Abstract
Purpose
Patients who are successfully resuscitated following out-of-hospital cardiac arrest (OHCA) are still at a high risk of neurological damage and death. Inflammation and brain injury are components of the post-cardiac arrest syndrome, and can be assessed by systemic interleukin 6 (IL-6) and neuron-specific enolase (NSE). Anti-inflammatory treatment with methylprednisolone may dampen inflammation, thereby improving outcome. This study aimed to determine if prehospital high-dose methylprednisolone could reduce IL-6 and NSE in comatose OHCA patients.
Methods
The STEROHCA trial was a randomized, blinded, placebo-controlled, phase II prehospital trial performed at two cardiac arrest centers in Denmark. Resuscitated comatose patients with suspected cardiac etiology were randomly assigned 1:1 to a single intravenous injection of 250 mg methylprednisolone or placebo. The co-primary outcome was reduction of IL-6 and NSE-blood levels measured daily for 72 h from admission. The main secondary outcome was survival at 180 days follow-up.
Results
We randomized 137 patients to methylprednisolone (n = 68) or placebo (n = 69). We found reduced IL-6 levels (p < 0.0001) in the intervention group, with median (interquartile range, IQR) levels at 24 h of 2.1 pg/ml (1.0; 7.1) and 30.7 pg/ml (14.2; 59) in the placebo group. We observed no difference between groups in NSE levels (p = 0.22), with levels at 48 h of 18.8 ug/L (14.4; 24.6) and 14.8 ug/L (11.2; 19.4) in the intervention and placebo group, respectively. In the intervention group, 51 (75%) patients survived and 44 (64%) in the placebo group.
Conclusion
Prehospital treatment with high-dose methylprednisolone to resuscitated comatose OHCA patients, resulted in reduced IL-6 levels after 24 h, but did not reduce NSE levels.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00134-023-07247-w.
Keywords: Out-of-hospital cardiac arrest, Intensive cardiovascular care, Post-cardiac arrest syndrome, Inflammation, Neuroprotection
Take-home message
| Prehospital high-dose methylprednisolone to patients resuscitated from out-of-hospital cardiac arrest resulted in a substantial decrease in interleukin 6 after 24 h but had no effect on neuron-specific enolase. The early anti-inflammatory intervention was not able to affect the possible link between inflammation and brain injury in these severely ill patients. |
Introduction
Out-of-hospital cardiac arrest (OHCA) is one of the leading causes of death in Europe, with an annual incidence between 67 and 170 per 100,000 inhabitants and a survival rate of ~ 10% [1]. Prehospital interventions such as early cardiopulmonary resuscitation and the use of an automated external defibrillator have improved outcomes. However, patients who remain unconscious after resuscitation have a higher risk of mortality compared to those who regain consciousness upon admission [2, 3]. OHCA induces global tissue hypoxia and reperfusion injury, triggering immunological cascades, which progress after the return of spontaneous circulation (ROSC), leading to the development of post-cardiac arrest syndrome (PCAS). PCAS is a complex condition characterized by a systemic inflammatory response, brain injury, and myocardial dysfunction [4, 5]. Hypoxic ischemic brain injury (HIBI) is the most common cause of death after OHCA [6, 7], and initial treatment of PCAS following resuscitation aims to reduce neurological damage by fever prevention and to achieve hemodynamic stabilization, as well as identifying and treating the precipitating pathology to the cardiac arrest [8].
Interleukin 6 (IL-6) is a pro-inflammatory cytokine associated with pathological processes in PCAS, including the development of neurological damage [9–12]. Neuron-specific enolase (NSE) reflects neuron cell body injury and is a guideline-recommended biomarker for neuroprognostication following OHCA [13, 14].
Methylprednisolone, a glucocorticoid that possesses a wide range of anti-inflammatory properties, is used in the treatment of various inflammatory diseases [15]. Methylprednisolone is associated with physiological effects that encompass attenuation of oxidative stress and potential anti-apoptotic properties [16].
This study hypothesized that high-dose methylprednisolone treatment in the prehospital setting would mitigate inflammatory injury, potentially preventing neurological deterioration and worsening of PCAS. Therefore, the aim of the STEROHCA randomized trial (STERoid treatment as anti-inflammatory and neuroprotective agent following OHCA) was to assess the potential anti-inflammatory and neuroprotective effects of early systemic glucocorticoid treatment, as measured by IL-6- and NSE levels in patients who were resuscitated from OHCA.
Methods
Design and setting
This study was an investigator-initiated, randomized, multicenter, blinded, placebo-controlled phase II clinical superiority trial. Prior to initiation, the trial was registered at https://clinicaltrials.gov (Unique Identifier: NCT04624776). The study protocol has previously been published [17]. The prehospital inclusion process is displayed in the appendix (supplementary Fig. 1). Study protocol and statistical code will be available on reasonable request.
Participants
The trial was conducted at two cardiac arrest centers in Denmark covering the Capital Region of Denmark (1.9 M out of 5.9 M inhabitants as of 2023 [18]) in collaboration with the Emergency Medical Services. The enrollment period spanned from the 10th of October 2020 to the 15th of July 2022.
Patients who suffered OHCA were considered eligible if they were adults (≥ 18 years), had cardiac arrest due to a suspected cardiac etiology, remained unconscious (Glasgow Coma Scale ≤ 8) following ROSC, and achieved ROSC for at least 5 min.
The exclusion criteria were: advanced life support termination-of-resuscitation exclusion criteria [19], asystole as first monitored rhythm, women of childbearing potential (judged by including physician), known treatment limitations (previous decision of no resuscitation), known allergy to methylprednisolone, known pre-arrest modified Rankin Scale (mRS) score ranging from 4 to 5, temperature below 30 °C upon randomization, or > 30 min to ROSC.
Screening of eligible patients was done by the prehospital physician manning the critical care unit attending the OHCA incident, and inclusion was based on the available information at the scene. In the protocol, we prespecified that included patients could be excluded in route to or upon arrival to hospital if criteria for inclusion were violated.
Following cardiac arrest, patients received standard of care in adherence to International post-resuscitation guidelines [8]. This involved targeted temperature management at 36° C in comatose patients, sedation with primarily propofol and fentanyl, vasopressor and inotropes as needed. In addition, all comatose patients received prophylactic antibiotic treatment with intravenous piperacillin/tazobactam or cefuroxime in case of β-lactam allergy, and continuous intravenous insulin for hyperglycaemia.
Randomization, group allocation, and concealment
The group allocation sequence for the study was generated using a random number generator randomizing patients in a 1:1 fashion in permuted blocks of four.
Study medicine and placebo were packed in identical opaque boxes numbered randomly according to allocation. The prehospital physician and accompanying medical assistant were unblinded after opening a medicine box. The prehospital staff were not involved further in the treatment of the patient or the study following admission. The patient, all hospital personel, and all study investigators and study staff were blinded for treatment allocation.
Study intervention
If eligible for inclusion, patients were randomized to receive a bolus injection of methylprednisolone 250 mg intravenously (2 × 125 mg/2 mL) or placebo (4 mL isotonic NaCl), both administered over 5 min. The dosage was the maximum allowed for methylprednisolone bolus injection in Denmark. The intervention was performed as soon as possible following resuscitation and a minimum of 5 min from ROSC in the prehospital setting. Injection of allocated medicine was completed before hospital arrival, and only allocation number was available at admission.
Outcomes
The co-primary outcome consisted of daily measurements of IL-6 and NSE from admission until 72 h from admission, with all available measurements included in the statistical analysis. NSE levels were also analyzed in the subset of patients who remained comatose at hospital arrival.
Secondary outcomes included survival and neurological function at hospital discharge and after 180 days. Survival was continuously updated with data from “The Medical Register of Births and Deaths” in Denmark. Neurological function, defined by cerebral performance category (CPC) score (range 1–5, higher scores indicating greater disability with 3 or 4 being severe disability, coma or vegetative state and 5 being death) and mRS score (range 0–6, to evaluate the degree of disability or dependence in daily activities with 0 being no symptoms and 6 being death) [20, 21]. CPC and mRS at discharge were determined by retrospective chart review and at 180 days through telephone interview. The anti-inflammatory impact of the intervention was further evaluated through the measurement of high-sensitive C-reactive protein (hsCRP) levels and leukocyte count. Additionally, the potential neuroprotective effect was assessed by quantifying neurofilament light chain (NfL) levels. To gauge markers of kidney and hepatic injury, we examined creatinine levels, alanine aminotransferase (ALAT), aspartate aminotransferase (ASAT), alkaline phosphatase (ALP), and bilirubin levels. Plasma fibrinogen served as an indicator for assessing the coagulation system. For potential cardiac protection, troponin T (TnT), troponin I (TnI), and creatine kinase MB (CKMB) levels were measured. Finally, within the setting of the intensive care unit (ICU), we monitored hemodynamic parameters, including mean arterial pressure (MAP), central venous pressure (CVP), mean pulmonary arterial pressure (PAPm), pulmonary capillary wedge pressure (PCWP) using a Swan-Ganz catheter, and arterial blood gas analyses were conducted to assess PaO2, PaCO2, and lactate levels.
Safety
Safety was assessed by the cumulative incidence of adverse events 180 days after randomization. As stated in the study protocol [17], the following was considered an adverse event: infection, bleeding, dialysis, electrolyte derangement (hypo- or hyperkalemia), metabolic derangement (hypo- or hyperglycemia), cardiac arrhythmia, seizure, and other (for example, other conditions leading to re-hospitalization or death). It was considered a serious adverse event if an adverse event led to prolonged hospitalization or a life-threatening condition requiring re-hospitalization or death. The sponsor evaluated all serious adverse events for the possibility of a serious adverse reaction or a suspected unexpected serious adverse reaction.
Biomarker assessment
Biological material to establish a research biobank was drawn from patients at admission and 24-, 48- and 72 h following admission. Biobank samples were subsequently spun at 2000 g for 10 min, aliquoted in four samples, and stored at – 80 °C. The co-primary outcome for inflammation, IL-6, was measured in ethylenediamine tetraacetic acid (EDTA) plasma samples with the 17-plex human cytokine assay (Bio-Rad). An IL-6 measurement below the lower limit of detection (LLD) were assigned 50% of the LLD; 0.025 pg/mL. The other co-primary outcome, NSE, was measured in serum samples along with creatinine, ALAT, ASAT, ALP, bilirubin, fibrinogen, TnI, TnT, and CKMB in a DS/EN ISO 15189 by a COBAS 8000. Finally, hsCRP was measured in EDTA plasma samples from the biobank by a COBAS 8000 and leukocytes were measured as routine biochemistry by a Sysmex XN.
Approvals, monitoring, and informed consent procedures
Approvals for the study were obtained from the Regional Ethics Committee (ID: H-20022320) and the Danish Medicines Agency (ID: 2,020,033,425), and a legal data handling agreement was provided by the Capital Region of Denmark (ID: 2020-866). According to Danish legislation, an independent primary trial guardian provided informed consent prior to the inclusion of a patient, with a secondary trial guardian subsequently confirming. Consent from a surrogate and the patient, if considered cognitively capable following regained consciousness, was obtained as soon as possible following admission. The trial was overseen by a Data and Safety Monitoring Board for safety and overall conduct and monitored for adherence to national and international guidelines by the Good Clinical Practice unit of Copenhagen.
Sample size calculation
Calculation of the target sample size was based on the co-primary outcome. We powered the trial towards a single measurement at 48 h due to the lack of supporting data for repeated biomarker measurements. The trial would achieve a 90% power to detect a 20% reduction in IL-6 levels if 112 patients were included and a 20% reduction in NSE levels if 114 patients were included at an alpha level of 0.025. Therefore, to ensure sufficient power, we planned to include 120 patients. Data from previous studies suggest that approximately 20% of resuscitated OHCA patients would die before assessment of the co-primary outcome, i.e., before 72 h after admission. We further expected approximately 10% post-randomization exclusions due to the acute nature of the study or if the consent could not be provided. Based on these estimates, we planned to continue the trial until 156 patients (120 + 24 + 12) were included or until 120 patients completed the co-primary outcome measurement prior to this.
Statistical analysis
We performed all analyses for primary and secondary outcomes on the modified intention-to-treat population, defined by patients not being excluded post-randomization with consent provided. Dichotomous variables were presented as numbers (n, %) and analyzed with the Chi-squared or Fisher’s exact test. Continuous variables were presented as median (25th percentile; 75th percentile) and difference between groups were tested with the Wilcoxon test. For continuous variables assessed at multiple time points, including the co-primary outcome, application of linear mixed models of unstructured covariance were applied, with logarithmic transformation for approximation of normal distribution. For these analyses, we used the ‘LMMstar’ package (Ozenne B, Forman J (2023). LMMstar: Repeated measurement models for discrete times. R package version 0.9.0). The values for IL-6 and NSE are presented as predicted geometric means and confidence limits after antilog. Missing values were estimated based on maximum likelihood inference, and multiple imputations would be performed at > 10% missingness. Assumptions for multiple imputations were assessed, but since the prespecified limit for missingness was not exceeded, multiple imputations were not performed.
The Kaplan–Meier estimator was applied to show differences in mortality for all included patients and the modified intention-to-treat population. Crude hazard ratio from a Cox regression model was reported. Additionally, a predefined multivariable analysis was made adjusting for sex, age, primary defibrillator rhythm, time to ROSC (duration from collapse or alarm call to the re-establishment of circulation), and primary percutaneous coronary intervention (pPCI) performed following investigating for interaction after fulfilling assumptions for proportional hazards. The selection of variables included in the multivariable analysis was done a priori guided by previous knowledge of factors known to influence survival following OHCA [22].
All statistical analyses were performed in R Studio, version 4.2.2 (RStudio Team [2020]. RStudio: Integrated Development for R. RStudio, PBC, Boston, MA; URL: http://www.rstudio.com/). For the co-primary outcome, a p value below 0.025 was considered statistically significant. In all other analyses, a p value below 0.05 was considered statistically significant.
Results
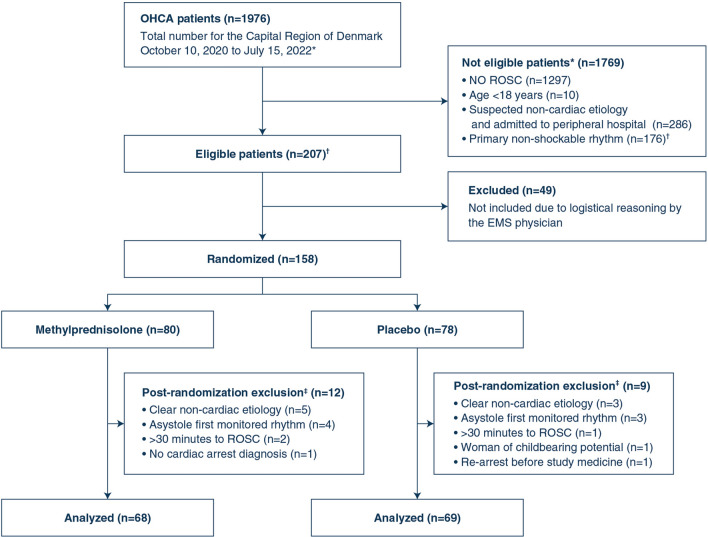
Between October 10, 2020, and July 15, 2022, 158 patients were randomized to methylprednisolone (n = 80) or placebo (n = 78), with 137 patients encompassing the modified intention-to-treat population (Fig. 1). Follow-up was completed by February 10, 2023. The trial ended after inclusion of 120 patients surviving > 72 h following hospital admission. All patients were followed for safety reasons and included in the safety analyses.
Fig. 1.
Consort flow diagram. *Screening was done retrospectively per-protocol through review of data from the Danish Cardiac Arrest Registry. †Based on previous data, patients with PEA as primary rhythm accounted for ~ 5% at our cardiac arrest centers, thus the number of eligible patients with PEA was estimated to 10% of patients with primary shockable rhythm. ‡Erronous inclusions, excluded en-route or upon arrival to hospital
Study participants
Baseline characteristics for the two groups were overall similar (Table 1), with a median age of 67 years (25th percentile: 56; 75th percentile: 75) and the majority being males (81%). The median time to ROSC was 18 min (13; 21) compared to 14 min (10; 20) in the intervention and the placebo group, respectively. Time-to-inclusion, defined as the time in minutes from ROSC to randomization, occurred at a median of 20 min (13; 29). All included patients received the full dosage (both ampoules) of the study medicine and placebo.
Table 1.
Baseline characteristics of the STEROHCA trial population
| Randomization | ||
|---|---|---|
| Methylprednisolone, N = 68 | Placebo, N = 69 | |
| Demographic characteristics | ||
| Age, years, median (IQR) | 67 (57, 74) | 66 (56, 75) |
| Male, n (%) | 56 (82%) | 56 (81%) |
| Medical history | ||
| Hypertension, n (%) | 30 (44%) | 29 (42%) |
| Hypercholesterolemia, n (%) | 16 (24%) | 22 (32%) |
| Heart failure, n (%) | 16 (24%) | 12 (17%) |
| Ischemic heart disease, n (%) | 11 (16%) | 18 (26%) |
| Atrial fibrillation, n (%) | 11 (16%) | 14 (20%) |
| Previous PCI or CABG, n (%) | 7 (10%) | 13 (19%) |
| Previous pacemaker or ICD, n (%) | 2 (3%) | 4 (6%) |
| Stroke, n (%) | 7 (10%) | 2 (3%) |
| Diabetes mellitus, n (%) | 9 (14%) | 6 (9%) |
| Chronic kidney disease, n (%) | 1 (2%) | 0 (0%) |
| COPD, n (%) | 4 (6%) | 2 (3%) |
| Current smoker, n (%) | 23 (35%) | 23 (35%) |
| Former smoker, n (%) | 30 (44%) | 28 (41%) |
| Characteristics of the cardiac arrest | ||
| Witnessed arrest, n (%) | 57 (84%) | 64 (93%) |
| Bystander CPR, n (%) | 60 (88%) | 56 (81%) |
| Place of arrest, n (%) | ||
| Residential | 28 (41%) | 33 (48%) |
| Public place | 40 (59%) | 36 (52%) |
| First monitored defibrillator rhythm shockablea, n (%) | 64 (94%) | 65 (94%) |
| Bystander defibrillation, n (%) | 14 (21%) | 15 (22%) |
| Time to EMS arrival, min, median (IQR) | 7 (5, 9) | 7 (5, 9) |
| CPR-related injury, n (%) | 15 (22%) | 14 (20%) |
| Number of defibrillations | 3 (2, 4) | 2 (1, 3) |
| Adrenaline administered, n (%) | 43 (63%) | 32 (46%) |
| Amiodarone administered, n (%) | 32 (47%) | 17 (25%) |
| Time to ROSC, min, median (IQR) | 18 (13, 20) | 14 (10, 19) |
| ROSC at admission, n (%) | 67 (99%) | 68 (99%) |
| Prehospital seizures, n (%) | 3 (4%) | 5 (7%) |
| Clinical characteristics at admission | ||
| LVEF at arrival, (%), median (IQR) | 40 (25, 45) | 40 (25, 50) |
| Post-resuscitation ECG rhythm, n (%) | ||
| Sinus rhythm | 48 (71%) | 53 (77%) |
| Atrial fibrillation | 12 (18%) | 13 (19%) |
| Otherb | 8 (12%) | 3 (4%) |
| Post-resuscitation ECG, signs of ischemia, n (%) | ||
| ST-elevation | 29 (43%) | 28 (41%) |
| LBBB or RBBB | 16 (24%) | 19 (28%) |
| Unspecific ischemia | 4 (6%) | 10 (14%) |
| No ischemia | 19 (28%) | 12 (17%) |
| Cardiogenic shock at arrival, n (%) | 2 (3%) | 8 (12%) |
| Acute CAG, n (%) | 38 (56%) | 42 (61%) |
| Acute PCI, n (%) | 23 (34%) | 25 (36%) |
| Acute CABG, n (%) | 0 (0%) | 0 (0%) |
| Patient consciousness at arrival, n (%) | ||
| Awake | 10 (15%) | 8 (12%) |
| Comatose | 58 (85%) | 61 (88%) |
CABG coronary arterial bypass grafting, COPD chronic obstructive pulmonary disease, CPR cardiopulmonary resuscitation, ECG electrocardiogram, EMS emergency medical services, ICD implantable cardioverter defibrillator, LBBB left bundle branch block, LVEF left ventricular ejection fraction, PCI percutaneous coronary intervention, RBBB right bundle branch block, ROSC return of spontaneous circulation
aIf an AED had provided a shock before EMS arrival, the primary rhythm was deemed shockable
bIncluding pace rhythm, nodal rhythm and sinus bradycardia
A total of 130 (95%) and 129 (94%) patients had at least one valid measurement of IL-6 or NSE during admission, respectively. Overall, the completeness of data for both co-primary outcomes were 92% for patients surviving > 72 h, including patients where blood samples were not possible to obtain (i.e., admission at a peripheral hospital).
Primary outcome
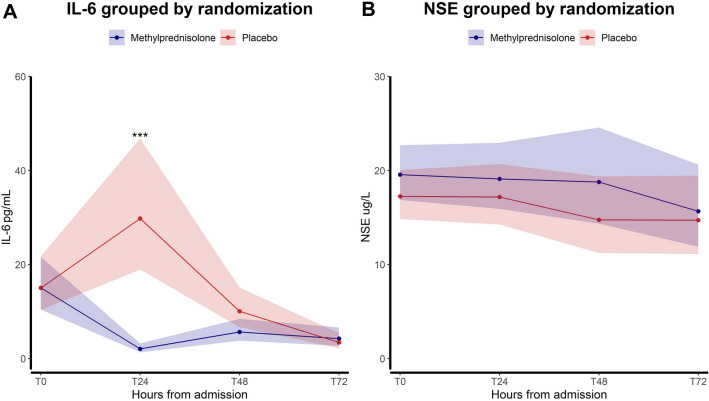
For the co-primary outcomes, we found that the first IL-6 level was almost identical in the two groups (15 pg/mL (95% confidence interval (CI) 10.4; 21.6) vs. 15 pg/mL (10.4; 21.7), p = 1), subsequently a reduction in IL-6 levels was observed in the intervention group with a significant treatment-by-time interaction, p < 0.0001 (Fig. 2A). The intervention group exhibited significantly lower IL-6 levels at 24 h compared to the placebo group: 2.1 pg/mL (1.3; 3.2) vs. 29.8 pg/mL (18.9; 46.8), p < 0.0001. The IL-6 levels at 48 h were: 5.7 pg/mL (3.8; 8.4) vs. 10.1 pg/mL (6.7; 15.1), p = 0.04, and at 72 h (4.3 pg/mL (2.7; 6.6) vs. 3.4 pg/mL (2.2; 5.4), p = 0.51).
Fig. 2.
Primary efficacy analyses: A Treatment-by-time interaction for IL-6 (pg/mL) depicting geometric means and 95% confidence intervals after antilog to each time point according to randomization; B Treatment-by-time interaction for NSE (ug/L) depicting geometric means and 95% confidence intervals after antilog to each time point according to randomization. The figure includes the measurements for the modified intention-to-treat population (n = 137)
There was no difference in NSE levels over time, p = 0.22 (Fig. 2B). NSE levels in the intervention group versus the placebo group were as follows for all time points (admission: 19.6 ug/L (16.9; 22.7) vs. 17.2 ug/L (14.8; 20, p = 0.11), 24 h: 19.1 ug/L (15.9; 22.9) vs. 17.2 ug/L (14.3; 20.7), p = 0.69), 48 h: 18.8 ug/L (14.4; 24.6) vs. 14.8 ug/L (11.2; 19.4), p = 0.58), and 72 h: 15.7 ug/L (11.9; 20.6) vs. 14.7 ug/L (11.1; 19.5), p = 0.82). When restricting the analysis to patients who were in a comatose state upon arrival at the hospital (n = 111), the findings remained consistent.
IL-6 and NSE levels, the co-primary outcomes, to all time points, including NSE levels in comatose patients only, are summarized in the supplementary material (supplementary Table 1).
Secondary outcomes
For the secondary outcomes, we found a significant treatment-by-time interaction for hsCRP, while there was no difference in NfL levels over time. The treatment-by-time interaction depicted for both biomarkers can be seen in the supplementary (supplementary Fig. 2A + B).
After 180 days, 51 (75%) patients vs. 44 (64%) patients were alive in the intervention and placebo arm, respectively (unadjusted hazard ratio 0.65 (0.35–1.2), p = 0.17, and adjusted hazard ratio 0.35 (0.18–0.67), p = 0.002, from a multivariable model including sex, age, primary defibrillator rhythm, time to ROSC, and pPCI), Fig. 3. The details from the multivariable model is included in the supplementary material (supplementary Table 2). When including all patients excluded from the modified intention-to-treat analysis in the Kaplan–Meier plot, the results were similar (supplementary Fig. 3).
Fig. 3.
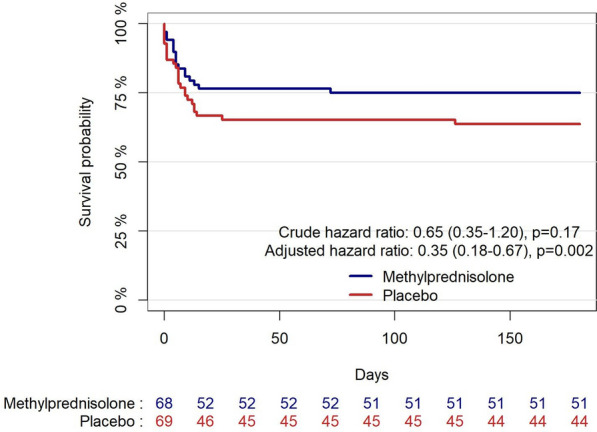
Kaplan–Meier plot of survival to day 180 after out-of-hospital cardiac arrest (modified intention-to-treat population), presenting the crude hazard ratio with 95% CI from a univariate Cox regression model, and the hazard ratio with 95% CI from a predefined multivariable Cox regression model adjusted for sex, age, primary defibrillator rhythm, time to ROSC, and primary percutaneous coronary intervention performed
CPC- and mRS-scores, evaluated a minimum of 180 days following OHCA, were similar in the two groups (supplementary Fig. 4). All other clinical outcomes can be found in Table 2.
Table 2.
Secondary clinical outcomes and serious adverse events
| Randomization | |||
|---|---|---|---|
| Methylprednisolone, N = 68 | Placebo, N = 69 | p value | |
| Secondary outcomes | |||
| Mortality, hospital discharge, n (%) | 16 (24%) | 24 (35%) | 0.15 |
| Mortality, 180 days, n (%) | 17 (25%) | 25 (36%) | 0.15 |
| CPCa score at discharge from hospital among patients alive at discharge, n (%) | 0.17 | ||
| 1 | 30 (58%) | 34 (76%) | |
| 2 | 15 (29%) | 7 (16%) | |
| 3 | 7 (13%) | 4 (8.9%) | |
| mRSb score at discharge from hospital among patients alive at discharge, n (%) | 0.65 | ||
| 0 | 14 (27%) | 13 (29%) | |
| 1 | 14 (27%) | 13 (29%) | |
| 2 | 10 (20%) | 11 (24%) | |
| 3 | 8 (16%) | 6 (13%) | |
| 4 | 5 (9.8%) | 1 (2.2%) | |
| 5 | 0 (0%) | 1 (2.2%) | |
| Serious adverse eventsc | |||
| Patients with ≥ 1 SAE, n (%) | 35 (51%) | 37 (54%) | 0.80 |
| Infection, n (%) | 5 (7.4%) | 4 (5.8%) | 0.74 |
| Bleeding, n (%) | 0 (0%) | 4 (5.8%) | 0.12 |
| Dialysis, n (%) | 2 (2.9%) | 1 (1.4%) | 0.62 |
| Electrolyte, n (%) | 2 (2.9%) | 1 (1.4%) | 0.62 |
| Metabolic, n (%) | 1 (1.5%) | 0 (0%) | 0.50 |
| Arrhytmia, n (%) | 7 (10%) | 10 (14%) | 0.46 |
| Seizures, n (%) | 12 (18%) | 13 (19%) | 0.86 |
CPC cerebral performance categories, mRS modified Rankin Score, SAE serious adverse event
aRange from 1 to 5 with higher scores indicating greater disability with 3 or 4 being severe disability, coma or vegetative state and 5 being death
bRange from 0 to 6 with higher scores indicating greater degree of disability or dependence in daily activities with 0 being no symptoms and 6 being death
cAdverse event leading to prolonged hospitalization or a life-threatening condition requiring re-hospitalization or death
Leukocyte counts were numerically higher in the intervention group with a statistically significant treatment-by-time interaction. Leukocyte counts are summarized in the supplementary along with markers of kidney injury (creatinine) and hepatic injury (ALAT, ASAT, ALP, and bilirubin), plasma fibrinogen, and cardiac enzymes (TnT, TnI and CKMB), supplementary Table 3.
Median lactate levels were higher in the intervention group, but ≤ 2 mmol/L at all time points expect from admission. Hemodynamic parameters can be seen in the supplementary material (supplementary Table 4).
Safety
All predefined serious adverse events were reported in Table 2 and an overview for all adverse events were reported in the appendix (supplementary Table 5). Overall, the incidence of adverse events and serious adverse events were similar between the two intervention groups (adverse events: 69 (86%) vs. 60 (77%); serious adverse events: 43 (54%) vs. 44 (56%)). In the intervention and placebo groups, hyperglycemia reported as an adverse event occurred in 30 (38%) and 12 (15%) patients, with no associated sequelae recorded during follow-up.
Discussion
In this randomized controlled trial assessing OHCA patients, we observed a reduction in IL-6 levels after 24 h, while NSE levels were unaffected, by an prehospital injection of high-dose methylprednisolone administered after ROSC.
Previous studies have associated elevated IL-6 and other inflammatory markers with unfavorable outcomes in resuscitated OHCA patients [23, 24]. Although the anti-inflammatory properties of methylprednisolone are well-known, there is limited evidence supporting its use after cardiac arrest [25]. In this trial, a substantial reduction in IL-6 levels was observed in the intervention group 24 h after admission, but at 72 h, there was no difference. In the present trial, the intervention was performed as early as possible in the prehospital setting to maximize efficacy. NSE levels were unaffected at all time points in this trial, and to our knowledge, there are no pharmacological intervention studies demonstrating reduced NSE levels in resuscitated OHCA patients. Previous studies report that IL-6 and NSE have central roles in neuroinflammation following cerebral ischemia [12, 26, 27]. NSE has a high predictive value for poor outcome following OHCA [14], whereas IL-6 has been found to have predictive value for mortality but not neurological outcome [28]. A study conducted by Hoiland et al. [29] demonstrated that cerebral IL-6 increases following resuscitated cardiac arrest in patients with brain tissue hypoxia. This finding raises the possibility of the involvement of IL-6 s in the previously described “two-hit model” wherein secondary HIBI develops following resuscitation [30]. But it is important to highlight, that while emerging evidence hints at a possible connection between inflammation and HIBI [12], the precise mechanistic effects are still to be unveiled, and that there is presently no substantiated evidence supporting the notion that inhibition of neuroinflammation following OHCA confers therapeutic benefits.
A prior study conducted at our institution, which explored the impact of in-hospital IL-6 blockage using Tocilizumab, revealed no significant difference in neurological outcomes. However, a notable reduction in hsCRP levels was observed [31]. Similar to the IL-6 reduction in the present study, hsCRP levels were reduced from 24 to 72 h. These biochemical results all support that the intervention dosage administered was sufficient to induce an anti-inflammatory response. Methylprednisolone also induced leukocytosis and hyperglycemia in the intervention group, both known physiological side effects to glucocorticoid treatment, during the initial three days of admission [15, 32]. Further, increased lactate levels were found in the intervention group, but besides admission lactate, the median levels were below 2 mmol/L at all time points. A previous ICU study found that treatment with high-dose dexamethasone increases lactate levels and suggested that this directly related to the increased glucose levels [33].
Two previous studies of limited size indicated improved survival and neurologic outcome in patients who received glucocorticoids along with vasopressin after in-hospital cardiac arrest [34, 35]. Another clinical study suggested increased rates of ROSC following glucocorticoid and vasopressin injections during cardiopulmonary resuscitation, but with no difference in mortality [36]. Although there was no difference in crude mortality, the adjusted analysis suggested lower risk of death with methylprednisolone. Blinded randomized controlled trials anticipate that patients in the two groups are comparable, but in this trial the intervention group had numeric longer time to ROSC, and received more adrenaline and amiodarone during resuscitation. Accordingly, after adjusting for predefined factors, including time to ROSC, we observed a potential benefit on survival within the intervention group. These findings are intriguing, but they can only serve to generate hypotheses for future studies adequately powered to investigate the impact on survival.
Effects of glucocorticoids are exerted through a slow genomic mechanism with gene expression alterations and a rapid non-genomic mechanism involving interaction with cellular membranes and non-specific membrane-bound receptors [32, 37]. In this study, we aimed to administer the intervention prehospital to leverage its potential rapid mechanism of action, and further to mitigate potential secondary ischemic/reperfusion injury after OHCA as early as possible. However, the treatment did not appear to reduce systemic IL-6 levels upon admission, which indicates that non-genomic pathways differ from the genomic pathways in reducing IL-6 levels, although we did not have baseline measurements available.
Enrolling OHCA patients in the prehospital setting presents challenges from both logistic and ethical perspectives. This prompts for an early and potentially important effect of the intervention, but also for possible erroneous inclusion of patients and the need for post-randomization exclusion. The modified intention-to-treat in this trial reflects this, with 21 patients excluded post-randomization. All these patients were followed for adverse events, including mortality, but could not be included in the co-primary outcome analysis since it was impossible to obtain biobank samples.
Finally, and importantly, methylprednisolone administered after OHCA prehospitally in the used dosage was deemed safe with a similar amount of serious adverse events in the two treatment arms. Steroid treatment has previously been found harmful after traumatic brain injury [38], but our safety results support that this does not apply to OHCA.
There are limitations to consider in this study. The co-primary outcomes were assessed using peripheral blood samples, whereas utilizing samples from the jugular vein might have provided more comprehensive insights into the neuroinflammatory process. The sample size in the trial was small, hence a risk of type II errors was present, and generally secondary outcomes should be cautiously interpreted. Further, a risk of selection bias was present when excluding patients post-randomization, but according to our sample size calculation of 120 patients completing the study, we expected a part of included patients to violate exclusion criteria. Inclusion of patients in the prehospital setting is challenging, and a number of potential eligible patients were not included.
In the STEROHCA trial, prehospital treatment with a single high-dose methylprednisolone injection to resuscitated comatose OHCA patients, resulted in reduced IL-6 levels after 24 h, but did not reduce NSE levels, during the first 72 h of admission.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank all prehospital physicians and trial guardians who were a part of the inclusion process, in particular daily leaders of the physician manned critical care units: Søren Loumann Nielsen, Steen Møiniche, Henrik Alstrøm, Lars Dahlgaard Hove, and Søren Hestad. Further, we thank Charlotte Barfod and Freddy Lippert from the Emergency Medical Services of the Capital Region of Denmark; Lia Bang from the Department of Cardiology, Rigshospitalet; and Christian Svane from the Trauma Center at Rigshospitalet, all Denmark. We thank all personnel at the Department of Cardiology—especially the Cardiac Intensive Care Unit—at Rigshospitalet, the Intensive Care Unit, Gentofte Hospital, and the Emergency Medical Services of the Capital Region of Denmark, all Copenhagen, Denmark. Further, we thank Mie Christa Larsen, Áslaug Karlsdóttir, and Lesli Hingstrup Larsen from the Clinical Research Unit, Rigshospitalet; Ann Kristine Thorsteinsson, Tung Thanh Phan, and Mette Krefeld Bentzen from the Department of Biochemistry, Rigshospitalet; and staff at the pharmacy of the Capital Region of Denmark, both Copenhagen, Denmark. Finally, we thank Pia Hornbeck from the Medical/Steno Aarhus Research Lab, Aarhus University for technical assistance.
Author contributions
All authors contributed to the critical revision and writing of the publication, and for the final approval to submit including accountability for the accuracy and integrity of the publication. LERO, SW, FF, JEM, JK, and CH contributed to the concept and design of the study. LERO and CH were responsible for the writing of the original draft and the decision to submit for publication, and were responsible for funding acquisition. LERO, RPB, and CH served as project administrators and were accountable for all visualisation in the study. LERO, RPB, MASM, and FTS were responsible for data curation. LERO, RPB, MASM, JG, BN, JJ, TM, ADM, FF, JEM, and JK were a part of the investigation process. LERO, RPB, MASM, JG, BN, JJ, MB, RFS, FF, JEM, JK, and CH provided study materials, and managed laboratory samples and analysis tools throughout the study. Formal analyses were done by LERO, RPB, MB, and RFS. Further, LERO, TM, ADM, FF, JEM, JK, and CH provided supervision during the study. Finally, validation was done by LERO, RPB, SW, FF, JEM, JK, and CH.
Funding
Open access funding provided by Royal Library, Copenhagen University Library. The study was supported by funding from Novo Nordisk Foundation (NNF20OC0064043), and the Research Foundation of Rigshospitalet (E-22652-04). The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the manuscript.
Data availability
The data supporting the findings of this study are available upon reasonable request to the corresponding author (LERO).
Declarations
Conflicts of interest
The salary of the corresponding author was supported by a grant from the Research Foundation of Rigshospitalet (E-22652-04). The study, inclusive salary of RPB, was supported by a grant from Novo Nordisk Foundation (NNF20OC0064043) in the name of CH. CH is also supported by an unrestricted grant from Lundbeck Foundation (R186-2015–2132), and received a speaker honorarium from Abiomed during the course of the study, and further is a board member of the European Society of Cardiology and serves as chair of the Danish Heart Foundation. JK is supported by an unrestricted grant from Novo Nordisk Foundation (NNF17OC0028706), and is a part of two Data Safety Monitoring Boards for two randomized controlled trials, The IVIO trial (chair) and the COCA trial, without any reimbursement. JEM received two research grants outside the current study from Abiomed and Novo Nordisk Foundation; further he received speaker honorariums from Abiomed, Abbott, and Boehringer Ingelheim, and attended meetings with the support of Abiomed. FF is supported by a research grant from Novo Nordisk Foundation (NNF19OC0055142). RFS received grants from Lundbeck Foundation, Danish Heart Foundation, and Sygeforsikringen Danmark Research Fund, all outside the current study. The remaining authors have nothing to disclose.
Ethical approval
Prior to initiation of the trial, permissions were obtained from the Regional Ethics Committee (ID: H-20022320) and the Danish Medicines Agency (ID: 2020033425), and a legal data handling agreement was approved by the Capital Region of Denmark (ID: P-2020–866).
Consent to participate
The trial was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from a primary trial guardian before inclusion and subsequently confirmed by a secondary trial guardian. Written informed consent from the nearest relatives was obtained as soon as possible following admission to the hospital and further from all patients upon regained capacity.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gräsner JT, Herlitz J, Tjelmeland IBM, Wnent J, Masterson S, Lilja G, et al. European resuscitation council guidelines 2021: epidemiology of cardiac arrest in Europe. Resuscitation. 2021;161:61–79. doi: 10.1016/j.resuscitation.2021.02.007. [DOI] [PubMed] [Google Scholar]
- 2.Sondergaard KB, Riddersholm S, Wissenberg M, Moller Hansen S, Barcella CA, Karlsson L, et al. Out-of-hospital cardiac arrest: 30-day survival and 1-year risk of anoxic brain damage or nursing home admission according to consciousness status at hospital arrival. Resuscitation. 2020;148:251–258. doi: 10.1016/j.resuscitation.2019.12.006. [DOI] [PubMed] [Google Scholar]
- 3.Yan S, Gan Y, Jiang N, Wang R, Chen Y, Luo Z, et al. The global survival rate among adult out-of-hospital cardiac arrest patients who received cardiopulmonary resuscitation: a systematic review and meta-analysis. Crit Care. 2020;24(1):8–13. doi: 10.1186/s13054-020-2773-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neumar RW, Nolan JP, Adrie C, Aibiki M, Berg RA, Böttiger BW, et al. Post-cardiac arrest syndrome epidemiology, pathophysiology, treatment, and prognostication a consensus statement from the international liaison committee on resuscitation (American Heart Association, Australian and New Zealand Stroke Foundation of Canada) Circulation. 2008;118:2452–2483. doi: 10.1161/CIRCULATIONAHA.108.190652. [DOI] [PubMed] [Google Scholar]
- 5.Penketh J, Nolan JP. Post-cardiac arrest syndrome. J Neurosurg Anesth. 2023;31(4):383–393. [Google Scholar]
- 6.Laver S, Farrow C, Turner D, Nolan J. Mode of death after admission to an intensive care unit following cardiac arrest. Intensive Care Med. 2004;30(11):2126–2128. doi: 10.1007/s00134-004-2425-z. [DOI] [PubMed] [Google Scholar]
- 7.Gräsner JT, Lefering R, Koster RW, Masterson S, Böttiger BW, Herlitz J, et al. EuReCa ONE—27 Nations, ONE Europe, ONE Registry: a prospective one month analysis of out-of-hospital cardiac arrest outcomes in 27 countries in Europe. Resuscitation. 2016;105:188–195. doi: 10.1016/j.resuscitation.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Nolan JP, Sandroni C, Böttiger BW, Cariou A, Cronberg T, Friberg H, et al. European resuscitation council and european society of intensive care medicine guidelines 2021: post-resuscitation care. Resuscitation. 2021;161:220–269. doi: 10.1016/j.resuscitation.2021.02.012. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki S, Tanaka K, Suzuki N. Ambivalent aspects of interleukin-6 in cerebral ischemia : inflammatory versus neurotrophic aspects. J Cereb Blood Flow Metab. 2009;29(3):464–479. doi: 10.1038/jcbfm.2008.141. [DOI] [PubMed] [Google Scholar]
- 10.Chong JY, Ahn HJ, Park JS, You Y, Min JH, Jeong W, et al. Interleukin-6 as a potential predictor of neurologic outcomes in cardiac arrest survivors who underwent target temperature management. J Emerg Med. 2023;59(6):828–835. doi: 10.1016/j.jemermed.2020.09.021. [DOI] [PubMed] [Google Scholar]
- 11.Bro-Jeppesen J, Kjaergaard J, Stammet P, Wise MP, Hovdenes J, Åneman A, et al. Predictive value of interleukin-6 in post-cardiac arrest patients treated with targeted temperature management at 33 °C or 36 °C. Resuscitation. 2016;98:1–8. doi: 10.1016/j.resuscitation.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 12.Sekhon MS, Stukas S, Hirsch-Reinshagen V, Thiara S, Schoenthal T, Tymko M, et al. Neuroinflammation and the immune system in hypoxic ischaemic brain injury pathophysiology after cardiac arrest. J Physiol. 2023 doi: 10.1113/JP284588. [DOI] [PubMed] [Google Scholar]
- 13.Sandroni C, Cronberg T, Sekhon M. Brain injury after cardiac arrest: pathophysiology, treatment, and prognosis. Intensive Care Med. 2021;47(12):1393–1414. doi: 10.1007/s00134-021-06548-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoiland RL, Rikhraj KJK, Thiara S, Fordyce C, Kramer AH, Skrifvars MB, et al. Neurologic prognostication after cardiac arrest using brain biomarkers a systematic review and meta-analysis. JAMA Neurol. 2022;79(4):390–398. doi: 10.1001/jamaneurol.2021.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids—new mechanisms for old drugs. N Engl J Med. 2005;353(16):1711–1723. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- 16.Varvarousi G, Stefaniotou A. Glucocorticoids as an emerging pharmacologic agent for cardiopulmonary resuscitation. Cardiovasc Drugs Ther. 2014;28:477–488. doi: 10.1007/s10557-014-6547-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Obling LER, Beske RP, Wiberg S, Folke F, Moeller JE, Kjaergaard J, et al. Steroid treatment as anti-inflammatory and neuroprotective agent following out-of-hospital cardiac arrest: a randomized clinical trial. Trials. 2022;23(952):1–12. doi: 10.1186/s13063-022-06838-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Statistics Denmark - Annual Report (2023) Available from: https://www.statbank.dk/statbank5a/SelectVarVal/Define.asp?Maintable=FOLK1A&PLanguage=1
- 19.Morrison LJ, Visentin LM, Kiss A, Theriault R, Eby D, Vermeulen M, et al. Validation of a rule for termination of resuscitation in out-of-hospital cardiac arrest. N Engl J Med. 2006;355(5):478–487. doi: 10.1056/NEJMoa052620. [DOI] [PubMed] [Google Scholar]
- 20.Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet (London, England) 1975;1(7905):480–484. doi: 10.1016/S0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- 21.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19(5):604–607. doi: 10.1161/01.STR.19.5.604. [DOI] [PubMed] [Google Scholar]
- 22.Martinell L, Nielsen N, Herlitz J, Karlsson T, Horn J, Wise MP, et al. Early predictors of poor outcome after out-of-hospital cardiac arrest. Crit Care. 2017;21(1):1–10. doi: 10.1186/s13054-017-1677-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bro-Jeppesen J, Kjaergaard J, Wanscher M, Nielsen N, Friberg H, Bjerre M, et al. Systemic inflammatory response and potential prognostic implications after out-of-hospital cardiac arrest: a substudy of the target temperature management trial. Crit Care Med. 2015;43(6):1223–1232. doi: 10.1097/CCM.0000000000000937. [DOI] [PubMed] [Google Scholar]
- 24.Peberdy MA, Andersen LW, Abbate A, Thacker LR, Gaieski D, Abella BS, et al. Inflammatory markers following resuscitation from out-of-hospital cardiac arrest: a prospective multicenter observational study. Resuscitation. 2016;103:117–124. doi: 10.1016/j.resuscitation.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 25.Penn J, Douglas W, Curran J, et al. Efficacy and safety of corticosteroids in cardiac arrest: a systematic review, meta-analysis and trial sequential analysis of randomized control trials. Crit Care. 2023;27(1):12. doi: 10.1186/s13054-022-04297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amantea D, Nappi G, Bernardi G, Bagetta G, Corasaniti MT. Post-ischemic brain damage : pathophysiology and role of inflammatory mediators. FEBS J. 2009;276:13–26. doi: 10.1111/j.1742-4658.2008.06766.x. [DOI] [PubMed] [Google Scholar]
- 27.Haque A, Polcyn R, Matzelle D, Banik NL. New insights into the role of neuron-specific enolase in neuroinflammation, neurodegeneration and neuroprotection. Brain Sci. 2018;8(2):33. doi: 10.3390/brainsci8020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akin M, Sieweke J, Garcheva V, Martinez CS, Adel J, Plank P, et al. Additive impact of interleukin 6 and neuron specific enolase for prognosis in patients with out-of-hospital cardiac arrest – experience from the Hannover cooling registry. Front Cardiovasc Med. 2022;9:1–10. doi: 10.3389/fcvm.2022.899583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoiland RL, Ainslie PN, Wellington CL, Cooper J, Stukas S, Thiara S, et al. Brain hypoxia is associated with neuroglial injury in humans post-cardiac arrest. Circ Res. 2021;129(5):583–597. doi: 10.1161/CIRCRESAHA.121.319157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sekhon MS, Ainslie PN, Griesdale DE. Clinical pathophysiology of hypoxic ischemic brain injury after cardiac arrest: a “two-hit” model. Crit Care. 2017;21(1):1–10. doi: 10.1186/s13054-017-1670-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meyer M, Wiberg S, Grand J, Meyer ASP, Obling LER, Frydland M, et al. Treatment effects of interleukin-6 receptor antibodies for modulating the systemic inflammatory response after out-of-hospital cardiac arrest (The IMICA trial) Circulation. 2021;143:1841–1851. doi: 10.1161/CIRCULATIONAHA.120.053318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Timmermans S, Souffriau J, Libert C. A general introduction to glucocorticoid biology. Front Immunol. 2019;10:1545. doi: 10.3389/fimmu.2019.01545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ottens TH, Nijsten MWN, Hofland J, Dieleman JM, Hoekstra M, van Dijk D, et al. Effect of high-dose dexamethasone on perioperative lactate levels and glucose control: a randomized controlled trial. Crit Care. 2015;19(1):1–13. doi: 10.1186/s13054-015-0736-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mentzelopoulos SD, Zakynthinos SG, Tzoufi M, Katsios N, Papastylianou A, Gkisioti S, et al. Vasopressin, epinephrine, and corticosteroids for in-hospital cardiac arrest. Arch Intern Med. 2009;169(1):15–24. doi: 10.1001/archinternmed.2008.509. [DOI] [PubMed] [Google Scholar]
- 35.Mentzelopoulos SD, Malachias S, Chamos C, Konstantopoulos D, Ntaidou T, Papastylianou A, et al. Vasopressin, steroids, and epinephrine and neurologically favorable survival after in-hospital cardiac arrest: a randomized clinical trial. JAMA - J Am Med Assoc. 2013;310(3):270–279. doi: 10.1001/jama.2013.7832. [DOI] [PubMed] [Google Scholar]
- 36.Andersen LW, Isbye D, Kjærgaard J, Kristensen CM, Darling S, Zwisler ST, et al. Effect of vasopressin and methylprednisolone vs placebo on return of spontaneous circulation in patients with in-hospital cardiac arrest: a randomized clinical trial. JAMA - J Am Med Assoc. 2021;326(16):1586–1594. doi: 10.1001/jama.2021.16628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Panettieri RA, Schaafsma D, Amrani Y, Koziol-White C, Ostrom R, Tliba O. Non-genomic effects of glucocorticoids: an updated view. Trends Pharmacol Sci. 2019;40(1):38–49. doi: 10.1016/j.tips.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roberts I, Yates D, Sandercock P, Farrell B, Wasserberg J, Lomas G, et al. Effect of intravenous corticosteroids on death within 14 days in 10008 adults with clinically significant head injury (MRC CRASH trial): randomised placebo-controlled trial. Lancet (London, England) 2004;364(9442):1321–1328. doi: 10.1016/S0140-6736(04)17188-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are available upon reasonable request to the corresponding author (LERO).