Abstract
Background
Transanal TME (taTME) combines abdominal and transanal dissection to facilitate sphincter preservation in patients with low rectal tumors. Few phase II/III trials report long-term oncologic and functional results. We report early results from a North American prospective multicenter phase II trial of taTME (NCT03144765).
Methods
100 patients with stage I–III rectal adenocarcinoma located ≤ 10 cm from the anal verge (AV) were enrolled across 11 centers. Primary and secondary endpoints were TME quality, pathologic outcomes, 30-day and 90-day outcomes, and stoma closure rate. Univariable regression analysis was performed to assess risk factors for incomplete TME and anastomotic complications.
Results
Between September 2017 and April 2022, 70 males and 30 females with median age of 58 (IQR 49–62) years and BMI 27.8 (IQR 23.9–31.8) kg/m2 underwent 2-team taTME for tumors located a median 5.8 (IQR 4.5–7.0) cm from the AV. Neoadjuvant radiotherapy was completed in 69%. Intersphincteric resection was performed in 36% and all patients were diverted. Intraoperative complications occurred in 8% including 3 organ injuries, 2 abdominal and 1 transanal conversion. The 30-day and 90-day morbidity rates were 49% (Clavien–Dindo (CD) ≥ 3 in 28.6%) and 56% (CD ≥ 3 in 30.4% including 1 mortality), respectively. Anastomotic complications were reported in 18% including 10% diagnosed within 30 days. Higher anastomotic risk was noted among males (p = 0.05). At a median follow-up of 5 (IQR 3.1–7.4) months, 98% of stomas were closed. TME grade was complete or near complete in 90%, with positive margins in 2 cases (3%). Risk factors for incomplete TME were ASA ≥ 3 (p = 0.01), increased time between NRT and surgery (p = 0.03), and higher operative blood loss (p = 0.003).
Conclusion
When performed at expert centers, 2-team taTME in patients with low rectal tumors is safe with low conversion rates and high stoma closure rate. Mid-term results will further evaluate oncologic and functional outcomes.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s00464-023-10266-9.
Keywords: Rectal cancer, Transanal total mesorectal excision, TME grade, Circumferential radial margin, Conversion, Anastomotic complication, Stoma-free rate
In conjunction with major advancements in neoadjuvant treatment strategies, worldwide adoption of total mesorectal excision (TME) technique in the curative resection of locally advanced rectal cancer over the past 3 decades has reduced 5-year local recurrence (LR) rates to 3.3–5.7% in large contemporary series [1–5]. Laparoscopy was a major advance in innovation for the surgical treatment of rectal cancer, although acceptance and adoption have been slowed by long learning curves and lingering concerns regarding inferior rate of circumferential resection margins (CRM) relative to open TME. By providing minimally invasive access to the pelvis, laparoscopic and robotic-assisted TME have improved short-term postoperative recovery without significantly impacting oncologic or functional outcomes relative to open proctectomy. As reflected in recent comparative trials, persistently high abdominoperineal resection (APR), CRM positive and conversional rates reported in male and obese patients relate to tumor location ≤ 6 cm from the anal verge [1, 6, 7]. Transanal TME (taTME) evolved from NOTES (Natural Orifice Transluminal Endoscopic Surgery) and is most commonly performed in a hybrid fashion in combination with abdominal laparoscopic assistance. Since the report of the first case in 2010, rapid adoption of taTME worldwide reflects the perceived benefits of direct in-line access to the distal rectum, augmented exposure, and navigation provided by pneumodistention and image guidance through multiport transanal endoscopic platforms in facilitating these complex procedures [8–11]. Large retrospective institutional and multicenter cohort studies have reported procedural and short-term oncologic results commensurate with those from laparoscopic trials, with notably lower conversion rates and higher rates of sphincter preservations [12, 13], but also a non-negligible incidence of procedure-specific adverse events including urethral injury and CO2 embolism [14–17]. Several national cancer audits, cohort studies, and the first recently published randomized trial of laparoscopic vs taTME have reported 3-year local recurrence rates ranging from 1.9% to 6.2% [18–27]. However, results from the 2019 Norwegian audit of 157 consecutive taTME cases have led several centers to abandon taTME, and recommend against adoption of the technique outside of centers with strict training pathways [28]. This posture was based on the 7.6% observed overall local recurrence rate, with 11.6 vs 2.4% estimated 2-year LR rate in the taTME vs non-taTME groups, in a cohort from the Norwegian Colorectal Cancer Registry, including a 5% reported incidence of multifocal pelvic side wall recurrences [28]. While variability in case selection, training and surgical technique are known to contribute to unfavorable outcomes during the early adoption phase of any new surgical procedure, ongoing randomized control trials (RCTs) will further clarify the impact of TME technique on oncologic results [29, 30]. In the US, based on the trend among experienced surgeons to favor taTME for tumors located in the distal rectum, a prospective phase II trial was designed to evaluate the safety, oncologic and functional results of taTME in a cohort of 100 patients with stage I–III rectal cancer when performed by experienced taTME surgeons beyond their learning curve. In this manuscript, postoperative morbidity at 30 and 90 days including outcomes of anastomotic complications, stoma closure rate, and pathologic outcomes are reported.
Methods
Study design
This is a phase II prospective multicenter trial conducted across 11 experienced taTME centers in the US and Canada. The primary endpoint was the rate of complete and near-complete mesorectal excision achieved with taTME, and secondary endpoints included detailed pathology assessment, 30 and 90-day complications, functional outcomes post-stoma reversal, and 3-year oncologic outcomes. The protocol was approved at each participating site IRB, and written informed consent was obtained from all study participants prior to enrollment (Protocol, Online Appendix 1). The trial was registered on clinicaltrial.gov (NCT03144765). The manuscript conforms to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [31].
Surgeon eligibility requirements included performance of ≥ 20 sphincter-preserving low anterior resections (LAR) including ≥ 5 taTME cases in the preceding 12 months and ≥ 20 transanal endoscopic resections by transanal surgeons in the preceding 24 months. Participating surgeons had been performing taTME procedures for a minimum of 3 years, and were required to submit recent unedited transanal video recordings of 2 cases, including one in a male patient, along with pathology reports and photographs of gross TME specimens to confirm technical proficiency. Study sites were required to follow National Accreditation Program in Rectal Cancer (NAPRC) standards with respect to staging and treatment protocols, multidisciplinary tumor board (MDT) review and consensus treatment planning of all rectal cancer patients [32].
Patients > 18 years old, with clinically staged I–III biopsy-confirmed adenocarcinoma, located ≤ 10 cm from the anal verge, who were candidates for minimally invasive curative sphincter-preserving low anterior resection with tumor-specific (TSME) or TME, were screened for study enrollment. Tumor height and relationship to the anal sphincters was based on digital rectal exam (DRE), and/or rigid proctosigmoidoscopy, and confirmed on staging MRI and operative reports. Staging was completed with CT scans of the chest, abdomen and pelvis and rectal cancer protocol pelvic MRI. Neoadjuvant and adjuvant treatment protocols were based on MDT consensus recommendations and included short-course radiotherapy (SCRT), long-course chemoradiotherapy (CRT), total neoadjuvant therapy (TNT) or chemotherapy only. Chemotherapy regimens included Capecitabine, 5-FU, FOLFOX, or Capecitabine in combination with Oxaliplatin. Oncologic surveillance scheduled followed NCCN guidelines. Clinical assessment was performed at 2 and 4–6 weeks, 5–7 months and 11–13 months postoperative visits in order to collect adverse events. Patients completed baseline preoperative functional questionnaires at least once prior to surgery, and at two postoperative time points post-stoma reversal, when applicable.
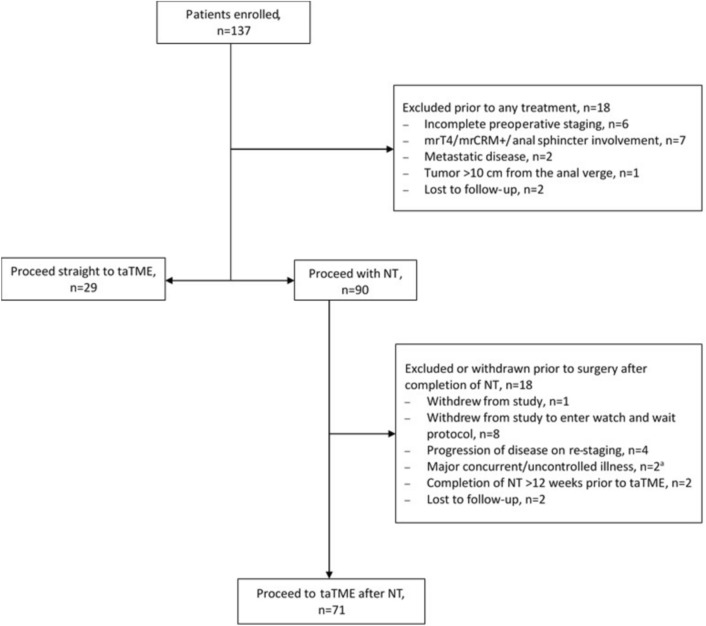
Enrollment occurred either prior to neoadjuvant treatment or prior to taTME (Fig. 1). All enrolled patients were reviewed at MDT at least once prior to taTME procedures and the decision to re-stage post-neoadjuvant treatment was up to site protocol and preferences. Exclusion criteria included metastatic or synchronous disease, severe intercurrent illness, T4 tumors and tumors with positive predicted circumferential margin (CRM) and/or involvement of anal sphincters on staging or post-treatment pelvic MRI, fecal incontinence, prior colorectal cancer or rectal resection and inflammatory bowel disease. Patients were also excluded if taTME was delayed more than 12 weeks post-completion of neoadjuvant treatment, and if they were unable to compete the functional questionnaire in English.
Fig. 1.
Patient enrollment flowchart. NT, neoadjuvant treatment; mrT4/mrCRM + , predicted T4 or positive circumferential margin on staging pelvic MRI; a1 patient with neurological decline after NT, 1 patient with concurrent urothelial malignancy
Study procedures and data collection
Preoperative bowel preparation and venous thrombosis prophylaxis were administered based on sites’ local protocols. Enhanced recovery protocols were used when possible with intraoperative anesthetic regimens including local, regional or intrathecal anesthesia. Surgeons were required to perform all taTME procedures with a 2-team approach using sequential or simultaneous abdominal and transanal dissection, and to video record all transanal procedures (Protocol, Online Appendix 1). Abdominal minimally invasive approach, distal vs proximal extent of abdominal vs transanal, and extent of anterior vs posterior TME dissection was left up to surgeons’ preferences. Typically, the anterior TME dissection was carried down to the anterior peritoneal reflection or mid-vagina/prostate, while posterior TME dissection is typically extended to the S1 or S2 level. For transanal procedures, the rectal lumen was occluded below the tumor with a single or double purse-string suture with air-tight closure prior to initiating taTME dissection. Alternatively, purse-string closure followed initial mucosectomy or partial intersphincteric resection, when performed. The choice of transanal endoscopic platform, splenic flexure mobilization, specimen extraction site, intraoperative bowel perfusion assessment by fluorescence angiography, anastomotic configuration, fecal diversion and pelvic drain placement were left to the surgeon’s preference and recorded. Gross specimens were photographed, inked and processed according to standard TME protocols [33–35].
Patient and tumor characteristics including demographics, comorbidities, tumor height, radiologic staging, neoadjuvant treatment received, and time interval between end of treatment and surgery were collected. Procedural details, intraoperative events, and length of hospital stay were recorded, in addition to postoperative complications reported during follow-up clinical visits, which were graded using Clavien–Dindo classification [36]. Anastomotic complications were reported based on clinical (by direct visualization or digital palpation, inspection of drain output), radiologic (CT scan, contrast enema) or endoscopic (sigmoidoscopy or colonoscopy) evidence of anastomotic leakage, ischemia, dehiscence or defect, peri-anastomotic cavity, sinus or fistula, as well as significant strictures (requiring more than single finger dilatation). Anastomotic leak (AL) refers to the early presentation of an anastomotic complication (identified within 30 days of surgery) and was reported based on clinical, radiologic, or endoscopic finding of an anastomotic defect, dehiscence, ischemia, and/or finding of pelvic abscess or phlegmon immediately adjacent to the anastomosis. Time to diagnosis, diagnostic modality, and interventions used to manage anastomotic complications were collected until ileostomy reversal or conversion to permanent stoma. Anastomotic complications were graded according to CD classification as well as the International Study Group of Rectal Cancer (ISGRC) classification [36, 37]. The protocol was amended in August 2018 to include collection of C-reactive protein level (CRP) on postoperative day (POD1-4), when applicable (Protocol, Online Appendix 1) [38].
Pathological assessment of specimens was collected from synoptic reports and included grade of completeness of the mesorectum, TNM stage, and assessment of circumferential radial margins, and proximal and distal margins [34]. Assignment of a grade of complete mesorectum required demonstration of a smooth surface of mesorectal fascia with the entire mesorectal envelope present and no defect deeper than 5 mm, while a near-complete mesorectum demonstrated an intact mesorectal envelope except for small irregularities and/or defects greater than 5 mm but not extending to the muscularis propria. In contrast, an incomplete mesorectum demonstrated deeper defects in the mesorectum with exposed muscularis propria and/or very irregular circumference. Positive proximal, distal, and circumferential resection margins were defined as viable tumor located ≤ 1 mm from the respective margin.
Urologic, sexual, and defecatory function was assessed at least once preoperatively and at 2 postoperative time points, 3–4 months and 9–12 months post-stoma reversal. Validated questionnaires were used, including COREFO (Colorectal Functional Outcome) [39], FIQL (Fecal Incontinence Quality of Life questionnaires) [40, 41], female sexual function Index (FSFI) [42], IIEF (International Index of Erectile Function questionnaires [43], and IPSS (International Prostate Symptom Score) [44]. Oncologic outcomes data will be collected up to 5 years following surgery including local and distant recurrence, disease-free (DFS) and overall survival (OS). Data entry was completed on REDCAp, a research electronic data capture platform, while photographs of TME specimens and unedited taTME videos were uploaded on an encrypted HIPAA compliant, password-protected Mount Sinai Hospital server.
Surgical and pathological quality control
Site initiation included review of standard TME protocols by site pathologists and pathology staff through webinars and a standard of operations (SOP) manual [33–35]. Quality assurance consisted of continuous remote monitoring to ensure accurate and timely data entry and reporting of adverse events and compliance with study procedures. Study sites underwent one on-site monitoring visit after the first 1–5 procedures to ensure compliance with study protocols. Quality assurance for pathology procedures included monitoring of compliance with standard TME protocol for specimen handling, processing and evaluation, central review of all TME gross photographs by central blinded pathologists to establish concordance in TME grade, and monitoring of the incidence of incomplete TME and/or positive resection margins. Quality improvement measures included (1) retraining of pathology staff on specimen handling and processing, (2) reviewing and reconciliation of major discordances in TME grading between study site and blinded central pathologists, (3) a stopping rule for sites with 2 cases of incomplete TME and/or positive surgical margins to allow for assessment of the adequacy of dissection based on blinded video review by an external taTME expert, with implementation of a corrective action plan. Interim analysis by a study data safety monitoring board was completed after the first 50 patients were enrolled.
Sample size and statistical analysis
The primary objective of this study was to determine whether taTME was non-inferior to standard LAR in terms of the proportion of subjects that achieve complete or near-complete mesorectal excision. With a sample size of 100, the one-sided binomial test will reject the null hypothesis that the success rate is ≤ 80% if the study procedure leads to efficacy of the total mesorectal excision for 87 or more subjects. This design achieves a power of 87% using one-sided binomial test for non-inferiority with 5% type 1 error assuming the true success rate is 90%. Patient demographic, disease-related, treatment-related, operative characteristics as well as pathologic outcomes and 90-day surgical complications were summarized for continuous variables as median and first and third quartiles, Q1–Q3, and for categorical variables as counts and percentages. Distributions of categorical variables were compared among group using the χ2 or Fisher’s exact test when appropriate. Univariable log-binomial regression was used to investigate associations between some of the aforementioned characteristics, identified through an extensive literature search as potential risk factors, and incomplete pathologic TME grade, reporting relative risks (RRs), corresponding 95% confidence intervals (CIs), and p-values. RRs were selected instead of odds ratios via logistic regression because the latter tends to overestimate the strength of the association when the incidence of the outcome is 10% or more, as it is in this study. Times from surgery to stoma closure were analyzed using the Kaplan–Meier (KM) method. Comparison of stoma closure KM distributions was made between groups with the log-rank test. Eighty-two patients were censored for anastomotic leak at the time of their ileostomy reversal or permanent colostomy creation. Two patients were censored for stoma closure at their permanent colostomy creation. Duration of follow-up for postoperative complications was calculated as the maximum of 90 days and days to ileostomy reversal or permanent colostomy creation, except for in one patient whose follow-up was only 70 days due to death. Univariable Cox proportional hazards regression was used to investigate associations between characteristics identified through literature search as potential risk factors for anastomotic leak and/or stoma closure, reporting hazard ratios (HRs), corresponding 95% confidence intervals (CIs), and p values. All statistical analyses were performed using SAS statistical software (version 9.4; SAS Institute). Hypothesis testing was conducted at the 5% level of significance. However, p values less than 10% were considered borderline significant in exploratory regression analyses.
Results
Accrual and Demographics
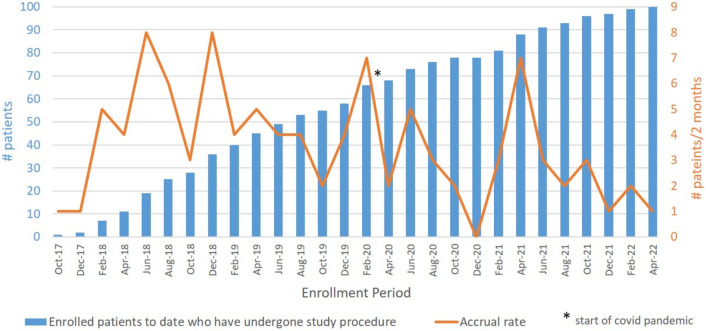
Between September 2017 and April 2022, 137 patients were enrolled across 11 sites in the United States and Canada. There were 37 screen failures due to exclusion criteria, withdrawal of consent or loss to follow-up. In 8 cases where clinical complete response was achieved post-neoadjuvant therapy, patients withdrew from the study to enter watch and wait protocols (Fig. 1). In total, 100 patients underwent taTME procedures with total accrual ranging from 2 to 17 subjects per study site (Fig. 2). Below target patient accrual at 5 study sites was due to site PI relocation, screen failures, and COVID-related interruptions. Between March 2020 and February 2021, COVID-related trial disruptions resulted in delays in accrual, in-person follow-up intervals, surveillance, and ileostomy reversal (Fig. 2). Among 100 taTME patients, 70 were male vs 30 female, 22% self-identified as other than Caucasian, median age was 58 (IQR 49–62), and median BMI was 27.8 (IQR 23.9–31.8) with 33% of patients with BMI ≥ 30 (Table 1).
Fig. 2.
Trial Accrual, bar graph with cumulative number of patients accrued on the taTME trial at 2-month intervals and line graph demonstrating the rate of accrual per 2 months
Table 1.
Demographic and clinical characteristics
| n = 100 patients | |
|---|---|
| Male | 70 (70.0%) |
| Age at index surgery, median [IQR] | 58 [49, 62] |
| Race | |
| Caucasian | 78 (78.0%) |
| Asian | 8 (8.0%) |
| Black | 5 (5.0%) |
| Hispanic | 5 (5.0%) |
| Othera | 4 (4.0%) |
| ASA score | |
| 1/2 | 60 (60.0%) |
| 3 | 40 (40.0%) |
| ECOG score | |
| 0 | 90 (90.0%) |
| 1 | 10 (10.0%) |
| BMI (kg/m2), median [IQR] | 27.8 [23.9, 31.8] |
| < 30 | 67 (67.0%) |
| ≥ 30 | 33 (33.0%) |
| Smokers | 7 (7.0%) |
| Comorbidities | 37 (37.0%) |
| Hypertension | 33 (33.0%) |
| Diabetes | 11 (11.0%) |
| Liver transplant | 1 (1.0%) |
| Previous unrelated abdominal surgery | 10 (10%) |
| Clinical AJCC stage | |
| I | 35 (35.0%) |
| II | 22 (22.0%) |
| III | 43 (43.0%) |
| Tumor distance from anal verge (cm), median [IQR] | 5.8 [4.5, 7.0] |
| ≤ 6 cm | 67 (67.0%) |
| > 6 cm | 33 (33.0%) |
| Clinical tumor stage | |
| cT1b | 5 (5.0%) |
| cT1b/2 | 9 (9.0%) |
| cT2 | 28 (28.0%) |
| cT2/3a | 1 (1.0%) |
| cT3 | 57 (57.0%) |
| Clinical node stage | |
| cN0 | 57 (57.0%) |
| cN1 | 31 (31.0%) |
| cN2 | 12 (12.0%) |
| CEA at diagnosis (ng/mL), median [IQR] | 1.9 [1.1, 4.1] |
| Neoadjuvant treatment | 71 (71.0%) |
| Long course CRT | 51 (51.0%) |
| Total neoadjuvant therapy | 17 (17.0%) |
| Chemotherapy alone | 2 (2.0%) |
| Short course RT | 1 (1.0%) |
| Restaging MRI post CRT | 57 (57.0%) |
| Time from end of RT to index surgery, days, median [IQR] | 76 [68–82] |
aIncludes Guyanese, Native American, Middle Eastern, and Pacific Islander
bCompletion TME was indicated in 8 patients following local excision due to the presence of high-risk histopathologic features
IQR interquartile range; ASA American Society of Anesthesiologists; BMI body mass index; AJCC American Joint Committee on Cancer; CEA carcinoembryonic antigen; CRT chemoradiation therapy, RT radiation therapy; MRI magnetic resonance imaging
Tumor characteristics
Median tumor height was 5.8 cm from the anal verge (IQR 4.5–7.0). Tumors were preoperatively staged as clinical stage I (35%), stage II (22%), or stage III (43%). Among stage I tumors, 8 cT1N0 or cT1/2N0 patients underwent completion taTME after local excision and uncovered high-risk histopathological features (poor differentiation, lymphovascular invasion, high tumor budding, and positive margin) warranting radical resection based on MDT consensus recommendations (Table 1). Neoadjuvant treatment was completed in 71% of patients, including 64 of the 65 stage II/III tumors and 7 stage I (T2N0) tumors. A significantly higher proportion of tumors located ≤ 6 cm from the AV were treated with neoadjuvant therapy than tumors > 6 cm (76.4% vs 25.4%, p = 0.011). Neoadjuvant regimens included long-course CRT in 51/71 (71.8%), TNT in 17/71 (23.9%), chemotherapy only in 2/71 (2.8%), and SCRT in 1/71 (1.4%). TNT strategy was employed by 6 of 11 sites during the study period, with 5 patients between 2017 and 2019 compared with 12 patients between 2021 and 2022 receiving TNT. Among 11 stage III, 5 stage II, and 1 stage I treated with TNT, 64.7% vs 35.3% received induction vs consolidation TNT. Overall, 80.3% (57/71) of tumors were re-staged post-neoadjuvant treatment. Median time between the end of neoadjuvant radiation and taTME was 76 (IQR 68–82) days (Table 1).
Surgical procedures
All procedures were completed using a 2-team approach with abdominal dissection performed by multiport (94%), robotic (4%), single port (1%), or hand-assisted laparoscopy (1%) with splenic flexure mobilization performed in 94 cases. Concurrent surgical procedures were performed in 8 patients (Table 2). Transanal platforms used included rigid (26%) or disposable endoscopic platforms (74%). In 38 cases where tumors were located a median of 4 cm from the AV, transanal mucosectomy (2) or partial intersphincteric resection (ISR, 36) was performed in combination with taTME. There were 11 intraoperative complications reported among 8 patients including 2 abdominal conversions to open surgery (ischemic colonic conduit, bleeding), 1 transanal conversion to abdominal robotic dissection (failure to progress), 1 suspected case of CO2 embolus, 2 vaginal injuries during transanal dissection, and 1 colotomy, 1 enterotomy, and 1 ureteral transection during abdominal dissection, all identified and repaired. Perfusion assessment of the colonic conduit and/or colorectal anastomosis was performed by fluorescence angiography using indocyanine green (ICG) in 55% (Table 2). Inadequate perfusion was observed in 9 cases and prompted intervention in 8 cases (change in proximal resection margin with additional mobilization, suture removal). TME specimens were extracted transanally in 52%, with handsewn coloanal anastomosis performed in 54%. Anastomotic reconstruction was completed end-end (89%), side-to-end (6%), or with coloanal J pouch (5%). All patients were diverted with a loop ileostomy and pelvic drains were placed in 80%. Median total operative time was 311.5 (IQR 265.5–380.5) minutes and median transanal dissection time was 101 (IQR 74.5–174) minutes. Median estimated blood loss was 100 (IQR 50–250) ml and blood transfusion was not required.
Table 2.
Intraoperative details
| n = 100 patients | |
|---|---|
| Operative time (minutes), median [IQR] | |
| Total | 311.5 [265.5, 380.5] |
| Transanal | 101 [74.5, 174] |
| EBL (mL), median [IQR] | 100 [50, 250] |
| Intraoperative analgesiaa | |
| Local anesthesia | 43 (43.0%) |
| TAP Block | 42 (42.0%) |
| Intrathecalb | 9 (9.0%) |
| Concurrent procedure | 8 (8.0%) |
| Salpingo-oophorectomy | 5 (5.0%) |
| Umbilical hernia repair | 2 (2.0%) |
| Cholecystectomy | 1 (1.0%) |
| Intraoperative complications | 8 (8.0%) |
| Conversion | 3 (3.0%) |
| Vaginotomy | 2 (2.0%) |
| Suspected CO2 embolus | 1 (1.0%) |
| Bleeding | 1 (1.0%) |
| Colotomy | 1 (1.0%) |
| Ischemic conduit | 1 (1.0%) |
| Ureteral injury | 1 (1.0%) |
| Enterotomy | 1 (1.0%) |
| Total number of complications | 11 |
| Approach to abdominal procedure | |
| Multiport laparoscopy | 94 (94.0%) |
| Robotic-assisted laparoscopy | 4 (4.0%) |
| Single incision laparoscopy | 1 (1.0%) |
| Hand-assisted laparoscopy | 1 (1.0%) |
| Splenic flexure mobilization | 94 (94.0%) |
| Abdominal converted to open | 2 (2.0%) |
| Transanal platform used | |
| GelPOINT® path | 74 (74.0%) |
| TEO® | 15 (15.0%) |
| TEM | 11 (11.0%) |
| Partial ISR or mucosectomy | 38 (38%) |
| Partial ISR | 36 (36.0%) |
| Mucosectomy | 2 (2.0%) |
| Transanal insufflator | |
| Airseal® | 92 (92.0%) |
| Unspecified high-pressure insufflator | 5 (5.0%) |
| Unspecified standard insufflator | 3 (3.0%) |
| Transanal conversion to abdominal | 1 (1.0%) |
| Specimen extraction site | |
| Transanal | 52 (52.0%) |
| Pfannenstiel incision | 26 (26.0%) |
| Periumbilical incision | 13 (13.0%) |
| Lower midline incision | 5 (5.0%) |
| Ileostomy site | 3 (3.0%) |
| Lower left quadrant | 1 (1.0%) |
| FA perfusion assessment with ICGc | 55 (55%) |
| FA perfusion assessment outcome, n (%) of those with FA | |
| Adequate | 46 (83.6%) |
| Inadequate | 9 (16.4%) |
| Action taken, n (%) of cases of inadequate perfusion | |
| Yes | 8 (88.9%) |
| No | 1 (11.1%) |
| Anastomotic technique | |
| Handsewn | 54 (54.0%) |
| Stapled | 46 (46.0%) |
| Stapler size, n (%) of those with stapled anastomosis | |
| 28 | 15 (32.6%) |
| 29 | 14 (30.4%) |
| 31 | 11 (23.9%) |
| 33 | 6 (13.0%) |
| Anastomotic reconstruction | |
| End-to-end | 89 (89.0%) |
| Side-to-end | 6 (6.0%) |
| Coloanal J pouch | 5 (5.0%) |
| Diverting loop ileostomy performed | 100 (100.0%) |
| Pelvic drain placed | 80 (80.0%) |
aAs single agent or in combination;
bSingle shot of intrathecal morphine given at start of case;
cSystems used for FA include PINPOINT (Novadaq Technologies ULC, Burnaby, BC, Canada); 1588 AIM System, 1688 AIM system, SPY-PHI (Stryker Corp, Kalamazoo, MI); NIR/ICG (Karl Storz Endoscopy America Inc, Auburn, MA); Firefly™ (Intuitive Surgical, Sunnyvale, CA)
EBL estimate blood loss; TAP transabdominal plane; GelPOINT Path (Applied Medical, Rancho Santo Margarita, CA); TEO®, transanal endoscopic operations (Karl Storz Endoscopy, Tuttlingen, Germany); TEM (Richard Wolf, Knittlingen, Germany), transanal endoscopic microsurgery; ISR intersphincteric resection; Airseal® (CONMED Corporation, Utica, NY, USA); FA fluorescence angiography; ICG Indocyanine Green; NIR near-infrared fluorescence
30- and 90-day postoperative outcomes
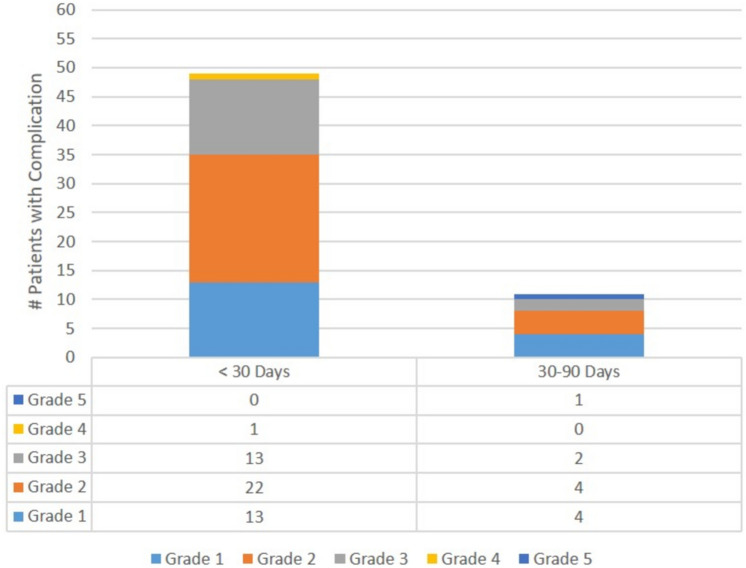
Median length of hospital stay was 5 (IQR 4–8) days. In total, 71 complications were reported in 49 patients within 30 days of surgery (CD3/4 in 28.6%) with a 17% readmission rate (Table 3, Fig. 3). Postoperative ileus with dehydration with or without high stoma output accounted for 35.2% (25/71) of 30-day complications and 47.1% (8/17) of readmissions. Ileus and high stoma output were associated with transient acute kidney injury in 4 (16%) patients. Urinary retention was reported in 19% with a 21.4 vs 13.3% incidence in male vs female patients (p = 0.344). Urinary retention was managed with medication in 68% of patients and resulted in prolonged bladder catheterization beyond discharge in 21% of patients. Other morbidities included anastomotic (10%), infectious (6%), and thrombotic (3%) complications, small bowel obstruction (SBO, 4%), pancreatic fistula (1%), bleeding duodenal ulcer (1%), myocardial infarction (1%), and intractable rectal pain (1%). Reoperation was required in 11/49 patients (22.4%), for anastomotic complications (n = 8), SBO (n = 2) and refractory rectal pain (n = 1). Between postoperative day 30 and 90, 11 new complications were recorded in 11 patients (CD1/2 in 72.7%, CD3/5 in 27.3%) including 1 death from suicide at POD70, 5 new anastomotic complications, 1 ileus with dehydration, 1 SBO, 1 DVT, and 1 stomal and 2 mucosal prolapse. Reoperation was required in 2/11 (18.2%) patients for 1 anastomotic complication and 1 stoma prolapse (Fig. 3).
Table 3.
Postoperative outcomes
| n = 100 patients | |
|---|---|
| Length of stay (days), median [IQR] | 5 [4, 8] |
| Length of follow-upa (months), median [min, Q1, Q3, max] | 5 [2.3, 3, 7, 23] |
| Postoperative complications (< 30 Days)b | 49 (49.0%) |
| Ileus ± stoma-related dehydrationc | 25 (25.0%) |
| With prolonged hospitalizationd | 10 (10.0%) |
| Urinary retentione | 19 (19.0%) |
| Anastomotic leaks/complications | 10 (10.0%) |
| SBO | 4 (4.0%) |
| UTI | 3 (3.0%) |
| SSI | 2 (2.0%) |
| DVT/PMVT | 2 (2.0%) |
| Pulmonary embolism | 1 (1.0%) |
| Pancreatic leak | 1 (1.0%) |
| C. diff infection | 1 (1.0%) |
| Myocardial infarction | 1 (1.0%) |
| Bleeding duodenal ulcer | 1 (1.0%) |
| Rectal pain | 1 (1.0%) |
| Total number of 30-day complications | 71 |
| CD classification < 30 daysf | |
| CD1/2 | 35 (35.0%) |
| CD3/4 | 14 (14.0%) |
| 30-day readmission | 17 (17.0%) |
| 30-day reoperation | 11 (11.0%) |
| Postoperative complications (30–90 days) | 11 (11.0%) |
| Anastomotic complications | 5 (5.0%) |
| Ileus ± stoma-related dehydrationc | 1 (1.0%) |
| SBO | 1 (1.0%) |
| DVT | 1 (1.0%) |
| Ileostomy prolapse | 1 (1.0%) |
| Mucosal prolapse | 1 (1.0%) |
| Death by suicide | 1 (1.0%) |
| Total number of 30–90-day complications | 11 |
| CD classification 30–90 days | |
| CD1/2 | 8 (8.0%) |
| CD3/5 | 3 (3.0%) |
| Total patients with postoperative complications < 90 daysg | 56 (56.0%) |
| Total number of < 90-day complications | 82 |
| CD classification < 90 days | |
| CD1/2 | 39 (39.0%) |
| CD ≥ 3 | 17 (17.0%) |
| POD3 CRP ≥ 145, among patients with CRP drawn POD3 | 15/67 (22.4%) |
aDuration of follow-up used for this analysis was the longer of either the 90-day postoperative complication monitoring period or days from surgery to ileostomy reversal/permanent colostomy creation; exception for 1 patient, whose follow-up was only 70 days due to death
bSome patients with multiple complications
cWith dehydration ± acute kidney injury ± high ileostomy output
dDefined as LOS ≥ 8 days
eFoley reinsertion and/or medical treatment
fHighest grade in those with multiple complications
gAccounts for those with complications occurring at both < 30 days and 30–90 days
CD Clavien-Dindo; SBO small bowel obstruction; DVT deep vein thrombosis; PMVT portal-mesenteric vein thrombosis; SSI surgical site infection; SBO small bowel obstruction; CRP c-reactive protein; LOS length of stay
Fig. 3.
Postoperative complications 30- and 90-day complications with highest CD grade per patient
Anastomotic complications and stoma reversal
At a median follow-up of 5 (IQR 3–7, range 2.3–23) months, 18 anastomotic complications were reported among 17 males and 1 female (p = 0.012), and included 10 early (diagnosed POD0-POD29), 5 late (POD30-POD90), and 3 very late anastomotic complications (> 90 days). Among 10 early anastomotic complications, 8 underwent early re-intervention and 2 were managed with percutaneous drainage and/or antibiotics (Tables 4 and 5, Fig. 4a). Among the 8 that underwent early re-intervention, 5 presented with pelvic sepsis requiring urgent pelvic washout with either transanal repair of anastomotic dehiscence (n = 3), or anastomotic takedown with end colostomy (n = 2), while 3 relatively asymptomatic patients underwent early transanal closure of small anastomotic defects. Overall, resolution of 6 anastomotic complications was achieved with 0–1 reinterventions within 30 days followed by ileostomy reversal between POD86 and POD372 (Table 5). Delayed anastomotic revision with redo-coloanal anastomosis was required in 1 patient, and Hartman’s reversal was performed in another patient who underwent early anastomotic takedown with end colostomy. Both patients underwent delayed ileostomy closure between POD271 and POD304. Two patients were permanently diverted including one who underwent anastomotic takedown due to conduit ischemia, and another who was converted to a permanent colostomy due to due to a refractory anastomotic defect later complicated by peritoneal carcinomatosis (Fig. 4a and b, Table 5).
Table 4.
Anastomotic complications and stoma outcomes
| n = 100 patients | |
|---|---|
| Early, < 30 days | 10 (10.0%) |
| Late, 30–90 days | 5 (5.0%) |
| Late, > 90 days | 3 (3.0%) |
| CD gradea | n = 18 |
| 1 | 3 (16.7%) |
| 2 | 2 (11.1%) |
| 3 | 13 (72.2%) |
| 3a | 1 (5.6%) |
| 3b | 12 (66.7%) |
| ISGRC gradea | n = 18 |
| A- no specific treatment | 3 (16.7%) |
| B- treatment other than laparotomy | 7 (38.9%) |
| C- laparotomy | 8 (44.4%) |
| All patients n = 100 |
No anastomotic complications n = 82 |
Anastomotic complications n = 18 |
p value | |
|---|---|---|---|---|
| Stoma closure | 98 (98.0%) | 82 (100%) | 16b (88.9%) | |
| Time to closure (days), median [IQR] | 154 [94–224.0] | 140 [91.0–213.0] | 226 [140–304] | 0.0015c |
| Closure delayed > 150 days | 49 (49.0%) | 38 (46.3%) | 11 (68.8%) | |
| Reason for delayd, in n (%) with delay | ||||
| Adjuvant treatment | 42 (85.7%) | 33 (86.8%) | 9 (81.8%) | |
| Postoperative complication | 16 (32.7%) | 5 (15.0%) | 11 (100%) | |
| Anastomotic complication | 11 (22.4%) | 0 (0.0%) | 11 (100%) | |
| Othere | 5 (10.2%) | 5 (13.2%) | 0 (0.0%) | |
| Unspecified | 2 (4.1%) | 4 (10.0%) | 0 (0.0%) |
aAccounts for intervention required with highest CD or ISGRC grade required for the anastomotic complication
bone patient converted to permanent end colostomy due to persistent anastomotic complication and development of disease recurrence
cLog-rank p-value
dFor some patients (n = 6), there are multiple reasons for stoma closure delays so does not sum to 100%
eIncludes patient preference, COVID, frailty, and diagnostic work-up of lung lesion
CD Clavien-Dindo
Table 5.
Details of anastomotic complications: diagnosis, management, and outcomes
| Patient | 0–30 days post-taTME |
30–90 days post-taTME |
> 90 days post-taTME |
Stoma reversal |
|---|---|---|---|---|
| Early anastomotic complications (< 30 days) | ||||
| 1 | POD4a: reop for rising CRP, transanal suture closure of small anastomotic defect | POD74: resolvedb | POD86 | |
| 2 |
POD8: abdominal and pelvic abscess, reop with transanal repair of defect and abdominal drainage |
Ongoing anastomotic cavity, no intervention | POD113: resolved | POD140 |
| 3 | POD11: asymptomatic defect on rectal contrast imaging, transanal repair | POD105: resolved | POD130 | |
| 4 |
POD4: fecal content in drain, AL, and pelvic abscess on CT with rectal contrast, reop with transanal repair of anastomotic dehiscence and abdominal washout POD11: no clinical improvement, persistent leak on CT with rectal contrast, 2nd reop with additional transanal drainage |
Ongoing pelvic abscess, no intervention | POD272: resolved | POD372 |
| 5 | POD6: symptomatic pelvic abscess treated with IV antibiotics | POD169: resolved | POD201 | |
| 6 | POD19: reop for anal pain, transanal suture repair of small posterior anastomotic dehiscence | POD45: pelvic abscess treated with oral antibiotics | POD91: EUA, resolved | POD107 |
| 7 |
POD3: fever, CT with pelvic collection, conservative management POD25: readmission with pelvic abscess, IV antibiotics and IR drainage |
POD109: endoscopy and enema study with anastomotic dehiscence and stricture, conservative management POD152: ongoing stricture POD194: anastomotic revision with coloanal pull-through POD242: resolved |
POD271 | |
| 8 |
POD14: readmission with pelvic abscess, IV antibiotics POD17: Persistent pain, CT enema with AL, reop with pelvic washout and drainage POD19: no improvement, reop with anastomotic takedown, end colostomy, and mucous fistula |
POD64: ongoing pelvic abscess, IR drainage | POD242: Hartman’s reversal | POD304 |
| 9 | POD4: fecal drainage from drain with sepsis, reop with transanal repair of anastomotic dehiscence, pelvic washout of abscess | Ongoing dehiscence, presacral cavity |
POD259, POD513: reop with EUA/debridement for ongoing dehiscence and large presacral cavity POD322: development of PC POD538: conversion of ileostomy to end colostomy at time of HIPEC and tumor debulking |
Never reversed |
| 10 | POD13: Outpatient EUA concerning for ischemic conduit, readmission, reop with pelvic washout of abscess, anastomotic takedown, resection of ischemic conduit, end colostomy, and mucous fistula | Never reversed | ||
| Delayed anastomotic complications (> 30 days) | ||||
| 11 | POD32: asymptomatic stricture and dehiscence on endoscopy, required 6 sequential endoscopic dilations |
POD151: endoscopy, resolved POD241: enema study, resolved |
POD249 | |
| 12 | POD56: small asymptomatic anastomotic defect on endoscopy, no intervention |
POD117: ongoing POD177: resolved |
POD177 | |
| 13 |
POD37: pelvic abscess, treated with oral antibiotics POD51: CT scan, resolved POD85: endoscopy, resolved |
POD189 | ||
| 14 | POD49: asymptomatic anastomotic dehiscence, endoscopy with conduit retraction, no intervention |
POD173: ongoing anastomotic stricture POD209: endoscopy dilation POD265: anastomotic revision by transanal circumferential stricture resection and sleeve mobilization POD338: endoscopy, resolved |
POD427 | |
| 15 |
POD58: small asymptomatic anastomotic defect on endoscopy, no intervention POD85: resolved |
POD103 | ||
| 16 | POD172: asymptomatic anastomotic stricture, operative dilation, resolved | POD226 | ||
| 17 |
POD95: asymptomatic anastomotic sinus on endoscopy, no intervention POD172: resolved |
POD250 | ||
| 18 |
POD349: asymptomatic stricture and dehiscence POD539: ongoing POD553: revision of anastomosis with coloanal pull-through |
POD695 | ||
aPOD refers to time (in days) from index surgery (taTME)
bResolution of anastomotic complication as determined by gastrograffin enema, sigmoidoscopy, anoscopy, or CT with rectal contrast
POD postoperative day; AL anastomotic leak; CT computed tomography; IV intravenous; IR interventional radiology; EUA exam under anesthesia; PC peritoneal carcinomatosis; HIPEC heated intraperitoneal chemotherapy
Fig. 4.
A. Management and outcomes of early anastomotic complications (< 30 days); B. Management and outcomes of late anastomotic complications (> 30 days). See Table 5 for details regarding each case. POD postoperative day; CD Clavien-Dindo; IR interventional radiology; IV intravenous; CAA coloanal anastomosis
Among late anastomotic complications diagnosed between POD30 and POD349, 3 were described as small defects or sinuses on endoscopy and resolved without any intervention, one was a pelvic abscess identified on POD37 and resolved with oral antibiotics, while one was an anastomotic stricture that required a single operative dilatation. In all 5 cases, stoma reversal was achieved between POD103 and POD250. In 3 patients with asymptomatic late anastomotic dehiscence and stricture identified on radiology and/or endoscopy, one resolved with serial dilatations with stoma closure on POD249, and 2 eventually required anastomotic revision with transanal sleeve advancement or coloanal pull-through, with delayed ileostomy closure between POD427 and POD695 (Tables 4 and 5, Fig. 4b).
Overall, 72.2% (13/18) of all anastomotic complications identified over the course of this trial required ≥ 1 surgical re-intervention including 90% of early complications, diagnosed within 30 days, and 50% of late complications, diagnosed after 30 days. Stoma closure was achieved in 80% of patients with early complications vs 100% of patients with late complications. Among interventions performed for anastomotic failure, anastomotic takedown was performed in 6 patients followed by immediate (4) or delayed reconstruction (1). Anastomotic failure resulted in permanent colostomy in 2 patients.
Anastomotic complications were reported among 21.8% (12/55) of patients who underwent intraoperative FA perfusion assessment vs 13.3% (6/45) among those who did not, and among 17.4% (8/46) vs 44% (4/9) of patients with adequate vs inadequate perfusion (Table 6). By univariable cox proportional hazards regression analysis, male sex (HR 7.52 [0.99, 56.75]; p = 0.0502) and inadequate bowel perfusion by FA (HR 3.3 [0.96, 11.32]; p = 0.0572) were associated with higher risk of anastomotic complications, but neither variable reached statistical significance (Table 6).
Table 6.
Univariate cox regression analysis of risk factors for anastomotic leak
| No anastomotic complication n = 82 |
Anastomotic complication n = 18 |
Hazard ratio [95% CI] | p value | |
|---|---|---|---|---|
| Age | ||||
| < 65 | 63 (76.8%) | 17 (94.4%) | Reference | 0.1217 |
| ≥ 65 | 19 (23.2%) | 1 (5.6%) | 0.20 [0.02, 1.52] | |
| Sex | ||||
| Female | 29 (35.4%) | 1 (5.6%) | Reference | 0.0502 |
| Male | 53 (64.6%) | 17 (94.4%) | 7.52 [0.99, 56.75] | |
| BMI | ||||
| < 30 | 56 (68.3%) | 11 (61.1%) | Reference | 0.7926 |
| ≥ 30 | 26 (31.7%) | 7 (38.9%) | 1.13 [0.43, 2.96] | |
| ASA | ||||
| < 3 | 50 (61.0%) | 10 (55.6%) | Reference | 0.5137 |
| ≥ 3 | 32 (39.0%) | 8 (44.4%) | 1.37 [0.53, 3.56] | |
| Diabetes | ||||
| No | 73 (89.0%) | 16 (88.9%) | Reference | 0.8870 |
| Yes | 9 (11.0%) | 2 (11.1%) | 0.89 [0.20, 3.98] | |
| Smoking | ||||
| No | 75 (91.5%) | 18 (100.0%) | Reference | Not-Est |
| Yes | 7 (8.5%) | 0 (0.0%) | Not-Est | |
| Clinical AJCC stage | ||||
| I | 30 (36.6%) | 5 (27.8%) | Reference | 0.4438 |
| II | 16 (19.5%) | 6 (33.3%) | 1.62 [0.47, 5.61] | |
| III | 36 (43.9%) | 7 (38.9%) | 1.13 [0.35, 3.57] | 0.8335 |
| Distance from anal verge (cm) | ||||
| ≤ 6 cm | 56 (68.3%) | 11 (61.1%) | Reference | 0.3828 |
| > 6 cm | 26 (31.7%) | 7 (38.9%) | 1.54 [0.58, 4.07] | |
| Neoadjuvant treatment | ||||
| No neoadjuvant treatment | 24 (29.3%) | 5 (27.8%) | Reference | Non-Est |
| Chemotherapy alone | 2 (2.4%) | 0 (0.0%) | Non-Est | 0.7145 |
| Long course CRT | 42 (51.2%) | 9 (50.0%) | 0.81 [0.26, 2.50] | Non-Est |
| Short course RT | 1 (1.2%) | 0 (0.0%) | Non-Est | 0.4715 |
| Total neoadjuvant therapy | 13 (15.9%) | 4 (22.2%) | 1.62 [0.43, 6.05] | |
| Days from end of neoadjuvant RT to surgery, per week increase | 75.5 [68, 82] | 70 [61, 79] | 1.00 [0.90, 1.11] | 0.9850 |
| EBL, per 100 mL increase | 100.0 [0.0, 660.0] | 105.0 [50.0,700.0] | 1.45 [0.81, 2.61] | 0.2063 |
| Partial ISR or mucosectomy | ||||
| No | 50 (61.0%) | 12 (66.7%) | Reference | 0.4548 |
| Yes | 32 (39.0%) | 6 (33.3%) | 0.69 [0.25, 1.85] | |
| FA perfusion assessment | ||||
| No | 39 (47.6%) | 6 (33.3%) | Reference | 0.2126 |
| Yes | 43 (52.4%) | 12 (66.7%) | 1.87 [0.70, 5.01] | |
| FA perfusion assessment outcome | ||||
| Adequate | 38 (46.3%) | 8 (44.4%) | Reference | 0.0572 |
| Inadequate | 5 (11.6%) | 4 (33.3%) | 3.30 [0.96, 11.32] | |
| Type of anastomosis | 0.4928 | |||
| Stapled | 37 (45.1%) | 9 (50.0%) | Reference | |
| Handsewn | 45 (54.9%) | 9 (50.0%) | 0.71 [0.27,1.85] | |
| Size of stapler (among stapled anastomosis) | ||||
| 28 | 13 (35.1%) | 2 (22.2%) | Reference | |
| 29 | 9 (24.3%) | 5 (55.6%) | 3.04 [0.59, 15.72] | 0.1834 |
| 31 | 9 (24.3%) | 2 (22.2%) | 1.20 [0.16, 8.63] | 0.8563 |
| 33 | 6 (16.2%) | 0 (0.0%) | Non-Est | Non-Est |
| Splenic flexure takedown | ||||
| No | 5 (6.1%) | 1 (5.6%) | Reference | 0.9406 |
| Yes | 77 (93.9%) | 17 (94.4%) | 1.08 [0.14, 8.14] | |
| Final TME grade | ||||
| Complete | 58 (70.7%) | 11 (61.1%) | Reference | |
| Incomplete | 8 (9.8%) | 2 (11.1%) | 1.40 [0.31, 6.38] | 0.6565 |
| Near complete | 16 (19.5%) | 5 (27.8%) | 1.26 [0.42, 3.74] | 0.6696 |
| CRP > = 145 on POD3 | ||||
| No | 45 (80.4%) | 7 (63.6%) | Reference | 0.3022 |
| Yes | 11 (19.6%) | 4 (36.4%) | 1.91 [0.56, 6.57] |
Non-Est not estimable due to 0 patients with incomplete TME with level of risk factor; ISR intersphincteric resection; FA fluorescence angiography
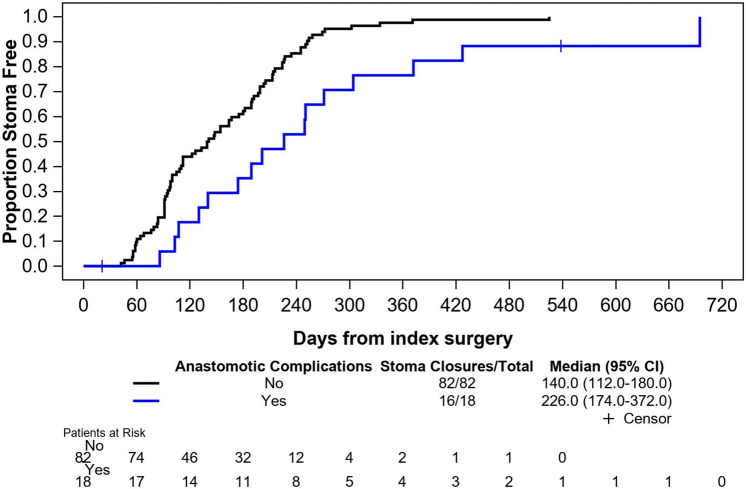
The overall stoma closure rate was 93% at 12 months and 98% at 23 months follow-up and median time to reversal was 154 (IQR 94–224) days (Table 4, Fig. 5). Stoma reversal rate was 89 vs 100% in patients with and without anastomotic complications, and median time to closure of 226 (IQR 140–304) days vs 140 (IQR 91–213) days, respectively (p = 0.0015). Factors contributing to delays in stoma reversal beyond 90 days were related to adjuvant treatment, anastomotic complications, COVID-related delays in elective surgery, patient preference, or other unclear reasons (Table 4).
Fig. 5.
Kaplan–Meier curve for time from taTME to stoma closure in patients with and without anastomotic complications (days)
Oncologic outcomes
Final pathologic staging demonstrated no residual disease (pT0N0) in 8 of 10 cases where completion taTME had been performed after polypectomy or local excision of a cT2 or cT1 tumor with high-risk histopathologic features. Complete pathologic response (pCR) was demonstrated in 25/71 (35.2%) of patients who received neoadjuvant treatment. The pCR rate was 41% (7/17) post TNT, 33% (17/51) post CRT, and 50% (1/2) post chemotherapy only (Table 7). The median number of harvested lymph nodes was 19 (IQR 14.5–26). Following reconciliation between site pathologists and central blinded reviewers, final TME grading was complete in 69%, near complete in 21%, and incomplete in 10% of specimens. There was no specimen perforation, but 1 case had positive CRM and 1 case had positive distal resection margin (DRM). After adjusting for the number of TME specimens with residual tumor, the positive margin rate was 3% (2/67). Adjuvant chemotherapy was completed in 46 patients, as recommended by MDT based on preoperative clinical stage or final pathology stage.
Table 7.
Pathology outcomes
| n = 100 patients | |
|---|---|
| TME grade | |
| Complete | 69 (69.0%) |
| Near complete | 21 (21.0%) |
| Incomplete | 10 (10.0%) |
| Pathology tumor stage | |
| pT0a/ypT0b | 8 (8.0%)/25 (25.0%) |
| pTis/ypTis | 0 (0.0%)/1 (1.0%) |
| pT1/ypT1 | 10 (10.0%)/11 (11.0%) |
| pT2/ypT2 | 8 (8.0%)/21 (21.0%) |
| pT3/ypT3 | 3 (3.0%)/13 (13.0%) |
| Pathology node stage | |
| pN0/ypN0 | 26 (26.0%)/56 (56.0%) |
| pN1/ypN1 | 2 (2.0%)/14 (14.0%) |
| pN2/ypN2 | 1 (1.0%)/1.0 (1.0%) |
| Tumor regression grade, among 71 treated tumors | |
| Grade 0 | 26 (36.6%) |
| Grade 1 | 22 (31.0%) |
| Grade 2 | 20 (28.2%) |
| Grade 3 | 3 (4.2%) |
| Lymphovascular invasion, among 67 residual tumors | |
| Absent | 51 (76.1%) |
| Present | 14 (20.9%) |
| Suspicious | 2 (3.0%) |
| Perineural invasion, among 67 residual tumors | |
| Absent | 53 (79.1%) |
| Present | 14 (20.9%) |
| Positive margin, among 67 residual tumors | |
| Any | 2 (3.0%) |
| DRM | 1 (1.5%) |
| CRM | 1 (1.5%) |
| Total number of nodes examined, median [IQR] | 19 [14.5, 26] |
| Adjuvant treatment | 46 (46.0%) |
aNo residual disease in patients who underwent previous local excision via TAMIS (n = 5) or polypectomy (n = 3)
bComplete pathological response to neoadjuvant therapy
TME total mesorectal excision; y, staging determined after neoadjuvant therapy; DRM distal resection margin; CRM circumferential resection margin
By univariable log-binomial regression analysis, ASA ≥ 3 (RR 13.50 [1.77, 102.47]; p = 0.0118), blood loss per 100 ml increase (RR 1.46 [1.14–1.87]; p = 0.0027), and longer time interval between end of neoadjuvant RT (NRT) to surgery (RR 1.07 [1.01, 1.14]; p = 0.0297, Table 8) were associated with a significant increase in the risk of incomplete TME (Table 8).
Table 8.
Univariate regression analysis of risk factors for incomplete TME
| Incomplete TME n = 10 | Complete/near-complete TME n = 90 | Relative risk [95% CI] |
p value | |
|---|---|---|---|---|
| Age | ||||
| < 65 | 7 (70.0%) | 73 (81.1%) | Reference | 0.4020 |
| ≥ 65 | 3 (30.0%) | 17 (18.9%) | 1.71 [0.48, 6.04] | |
| Sex | ||||
| Female | 3 (30.0%) | 27 (30.0%) | Reference | 1.0000 |
| Male | 7 (70.0%) | 63 (70.0%) | 1.00 [0.27, 3.60] | |
| BMI | ||||
| < 30 | 5 (50.0%) | 62 (68.9%) | Reference | 0.2344 |
| ≥ 30 | 5 (50.0%) | 28 (31.1%) | 2.03 [0.63, 6.52] | |
| ASA | ||||
| < 3 | 1 (10.0%) | 59 (65.6%) | Reference | 0.0118* |
| ≥ 3 | 9 (90.0%) | 31 (34.4%) | 13.50 [1.77, 102.47] | |
| Diabetic | ||||
| No | 9 (90.0%) | 80 (88.9%) | Reference | |
| Yes | 1 (10.0%) | 10 (11.1%) | 0.89 [0.12, 6.43] | 0.9156 |
| Smoking | ||||
| No | 10 (100.0%) | 83 (92.2%) | Reference | |
| Yes | 0 (0.0%) | 7 (7.8%) | 0.53 [0.02, 12.10] | 0.6908 |
| Clinical AJCC stage | ||||
| I | 2 (20.0%) | 33 (36.7%) | Reference | |
| II | 2 (20.0%) | 20 (22.2%) | 1.59 [0.24,10.48] | 0.6294 |
| III | 6 (60.0%) | 37 (41.1%) | 2.44 [0.52, 11.35] | 0.2549 |
| Distance from anal verge (cm) | ||||
| ≤ 6 cm | 6 (60%) | 61 (67.8%) | Reference | |
| > 6 cm | 4 (40%) | 29 (32.2%) | 1.35 [0.41, 4.47] | 0.6194 |
| Neoadjuvant treatment | ||||
| No neoadjuvant treatment | 2 (20.0%) | 27 (30.0%) | Reference | |
| Neoadjuvant treatment | 8 (80.0%) | 63 (70.0%) | 1.63 [0.37, 7.24] | 0.5179 |
| Neoadjuvant treatment | ||||
| No neoadjuvant treatment | 2 (20.0%) | 27 (30.0%) | Reference | |
| Long course CRT | 3 (30%) | 48 (53.3%) | 0.85 [0.15, 4.81] | 0.8570 |
| Chemotherapy alone | 0 (0%) | 2 (2.2%) | Non-Est | Non-Est |
| Short course RT | 0 (0%) | 1 (1.1%) | Non-Est | Non-Est |
| Total neoadjuvant therapy | 5 (50%) | 12 (13.3%) | 4.26 [0.93, 19.63] | 0.0626 |
| Days from end of neoadjuvant RT to surgery, per week increase | 85.5 [77, 147] | 74 [66, 80] | 1.07 [1.01, 1.14] | 0.0297* |
| EBL, per 100 mL increase | 200.0 [40.0, 700.0] | 100 [0.0, 660.0] | 1.46 [1.14, 1.87] | 0.0027* |
| Type of anastomosis | ||||
| Stapled | 5 (50.0%) | 41 (45.6%) | Reference | |
| Handsewn | 5 (50.0%) | 49 (54.4%) | 0.85 [0.26, 2.76] | 0.7892 |
| Pathology T stage | ||||
| (y)pT0 | 4 (40.0%) | 29 (32.2%) | Reference | |
| (y)pT1/Tis | 4 (40.0%) | 18 (20.0%) | 1.50 [0.42, 5.38] | 0.5336 |
| (y)pT2 | 1 (10.0%) | 28 (31.1%) | 0.28 [0.03, 2.40] | 0.2482 |
| (y)pT3 | 1 (10.0%) | 15 (16.7%) | 0.52 [0.06, 4.25] | 0.5381 |
| Pathology N stage | ||||
| (y)pN0 | 9 (90.0%) | 73 (81.1%) | Reference | |
| (y)pN1 | 1 (10.0%) | 15 (16.7%) | 0.57 [0.08, 4.19] | 0.5802 |
| (y)pN2 | 0 (0%) | 2 (2.2%) | Non-Est | |
| Positive margins in patients with residual tumor | ||||
| No | 6 (60.0%) | 59 (65.6%) | Reference | |
| Yes | 0 (0.0%) | 2 (2.2%) | 1.83 [0.04, 82.42] | 0.7554 |
| Partial ISR or mucosectomy | ||||
| No | 6 (60.0%) | 56 (62.2%) | Reference | |
| Yes | 4 (40.0%) | 34 (37.8%) | 1.08 [0.32, 3.60] | 0.8907 |
CI confidence interval; Non-Est not estimable due to 0 patients with incomplete TME with level of risk factor; ISR intersphincteric resection
*p < 0.05
Discussion
This phase II multicenter North American trial is the most recent large prospective trial to confirm the procedural safety, favorable pathologic outcomes, and low rates of conversion achieved with taTME when performed by experienced surgeons using a 2-team hybrid approach. The 2% abdominal open conversion rate is consistent with the 0–5.1% conversion rates reported in large taTME series of patients, and was achieved despite a predominantly male cohort with the highest proportion of patients with BMI ≥ 30 reported to date (33%) and in whom 67% of tumors were located ≤ 6 cm from the AV [14, 19, 22, 45–48]. The current trial results validate prior findings that combining taTME with a laparoscopic or robotic abdominal approach enables completion of minimally invasive sphincter-preserving TME, particularly in obese patients with low rectal tumors. Intraoperative complications related to transanal dissection are rare and included 2 vaginal injuries but no rectal perforation or urethral injury, which underscores the experience of participating surgeons [15].
The primary endpoint of the trial was mesorectal TME grade, an important pathologic endpoint and strong predictor of local recurrence and disease-free survival that also serves as an accurate marker of surgical quality [34, 49–51]. Complete and near-complete TME grade was achieved in 90% with a 10% rate of incomplete TME and 3% rate of positive margins. These results are in line with the 1.5–11% rates of incomplete TME reported in large retrospective taTME series [22, 46, 47, 52], and the 8% and 9.3% rates reported in the laparoscopic arm of the ACOSOG RCT and the robotic arm of the ROLAAR RCT, respectively [53, 54]. But this rate is significantly higher than the recently reported 0% rate of incomplete TME in the taTME arms of the 2 laparoscopic vs taTME RCT in China and Spain [55, 56]. Relative to the RCT by Liu et al. in which tumors were similarly located (5.0 vs 5.8 cm from the AV) in patients with higher BMI (22.9 vs 27.8), our higher rate of incomplete TME may reflect a more challenging dissection in a previously radiated field, given that 69% of our taTME cohort was treated with NRT vs only 14% of the Chinese cohort. Among 10 incomplete TME specimens in our trial, 8 occurred in patients treated with neoadjuvant long-course CRT or TNT, and for every 1 week increase in time from end of NRT to surgery, the risk of incomplete TME increased by 7%.
There is a paucity of data regarding the impact of delayed time to TME following completion of NRT on TME grade, and this has not been described in prior taTME series or trials. The 2015 TIMING phase II trial assigned patients with locally advanced tumors into 4 groups treated with CRT: CRT followed by 2, 4, or 6 cycles of mFOLFOX6, effectively increasing the interval to TME by 6 up to 20 weeks [57]. While surgeons reported an increase in pelvic fibrosis dissection with longer delays to surgery, they reported no differences in technical difficulty, surgical complications, R1 resection rate, or sphincter preservation. However, the authors did not analyze the impact of longer delays on TME grade. The UNICANCER-PRODIGE23 RCT comparing standard CRT to FOLFIRINOX followed by CRT for patients with locally advanced cancer, reported no difference in mesorectal grade between cohorts (5 vs 8% rate of incomplete TME). However, given that TNT was administered as induction treatment, there was no difference in the time interval between the end of NRT and surgery, which was relatively short in both groups (54.5 and 55 days, respectively) [58]. Given the increasing adoption of TNT worldwide in an effort to increase the rate of non-operative management, the impact of this delay on perioperative morbidity as well as TME quality will need to be further examined.
In the Spanish laparoscopic vs taTME RCT where a 0% rate of incomplete TME was reported in the taTME arm, a similar proportion of patients were treated with NRT (69.1% vs 69%) but the NRT-to-TME interval was not specified. The difference in rate of incomplete TME may reflect a higher level of technical difficulty during transanal dissection based on differences in median tumor height (8.0 cm vs 5.8 cm), and in the proportion of cases where ISR was performed (0% vs 36%) in the Spanish vs North American taTME cohorts, respectively [56]. In this phase II trial, ASA ≥ 3 and increased blood loss were identified as significant risk factors for incomplete TME, with increased blood loss generally expected during more difficult pelvic dissections. Lastly, higher rates of incomplete TME grading in this trial likely reflect more stringent quality control measures used for pathological specimen assessment. Concordance in TME grading between site pathologists and blinded central reviewers was evaluated, and major discrepancies in TME grading were resolved with final grade assigned based on consensus agreement. Neither of the recent taTME RCTs specifically incorporated quality control measures to validate TME grading. Importantly, Liu et al. in reporting a 0% rate of incomplete TME acknowledged that “an overestimation bias for successful resection still remains a certain possibility” [55].
Regarding other pathologic endpoints, our 1.5% positive CRM and 1.5% positive DRM rates compare favorably with the 1.4–12.7% CRM and 0–2.5% DRM-positive rates published in large taTME series [19, 22, 23, 59] and with the 0.9%/0%, and 0.4%/12.7% DRM-positive rates in the Liu et al. and Serra et al. taTME vs lap TME RCTs, respectively [55, 56]. TaTME was combined with ISR in 36% of patients whose tumors were located at a median of 4 cm from the AV in our North American trial, compared with 14.9% and 0% of the Chinese and Spanish taTME cohorts where median tumor height was 5 and 8 cm, from the AV, respectively [55, 56]. These results highlight the role that ISR will continue to play in facilitating sphincter preservation as well as R0 resection for low rectal tumors, without adverse impact on oncologic outcomes [59–64].
This prospective trial is unique in that it provided extended capture of early and late postoperative complications following taTME, including outcomes of all anastomoses until primary stoma closure or permanent stoma creation. Overall, 71 complications among 49 patients and 82 complications among 56 patients were reported at 30 and 90 days, respectively, which is relatively high relative to other taTME publications; but the majority were minor, with severe (CD ≥ 3) complications only reported in 14 and 17 patients at 30 and 90 days, respectively. Ileus and stoma-related dehydration accounted for 35% of 30-day complications and 47% of readmissions, and were a direct consequence of loop ileostomy creation. Although published rates of diversion following sphincter-preserving taTME vary from 55.7 to 100% [14, 21, 22, 55, 65], our trial reflects standard practice in North American centers where high-risk colorectal and all coloanal anastomoses are routinely diverted [22, 53]. Fecal diversion is particularly prevalent when ISR and handsewn anastomoses are performed, and in patients receiving extended courses of radiotherapy [13, 14, 66]. Urinary retention contributed to 27% of 30-day complications and occurred at a higher rate than the reported 2–13% incidence in other taTME series [9, 21, 67], which may be related to the timing of catheter removal. The impact of taTME procedures on long-term urinary and sexual functional will be reported in a later publication.
Anastomotic complications accounted for 10% of the trial 30-day morbidity. Given the lack of agreement with standardized definitions and diagnostic criteria for anastomotic leaks, we opted to report all anastomotic complications identified and treated throughout the trial clinical follow-up period. This broader framework for reporting early and late anastomotic complications, which also incorporates stoma closure and non-closure rates, has been used by other groups when reporting outcomes of restorative proctectomy from large colorectal cancer registries, clinical trials, institutional series, and the international taTME registry [13, 14, 68–72]. While the reported 30-day anastomotic leak rates range between 2 and 10% in most large TME and taTME series [9, 21, 53, 55, 56], these rates are not inclusive of pelvic abscess or phlegmon adjacent to anastomoses, nor of leaks or sequelae of leaks (sinus, fistula, stricture) identified beyond 30 days of surgery. In contrast, the TME registries and trials that intentionally report early and late-occurring anastomotic complications up to 1 year post TME, report rates as high as 20% [13, 68–71]. Using this anastomotic complication reporting framework, at a median follow-up of 5 (IQR 3–7) months, anastomotic complications were identified in 10% by 30 days, 15% by 90 days, and 18% by 349 days in our cohort. The 10% early leak rate is consistent with the 6.3–11% 30-day leak rates reported in large taTME series [20, 22, 24, 28, 38, 55, 59] and the 18% overall anastomotic complication rate is similar to the 15.7%-17.7% rates reported at 5–12 months post-taTME in the international registry and other retrospective series [13, 14]. Although the incidence of anastomotic complications was higher in male patients (24.3 vs 3.3%, p = 0.0502), this did not reach statistical significance in this study, but it has been identified as a risk factor in other taTME studies [21–23, 26, 38, 52, 59]. The role of intraoperative perfusion assessment on anastomotic failure had not been previously reported in the setting of a prospective phase II TME trial. In the current study, intraoperative fluorescence angiography (FA) was used at 4 of the 11 study sites, with the decision to use FA made on a case-by-case basis. Perfusion assessment was reported to have altered the operative plan in 14.5% of cases, which is consistent with 2 prior retrospective taTME series demonstrating a change in planned proximal resection margin in 18 and 28.7% of patients when FA was used [45, 73]. Despite active intervention to correct inadequate perfusion identified on FA, the incidence of anastomotic complications was higher in the FA group, which likely reflects selection bias with preferential use of FA in cases where ischemia was suspected. Although documentation of adequate bowel perfusion by FA was not entirely protective against anastomotic complications, it was associated with a lower risk of anastomotic complication, although this did not reach statistical significance.
This trial highlighted emerging trends in the management of anastomotic complications among North American rectal cancer practices, including the use of transanal rescue approaches to manage early symptomatic and asymptomatic leaks, and anastomotic revision with coloanal anastomosis for refractory anastomotic complications that failed initial interventions. Among early anastomotic complications, given that all anastomoses had been diverted, some leaks were diagnosed in patients who were otherwise asymptomatic. One leak was suspected based on CRP elevation and another diagnosed on CT scan with rectal contrast performed to evaluate for possible early ileostomy closure. Whether symptomatic or not, early leaks were managed within 30 days with transanal repair attempted in 75% of reoperations. Early re-intervention led to resolution and ileostomy reversal in 80% patients with ≤ 1 additional re-intervention. All anastomotic complications diagnosed > 30 days postoperatively were managed with 0–1 major surgical intervention, and stoma reversal was achieved in all cases. Overall, 2 patients with anastomotic complications were converted to permanent colostomy due to colonic conduit ischemia and refractory anastomotic dehiscence in the setting of disease progression, respectively. There was no mortality related to anastomotic complications and 89% stoma-free rate was achieved at 2 years in patients with anastomotic complications. The trial’s overall 93% 1-year and 98% 2-year stoma reversal rates are higher than those in other trials that report long-term outcomes of sphincter-preserving TME with or without a primary fecal diversion [74]. Among 595 patients in the 2015–2017 Dutch ColoRectal Audit (DCRA) that underwent sphincter-preserving TME, the 1-year stoma closure was 81.3 with 19.7% of patients requiring a permanent stoma [72]. In a meta-analysis that included 10 studies, the pooled rate of non-closure of diverting stomas following LAR for rectal cancer was 19% (95% CI 12–24%; p < 0.001) at follow-up ranging from 13 months to 7.1 years. Risks factors for non-closure included surgical complications, anastomotic leakage, stage IV tumors, and local recurrence [60]. Long-term stoma closure rates have been reported in 7 retrospective taTME series and large registries, and range from 56.5% at 4.73 months to 89.5% at 27 months [9, 21, 22, 28, 59, 61, 62, 65]. However, long-term stoma closure rates have not been previously reported in the context of a prospective rectal cancer trial. The high stoma closure rate in the current trial is most likely attributed to early management of symptomatic leaks, which helped minimize leak-related morbidity and mortality, as well as the aggressive management for refractory anastomotic defects and sequelae. Redo-coloanal anastomosis was performed in 3 cases with refractory anastomotic dehiscence or stricture 7–18 months post-taTME with successful ileostomy reversal in all 3 cases. Delayed anastomotic reconstruction for failed anastomoses has been reported in very few small TME series [63, 64, 75]. In a recent systematic review of 9 studies and 291 patients, redo-anastomosis was performed at a median 14–41 months post the index procedure, with a 79% rate of stoma closure and 16% morbidity rate [64, 75].
A limitation of the trial concerns strict inclusion and exclusion criteria for enrollment. Similar to the two recent RCT’s of lap vs taTME, cT3b tumors with predicted positive CRM, sphincter invasion or cT4 tumors on preoperative staging were excluded, even when downstaging was achieved with neoadjuvant treatment and restaging. While this was necessary in order to establish the feasibility and safety of taTME on a homogenous cohort of resectable cancer cases, our cohort does not entirely reflect current practice where taTME has become the preferred approach for tumors with incomplete response or tumor regrowth following neoadjuvant treatment. While ongoing RCTs will validate the long-term oncologic safety of taTME and evaluate functional results relative to laparoscopic TME, it will become important to understand the role that taTME plays in contemporary rectal cancer practices in the era of robotic surgery, TNT and an increased demand for sphincter preservation among young patients.
Conclusions
In this prospective phase II multicenter North American trial, the procedural and preliminary oncologic safety of taTME was demonstrated. In a predominantly male cohort with low rectal tumors and median BMI 27.8, a 2-team taTME approach achieved acceptable rates of complete and near-complete mesorectal grade with a low rate of open conversion. Continued monitoring and management of anastomotic complications resulted in high stoma closure rates.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to acknowledge the following collaborators for their contribution to this trial: Bachir Taouli MD, Peter Kozuch MD, Michael Buckstein MD, Elisabeth Hain MD, Nick West MBChB, PhD, FRCPath, FHEA, Dan Popowich MD, Joseph Martz MD, Jennifer Davids MD, Paul Sturrock MD, Tess H Aulet MD, Fayez Quereshy MD, Raj Vajpeyi MD, Urooq Taqvi MSc, and Taariq Shaik MBA.
Funding
This trial was funded by a Research Grant from the American Society of Colon and Rectal Surgery (ASCRS) Research Foundation. The Society of Gastrointestinal and Endoscopic Surgeons (SAGES) provided administrative support for the trial. Additional funding was received by the ASCRS Research Foundation in support of the trial from Applied Medical, and by SAGES from Karl Storz, Richard Wolf, Ethicon, Medtronic, Cooper Surgical, Stryker, Novadaq, Conmed, Olympus, and Intuitive.
Declarations
Disclosures
Dr. Patricia Sylla reports consultant payments from Ethicon, Medtronic, Safeheal, Olympus, Stryker, RedDress, and GI windows. Dr. Alexandros Polydorides reports royalties or licenses with Elsevier, Med Learning Group, payment or honoraria from Johns Hopkins and USCAP, and payment for various expert testimony. Dr. John Marks serves as a consultant for Stryker and Intuitive, reports payment or honoraria from Stryker and Medtronic, and participates on the data safety monitoring board (DSMB) or advisory board for Virtual Incision. Dr. Elisabeth McLemore serves as a chair on the SAGES Colorectal Surgery Committee. Dr. Justin Maykel reports consultant fees for Olympus, Takeda, Safeheal, RedDress, and Applied Medical, and payment for expert testimony for medical legal review. Dr. Karim Alavi reports consultant fees for Safeheal and Olympus, payment for expert testimony for Crico, and serves on the DCR editorial board and as an Operative Competency Committee Chair of ASCRS. Dr. Karen Zaghiyan reports grants received by Surgical Research Network and Crohn’s & Colitis Foundation, consultant fees for Takeda and Intuitive, and payment or honoraria from Natera. Dr. Sami Chadi serves as a consultant for Stryker and Noah Medical and receives payment or honoraria from Stryker. Dr. Alessio Pigazzi reports receiving a Research grant from Vioptix, royalties with Xodus Medical, and serves as a consultant for Medtronic, Ethicon, and Intuitive. Dr. Matt Albert reports grants or contracts from Applied Medical, Stryker Endoscopy, Conmed, Human Extensions, Endoquest, Livsmed, Astellas Pharmaceuticals, Distal Motion, Proximie, and Noah, serves as a consultant and receives payment or honoraria from Applied Medical, Stryker, Distal Motion, EndoQuest, Astellas Pharmaceuticals, Livsmed, and ConMed, participates on the DSMB or Advisory board for Proximie and Distal Motion, and holds stock or stock options for Applied Medical, Proximie, and Human Extension. Dr. Teresa deBeche-Adams receives personal honoraria for educational courses with support for travel expenses from Applied Medical. Erin Moshier reports a grant received for biostatistical support by the National Cancer Institute Cancer Center Support Grant P30CA196521-01, which was awarded to the Tisch Cancer Institute of the Icahn School of Medicine at Mount Sinai. Dr. Steven Wexner receives consulting fees from ARC/Corvus, Baxter, Becton Dickinson, GI Supply, ICON Clinical Research Limited, Intuitive Surgical, Leading BioSciences, Leading Biosciences/Pallisade Bio, Livsmed, Medtronic, Olympus, Stryker, and Takeda and receives royalties from Intuitive Surgical, Karl Storz Endoscopy America Inc, Medtronic, and Unique Surgical Innovations LLC. Dr. Dana Sands, Dr. Alison Ricardo, Dr. Antoinette Bonaccorso, Dr. Mariana Berho, Dr. Mark Whiteford, Dr. Sherief Shawki, and Dr. Scott Steele have no financial disclosures to report.
Footnotes
Abstract presented at the Society of Gastrointestinal and Endoscopic Surgeons Annual Meeting in March 2023, Montréal, Canada, Program Number S191.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fleshman J, Branda ME, Sargent DJ, Boller AM, George VV, Abbas MA, Peters WR, Jr, Maun DC, Chang GJ, Herline A, Fichera A, Mutch MG, Wexner SD, Whiteford MH, Marks J, Birnbaum E, Margolin DA, Larson DW, Marcello PW, Posner MC, Read TE, Monson JRT, Wren SM, Pisters PWT, Nelson H. Disease-free survival and local recurrence for laparoscopic resection compared with open resection of stage II to III rectal cancer: follow-up results of the ACOSOG Z6051 randomized controlled trial. Ann Surg. 2019;269:589–595. doi: 10.1097/SLA.0000000000003002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fukui Y, Hida K, Hoshino N, Song SH, Park SY, Choi GS, Maeda Y, Matoba S, Kuroyanagi H, Bae SU, Jeong WK, Baek SK, Sakai Y. Oncologic benefit of adjuvant chemotherapy for locally advanced rectal cancer after neoadjuvant chemoradiotherapy and curative surgery with selective lateral pelvic lymph node dissection: an international retrospective cohort study. Eur J Surg Oncol. 2022;48:1631–1637. doi: 10.1016/j.ejso.2022.01.030. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y, Sun Y, Xu Z, Chi P, Lu X. Is neoadjuvant chemoradiotherapy always necessary for mid/high local advanced rectal cancer: a comparative analysis after propensity score matching. Eur J Surg Oncol. 2017;43:1440–1446. doi: 10.1016/j.ejso.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 4.Saadoun JE, Meillat H, Zemmour C, Brunelle S, Lapeyre A, de Chaisemartin C, Lelong B. Nomogram to predict disease recurrence in patients with locally advanced rectal cancer undergoing rectal surgery after neoadjuvant therapy: retrospective cohort study. BJS Open. 2022 doi: 10.1093/bjsopen/zrac138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park JK, Kim YW, Hur H, Kim NK, Min BS, Sohn SK, Choi YD, Kim YT, Ahn JB, Roh JK, Keum KC, Seong JS. Prognostic factors affecting oncologic outcomes in patients with locally recurrent rectal cancer: impact of patterns of pelvic recurrence on curative resection. Langenbecks Arch Surg. 2009;394:71–77. doi: 10.1007/s00423-008-0391-6. [DOI] [PubMed] [Google Scholar]
- 6.Bonjer HJ, Deijen CL, Abis GA, Cuesta MA, van der Pas MH, de Lange-de Klerk ES, Lacy AM, Bemelman WA, Andersson J, Angenete E, Rosenberg J, Fuerst A, Haglind E. A randomized trial of laparoscopic versus open surgery for rectal cancer. N Engl J Med. 2015;372:1324–1332. doi: 10.1056/NEJMoa1414882. [DOI] [PubMed] [Google Scholar]
- 7.Stevenson ARL, Solomon MJ, Brown CSB, Lumley JW, Hewett P, Clouston AD, Gebski VJ, Wilson K, Hague W, Simes J. Disease-free survival and local recurrence after laparoscopic-assisted resection or open resection for rectal cancer: the Australasian laparoscopic cancer of the rectum randomized clinical trial. Ann Surg. 2019;269:596–602. doi: 10.1097/SLA.0000000000003021. [DOI] [PubMed] [Google Scholar]
- 8.Sylla P, Rattner DW, Delgado S, Lacy AM. NOTES transanal rectal cancer resection using transanal endoscopic microsurgery and laparoscopic assistance. Surg Endosc. 2010;24:1205–1210. doi: 10.1007/s00464-010-0965-6. [DOI] [PubMed] [Google Scholar]
- 9.Lacy AM, Tasende MM, Delgado S, Fernandez-Hevia M, Jimenez M, De Lacy B, Castells A, Bravo R, Wexner SD, Heald RJ. Transanal total mesorectal excision for rectal cancer: outcomes after 140 patients. J Am Coll Surg. 2015;221:415–423. doi: 10.1016/j.jamcollsurg.2015.03.046. [DOI] [PubMed] [Google Scholar]
- 10.Veltcamp Helbach M, Deijen CL, Velthuis S, Bonjer HJ, Tuynman JB, Sietses C. Transanal total mesorectal excision for rectal carcinoma: short-term outcomes and experience after 80 cases. Surg Endosc. 2016;30:464–470. doi: 10.1007/s00464-015-4221-y. [DOI] [PubMed] [Google Scholar]
- 11.Rasulov AO, Mamedli ZZ, Gordeyev SS, Kozlov NA, Dzhumabaev HE. Short-term outcomes after transanal and laparoscopic total mesorectal excision for rectal cancer. Tech Coloproctol. 2016;20:227–234. doi: 10.1007/s10151-015-1421-3. [DOI] [PubMed] [Google Scholar]
- 12.Rutgers ML, Detering R, Roodbeen SX, Crolla RM, Dekker JWT, Tuynman JB, Sietses C, Bemelman WA, Tanis PJ, Hompes R. Influence of minimally invasive resection technique on sphincter preservation and short-term outcome in low rectal cancer in the Netherlands. Dis Colon Rectum. 2021;64:1488–1500. doi: 10.1097/DCR.0000000000001906. [DOI] [PubMed] [Google Scholar]
- 13.Hol JC, Bakker F, van Heek NT, de Jong GM, Kruyt FM, Sietses C. Morbidity and costs of diverting ileostomy in transanal total mesorectal excision with primary anastomosis for rectal cancer. Tech Coloproctol. 2021;25:1133–1141. doi: 10.1007/s10151-021-02498-5. [DOI] [PubMed] [Google Scholar]
- 14.Penna M, Hompes R, Arnold S, Wynn G, Austin R, Warusavitarne J, Moran B, Hanna GB, Mortensen NJ, Tekkis PP. Transanal total mesorectal excision: international registry results of the first 720 cases. Ann Surg. 2017;266:111–117. doi: 10.1097/SLA.0000000000001948. [DOI] [PubMed] [Google Scholar]
- 15.Sylla P, Knol JJ, D'Andrea AP, Perez RO, Atallah SB, Penna M, Hompes R, Wolthuis A, Rouanet P, Fingerhut A. Urethral injury and other urologic injuries during transanal total mesorectal excision: an international collaborative study. Ann Surg. 2021;274:e115–e125. doi: 10.1097/SLA.0000000000003597. [DOI] [PubMed] [Google Scholar]
- 16.Harnsberger CR, Alavi K, Davids JS, Sturrock PR, Zayaruzny M, Maykel JA. CO(2) embolism can complicate transanal total mesorectal excision. Tech Coloproctol. 2018;22:881–885. doi: 10.1007/s10151-018-1897-8. [DOI] [PubMed] [Google Scholar]
- 17.Rouanet P, Mourregot A, Azar CC, Carrere S, Gutowski M, Quenet F, Saint-Aubert B, Colombo PE. Transanal endoscopic proctectomy: an innovative procedure for difficult resection of rectal tumors in men with narrow pelvis. Dis Colon Rectum. 2013;56:408–415. doi: 10.1097/DCR.0b013e3182756fa0. [DOI] [PubMed] [Google Scholar]
- 18.Simo V, Tejedor P, Jimenez LM, Hernan C, Zorilla J, Arrredondo J, Lapuente F, Pastor C. Oncological safety of transanal total mesorectal excision (TaTME) for rectal cancer: mid-term results of a prospective multicentre study. Surg Endosc. 2021;35:1808–1819. doi: 10.1007/s00464-020-07579-4. [DOI] [PubMed] [Google Scholar]
- 19.Caycedo-Marulanda A, Lee L, Chadi SA, Verschoor CP, Crosina J, Ashamalla S, Brown CJ. Association of transanal total mesorectal excision with local recurrence of rectal cancer. JAMA Netw Open. 2021;4:e2036330. doi: 10.1001/jamanetworkopen.2020.36330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hol JC, van Oostendorp SE, Tuynman JB, Sietses C. Long-term oncological results after transanal total mesorectal excision for rectal carcinoma. Tech Coloproctol. 2019;23:903–911. doi: 10.1007/s10151-019-02094-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gloor S, Pozza G, Troller R, Wehrli M, Adamina M. Surgical outcomes, long-term recurrence rate, and resource utilization in a prospective cohort of 165 patients treated by transanal total mesorectal excision for distal rectal cancer. Cancers (Basel) 2023 doi: 10.3390/cancers15041190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perdawood SK, Kroeigaard J, Eriksen M, Mortensen P. Transanal total mesorectal excision: the Slagelse experience 2013–2019. Surg Endosc. 2021;35:826–836. doi: 10.1007/s00464-020-07454-2. [DOI] [PubMed] [Google Scholar]
- 23.de Lacy FB, Roodbeen SX, Ríos J, van Laarhoven J, Otero-Piñeiro A, Bravo R, Visser T, van Poppel R, Valverde S, Hompes R, Sietses C, Castells A, Bemelman WA, Tanis PJ, Lacy AM. Three-year outcome after transanal versus laparoscopic total mesorectal excision in locally advanced rectal cancer: a multicenter comparative analysis. BMC Cancer. 2020;20:677. doi: 10.1186/s12885-020-07171-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lau S, Kong J, Bell S, Heriot A, Stevenson A, Moloney J, Hayes J, Merrie A, Eglinton T, Guest G, Clark D, Warrier S. Transanal mesorectal excision: early outcomes in Australia and New Zealand. Br J Surg. 2021;108:214–219. doi: 10.1093/bjs/znaa098. [DOI] [PubMed] [Google Scholar]
- 25.Zeng Z, Liu Z, Luo S, Liang Z, Huang L, Ruan L, Chen J, Jie H, Liang W, Liu H, Kang L. Three-year outcomes of transanal total mesorectal excision versus standard laparoscopic total mesorectal excision for mid and low rectal cancer. Surg Endosc. 2022;36:3902–3910. doi: 10.1007/s00464-021-08707-4. [DOI] [PubMed] [Google Scholar]
- 26.Kang L, Chen YG, Zhang H, Zhang HY, Lin GL, Yang YC, Chen WH, Luo SL, Chen N, Tong WD, Shen ZL, Xiong DH, Xiao Y, Zhang ZT, Wang JP. Transanal total mesorectal excision for rectal cancer: a multicentric cohort study. Gastroenterol Rep (Oxf) 2020;8:36–41. doi: 10.1093/gastro/goz049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.González-Abós C, de Lacy FB, Guzmán Y, Nogueira ST, Otero-Piñeiro A, Almenara R, Lacy AM. Transanal total mesorectal excision for stage II or III rectal cancer: pattern of local recurrence in a tertiary referral center. Surg Endosc. 2021;35:7191–7199. doi: 10.1007/s00464-020-08200-4. [DOI] [PubMed] [Google Scholar]
- 28.Wasmuth HH, Faerden AE, Myklebust T, Pfeffer F, Norderval S, Riis R, Olsen OC, Lambrecht JR, Kørner H, Larsen SG, Forsmo HM, Baekkelund O, Lavik S, Knapp JC, Sjo O, Rashid G. Transanal total mesorectal excision for rectal cancer has been suspended in Norway. Br J Surg. 2020;107:121–130. doi: 10.1002/bjs.11459. [DOI] [PubMed] [Google Scholar]
- 29.Deijen CL, Velthuis S, Tsai A, Mavroveli S, de Lange-de Klerk ES, Sietses C, Tuynman JB, Lacy AM, Hanna GB, Bonjer HJ. COLOR III: a multicentre randomised clinical trial comparing transanal TME versus laparoscopic TME for mid and low rectal cancer. Surg Endosc. 2016;30:3210–3215. doi: 10.1007/s00464-015-4615-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lelong B, de Chaisemartin C, Meillat H, Cournier S, Boher JM, Genre D, Karoui M, Tuech JJ, Delpero JR. A multicentre randomised controlled trial to evaluate the efficacy, morbidity and functional outcome of endoscopic transanal proctectomy versus laparoscopic proctectomy for low-lying rectal cancer (ETAP-GRECCAR 11 TRIAL): rationale and design. BMC Cancer. 2017;17:253. doi: 10.1186/s12885-017-3200-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, Poole C, Schlesselman JJ, Egger M. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. PLoS Med. 2007;4:e297. doi: 10.1371/journal.pmed.0040297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.American College of Surgeons (n.d.) The National Accreditation Program for Rectal Cancer Standards Manual. https://www.facs.org/quality-programs/cancer-programs/national-accreditation-program-for-rectal-cancer/standards-and-resources/
- 33.Washington MK, Berlin J, Branton P, Burgart LJ, Carter DK, Fitzgibbons PL, Halling K, Frankel W, Jessup J, Kakar S, Minsky B, Nakhleh R, Compton CC. Protocol for the examination of specimens from patients with primary carcinoma of the colon and rectum. Arch Pathol Lab Med. 2009;133:1539–1551. doi: 10.5858/133.10.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nagtegaal ID, van de Velde CJ, van der Worp E, Kapiteijn E, Quirke P, van Krieken JH. Macroscopic evaluation of rectal cancer resection specimen: clinical significance of the pathologist in quality control. J Clin Oncol. 2002;20:1729–1734. doi: 10.1200/JCO.2002.07.010. [DOI] [PubMed] [Google Scholar]
- 35.Campa-Thompson M, Weir R, Calcetera N, Quirke P, Carmack S. Pathologic processing of the total mesorectal excision. Clin Colon Rectal Surg. 2015;28:43–52. doi: 10.1055/s-0035-1545069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rahbari NN, Weitz J, Hohenberger W, Heald RJ, Moran B, Ulrich A, Holm T, Wong WD, Tiret E, Moriya Y, Laurberg S, den Dulk M, van de Velde C, Büchler MW. Definition and grading of anastomotic leakage following anterior resection of the rectum: a proposal by the International Study Group of Rectal Cancer. Surgery. 2010;147:339–351. doi: 10.1016/j.surg.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 38.Chernyshov S, Alexeev M, Rybakov E, Tarasov M, Shelygin Y, Zarodniuk I, Sukhina M. Risk factors and inflammatory predictors for anastomotic leakage following total mesorectal excision with defunctioning stoma. Pol Przegl Chir. 2018;90:31–36. doi: 10.5604/01.3001.0011.8169. [DOI] [PubMed] [Google Scholar]
- 39.Bakx R, Sprangers MA, Oort FJ, van Tets WF, Bemelman WA, Slors JF, van Lanschot JJ. Development and validation of a colorectal functional outcome questionnaire. Int J Colorectal Dis. 2005;20:126–136. doi: 10.1007/s00384-004-0638-9. [DOI] [PubMed] [Google Scholar]
- 40.Rockwood TH, Church JM, Fleshman JW, Kane RL, Mavrantonis C, Thorson AG, Wexner SD, Bliss D, Lowry AC. Fecal incontinence quality of life scale: quality of life instrument for patients with fecal incontinence. Dis Colon Rectum. 2000;43:9–16. doi: 10.1007/BF02237236. [DOI] [PubMed] [Google Scholar]
- 41.Rockwood TH, Church JM, Fleshman JW, Kane RL, Mavrantonis C, Thorson AG, Wexner SD, Bliss D, Lowry AC. Patient and surgeon ranking of the severity of symptoms associated with fecal incontinence: the fecal incontinence severity index. Dis Colon Rectum. 1999;42:1525–1532. doi: 10.1007/BF02236199. [DOI] [PubMed] [Google Scholar]
- 42.Rosen R, Brown C, Heiman J, Leiblum S, Meston C, Shabsigh R, Ferguson D, D'Agostino R., Jr The female sexual function index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26:191–208. doi: 10.1080/009262300278597. [DOI] [PubMed] [Google Scholar]
- 43.Rosen RC, Cappelleri JC, Gendrano N., 3rd The international index of erectile function (IIEF): a state-of-the-science review. Int J Impot Res. 2002;14:226–244. doi: 10.1038/sj.ijir.3900857. [DOI] [PubMed] [Google Scholar]
- 44.Barry MJ, Fowler FJ, Jr, O'Leary MP, Bruskewitz RC, Holtgrewe HL, Mebust WK, Cockett AT. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148:1549–1557. doi: 10.1016/S0022-5347(17)36966-5. [DOI] [PubMed] [Google Scholar]
- 45.Otero-Piñeiro AM, de Lacy FB, Van Laarhoven JJ, Martín-Perez B, Valverde S, Bravo R, Lacy AM. The impact of fluorescence angiography on anastomotic leak rate following transanal total mesorectal excision for rectal cancer: a comparative study. Surg Endosc. 2021;35:754–762. doi: 10.1007/s00464-020-07442-6. [DOI] [PubMed] [Google Scholar]
- 46.Roodbeen SX, Penna M, van Dieren S, Moran B, Tekkis P, Tanis PJ, Hompes R. Local recurrence and disease-free survival after transanal total mesorectal excision: results from the International TaTME registry. J Natl Compr Canc Netw. 2021;19:1232–1240. doi: 10.6004/jnccn.2021.7012. [DOI] [PubMed] [Google Scholar]
- 47.Roodbeen SX, Spinelli A, Bemelman WA, Di Candido F, Cardepont M, Denost Q, D'Hoore A, Houben B, Knol JJ, Martín-Pérez B, Rullier E, Sands D, Setton I, Van de Steen K, Tanis PJ, Wexner SD, Hompes R, Wolthuis AM. Local recurrence after transanal total mesorectal excision for rectal cancer: a multicenter cohort study. Ann Surg. 2021;274:359–366. doi: 10.1097/SLA.0000000000003757. [DOI] [PubMed] [Google Scholar]
- 48.Yao H, An Y, Zhang H, Ren M, Chen CC, Xu Q, Wang Q, Zhang Z. Transanal total mesorectal excision: short-term outcomes of 1283 cases from a nationwide registry in China. Dis Colon Rectum. 2021;64:190–199. doi: 10.1097/DCR.0000000000001820. [DOI] [PubMed] [Google Scholar]
- 49.Nagtegaal ID, Quirke P. What is the role for the circumferential margin in the modern treatment of rectal cancer? J Clin Oncol. 2008;26:303–312. doi: 10.1200/JCO.2007.12.7027. [DOI] [PubMed] [Google Scholar]
- 50.Quirke P, Steele R, Monson J, Grieve R, Khanna S, Couture J, O'Callaghan C, Myint AS, Bessell E, Thompson LC, Parmar M, Stephens RJ, Sebag-Montefiore D. Effect of the plane of surgery achieved on local recurrence in patients with operable rectal cancer: a prospective study using data from the MRC CR07 and NCIC-CTG CO16 randomised clinical trial. Lancet. 2009;373:821–828. doi: 10.1016/S0140-6736(09)60485-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maslekar S, Sharma A, Macdonald A, Gunn J, Monson JR, Hartley JE. Mesorectal grades predict recurrences after curative resection for rectal cancer. Dis Colon Rectum. 2007;50:168–175. doi: 10.1007/s10350-006-0756-2. [DOI] [PubMed] [Google Scholar]
- 52.Xu F, Li H, Guo C, Yang Z, Gao J, Zhang X, Wei Q, Meng C, Sun L, Wu G, Yao H, Zhang Z. Incidence and risk factors of surgical complications and anastomotic leakage after transanal total mesorectal excision for middle and low rectal cancer. J Gastrointest Surg. 2023;27:373–381. doi: 10.1007/s11605-022-05546-z. [DOI] [PubMed] [Google Scholar]
- 53.Fleshman J, Branda M, Sargent DJ, Boller AM, George V, Abbas M, Peters WR, Jr, Maun D, Chang G, Herline A, Fichera A, Mutch M, Wexner S, Whiteford M, Marks J, Birnbaum E, Margolin D, Larson D, Marcello P, Posner M, Read T, Monson J, Wren SM, Pisters PW, Nelson H. Effect of laparoscopic-assisted resection vs open resection of stage II or III rectal cancer on pathologic outcomes: the ACOSOG Z6051 randomized clinical trial. JAMA. 2015;314:1346–1355. doi: 10.1001/jama.2015.10529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jayne D, Pigazzi A, Marshall H, Croft J, Corrigan N, Copeland J, Quirke P, West N, Rautio T, Thomassen N, Tilney H, Gudgeon M, Bianchi PP, Edlin R, Hulme C, Brown J. Effect of robotic-assisted vs conventional laparoscopic surgery on risk of conversion to open laparotomy among patients undergoing resection for rectal cancer: the ROLARR randomized clinical trial. JAMA. 2017;318:1569–1580. doi: 10.1001/jama.2017.7219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu H, Zeng Z, Zhang H, Wu M, Ma D, Wang Q, Xie M, Xu Q, Ouyang J, Xiao Y, Song Y, Feng B, Xu Q, Wang Y, Zhang Y, Hao Y, Luo S, Zhang X, Yang Z, Peng J, Wu X, Ren D, Huang M, Lan P, Tong W, Ren M, Wang J, Kang L. Morbidity, mortality, and pathologic outcomes of transanal versus laparoscopic total mesorectal excision for rectal cancer short-term outcomes from a multicenter randomized controlled trial. Ann Surg. 2023;277:1–6. doi: 10.1097/SLA.0000000000005523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Serra-Aracil X, Zarate A, Bargalló J, Gonzalez A, Serracant A, Roura J, Delgado S, Mora-López L. Transanal versus laparoscopic total mesorectal excision for mid and low rectal cancer (Ta-LaTME study): multicentre, randomized, open-label trial. Br J Surg. 2023;110:150–158. doi: 10.1093/bjs/znac324. [DOI] [PubMed] [Google Scholar]
- 57.Garcia-Aguilar J, Chow OS, Smith DD, Marcet JE, Cataldo PA, Varma MG, Kumar AS, Oommen S, Coutsoftides T, Hunt SR, Stamos MJ, Ternent CA, Herzig DO, Fichera A, Polite BN, Dietz DW, Patil S, Avila K. Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: a multicentre, phase 2 trial. Lancet Oncol. 2015;16:957–966. doi: 10.1016/S1470-2045(15)00004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Conroy T, Bosset JF, Etienne PL, Rio E, François É, Mesgouez-Nebout N, Vendrely V, Artignan X, Bouché O, Gargot D, Boige V, Bonichon-Lamichhane N, Louvet C, Morand C, de la Fouchardière C, Lamfichekh N, Juzyna B, Jouffroy-Zeller C, Rullier E, Marchal F, Gourgou S, Castan F, Borg C. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:702–715. doi: 10.1016/S1470-2045(21)00079-6. [DOI] [PubMed] [Google Scholar]
- 59.Penna M, Hompes R, Arnold S, Wynn G, Austin R, Warusavitarne J, Moran B, Hanna GB, Mortensen NJ, Tekkis PP. Incidence and risk factors for anastomotic failure in 1594 patients treated by transanal total mesorectal excision: results from the International TaTME Registry. Ann Surg. 2019;269:700–711. doi: 10.1097/SLA.0000000000002653. [DOI] [PubMed] [Google Scholar]
- 60.Zhou X, Wang B, Li F, Wang J, Fu W. Risk factors associated with nonclosure of defunctioning stomas after sphincter-preserving low anterior resection of rectal cancer: a meta-analysis. Dis Colon Rectum. 2017;60:544–554. doi: 10.1097/DCR.0000000000000819. [DOI] [PubMed] [Google Scholar]
- 61.Van Oostendorp SE, Belgers HJE, Hol JC, Doornebosch PG, Belt EJT, Oosterling SJ, Kusters M, Bonjer HJJ, Sietses C, Tuynman JB. The learning curve of transanal total mesorectal excision for rectal cancer is associated with local recurrence: results from a multicentre external audit. Colorectal Dis. 2021;23:2020–2029. doi: 10.1111/codi.15722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hallam S, Ahmed F, Gouvas N, Pandey S, Nicol D. Oncological outcomes and stoma-free survival following TaTME, a prospective cohort study. Tech Coloproctol. 2021;25:439–447. doi: 10.1007/s10151-020-02390-8. [DOI] [PubMed] [Google Scholar]
- 63.Calmels M, Collard MK, O'Connell L, Voron T, Debove C, Chafai N, Parc Y, Lefevre JH. Redo-surgery after failed colorectal or coloanal anastomosis: Morbidity, mortality and factors predictive of success. A retrospective study of 200 patients. Colorectal Dis. 2022;24:511–519. doi: 10.1111/codi.16025. [DOI] [PubMed] [Google Scholar]
- 64.Westerduin E, Klaver CEL, van Geloven AAW, Westerterp M, Bemelman WA, Tanis PJ. Outcome after redo surgery for complicated colorectal and coloanal anastomosis: a systematic review. Dis Colon Rectum. 2018;61:988–998. doi: 10.1097/DCR.0000000000001129. [DOI] [PubMed] [Google Scholar]
- 65.Hol JC, Burghgraef TA, Rutgers MLW, Crolla R, van Geloven NAW, Hompes R, Leijtens JWA, Polat F, Pronk A, Smits AB, Tuynman JB, Verdaasdonk EGG, Consten ECJ, Sietses C. Comparison of laparoscopic versus robot-assisted versus transanal total mesorectal excision surgery for rectal cancer: a retrospective propensity score-matched cohort study of short-term outcomes. Br J Surg. 2021;108:1380–1387. doi: 10.1093/bjs/znab233. [DOI] [PubMed] [Google Scholar]
- 66.Talboom K, Vogel I, Blok RD, Roodbeen SX, Ponsioen CY, Bemelman WA, Hompes R, Tanis PJ. Highly selective diversion with proactive leakage management after low anterior resection for rectal cancer. Br J Surg. 2021;108:609–612. doi: 10.1093/bjs/znab018. [DOI] [PubMed] [Google Scholar]
- 67.Kagami S, Funahashi K, Koda T, Ushigome T, Kaneko T, Suzuki T, Miura Y, Nagashima Y, Yoshida K, Kurihara A. Transanal down-to-up dissection of the distal rectum as a viable approach to achieve total mesorectal excision in laparoscopic sphincter-preserving surgery for rectal cancer near the anus: a study of short- and long-term outcomes of 123 consecutive patients from a single Japanese institution. World J Surg Oncol. 2022;20:363. doi: 10.1186/s12957-022-02826-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Matthiessen P, Hallböök O, Rutegård J, Simert G, Sjödahl R. Defunctioning stoma reduces symptomatic anastomotic leakage after low anterior resection of the rectum for cancer: a randomized multicenter trial. Ann Surg. 2007;246:207–214. doi: 10.1097/SLA.0b013e3180603024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jutesten H, Draus J, Frey J, Neovius G, Lindmark G, Buchwald P, Lydrup ML. Late leakage after anterior resection: a defunctioning stoma alters the clinical course of anastomotic leakage. Colorectal Dis. 2018;20:150–159. doi: 10.1111/codi.13914. [DOI] [PubMed] [Google Scholar]
- 70.Borstlap WAA, Westerduin E, Aukema TS, Bemelman WA, Tanis PJ. Anastomotic leakage and chronic presacral sinus formation after low anterior resection: results from a large cross-sectional study. Ann Surg. 2017;266:870–877. doi: 10.1097/SLA.0000000000002429. [DOI] [PubMed] [Google Scholar]
- 71.Blok RD, Stam R, Westerduin E, Borstlap WAA, Hompes R, Bemelman WA, Tanis PJ. Impact of an institutional change from routine to highly selective diversion of a low anastomosis after TME for rectal cancer. Eur J Surg Oncol. 2018;44:1220–1225. doi: 10.1016/j.ejso.2018.03.033. [DOI] [PubMed] [Google Scholar]
- 72.Hol JC, Burghgraef TA, Rutgers MLW, Crolla R, van Geloven AAW, de Jong GM, Hompes R, Leijtens JWA, Polat F, Pronk A, Smits AB, Tuynman JB, Verdaasdonk EGG, Consten ECJ, Sietses C. Impact of a diverting ileostomy in total mesorectal excision with primary anastomosis for rectal cancer. Surg Endosc. 2023;37:1916–1932. doi: 10.1007/s00464-022-09669-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mizrahi I, de Lacy FB, Abu-Gazala M, Fernandez LM, Otero A, Sands DR, Lacy AM, Wexner SD. Transanal total mesorectal excision for rectal cancer with indocyanine green fluorescence angiography. Tech Coloproctol. 2018;22:785–791. doi: 10.1007/s10151-018-1869-z. [DOI] [PubMed] [Google Scholar]
- 74.Hazen SJA, Vogel I, Borstlap WAA, Dekker JWT, Tuynman JB, Tanis PJ, Kusters M. Long-term stoma-related reinterventions after anterior resection for rectal cancer with or without anastomosis: population data from the Dutch snapshot study. Tech Coloproctol. 2022;26:99–108. doi: 10.1007/s10151-021-02543-3. [DOI] [PubMed] [Google Scholar]
- 75.Westerduin E, Borstlap WAA, Musters GD, Westerterp M, van Geloven AAW, Tanis PJ, Wolthuis AM, Bemelman WA, D'Hoore A. Redo coloanal anastomosis for anastomotic leakage after low anterior resection for rectal cancer: an analysis of 59 cases. Colorectal Dis. 2018;20:35–43. doi: 10.1111/codi.13844. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.