Abstract
Objectives
Cholangiocarcinoma (CHOL) is a malignant tumor from extrahepatic bile duct with poor prognosis. The critical roles of long non-coding RNAs (lncRNAs) in cancers including CHOL have been unveiled in recent decades. The present study was aimed to investigate the role and mechanism of a certain lncRNA, namely, hepatocellular carcinoma (HCC) associated long non-coding RNA (HANR) in CHOL.
Methods
Reverse transcription quantitative polymerase chain reaction (RT-qPCR) was applied for detecting gene expression. Functional assays were done for assessing CHOL cell malignancy and mechanistic assays were conducted for analyzing correlation between HANR and Notch signal pathway, as well as the relation between HANR and Notch intracellular domain (NICD) in CHOL cells.
Results
HANR was detected to be significantly overexpressed in CHOL cell lines. HANR silence inhibited cell proliferation, migration and stemness. Besides, HANR could positively regulate the Notch signaling pathway through modulating RBP-JK. HANR could bind to NICD and affect the transcriptional activity of RBP-JK. Furthermore, p-Notch1-NICD-r could wholly countervail the inhibitory effects of HANR silence on CHOL cell proliferation, migration and stemness.
Conclusion
HANR could activate Notch pathway by regulating the RBP-JK transcriptional activity, thus contributing to exacerbated malignant behaviors of CHOL cells.
Keywords: HANR, Cholangiocarcinoma, Notch pathway, NICD
1. Introduction
Cholangiocarcinoma (CHOL) is a type of invasive and metastatic tumor that arises from cholangiocytes with increasing incidence all over the world,which is the second most common hepatobiliary malignancy after hepatocellular carcinoma (HCC) [1] 【30207593】. CHOL can be divided as intrahepatic and extrahepatic types [2]. Although most CHOL is considered to be a de novo disease with no apparent cause, there are some identified risk factors, such as infection, inflammatory liver and biliary disease, chemical injury, and others [22420979]. Numerous epidemiological and clinical data indicate that infection with hepatitis viruses HBV and HCV is a definite risk factor for the development of cholangiocarcinoma, the detailed pathogenesis of CHOL still remains undefined [3,4]. Due to the insidious onset of CHOL, the lack of typical symptoms and specific diagnostic indicators in the early stage, and the special anatomical location of the bile ducts, the cancer cells are prone to metastasise to the surrounding blood vessels and lymphatic tissues, which leads to the majority of patients being in the middle and late stages when diagnosed, accompanied by distant metastases, but the recurrence rate after surgery is as high as 60%–65 % [5]. Gemcitabine and cisplatin are currently the standard chemotherapy regimen for patients in the progressive phase, but patients still have a median survival of less than 1 year[20375404], CHOL has an extremely poor prognosis, with a 5-year survival rate of only 15% in the United States and Asia[27000463, 30820787] [6].(删除). Considering the low 5-year survival rate [7], it is urgent to seek other effective treatments and therapeutic targets for CHOL[32418766]. With the prevalence of molecular targeted therapy, some non-coding RNA (ncRNA) associated with cancer have been widely used as available therapeutic and prognostic biomarkers [8]. Hence we also devoted to further exploring the carcinogenesis and development of CHOL to find more effective therapeutic targets.
Long non-coding RNAs (lncRNAs) are RNA molecules with over 200 nucleotides in length, which lack the protein-coding ability [9]. Moreover, relevant reports have suggested that lncRNAs can function as critical effectors in diverse biological processes including gene expression, chromatin remodeling and cellular differentiation [10]. Moreover, lncRNAs play critical roles in the tumorigenesis and progression of different diseases including CHOL [11]. For instance, lncRNA UCA1 up-regulates PD-L1 expression by inhibiting miR-26a/b, miR-193a and miR-214, and promotes the proliferation, migration and immune escape of gastric cancer cells【31272462】. Abnormal up-regulation of lncRNA-cCSC1 in colorectal cancer promotes cell proliferation by up-regulating CD44 proliferation【33887588】. LncRNA MEG3 was down-regulated in prostate cancer tissues and cells, and overexpression of MEG3 inhibited the proliferation, migration and invasive ability of prostate cancer cells【30565858】. In addition, high expression of TP73-AS1 promotes the migration as well as invasion of CHOL cells [12]. Likewise, lncRNA TP73-AS1 also exerts oncogenic effects in CHOL [13]. However, The expression pattern and function of hepatocellular carcinoma associated long non-coding RNA (HANR) and its specific functions in CHOL are unknown. This study reports for the first time the expression pattern and potential mechanistic impact of HANR in CHAL, which may provide new targets or strategies for the treatment of CHAL.
Furthermore, relevant studies have suggested that lncRNA can regulate Notch signaling pathway by modulating expressions of its receptors and ligands [14]. Notch signaling pathway, as an evolutionarily conservative pathway, is composed of receptors, ligands as well as intracellular proteins. The notch receptors-Notch1, Notch2, Notch 3 and Notch4 are single-pass transmembrane proteins composed of an extracellular (NECD), a transmembrane (TM), and an intracellular domain (NICD) [15]. Notch ligands (DLL-1, DLL-3, DLL4, Jagged-1 and Jagged-2) combine with NECD to liberate NICD that enters nucleus to modify gene expression [16]. Moreover, recombination signal-binding protein 1 for J-Kappa, namely RBP-JK, is a transcription factor of the Notch signaling pathway, which can bind with other factors to form a complex and play important roles in the cellular processes [17]. In addition, Notch signaling pathway has been verified as a crucial effector in modulating progression of several malignancies including liver cancer, bladder cancer and prostate cancer [18]. Therefore, it possibly emerges as an underlying target in treatment of different cancers. Through current investigation, it was verified that Notch signaling pathway could be inactivated by HANR inhibition. Thus, the involvement of Notch pathway in HANR modulatory mechanism in CHOL cells was further investigated.
In this study, we initially elucidated the expression of HANR in CHOL and its effects on CHOL cell proliferation, migration and stemness. In addition, we further analyzed the inner relationship between HANR and Notch signaling pathway.
2. Materials and methods
2.1. Cell culture
Human cholangiocarcinoma RBE cell line was procured from Procell Life Science & Technology Co., Ltd. (Wuhan, China). TFK-1 cell line was bought from DSMZ-German Collection of Microorganisms and Cell Cultures GmbH (Braunschweig, Germany). HuCC-T1 cell line was purchased from Japanese Collection of Research Bioresources (JCRB) Cell Bank (Osaka, Japan) and SSP-25 cell line was procured from Shanghai Honsun Biological Technology Co., Ltd (Shanghai, China). The above-mentioned cells and human normal intestinal epithelial cell line HIEC (ATCC, Manassas, VA, USA) were incubated in an environment with 5 % CO2 at 37 °C. RPMI-1640 medium (GIBCO, Rockville, MD, USA) supplemented with 10 % fetal bovine serum (FBS; GIBCO) was commercially acquired for cell culture.
2.2. Cell transfection
HANR-specific shRNAs were designed and constructed by GenePharma (Shanghai, China), with the nonspecific shRNAs used as negative control (NC). Moreover, p-Notch1-NICD-r plasmid was bought from Shanghai Zhen Biotechnology Co., Ltd. (Shanghai, China). Cells were transfected with the indicated plasmids with application of Lipofectamine 3000 (Invitrogen). After 48 h, transfected cells were harvested for analysis. The experiment was carried out in triplicate.
2.3. Reverse transcription quantitative polymerase chain reaction (RT-qPCR)
The total RNA was extracted from cultured cell lines with application of TRIzol Reagent (Invitrogen, Carlsbad, CA, USA) for cDNA synthesis. PrimeScript Reverse Transcriptase Kit was utilized as guided by provider (Takara, Shiga, Japan). Next, SYBR Green PCR Kit (Takara) was applied for implementing qPCR, followed by analysis of comparative change-in-cycle method (2−ΔΔCt). For quantitation, GAPDH mRNA was used as the internal reference. The experiment was done in triplicate.
2.4. Cell counting kit-8 (CCK-8) assay
After 48-h transfection, TFK-1 and RBE cells were reaped and seeded into 96-well plates (5 × 103 cell per well). 10 μL of CCK-8 solution was added into plates for 2-h cultivation, as instructed by the manufacturer (Dojindo Laboratories, Kumamoto, Japan). The spectrophotometer was used for detection of OD values at 450 nm with the protocol strictly followed (Thermo Fisher Scientific, Waltham, MA, USA). The quantified result is equal to (OD measurement of the experimental group - OD measurement of the control group))/(negative control-Mock).The experiment was repeated for at least three times.
2.5. Colony formation
Transfected TFK-1 and RBE cells (800) were seeded in 6-well plates for colony formation. After 14 days, the colonies were fixed with methanol for 30 min, and then stained with 0.5 % crystal violet for 30 min. The visible clones were manually counted. The experiment was repeated at least three times.
2.6. Wound healing
Transfected TFK-1 and RBE cells (1 × 106) were cultured in 6-well plates until cells adhered to plates. On the following day, cells were scraped by 200-μL pipette tip and cultured in serum-free medium for 24 h. Images of wound closure were captured at 0 and 24 h to analyze cell migration. The results were quantified using ImageJ. Bio-triple repeats were required for this assay.
2.7. Transwell migration assay
Transwell chamber (Corning, Corning, NY, USA) was applied for testing cell migration. The upper chamber was added with 1 × 106 transfected cells (TFK-1 and RBE), and the complete culture medium was added into the lower chamber. After 24-h incubation, cells migrated to the bottom were fixed by 4 % paraformaldehyde. The migrated cells in five random fields were observed under optical microscope (Olympus, Tokyo, Japan) after crystal violet staining. The results were quantified using ImageJ. Bio-triple repeats were required for the experiment.
2.8. Luciferase reporter assay
Luciferase reporter assay was done with TFK-1 and RBE cells co-transfected with indicated luciferase reporter vectors and sh-HANR or sh-NC for 48 h. RBP-JK reporter kit (SABiosciences, Frederic, MA, USA) was used to analyze Notch pathway; HIF-1 Luciferase Reporter Vector (Shanghai Yu Bo Biotech Co., Ltd., Shanghai, China) was acquired for analyzing Hypoxia pathway activity; E2F luciferase reporter plasmid (Baiaolaibo, Beijing, China) was obtained for pRb-E2F pathway; NF-κB luciferase reporter plasmid (Yeasen Biotech Co., Ltd., Shanghai, China) was applied for NF-κB pathway; SMAD luciferase reporter plasmid (Genomeditech, Shanghai, China) was applied for TGF-β pathway; TOP/FOP-Flash luciferase reporter vector (Millipore, Billerica, MA, USA) was applied for Wnt pathway; p53 luciferase reporter plasmid (Genomeditech) was applied for p53 pathway; TEAD 8xGTIIC-luciferase reporter (YouBio, Changsha, China) was applied for Hippo pathway. Luciferase activity was measured with Dual-luciferase reporter assay system (Promega, Madison, WI, USA). The experiment was repeated at least three times.
2.9. Sphere formation assay
Transfected cells (TFK-1 and RBE) were cultured with sphere medium in 96-well ultralow attachment plates (Corning; 10 cells per well). After 7-day incubation, cell clusters with diameter more than 75 μm were defined as spheres, and counted for assessing sphere formation. The experiment was repeated at least three times.
2.10. Subcellular fractionation assay
The nuclear fraction and cytoplasmic fraction were separately isolated from TFK-1 and RBE cells with PARIS™ Kit (Invitrogen). Cell fractionation buffer was added into cell samples before centrifugation. The content of HANR in cell fractions was analyzed by RT-qPCR. GAPDH and U6 were respectively taken as the cytoplasmic and nuclear controls. Bio-triple repeats were required for the experiment.
2.11. RNA immunoprecipitation (RIP)
RIP assay was implemented with NICD antibody, human Ago2 antibody and control IgG antibody, as well as Magna RIP™ RNA-Binding Protein Immunoprecipitation Kit (Millipore, Bedford, MA, USA). Cell lysates were treated in RIP buffer(Solarbio, Beijing, China). Subsequently, lysates were conjugated with anti-Ago2 (Abcam) antibody or anti-IgG (Abcam) antibody, and then incubated with the antibody-bound magnetic beads for 6 h. After that, the precipitated RNAs were purified and subjected to RT-qPCR. The experiment was repeated at least three times.
2.12. Statistical analyses
Each experiment was implemented thrice, and experimental data were all expressed as the means ± SD. Data was analyzed to produce scientific graphs with the application of GraphPad PRISM 6 (GraphPad, San Diego, CA, USA). Comparisons between two or more groups were assessed with the employment of Student's t-test or ANOVA. The difference was considered to be statistically significant when p < 0.05.
3. Results
3.1. LncRNA HANR facilitates CHOL cell proliferation and migration in vitro
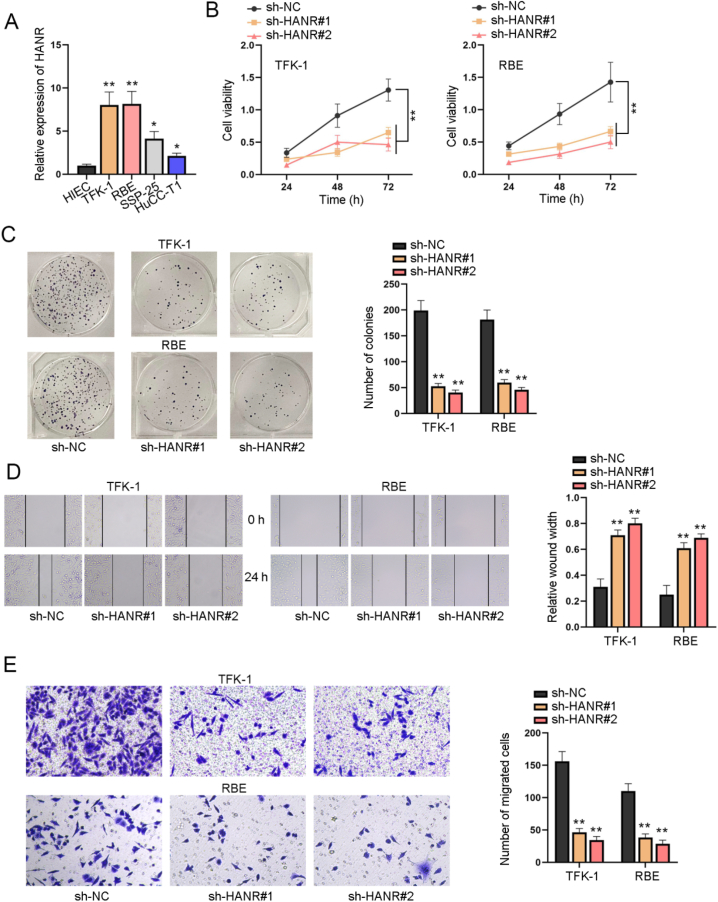
Aiming to explore the specific role of HANR in CHOL cells, we first searched on GEPIA2 (http://gepia2.cancer-pku.cn/#index) for its expression profile and found that HANR was aberrantly up-regulated in CHOL tissues in comparison with normal tissues (Fig. S1A). We also measured HANR expression level in CHOL cells (TFK-1, RBE, SSP-25 and HuCC-T1) and normal human intestinal epithelial cells (HIEC) by RT-qPCR. Compared with HIEC, HANR was detected to display significantly higher expression in CHOL cells, especially in TFK-1 and RBE cells (Fig. 1A). On this basis, we selected TFK-1 and RBE cells with the highest expression in CHOL cells for subsequent experiments. We transfected sh-RNA plasmids targeting HANR into TFK-1 and RBE cells to silence HANR. As was hinted by the results of CCK-8 assay, it was found that the viability of TFK-1 and RBE cells was overtly weakened by transfection of sh-HANR#1 or sh-HANR#2 (Fig. 1B). Besides, the inhibitory influence of sh-HANR#1 and sh-HANR#2 on proliferation of TFK-1 and RBE cells was confirmed, as evidenced by the decline in colony number upon sh-HANR#1/2 transfection (Fig. 1C). In wound healing assay, the wound width at 24h after scratch was larger in sh-HANR#1/2 group than the sh-NC group, suggesting depletion of HANR inhibited the migration of CHOL cells (Fig. 1D). The same conclusion could be drawn based on the result of transwell migration assay, as sh-HANR#1/2 transfection reduced the number of successfully migrated cells (Fig. 1E)were transfected. To sum up, HANR up-regulation facilitated CHOL cell proliferation and migration in vitro.
Fig. 1.
LncRNA HANR facilitates CHOL cell proliferation and migration in vitro A. RT-qPCR analysis measured HANR expression in CHOL cells (TFK-1, RBE and SSP-25, HuCC-T1) and normal human intestinal epithelial cells HIEC. Functional assays were done with CHOL cells with transfection of sh-NC, sh-HANR#1 or sh-HANR#2. B. CCK-8 assay evaluated the viability of CHOL cells at 24h, 48h and 72h. C. The proliferation of transfected CHOL cells was measured via 14-day colony formation assay. D. The migration of transfected CHOL cells was testified by measurement of wound width in wound healing assay (magnification × 100). E. CHOL cell migration was assessed by transwell assay after HANR was stably knocked down (magnification × 100). Data acquired from three independent experiments were exhibited as the mean ± SD. *P < 0.05, **P < 0.01.
3.1.1. HANR regulates notch pathway and promotes cell stemness
Growing evidences have indicated that lncRNA is closely correlated with different signaling pathways to affect the progression of cancers [19,20]. We assumed that HANR might regulate a certain signaling pathway to affect the progression of CHOL. In order to prove the hypothesis, we conducted luciferase reporter assay in sh-HANR transfected CHOL cells. The results showed that HANR silence weakened the luciferase activity of Notch signal pathway, but did not impact that of Hypoxia, pRb-E2F, NF-κB, TGF-β, Wnt, p53 and Hippo pathways (Fig. 2A). Further, it was found that the RBP-JK luciferase activity was impaired upon HANR silence in TFK-1 and RBE cells, further confirming that HANR knockdown inactivated Notch pathway via suppressing the transcriptional activity of RBP-JK (Fig. 2B). Given the evidence that Notch pathway could regulate stemness property of cancer cells [21,22], we conducted sphere formation assay, the result of which revealed the sphere formation efficiency of TFK-1 and RBE cells was sharply reduced after sh-HANR#1/2 transfection (Fig. 2C). Collectively, the data showed that HANR regulated Notch pathway and promoted cell stemness in CHOL.
Fig. 2.
HANR regulates Notch pathway and promotes cell stemness in CHOL A. Luciferase reporter assay assessed the luciferase activity of different signaling pathways (Hypoxia, Notch, pRb-E2F, NF-κB, TGF-β, Wnt, p53 and Hippo pathways) in CHOL cells with HANR depletion or not. B. RBP-JK luciferase reporter assay was done in sh–NC– or sh-HANR#1-transfected CHOL cells. C. Sphere formation assay tested sphere formation efficiency of TFK-1 and RBE cells transfected with sh-NC or sh-HANR#1/2 (magnification × 100). Above data obtained from three independent experiments were exhibited as the mean ± SD. **P < 0.01.
3.2. HANR binds to NICD in CHOL cells
To determine the underlying mechanism of HANR on regulating Notch pathway in CHOL, the expression of receptors Notch1, Notch2 and Notch3 as well as that of ligands Jagged-1, Jagged-2, DLL-1, DLL-3 and DLL-4 was tested in CHOL cells with HANR knockdown. As shown in Fig. 3A, HANR silence could not impact expression of any receptor or ligand in TFK-1 and RBE cells. Subcellular fractionation assay revealed that HANR accumulated more in cytoplasm than in nucleus of TFK-1 and RBE cells, implying that HANR might exert its downstream genes by post-transcriptional modulation (Fig. 3B). To verify whether HANR could function as a competing endogenous RNA (ceRNA) in CHOL cells, we conducted Ago2-RIP assay and uncovered that HANR enrichment in Anti-Ago2 group or Anti-IgG group presented no obvious changes, excluding the potential involvement of a ceRNA network (Fig. 3C). Interestingly, the enrichment of HANR was significantly higher in Anti-NICD in comparison with the control Anti-IgG group, which clearly indicated that HANR directly bound with NICD (Fig. 3D). In a nutshell, HANR bound to NICD in CHOL cells.
Fig. 3.
HANR binds to NICD in CHOL cells A. RT-qPCR measured the expression levels of Notch receptors and ligands in CHOL cells with HANR silence. B. Subcellular fractionation analyzed subcellular accumulation of HANR in TFK-1 and RBE cells, with U6 and GAPDH serving as the respective nuclear and cytoplasmic controls. C. RIP experiment was performed with Anti-Ago2 and Anti-IgG and enrichment of HANR in antibodies was assessed via RT-qPCR. D. After RIP assay, HANR enrichment in Anti-NICD group was measured via RT-qPCR. All data obtained from three independent experiments were displayed as the mean ± SD. **P < 0.01.
3.3. HANR promotes CHOL cell proliferation, migration and stemness by the activation of notch pathway
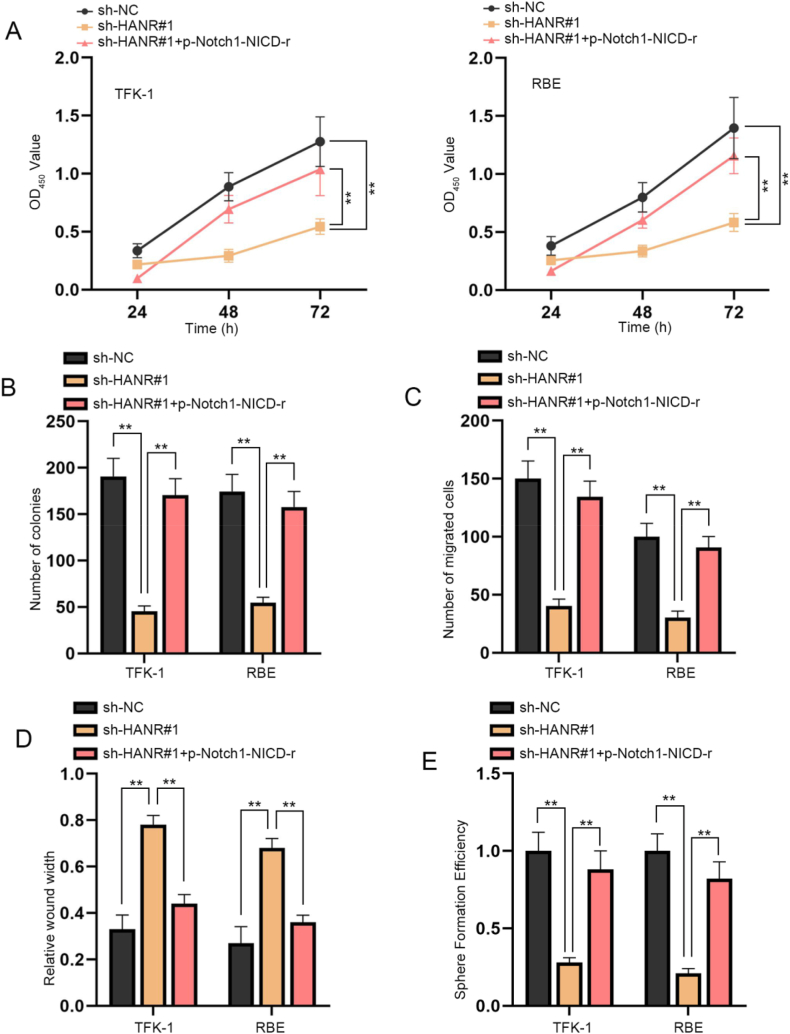
To explore the validity of the regulatory mechanism involving HANR and Notch pathway in CHOL cells, rescue experiments were done with TFK-1 and RBE cells respectively transfected with sh-NC, sh-HANR#1, or sh-HANR#1+p-Notch1-NICD-r. According to the results of CCK-8 and colony formation assays, the inhibitory influences of HANR silence on the proliferation of TFK-1 and RBE cells were countervailed by p-Notch1-NICD-r co-transfection (Fig. 4A and B). Moreover, we also assessed cell migration ability in the indicated transfection groups. As depicted in transwell assay, the decline in number of successfully migrated cells resulting from HANR knockdown was detected to be recovered by p-Notch1-NICD-r (Fig. 4C). Besides, wound healing assay also revealed that wider wound width induced by sh-HANR#1 was rescued by p-Notch1-NICD-r (Fig. 4D). The findings together reflected that the suppressive effects of HANR silence on CHOL cell migration could be counteracted by activation of Notch pathway induced by p-Notch1-NICD-r transfection. Furthermore, sphere formation assay showed HANR depletion inhibited sphere formation efficiency of TFK-1 and RBE cells, which was fully reversed by p-Notch1-NICD-r co-transfection (Fig. 4E). In summary, HANR promoted CHOL cell proliferation, migration and stemness by activating Notch pathway.
Fig. 4.
HANR promotes CHOL cell proliferation, migration and stemness by activation of Notch pathway
TFK-1 and RBE cells were transfected with different plasmids including sh-NC, sh-HANR#1 or sh-HANR#1+p-Notch1-NICD-r. A. CCK-8 assay assessed viability of cells in different groups. B. The effects of HANR knockdown and p-Notch1-NICD-r on cell colony formation ability were analyzed. C. Transwell assay assessed the migratory abilities of TFK-1 and RBE cells with different transfection. D. The migration of CHOL cells under different transfection conditions was evaluated through wound healing assays. E. The effects of HANR silence and p-Notch1-NICD-r on cell stemness were examined via sphere formation assay. Data from three independent experiments were demonstrated as the mean ± SD. **P < 0.01.
4. Discussion
Due to the lack of specific indicators for early diagnosis of CHOL, most of the patients are often in the middle to late stage with distant metastasis when diagnosed. Surgery is considered the main treatment strategy for advanced CHOL, but the recurrence rate of patients after surgery is still very high. Although many scholars have made great efforts to improve the therapeutic outcome of CHOL, CHOL is still one of the deadliest diseases in human beings. Therefore, finding effective biomarkers and therapeutic targets for early diagnosis and early treatment is particularly important to improve the therapeutic outcome of CHOL. The discovery of some tumor markers provides some help for the early diagnosis of tumors. For example, DDX1 has been diagnosed as a biomarker for a variety of cancers, including CHOL, and is associated with prognosis[PMID: 36939765]. Currently, some studies have claimed that HANR also plays a key regulatory role in the occurrence and development of tumors via multiple mechanisms. Specifically, HANR has been shown to bind to GSPP to regulate GSK3β phosphorylation in HCC [23]. Recent literature also unveils the close correlation between HANR up-regulation and poor prognosis of patients with glioma, and HANR aggravates the malignant behaviors of glioma cells via negatively regulating miRNA-335 [24]. Moreover, a ceRNA network involving HANR, miR-29b and ATG9A has been verified to contribute to enhanced sorafenib resistance in HCC cells [25]. Supported by bioinformatics analysis and RT-qPCR analysis, the aberrantly high expression of HANR in CHOL was detected in this study, implying that HANR might participate in CHOL development and progression. Consistent with previous literature, HANR silence was verified to exert inhibitory influences on cell proliferation, migration and stemness in CHOL.
Accumulating reports have uncovered the critical involvement of the interaction between lncRNAs and signaling pathways in regulating multiple cellular processes and progression of malignancies, and the significance of signaling pathways in CHOL tumor onset and growth has also been unveiled [26,27]. For instance, lncRNA MIR22HG inhibits Wnt/β-catenin to suppress CHOL cell proliferation and migration [28]. LncRNA ASAP1-IT1 functions as an oncogenic factor in CHOL via positively modulating Hedgehog pathway [29]. Herein, it was validated in current study that HANR could activate Notch signaling pathway in CHOL cells. In the canonical signaling pathway, the Notch receptor is cleaved following ligand binding, resulting in the release and nuclear translocation of NICD, which could activate the transcription of target genes by forming a ternary complex with the DNA binding protein CBF1/RBP-JK, Suppressor of Hairless, Lag1, and Maml [30]. In this study, it was manifested in luciferase reporter assay that HANR depletion led to a decline in RBP-JK luciferase activity. Thereafter, we detected the influence of HANR silence on Notch components [31]. However, we found that HANR could not influence the expression of neither Notch receptors nor ligands. Additionally, the major accumulation of HANR in cell cytoplasm reflected that HANR might function by post-transcriptional regulation on downstream genes, but little enrichment of HANR in Anti-Ago2 ruled out the possibility of the ceRNA mechanism. Instead, the binding between HANR and NICD was verified via RIP assay. Thus, it could be concluded that HANR modulated the activity of Notch pathway via binding with NICD. Finally, we found that the suppressive influences of HANR silence on CHOL cell proliferation, migration and stemness could be countervailed by p-Notch1-NICD-r. In this study, we revealed the role of HANR in CHOL cell proliferation, migration and tumour stemness through HANR/NICD/Notch signaling. For the first time, we deciphered the novel mechanism of HANR-mediated CHOL progression. Most importantly, these findings provide a deeper understanding of CHOL.
There are some limitations of this study. The clinical relevance and significance were not investigated due to the lack of sufficient clinical samples.The carcinogenic effect of HARN in CHOL needs to be further confirmed by animal experiments. In the future, we will further reveal the mechanism of NICD in cholangiocarcinoma through molecular biology experiments and animal model experiments, with the aim of providing more guidance to improve the clinical diagnosis and treatment of head and neck cholangiocarcinoma from the perspective of lncRNA. Furthermore, as it was learnt from previous literature that lncRNA could regulate the interaction between NICD and RBP-JK [32], it was assumed that HANR might also exert influence on Notch signaling pathway via affecting the formation of RBP-JK/NICD transcription complex, which needs to be elucidated in future study.
To conclude, HANR, up-regulated in CHOL tissues and cells, could facilitate cell proliferation, migration and stemness in CHOL via binding to NICD and activating Notch signaling pathway.
Data availability statement
The data used to support the findings of this study are included within the article.
CRediT authorship contribution statement
Guoqing Zhou: Writing – review & editing, Writing – original draft, Visualization, Supervision, Software, Resources, Methodology, Investigation, Formal analysis, Data curation. Hongwei He: Writing – original draft, Supervision, Project administration, Formal analysis. Xu'an Wang: Writing – original draft, Data curation. Qiyun Gu: Writing – review & editing, Writing – original draft, Supervision, Funding acquisition, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Acknowledgements
The Jinshan District Science and Technology Fund, (No.: 2015-3-20); The National Natural Science Foundation Of China, (No.: 81602486); The Jinshan District Science and Technology Fund, (No.: 2022-WS-22); Program of Shanghai Jinshan District National Health Commission, (No.: JSKJ-KTMS-2019-08).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e22087.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Razumilava N., Gores G.J. Cholangiocarcinoma. Lancet. 2014;383(9935):2168–2179. doi: 10.1016/S0140-6736(13)61903-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Esnaola N.F., et al. Evaluation and management of intrahepatic and extrahepatic cholangiocarcinoma. Cancer. 2016;122(9):1349–1369. doi: 10.1002/cncr.29692. [DOI] [PubMed] [Google Scholar]
- 3.Jeong S., et al. Impact of viral hepatitis B status on outcomes of intrahepatic cholangiocarcinoma: a meta-analysis. Hepatol Int. 2018;12(4):330–338. doi: 10.1007/s12072-018-9881-y. [DOI] [PubMed] [Google Scholar]
- 4.Jeong S., et al. Hepatitis B virus-associated intrahepatic cholangiocarcinoma: a malignancy of distinctive characteristics between hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Oncotarget. 2017;8(10):17292–17300. doi: 10.18632/oncotarget.14079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rizvi S., et al. Cholangiocarcinoma - evolving concepts and therapeutic strategies. Nat. Rev. Clin. Oncol. 2018;15(2):95–111. doi: 10.1038/nrclinonc.2017.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rizvi S., Gores G.J. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology. 2013;145(6):1215–1229. doi: 10.1053/j.gastro.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chun Y.S., Javle M. Systemic and adjuvant therapies for intrahepatic cholangiocarcinoma. Cancer Control. 2017;24(3) doi: 10.1177/1073274817729241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rizvi S., Gores G.J. Emerging molecular therapeutic targets for cholangiocarcinoma. J. Hepatol. 2017;67(3):632–644. doi: 10.1016/j.jhep.2017.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deniz E., Erman B. Long noncoding RNA (lincRNA), a new paradigm in gene expression control. Funct. Integr. Genomics. 2017;17(2–3):135–143. doi: 10.1007/s10142-016-0524-x. [DOI] [PubMed] [Google Scholar]
- 10.Ransohoff J.D., Wei Y., Khavari P.A. The functions and unique features of long intergenic non-coding RNA. Nat. Rev. Mol. Cell Biol. 2018;19(3):143–157. doi: 10.1038/nrm.2017.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peng W.X., Koirala P., Mo Y.Y. LncRNA-mediated regulation of cell signaling in cancer. Oncogene. 2017;36(41):5661–5667. doi: 10.1038/onc.2017.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yao Y., et al. Enhanced expression of lncRNA TP73-AS1 predicts adverse phenotypes for cholangiocarcinoma and exerts oncogenic properties in vitro and in vivo. Biomed. Pharmacother. 2018;106:260–266. doi: 10.1016/j.biopha.2018.06.045. [DOI] [PubMed] [Google Scholar]
- 13.Shi X., et al. LncRNA AFAP1-AS1 promotes growth and metastasis of cholangiocarcinoma cells. Oncotarget. 2017;8(35):58394–58404. doi: 10.18632/oncotarget.16880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reicher A., et al. Crosstalk between the Notch signaling pathway and long non-coding RNAs. Cancer Lett. 2018;420:91–96. doi: 10.1016/j.canlet.2018.01.070. [DOI] [PubMed] [Google Scholar]
- 15.Ehebauer M., Hayward P., Martinez-Arias A. Notch signaling pathway. Sci. STKE. 2006;2006(364):cm7. doi: 10.1126/stke.3642006cm7. [DOI] [PubMed] [Google Scholar]
- 16.Bi P., Kuang S. Notch signaling as a novel regulator of metabolism. Trends Endocrinol Metab. 2015;26(5):248–255. doi: 10.1016/j.tem.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeCotiis J.L., Lukac D.M. KSHV and the role of notch receptor dysregulation in disease progression. Pathogens. 2017;6(2) doi: 10.3390/pathogens6030034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teoh S.L., Das S. Notch signalling pathways and their importance in the treatment of cancers. Curr. Drug Targets. 2018;19(2):128–143. doi: 10.2174/1389450118666170309143419. [DOI] [PubMed] [Google Scholar]
- 19.Si Y., et al. LncRNA PlncRNA1 regulates proliferation and differentiation of hair follicle stem cells through TGFbeta1mediated Wnt/betacatenin signal pathway. Mol. Med. Rep. 2018;17(1):1191–1197. doi: 10.3892/mmr.2017.7944. [DOI] [PubMed] [Google Scholar]
- 20.Wei G.H., Wang X. lncRNA MEG3 inhibit proliferation and metastasis of gastric cancer via p53 signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2017;21(17):3850–3856. [PubMed] [Google Scholar]
- 21.Perdigoto C.N., Bardin A.J. Sending the right signal: notch and stem cells. Biochim. Biophys. Acta. 2013;1830(2):2307–2322. doi: 10.1016/j.bbagen.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 22.Karamboulas C., Ailles L. Developmental signaling pathways in cancer stem cells of solid tumors. Biochim. Biophys. Acta. 2013;1830(2):2481–2495. doi: 10.1016/j.bbagen.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 23.Xiao J., et al. LncRNA HANR promotes tumorigenesis and increase of chemoresistance in hepatocellular carcinoma. Cell. Physiol. Biochem. 2017;43(5):1926–1938. doi: 10.1159/000484116. [DOI] [PubMed] [Google Scholar]
- 24.Wang W.J., et al. LncRNA HANR aggravates the malignant progression of glioma via targeting miRNA-335. Eur. Rev. Med. Pharmacol. Sci. 2020;24(2):758–765. doi: 10.26355/eurrev_202001_20056. [DOI] [PubMed] [Google Scholar]
- 25.Shi Y., et al. HANR enhances autophagy-associated sorafenib resistance through miR-29b/ATG9A Axis in hepatocellular carcinoma. OncoTargets Ther. 2020;13:2127–2137. doi: 10.2147/OTT.S229913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu Y., et al. PI3K/AKT/mTOR pathway-related long non-coding RNAs: roles and mechanisms in hepatocellular carcinoma. Pharmacol. Res. 2020;160 doi: 10.1016/j.phrs.2020.105195. [DOI] [PubMed] [Google Scholar]
- 27.Papoutsoglou P., Louis C., Coulouarn C. Transforming growth factor-beta (TGFβ) signaling pathway in cholangiocarcinoma. Cells. 2019;8(9) doi: 10.3390/cells8090960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu X., et al. Long non-coding RNA MIR22HG inhibits cell proliferation and migration in cholangiocarcinoma by negatively regulating the Wnt/β-catenin signaling pathway. J. Gene Med. 2019;21(5):e3085. doi: 10.1002/jgm.3085. [DOI] [PubMed] [Google Scholar]
- 29.Guo L., et al. LncRNA ASAP1-IT1 positively modulates the development of cholangiocarcinoma via hedgehog signaling pathway. Biomed. Pharmacother. 2018;103:167–173. doi: 10.1016/j.biopha.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 30.Zanotti S., Canalis E. Notch regulation of bone development and remodeling and related skeletal disorders. Calcif. Tissue Int. 2012;90(2):69–75. doi: 10.1007/s00223-011-9541-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Batista M.R., et al. Notch signaling in mouse blastocyst development and hatching. BMC Dev. Biol. 2020;20(1):9. doi: 10.1186/s12861-020-00216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu Q., et al. LncRNA HOXA-AS2 activates the notch pathway to promote cervical cancer cell proliferation and migration. Reprod. Sci. 2021;28(10):3000–3009. doi: 10.1007/s43032-021-00626-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used to support the findings of this study are included within the article.