 post-contrast and (D) T2 fat-saturated images of the optic nerves showing lack of optic nerve enhancement and absent abnormal T2 signal, respectively.
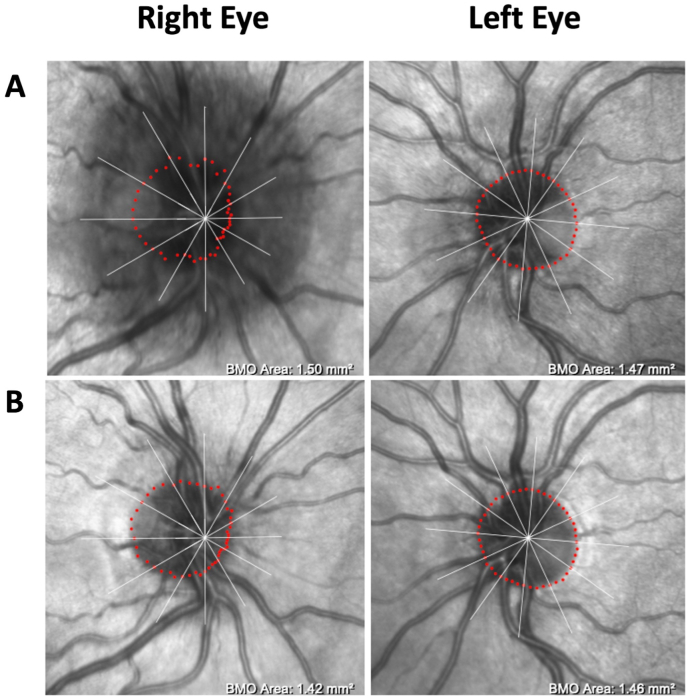
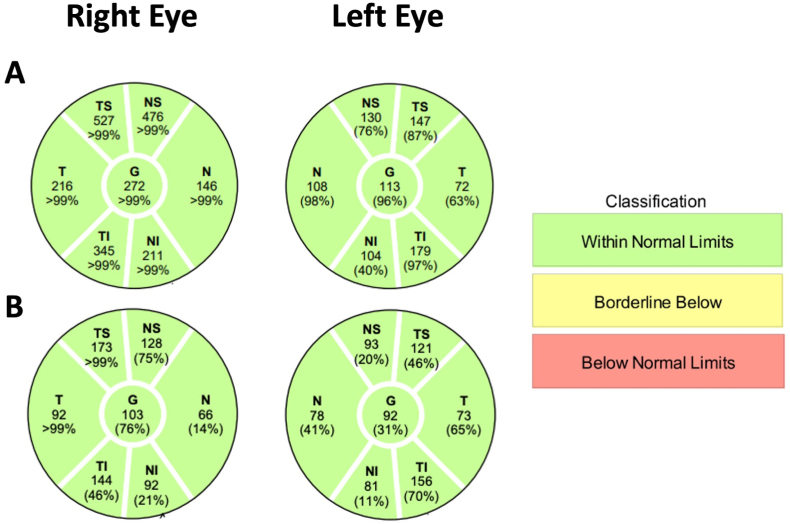
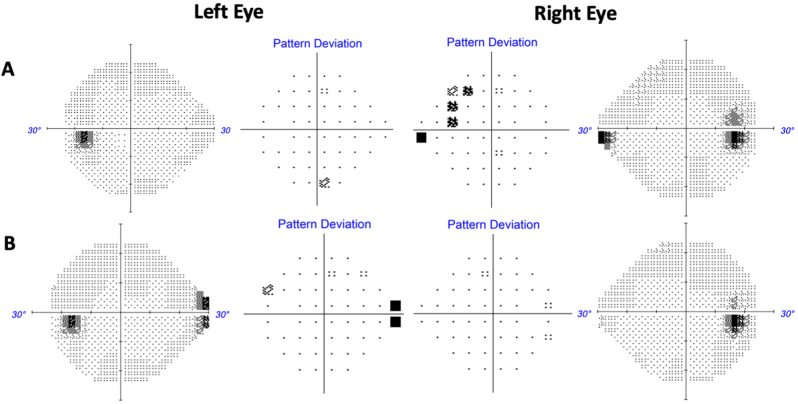
On his initial visit with us, his only complaint was mild persistent blurriness in the inferonasal visual field of his right eye. He denied other relevant symptoms for raised ICP, such as headaches, double vision, pulsatile tinnitus, nor transient visual obscurations. Best corrected visual acuity was 20/20 in the right eye and 20/20 in the left eye. Color vision testing with Ishihara plates was full in both eyes. There were no afferent pupillary defects. Slit lamp exam did not reveal any signs of ocular inflammation. Dilated funduscopic examination (DFE) revealed 360-degree blurring of the right optic disc margin as well as 270-degree disc margin blurring (sparing the temporal sector) in the left eye, which was also seen on optical coherence tomography (OCT) infra-red imaging (Fig. 2A). Optical coherence tomography of the retinal nerve fiber layer (OCT-RNFL) further confirmed evidence of bilateral optic nerve edema with a global RNFL thickness of 272 μm in the right eye and 113 μm in the left eye (Fig. 3A). Humphrey visual field (HVF) revealed a mild nasal step and enlarged blind spot in the right eye and a full visual field in the left eye (Fig. 4A).
Fig. 2.
Ocular Coherence Tomography (OCT) infra-red images. (A) OCT infra-red image at the initial visit with evidence of optic disc edema, right eye worse than the left eye. (B) Follow-up OCT infra-red image after three months of acetazolamide, showing significant resolution of optic disc edema in both eyes. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 3.
Ocular Coherence Tomography (OCT) retinal nerve fiber layer (RNFL) analysis. (A) OCT RNFL measurements at the initial visit with elevated global thickness of 272 μm in the right eye and 113 μm in the left eye. (B) Follow-up OCT RNFL measurements after 3 months of acetazolamide showing improvement of RNFL thickness in all sectors in both eyes.
Fig. 4.
Humphrey Visual Field (HVF) results. (A) Initial diagnostic HVF grayscale and pattern deviation maps consistent with patient's subjective report of mild inferonasal defect in the right eye. There was also enlarged blind spot in the right eye. Visual field was full in the left eye. (B) HVF grayscale and pattern deviation maps after three months of acetazolamide demonstrating improvement of the enlarged blind spot and resolution of the inferonasal defect in the right eye.
At this point, potential ponatinib induced papilledema was suspected for this patient's optic nerve edema. Although the opening pressure was only at the upper limit of normal, there were no evidence of other likely etiologies based on our work-up and exams, such as infiltrative/neoplastic, infectious, or inflammatory causes (given normal CSF analysis, normal slit lamp and fundus exam, and the exclusion of intracranial mass or optic nerve enhancement on MRI). Another possibility on the differential diagnosis was potential papilledema from steroid withdrawal, though the steroid courses were too short to result in optic disc edema. Therefore, we treated the patient as probable papilledema due to ponatinib with acetazolamide 500 mg twice daily. No changes to the ponatinib regimen were made. At three-month follow up, the patient reported resolution of his right eye blurriness and the repeat HVF revealed resolution of his prior visual field defects in the right eye (Fig. 4B) This symptomatic improvement was consistent with improved right optic nerve edema and complete resolution of left optic nerve edema on fundus exam and OCT infra-red imaging (Fig. 2B). The improvement in OCT-RNFL thickness further substantiated edema resolution from acetazolamide treatment (Fig. 3B). Given the resolution of his symptoms and disc edema with acetazolamide initiation, we therefore concluded that his disc edema was likely ponatinib-induced papilledema. Moreover, the stability of his macular ganglion cell layer volume over the time suggest that the improvement of the papilledema was not due to optic atrophy (data not shown).
3. Discussion
Although papilledema secondary to sunitinib, dasatinib, and imatinib (other tyrosine kinase inhibitors), and recently cabozantinib has been reported in the literature,1, 2, 3, 4 this is to our knowledge the first case of probable papilledema secondary to ponatinib treatment. Our patient presented with a minor inferonasal visual defect in his right eye without other ocular or neurologic symptoms suggestive of raised ICP. Bilateral optic nerve edema was discovered, right being worse than the left. Making a diagnosis was challenging, as the MRI only revealed a nonspecific sign of raised ICP - an empty sella, and the opening pressure from lumbar puncture was only at the high end of normal (23). Ultimately, ponatinib-induced papilledema was thought to be the underlying etiology given the resolution of his visual field defect and disc edema with initiation of acetazolamide. Though optic neuritis was a possibility, the lack of eye pain or pain with eye movement as well as lack of optic nerve enhancement on MRI imaging argued against it.
In both scientific literature and public media, there have been extensive reports on ponatinib induced cerebrovascular, cardiovascular, and peripheral vascular thrombotic events, sometimes fatal, which even led to a temporary hold on the drug in 2013 and the cancellation of the EPIC trial.5, 6, 7 In terms of ophthalmic side-effects, ponatinib has been described in both the OPTIC (11% of patients)8 and PACE (30% of patients)9 clinical trials causing most commonly dry eye, blurred vision, and eye pain or, less commonly, serious retinal toxicities. There has also been one case of ponatinib inducing pan-uveitis, choroidal effusion, and retinal detachment.10 However, to our knowledge, ponatinib-associated papilledema has not been reported.
Neuro-ophthalmic side effects of targeted cancer therapies have been described in the literature especially as molecular therapies have seen a dramatic increase over the past two decades.11 Papilledema, though rare, has been described for ponatinib's predecessor BCR-ABL TKI, imatinib, as well as sunitinib and dasatinib.1, 2, 3 Imatinib specifically has been reported to be associated with rare cases of optic disc edema.12,13 A case report has hypothesized that optic disc edema secondary to TKIs may be due to platelet-derived growth factor receptor beta inhibition and subsequent fluid retention that also results in the more common side effect of periorbital edema,14 but the pathophysiology remains unclear and warrants further investigation. Secondary papilledema has also been reported in the setting of leukemia due to various other treatments such as all-trans retinoic acid, cyclosporine, and fludarabine.15 Lastly, the TKI imatinib also has one reported case of inducing optic neuritis.16
In summary, visual disturbances in a patient taking targeted molecular drugs or undergoing leukemia treatment, particularly with TKI, should receive an ophthalmologic referral and full work-up. Although progressive painless vision loss with headache is the classic presentation, raised ICP can present in various forms including but not limited to pulsatile tinnitus, diplopia, psychiatric changes, or completely asymptomatically. If there are signs of optic disc edema, then further evaluation for edema with neuro-imaging and LP with cerebrospinal fluid analysis should be pursued. Diagnosis of drug-induced papilledema is considered when there is bilateral optic nerve swelling, neuro-imaging with normal brain parenchyma and no evidence of hydrocephalus, opening pressure of >25 cm H2O but normal CSF composition, and a medication as the offending agent.17,18 If a diagnosis of drug-induced papilledema due to a cancer drug is reached, the goal should be to quickly address this life altering side effect. The primary intervention can be temporarily or permanently, if possible, removing the offending drug and treating papilledema per typical management.3 In our case, ponatinib discontinuation was not favored by the oncologist as it is a last-line therapy for BCR-ABL leukemia patients who have cross resistant mutations such as the T315I mutation-based resistance.19 In the case that discontinuing the offending agent may cause more harm than good, we present treating the papilledema with acetazolamide as a viable option. Lastly, physicians managing cancer care involving ponatinib should consider referring patients for baseline and periodic comprehensive eye exams during treatment.5
4. Conclusion
The TKI ponatinib can induce secondary papilledema. This case illustrates that, though very rare, it is important to recognize the varying signs of increased ICP in patients undergoing ponatinib therapy and to initiate a combination of drug discontinuation, if possible, and/or acetazolamide treatment. Baseline and regular eye exams can help monitor for signs of common and rare ocular side effects in ponatinib patients.
Patient consent
The patient consented to publication of the case in writing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements and Disclosures
No funding or grant support. The following authors have no financial disclosures: JAM, ECB, MHA. All authors attest that they meet the current ICMJE criteria for Authorship.
Contributor Information
Jared A. Moon, Email: jaredmoon@utexas.edu.
Eileen C. Bowden, Email: eileen.bowden@austin.utexas.edu.
Moe H. Aung, Email: moe.aung@austin.utexas.edu.
References
- 1.Yoong J., Chong G., Hamilton K. Bilateral papilledema on sunitinib therapy for advanced renal cell carcinoma. Med Oncol. 2011;28(Suppl 1):S395–S397. doi: 10.1007/s12032-010-9719-5. [DOI] [PubMed] [Google Scholar]
- 2.Haddad F., Kantarjian H., Issa G.C., Jabbour E., Sasaki K. Intracranial hypertension associated with BCR-ABL1 tyrosine kinase inhibitors in chronic myeloid leukemia. Leuk Lymphoma. 2022;63(7):1714–1717. doi: 10.1080/10428194.2022.2045599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lipton N.J., Lipton J.H. Making the case for the case report - informing physicians of intracranial hypertension as an adverse event in tyrosine kinase inhibitor treated chronic myeloid leukemia patients. Leuk Lymphoma. 2022;63(7):1522–1523. doi: 10.1080/10428194.2022.2056180. [DOI] [PubMed] [Google Scholar]
- 4.Huang Y.T., Lin C.J., Tsai Y.Y., Hsia N.Y. Bilateral optic disc edema as a possible complication of cabozantinib use-a case report. Eur J Ophthalmol. May 2023;33(3):NP56–NP59. doi: 10.1177/11206721221078675. [DOI] [PubMed] [Google Scholar]
- 5.Iclusig ® (Ponatinib Hydrochloride) Tablets Prescribing Information. 2012. [Google Scholar]
- 6.Carroll J. FierceBiotech; 2013. Ariad Hammered on Toxicity Concerns for Leukemia Drug Iclusig. Oct. 9th. [Google Scholar]
- 7.Pollack A. NY Times; 2013. After Brief Halt, F.D.A. Allows Sales of Drug for Cancer to Resume. Dec. 20th. [Google Scholar]
- 8.Cortes J., Apperley J., Lomaia E., et al. Ponatinib dose-ranging study in chronic-phase chronic myeloid leukemia: a randomized, open-label phase 2 clinical trial. Blood. 2021;138(21):2042–2050. doi: 10.1182/blood.2021012082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cortes J.E., Kim D.W., Pinilla-Ibarz J., et al. A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. N Engl J Med. Nov 07 2013;369(19):1783–1796. doi: 10.1056/NEJMoa1306494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patil A.D., Backhouse O.C., McGonagle D., Bhan K. Ponatinib inducing a panuveitis with choroidal effusions and neurosensory retinal detachment in a patient with chronic myeloid leukaemia. Ocul Immunol Inflamm. 2022;30(5):1186–1189. doi: 10.1080/09273948.2020.1866618. [DOI] [PubMed] [Google Scholar]
- 11.Bhatti M.T., Salama A.K.S. Neuro-ophthalmic side effects of molecularly targeted cancer drugs. Eye (Lond) 2018;32(2):287–301. doi: 10.1038/eye.2017.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kwon S.I., Lee D.H., Kim Y.J. Optic disc edema as a possible complication of Imatinib mesylate (Gleevec) Jpn J Ophthalmol. 2008 Jul-Aug 2008;52(4):331–333. doi: 10.1007/s10384-008-0561-7. [DOI] [PubMed] [Google Scholar]
- 13.DeLuca C., Shenouda-Awad N., Haskes C., Wrzesinski S. Imatinib mesylate (Gleevec) induced unilateral optic disc edema. Optom Vis Sci. Oct 2012;89(10):e16–e22. doi: 10.1097/OPX.0b013e318269111d. [DOI] [PubMed] [Google Scholar]
- 14.Kusumi E., Arakawa A., Kami M., et al. Visual disturbance due to retinal edema as a complication of imatinib. Leukemia. Jun 2004;18(6):1138–1139. doi: 10.1038/sj.leu.2403364. [DOI] [PubMed] [Google Scholar]
- 15.Mejia-Vergara A.J., Arnold A.C., Bonelli L., Raviskanthan S., Lee A.G. Papilledema and intracranial hypertension in leukemia: case series and review. Can J Ophthalmol. 2022;57(2):e54–e56. doi: 10.1016/j.jcjo.2021.06.022. [DOI] [PubMed] [Google Scholar]
- 16.Govind Babu K., Attili V.S., Bapsy P.P., Anupama G. Imatinib-induced optic neuritis in a patient of chronic myeloid leukemia. Int Ophthalmol. Feb 2007;27(1):43–44. doi: 10.1007/s10792-007-9038-9. [DOI] [PubMed] [Google Scholar]
- 17.Xie J.S., Donaldson L., Papilledema Margolin E. A review of etiology, pathophysiology, diagnosis, and management. Surv Ophthalmol. 2022;67(4):1135–1159. doi: 10.1016/j.survophthal.2021.11.007. [DOI] [PubMed] [Google Scholar]
- 18.Friedman D.I., Liu G.T., Digre K.B. Revised diagnostic criteria for the pseudotumor cerebri syndrome in adults and children. Neurology. Sep 24 2013;81(13):1159–1165. doi: 10.1212/WNL.0b013e3182a55f17. [DOI] [PubMed] [Google Scholar]
- 19.O'Hare T., Shakespeare W.C., Zhu X., et al. AP24534, a pan-BCR-ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutation-based resistance. Cancer Cell. Nov 06 2009;16(5):401–412. doi: 10.1016/j.ccr.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]