Highlights
-
•
SCM1A-gene mutations can cause a Developmental and Epileptic Encephalopathy.
-
•
Early onset SCM1A-related epilepsy can resemble a PCDH19-related epilepsy phenotype.
-
•
The association of this epileptic phenotype and Rett-like regression is possible.
Keywords: SMC1A, Developmental and epileptic encephalopathy, Epilepsy, Neurodevelopmental disorder, Developmental regression, PCDH19-related epilepsy
Abstract
Developmental and epileptic encephalopathies (DEE) are conditions in which a mutated gene may cause abnormal functioning of the central nervous system, resulting in both encephalopathy and epileptogenesis. We present a case of a girl with a DEE characterized by a Rett-like phenotype in association with febrile and afebrile clusters of focal seizures. The girl presented typical development until the age of 18 months, followed by regression. The first febrile bilateral tonic-clonic seizure was observed at 30 months of age, and the following month seizures recurred in clusters of several episodes per day every 10 days. These seizures were characterized by behavioural arrest, emotional symptoms, head turning, and followed by bilateral tonic-clonic seizures. The administration of valproic acid and levetiracetam led to prolonged seizure control. However, from the age of 7 years, she had monthly recurrent clusters of focal seizures and non-convulsive status epilepticus which occurred at different ages. Brain and spinal cord MRI showed mild non-progressive hemispheric cerebellar atrophy. A next generation sequencing panel for epilepsy identified the de novo splicing mutation c.2973+1G>A of the SMC1A gene.
1. Introduction
Approximately 22% of children with intellectual disability manifest epilepsy as a comorbidity [1]. The association of the two conditions is named “Developmental and epileptic encephalopathy (DEE)” [2]. Recognized by the International League Against Epilepsy classification [3], this terminology allows to overcome the previous term “Epileptic Encephalopathy” [4]. DEE can refer to developmental encephalopathy characterized by developmental impairment without frequent epileptic activity; epileptic encephalopathy without pre-existing developmental delay in which the genetic mutation is not thought to cause developmental impairment; and developmental and epileptic encephalopathy where both factors are involved [2]. Nowadays, new DNA analysis techniques allow physicians to discover a genetic mutation responsible for DEE. The mutated gene determines an abnormal functioning of the central nervous system, and consequently both encephalopathy and epileptogenesis [5]. In clinical practice, however, the recognition of specific clinical and electroclinical patterns is of paramount importance, as it allows clinicians to identify the possible differential diagnoses. We present the case of a 15-year-old girl with DEE and an interesting clinical picture characterized by a Rett-like phenotype associated with febrile and afebrile clusters of focal seizures and mild physical signs carrying a SMC1A gene mutation. The SMC1A gene is located on the X chromosome, codes for the SMC1A protein, and is a component of the ring-shaped cohesion complex which forms the heterodimers with RAD21 and SMC3. It is involved in chromosome segregation, DNA repair, and regulation of chromosomal architecture. Heterozygous de novo SMC1A loss-of-function mutations have been recently described in females with a more severe DEE clinical phenotype [6], [7].
2. Case report
The patient is a 15-year-old girl, born at term (39 weeks gestational age) from nonconsanguineous parents after an uncomplicated pregnancy. Early development was typical until the age of 18 months: at 1 year old she walked, said single words, and understood simple commands. In the following months, she developed a vocabulary of approximately 50 words. However, from the age of 18 months, an unexplained regression was observed: a failure in the acquisition of new words was followed by a gradual reduction in the use of meaningful words. At 24 months of age her language consisted of few words and did not change over the following years. Eye contact and interpersonal relations, on the other hand, were unaffected. No consistent loss of hand movements, both intentional and stereotyped, was observed. Starting from 8 years of age, her gait gradually became unsteady and awkward. The patient slowly reduced stride length and presented with a very stiff and wide gait and was only able to walk for a few minutes at a time, covering a distance of a few meters. From 10 to 12 years of age, she was assisted with the use of a wheelchair. On physical examination she presented with spasticity of the legs, brisk reflexes, and bilateral plantar extensor response. Despite the progressive development of a paraparetic gait, several MRI brain scans (performed at 3, 7 and 14 years of age) and an MRI of the spinal cord (performed at 14 years of age) revealed no significant abnormalities. The last scan revealed only mild cerebellar hemispheric atrophy (Fig. 1). Physical examination also showed generalized hirsutism, a normal head circumference and dysmorphic features consisting of mild synophrys, long eyelashes, full lips, prominent nasal root and bridge, hypoplastic nasal wings, short philtrum, proximal placement of thumbs and bilateral fifth finger clinodactyly. Together, these physical features did not suggest a specific syndromic diagnosis, and initial genetic tests consisting of conventional karyotype, molecular analysis for fragile X syndrome (FMR1-testing) and array-CGH, performed when the patient was 3–4 years of age, were negative. At 5 years old, a methylation test of the SNRPN gene did not detect abnormalities, while a search for mutation in the UBE3A gene also yielded negative results. Considering the evolution of the clinical picture, which resembled that observed in girls with Rett syndrome, a search for a mutation in the MECP2 gene was recommended, but the family refused further genetic tests.
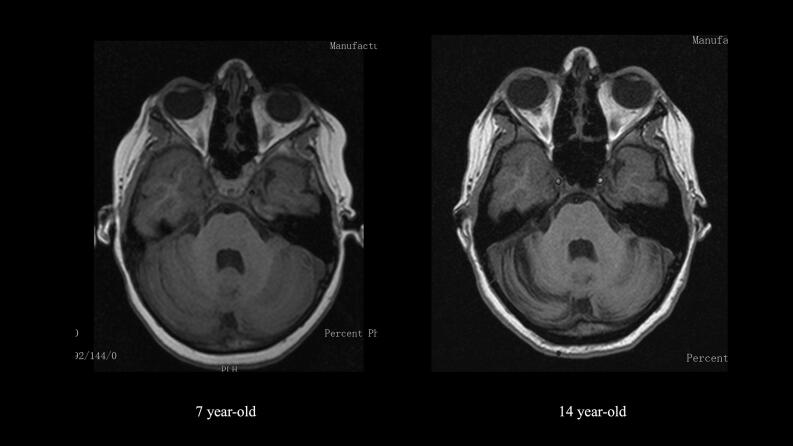
Fig. 1.
Brain MRI of the patient at 7 years (left) and 14 years (right) - T1 weighted, axial view at cerebellum level showing cerebellar hemispheric mild atrophy at 14 years of age.
The first epileptic seizure which occurred at the age of 30 months was a bilateral tonic-clonic seizure during sleep, whilst the patient had an intercurrent febrile illness. One month later, the first afebrile seizure was observed: the patient ran to her mother, presenting as afraid and wanting to be held, then a bilateral tonic-clonic seizure followed. The event lasted about 30 s and was followed by sleep. One week later, the patient presented with a cluster of afebrile seizures. A first focal to bilateral tonic-clonic seizure occurred during sleep followed by three more seizures the next day. She was therefore admitted to the local hospital and subsequently transferred to the regional hospital, where an EEG was performed and showed generalized polyspikes and waves with frontal emphasis and variable lateralization. Anti-seizure treatment with valproic acid was initiated and the patient reached the therapeutic range within 10 days. Despite anti-seizure therapy, she had a bilateral tonic-clonic seizure 10 days later during sleep, followed by another cluster of three bilateral tonic-clonic seizures in 90 min during sleep after another 10 days, which required acute treatment with rectal diazepam. Her plasma valproic acid level was above therapeutic range (148 mg/dl) and the dosage was therefore reduced. Additional treatment with levetiracetam was initiated and over a week was increased to a dose of 38 mg/kg/day. With the combined therapy of valproic acid and levetiracetam the patient achieved adequate seizure control, which was maintained for almost 4 years.
At 7 years old, without changing the anti-seizure medications, a recurrence of clusters of 2–3 seizures in sleep was observed, characterized by awakening from sleep followed by sudden eyes and head deviation to the right with fearful expression or laughter. The duration of each single seizure was brief (approximately 30 s) and the patient maintained awareness. The seizures recurred every night for three nights; the patient was then brought to the emergency room after a fourth night that was characterized by similar episodes, when a seizure occurred on awakening. A typical event was documented during a subsequent video-EEG recording. Clinically, she had an arrest in behaviour, then a forced rotation of the eyes and head to the left for 10–15 s; the head then returned to midline, and she laughed. This clinical event correlated with a change to alpha activity over the right fronto-temporal region, followed by rapid involvement of the left leads and further change in frequency to theta activity. The seizure lasted approximately about 70 s. Acute oral treatment with Lorazepam 2.5 mg was administered after the resolution of the event, allowing for seizure control that lasted several hours. Over the next few days, the frequency of the seizures increased to multiple events per hour (up to 2–3 seizures/hour), with the patient not responding to increasing doses of valproic acid and levetiracetam. Acute treatment with a loading IV dose of phenytoin (20 mg/kg) and a loading oral dose of topiramate (10 mg/kg) were administred. Sometimes, the seizures clustered every few seconds to 15 min (Fig. 2) and could only be controlled with midazolam infusion. Seizures remained very brief and were not accompanied by significant oxygen desaturation. Control of seizure clusters was finally achieved after two weeks with a combination of topiramate and lacosamide. No acute changes were observed in the brain MRI scan performed at 7 years of age.
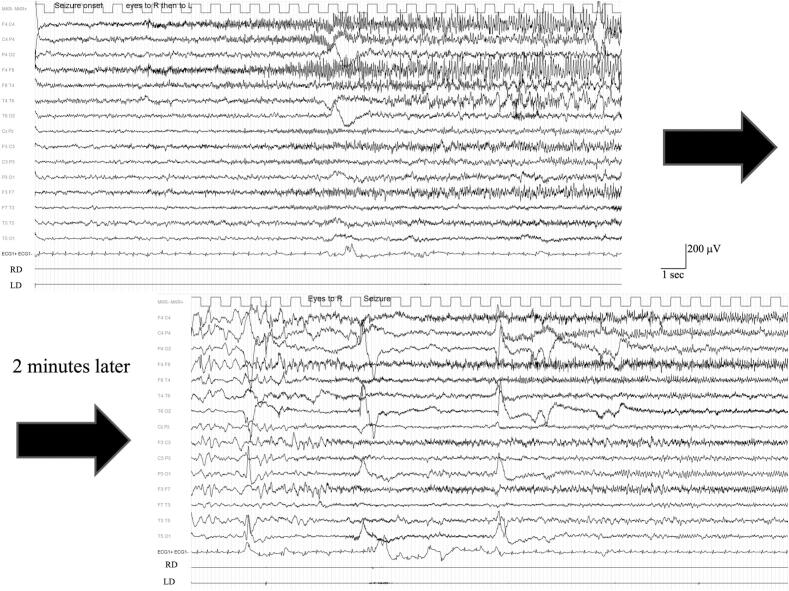
Fig. 2.
Recording of a cluster of seizures during an episode of non-convulsive status epilepticus at the age of 7 years. Seizures recurred every 2–4 min characterized firstly by head turning to the right followed by turning to the opposite side. EEG shows a fast rhythmic activity involving frontal leads with emphasis on the right.
Considering the clinical picture characterized by severe epilepsy with predominantly focal febrile and afebrile seizures from the age of 30 months in a girl with severe intellectual disability, PCDH19-associated epilepsy was suspected but a search for this yielded negative results.
From the age of 11 years, seizures reappeared every 3 days to a week, resistant to several other anti-seizure medications used in different combinations such as rufinamide, perampanel, clobazam, nitrazepam, zonisamide, and recently cannabidiol. In addition, nonconvulsive status epilepticus similar to that described at age 7 occurred at 12 and 14 years of age, controlled with a combination of intravenous phenytoin and midazolam.
The clinical history, in addition to the epilepsy phenotype and the absence of acquired cerebral lesions, led to the speculation of a genetic cause to this patient’s epilepsy. A next generation panel of 149 known epilepsy genes was performed which revealed the de novo splicing mutation c.2973+1G>A of the SMC1A gene.
3. Discussion
We have presented a 15-year-old girl affected by severe intellectual disability, progressive gait disorder and drug resistant focal epilepsy . The patient had a typical development pattern until 18 months of age, followed bya plateau and regression. From the age of 30 months, she developed drug resistant focal epilepsy, supporting the diagnosis of a DEE. Several clinical syndromes should be considered in the differential diagnosis and the most relevant are reported in Table 1.
Table 1.
Differential diagnosis. Key: ✓, feature present; X, feature not present; DEE, developmental and epileptic encephalopathy; CDLS2, Cornelia de Lange Syndrome type 2.
| Our Patient | Angelman syndrome | Rett syndrome | Pitt-Hopkins syndrome | PCDH19-related epilepsy | SCN1A-related DEE | CDLS2 | |
|---|---|---|---|---|---|---|---|
| Normal development until 18 months | X | ✓ | X | ✓ | ✓ | X | |
| Regression and loss of speech | X | ✓ | ✓/X | X | X | ✓ | |
| Progressive paraparesis from 8 years | X | ✓ | X | X | X | X | |
| Febrile related seizures | ✓/X | X | X | ✓ | ✓ | ✓/X | |
| Clustered focal seizures with affective component | X | X | X | ✓ | X | X | |
| Physical phenotype |
Hirsutism | X | X | X | X | X | ✓ |
| Thick Eyebrows | X | X | X | X | X | ✓ | |
| Synophrys | X | X | X | X | X | ✓ | |
| Proximally placed thumb | X | X | X | X | X | ✓ | |
| 5th digit clinodactyly | X | X | ✓ | X | X | ✓ | |
The clinical picture of our patient strongly resembled the well-defined stage evolution observed in Rett syndrome [8]. However, only criteria compatible with an atypical form of Rett syndrome were present [9]. Epilepsy is a frequent feature in Rett syndrome, being reported in 60–80% of patients [10]. Despite its frequency, the characterization of a specific epilepsy phenotype in Rett syndrome is lacking and all seizure types are reported [8]. Moreover, mutations of the SMC1A gene are reported to cause Cornelia de Lange Syndrome-2 (CDLS2) and a phenotype strongly resembling Rett syndrome [11]. The SMC1A gene located on the short arm of X chromosome (Xp11.22) is ubiquitously expressed, and all known pathological phenotypes caused by mutations of this gene are transmitted as X-linked dominant diseases. SMC1A protein is one of the components of the ring-shaped cohesion complex, which functions by forming heterodimers with RAD21 and SMC3. This core subunit orchestrates long-range DNA interactions to mediate sister chromatid cohesion during the cell cycle, which is essential for accurate chromosome segregation, DNA repair, and regulation of chromosomal architecture [6], [7]. Heterozygous de novo SMC1A loss-of-function mutations have recently been described in a limited number of affected females with a more severe DEE clinical phenotype. The etiology of the epilepsy due to SMC1A-mutations has yet to be studied, due to limited numbers of patients and a lack of studies on neuronal cells derived from induced pluripotent stem cell lines obtained from patients. According to a very intriguing hypothesis, a differential SMC1A insufficiency in SMC1A-DEE individuals with random X-chromosome inactivation could lead to cellular interference (i.e., interference between cells with differential levels of SMC1A) at tissue levels, similar to the proposed pathogenetic mechanism for the PCDH19-related epilepsy. Interestingly, SMC1A-DEE and PCDH19-related epilepsy share some epileptic features, e.g. drug resistance and seizure clustering [7].
Our patient’s epilepsy phenotype was particularly interesting, starting with a simple febrile seizure at 30 months of age shortly followed by the emergence of clusters of brief focal seizures presenting with turning of the eyes and head in combination with emotional symptoms (fear or laughter). The association of clustered and fever-induced seizures observed in girls during the first years of life is typical of PCDH19-related epilepsy [12]. Motor seizures with a tonic component accompanied by fearful expression have been observed in the majority of patients (82%) [12]. Escalation to focal status epilepticus is described in approximately 30% of patients [12]. These clinical features were present in our patient and suggested the diagnosis of PCDH19-related epilepsy. However, some characteristics were atypical: a) most patients presenting with PCDH19-related epilepsy improve by age 10 years [12], while our patient continued to have clusters of seizures until the last clinical follow-up at 15 years old; b) cognitive impairment is usually present but is not very severe [13]; c) patients with clinical phenotype suggesting the diagnosis of Rett syndrome are very unusual among those affected by PCDH19-related epilepsy [14].
Different cohorts of patients bearing missense mutations of the SMC1A gene and presenting with an epilepsy syndrome resembling PCDH19-related epilepsy have been reported [15], [16]. However, none of the reported patients, presented with a development regression resembling Rett syndrome phenotype, with the only exception of a few patients recently reported by Bozarth et al [7]. One child described in the cohort of Symonds et al [16], developed babbling between 1 and 2 years old, which ceased after a status epilepticus at the age of 3.
The mild physical features observed in our patient could not suggest a specific syndrome, but they should be considered for the differential diagnosis, to select the Human Phenotype Ontology Terms to be included in next generation sequencing analyses.
4. Conclusion
In conclusion, our patient presented with a molecularly confirmed diagnosis of CDLS2 with compatible phenotype and peculiar epileptic and neurodevelopmental features. In particular, the patient showed a normal development up to 18 months, followed by plateau and regression, like Rett syndrome, and febrile and afebrile clustered seizures, starting at 30 months, partially overlapping with PCDH19-related epilepsy. Rett-like and PCDH19-related epilepsy phenotypes have already been described in some female patients bearing a mutation of the SMC1A gene, but their association in the same patients has been only poorly defined [7], [16]. Therefore, it would be advisable to suspect a mutation of the SMC1A gene in girls with a complex phenotypic overlap, like the patient described.
Ethical statement
Informed consent was obtained from the patient and family.
Funding
None is supported in this work.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to thank Dr. Mila Ann Kalapurackal and Emily Catherine Butterworth for reviewing the English content of the manuscript.
Contributor Information
L. Parmeggiani, Email: lucio.parmeggiani@sabes.it.
F. Stanzial, Email: franco.stanzial@sabes.it.
E. Menna, Email: elisa.menna@sabes.it.
E. Boni, Email: elisa.boni@sabes.it.
F. Manzoni, Email: francesca.manzoni@sabes.it.
F. Benedicenti, Email: francesco.benedicenti@sabes.it.
S. Pellegrin, Email: serena.pellegrin@sabes.it.
References
- 1.McTague A., Howell K.B., Cross J.H., Kurian M.A., Scheffer I.E. The genetic landscape of the epileptic encephalopathies of infancy and childhood. Lancet Neurol. 2016;15(3):304–316. doi: 10.1016/S1474-4422(15)00250-1. [DOI] [PubMed] [Google Scholar]
- 2.Scheffer I.E., Berkovic S., Capovilla G., et al. ILAE classification of the epilepsies: Position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58(4):512–521. doi: 10.1111/epi.13709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Specchio N., Wirrell E.C., Scheffer I.E., et al. International League Against Epilepsy classification and definition of epilepsy syndromes with onset in childhood: Position paper by the ILAE Task Force on Nosology and Definitions. Epilepsia. 2022;63(6):1398–1442. doi: 10.1111/epi.17241. [DOI] [PubMed] [Google Scholar]
- 4.Berg A.T., Berkovic S.F., Brodie M.J., et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia. 2010;51:676–685. doi: 10.1111/j.1528-1167.2010.02522.x. [PubMed: 20196795] [DOI] [PubMed] [Google Scholar]
- 5.Specchio N., Curatolo P. Developmental and epileptic encephalopathies: what we do and do not know. Brain. 2021;144(1):32–43. doi: 10.1093/brain/awaa371. [DOI] [PubMed] [Google Scholar]
- 6.Huisman S., Mulder P.A., Redeker E., et al. Phenotypes and genotypes in individuals with SMC1A variants. Am J Med Genet A. 2017;173(8):2108–2125. doi: 10.1002/ajmg.a.38279. [DOI] [PubMed] [Google Scholar]
- 7.Bozarth X.L., Lopez J., Fang H., Lee-Eng J., Duan Z., Deng X. Phenotypes and genotypes in patients with SMC1A-related developmental and epileptic encephalopathy. Genes (Basel) 2023;14(4):852. doi: 10.3390/genes14040852. Published 2023 Mar 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Operto F.F., Mazza R., Pastorino G.M.G., Verrotti A., Coppola G. Epilepsy and genetic in Rett syndrome: a review. Brain Behav. 2019;9:e01250. doi: 10.1002/brb3.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neul J.L., Kaufmann W.E., Glaze D.G., et al. Rett syndrome: revised diagnostic criteria and nomenclature. Ann Neurol. 2010;68(6):944–950. doi: 10.1002/ana.22124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glaze D.G., Percy A.K., Skinner S., et al. Epilepsy and the natural history of Rett syndrome. Neurology. 2010;74(11):909–912. doi: 10.1212/WNL.0b013e3181d6b852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borrie M.S., Campor J.S., Joshi H., Gartenberg M.R. Binding, sliding, and function of cohesion during transcriptional activation. Proc Natl Acad Sci USA. 2017;114:E1062–E1071. doi: 10.1073/pnas.1617309114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trivisano M., Pietrafusa N., Terraciano A., Marini C., Mei D., Darra F., et al. Defining the electroclinical phenotype and outcome of PCDH19-related epilepsy: A multicenter study. Epilepsia. 2018;59:2260–2271. doi: 10.1111/epi.14600. [DOI] [PubMed] [Google Scholar]
- 13.Debopam S. PCDH19-related epilepsy syndrome: a comprehensive clinical review. Pediatr Neurol. 2020;105:3–9. doi: 10.1016/j.pediatrneurol.2019.10.009. [DOI] [PubMed] [Google Scholar]
- 14.Hynes K., Tarpey P., Dibbens L.M., Bayley M.A., Berkovic S.F., Smith R., et al. Epilepsy and mental retardation limited to females with PCDH19 mutations can present de novo or in single generation families. J Med Genet. 2010;47:211–226. doi: 10.1136/jmg.2009.068817. [DOI] [PubMed] [Google Scholar]
- 15.Oguni H., Nishikawa A., Sato Y., Otani Y., Ito S., Nagata S., et al. A missense variant of SMC1A causes periodic pharmaco-resistant cluster seizures similar to PCDH19-related epilepsy. Epilepsy Res. 2019;155:1–5. doi: 10.1016/j.eplepsyres.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 16.Symonds J.D., Joss S., Metcalfe K.A., Somarathi S., Cruden J., Devlin A.M., et al. Heterozygous truncation mutations of the SMC1A gene cause a severe early onset epilepsy with cluster seizures in females: detailed phenotyping of 10 new cases. Epilepsia. 2017;58:565–575. doi: 10.1111/epi.13669. [DOI] [PubMed] [Google Scholar]