Abstract
Background
We conducted a systematic review and meta-analysis to study the association between non-alcoholic fatty liver disease (NAFLD) and incident cardiovascular disease (CVD).
Methods
We searched Medline, Embase, Cochrane database and TRIP database. Random-effects model meta-analyses were used to obtain pooled effect sizes and 95% confidence intervals. The certainty in evidence was rated using the GRADE tool.
Results
Altogether 36 studies including a total of 7,068,007 participants were included in the systematic review and meta-analysis. Pooled data from 19 cohort studies demonstrated a significant increase in the risk of non-fatal CVD events in patients with NAFLD (HR 1.57, 95% CI 1.33–1.85, I2 = 95%). Pooled data from eight studies showed a significant increase in fatal CVD (HR 1.40, 95% CI 1.24–1.57, I2 =27%), and eight cohort studies suggested a significant increase in combined non-fatal and fatal CVD (HR 1.41, 95% CI 1.13–1.76, I2 =80%). Meta-analysis of studies reporting adjusted estimates in NAFLD patients with fibrosis revealed a significant increase in CVD events with acceptable level of heterogeneity (HR 1.64, 95% CI 1.25–2.16, I2 = 31%). The anticipated absolute increase in the risk of combined fatal and non-fatal CVD was estimated to be 29 more per thousand with NAFLD; that of fatal CVD events 16 more per thousand and that of non-fatal CVD events 19 more per thousand with NAFLD. The GRADE rating ranged from very low to low for overall and subgroup analyses.
Conclusion
The present systematic review suggests that NAFLD increases the risk of incident CVD. Cohort studies with the ability to analyze subgroup effects based on severity, along with randomized controlled trials that provide experimental evidence demonstrating a decrease in cardiovascular disease events through the treatment of non-alcoholic fatty liver disease, are necessary to validate and reinforce these findings.
Keywords: non-alcoholic fatty liver disease, cardiovascular disease, meta-analysis, GRADE
Non-alcoholic fatty liver disease (NAFLD), the most common chronic liver disease worldwide, is estimated to affect one in three adults worldwide.1 The prevalence of NAFLD is expected to increase further, driven by lifestyle and demographic factors, along with the associated risk of liver related mortality and morbidi-ty.2
Over the past decade, it has become increasingly clear that NAFLD is a multisystem disease that affects a variety of extra-hepatic organ systems, including the cardiovascular system. There is increased cognizance of the close bi-directional link that NAFLD has with other non-communicable diseases, such as cardiovascular diseases (CVD), and that degree of fibrosis further predicts higher risk of incident CVD.3 . A strong association of NAFLD with dyslipidaemia, hypertension and coronary artery disease has been shown in several studies across the globe.4 However, some studies have not found the association.5 It is pertinent to study this association since it will influence strategies for management of NAFLD patients. It also has important implications for risk stratification for CVD and its prevention. Moreover, the absolute effect of NAFLD on incident CVD, independent of conventional risk factors, is unclear and has not been reported.
Previous studies have yielded disparate findings on the association of NAFLD and incident cardiovascular disease.6 Lazo et al. reported that NAFLD was not associated with increased all-cause and cause-specific (CVD, cancer, and liver) mortality.7 On the other hand, numerous studies have reported an increased risk of CVD with NAFLD.8
Previous reviews that have attempted to summarize the evidence and study these discrepancies have limited clinical applicability due to the reporting of surrogate outcomes like increased carotid intima media thickness, reduced endothelial function, increased coronary artery calcification, and increased arterial stiffness, rather than clinical cardiovascular events.9 Pertinent methodological issues in the interpretation of pooled results have not been given due attention in previous systematic reviews. It is possible that application of the GRADE (Grading of Recommendations, Assessment, Development and Evaluations) tool may give adequate consideration to issues that affect the certainty in evidence. Moreover, calculation of anticipated absolute effects for this association will help to delineate more clearly the burden of NAFLD as risk factor for CVD.
An updated systematic summary of the current evidence on this association is needed. Therefore, we conducted a GRADE evaluation using a comprehensive and updated systematic review and meta-analysis to study the association between NAFLD and risk of developing incident cardiovascular disease. The primary objective was to find the association of NAFLD with incident fatal and non-fatal CVD, and anticipated absolute effects for this association. The secondary objective was to study this association in the subgroup of NAFLD patients with advanced fibrosis.
Methods
We adhered to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) and meta-analysis of observational studies in epidemiology (MOOSE) statements for the present review.10,11 We developed a protocol before beginning this systematic review and registered it in on PROSPERO (registration number CRD42021241961). The protocol mentioned the search methods planned for identification of studies, as well as the methods of screening titles, abstracts and full texts. The protocol also pre-specified the subgroup analyses that were planned for investigation of heterogeneity, and postulated the hypothesis that greater effect size will be seen in patients with fibrosis as compared to those without.
Inclusion Criteria
We included cohort studies that compared adult patients with NAFLD diagnosed by histology or imaging to adults without NAFLD. The outcomes of interest were coronary artery disease (including ischemic heart disease and angina pectoris), stroke and death from cardiovascular causes that occurred during the follow up period of the cohort study, i.e., they were incident cases of cardiovascular disease. We included both prospective and retrospective cohort studies.
We excluded studies that diagnosed NAFLD using methods other than histology or imaging. We also excluded case–control studies, cross-sectional studies, reviews, commentaries and editorials.
Definitions
Fatal and non-fatal CVD: composite outcome of coronary artery disease (including ischemic heart disease and angina pectoris), stroke and death from cardiovascular causes.
NAFLD: NAFLD diagnosed on histology or imagining using standard criteria as mentioned in the original studies.
Fibrosis: Fibrosis was defined as per the criteria mentioned in the included study, defined as: Fibrosis stage classified as F3 or F4 on histology or liver stiffness measure more than 9.4 kPa on transient elastography or risk stratification as advanced fibrosis using Fibrosis-4 score (>2.67). In case the study did not report the estimate for advanced fibrosis separately from that of indeterminate, these two strata were together considered as fibrosis.
Database Searches
We searched the following databases for articles published till 25th June 2023: Medline, Embase, Cochrane database and TRIP database. Search terms, a combination of MeSH terms and text words, for NAFLD included “nonalcoholic fatty liver disease,” “non-alcoholic fatty liver disease” “NAFLD,” “fatty liver,” “nonalcoholic steatohepatitis,” “NASH,” or “hepatic steatosis”; for CVD included “myocardial infarct,” “heart attack,” “cardiovascular outcomes,” “CVD” “myocardial ischemia,” “cardiovascular disease,” “coronary heart disease,” “cardiac dysfunction,” or “coronary artery disease”; for stroke included “stroke,” “transient ischemic attack,” “ischemic cerebral infarction,” “cerebral infarction” “CVA” and “cerebrovascular accident.”
Editorials, letters, news, reviews, expert opinions, case reports, and studies without original data were excluded.
We screened the reference lists of pertinent original articles and reviews to search for relevant articles. The whole process of study selection, data abstraction and risk of bias assessment was carried out independently by two reviewers (MP and SG).
Selection of Studies
Two reviewers (MP and SG) screened titles and abstracts in duplicate. Full texts were procured for those that either reviewer deemed potentially suitable. The eligibility of articles was ascertained based on their full texts. Additionally, data extraction was independently carried out by the two reviewers, and an evaluation of bias risk was conducted. Throughout all stages of the project, any disagreements were resolved through discussion until a consensus was achieved.
Data Extraction
The following data were extracted from each study independently by two reviewers (MP and SG): surname of the first author, year of publication, country, population characteristics, diagnostic method for NAFLD, follow up period, sample size, method of adjustment, variables adjusted and outcomes reported (myocardial infarction/stroke/deaths due to cardiovascular causes in NAFLD and non-NAFLD groups). Disagreements were resolved by discussion.
Risk of Bias Assessment
Risk of bias was assessed using the modified version of New Castle Ottawa scale.12 Each criterion was judged as definitely or probably low risk of bias, or probably or definitely high risk of bias. Two review authors independently assessed the study risk of bias with disagreements resolved by discussion.
Data Synthesis and Statistical Analysis
Random effects model meta-analyses were performed to obtain pooled effect sizes and 95% confidence intervals, using the Generic Inverse variance method. We carried out all statistical analyses using Review Manager 5.3. We used adjusted estimates, wherever available, to pool data.
We assessed heterogeneity using the I2 statistic, and by visual inspection of forest plot to look for overlap of confidence intervals and closeness of point estimates.
We attempted to explain the heterogeneity observed through a subgroup analysis by severity. Studies that reported associations in NAFLD patients with fibrosis were included in the subgroup, as defined above. Metaregression was also performed to explore heterogeneity by assessing the effect of severity as a covariate.
GRADE
The GRADE methodology was employed to assign levels of confidence in the evidence for each outcome, categorized as high, moderate, low, or very low. Comprehensive GRADE guidelines were followed to evaluate the overall risk of bias, precision, inconsistency, indirectness, and publication bias. The findings were then synthesized into an evidence profile.13
Results
Study Selection
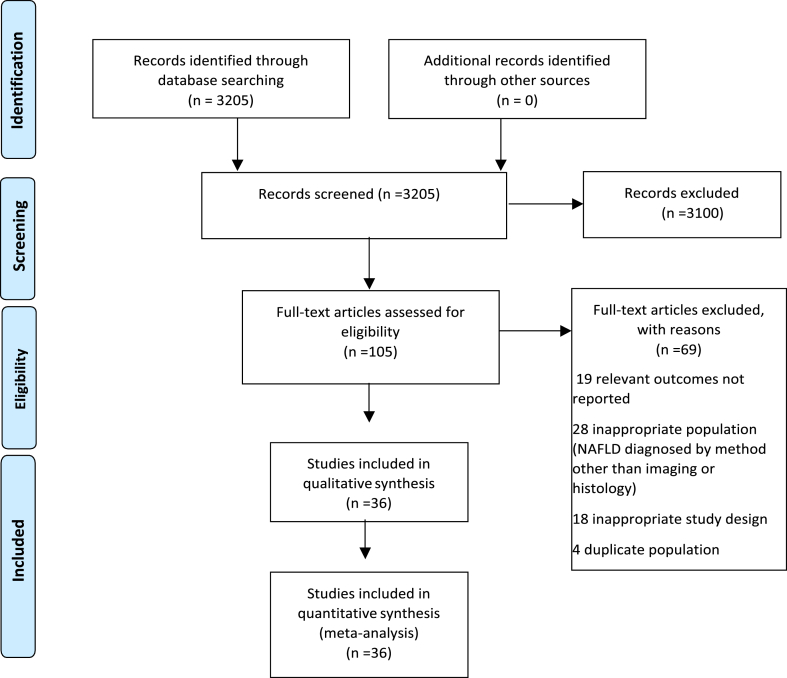
A total of 3205 titles and abstracts were obtained through our search, all of which were identified from the electronic database search. Among these, 3100 articles were excluded after reviewing their titles and abstracts, resulting in 105 articles undergoing a full-text review. From this pool, 69 articles were subsequently excluded: 19 due to reporting of irrelevant outcomes, 28 due to an unsuitable population, 18 due to an inappropriate study design, and four due to investigation of a duplicate population. Ultimately, 36 studies were deemed eligible. Overall, there was good inter-rater agreement for study selection (κ =0.82). These 36 studies that included 7,068,007 participants, were included in the systematic review and meta-analysis14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46,54, 55, 56 (Figure 1).
Figure 1.
PRISMA Flow Diagram for study selection.
Study Characteristics
Most of the studies were conducted in the USA or Europe. Twenty out of the 36 studies were prospective cohort studies. Most of the included study participants were in the age group 50–60 years. Sixteen studies reported outcomes for non-fatal CVD events, most of which were a composite MI, angina, stroke and need for revascularization. Eight studies reported only fatal CVD events and eight studies included CVD deaths in the composite end point. All except one study carried out adjustment for potential confounders. The most common adjustment method was Cox proportional hazards regression. Most studies adjusted for age, sex and smoking. Seven studies adjusted for blood pressure and five for BMI at baseline. 15 studies adjusted for hypertension. One study54 adjusted for time-varying covariates also. The variables adjusted and other characteristics of the included studies are presented in Appendix I table 1.
Risk of Bias Assessment
Across all outcomes examined in the cohort studies, the risk of bias pertaining to the selection of both exposed and non-exposed populations, as well as exposure assessment, was determined to be low. For all studies, a low risk of bias was ascribed to the presence of the outcome at the commencement of the study, with a definite low risk assigned to studies reporting mortality outcomes. Nevertheless, one study lacked sufficient adjustment and evaluation of prognostic factors. Seven studies were identified as probably having a high risk of bias due to inadequate follow-up (Appendix II).
Pooled Effects of the Association of NAFLD with Incident CVD Events
Non-fatal CVD events
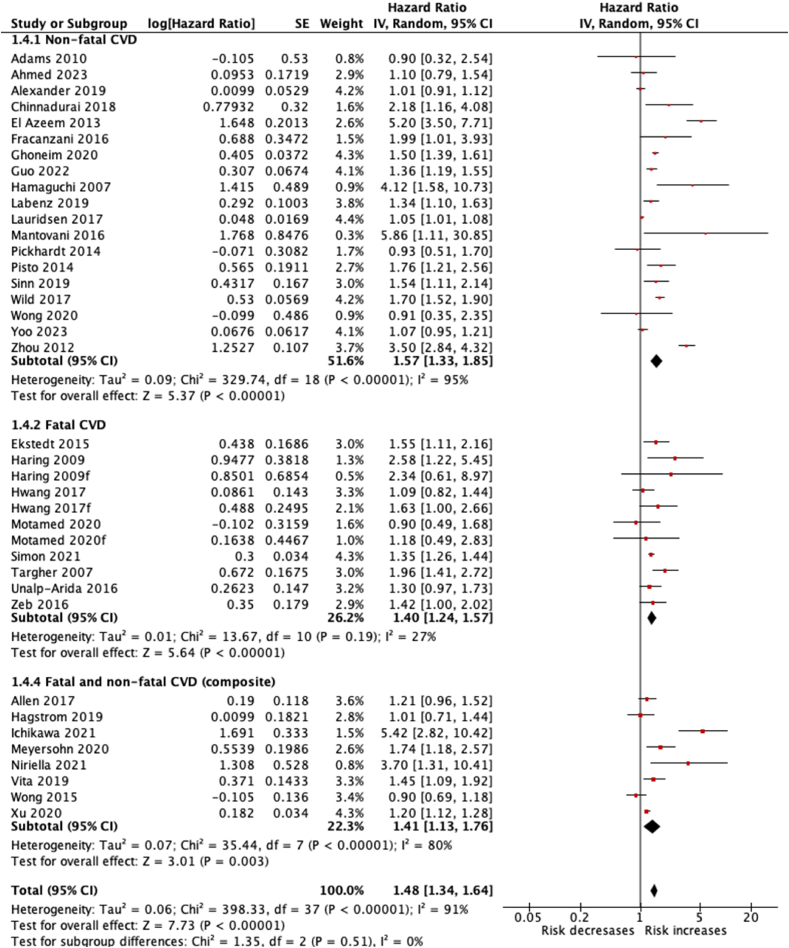
Pooled data from 19 cohort studies14,15,18,20, 21, 22,24,28, 29, 30,34,35,37,41,42,46,54, 55, 56 suggested a statistically significant increase in the risk of non-fatal CVD events in patients with NAFLD compared to controls (HR 1.57, 95% CI 1.33–1.85, I2 = 95%).
Fatal CVD events
Pooled data from eight cohort studies19,25,26,32,36,38,39,45 suggested a statistically significant increase in the risk of fatal CVD events in patients with NAFLD compared to controls (HR 1.40, 95% CI 1.24–1.57, I2 = 27%).
Fatal and non-fatal CVD events (combined)
Pooled data from eight cohort studies16,23,27,31,33,40,42,44 suggested a statistically significant increase in the risk of non-fatal and fatal CVD events in patients with NAFLD compared to controls (HR 1.41, 95% CI 1.13–1.76, I2 = 80%) (Figure 2).
Figure 2.
Forest plot for the association of NAFLD with incident CVD events.
Examination of funnel plots revealed no obvious asymmetry (Appendix III).
Subgroup analysis
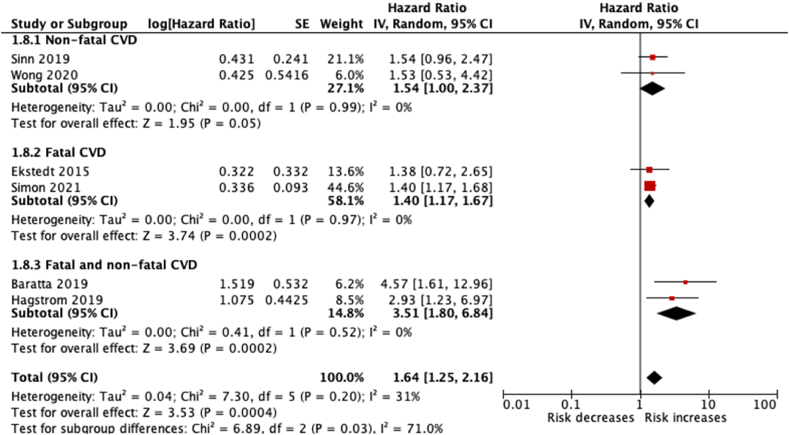
Meta-analysis of six studies17,19,23,36,37 reporting estimates in NAFLD patients with fibrosis revealed a statistically significant increase in the risk for CVD events. The pooled estimates for combined, non-fatal and fatal CVD events were HR 3.51 (95% CI 1.80–6.84), HR 1.54 (95% CI 1.00–2.37) and HR 1.40 (95% CI 1.17–1.67), respectively. There was acceptable level of heterogeneity for all these outcomes (I2 = 0%) (Figure 3).
Figure 3.
Forest plot for the association of NAFLD with advanced fibrosis with incident CVD events.
In the subgroup analysis, results were consistent with our prior hypothesis that effects would be larger in studies that reported estimates for patients with fibrosis. However, on meta-regression, the relationship between presence of fibrosis and effect size for each study was not statistically significant (P = 0.632, co-efficient = 0.115, 95% CI = −0.355–0.584).
GRADE assessments for certainty of evidence
The overall rating for certainty in estimates was very low for the outcomes non-fatal CVD and combined fatal and non-fatal CVD. The certainty in estimates was rated down for high level of heterogeneity for these outcomes. However, with the outcome fatal CVD, and in the subgroup of severe NAFLD patients, the certainty in estimates was low as these meta-analyses demonstrated acceptable levels of heterogeneity. The anticipated absolute effects for these outcomes, along with the GRADE assessments are reported in Table 1.
Table 1.
GRADE Assessments and Summary of Findings Table.
| Certainty assessment |
Summary of findings |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Studies | Risk of bias | Inconsistency | Indirectness | Imprecision | Other bias | Overall certainty of evidence | Relative effect (95% CI) | Anticipated absolute effects |
|
| Risk with no NAFLDa | Risk difference with NAFLD | ||||||||
| Non-fatal CVD events | |||||||||
| 19 cohort studies | Not serious | Very seriousb | Not serious | Not serious | All plausible residual confounding would suggest spurious effect, while no effect was observed | ⨁◯◯◯ VERY LOW | HR 1.57 (1.33–1.85) | 34 per 1000 | 19 more per 1000 (from 11 more to 28 more) |
| Fatal CVD events | |||||||||
| 8 cohort studies | Not serious | Not seriousc | Not serious | Not serious | All plausible residual confounding would suggest spurious effect, while no effect was observed | ⨁⨁◯◯ LOW |
HR 1.40 (1.24–1.57) | 41 per 1000 | 16 more per 1000 (from 10 more to 23 more) |
| Fatal and non-fatal CVD events | |||||||||
| 8 cohort studies | Not serious | Very seriousd | Not serious | Not serious | All plausible residual confounding would suggest spurious effect, while no effect was observed | ⨁◯◯◯ VERY LOW |
HR 1.41 (1.13–1.76) | 76 per 1000 | 29 more per 1000 (from 9 more to 54 more) |
| Non-fatal CVD events: fibrosis subgroup | |||||||||
| 2 cohort studies | Not serious | Not serious | Not serious | Serious | All plausible residual confounding would suggest spurious effect, while no effect was observed | ⨁⨁◯◯ LOW |
HR 1.54 (1.00–2.37) | 34 per 1000 | 18 more per 1000 (from 0 fewer to45 more) |
| Fatal CVD events: fibrosis subgroup | |||||||||
| 2 cohort studies | Not serious | Not serious | Not serious | Not serious | All plausible residual confounding would suggest spurious effect, while no effect was observed | ⨁⨁◯◯ LOW |
HR 1.40 (1.17–1.67) | 41 per 1000 | 16 more per 1000 (from 7 more to 27 more) |
| Fatal and non-fatal CVD events: fibrosis subgroup | |||||||||
| 2 cohort studies | Not serious | Not serious | Not serious | Not serious | All plausible residual confounding would suggest spurious effect, while no effect was observed | ⨁⨁◯◯ LOW |
HR 3.51 (1.80–6.84) | 76 per 1000 | 166 more per 1000 (from 57 more to 342 more) |
CI: confidence interval; HR: hazard ratio.
Baseline risk comes from the emerging risk factors collaboration, with a median of 10.8 y of follow-up for a total of 102 international cohorts (https://doi.org/10.1016/S0140-6736(1060484-9).
I2 = 95%.
I2 = 27%.
I2 = 80%.
Discussion
Principal Findings
The present GRADE evaluation suggests that NAFLD is associated with increased risk of incident CVD events. The body of evidence arises from cohort studies, almost all of which adjusted for potential confounding variables. The anticipated absolute increase in the risk of combined fatal and non-fatal CVD was estimated to be 29 more per thousand with NAFLD; and that of non-fatal CVD events 19 more per thousand with NAFLD. The GRADE rating ranged from very low to low for overall and subgroup analyses.
Heterogeneity observed with the overall analysis was high. However, on conducting a subgroup analysis with studies reporting estimates for NAFLD patients with fibrosis, we observed an acceptable level of heterogeneity. Thus, it is conceivable that the high heterogeneity in the overall meta-analysis may be a result of diverse clinical characteristics of included patients.
Implications of Findings
CVD is recognized as the primary cause of mortality among individuals with NAFLD.47 However, investigating the link between NAFLD and the occurrence of CVD is made complex due to the shared pathways involving factors like obesity.29 Notably, it has been documented that NAFLD-associated fibrosis is independently linked to various cardiovascular risk factors, encompassing obesity, metabolic syndrome, diabetes, hypertension, and high-density lipoprotein cholesterol.48 These interconnected relationships create challenges in differentiating between causal relationships and distorted effects. The deficiency of substantial evidence to definitively establish NAFLD's role in CVD pathogenesis is evident in the divergent conclusions of prior investigations. Extensive cohort studies, such as Labenz et al.'s research,28 have detected a correlation between NAFLD and CVD within a vast administrative database of primary care practices. In contrast, the mendelian randomization study conducted by Lauridsen et al.,29 which employed the PNPLA3 gene as an instrumental variable, concluded that elevated liver fat content was not causally linked to coronary heart disease (CHD) risk. This study suggested that the observed association might be attributed to confounding factors or reverse causation.
While establishing causation remains challenging with the existing body of evidence, this current review directs focus towards the role of NAFLD role as an emerging contributor to cardiovascular disease risk. The underlying biological mechanism linking NAFLD and CVD is posited to originate within the expanded visceral adipose tissue. This process involves chronic inflammation that results in heightened circulation of pro-atherogenic mediators like free fatty acids, interleukin-6, and other pro-inflammatory cytokines. Additionally, the activation of two key intracellular transcription factor-signaling pathways, namely the nuclear factor kB and JNK pathways, leads to the development of insulin resistance.48
Considering the high prevalence of NAFLD, this is an association with serious public health implications. These findings would have important implications for cardiovascular risk stratification. Patients of NAFLD would be candidates for more aggressive and early treatment of associated cardiovascular risk factors. Policy decisions on population level interventions, such as universal screening, will be governed by the establishment of NAFLD as an independent risk factor for CVD.
Metabolic dysfunction, characterized by obesity, diabetes, or the presence of various components of the metabolic syndrome, is frequently observed in individuals with fatty liver, irrespective of alcohol consumption. The acronym NAFLD does not adequately capture this correlation. As a result, the adoption of the terms metabolic (dysfunction) associated fatty liver disease (MAFLD) and metabolic dysfunction-associated steatotic liver disease (MASLD), which frame the disease more inclusively, have been proposed as being more appropriate.
Comparison to Other Reviews
The results of the present systematic review are consistent with that of Targher et al.,49 who also reported a statistically significant increase in the risk of CVD in NAFLD. The magnitude of effect reported by Targher et al. is similar to that of the present study for non-fatal and combined effects. The present review includes 20 recently published cohort studies in addition to the ones included in the review by Targher et al. The systematic reviews by Lu et al., Veracruz et al. and Wu et al.50, 51, 52 also reported an increased risk; however, they included cross-sectional studies in their meta-analysis. We intended to study the temporal association, and hence excluded cross-sectional and case control studies. The review by Mahfood Haddad et al. included six cohort studies and reported a consistent increase in risk of clinical CVE with NAFLD.53
Though the present review corroborates the findings of Mantovani et al.,57 there are additional findings reported in the present review that are important from the perspective of clinical decision-making and public health. In primary studies and systematic reviews focusing on risk factors, it's customary to examine and present relative measures of association between the factor and the outcome under study. This approach is primarily adopted due to the consistent nature of relative measures across different levels of baseline risk. Nonetheless, in the context of decision-making, healthcare providers directly involved in patient care, guideline developers, and policymakers ultimately require the absolute risk values for both those possessing the risk factor in question and those without it.13 Calculation of anticipated absolute effects for this association will help to delineate more clearly the burden of NAFLD as risk factor for CVD. The present review, in addition to reporting pooled effect in relative measures, also reports the how the findings translate in absolute terms. That is, the burden of CVD that will reduce on elimination of the risk contribution of NAFLD is derived from the findings. This is in keeping with the GRADE guidance that the present review adheres to, which also entails increased credence to the implications of methodological issues in the body of evidence. Furthermore, previous meta-analyses and reviews have pooled clinical cardiovascular disease (CVD) with surrogate outcomes, such as coronary artery stenosis of 50% without documented clinical events, coronary artery calcium, carotid intimal medial thickness, and so forth.6 We excluded studies that only reported these surrogate outcomes in order to study the true association with clinical events and thus increase the clinical utility of the results. Moreover, we reported anticipated absolute effects as a summary of findings.
Strengths and Limitations
The current systematic review possesses several notable strengths. It encompasses a robust and extensive search across three major databases. To enhance validity, we exclusively considered studies that employed imaging or histology to diagnose NAFLD. The processes of screening, data extraction, and risk of bias assessment were all carried out independently by two reviewers. In an attempt to address heterogeneity, we conducted subgroup analyses that were specified a priori. Moreover, we employed the GRADE methodology to categorize the certainty of evidence as very low, low, moderate, or high. This allowed us to meticulously address methodological concerns like imprecision, inconsistency, and bias risk. We also present absolute effects concerning the additional burden of cardiovascular disease attributed to NAFLD. As far as we are aware, this represents the most up-to-date and comprehensive systematic review investigating the correlation between NAFLD and the occurrence of cardiovascular diseases.
Nevertheless, the limitations of this review are partly a result of the constraints within the primary studies that were included. The included studies varied considerably in the outcome reported, commonly different composite outcomes and in some cases cause specific CVD outcomes. This resulted in there being considerable heterogeneity in the meta-analysis, part of which could be explained by subgroup analyses by severity. Heterogeneity might also have been due to the kind of patients included, which were in fact quite diverse clinically in terms of race, age and co-morbidities. Some studies failed to adequately adjust for potential confounders, with one study even reporting unadjusted estimates.42 The operational definition for fibrosis used for the subgroup analysis was as per the included study; however, this may pose as a challenge in interpretation with regard to varying value judgments as to what constitutes an appropriate definition of fibrosis, particularly for cut-offs used in transient elastography and non-invasive bioma-rker tools.
Recommendations
The ongoing debate regarding whether the association between NAFLD and incident CVD is truly independent or is influenced by confounding due to shared risk factors necessitates evidence from studies that are both adequately powered and methodologically robust. Observational studies should possess the capacity to effectively address potential confounding by including adjustments for such covariates. While utilizing epidemiological techniques like mendelian randomization proves to be valuable in exploring such associations, a step closer to establishing causality could potentially be taken through the implementation of randomized controlled trials that treat NAFLD and report clinically meaningful outcomes such as cardiovascular disease occurrences. Additionally, further investigation is required to determine at which point along the spectrum, ranging from simple steatosis to non-alcoholic steatohepatitis (NASH), the risk of cardiovascular disease begins to escalate. Moreover, seeing that the principal cause of death in NAFLD patients is CVD, clinical trials that evaluate the effect of drugs for NAFLD and NASH should perhaps study agents that also have a cardioprotective effect.
The present systematic review and meta-analysis supports the hypothesis that NAFLD is independently associated with CVD. The included studies demonstrate that there is growing body of evidence of an increased risk of incident cardiovascular disease with NAFLD, which is independent of the risk conferred by traditional risk factors Cohort studies with the ability to analyze subgroup effects based on severity, along with randomized controlled trials that provide experimental evidence demonstrating a decrease in cardiovascular disease events through the treatment of non-alcoholic fatty liver disease, are necessary to validate and reinforce these findings.
Credit authorship contribution statement
Manya Prasad: Conceptualization; Data curation; Formal analysis; Project administration; Resources; Software; Validation; Visualization; Roles/Writing—original draft; Writing—review & editing;
Sunanda Gupta: Data curation; Validation; Writing—review.
Shiv Sarin: Supervision, Writing—review and editing.
Conflicts of interest
All authors have none to declare.
Funding
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jceh.2023.08.013.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
References
- 1.Younossi Z.M., Koenig A.B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016 Jul;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 2.Li J., Zou B., Yeo Y.H., et al. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999-2019: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2019 May;4:389–398. doi: 10.1016/S2468-1253(19)30039-1. [DOI] [PubMed] [Google Scholar]
- 3.Lonardo A., Ballestri S., Marchesini G., Angulo P., Loria P. Nonalcoholic fatty liver disease: a precursor of the metabolic syndrome. Dig Liver Dis. 2015 Mar;47:181–190. doi: 10.1016/j.dld.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 4.Long M.T., Zhang X., Xu H., et al. Hepatic fibrosis ASSOCIATES WITH MULTIPLE cardiometabolic disease risk factors: the Framingham Heart study. Hepatology. 2021 Feb;73:548–559. doi: 10.1002/hep.31608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feitosa M.F., Reiner A.P., Wojczynski M.K., et al. Sex-influenced association of nonalcoholic fatty liver disease with coronary heart disease. Atherosclerosis. 2013 Apr;227:420–424. doi: 10.1016/j.atherosclerosis.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu H., Lu H.Y. Nonalcoholic fatty liver disease and cardiovascular disease. World J Gastroenterol. 2014 Jul 14;20:8407–8415. doi: 10.3748/wjg.v20.i26.8407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lazo M., Hernaez R., Bonekamp S., et al. Non-alcoholic fatty liver disease and mortality among US adults: prospective cohort study. BMJ. 2011 Nov 18;343:d6891. doi: 10.1136/bmj.d6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luo J., Xu L., Li J., Zhao S. Nonalcoholic fatty liver disease as a potential risk factor of cardiovascular disease. Eur J Gastroenterol Hepatol. 2015 Mar;27:193–199. doi: 10.1097/MEG.0000000000000254. [DOI] [PubMed] [Google Scholar]
- 9.Oni E.T., Agatston A.S., Blaha M.J., et al. A systematic review: burden and severity of subclinical cardiovascular disease among those with nonalcoholic fatty liver; should we care? Atherosclerosis. 2013 Oct;230:258–267. doi: 10.1016/j.atherosclerosis.2013.07.052. [DOI] [PubMed] [Google Scholar]
- 10.Moher D., Shamseer L., Clarke M., et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015 Jan 1;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stroup D.F., Berlin J.A., Morton S.C., et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000 Apr 19;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 12.Tool to Assess Risk of Bias in Cohort Studies.pdf [Internet]. [cited 2020 May 29]. Available from: https://methods.cochrane.org/sites/methods.cochrane.org.bias/files/public/uploads/Tool%20to%20Assess%20Risk%20of%20Bias%20in%20Cohort%20Studies.pdf.
- 13.Guyatt G., Oxman A.D., Akl E.A., et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011 Apr;64:383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 14.Adams L.A., Harmsen S., St Sauver J.L., et al. Nonalcoholic fatty liver disease increases risk of death among patients with diabetes: a community-based cohort study. Am J Gastroenterol. 2010;105:1567–1573. doi: 10.1038/ajg.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alexander M., Loomis A.K., van der Lei J., et al. Non-alcoholic fatty liver disease and risk of incident acute myocardial infarction and stroke: findings from matched cohort study of 18 million European adults. BMJ. 2019;367:l5367. doi: 10.1136/bmj.l5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allen A.M., Therneau T.M., Larson J.J., Coward A., Somers V.K., Kamath P.S. Nonalcoholic fatty liver disease incidence and impact on metabolic burden and death: a 20 year-community study. Hepatology. 2018;67:1726–1736. doi: 10.1002/hep.29546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baratta F., Pastori D., Angelico F., et al. Nonalcoholic fatty liver disease and fibrosis associated with increased risk of cardiovascular events in a prospective study. Clin Gastroenterol Hepatol. 2020 Sep;18:2324–2331.e4. doi: 10.1016/j.cgh.2019.12.026. [DOI] [PubMed] [Google Scholar]
- 18.Chinnadurai R., Ritchie J., Green D., Kalra P.A. Non-alcoholic fatty liver disease and clinical outcomes in chronic kidney disease. Nephrol Dial Transplant. 2019;34:449–457. doi: 10.1093/ndt/gfx381. [DOI] [PubMed] [Google Scholar]
- 19.Ekstedt M., Hagström H., Nasr P., et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61:1547–1554. doi: 10.1002/hep.27368. [DOI] [PubMed] [Google Scholar]
- 20.El Azeem H.A., Khalek E.-S.A., El-Akabawy H., Naeim H., Khalik H.A., Alfifi A.A. Association between nonalcoholic fatty liver disease and the incidence of cardiovascular and renal events. J Saudi Heart Assoc. 2013;25:239–246. doi: 10.1016/j.jsha.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fracanzani A.L., Tiraboschi S., Pisano G., et al. Progression of carotid vascular damage and cardiovascular events in non-alcoholic fatty liver disease patients compared to the general population during 10 years of follow-up. Atherosclerosis. 2016;246:208–213. doi: 10.1016/j.atherosclerosis.2016.01.016. [DOI] [PubMed] [Google Scholar]
- 22.Ghoneim S., Dhorepatil A., Shah A.R., et al. Non-alcoholic steatohepatitis and the risk of myocardial infarction: a population-based national study. World J Hepatol. 2020;12:378–388. doi: 10.4254/wjh.v12.i7.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hagström H., Nasr P., Ekstedt M., et al. Cardiovascular risk factors in non-alcoholic fatty liver disease. Liver Int. 2019;39:197–204. doi: 10.1111/liv.13973. [DOI] [PubMed] [Google Scholar]
- 24.Hamaguchi M., Kojima T., Takeda N., et al. Nonalcoholic fatty liver disease is a novel predictor of cardiovascular disease. World J Gastroenterol. 2007;13:1579–1584. doi: 10.3748/wjg.v13.i10.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haring R., Wallaschofski H., Nauck M., Dörr M., Baumeister S.E., Völzke H. Ultrasonographic hepatic steatosis increases prediction of mortality risk from elevated serum gamma-glutamyl transpeptidase levels. Hepatology. 2009 Nov;50:1403–1411. doi: 10.1002/hep.23135. [DOI] [PubMed] [Google Scholar]
- 26.Hwang Y.C., Ahn H.Y., Park S.W., Park C.Y. Nonalcoholic fatty liver disease associates with increased overall mortality and death from cancer, cardiovascular disease, and liver disease in women but not men. Clin Gastroenterol Hepatol. 2018 Jul;16:1131–1137.e5. doi: 10.1016/j.cgh.2017.11.026. [DOI] [PubMed] [Google Scholar]
- 27.Ichikawa K., Miyoshi T., Osawa K., et al. Prognostic value of non-alcoholic fatty liver disease for predicting cardiovascular events in patients with diabetes mellitus with suspected coronary artery disease: a prospective cohort study. Cardiovasc Diabetol. 2021;20:8. doi: 10.1186/s12933-020-01192-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Labenz C., Prochaska J.H., Huber Y., et al. Cardiovascular risk categories in patients with nonalcoholic fatty liver disease and the role of low-density lipoprotein cholesterol. Hepatol Commun. 2019;3:1472–1481. doi: 10.1002/hep4.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lauridsen B.K., Stender S., Kristensen T.S., et al. Liver fat content, non-alcoholic fatty liver disease, and ischaemic heart disease: mendelian randomization and meta-analysis of 279 013 individuals. Eur Heart J. 2018 Feb 1;39:385–393. doi: 10.1093/eurheartj/ehx662. [DOI] [PubMed] [Google Scholar]
- 30.Mantovani A., Mingolla L., Rigolon R., et al. Nonalcoholic fatty liver disease is independently associated with an increased incidence of cardiovascular disease in adult patients with type 1 diabetes. Int J Cardiol. 2016;225 doi: 10.1016/j.ijcard.2016.10.040. (Mantovani A., alessandro.mantovani24@gmail.com; Mingolla L.; Rigolon R.; Pichiri I.; Cavalieri V.; Zoppini G.; Bonora E.; Targher G.) Section of Endocrinology, Diabetes and Metabolism, Department of Medicine, University and Azienda Ospedaliera Integrata of Verona, Verona, Italy):387–91. [DOI] [PubMed] [Google Scholar]
- 31.Meyersohn N.M., Mayrhofer T., Corey K.E., et al. Association of hepatic steatosis with major adverse cardiovascular events, independent of coronary artery disease. Clin Gastroenterol Hepatol. 2020 Jul 21;S1542–3565:30992–30997. doi: 10.1016/j.cgh.2020.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Motamed N., Ajdarkosh H., Ahmadi M., et al. Non-alcoholic fatty liver disease is not independent risk factor for cardiovascular disease event: a cohort study. World J Hepatol. 2020;12:323–331. doi: 10.4254/wjh.v12.i6.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Niriella M.A., Ediriweera D.S., Kasturiratne A., et al. Outcomes of NAFLD and MAFLD: results from a community-based, prospective cohort study. PLoS One. 2021;16 doi: 10.1371/journal.pone.0245762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pickhardt P.J., Hahn L., Muñoz del Rio A., Park S.H., Reeder S.B., Said A. Natural history of hepatic steatosis: observed outcomes for subsequent liver and cardiovascular complications. AJR Am J Roentgenol. 2014;202:752–758. doi: 10.2214/AJR.13.11367. [DOI] [PubMed] [Google Scholar]
- 35.Pisto P., Santaniemi M., Bloigu R., Ukkola O., Kesäniemi Y.A. Fatty liver predicts the risk for cardiovascular events in middle-aged population: a population-based cohort study. BMJ Open. 2014;4 doi: 10.1136/bmjopen-2014-004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simon T.G., Roelstraete B., Khalili H., Hagström H., Ludvigsson J.F. Mortality in biopsy-confirmed nonalcoholic fatty liver disease: results from a nationwide cohort. Gut. 2021 Jul;70:1375–1382. doi: 10.1136/gutjnl-2020-322786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sinn D.H., Kang D., Chang Y., et al. Non-alcoholic fatty liver disease and the incidence of myocardial infarction: a cohort study. J Gastroenterol Hepatol. 2020;35:833–839. doi: 10.1111/jgh.14856. [DOI] [PubMed] [Google Scholar]
- 38.Targher G., Bertolini L., Rodella S., et al. Nonalcoholic fatty liver disease is independently associated with an increased incidence of cardiovascular events in type 2 diabetic patients. Diabetes Care. 2007 Aug;30:2119–2121. doi: 10.2337/dc07-0349. [DOI] [PubMed] [Google Scholar]
- 39.Unalp-Arida A., Ruhl C.E. Noninvasive fatty liver markers predict liver disease mortality in the U.S. population. Hepatology. 2016 Apr;63:1170–1183. doi: 10.1002/hep.28390. Epub 2016 Jan 22. PMID: 26663021; PMCID: PMC4805455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vita T., Murphy D.J., Osborne M.T., et al. Association between nonalcoholic fatty liver disease at CT and coronary microvascular dysfunction at myocardial perfusion PET/CT. Radiology. 2019;291:330–337. doi: 10.1148/radiol.2019181793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wild S.H., Walker J.J., Morling J.R., et al. Cardiovascular disease, cancer, and mortality among people with type 2 diabetes and alcoholic or nonalcoholic fatty liver disease hospital admission. Diabetes Care. 2018;41:341–347. doi: 10.2337/dc17-1590. [DOI] [PubMed] [Google Scholar]
- 42.Wong V.W.-S., Wong G.L.-H., Yeung J.C.-L., et al. Long-term clinical outcomes after fatty liver screening in patients undergoing coronary angiogram: a prospective cohort study. Hepatology. 2016;63:754–763. doi: 10.1002/hep.28253. [DOI] [PubMed] [Google Scholar]
- 43.Wong S.-W., Chan W.-K., Mohamed R. Fatty liver is associated with advanced fibrosis but does not predict adverse outcomes in patients with chronic hepatitis B. J Viral Hepat. 2020;27:1297–1305. doi: 10.1111/jvh.13361. [DOI] [PubMed] [Google Scholar]
- 44.Xu J., Dai L., Zhang Y., et al. Severity of nonalcoholic fatty liver disease and risk of future ischemic stroke events. Stroke. 2021;52:103–110. doi: 10.1161/STROKEAHA.120.030433. [DOI] [PubMed] [Google Scholar]
- 45.Zeb I., Li D., Budoff M.J., et al. Nonalcoholic fatty liver disease and incident cardiac events: the multi-ethnic study of atherosclerosis. J Am Coll Cardiol. 2016;67:1965–1966. doi: 10.1016/j.jacc.2016.01.070. [DOI] [PubMed] [Google Scholar]
- 46.Zhou Y.J., Li Y.Y., Nie Y.Q., Huang C.M., Cao C.Y. Natural course of nonalcoholic fatty liver disease in southern China: a prospective cohort study. J Dig Dis. 2012;13:153–160. doi: 10.1111/j.1751-2980.2011.00571.x. [DOI] [PubMed] [Google Scholar]
- 47.Stefan N., Häring H.U., Cusi K. Non-alcoholic fatty liver disease: causes, diagnosis, cardiometabolic consequences, and treatment strategies. Lancet Diabetes Endocrinol. 2019 Apr;7:313–324. doi: 10.1016/S2213-8587(18)30154-2. [DOI] [PubMed] [Google Scholar]
- 48.Targher G., Day C.P., Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010 Sep 30;363:1341–1350. doi: 10.1056/NEJMra0912063. [DOI] [PubMed] [Google Scholar]
- 49.Targher G., Byrne C.D., Lonardo A., Zoppini G., Barbui C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: a meta-analysis. J Hepatol. 2016 Sep;65:589–600. doi: 10.1016/j.jhep.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 50.Lu H., Liu H., Hu F., Zou L., Luo S., Sun L. Independent association between nonalcoholic fatty liver disease and cardiovascular disease: a systematic review and meta-analysis. Int J Endocrinol. 2013;2013 doi: 10.1155/2013/124958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Veracruz N., Hameed B., Saab S., Wong R.J. The association between nonalcoholic fatty liver disease and risk of cardiovascular disease, stroke, and extrahepatic cancers. J Clin Exp Hepatol. 2021;11:45–81. doi: 10.1016/j.jceh.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu S., Wu F., Ding Y., Hou J., Bi J., Zhang Z. Association of non-alcoholic fatty liver disease with major adverse cardiovascular events: a systematic review and meta-analysis. Sci Rep. 2016 Sep 16;6 doi: 10.1038/srep33386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mahfood Haddad T., Hamdeh S., Kanmanthareddy A., Alla V.M. Nonalcoholic fatty liver disease and the risk of clinical cardiovascular events: a systematic review and meta-analysis. Diabetes Metab Syndr. 2017 Nov;11(suppl 1):S209–S216. doi: 10.1016/j.dsx.2016.12.033. [DOI] [PubMed] [Google Scholar]
- 54.Ahmed H.S., Wang N., Carr J.J., et al. The association between hepatic steatosis and incident cardiovascular disease, cancer, and all-cause mortality in a US multicohort study. Hepatology. 2023 Jun 1;77:2063–2072. doi: 10.1097/HEP.0000000000000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yoo T.K., Lee M.Y., Kim S.H., et al. Comparison of cardiovascular mortality between MAFLD and NAFLD: a cohort study. Nutr Metab Cardiovasc Dis. 2023 May;33:947–955. doi: 10.1016/j.numecd.2023.01.013. [DOI] [PubMed] [Google Scholar]
- 56.Guo Y., Yang J., Ma R., et al. Metabolic dysfunction-associated fatty liver disease is associated with the risk of incident cardiovascular disease: a prospective cohort study in Xinjiang. Nutrients. 2022 Jun 7;14:2361. doi: 10.3390/nu14122361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mantovani A., Csermely A., Petracca G., et al. Non-alcoholic fatty liver disease and risk of fatal and non-fatal cardiovascular events: an updated systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2021 Nov;6:903–913. doi: 10.1016/S2468-1253(21)00308-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.