Abstract
Cork taint provides off-odors and changes negatively wine composition. In fact, it is one of the most important causes of discarding bottled wine. 2,4,6-Trichloroanisole (TCA) is the most known molecule responsible of that problem. In this study, cork stoppers were artificially contaminated with a multi-pattern solution which contained different chloroanisoles and chlorophenols. Contaminated corks were immersed for 3 h in four Plasma Activated Water (PAW) generated during 1.5 min, 5 min, 15 min and 30 min. The products of OH•, NO• and NO2• with phenol were determined by HPLC for each PAW. After treating contaminated corks with PAW generated during 5 min, more than 72 % of TCA was removed and it was suggested OH• as the main reactive species decomposing TCA. Finally, other chloroanisole and chlorophenol molecules were examined after PAW treatments showing successful reductions in almost every molecule. Thus, it was presented PAW treatment as an easy solution for solving cork taint problems in wine industry.
Keywords: Cork taint; 2,4,6-Trichloroanisole; Plasma activated water; Wine; OH•
Graphical abstract
Highlights
-
•
2,4,6-Trichloroanisole in corks were decomposed using plasma activated water.
-
•
PAW generated during 5 min removed 75.2 % of TCA from contaminated corks.
-
•
OH· plays the main role decomposing 2,4,6-Trichloroanisole in cork stoppers.
-
•
PAW could be a promising method to remove TCA from corks.
1. Introduction
Over the last year, the Spanish wine sector increased 4.7 % which makes wine one of the most important markets of the Spanish economy (Observatorio Español del Mercado del Vino, 2022). Wines are classified by parameters such as color, alcohol content, age and sweetness. However, there are some wines that need to be distinguished by more complex characteristics. In this sense, the definitions of aroma and bouquet are of great importance. The first one is related to the mixture of complex nuances that arise during its production and packaging; the latter is related to the natural taste and smell imparted by the grapes (Buja, 2022). Thus, wine is such a complex aqueous solution that involve more than 500 chemical constituents. Nevertheless, this combination could be spoiled by undesirable compounds causing wine faults (Tarasov et al., 2017).
Cork stoppers are highly effective as wine sealers, allowing wine to develop and age over time. However, they can suffer from chemical and biological contamination. This could lead to the formation of haloanisoles, being 2,4,6-Trichloroanisole (TCA) the most known molecule due to the capacity to produce the so-called cork taint, a moldy and musty off-odor and consequently affecting wine composition (about 80–85 % of total wine) (Sefton and Simpson, 2005; Silva Pereira, Figueiredo Marques and San Romão, 2000). This molecule has a very low threshold in wines, 1–2 ng/L (Sefton and Simpson, 2005; Soleas et al., 2002). The presence of TCA above these concentrations can spoil the wine organoleptic properties. It is a severe problem in wineries because between 1 and 7 % of bottled wines are discarded (Coque et al., 2006; Martín, 2018; Sefton and Simpson, 2005).
There are several publications indicating different pathways for the origin of TCA in cork bark and corks: phenol and chlorine-derived sources, chlorophenolic biocides, biosynthesis of chlorophenolic compounds in the forest, biosynthesis of chlorophenolic compounds after harvest of the cork bark, etc. (Monteiro et al., 2022; Simpson and Sefton, 2007). However, the formation of TCA by biomethylation of 2,4,6-Trichlorophenol (TCP) is the only scientifically proven. They are produced by the reaction of chlorines and phenols commonly found in town water suppliers and drainage systems and they can be formed in the environment, at room temperature (RT) in aqueous solutions (Simpson and Sefton, 2007). Once the TCP is in trunks of trees or in corks, the microflora such as Penicillium spp, Aspergillus spp, Actinomyces spp and Streptomyces spp, among others, presented on corks transform TCP into TCA through the enzyme chlorophenol O-methyltransferase by a methylation process (Cravero, 2020). A review carried out by (Simpson and Sefton, 2007) shows a complete table with different possible origins of TCA in wine corks suggesting that most of the TCA found in corks comes from trees.
In this sense, the cork industry has tried to prevent, control or even eradicate TCA. Thus, most researches are focused on the prevention of these defects more than in its elimination, because it is a tricky action. Moreover, it is important to follow several cleaning procedures in wine cellars. For example, the sanitizers used need to be free of chlorine and the microflora has to be controlled throughout the humidity, the temperature and the room ventilation. Furthermore, the regular checking of corks, dyes, sanitizers, inks and lubricants is vital for preventing this problem.
Several studies have been carried out on the removal of TCA but they mostly focus on TCA in a liquid solution. Valdés et al. (2018) studied if some polymers (polyanilines) could reduce the TCA content in whisky. They found that 75 % of TCA was eliminated without affecting negatively the aromatic profile and phenolic content of the whisky sample. Moreover, this group used the same polymeric materials to retain TCA from wine, achieving more than 75 % of reduction of this molecule. In other work it was shown a reduction of 91 % of TCA from wine using air-depleted solvent-impregnated cork powder. However, the applied treatment affected negatively the alkyl esters and acids of wine (Cosme et al., 2022). Besides, 10 min catalytic ozonization by raw bauxite was effective in the reduction of TCA in drinking water up to 86 % (Qi et al., 2009).
Regarding TCA elimination in corks, there are some patents and articles published. Different methods and technologies were used for that purpose: a flow of ethanol and water, the supercritical fluid technology, superheated steam or chemical and electrochemical methods were some of them (Cabral, 2006; Martín, 2018; Palacios et al., 2012; Ponte et al., 2013; Guedes, Mateus, Fernandes and Ribeiro, 2019a; Recio, Álvarez-Rodríguez, Rumbero, Garzón and Coque, 2011a; Viguera et al., 2018).
Atmospheric pressure cold plasma (APCP) is an approach for different purposes such as inactivating microorganisms. It is known that reactive species produced by APCPs are brought from the gas phase through the interphase into the bulk of the water, resulting in a liquid called plasma activated water (PAW) which is being investigated (Zhou et al., 2020). Among the most important applications, sterilization, microorganisms’ inactivation, either in biofilm or planktonic stage, seed germination or cancer therapy are the most investigated.
This treated liquid is commonly known to be efficient in all these applications due to the interaction and synergistic effect of different reactive species. These include reactive oxygen species (ROS), such as hydroxyl radicals (OH∙), atomic oxygen, superoxide (O2−), ozone (O3) and hydrogen peroxide (H2O2), as well as reactive nitrogen species (RNS) including atomic nitrogen, peroxynitrite, nitric oxide and nitrites (NO2−). Some of these reactive species are stable in the treated liquid (H2O2, NO2−) while the life-time of other ones is short. These last reactive species are supposed to be generated inside the treated liquid through secondary reactions under specific conditions (i.e. low pH).
Considering all the above, a procedure to eliminate TCA from cork stoppers is needed since the procedures and technologies evaluated up to now request high temperatures and pressures, long times, most of them cause irreversible deformations in corks and are not able to eliminate TCA spoilage. Thus, PAW could be a real solution in order to solve TCA problems in wineries and cork industry.
To the best of our knowledge, PAW was never tested for TCA removal from contaminated cork stoppers. In this study, different PAW were put into contact with cork stoppers artificially contaminated with a standard solution that included different chloroanisole and chlorophenol molecules. The aim was to demonstrate the capacity of PAW for decomposing and eliminating TCA, thus providing a new decontamination strategy to combat this known problem in wine industry.
2. Materials and methods
2.1. Cork samples, chloroanisole and chlorophenol solution
Natural cork stoppers (Ø24 × 45 mm) guaranteed free of chloroanisoles and chlorophenols were acquired in Lafitte Cork Portugal (Paços de Brandão, Portugal).
The stock solution (CPA Chem Ltd., Stara Zagora, Bulgaria) containing 0,40 ng/L of each chloroanisole and chlorophenol molecule was prepared as a standard solution in ethanol (96 % purity, Labbox Labware S.L., Premià de Dalt, Barcelona, Spain). This stock solution was diluted at 400 ng/L in deionized water for cork contamination. The chloroanisole molecules found in stock solution were: 2,4,6-Trichloroanisole; 2,4,5-Trichloroanisole; 2,3,4,5-Tetrachloroanisole; 2,3,4,6-Tetrachloroanisole; 2,3,5,6Tetrachloroanisole and Pentachloroanisole. The chlorophenol molecules: 2,4,6-Trichlophenol; 2,3,4-Trichlophenol; 2,3,4,5-Tetrachlorophenol; 2,3,4,6-Tetrachlorophenol and 2,3,5,6-Tetrachlorophenol.
Purified water (PW) for PAW generation and for preparing the 400 ng/L solution of chloroanisoles and chlorophenols was obtained from a water purification system (Elix® Essential, Merck Millipore, Burlington, Massachusetts, United States) with an electrical conductivity of <0.2 μS/cm and natural pH of >7.00 at 25 °C.
2.2. Chloroanisole and chlorophenol contamination procedure of cork stoppers and quantification method
Natural cork stoppers were individually immersed in glass jars (Ø44 × 94.8 mm) containing 80 mL of the 400 ng/L solution of chloroanisoles and chlorophenols. During 4 h the glass jars were in a rotating stirrer at 50 rpm (Multi Bio RS-24, Biosan, Riga, Latvian) (Fig. 1). Then, each cork stopper was dried at RT during 24 h. After that, the twelve different chloroanisoles and chlorophenols were analyzed following two chemical methods: OIV-MA-AS315-16 (determination of 2,4,6-Trichloroanisole in wine given from the cork stoppers; Resolution OIV/OENO 296/2009) and OIV-MA-AS315-17 (determination of polychlorophenols and polychloroanisoles in wines, cork stoppers, wood and bentonites used as adsorbent of those compounds in the atmosphere; Resolution OIV/OENO 374/2009) (International Organization of Vine and Wine -OIV-) (OIV, 2009). These methods simulate the migration of 2,4,6-Trichloroanisole, 2,4,6-Trichlorophenol, 2,3,4,6-Tetrachloroanisole, 2,3,4,6-Tetrachlorophenol, Pentachloroanisole and Pentachlorophenol susceptible of being produced between bottled wine and cork stoppers. Firstly, an alcoholic extraction with a by solid-phase microextraction system (SPME Fibras ARROW: 50/30μm DVB/CAR/PDMS) with a “liner” (SPME Injection Sleeve 0,75 mmID) was carried out. Then, samples were analyzed by gas chromatography (AGILENT TECHNOLOGIES. Model 8890; Injector: Agilent Technologies. PAL-System Model PAL III Series 2) with detection by mass spectrometer (Agilent Technologies. Model 7000C).
Fig. 1.
Scheme of the cork treatment process: [a] cork contamination, [b] PAW generation and cork decontamination and [c] PAW and cork characterization.
2.3. Preparation of PAW solutions
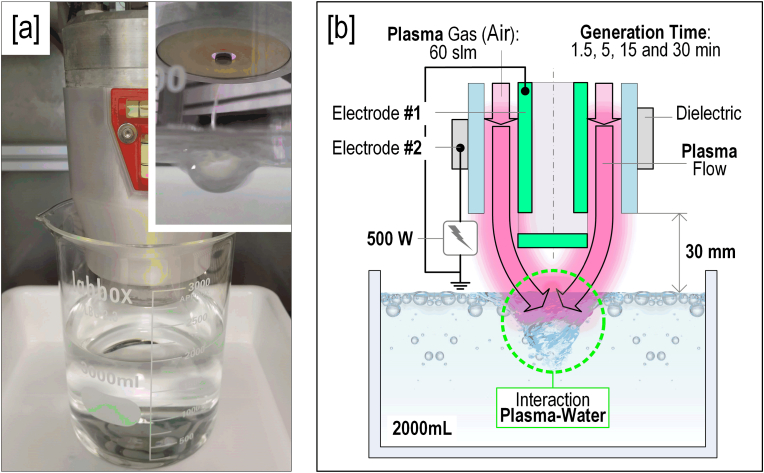
The APCP equipment used in this study was PlasmaSpot500 (Molecular Plasma Group, Foetz, Luxemburg). It consists of an internal grounded electrode, an external electrode linked to a high voltage source and an aluminum oxide dielectric tube between both electrodes.
PAW was generated by exposing PW to the plasma jet as shown in Fig. 2. After filling a 3000 mL beaker with 2000 mL of PW, plasma was turned on during four different times (1.5, 5, 15 and 30 min; samples referred to as PAW-1.5, PAW-5, PAW-15 and PAW-30, respectively). For all PAW generation treatments, air was used as plasma gas at 60 standard liter per minute (slm) and plasma power was 500 W. The distance between the end of the plasma nozzle and the PW surface was constant at 30 mm to optimize the transport of RONS from the plasma jet to the water volume while lowering the water losses.
Fig. 2.
[a] PAW generation system and detail of the interaction plasma-water and [b] PAW generation scheme.
2.4. Characterization of physicochemical properties of PAW
Physicochemical parameters of PAW were measured by different methods.
For pH, oxidation-reduction potential (ORP) and electrical conductivity (EC) a portable multimeter sensION MM150 DL with a 50 48 probe (Hach Company, United States of America) was used. Nitrates (NO3−) in PAW were quantified with a portable Imacimus® MultiIon analyser (NTsensors S.L., Spain). Nitrites (NO2−) were measured following the colorimetric Griess assay by spectrophotometry. Through this procedure nitrites were determined by reaction with sulfanilic acid under acidic conditions to form a diazonium ion, which couples to α-naphthylamine, forming a magenta colored dye that can be spectrophotometrically quantified based on its absorbance at 548 nm (Jablonowski and von Woedtke, 2015). The concentration of hydrogen peroxide (H2O2) was determined by measuring the absorbance of titanium peroxide at 407 nm. This method was based on the reaction of titanyl sulfate to yellow-colored peroxotitanyl sulfate. For all photometric measurements a Onda V-11 SCAN spectrophotometer (Giorgio Bormac s.r.l., Italy) was used. Griess reagent and Titanium (IV) oxysulfate were purchased from Sigma Aldrich (USA). All these parameters were measured at RT after PAW generation.
Finally, in order to detect secondary RONS, a method based on the reaction between phenol (C6H5-OH) and radicals OH•, NO• or NO2• was used (P. Lukes et al., 2014). The concentrations of phenol and its primary degradation by-products (benzoquinone (phenol + OH•), 4-nitrosophenol (phenol + NO•) and 2-nitrophenol (phenol + NO2•)) were analyzed using HPLC system with UV detection (Agilent 1100 Series, Agilent Technologies, Spain). Analyses were made using a 5 μm reversed phase Supelcosil C-18 column (25 cm × 2.4 mm; Supelco Inc. USA). The detection limit for the HPLC analysis was 0.01–0.1 μM (depending on the compound and the detection used). A solution of 2·10−2 M of phenol (Monplet & Esteban S.A., Spain) was prepared in water; 5.0 mL of this solution were mixed with 95 mL of PAW and incubated at 50 °C for 24 h. Then, the solution was filtered (0.45 μm) and measured by HPLC analysis using a gradient elution (Table 1) and the following conditions: injection volume of 20 μL, flow rate at 1.0 mL/min mobile phase; total time 16 min; P: ≈90 bar at 90-10, ≈60 bar at 60-40; and a detector at 260 nm (reference: 699 nm). The retention times were as follows: benzoquinone 5.0 min, 4-nitrosophenol 5.4 min, phenol 8.2 min, and 2-nitrophenol 11.2 min (Table 2).
Table 1.
Gradient elution for HPLC analysis.
| t (min) | Acetic acid:1 % (V/V) | Acetonitrile |
|---|---|---|
| 0 | 90 | 10 |
| 7 | 60 | 40 |
| 13 | 90 | 10 |
Table 2.
Calibration curves for phenol by-products quantification.
| Degradation by-product | Calibration curve | R2 |
|---|---|---|
| benzoquinone | A (mAU) = 0.09 + 6.99 × 105c (M) | 0.9996 |
| 4-nitrosophenol | A (mAU) = −0.24 + 1.50 × 105c (M) | 0.9997 |
| 2-nitrophenol | A (mAU) = −1.00 + 3.78 × 105c (M) | 0.9997 |
In order to quantify the degradation by-products, different calibration curves were prepared with solutions of known concentration of benzoquinone (Sigma Aldrich, USA) (1.35·10−5 M), 4-nitrosophenol (TCI Chemicals, Japan) (2.54·10−4 M) and 2-nitrophenol (Sigma Aldrich, USA) (2.54·10−4 M).
2.5. Chloroanisole and chlorophenol decontamination procedure from cork stoppers
Contaminated cork stoppers were individually immersed during 3 h in glass jars containing 80 mL of each type of PAW or PW (Fig. 1). After that, each cork stopper was dried at RT during 24 h and sent to the laboratory to analyze the concentration of the different chloroanisoles and chlorophenols following the procedure of section 2.2.
2.6. Statistical analysis
All experiments were conducted in triplicate. Statistical analysis of the data was performed using Statgraphics Centurion software (v19, The Plains, USA). p value < 0.05 was used to analyze statistically significant differences.
3. Results and discussion
3.1. Characterization of PAW
The physicochemical parameters of PAW (pH, EC, ORP, NO3− and NO2−) were characterized as a function of plasma activation time (Table 3).
Table 3.
Physicochemical parameters of each PAW at RT.
| PAW | pH | EC (μS/cm) | ORP (mV) | NO3− (mg/L) | NO2− (mg/L) |
|---|---|---|---|---|---|
| PAW-1.5 | 4.46 ± 0.15 | 25 ± 5 | 315 ± 13 | 3.67 ± 0.31 | 0.74 ± 0.05 |
| PAW-5 | 4.01 ± 0.17 | 52 ± 7 | 355 ± 10 | 4.13 ± 0.15 | 2.58 ± 0.27 |
| PAW-15 | 3.58 ± 0.09 | 103 ± 13 | 383 ± 9 | 9.90 ± 1.30 | 3.47 ± 0.13 |
| PAW-30 | 3.10 ± 0.12 | 190 ± 15 | 408 ± 21 | 17.90 ± 2.05 | 5.01 ± 0.87 |
The pH of PW dropped from 7.00 to 4.46 after exposure to plasma for 1.5 min and lowered to 3.10 after 30 min, showing plasma treatment acidified the water. A linear increase of EC over plasma activation time was observed, which raised from 5 μS/cm to 25 μS/cm after 1.5 min of treatment and increased to 190 μS/cm after 30 min. The ORP value increased from 299 mV to 315 mV after plasma activation for 1.5 min and reached 408 mV after 30 min. Related to the chemical analysis of PAW for NO3- and NO2-, there was a significant increase in the NO3- and NO2- concentrations during plasma activation time. Thus, the highest concentrations were reached for PAW-30 being 17.90 mg/L (NO3-) and 5.01 mg/L (NO2-), respectively. Finally, H2O2 was not detected in any of the PAW analyzed. It is supposed that in case of NO2- excess, H2O2 was completely reacted.
It is in accordance with the investigation carried out by (Rathore and Nema, 2021) who said that while the plasma activation time increased, a significant raise in EC and ORP and a pH reduction was observed. This trend is mainly due to the oxidizing species and active ions (such as H+, NO2-, NO3-) generated during PAW generation time. Moreover, other researchers have come to the same conclusion (El Shaer et al., 2020; Pan et al., 2017; Q. N. Zhang et al., 2016).
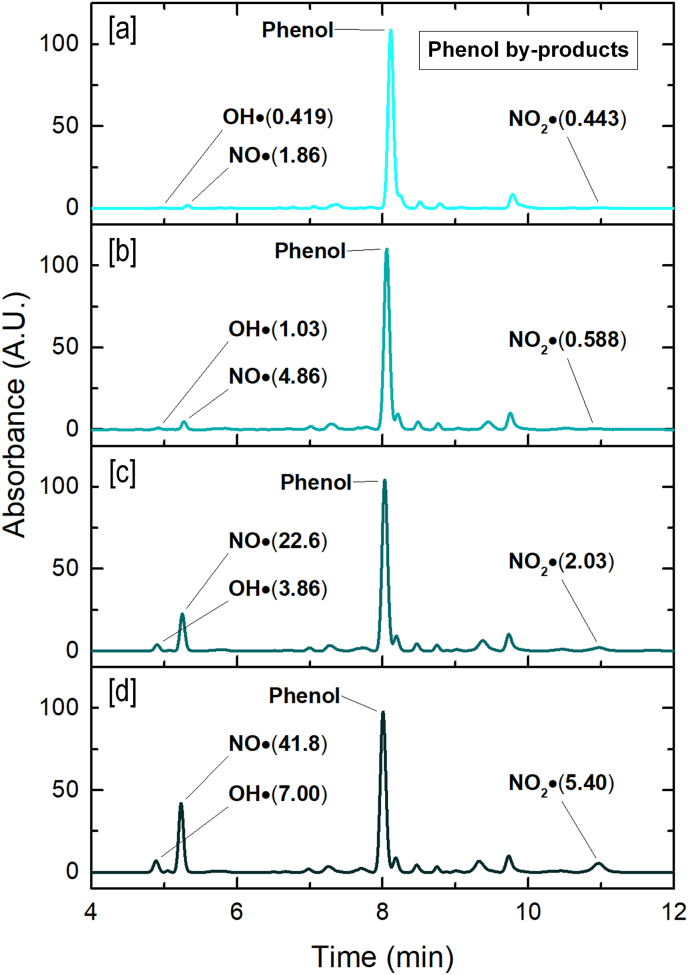
In order to further analyze other reactive species that could be present in PAW, HPLC chromatography was performed. Thus, the detection of phenol and its degradation by-products was used as an indirect analytical method to evaluate the presence of OH•, NO• and NO2•. The chromatograms showed the identified by-products after the reaction of OH•, NO• and NO2• with phenol (benzoquinone, 4-nitrosophenol and 2 nitrophenol, respectively) (Fig. 3).
Fig. 3.
HPLC chromatograms of each PAW: [a] PAW-1.5; [b] PAW-5; [c] PAW-15 and [d] PAW-30.
Table 4 indicates the concentration of the phenol by-products for each analyzed PAW. It was observed a common trend, since the three by products showed an increase with longer plasma activation times. Talking about benzoquinone, PAW-1.5 generated 8.2 μg/L in comparison to the 168.1 μg/L of PAW-30. Besides, the concentration of 4-nitrosophenol ranged from 227.0 μ/L to 3404.8 μg/L. Finally, 2-nitrophenol, varied from 209.5 μg/L to 1729.6 μg/L when the treatment time increased from 1.5 to 30 min. These products give evidence about the formation OH·, NO· and NO2·. There are few articles where those reactive species were found and quantified in PAW (P. Lukes et al., 2014; Tarabová et al., 2018).
Table 4.
Phenol by-products quantification.
| PAW | Phenol by-product concentration (μg/L) |
||
|---|---|---|---|
| Benzoquinone (OH•) | 4-nitrosophenol (NO•) | 2-nitrophenol (NO2•) | |
| PAW-1.5 | 8.2 | 227.0 | 209.5 |
| PAW-5 | 23.1 | 465.1 | 253.9 |
| PAW-15 | 91.7 | 1873.0 | 696.1 |
| PAW-30 | 168.1 | 3404.8 | 1729.6 |
Two possible theories about the origin of the reactive species identified by chromatography have been identified: (1) instability of acidified nitrites at acid pH and (2) photochemical reactions induced by UV radiation from plasma with ozone, nitrates and nitrites. On the other hand, it is known the short lifetime (nanoseconds) of these reactive species; however, they are implicated in cyclic reactions which could justify their presence in PAW several hours after the generation (Liu et al., 2020; Ptr Lukes et al., 2017).
3.2. TCA degradation in corks by PAW
Different methods and technologies have been tested trying to eliminate TCA from cork. For example, the use of an ethanol and water flow at 0.1 mbar and 100 °C during 10 h showed a reduction of 76 % of TCA (Palacios et al., 2012). When using a steam distillation system in rotative mode (100-125 °C, 0.2–0.8 bar, 6–65 min and 1–10 rpm) a similar percentage of TCA reduction (70 %) was achieved (Cabral, 2006). In this line, a device that use superheated steam at 125-135 °C and 4–8 bar was patented to reduce TCA from cork (Martín, 2018). Moreover, chemical methods like hydrogen peroxide as an oxidant were test removing 86 % of TCA from the surface of natural cork stoppers (Recio, Álvarez-Rodríguez, Rumbero, Garzón and Coque, 2011b). Guedes et al. (2019a, b) investigated different electrochemical reactors achieving 41 % decrease of TCA in cork disks after 24 h immersion in saline solution. Finally, supercritical fluids technology has been applied with results below the TCA detection limit (Ponte et al., 2013; Viguera et al., 2018). However, for most technologies, very expensive and complex equipment operating at elevated pressures and temperatures are needed.
Considering that the chemical compound 2,4,6-Trichloroanisole was identified as the main cause of cork taint in wine, first experiments were focused on its removal. For this purpose, four different PAW were tested against artificially contaminated corks. PW was applied to assess possible washing effects and three artificially contaminated corks were used as control sample.
Fig. 4 shows the average TCA concentration in cork samples after 3 h of contact with PAW and PW and the TCA concentration of dried contaminated corks (as control). For the shortest PAW generation time (PAW-1.5) only 18.1 % of TCA was removed. For PAW-5 and PAW-30 there were no statistically significant differences reaching 75.2 % and 72.6 % of removal, respectively; for PAW-15 the degradation was a little lower, 65.0 %. Finally, corks treated with PW did not show significative changes in TCA content with respect to the control which means that a washing effect did not take place.
Fig. 4.
Average TCA concentration (ng/L) of cork samples after 3 h in contact with different PAW and PW and contaminated corks (control). Different letters indicate statistically significant differences (p < 0.05).
There are no researchers who have investigated PAW technology in order to decompose or degrade TCA chemical molecule. However, some authors have studied how to decompose TCA molecules by other technologies, mainly ozonation. Those researchers have suggested OH• as the main reactive species decomposing TCA. Peter and Von Gunten (2007) observed higher TCA decomposition when increasing TCA exposure in water during off-flavors elimination by ozonation. In other work, (Qi et al., 2009) found 86 % of TCA decomposition in drinking water after 10 min ozonation with raw bauxite as catalyst. They indicated that the high percentage of reduction when using this catalyst could be attributed to the generation of more OH•. They carried out a scavenging experiment that confirmed the higher OH• generation accounted for the enhancement of the degradation of TCA. The same authors achieved almost 100 % of TCA degradation using iron modified bauxite and being attributed to the promotion of the OH• formation (Qi et al., 2012). Besides, (Xu and Qi, 2016) studied the elimination of TCA in catalytic ozonation by g-AlOOH in water obtaining 79.3 % of reduction and suggesting that OH• was the main reactive species which attacks TCA.
Considering the abovementioned, OH• was the reactive species proposed to play the main role in TCA decomposition. Moreover, (X. Zhang et al., 2018) quantified the products of reaction of OH• with salicylic acid. They indicated the measurement of 2,3-DHBA and 2,5-DHBA should provide an accurate estimation of the amount of OH• and they found highest concentrations when using air and oxygen as plasma gas and longer treatment times.
Thus, it is suggested that the main cause for TCA removal could be a result of the OH• found in each PAW. The lower values of TCA decomposition were obtained with PAW-1.5 because the OH• level was the minimum (Table 4). On the other hand, the highest decomposition could be expected for PAW-30 treatment, however, it seems that a huge amount of OH• did not produce the maximum decomposition. In this regard, (Qi et al., 2012) indicated that an excess in OH• generation resulted in OH• quenching. This is what probably occurs for PAW generated during the highest activation times, 15 and 30 min. Thereby, PAW-5 achieved the best TCA decomposition.
Furthermore, it is important to investigate how OH• attacks TCA molecules. In this context, OH• has a high oxidative potential and is known to oxidize molecules that other radicals cannot even react. Taking notice of what (Benitez et al., 2000) said, the first attack of OH• to TCA molecule is in the sites where chlorine is not bonded. Thus, the -OCH3 group is proposed to be the target. This is in line with (N. Q. Zhang et al., 2016), who showed the reaction between OH• and -OCH3 group by H abstraction, followed by a reaction with O2 forming peroxyl radicals and finally a disproportionation maybe through Russell mechanism. Besides, (Trewick et al., 2005) suggested a demethylation by hydroxylation; thus, OH• could be capable of eliminating methyl group from TCA. Once -OCH3 group is attacked, OH• goes for chlorine bonds (Kavitha and Palanivelu, 2016; Trewick et al., 2005). As far as we are concerned, there are two possible mechanisms to attack Cl atoms. The first one is when the three Cl atoms are replaced with OH• (L. L. Zhang et al., 2011). The second one is characterized by a dissociation of Cl atoms (Benitez et al., 2000).
3.3. Other chlorine molecules degradation in corks by PAW
After successfully proving the power of PAW to remove TCA from corks, we tried to know if other chloroanisole and chlorophenol molecules could also be decomposed by PAW. Since it was confirmed that PW has no effect in eliminating this type of chemical molecules, PW was not tested within this part of the study. In this case, ten molecules of chloroanisole and chlorophenol were analyzed after PAW treatment in corks.
Fig. 5 illustrates the average concentration of chloroanisole molecules after 3 h in contact with PAW and dried contaminated cork control. In general, it was observed high levels of decomposition regardless the chloroanisole analyzed or the PAW used.
Fig. 5.
Average concentration (ng/L) of different chloroanisole molecules of cork samples after 3 h in contact with different PAW and dried contaminated corks (control). Different letters indicate statistically significant differences (p < 0.05).
Fig. 6 shows the average concentration of chlorophenol molecules after 3 h in contact with PAW and dried contaminated corks (control). It could be confirmed, similarly to the results for chloroanisoles, that PAW treatments were successful in decomposing almost the total chlorophenol molecules analyzed. Comparing Fig. 5, Fig. 6, it can be observed a higher degree of decomposition for chlorophenols than chloroanisoles. This fact could be explained because –OH group of phenol molecule is more reactive towards polar molecules, such as OH•, than –OCH3 group of anisole (Revolution, 2021). Among all the chlorophenol molecules studied, the most known and important one is TCP since it is the precursor of TCA formation. Thus, we found two articles that investigate its removing. Saritha et al. (2009) compared four advanced oxidation processes for TCP removal (UV, UV/H2O2, Fenton, UV/Fenton and UV/TiO2). UV/Fenton was the best oxidation process since it achieved 90 % of TCP eliminated. In the same way, (Kavitha and Palanivelu, 2016) observed TCP decomposition after photo-Fenton process of almost 100 % suggesting a critical role of OH•.
Fig. 6.
Average concentration (ng/L) of different chlorophenol molecules of cork samples after 3 h in contact with different PAW and dried contaminated corks (control). Different letters indicate statistically significant differences (p < 0.05).
Finally, it is worth mentioning that the percentage of chloroanisole and chlorophenol molecules reduction were quite similar when applying a treatment with PAW generated during a plasma activation time equal or higher than 5 min (PAW-5, PAW-15 and PAW-30). Considering that the energy and time consumed when generating each PAW were different, PAW-5 was chosen as the best PAW from the economical and the ecofriendly points of view.
4. Conclusions
The huge percentage of wine bottles discarded each year due to the so-called cork taint is an important issue for wineries. Different chloroanisole and chlorophenol molecules seem to be responsible of such a big problem, specially the known 2,4,6-Trichloroanisole molecule.
In this research, it was confirmed PAW treatment as an effective method to eliminate TCA from artificially contaminated corks. It was also showed the activity of PAW to decompose other chloroanisole and chlorophenol molecules. The best treatment was the one using PAW generated during 5 min of plasma activation time (PAW-5) in which contaminated corks were individually immersed for 3 h.
Moreover, it was determined that the reactive species which plays the main role decomposing TCA and probably other chloroanisole and chlorophenol molecules, was OH•. It was suggested the mechanisms by the one OH• degrades TCA molecule; first a demethylation by hydroxylation reaction is produced, then OH• attacks Cl atoms.
In conclusion, decomposing chloroanisole and chlorophenol molecules with PAW could be an emerging solution to reduce the number of wine bottles discarded in wineries due to this major problem.
Funding
This work has been funded by MCIN/AEI/10.13039/501100011033 and the “European Union NextGenerationEU/PRTR” through grants PID2019-105367RB, PID2020-11365RB-C21 and PDC2022-133242-I00.
CRediT authorship contribution statement
Ana Sainz-García: Conceptualization, Data curation, Formal analysis, Investigation, Validation, Visualization, Writing – original draft, Writing – review & editing. Ana González-Marcos: Data curation, Funding acquisition, Supervision, Validation, Writing – review & editing. Rodolfo Múgica-Vidal: Conceptualization, Data curation, Formal analysis, Investigation, Visualization, Writing – review & editing. Ignacio Muro-Fraguas: Conceptualization, Data curation, Formal analysis, Investigation, Writing – review & editing. Félix Gallarta-González: Data curation, Resources, Validation, Writing – review & editing. Lucía González-Arenzana: Data curation, Investigation, Writing – review & editing. Isabel López-Alfaro: Conceptualization, Resources, Writing – review & editing. Pilar Santamaría: Conceptualization, Resources, Writing – review & editing. Rocío Escribano-Viana: Data curation, Investigation, Writing – review & editing. Elisa Sainz-García: Conceptualization, Data curation, Formal analysis, Investigation, Supervision, Writing – original draft, Writing – review & editing. Fernando Alba-Elías: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Supervision, Visualization, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The author Ana Sainz-García is thankful to the program of pre-doctoral contracts for the training of research staff funded by the Spanish Ministry of Science and Innovation.
The author Ignacio Muro-Fraguas, thanks the program of post-doctoral orientation contracts for the training of research staff funded by the autonomous community of La Rioja.
The author Rocío Escribano-Viana as postdoctoral researcher of the University of La Rioja, thanks the postdoctoral training program funded by the Ministry of Universities.
Handling editor: Maria Corradini
Contributor Information
Ana Sainz-García, Email: ana.sainz@unirioja.es.
Ana González-Marcos, Email: ana.gonzalez@unirioja.es.
Rodolfo Múgica-Vidal, Email: rodolfo.mugica@unirioja.es.
Ignacio Muro-Fraguas, Email: ignacio.muro@unirioja.es.
Félix Gallarta-González, Email: felix.gallarta@unirioja.es.
Lucía González-Arenzana, Email: lucia.gonzalez@unirioja.es.
Isabel López-Alfaro, Email: isabel.lopez@unirioja.es.
Pilar Santamaría, Email: psantamaria@larioja.org.
Rocío Escribano-Viana, Email: roescrv@unirioja.es.
Elisa Sainz-García, Email: elisa.sainzg@unirioja.es.
Fernando Alba-Elías, Email: fernando.alba@unirioja.es.
Data availability
Data will be made available on request.
References
- Benitez F.J., Beltran-Heredia J., Acero J.L., Rubio F.J. Contribution of free radicals to chlorophenols decomposition by several advanced oxidation processes. Chemosphere. 2000;41(8):1271–1277. doi: 10.1016/S0045-6535(99)00536-6. [DOI] [PubMed] [Google Scholar]
- Buja L.M. The history, science, and art of wine and the case for health benefits: perspectives of an oenophilic cardiovascular pathologist. Cardiovasc. Pathol. 2022;60 doi: 10.1016/j.carpath.2022.107446. [DOI] [PubMed] [Google Scholar]
- Cabral, M. (2006). 2 268 459.
- Coque J.J.R., Pérez E.R., Goswami M., Martínez R.F., García S.C., Rodríguez M.L.A., Martín J.F.M. Wine Contamination by Haloanisoles: towards the development of biotechnological strategies to remove chloroanisoles from cork stoppers. Inbiotec. 2006;3–51 https://www.apcor.pt/wp-content/uploads/2015/08/Inbiotec-index.pdf Retrieved from. [Google Scholar]
- Cosme F., Gomes S., Vilela A., Filipe-Ribeiro L., Nunes F.M. Air-depleted and solvent-impregnated cork powder as a new natural and sustainable fining agent for removal of 2,4,6-trichloroanisole (TCA) from red wines. Molecules. 2022;27(14) doi: 10.3390/molecules27144614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravero M.C. Musty and moldy taint in wines: a review. Beverages. 2020;6(2):1–13. doi: 10.3390/beverages6020041. [DOI] [Google Scholar]
- El Shaer M., Eldaly M., Heikal G., Sharaf Y., Diab H., Mobasher M., Rousseau A. Antibiotics degradation and bacteria inactivation in water by cold atmospheric plasma discharges above and below water surface. Plasma Chem. Plasma Process. 2020;40(4):971–983. doi: 10.1007/s11090-020-10076-0. [DOI] [Google Scholar]
- Español del Mercado del Vino Observatorio. 2022. Exportaciones españolas de vino y productos vitivinícolas. [Google Scholar]
- Guedes P., Mateus E.P., Fernandes J.P., Ribeiro A.B. Electro-technologies for the removal of 2,4,6-trichloroanisole from naturally contaminated cork discs: reactor design and proof of concept. Chem. Eng. J. 2019;361(December 2018):80–88. doi: 10.1016/j.cej.2018.12.040. [DOI] [Google Scholar]
- Guedes P., Mateus E.P., Fernandes J.P., Ribeiro A.B. Electro-technologies for the removal of 2,4,6-trichloroanisole from naturally contaminated cork discs: reactor design and proof of concept. Chem. Eng. J. 2019;361:80–88. doi: 10.1016/j.cej.2018.12.040. August 2018. [DOI] [Google Scholar]
- Jablonowski H., von Woedtke T. Research on plasma medicine-relevant plasma-liquid interaction: what happened in the past five years? Clinical Plasma Medicine. 2015;3(2):42–52. doi: 10.1016/j.cpme.2015.11.003. [DOI] [Google Scholar]
- Kavitha V., Palanivelu K. Degradation of phenol and trichlorophenol by heterogeneous photo-Fenton process using Granular Ferric Hydroxide®: comparison with homogeneous system. Int. J. Environ. Sci. Technol. 2016;13(3):927–936. doi: 10.1007/s13762-015-0922-y. [DOI] [Google Scholar]
- Liu K., Liu S.T., Ran C.F. The effect of air-water-plasma-jet-activated water on Penicillium: the reaction of HNO2 and H2O2 under acidic condition. Frontiers in Physics. 2020;8(2):1–12. doi: 10.3389/fphy.2020.00242. [DOI] [Google Scholar]
- Lukes P., Dolezalova E., Sisrova I., Clupek M. Aqueous-phase chemistry and bactericidal effects from an air discharge plasma in contact with water: evidence for the formation of peroxynitrite through a pseudo-second-order post-discharge reaction of H2O 2 and HNO2. Plasma Sources Sci. Technol. 2014;23(1) doi: 10.1088/0963-0252/23/1/015019. [DOI] [Google Scholar]
- Lukes Ptr, Akiyama H., Jiang C., Doria A., Gallerano G.P., Ramundo-Orlando A., et al. Bioelectrics. 2017. Special electromagnetic agents: from cold plasma to pulsed electromagnetic radiation. [DOI] [Google Scholar]
- Martín, R. B. (2018). 2 726 598.
- Monteiro S., Bundaleski N., Malheiro A., Cabral M., Teodoro O.M.N.D. Cross contamination of 2,4,6-trichloroanisole in cork stoppers. J. Agric. Food Chem. 2022;70(22):6747–6754. doi: 10.1021/acs.jafc.2c02493. [DOI] [PubMed] [Google Scholar]
- OIV . vol. 2. International Organisation of Vine and Wine; 2009. (Compendium of International Methods of Analysis-OIV Alkalinity of Ash). [Google Scholar]
- Palacios, F. S., Misiego, C. I., Montero, M. J. S., García, J. M., & Sánchez, N. M. (2012). 2 423 255.
- Pan J., Li Y.L., Liu C.M., Tian Y., Yu S., Wang K.L., et al. Investigation of cold atmospheric plasma-activated water for the dental unit waterline system contamination and safety evaluation in vitro. Plasma Chem. Plasma Process. 2017;37(4):1091–1103. doi: 10.1007/s11090-017-9811-0. [DOI] [Google Scholar]
- Peter A., Von Gunten U. Oxidation kinetics of selected taste and odor compounds during ozonation of drinking water. Environ. Sci. Technol. 2007;41(2):626–631. doi: 10.1021/es061687b. [DOI] [PubMed] [Google Scholar]
- Ponte, M. L. D. M. N. Da, Lopes, J. A. D. S., Vesna, N.-V., Manic, M., Mesquita, A. C. D. A. L. C., Silva, R. P. M. Da, & Montenegro, I. M. D. Q. (2013). 2 402 890.
- Qi F., Xu B., Chen Z., Ma J., Sun D., Zhang L., Wu F. Ozonation catalyzed by the raw bauxite for the degradation of 2,4,6-trichloroanisole in drinking water. J. Hazard Mater. 2009;168(1):246–252. doi: 10.1016/j.jhazmat.2009.02.037. [DOI] [PubMed] [Google Scholar]
- Qi F., Xu B., Zhao L., Chen Z., Zhang L., Sun D., Ma J. Comparison of the efficiency and mechanism of catalytic ozonation of 2,4,6-trichloroanisole by iron and manganese modified bauxite. Appl. Catal. B Environ. 2012;121–122:171–181. doi: 10.1016/j.apcatb.2012.04.003. [DOI] [Google Scholar]
- Rathore V., Nema S.K. Optimization of process parameters to generate plasma activated water and study of physicochemical properties of plasma activated solutions at optimum condition. J. Appl. Phys. 2021;129(8) doi: 10.1063/5.0033848. [DOI] [Google Scholar]
- Recio E., Álvarez-Rodríguez M.L., Rumbero A., Garzón E., Coque J.J.R. Destruction of chloroanisoles by using a hydrogen peroxide activated method and its application to remove chloroanisoles from cork stoppers. J. Agric. Food Chem. 2011;59(23):12589–12597. doi: 10.1021/jf2035753. [DOI] [PubMed] [Google Scholar]
- Recio E., Álvarez-Rodríguez M.L., Rumbero A., Garzón E., Coque J.J.R. Destruction of chloroanisoles by using a hydrogen peroxide activated method and its application to remove chloroanisoles from cork stoppers. J. Agric. Food Chem. 2011;59(23):12589–12597. doi: 10.1021/jf2035753. [DOI] [PubMed] [Google Scholar]
- Revolution E. Why OH group shows MORE +R than OCH3. 2021. https://edurev.in/question/264878/Why-OH-group-shows-MORE-R-than-OCH3#:~:text=The OH group is more acidic%2C nucleophilic%2C and can form,versatile than the OCH3 group Retrieved June 1, 2023, from.
- Saritha P., Raj D.S.S., Aparna C., Laxmi P.N.V., Himabindu V., Anjaneyulu Y. Degradative oxidation of 2,4,6 trichlorophenol using advanced oxidation processes - a comparative study. Water Air Soil Pollut. 2009;200(1–4):169–179. doi: 10.1007/s11270-008-9901-y. [DOI] [Google Scholar]
- Sefton M.A., Simpson R.F. Compounds causing cork taint and the factors affecting their transfer from natural cork closures to wine - a review. Aust. J. Grape Wine Res. 2005;11(2):226–240. doi: 10.1111/j.1755-0238.2005.tb00290.x. [DOI] [Google Scholar]
- Silva Pereira C., Figueiredo Marques J.J., San Romão M.V. Cork taint in wine: scientific knowledge and public perception - a critical review. Crit. Rev. Microbiol. 2000;26(3):147–162. doi: 10.1080/10408410008984174. [DOI] [PubMed] [Google Scholar]
- Simpson R.F., Sefton M. Origin and fate of 2,4,6-trichloroanisole in cork bark and wine corks. Aust. J. Grape Wine Res. 2007;13(2):106–116. doi: 10.1111/j.1755-0238.2007.tb00241.x. [DOI] [Google Scholar]
- Soleas G.J., Yan J., Seaver T., Goldberg D.M. Method for the gas chromatographic assay with mass selective detection of trichloro compounds in corks and wines applied to elucidate the potential cause of cork taint. J. Agric. Food Chem. 2002;50(5):1032–1039. doi: 10.1021/jf011149c. [DOI] [PubMed] [Google Scholar]
- Tarabová B., Lukeš P., Janda M., Hensel K., Šikurová L., Machala Z. Specificity of detection methods of nitrites and ozone in aqueous solutions activated by air plasma. Plasma Process. Polym. 2018;15(6) doi: 10.1002/ppap.201800030. [DOI] [Google Scholar]
- Tarasov A., Rauhut D., Jung R. “Cork taint” responsible compounds. Determination of haloanisoles and halophenols in cork matrix: a review. Talanta. 2017;175(March):82–92. doi: 10.1016/j.talanta.2017.07.029. [DOI] [PubMed] [Google Scholar]
- Trewick S.C., McLaughlin P.J., Allshire R.C. Methylation: lost in hydroxylation? EMBO Rep. 2005;6(4):315–320. doi: 10.1038/sj.embor.7400379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdés O., Marican A., Avila-Salas F., Castro R.I., Amalraj J., Laurie V.F., Santos L.S. Polyaniline based materials as a method to eliminate haloanisoles in spirits beverages. Ind. Eng. Chem. Res. 2018;57(24):8308–8318. doi: 10.1021/acs.iecr.8b01139. [DOI] [Google Scholar]
- Viguera M., Prieto C., Casas J., Casas E., Cabañas A., Calvo L. The parameters that affect the supercritical extraction OF 2,4,6-trichloroanisol from cork. J. Supercrit. Fluids. 2018;141(March):137–142. doi: 10.1016/j.supflu.2018.03.017. [DOI] [Google Scholar]
- Xu B., Qi F. Reaction mechanism of 2-methylisoborneol and 2,4,6-trichloroanisole in catalytic ozonation by γ-AlOOH: role of adsorption. Clean. 2016;44(9):1099–1105. doi: 10.1002/clen.201500749. [DOI] [Google Scholar]
- Zhang L.L., Leng S.Q., Zhu R.Y., Chen J.M. Degradation of chlorobenzene by strain Ralstonia pickettii L2 isolated from a biotrickling filter treating a chlorobenzene-contaminated gas stream. Appl. Microbiol. Biotechnol. 2011;91(2):407–415. doi: 10.1007/s00253-011-3255-x. [DOI] [PubMed] [Google Scholar]
- Zhang N., Geronimo I., Paneth P., Schindelka J., Schaefer T., Herrmann H., et al. Analyzing sites of OH radical attack (ring vs. side chain) in oxidation of substituted benzenes via dual stable isotope analysis (δ13C and δ2H) Sci. Total Environ. 2016;542:484–494. doi: 10.1016/j.scitotenv.2015.10.075. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Ma R., Tian Y., Su B., Wang K., Yu S., et al. Sterilization efficiency of a novel electrochemical disinfectant against Staphylococcus aureus. Environ. Sci. Technol. 2016;50(6):3184–3192. doi: 10.1021/acs.est.5b05108. [DOI] [PubMed] [Google Scholar]
- Zhang X., Zhou R., Bazaka K., Liu Y., Zhou R., Chen G., et al. Quantification of plasma produced OH radical density for water sterilization. Plasma Process. Polym. 2018;15(6):1–12. doi: 10.1002/ppap.201700241. [DOI] [Google Scholar]
- Zhou R., Zhou R., Wang P., Xian Y., Mai-Prochnow A., Lu X., et al. Plasma-activated water: generation, origin of reactive species and biological applications. J. Phys. Appl. Phys. 2020;53(30) doi: 10.1088/1361-6463/ab81cf. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.