Abstract
The changing global climate have given rise to abiotic stresses that adversely affect the metabolic activities of plants, limit their growth, and agricultural output posing a serious threat to food production. The abiotic stresses commonly lead to production of reactive oxygen species (ROS) that results in cellular oxidation. Over the course of evolution, plants have devised efficient enzymatic and non-enzymatic anti-oxidative strategies to counteract harmful effects of ROS. Among the emerging non-enzymatic anti-oxidative technologies, the chloroplast lipophilic antioxidant vitamin A (Tocopherol) shows great promise. Working in coordination with the other cellular antioxidant machinery, it scavenges ROS, prevents lipid peroxidation, regulates stable cellular redox conditions, simulates signal cascades, improves membrane stability, confers photoprotection and enhances resistance against abiotic stresses. The amount of tocopherol production varies based on the severity of stress and its proposed mechanism of action involves arresting lipid peroxidation while quenching singlet oxygen species and lipid peroxyl radicals. Additionally, studies have demonstrated its coordination with other cellular antioxidants and phytohormones. Despite its significance, the precise mechanism of tocopherol action and signaling coordination are not yet fully understood. To bridge this knowledge gap, the present review aims to explore and understand the biosynthesis and antioxidant functions of Vitamin E, along with its signal transduction and stress regulation capacities and responses. Furthermore, the review delves into the light harvesting and photoprotection capabilities of tocopherol. By providing insights into these domains, this review offers new opportunities and avenues for using tocopherol in the management of abiotic stresses in agriculture.
Keywords: Vitamin E, Reactive oxygen species (ROS), Stress regulator, Tocopherol, Climate change
Introduction
The global climate crisis has led to an increase in a variety of biotic and abiotic stresses such as pathogens, salinity, drought, heavy metals, and high light intensity which cause the disruption of biochemical and evolutionary mechanisms in plants (Surabhi 2018; Kerchev et al. 2020; Mohammadi et al. 2020; Ghosh et al. 2021). Plants exposed to these stresses produce a high level of reactive oxidative species (ROS) which gets largely accumulated in plant leaves and induces peroxidation of lipids and oxidation of other cellular components such as chlorophyll and proteins (Arif et al. 2016; Singh et al. 2017; Nguyen et al. 2018). The accumulation of ROS in plant cells gets generally increased during unfavourable environmental conditions which can cause oxidative damage and death of plant cells. The increase of ROS accumulation in plants can lead to an increase in the ion discharge and hence level of malondialdehyde, an end-product of the oxidative breakdown of lipids and the cell wall damage (Sharma et al. 2012). To combat the harmful effects induced by drought, salinity, heavy metal, temperature and other abiotic stresses, plants activate multitude of stress responses (He et al. 2018) including aggregation of antioxidants, desaturation of lipids, phytohormones production, and activation of transcription factors (Atkinson et al. 2013; Wani et al. 2016). In addition to these, plants have gradually evolved two subtle enzymatic and non-enzymatic defensive mechanisms (Miller et al. 2010) in which vitamin E also known as tocopherol plays a significant role to stabilize the ROS generation and scavenging (Alscher et al. 2002).
Biosynthesis and aggregation of tocopherol has been considered as the major host plant responses to cope with the harmful effects caused during oxidative stress (Lushchak and Semchuk 2012). Vitamin E is a fat-soluble or lipophilic substance which is part of a family consisting of eight members including α, β, γ, and δ tocopherols. The precursors of tocopherols known as tocotrienols possess strong antioxidant properties, which provide defence to plants against stress via distinct metabolic processes (Hasegawa et al. 2000). Tocopherols have a key role in regulating a stable cellular redox condition and with their natural antioxidant defense systems; these are considered as a primary conserved system to impart resistance against stress in plants. The key role of tocopherols as an antioxidant involves firmly holding the phospholipid bilayer of polyunsaturated fatty acyl chain (Sattler et al. 2003). Furthermore, tocopherol protects plants in defiance of ultraviolet-B radiation stress (Bosch and Alegre 2002). The component vitamin E compound in plant leaves is known as α- tocopherol, a lipophilic compound present in the chloroplast envelope and thylakoid membranes that is accounted for more than 90% of the thylakoids (Havaux et al. 2000; Saffrané and Pellaud 2017). It has been documented that in various plant species, plastids based α- tocopherol decreases ROS, peroxidation of lipids, and H2O2, and provides strength to cell membrane integrity under ultraviolet-B radiation (Sharma et al. 2012).
There are only a few photosynthetic organisms including plants, algae, and cyanobacteria that produce tocopherols (Quadrana et al. 2013). The proportion of tocopherol varies in plant organs and a remarkably moderate to high level is found in the seeds and leaves (Bosch and Alegre 2002; Badrhadad et al. 2013). During recent years, several studies have found that besides being an antioxidant, which protects cellular membranes in both animals and plants (Bosch et al. 2007; Li et al. 2008). Although tocopherols have been rarely found in waxy cuticles 1–2% of total wax of Ginkgo biloba and Rubus have been represented by γ-, and δ- tocopherols (Robertson et al. 1991; Gülz et al. 1992; Shepherd et al. 1999). Minute traces of α-tocopherol have been detected in the green leaves of some plants (Franzen et al. 1991). In plants, the concentration of α-tocopherol is regulated by the environmental stresses and it provides protection against the photo-oxidative and salinity stress (Havaux et al. 2005; Queirós et al. 2011). In recent years, there has been effective implementation of biofortification to enhance vitamin E in various plant species (Saffrané and Pellaud 2017). Among the bioactive E-vitamins, α-tocopherol is considered as the most effective form, and vital vitamin E resources include vegetable oils with high levels of α-tocopherol and γ-tocopherol (Eitenmiller 1997). α-tocopherol is considered to have higher bioactivity than γ-tocopherol.
α-Tocopherol can assist in membrane protection by enhancing the activity of antioxidative enzymes and the concentration of non-enzymatic antioxidants with improved water relations (Ali et al. 2020). In an experiment with carrot plants exposed to abiotic stress conditions; α-tocopherol with varying levels of 50 and 100 mg L−1 was used by seed-priming. It was found that a concentration of 100 mg L−1 α-tocopherol resulted in improved plant development, increased level of osmoprotectants and a strengthened cellular defense system in carrot plants when faced with oxidative stress. The improvement in stress mitigation was attributed to increased uptake of essential nutrients, up-regulation of the oxidative defense system, water status, stomatal functioning, and improved rate of photosynthetic attributes (Hameed et al. 2021).
It has been found that while α-tocopherol is concentrated into leaves and flowers, seeds have enhanced levels of γ-tocopherol (Velasco et al. 2013). Experimental findings suggested that α-tocopherol can be administered externally to boost the plants’ tolerance against stress conditions (Uchendu et al. 2010). Due to distinctive characteristics of α-tocopherol such as prevention of lipid peroxidation, stimulation of signal cascade interactions that transfer biotic and abiotic signals, intracellular signaling, photosystem-II, membrane stability, and photo-protection, it is referred to as an essential element for plants and functions as an antioxidant in response to environmental stresses (Espinoza et al. 2013).
On the other hand, γ-tocopherol is the most common form of vitamin E in plant tissues, which has been studied more frequently than other tocopherols (Sen et al. 2007). In Arabidopsis thaliana, γ-tocopherol play a main role in seed dispersal resilience by acting against the oxidation of polyunsaturated fatty acids thus enhancing seed viability (Sattler et al. 2004). Plastidic γ-tocopherol methyltransferase can convert γ-tocopherol to α-tocopherol form, which is later preserved in the plastid micro compartments and thylakoid membranes (Szarka et al. 2012). Tocopherol provides protection to PSΙΙ against singlet oxygen (Bosch 2005; Liszkay and Trebst 2006).
Tocopherols act as annihilators in chain reaction that eliminate polyunsaturated fatty acids by scavenging and quenching of oxygen (Bosch and Falk 2004). Among the various kinds of tocopherols, α-tocopherol possesses the most efficient antioxidant properties. On the other hand, β- and γ-tocopherols have limited antioxidant properties, while, δ-tocopherol possesses the lowest antioxidant properties (Kapoor et al. 2015). Overall, the observed antioxidant activities of tocopherols with respect to the peroxidation of lipids in in vivo are α > β > γ > δ (Fukuzawa et al. 1982), while when examined in laboratory conditions, the activity levels were transposed to δ > γ > β > α (Eldin and Appelqvist 1996). Over the last few decades, the primary role of tocopherols has been contemplated as an antioxidant in prolonging membrane integrity when encountered with stress though additional roles have also been earmarked to them (Saeidnejad and Rajaei 2015; Fang et al. 2019). This review article provides a comprehensive summary of the stress amelioration role of vitamin E in crop plants, particularly its effectiveness against the environmental stresses such as salt, drought, and heavy metal stress responses. Despite its proven antioxidative potential, the exact mechanism of tocopherol action in plants remains unclear. This review seeks to address this gap by exploring and understanding the Vitamin E-mediated stress signaling pathways, antioxidative properties through involvement in biosynthetic pathways, and its contribution to enhancing light harvesting and photoprotection in plants. The insights from this review offer new possibilities of using tocopherol in managing abiotic stresses in agriculture.
Vitamin E biosynthesis in plants
Radio-trace research in the middle of 1980s led to the discovery of production mechanism of tocopherols in autotrophs (Grusak and DellaPenna 1999). Basically, two mechanisms namely, cytosolic shikimate mechanism and plastid methylerythritol 4-phosphate (MEP) that involve a chromanol head and a hydrophobic polyprenyl tail have been shown to be involved in the biosynthesis of tocopherols in plants (Szarka et al. 2012). These pathways are regulated by about 25 different enzymes (Vinutha et al. 2017) and tocopherols are synthesized with a chromosomal ring and a 15-carbon tail derived from homogentisate and phytyl diphosphate (Herrmann and Weaver 1999). The initial reaction for tocopherol biosynthesis occurs in the cytoplasm that finally culminates in plastids (Soll et al. 1980).
Cytosolic shikimate pathway
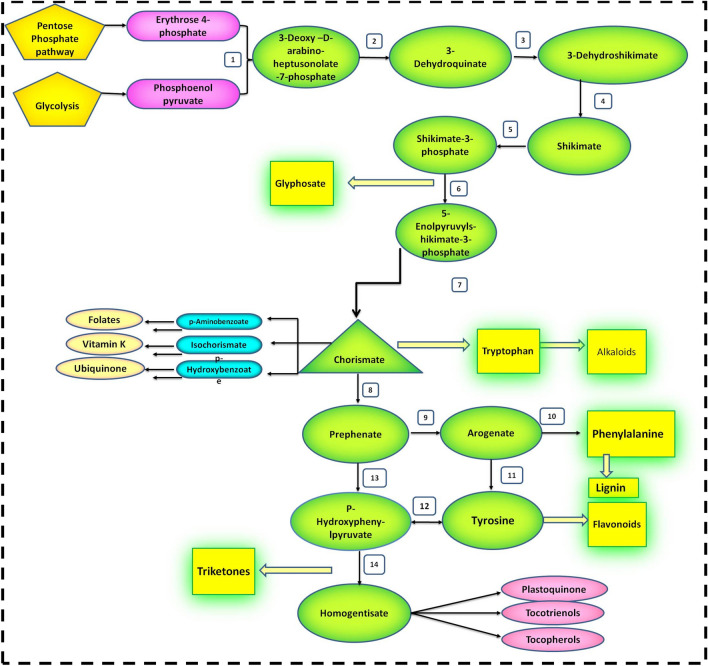
The shikimate pathway has been reported in several microorganisms and seed plants. This pathway is also entailed in several aromatic amino acids’ production including precursors for a wide selection of natural products including vitamins, pigments, etc. (Herrmann and Weaver 1999). The pathway comprises of seven reactions in which Erythrose-4-phosphate and phosphoenol pyruvate react to form the end product Chorismate, which is later used as a precursor by broad range of metabolites namely, aromatic amino acids, tocopherols, quinones, etc. (Lushchak and Semchuk 2012). 5-Enolpyruvylshikimate-3-phosphate (EPSP) (Fig. 1) synthase catalyzes the reversible formation of EPSP which is known as the rate-determining step in shikimate pathway. After the Chorismate is formed, it is further catalyzed into Prephenate by an enzyme chorismate mutase yielding two diverse pathways, which results in phenylalanine and tyrosine production. Next step involves the formation of P-Hydroxyphenyl pyruvate (HPP) from prephenate, a reaction catalyzed by prephenate dehydrogenase, an important step in the biosynthesis of tocopherols and tocotrienols (Lushchak and Semchuk 2012). The interface between arogenate and tyrosine lead to the synthesis of HPP in green plants. A fixed carbon from tyrosine is then supplied to produce HPP and homogentisate (a precursor of tocochromanol) upon action of tyrosine aminotransferase enzyme (Sterkel and Oliveira 2017). Simultaneously, phenylalanine is utilized in the production of lignin, flavonoids, and other phenylpropanoids.
Fig. 1.
The Shikimate pathway of homogentisate biosynthesis in photosynthetic organisms. The numbers in the reaction correspond the enzymes as follows: 1. 3-deoxy-D-arabino-heptulosonate 7-phosphate synthase; 2. 3-dehydroquinate synthase; 3. 3-dehydroquinate dehydratase; 4. shikimate dehydrogenase; 5. shikimate kinase; 6. 5-enolpyruvylshikimate 3-phosphate synthase; 7. chorismate synthase; 8. chorismate mutase; 9. prephenate aminotransferase; 10. arogenate dehydratase; 11. arogenate dehydrogenase; 12. tyrosine aminotransferase; 13. prephenate dehydrogenase; 14. p-hydroxyphenylpyruvate dioxygenase
Plastid methylerythritol 4-phosphate (MEP) pathway
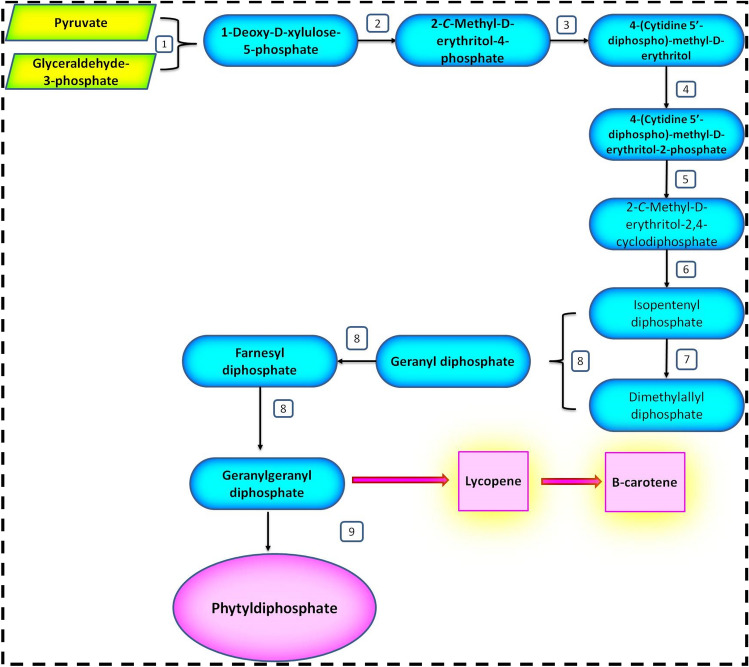
Hydrophobic prenyl tail is obtained from Phytyl diphosphate (PDP), which is produced from the precursor molecules isopentenyl diphosphate (IPP) along with its isomer dimethylallyl diphosphate (DMAPP). Both, IPP and DMAPP are synthesized from Mevalonate (MVA) and methyl erythritol phosphate (MEP) pathways. The Mevalonate pathway occurs in the cytoplasm which transforms sesquiterpenes (C15) into triterpenes (C30). Meanwhile, MEP pathway occurs in plastids, yields IPP which is further utilized in the formation of isoprenes, monoterpenes (C10), diterpenes (C20), carotenoids, plastoquinones, and phytol conjugates including chlorophylls, and tocopherols (Estévez et al. 2001).
In MEP pathway, the first step comprises of the precipitation of pyruvate catalyzed by transketolase enzyme and glyceraldehyde 3-phosphate, producing 1-deoxy-D-xylulose-5-phosphate (DOXP) (Fig. 2), by the action of DOXP synthase, a vital enzyme for the synthesis of plastidic IPP (Lichtenthaler 2000; Wanke et al. 2001; Rohmer 2003). Geranylgeranyl diphosphate (GGPP) is a 20C organic compound made up off 4 IPP molecules (Bosch and Alegre 2002). This GGPP along with several other terpenoid metabolic products including carotenoids, chlorophyll, gibberellin, and phytyl diphosphate serves as a precursor compound in the formation of tocotrienols (Ischebeck et al. 2006).
Fig. 2.
Methylerythritol pathway of phytyl diphosphate biosynthesis in the plastids of higher plants. The enzymes involved are 1. 1-deoxy-D-xylulose-5-phosphate (DOXP) synthase; 2. DOXP reductoisomerase; 3. 4-(cytidine 5’-diphospho)-methyl-D-erythritol (CDP-ME) synthase; 4. CDP-ME kinase; 5. ME cyclodiphosphate synthase; 6. multistep reaction catalyzed by two reductases and two dehydratases; 7. isomerase; 8. geranylgeranyl reductase; 9. chlorophyll synthase
It has been found that there is a non-equivalence relation between chlorophyll content and tocopherol in plants, because of which biosynthesis of tocopherols requires phytol in the form of a precursor, derived from chlorophyll (Valentin et al. 2006). This phytol has been observed to significantly enhance the tocopherol content in Arabidopsis seedlings and Sunflower cell culture (Ischebeck et al. 2006). The addition of phosphate group to phytol also results in the formation of phytyl phosphate and diphosphate (Ischebeck et al. 2006). The over expression of CLA1 gene in Arabidopsis leads to an increase in the levels of chlorophylls, carotenoids, gibberellins, abscisic acid, and tocopherols, while, a decrease in the levels of DOXP synthase resulted in reduction of their levels (Estévez et al. 2001). The next step catalyzed by enzyme homogentisate involves the synthesis of tocopherol and includes the precipitation of the preceding compound, Homogentisate, and Phytyl diphosphate, to produce 2-methyl-6-phytyl-1,4-benzoquinone (MPBQ). MPBQ methyltransferase further methylates MPBQ to 2,3-Dimethyl-5-phytyl-1,4-benzoquinol (DMPBQ). DMPBQ and MPBQ act as substrates in reactions catalyzed by tocopherol cyclase to yield γ-tocopherol and δ-tocopherol, respectively. Further methylation of γ- and δ-tocopherols to α-and β-tocopherols is carried out by the last enzyme of the pathway known as γ-tocopherol methyltransferase, respectively. Throughout the whole process, homogentisate phytyltransferase, p-hydroxyphenylpyruvate dioxygenase, and methyl transferases, the key regulatory enzymes play a significant role (Lakshmaiah et al. 2022).
Integration of vitamin E in abiotic stress responses
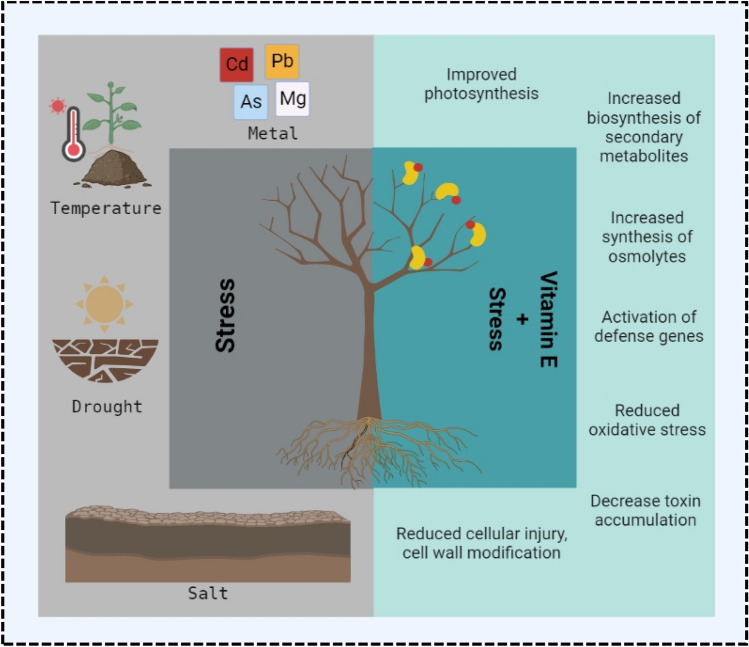
Plants being sessile are continually exposed to environmental challenges in the form of abiotic stresses including drought, salinity, extremely high or low temperatures, waterlogging, metal toxicity, and radiations. Since abiotic stresses interfere with plant growth, physiology, and productivity, these pose a direct threat to global food security (Hasanuzzaman et al. 2012a, b, 2013a, 2014; b). The statistics suggest that various abiotic stressors can reduce the agricultural output by as much as 50% (Rodríguez et al. 2005; Blohm 2007). All abiotic stimuli that can severely harm cells involve oxidative stress, which is attributable to reactive oxygen species (ROS) including singlet oxygen (1O2), hydrogen peroxide (H2O2), superoxide (O2•‾) and hydroxyl radical (OH•) (Fig. 3) (Gill and Tuteja 2010; Ali and Ashraf 2011). The transmission of excited electrons throughout photosynthesis and respiration processes, which directly influences cellular membranes through lipid peroxidation, causes a dramatic rise in ROS levels under environmental stresses (Ali et al. 2020). To counter the severe effects of oxidative stress, plants have evolved a highly developed antioxidant defence system comprising both enzymatic and non-enzymatic antioxidants, including Superoxide dismutase (SOD), Catalase (CAT), Peroxidase (POX), Glutathione reductase (GR), Glutathione (GSH), Ascorbate (AsA), Tocopherol (Toc) and alkaloids. These antioxidants, which are distributed across many cell organelles, cooperate to prevent oxidative damage by lowering the ROS level below a hazardous level (Mittler et al. 2006; Gill and Tuteja 2010; Ali et al. 2016, 2018). Vitamin E (primarily α-tocopherol) in conjunction with other antioxidants (such as AsA) efficiently reduces ROS generation (mostly 1O2 and OH·). It has been clearly established now that the tocopherol biosynthesis gets enhanced under extreme stress and improves oxidative stress defence by decreasing ROS production (Lushchak and Semchuk 2012; Jin and Daniell 2014; Chen et al. 2018). Numerous studies on plants have demonstrated that high levels of tocopherol can help plants withstand a variety of abiotic stresses, including salt stress (Ouyang et al. 2011; Farouk 2011; Chen et al. 2018; Naqve et al. 2021), drought (Cela et al. 2011; Espinoza et al. 2013; Ali et al. 2019; Shah et al. 2021); temperature extremes (Kanayama et al. 2013; Kumar et al. 2013; Siger et al. 2017), metal toxicity (Yusuf et al. 2010; di Toppi et al. 2012; Ali et al. 2020; Hashish et al. 2015; Kasperczyk et al. 2017), elevated ozone (Guo et al. 2009), and UV radiation (Bosch and Alegre 2002). All theses stresses are described in detail below.
Fig. 3.
Overview of the effects of vitamin E to decrease toxicity under abiotic stresses
Drought stress
Among the various abiotic stresses, drought stress is a significant constraint that reduces the crop productivity worldwide upto 45%. Drought occurs due to a combination of reduction in soil–water levels and evaporation under drier climatic spells. Under drought stress, plants face scarcity of water and nutrients, and toxicity of ions leading to reduced growth and crop yield due to disruption of photosynthetic attributes (Fig. 3). Moreover drought also hinders the morphological, physiological and biochemical processes of the plants and reduces the grain yield. However, tocopherol helps to ameliorate the toxicity caused by drought stress by scavenging the lipid peroxides, oxygen radicals, and singlet oxygen, thereby, detoxifying the reactive oxygen species. Tocopherols possess great antioxidative capacities and guard plants from abiotic stress through a number of metabolic processes (Hasegawa et al. 2000). Among the various known tocopherols (α, β, γ, and δ), α-tocopherol is recognized as vitamin E and stands out for its higher antioxidative capacity as compared to others.
Salt stress
The degree of salt stress as well as plant species influences the modulation of endogenous tocopherol. In various studies, exogenous tocopherol has proven effective against a variety of abiotic stresses (Guo et al. 2009; Espinoza et al. 2013). Furthermore, tocopherols levels fluctuate during salt stress, indicating their crucial role as antioxidants and as a measure of a plant’s stress tolerance (Ali et al. 2022). Application of tocopherol reduces the toxicity of salt stress and restores normal growth and development (Fig. 3). Under salt stress, application of tocopherol enhances the photosynthetic attributes such as net photosynthetic rate, CO2 assimilation, stomatal conductance and water use efficiency resulting in plant growth. Similar results on salt stress mitigation through tocopherol has been observed in Triticum aestivum and Zea mays by Ali et al. (2019, 2020).
Under salt stress, α-tocopherol gets altered in two stages in plants. In the first phase, the production of tocopherol rises to reduce the ROS, improving protection by mitigating oxidative damage. However, in the second phase, net tocopherol loss occurs due to increased breakdown of tocopherol, which surpasses its synthesis. If α-tocopherol deficiency does not get compensated by fast production or replenishment from exogenous administration of tocopherol, cell death results from increased lipid peroxidation. The first phase generally appears in stress-tolerant plants, while, the second phase has been seen in stress-sensitive ones (Bosch 2005).
In case of Triticum aestivum growing under salt stress, spraying α-tocopherol greatly increases the activities of antioxidant enzymes, leading to accumulation of AsA, phenolics and carotenoids enhancing membrane permeability, and reducing hydrogen peroxide and lipid peroxidation. This foliar application of α-tocopherol also lowers levels of chloride and sodium, while there is a buildup of potassium, magnesium and calcium. As a result of this, senescence gets delayed and salt tolerance increases (Farouk 2011).
Heavy metal stress
Heavy metals (HMs) toxicity is a serious and challenging problem in the world arising from both natural processes as well as anthropogenic activities. Out of the various strategies employed for HM removal, tocopherol-mediated HMs mitigation is a promising approach (Fig. 3). Tocopherol plays important role in the tolerance of oxidative stress induced by Cu and Cd in Arabidopsis plants. Furthermore, tocopherols may aid in the removal of hydroxyl radicals generated by the Fenton and Haber–Weiss reactions in chloroplasts when the ascorbate–glutathione cycle is impaired or overloaded by metal accumulation (Navarrete et al. 2005; Stoiber et al. 2013; Collin et al. 2008; Aranjuelo et al. 2014).
Vitamin E-mediated stress signaling pathway
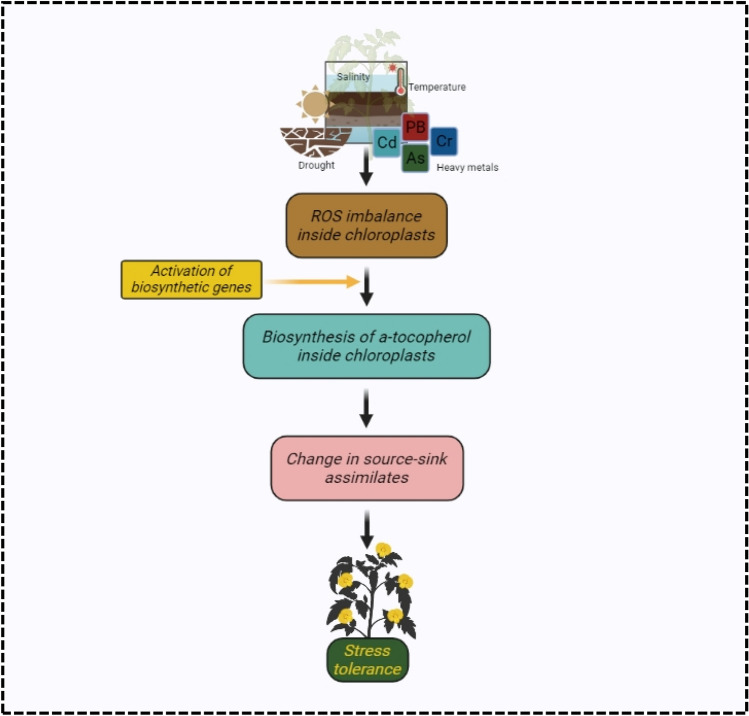
In plants, cellular signaling is a complex process involving many interconnected pathways. Tocopherol plays a crucial role in transmitting abiotic stress signals and facilitating better development and stress tolerance through participation in signal cascade (Fig. 4) (Sattler et al. 2004). The action of vitamin E (tocopherol) in stress sensing and signaling is coupled with the modulation of lipid peroxidation and photoprotection in thylakoid membranes as observed in the mechanisms in chloroplast-to-nucleus retrograde signaling (Maruta et al. 2012; Foyer 2018; Gläßer et al. 2014; D’Alessandro et al. 2018). α-tocopherol stimulates diverse signaling factors associated with growth stimulation (Naqve et al. 2021).
Fig. 4.
Schematic representation of α-tocopherol mediated stress tolerance in plants
During oxidative stress, lipid peroxidation in membranes produces free radicals that attack the double bonds of polyunsaturated fatty acids (PUFAs), resulting in the removal of an electron and generation of a lipid radical (PUFA*),. This radical then reacts with oxygen to create a lipid peroxyl radical (PUFA-OO*).
In return, these lipid peroxyl radicals repeatedly invade PUFAs in proximity, hence propagating lipid peroxidation chain reaction through the membrane. Essential fatty acids like linolenate (ω-3) and linoleate (ω-6) are predominantly available in plant membranes and are principal reactants in non-enzymatic lipid peroxidation. After the generation of linolenic (18:3) and linoleic (18:2) peroxyl radicals’, these undergo a cascade of reactions, thereby, leading to the generation of MDA and other signaling molecules as jasmonic acid (JA) (Farmer 2007). Jasmonic acid is known to be associated with the regulation of hydroxyphenolpyruvate dioxygenase (HPPD) enzyme of tocopherol biosynthetic pathway (Sandorf and Czytko 2002), indicating its participation in cell signaling. Tocopherols protect fatty acids from lipid peroxidation in plastids by efficiently scavenging lipid peroxyl radicals and quenching ROS (Leng et al. 2015; Broznić et al. 2016; Boonnoy et al. 2017; Fritsche et al. 2017).
Also, tocopherol isoforms are known to defend up to 220 (α-), 120 (β-), 100 (γ-), and 30 (δ-) lipid molecules (Fukuzawa et al. 1982). Tocopherol obstructs the propagation of lipid peroxidation in the membranes by sacricing a hydrogen (H) atom to the lipid peroxyl radicals. The resulting lipid peroxides are then transformed either spontaneously or enzymatically into inert hydroxy fatty acids or to other products (Eldin and Appelqvist 1996). The donation of a hydrogen atom results in the formation of a tocopheroxyl radical that is recycled back to the original tocopherol through its reduction by ascorbic acid (AsA) which itself is converted to dehydroascorbate (DHA) during this process (Zsigmond et al. 2011).
Furthermore, Glutathione provides electrons for GSSG production and reduces DHA to ascorbic acid (Azzi and Stocker 2000). Alternatively, tocopheroxyl radicals are converted to tocopherol by coenzyme Q (Wang and Quinn 1999). On the other hand, PUFA* and PUFA-OO* generated throughout the reactions either react with each other producing inert products. Numerous electron transport systems are deactivated by environmental stress, including photoinhibition of photosynthesis and malfunction of mitochondrial electron transport in plants due to excessive production and accumulation of reactive oxygen species (ROS). ROS can harm nucleic acids, proteins, and lipids if they are produced continuously, however, when they concentrate momentarily, they take part in retrograde signaling, which is crucial for mediating both developmental and acclimatization processes (Verdeja and Strand 2018; Cardamone et al. 2018).
Vitamin E as an antioxidative agent
Vitamin E, a lipophilic non-enzymatic antioxidant plays a crucial role in enabling plants to withstand abiotic stresses. However, plant species, level of stress and physiological state play a significant role in the vitamin E-induced stress tolerance (Bosch 2005). Eight members of the vitamin E family including four tocopherols (in α, β, γ and δ forms) and their respective precursors (four tocotrienols) possess significant antioxidant activity and shield plants form stress through several metabolic processes (Surówka et al. 2020). Since, it shields photosystem II and the lipid membranes in chloroplasts from environmental stress, α-tocopherol, also known as vitamin E, has the highest antioxidant capacity of all tocopherol isoforms (Naqve et al. 2021). Vitamin E’s ability to prevent membrane lipid peroxidation is due to the hydroxyl group on the chromanol head’s ability to donate a proton. Tocopherols may recycle themselves as antioxidants and can scavenge ROS, which helps to minimize lipid peroxidation.
By halting the spread of lipid peroxidation and inducing the production of α-tocopherol radicals, which are then converted into α-tocopherol via the ascorbate–glutathione cycle, α-tocopherol quenches lipid peroxyl radicals (LOO·) (Szarka et al. 2012; Hussain et al. 2013; Orabi and Abdelhamid 2016). Tocotrienols and γ -tocopherol are present in the seeds of many dicots and monocots, respectively, while α-tocopherol is found throughout the whole plant kingdom and is the primary vitamin E form in photosynthetic tissues (Esteban et al. 2009; Siles et al. 2013; Diepenbrock et al. 2017). Tocopherol has been demonstrated to play an antioxidant role not only in leaves (Havaux et al. 2005), but also in some fruits (Gramegna et al. 2019) and flowers due to their capacity to quench and scavenge singlet oxygen (1O2) and regulate the extent of lipid peroxidation in chloroplasts (Fernandes et al. 2018; Muñoz et al. 2018).
Vitamin E mediated enhancement in light harvesting and photoprotection
Environmental stress may affect photosynthesis by preventing electron transport and deactivating reaction centers of photosystem II (PSII) (Mehta et al. 2010). By quenching ROS, Vitamin E protects chloroplasts from stress-induced photoinhibition, maintaining redox equilibrium and increasing plant photosynthetic productivity (CR120-Sh SM 2014; Hasanuzzaman et al. 2020). α-tocopherol is produced in plastids and being an antioxidant, it helps to increase the production of chlorophyll pigments and preventing stress-related oxidative bursts (Surówka et al. 2020). They remove ROS (mostly 1O2 and OH·) from photosynthetic membranes and convert lipid peroxyl radicals (LOO·) to their equivalent hydro peroxides via reduction (Maeda et al. 2005).
Singlet oxygen (1O2), the most unstable ROS and the initial product of photoinhibition, is thought to act as the catalyst for the process (Tikkanen et al. 2014). When energy transmission from chlorophylls to downstream acceptors is not efficient, it results in their reduction and 1O2 is formed in photosystems (PSII) and (PSI) (Takagi et al. 2016). As electron spins transition into a low-energy excited state happens due to photoactivation, and triple-state chlorophylls (3Chl) are formed (Prasad et al. 2018). Consequently, the interaction of triplet oxygen (3O2) with 3Chl can lead to an increase in 1O2 levels (Muñoz and Bosch 2019). Vitamin E can prevent the generation of 1O2 in this situation (Gruszka et al. 2008). It has been demonstrated that tocopherol is extremely effective at quenching and scavenging 1O2 in biological membranes (Qin et al. 2009). According to Fahrenholzt et al. (1974), tocopherols are capable to dismutase 1O2, with one molecule of tocopherol having the potential to dismutase roughly 120 molecules of 1O2. In the presence of ascorbate and glutathione, the latter reaction produces tocopherol quinones, which can then be recycled by the subsequent actions of tocopherol cyclase (VTE1) and NAD(P)H-dependent quinine oxidoreductase (NDC1), an unidentified dehydratase (Kobayashi and Dellapenna 2008). Therefore, it has been demonstrated that vitamin E, and in particular α-tocopherol, is crucial for photoprotection when leaves are exposed to photo-oxidative stress, which can be stimulated by either extreme light or other environmental stressors such as extreme temperatures, salinity, water deficiency or metal toxicity (Havaux et al. 2005; Jin and Daniell 2014).
The capacities of light capturing and absorption processes, as well as the number of photosynthetic pigments in the plant both have a direct impact on the rate of photosynthetic activity in the leaves (Ali et al. 2019; Taiz et al. 2015). Different plant species and even cultivars within the same species have varying capacities to withstand harsh environments (Foyer and Noctor 2000). Reduced photosynthetic pigment and altered water relations are linked to stress-induced reductions in biomass (Ali and Ashraf 2011). According to a study conducted under water stress, foliar application of α-Tocopherol significantly increased the plant’s endogenous levels and improved growth both in stressed and non-stressed conditions (Ali et al. 2020). The improvement in plant water relations and the formation of biosynthetic pigments like chlorophyll and carotenoids under the impact of α-Tocopherol foliar spray are favorably correlated with increases in plant biomass output. The increase in plant water status may be attributed to the effect of α-Tocopherol on H-ATPase system, which demonstrates its function in cellular osmotic adjustment because it is an essential component of cellular membranes. Because of its involvement in cellular osmotic adjustment, α-Tocopherol plays a crucial part in preserving cellular water balance under challenging circumstances. Because better plant water content is required to govern stomatal regulation for better photosynthesis, this increase in plant water relations also contributes to an improvement in the net photosynthetic efficiency by leaves (Taiz et al. 2015; Ali et al. 2020).
Conclusion and future prospective
In conclusion, the present review has highlighted the vital role of vitamin E in increasing agricultural crop yield. Vitamin E acts as annihilator in chain reaction by eliminating polyunsaturated fatty acids by scavenging and quenching oxygen. It is synthesized through two different mechanisms and regulated by approximately 25 different enzymes. Functionally, it helps in lipid peroxidation, stimulation of signal cascade interactions, intracellular signaling, membrane stability, and photo-protection. Being a potent antioxidant, Vitamin E not only contributes to the nutritive value of cereal grains, but also enhances the resistance of plants to abiotic stresses improving their growth under stressful environmental conditions. Furthermore, Vitamin E has important protective role in ameliorating the oxidative stresses in crop plants.
Despite its immense significance, the precise mechanistic functions and regulation of key genes and pathways influenced by vitamin E under abiotic stress conditions require further molecular investigations to unravel the involved intricacies. Nevertheless, by shedding light on important domains of biosynthesis, functions and mechanism of action of vitamin E, the present review has presented fresh possibilities and pathways for effective utilization of tocopherol in agriculture to combat abiotic stresses and increase crop productivity.
Acknowledgements
This study is supported via funding from Prince Sattam bin Abdulaziz University project number (PSAU/2023/R/1444). VDR and TM acknowledge the support from the Ministry of Science and Higher Education of the Russian Federation, agreement No. 075-15-2023-587.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Mohammad Faizan and Pravej Alam have contributed equally to this work.
Contributor Information
Mohammad Faizan, Email: faizanetawah8@gmail.com.
Pravej Alam, Email: alamprez@gmail.com.
References
- Ali Q, Ashraf M. Induction of drought tolerance in maize (Zea mays L.) due to exogenous application of trehalose: growth, photosynthesis, water relations and oxidative defence mechanism. J Agron Crop Sci. 2011;197(4):258–271. [Google Scholar]
- Ali Q, Haider MZ, Iftikhar W, Jamil S, Tariq Javed M, Noman A, Iqbal M, Perveen R. Drought tolerance potential of Vigna mungo L. lines as deciphered by modulated growth, antioxidant defense, and nutrient acquisition patterns. Braz. J. Bot. 2016;39(3):801–812. [Google Scholar]
- Ali Q, Javed MT, Noman A, Haider MZ, Waseem M, Iqbal N, Waseem M, Shah MS, Shahzad F, Perveen R. Assessment of drought tolerance in mung bean cultivars/lines as depicted by the activities of germination enzymes, seedling’s antioxidative potential and nutrient acquisition. Arch. Agron. Soil Sci. 2018;64(1):84–102. [Google Scholar]
- Ali Q, Ali S, Iqbal N, Javed MT, Rizwan M, Khaliq R, Shahid S, Perveen R, Alamri SA, Alyemeni MN, Wijaya L, Ahmad P. Alpha-tocopherol fertigation confers growth physio-biochemical and qualitative yield enhancement in field grown water deficit wheat (Triticum aestivum L.) Sci Rep. 2019;9(1):1–15. doi: 10.1038/s41598-019-49481-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali Q, Tariq Javed M, Haider MZ, Habib N, Rizwan M, Perveen R, Ali S, Alyemeni MN, El-Serehy HA, Al-Misned FA. α-Tocopherol foliar spray and translocation mediates growth, photosynthetic pigments, nutrient uptake, and oxidative defense in maize (Zea mays L.) under drought stress. Agronomy. 2020;10(9):1235. [Google Scholar]
- Ali E, Hussain S, Hussain N, Kakar KU, Shah JM, Zaidi SHR, Jan M, Zhang K, Khan MA, Imtiaz M. Tocopherol as plant protector: an overview of Tocopherol biosynthesis enzymes and their role as antioxidant and signaling molecules. Acta Physiol Plant. 2022;44(2):1–11. [Google Scholar]
- Alscher RG, Erturk N, Heath LS. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J Exp Bot. 2002;53(372):1331–1341. [PubMed] [Google Scholar]
- Aranjuelo I, Doustaly F, Cela J, Porcel R, Müller M, Aroca R, Munné-Bosch S, Bourguignon J. Glutathione and transpiration as key factors conditioning oxidative stress in Arabidopsis thaliana exposed to uranium. Planta. 2014;239(4):817–830. doi: 10.1007/s00425-013-2014-x. [DOI] [PubMed] [Google Scholar]
- Arif N, Yadav V, Singh S, Kushwaha BK, Singh S, Tripathi DK, Vishwakarma K, Sharma S, Dubey NK, Chauhan DK (2016) Assessment of antioxidant potential of plants in response to heavy metals. In: Plant responses to xenobiotics. Springer, Singapore, pp 97–125
- Atkinson NJ, Lilley CJ, Urwin PE. Identification of genes involved in the response of Arabidopsis to simultaneous biotic and abiotic stresses. Plant Physiol. 2013;162(4):2028–2041. doi: 10.1104/pp.113.222372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzi A, Stocker A. Vitamin E: non-antioxidant roles. Prog Lipid Res. 2000;39(3):231–255. doi: 10.1016/s0163-7827(00)00006-0. [DOI] [PubMed] [Google Scholar]
- Badrhadad A, Piri K, Ghiasvand T. Increase alpha-tocopherol in cell suspension cultures Elaeagnus angustifolia L. Int J Agri Crop Sci. 2013;5:1–4. [Google Scholar]
- Boonnoy P, Karttunen M, Wong-Ekkabut J. Alpha-tocopherol inhibits pore formation in oxidized bilayers. Phys Chem Chem Phys. 2017;19(8):5699–5704. doi: 10.1039/c6cp08051k. [DOI] [PubMed] [Google Scholar]
- Broznić D, Čanadi Jurešić G, Milin Č. Involvement of α-, γ-and δ-tocopherol isomers from pumpkin (Cucurbita pepo L.) seed oil or oil mixtures in the biphasic DPPH disappearance kinetics. Food Technol Biotechnol. 2016;54(2):200–210. doi: 10.17113/ftb.54.02.16.4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardamone MD, Tanasa B, Cederquist CT, Huang J, Mahdaviani K, Li W, Rosenfeld MG, Liesa M, Perissi V. Mitochondrial retrograde signaling in mammals is mediated by the transcriptional cofactor GPS2 via direct mitochondria-to-nucleus translocation. Mol Cell. 2018;69(5):757–772. doi: 10.1016/j.molcel.2018.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cela J, Chang C, Munné-Bosch S. Accumulation of γ-rather than α-tocopherol alters ethylene signaling gene expression in the vte4 mutant of Arabidopsis thaliana. Plant Cell Physiol. 2011;52(8):1389–1400. doi: 10.1093/pcp/pcr085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Zhang L, Miao X, Hu X, Nan S, Wang J, Fu H. Effect of salt stress on fatty acid and α-tocopherol metabolism in two desert shrub species. Planta. 2018;247(2):499–511. doi: 10.1007/s00425-017-2803-8. [DOI] [PubMed] [Google Scholar]
- Collin VC, Eymery F, Genty B, Rey P, Havaux M. Vitamin E is essential for the tolerance of Arabidopsis thaliana to metal-induced oxidative stress. Plant Cell Environ. 2008;31(2):244–257. doi: 10.1111/j.1365-3040.2007.01755.x. [DOI] [PubMed] [Google Scholar]
- D’alessandro S, Ksas B, Havaux M. Decoding β-cyclocitral-mediated retrograde signaling reveals the role of a detoxification response in plant tolerance to photooxidative stress. Plant Cell. 2018;30(10):2495–2511. doi: 10.1105/tpc.18.00578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diepenbrock CH, Kandianis CB, Lipka AE, Magallanes-Lundback M, Vaillancourt B, Góngora-Castillo E, Wallace JG, Cepela J, Mesberg A, Bradbury PJ, Ilut DC, DellaPenna D. Novel loci underlie natural variation in vitamin E levels in maize grain. Plant Cell. 2017;29(10):2374–2392. doi: 10.1105/tpc.17.00475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eitenmiller RR. Vitamin E content of fats and oils–nutritional implications. Food Technol. 1997;51:78–81. [Google Scholar]
- Espinoza A, San Martín A, López-Climent M, Ruiz-Lara S, Gómez-Cadenas A, Casaretto JA. Engineered drought-induced biosynthesis of α-tocopherol alleviates stress-induced leaf damage in tobacco. J Plant Physiol. 2013;170(14):1285–1294. doi: 10.1016/j.jplph.2013.04.004. [DOI] [PubMed] [Google Scholar]
- Esteban R, Olano JM, Castresana J, Fernández-Marín B, Hernández A, Becerril JM, García-Plazaola JI. Distribution and evolutionary trends of photoprotective isoprenoids (xanthophylls and tocopherols) within the plant kingdom. Physiol Plant. 2009;135(4):379–389. doi: 10.1111/j.1399-3054.2008.01196.x. [DOI] [PubMed] [Google Scholar]
- Estévez JM, Cantero A, Reindl A, Reichler S, León P. 1-Deoxy-D-xylulose-5-phosphate synthase, a limiting enzyme for plastidic isoprenoid biosynthesis in plants. J Biol Chem. 2001;276(25):22901–22909. doi: 10.1074/jbc.M100854200. [DOI] [PubMed] [Google Scholar]
- Fahrenholzt SR, Doleiden FH, Lamola AA. On the quenching of singlet oxygen by α-Tocopherol. Photochem Photobiol. 1974;20(6):505–509. doi: 10.1111/j.1751-1097.1974.tb06610.x. [DOI] [PubMed] [Google Scholar]
- Fang X, Zhao G, Zhang S, Li Y, Gu H, Li Y, Zhao Q, Qi Y. Chloroplast-to-nucleus signaling regulates microRNA biogenesis in Arabidopsis. Dev Cell. 2019;48(3):371–382. doi: 10.1016/j.devcel.2018.11.046. [DOI] [PubMed] [Google Scholar]
- Farmer EE. Plant biology: jasmonate perception machines. Nature. 2007;448:659–660. doi: 10.1038/448659a. [DOI] [PubMed] [Google Scholar]
- Farouk S. Ascorbic acid and α-tocopherol minimize salt-induced wheat leaf senescence. J Stress Physiol Biochem. 2011;7(3):58–79. [Google Scholar]
- Fernandes L, Ramalhosa E, Pereira JA, Saraiva JA, Casal S. The unexplored potential of edible flowers lipids. Agriculture. 2018;8(10):146. [Google Scholar]
- Foyer CH. Reactive oxygen species, oxidative signaling and the regulation of photosynthesis. Environ Exp Bot. 2018;154:134–142. doi: 10.1016/j.envexpbot.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer CH, Noctor G. Tansley Review No. 112 Oxygen processing in photosynthesis: regulation and signalling. New Phytol. 2000;146(3):359–388. [Google Scholar]
- Franzen J, Bausch J, Glatzle D, Wagner E. Distribution of vitamin E in spruce seedling and mature tree organs, and within the genus. Phytochemistry. 1991;30(1):147–151. [Google Scholar]
- Fritsche S, Wang X, Jung C. Recent advances in our understanding of tocopherol biosynthesis in plants: an overview of key genes, functions, and breeding of vitamin E improved crops. Antioxidants. 2017;6(4):99. doi: 10.3390/antiox6040099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuzawa K, Tokumura A, Ouchi S, Tsukatani H. Antioxidant activities of tocopherols on Fe2+-ascorbate-induced lipid peroxidation in lecithin liposomes. Lipids. 1982;17(7):511–513. doi: 10.1007/BF02535334. [DOI] [PubMed] [Google Scholar]
- Ghosh UK, Islam MN, Siddiqui MN, Khan MAR. Understanding the roles of osmolytes for acclimatizing plants to changing environment: a review of potential mechanism. Plant Signal Behav. 2021;16(8):1913306. doi: 10.1080/15592324.2021.1913306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem. 2010;48(12):909–930. doi: 10.1016/j.plaphy.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Gläßer C, Haberer G, Finkemeier I, Pfannschmidt T, Kleine T, Leister D, Dietz KJ, Häusler RE, Grimm B, Mayer KFX. Meta-analysis of retrograde signaling in Arabidopsis thaliana reveals a core module of genes embedded in complex cellular signaling networks. Mol Plant. 2014;7(7):1167–1190. doi: 10.1093/mp/ssu042. [DOI] [PubMed] [Google Scholar]
- Gramegna G, Rosado D, Sanchez Carranza AP, Cruz AB, Simon-Moya M, Llorente B, Rodríguez-Concepcíon M, Freschi L, Rossi M. PHYTOCHROME-INTERACTING FACTOR 3 mediates light-dependent induction of tocopherol biosynthesis during tomato fruit ripening. Plant Cell Environ. 2019;42(4):1328–1339. doi: 10.1111/pce.13467. [DOI] [PubMed] [Google Scholar]
- Grusak MA, DellaPenna D. Improving the nutrient composition of plants to enhance human nutrition and health. Annu Rev Plant Biol. 1999;50(1):133–161. doi: 10.1146/annurev.arplant.50.1.133. [DOI] [PubMed] [Google Scholar]
- Gruszka J, Pawlak A, Kruk J. Tocochromanols, plastoquinol, and other biological prenyllipids as singlet oxygen quenchers—determination of singlet oxygen quenching rate constants and oxidation products. Free Radical Biol Med. 2008;45(6):920–928. doi: 10.1016/j.freeradbiomed.2008.06.025. [DOI] [PubMed] [Google Scholar]
- Gülz PG, Müller E, Schmitz K, Marner FJ, Güth S. Chemical composition and surface structures of epicuticular leaf waxes of Ginkgo biloba, Magnolia grandiflora and Liriodendron tulipifera. Z Naturforschung C. 1992;47(7–8):516–526. [Google Scholar]
- Guo J, Li XF, Qi DM, Chen SY, Li ZQ, Nijs I, Li YG, Liu GS. Effects of ozone on wild type and transgenic tobacco. Biol Plant. 2009;53(4):670–676. [Google Scholar]
- Hameed A, Akram NA, Saleem MH, Ashraf M, Ahmed S, Ali S, Abdullah Alsahli A, Alyemeni MN. Seed treatment with α-tocopherol regulates growth and key physio-biochemical attributes in carrot (Daucus carota L.) plants under water limited regimes. Agronomy. 2021;11(3):469. [Google Scholar]
- Hasanuzzaman M, Hossain MA, Silva JA, Fujita M (2012a) Plant response and tolerance to abiotic oxidative stress: antioxidant defense is a key factor. In: Crop stress and its management: perspectives and strategies. Springer, Dordrecht, pp 261–315
- Hasanuzzaman M, Nahar K, Alam MM, Fujita M. Exogenous nitric oxide alleviates high temperature induced oxidative stress in wheat ('Triticum aestivum' L.) seedlings by modulating the antioxidant defense and glyoxalase system. Aust J Crop Sci. 2012;6(8):1314–1323. [Google Scholar]
- Hasanuzzaman M, Nahar K, Fujita M (2013a) Plant response to salt stress and role of exogenous protectants to mitigate salt-induced damages. In: Ecophysiology and responses of plants under salt stress. Springer, New York, pp 25–87
- Hasanuzzaman M, Nahar K, Fujita M, Ahmad P, Chandna R, Prasad MNV, Ozturk M (2013b) Enhancing plant productivity under salt stress: relevance of poly-omics. Salt Stress Plants 113–156
- Hasanuzzaman M, Nahar K, Fujita M (2014) Role of tocopherol (vitamin E) in plants: abiotic stress tolerance and beyond. In: Emerging technologies and management of crop stress tolerance. Academic Press, pp 267–289
- Hasanuzzaman M, Bhuyan MB, Zulfiqar F, Raza A, Mohsin SM, Mahmud JA, Fujita M, Fotopoulos V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: revisiting the crucial role of a universal defense regulator. Antioxidants. 2020;9(8):681. doi: 10.3390/antiox9080681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ. Plant cellular and molecular responses to high salinity. Annu Rev Plant Biol. 2000;51(1):463–499. doi: 10.1146/annurev.arplant.51.1.463. [DOI] [PubMed] [Google Scholar]
- Hashish EA, Elgaml SA, El-Murr A, Khalil R. Nephroprotective and antioxidant significance of selenium and α-tocopherol on lead acetate-induced toxicity of Nile Tilapia (Oreochromis niloticus) Fish Physiol Biochem. 2015;41(3):651–660. doi: 10.1007/s10695-015-0035-z. [DOI] [PubMed] [Google Scholar]
- Havaux M, Bonfils JP, Lutz C, Niyogi KK. Photodamage of the photosynthetic apparatus and its dependence on the leaf developmental stage in the npq1 Arabidopsis mutant deficient in the xanthophyll cycle enzyme violaxanthin de-epoxidase. Plant Physiol. 2000;124(1):273–284. doi: 10.1104/pp.124.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havaux M, Eymery F, Porfirova S, Rey P, Dörmann P. Vitamin E protects against photoinhibition and photooxidative stress in Arabidopsis thaliana. Plant Cell. 2005;17(12):3451–3469. doi: 10.1105/tpc.105.037036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M, He CQ, Ding NZ. Abiotic stresses: general defenses of land plants and chances for engineering multistress tolerance. Front Plant Sci. 2018;9:1771. doi: 10.3389/fpls.2018.01771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Verdeja T, Strand Å. Retrograde signals navigate the path to chloroplast development. Plant Physiol. 2018;176(2):967–976. doi: 10.1104/pp.17.01299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann KM, Weaver LM. The shikimate pathway. Annu Rev Plant Biol. 1999;50:473. doi: 10.1146/annurev.arplant.50.1.473. [DOI] [PubMed] [Google Scholar]
- Hussain N, Irshad F, Jabeen Z, Shamsi IH, Li Z, Jiang L. Biosynthesis, structural, and functional attributes of tocopherols in planta; past, present, and future perspectives. J Agric Food Chem. 2013;61(26):6137–6149. doi: 10.1021/jf4010302. [DOI] [PubMed] [Google Scholar]
- Ischebeck T, Zbierzak AM, Kanwischer M, Dörmann P. A salvage pathway for phytol metabolism in Arabidopsis. J Biol Chem. 2006;281(5):2470–2477. doi: 10.1074/jbc.M509222200. [DOI] [PubMed] [Google Scholar]
- Jin S, Daniell H. Expression of γ-tocopherol methyltransferase in chloroplasts results in massive proliferation of the inner envelope membrane and decreases susceptibility to salt and metal-induced oxidative stresses by reducing reactive oxygen species. Plant Biotechnol J. 2014;12(9):1274–1285. doi: 10.1111/pbi.12224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamal-Eldin A, Appelqvist LÅ. The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids. 1996;31(7):671–701. doi: 10.1007/BF02522884. [DOI] [PubMed] [Google Scholar]
- Kanayama Y, Sato K, Ikeda H, Tamura T, Nishiyama M, Kanahama K. Seasonal changes in abiotic stress tolerance and concentrations of tocopherol, sugar, and ascorbic acid in sea buckthorn leaves and stems. Sci Hortic. 2013;164:232–237. [Google Scholar]
- Kapoor D, Sharma R, Handa N, Kaur H, Rattan A, Yadav P, Gautam V, Kaur R, Bhardwaj R. Redox homeostasis in plants under abiotic stress: role of electron carriers, energy metabolism mediators and proteinaceous thiols. Front Environ Sci. 2015;3:13. [Google Scholar]
- Kasperczyk S, Dobrakowski M, Kasperczyk A, Nogaj E, Boroń M, Szlacheta Z, Birkner E. α-Tocopherol supplementation and the oxidative stress, homocysteine, and antioxidants in lead exposure. Arch Environ Occup Health. 2017;72(3):153–158. doi: 10.1080/19338244.2016.1182112. [DOI] [PubMed] [Google Scholar]
- Kerchev P, van der Meer T, Sujeeth N, Verlee A, Stevens CV, Van Breusegem F, Gechev T. Molecular priming as an approach to induce tolerance against abiotic and oxidative stresses in crop plants. Biotechnol Adv. 2020;40:107503. doi: 10.1016/j.biotechadv.2019.107503. [DOI] [PubMed] [Google Scholar]
- Kobayashi N, DellaPenna D. Tocopherol metabolism, oxidation and recycling under high light stress in Arabidopsis. Plant J. 2008;55(4):607–618. doi: 10.1111/j.1365-313X.2008.03539.x. [DOI] [PubMed] [Google Scholar]
- Krieger-Liszkay A, Trebst A. Tocopherol is the scavenger of singlet oxygen produced by the triplet states of chlorophyll in the PSII reaction centre. J Exp Bot. 2006;57(8):1677–1684. doi: 10.1093/jxb/erl002. [DOI] [PubMed] [Google Scholar]
- Kumar S, Singh R, Nayyar H. α-Tocopherol application modulates the response of wheat (Triticum aestivum L.) seedlings to elevated temperatures by mitigation of stress injury and enhancement of antioxidants. J Plant Growth Regul. 2013;32(2):307–314. [Google Scholar]
- Lakshmaiah VV, Joseph BV, Bhaskar R, Ulhas RS, Al-Khayri JM, Nagella P (2022) In Vitro Production of Tocopherols. In: Nutraceuticals production from plant cell factory. Springer, Singapore, pp 287–319
- Leng X, Kinnun JJ, Marquardt D, Ghefli M, Kučerka N, Katsaras J, Atkinson J, Harroun TA, Feller SE, Wassall SR. α-Tocopherol is well designed to protect polyunsaturated phospholipids: MD simulations. Biophys J. 2015;109(8):1608–1618. doi: 10.1016/j.bpj.2015.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wang Z, Sun X, Tang K. Current opinions on the functions of tocopherol based on the genetic manipulation of tocopherol biosynthesis in plants. J Integr Plant Biol. 2008;50(9):1057–1069. doi: 10.1111/j.1744-7909.2008.00689.x. [DOI] [PubMed] [Google Scholar]
- Lichtenthaler HK. Non-mevalonate isoprenoid biosynthesis: enzymes, genes, and inhibitors. Biochem Soc Trans. 2000;28(6):785–789. [PubMed] [Google Scholar]
- Lushchak VI, Semchuk NM. Tocopherol biosynthesis: chemistry, regulation, and effects of environmental factors. Acta Physiol Plant. 2012;34(5):1607–1628. [Google Scholar]
- Maeda H, Sakuragi Y, Bryant DA, DellaPenna D. Tocopherols protect Synechocystis sp. strain PCC 6803 from lipid peroxidation. Plant Physiol. 2005;138(3):1422–1435. doi: 10.1104/pp.105.061135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruta T, Noshi M, Tanouchi A, Tamoi M, Yabuta Y, Yoshimura K, Ishikawa T, Shigeoka S. H2O2-triggered retrograde signaling from chloroplasts to nucleus plays specific role in response to stress. J Biol Chem. 2012;287(15):11717–11729. doi: 10.1074/jbc.M111.292847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta P, Jajoo A, Mathur S, Bharti S. Chlorophyll a fluorescence study revealing effects of high salt stress on Photosystem II in wheat leaves. Plant Physiol Biochem. 2010;48(1):16–20. doi: 10.1016/j.plaphy.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Mène-Saffrané L, Pellaud S. Current strategies for vitamin E biofortification of crops. Curr Opin Biotechnol. 2017;44:189–197. doi: 10.1016/j.copbio.2017.01.007. [DOI] [PubMed] [Google Scholar]
- Miller GAD, Suzuki N, Ciftci-Yilmaz SULTAN, Mittler RON. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010;33(4):453–467. doi: 10.1111/j.1365-3040.2009.02041.x. [DOI] [PubMed] [Google Scholar]
- Mittler R, Kim Y, Song L, Coutu J, Coutu A, Ciftci-Yilmaz S, Lee H, Stevenson B, Zhu JK. Gain-and loss-of-function mutations in Zat10 enhance the tolerance of plants to abiotic stress. FEBS Lett. 2006;580(28–29):6537–6542. doi: 10.1016/j.febslet.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi H, Hazrati S, Ghorbanpour M (2020) Tolerance mechanisms of medicinal plants to abiotic stresses. In: Plant life under changing environment. Academic Press, pp 663–679
- Munné-Bosch S. The role of α-tocopherol in plant stress tolerance. J Plant Physiol. 2005;162(7):743–748. doi: 10.1016/j.jplph.2005.04.022. [DOI] [PubMed] [Google Scholar]
- Munné-Bosch S, Alegre L. The function of tocopherols and tocotrienols in plants. Crit Rev Plant Sci. 2002;21(1):31–57. [Google Scholar]
- Munné-Bosch S, Falk J. New insights into the function of tocopherols in plants. Planta. 2004;218(3):323–326. doi: 10.1007/s00425-003-1126-0. [DOI] [PubMed] [Google Scholar]
- Munné-Bosch S, Weiler EW, Alegre L, Müller M, Düchting P, Falk J. α-Tocopherol may influence cellular signaling by modulating jasmonic acid levels in plants. Planta. 2007;225(3):681–691. doi: 10.1007/s00425-006-0375-0. [DOI] [PubMed] [Google Scholar]
- Muñoz P, Munné-Bosch S. Vitamin E in plants: biosynthesis, transport, and function. Trends Plant Sci. 2019;24(11):1040–1051. doi: 10.1016/j.tplants.2019.08.006. [DOI] [PubMed] [Google Scholar]
- Muñoz P, Briones M, Munné-Bosch S. Photoinhibition and photoprotection during flower opening in lilies. Plant Sci. 2018;272:220–229. doi: 10.1016/j.plantsci.2018.04.023. [DOI] [PubMed] [Google Scholar]
- Naqve M, Wang X, Shahbaz M, Mahmood A, Bibi S, Fiaz S. Alpha tocopherol-induced modulations in the morphophysiological attributes of okra under saline conditions. Front Plant Sci. 2021;12:800251–800251. doi: 10.3389/fpls.2021.800251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete M, Rangel C, Corchado JC, Espinosa-Garcia J. Trapping of the OH radical by α-tocopherol: a theoretical study. J Phys Chem A. 2005;109(21):4777–4784. doi: 10.1021/jp050717e. [DOI] [PubMed] [Google Scholar]
- Nguyen HC, Lin KH, Ho SL, Chiang CM, Yang CM. Enhancing the abiotic stress tolerance of plants: from chemical treatment to biotechnological approaches. Physiol Plant. 2018;164(4):452–466. doi: 10.1111/ppl.12812. [DOI] [PubMed] [Google Scholar]
- Orabi SA, Abdelhamid MT. Protective role of α-tocopherol on two Vicia faba cultivars against seawater-induced lipid peroxidation by enhancing capacity of anti-oxidative system. J Saudi Soc Agric Sci. 2016;15(2):145–154. [Google Scholar]
- Ouyang S, He S, Liu P, Zhang W, Zhang J, Chen S. The role of tocopherol cyclase in salt stress tolerance of rice (Oryza sativa) Sci China Life Sci. 2011;54(2):181–188. doi: 10.1007/s11427-011-4138-1. [DOI] [PubMed] [Google Scholar]
- Prasad A, Sedlářová M, Pospíšil P. Singlet oxygen imaging using fluorescent probe Singlet Oxygen Sensor Green in photosynthetic organisms. Sci Rep. 2018;8(1):1–13. doi: 10.1038/s41598-018-31638-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin SS, Yu ZW, Yu YX. Structural and kinetic properties of α-tocopherol in phospholipid bilayers, a molecular dynamics simulation study. J Phys Chem B. 2009;113(52):16537–16546. doi: 10.1021/jp9074306. [DOI] [PubMed] [Google Scholar]
- Quadrana L, Almeida J, Otaiza SN, Duffy T, Corrêa da Silva JV, de Godoy F, Asís R, Bermúdez L, Fernie AR, Carrari F, Rossi M. Transcriptional regulation of tocopherol biosynthesis in tomato. Plant Mol Biol. 2013;81(3):309–325. doi: 10.1007/s11103-012-0001-4. [DOI] [PubMed] [Google Scholar]
- Queirós F, Rodrigues JA, Almeida JM, Almeida DP, Fidalgo F. Differential responses of the antioxidant defence system and ultrastructure in a salt-adapted potato cell line. Plant Physiol Biochem. 2011;49(12):1410–1419. doi: 10.1016/j.plaphy.2011.09.020. [DOI] [PubMed] [Google Scholar]
- Robertson GW, Griffiths DW, Birch ANE, Jones AT, McNicol JW, Hall JE. Further evidence that resistance in raspberry to the virus vector aphid, Amphorophora idaei, is related to the chemical composition of the leaf surface. Ann Appl Biol. 1991;119(3):443–449. [Google Scholar]
- Rodríguez M, Canales E, Borrás-Hidalgo O. Molecular aspects of abiotic stress in plants. Biotecnol Apl. 2005;22(1):1–10. [Google Scholar]
- Rohmer M. Mevalonate-independent methylerythritol phosphate pathway for isoprenoid biosynthesis. Elucidation and distribution. Pure Appl Chem. 2003;75(2–3):375–388. [Google Scholar]
- Saeidnejad AH, Rajaei P. Antioxidative responses to drought and salinity stress in plants, a comprehensive review. Int J Life Sci. 2015;9(2):1–8. [Google Scholar]
- Sandorf I, Holländer-Czytko H. Jasmonate is involved in the induction of tyrosine aminotransferase and tocopherol biosynthesis in Arabidopsis thaliana. Planta. 2002;216(1):173–179. doi: 10.1007/s00425-002-0888-0. [DOI] [PubMed] [Google Scholar]
- Sanità di Toppi L, Vurro E, De Benedictis M, Falasca G, Zanella L, Musetti R, Lenucci MS, Dalessandro G, Altamura MM. A bifasic response to cadmium stress in carrot: early acclimatory mechanisms give way to root collapse further to prolonged metal exposure. Plant Physiol Biochem. 2012;58:269–279. doi: 10.1016/j.plaphy.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Sattler SE, Cahoon EB, Coughlan SJ, DellaPenna D. Characterization of tocopherol cyclases from higher plants and cyanobacteria. Evolutionary implications for tocopherol synthesis and function. Plant Physiol. 2003;132(4):2184–2195. doi: 10.1104/pp.103.024257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler SE, Gilliland LU, Magallanes-Lundback M, Pollard M, DellaPenna D. Vitamin E is essential for seed longevity and for preventing lipid peroxidation during germination. Plant Cell. 2004;16(6):1419–1432. doi: 10.1105/tpc.021360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen CK, Khanna S, Rink C, Roy S. Tocotrienols: the emerging face of natural vitamin E. Vitam Horm. 2007;76:203–261. doi: 10.1016/S0083-6729(07)76008-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sh SM. Role of ascorbic acid and α tocopherol in alleviating salinity stress on flax plant (Linum usitatissimum L.) J Stress Physiol Biochem. 2014;10(1):93–111. [Google Scholar]
- Shah W, Ullah S, Ali S, Idrees M, Khan MN, Ali K, Younas F. Effect of exogenous alpha-tocopherol on physio-biochemical attributes and agronomic performance of lentil (Lens culinaris Medik.) under drought stress. PLoS ONE. 2021;16(8):e0248200. doi: 10.1371/journal.pone.0248200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P, Jha AB, Dubey RS, Pessarakli M (2012) Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot
- Shepherd T, Robertson GW, Griffiths DW, Birch ANE. Epicuticular wax composition in relation to aphid infestation and resistance in red raspberry (Rubus idaeus L.) Phytochemistry. 1999;52(7):1239–1254. doi: 10.1016/s0031-9422(00)00250-8. [DOI] [PubMed] [Google Scholar]
- Siger A, Józefiak M, Górnaś P. Cold-pressed and hot-pressed rapeseed oil: the effects of roasting and seed moisture on the antioxi-dant activity, canolol, and tocopherol level. Acta Sci Pol Technol Aliment. 2017;16(1):69–81. doi: 10.17306/J.AFS.2017.0458. [DOI] [PubMed] [Google Scholar]
- Siles L, Cela J, Munné-Bosch S. Vitamin E analyses in seeds reveal a dominant presence of tocotrienols over tocopherols in the Arecaceae family. Phytochemistry. 2013;95:207–214. doi: 10.1016/j.phytochem.2013.07.008. [DOI] [PubMed] [Google Scholar]
- Singh S, Tripathi DK, Singh S, Sharma S, Dubey NK, Chauhan DK, Vaculík M. Toxicity of aluminium on various levels of plant cells and organism: a review. Environ Exp Bot. 2017;137:177–193. [Google Scholar]
- Soll J, Kemmerling M, Schultz G. Tocopherol and plastoquinone synthesis in spinach chloroplasts subfractions. Arch Biochem Biophys. 1980;204(2):544–550. doi: 10.1016/0003-9861(80)90066-1. [DOI] [PubMed] [Google Scholar]
- Sterkel M, Oliveira PL. Developmental roles of tyrosine metabolism enzymes in the blood-sucking insect Rhodnius prolixus. Proc R Soc b: Biol Sci. 2017;284(1854):20162607. doi: 10.1098/rspb.2016.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoiber TL, Shafer MM, Armstrong DE. Induction of reactive oxygen species in Chlamydomonas reinhardtii in response to contrasting trace metal exposures. Environ Toxicol. 2013;28(9):516–523. doi: 10.1002/tox.20743. [DOI] [PubMed] [Google Scholar]
- Surabhi GK. Update in root proteomics with special reference to abiotic stresses: achievements and challenges. J Protein Proteomics. 2018;9(1):31–35. [Google Scholar]
- Surówka E, Potocka I, Dziurka M, Wróbel-Marek J, Kurczyńska E, Żur I, Maksymowicz A, Gajewska E, Miszalski Z. Tocopherols mutual balance is a key player for maintaining Arabidopsis thaliana growth under salt stress. Plant Physiol Biochem. 2020;156:369–383. doi: 10.1016/j.plaphy.2020.09.008. [DOI] [PubMed] [Google Scholar]
- Szarka A, Tomasskovics B, Bánhegyi G. The ascorbate-glutathione-α-tocopherol triad in abiotic stress response. Int J Mol Sci. 2012;13(4):4458–4483. doi: 10.3390/ijms13044458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taiz L, Zeiger E, Møller IM, Murphy A (2015) Plant physiology and development (No. Ed. 6). Sinauer Associates Incorporated
- Takagi D, Takumi S, Hashiguchi M, Sejima T, Miyake C. Superoxide and singlet oxygen produced within the thylakoid membranes both cause photosystem I photoinhibition. Plant Physiol. 2016;171(3):1626–1634. doi: 10.1104/pp.16.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikkanen M, Mekala NR, Aro EM. Photosystem II photoinhibition-repair cycle protects Photosystem I from irreversible damage. Biochim Biophys Acta (BBA) Bioenerg. 2014;1837(1):210–215. doi: 10.1016/j.bbabio.2013.10.001. [DOI] [PubMed] [Google Scholar]
- Uchendu EE, Leonard SW, Traber MG, Reed BM. Vitamins C and E improve regrowth and reduce lipid peroxidation of blackberry shoot tips following cryopreservation. Plant Cell Rep. 2010;29(1):25–35. doi: 10.1007/s00299-009-0795-y. [DOI] [PubMed] [Google Scholar]
- Valentin HE, Lincoln K, Moshiri F, Jensen PK, Qi Q, Venkatesh TV, Karunanandaa B, Baszis SR, Norris SR, Savidge B, Last RL. The Arabidopsis vitamin E pathway gene5-1 mutant reveals a critical role for phytol kinase in seed tocopherol biosynthesis. Plant Cell. 2006;18(1):212–224. doi: 10.1105/tpc.105.037077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veatch-Blohm ME. Principles of plant genetics and breeding. Crop Sci. 2007;47(4):1763. [Google Scholar]
- Velasco L, García-Navarro E, Pérez-Vich B, Fernández-Martínez JM. Selection for contrasting tocopherol content and profile in Ethiopian mustard. Plant Breed. 2013;132(6):694–700. [Google Scholar]
- Vinutha T, Bansal N, Kumari K, Prashat GR, Sreevathsa R, Krishnan V, Kumari S, Dahuja A, Lal S, Sachdev A, Praveen S. Comparative analysis of tocopherol biosynthesis genes and its transcriptional regulation in soybean seeds. J Agric Food Chem. 2017;65(50):11054–11064. doi: 10.1021/acs.jafc.7b03448. [DOI] [PubMed] [Google Scholar]
- Wang X, Quinn PJ. Vitamin E and its function in membranes. Prog Lipid Res. 1999;38(4):309–336. doi: 10.1016/s0163-7827(99)00008-9. [DOI] [PubMed] [Google Scholar]
- Wani SH, Kumar V, Shriram V, Sah SK. Phytohormones and their metabolic engineering for abiotic stress tolerance in crop plants. Crop J. 2016;4(3):162–176. [Google Scholar]
- Wanke M, Skorupinska-Tudek K, Swiezewska E. Isoprenoid biosynthesis via 1-deoxy-D-xylulose 5-phosphate/2-C-methyl-D-erythritol 4-phosphate (DOXP/MEP) pathway. Acta Biochim Pol. 2001;48(3):663–672. [PubMed] [Google Scholar]
- Yusuf MA, Kumar D, Rajwanshi R, Strasser RJ, Tsimilli-Michael M, Sarin NB. Overexpression of γ-tocopherol methyl transferase gene in transgenic Brassica juncea plants alleviates abiotic stress: physiological and chlorophyll a fluorescence measurement. Biochim Biophys Acta (BBA) Bioenerg. 2010;1797(8):1428–1438. doi: 10.1016/j.bbabio.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Zsigmond L, Tomasskovics B, Deák V, Rigó G, Szabados L, Bánhegyi G, Szarka A. Enhanced activity of galactono-1, 4-lactone dehydrogenase, and ascorbate–glutathione cycle in mitochondria from complex III deficient Arabidopsis. Plant Physiol Biochem. 2011;49(8):809–815. doi: 10.1016/j.plaphy.2011.04.013. [DOI] [PubMed] [Google Scholar]