Abstract
The cooperative role of vital components of the antioxidative defense pathway in addition to redox couples was studied in a growth-phase dependent manner at 20, 30, and 40 days after subculturing (DAS) in five different euryhaline microalgal strains (EMS) Scenedesmus MKB (B-S), Spirulina subsalsa (B-6), Anabaena sp. (B-7), Chlorella sp. (B-8), and Chlorosarcinopsis eremi (B-18) collected from waterlogged areas of Punjab, India and in two freshwater microalgal strains (FMS). EMS surpasses to maintain a high redox couple’s ratio ascorbic acid/dehydroascorbate (AsA/DHA), and reduced glutathione/oxidized glutathione (GSH/GSSG) through a close-knit pattern of antioxidative enzymes including high specific activities of ascorbate peroxidase (APX), monodehydroascorbate reductase (MDHAR), glutathione reductase (GR), dehydroascorbate reductase (DHAR) and less specific activity of glutathione peroxidase (GPX). While FMS struggled for the same irrespective of near similar total glutathione and higher specific activity of GPX might be answerable for the lesser redox ratio than EMS. However, high specific activity of catalase (CAT) might be sought to compensate for the less increase of APX in FMS. The fact significantly less H2O2, and malondialdehyde (MDA) with DAS in EMS than in FMS and higher redox ratios exquisitely elevate their tolerance ability making EMS a captivating prospect for cultivation in waterlogged areas. Additionally, their abundance of potent antioxidants further highlights the potential of EMS as an excellent source of these beneficial compounds.
Graphical Abstract
Keywords: Antioxidative enzymes, Ascorbate–glutathione cycle, Microalgal strains, ROS, Salt tolerant
Introduction
Microalgae are incredible organisms that can survive in any place and in extreme surroundings (Siddiki et al. 2022). The inimitable ability of microalgae to utilize sunlight more effectively than land plants permits them to produce more bioactive compounds (Sarwer et al. 2022). In recent ages, progressively more devotion was devoted to microalgae due to their unique potential. The great adaptation strategy of microalgae to live in innumerable physicochemical environments is the major advantage of this nutrient resource. They could act as beginning material for the food, feed, drug, clinical, and restorative industries (Kaur et al. 2023; Koyande et al. 2019). Salinity is a universal problem that influences nearly 20% of the entire arable land, and 33% of irrigated lands (Devkota et al. 2022) with 1 billion ha of salt-affected areas globally ( Omar et al. 2023) and is a continuous issue in numerous districts of the reality where the water system is important to support effective agricultural production. Furthermore, the saline zones are growing annually at a 10% rate for various reasons, counting high surface evaporation, slight rainfall, native rocks weathering, impoverished cultural performances, or salty water irrigations (Ullah et al. 2021).
In Punjab, India, the Southwest zone which is about 34% of the total area suffers from erratic and scanty rainfall problems. The shallow groundwater of these districts is alkaline and is moderate to highly saline, and is affected by waterlogging (Krishan et al. 2022). Waterlogging and increased soil salinity fosters land degradation and desertification, substantially diminishing agricultural practices and crop yields in affected areas (Prajapati et al. 2021). But these areas can be efficiently used for salt-tolerant microalgae cultivation. However, salinity may induce osmotic stress and trigger ROS overproduction which ultimately damages cells and biomass production (Hasanuzzaman et al. 2021). Saltiness-prompted ROS overgeneration is one of the first purposes behind hindering the morpho-physiological and biochemical activities which can be mostly re-established through upgrading the cell reinforcement protection framework that detoxifies ROS.
Ascorbic acid (AsA) and dehydroascorbate (DHA) are vital in redox state-based signaling, detoxifying ROS, and transmitting signals (Miret et al. 2017). Environmental cues modulate the AsA/DHA redox pair's pool and ratio, impacting enzyme and protein levels, resulting in enhanced stress tolerance (Xiao et al. 2021). Glutathione, a tripeptide (glutamic-glycine-cysteine), serves as a vital antioxidant in reduced (GSH) and oxidized (GSSG) forms (Al-Temimi et al. 2023). GSH protects against oxidative stress and maintains reduced molecules. The GSH/GSSG ratio influences cellular homeostasis and signaling, potentially impacting ROS perception. A lower GSH/GSSG ratio indicates higher oxidative stress, while unstressed cells typically have around 90% GSH and 10% GSSG (Fotopoulos et al. 2010). The escalating interest in the AsA/DHA and GSH/GSSG redox couples reveals profound insights into adaptive responses and stress tolerance against diverse challenges. Recent studies have concentrated on the various new types of microalgae, their bioproducts, nutritional properties, and applications. However, only limited literature is available regarding their antioxidant defense system under salinity-cum-alkalinity and their oxidative stability. Moreover, the impact of different antioxidative components in relation to their better survival is unclear. In accordance with these explanations, the current study attempted to comprehend the growth-phase-dependent cooperative role of various antioxidative enzymes, antioxidants and redox couple ratio’s for ameliorating salty conditions (EC 436–3510 siemen/cm and pH 8–11) in EMS isolated from natural water-logged areas of Punjab. This research could be beneficial to utilize EMS as the opportunity to be a profitable route to producing bioactive metabolites.
Materials and methods
Maintenance and harvesting of pure cultures of microalgal strains
The five euryhaline microalgal strains, Scenedesmus MKB (B-S) (Accession Number MN796425), Spirulina subsalsa (B-6), Anabaena sp. (B-7), Chlorella sp. (B-8) (Accession no. MW443083), and Chlorosarcinopsis eremi (B-18) (Accession Number MN832495) collected from natural waterlogged areas of Punjab, India and two freshwater microalgal strains Spirulina NCIM (S–N) and Chlorella NCIM (C-N) were obtained from Biogas Laboratory, Department of Renewable Energy Engineering, Punjab Agricultural University, India. The collected microalgal strains were photographed at 40 × magnification using the Magnus Icon 528,293 Freedom Model Microscope using the Toup view software program (Olympus, Tokyo, Japan) for identification. The culture conditions were optimized and were grown on suitable media i.e., B-S on Algal Culture Media (ACM) (Lembi and Waaland 1988) at pH (11.5–12.0), B-6 on ACM at pH (9.5–10.5), B-7 on BG11 at pH (9.5–10), B-8 on Bolds Basal Media (BBM) at pH (8.5–9.5), and B-18 on BBM at pH (10.5) in 50 L capacity covered fish tanks (Fig. 1 A-G). For subculturing, 300 µl dense cultures of each strain after autoclaving (25 min, 1 atm, 125˚C) were transferred in 500 ml wide mouth glass flasks loaded with 300 ml of desired fresh culture media placed under the standard laboratory conditions (25 °C temperature, 40 µmolm−2 s−1 light intensity, and 12:12 h light and dark photoperiod) capped with a cotton wool plug for proper aeration or gas exchange (Fig. 1 a–g). Then from these flasks, 10 ml aliquots of the culture were taken at 20, 30 and 40 days after subculturing (DAS), centrifuged for 5 min at 8000 g, and the pellet was stored (− 20 °C) for biochemical analysis.
Fig. 1.
Culturing of microalgal strains (A, a: B-6, B, b: B-7, C, c: B-8, D, d: B-18, E, e: B-S, F, f: S–N, G, g: C-N). Capital letters indicated culturing in covered fish tanks and small letters indicated subculturing in wide-mouth glass flasks
Enzyme assays
The homogenization of biomass (100 mg) was done in 2 mL potassium phosphate buffer (0.1 M, pH 7.5) comprising EDTA (1 mM), β-mercaptoethanol (10 mM), and polyvinylpyrrolidine (PVP) (1% w/v) by chilled pestle and mortar. The supernatant after centrifugation at 10,000 × g (4 °C, 25 min) was used in the assay of antioxidant enzymes superoxide dismutase (SOD), catalase (CAT), and glutathione reductase (GR). For ascorbate peroxidase (APX), 2 mM ascorbate was also added to the above extraction mixture. For glutathione peroxidase (GPX), 100 mg biomass homogenized in 2 ml potassium phosphate extraction buffer (50 mM) containing EDTA (0.6 mM), KCl (100 mM), ascorbate (1%) along with glycerol (10%), and the supernatant obtained after centrifugation (11,000 × g, 4 °C, and 20 min) used for estimation. SOD (EC 1.15.1.1) was estimated using the method of Marklund and Marklund (1974) with 0.1 M Tris–HCl buffer (pH 8.2), 6 mM EDTA, and pyrogallol (0.6 mM). CAT (EC 1.11.1.6) was estimated using H2O2 solution, 50 mM sodium phosphate buffer (pH 7.5), and enzyme extract (Chance and Maehly 1955). APX (EC 1.11.1.1) was estimated using 50 mM potassium phosphate buffer (pH 7.0), ascorbate (0.5 mM), H2O2 (0.4 mM), and enzyme extract (Nakano and Asada 1981). GR (EC 1.6.4.2) was estimated using reaction mixture containing 0.1 M potassium phosphate buffer (pH 7.5), 1.5 mM MgCl2, 2 mM EDTA, NADPH (0.025 mM), enzyme extract and oxidized glutathione (0.25 mM) (Smith et al. 1988). The GPX-specific activity was estimated following method of Elia et al. (2003) with enzyme extract, 50 mM potassium phosphate buffer (pH 7.0), NaN3 (0.1 mM), GSH (2 mM), NADPH (0.12 mM), H2O2 (0.6 mM), and one unit of GR.
The biomass (100 mg) was mixed with 2 mL of phosphate buffer (pH 7.5, 100 mM) containing PVP (2%), AsA (12 mM), and EDTA (1 mM). The mixture was homogenized, then centrifuged at 4 °C and 10,000 × g for 25 min, and the above extract was used for the estimation of monodehydroascorbate reductase (MDHAR) and dehydroascorbate reductase (DHAR) Hossain and Asada (1984). For MDHAR (EC 1.6.5.4), the reaction mixture contained 2 mL of 50 mM Tris–HCl buffer, 0.2 mM NADPH, 2.5 mM ascorbate, 0.2 mL enzyme extract, and one unit of ascorbate oxidase. For DHAR (EC 1.8.5.1), the reaction mixture consisted of 1 mL of 50 mM potassium phosphate buffer (pH 7.0), 0.5 mM dehydroascorbic acid (DHA), 2.5 mM GSH, and enzyme extract.
Extraction and Estimation of ascorbic acid (AsA), dehydroascorbate (DHA), total glutathione, reduced glutathione (GSH), oxidized glutathione (GSSG), and total glutathione (T. Glu.)
The biomass (100 mg) was homogenized in 2 ml chilled metaphosphoric acid (5%) and the supernatant obtained after centrifugation (10 min at 10,000 × g) was used for AsA and DHA estimation. For AsA the reaction mixture included supernatant, 5 mM EDTA, 1.7% TCA, 16 mM FeCl3 in 100 mM potassium phosphate buffer (pH 7.5), 7.6% o-phosphoric acid, and 44 mM bipyridyl (Law et al. 1983). For DHA the reaction mixture containing supernatant and 2% 2,4-dinitrophenylhydrazine in 9 N H2SO4 was used (Joseph et al. 1990). The biomass (200 mg) was homogenized with 2 ml of 5% metaphosphoric acid and centrifuged at 10,000 g for 25 min and the supernatant was used to measure total glutathione, GSH, and GSSG (Bashir et al. 2013). To estimate GSH, 0.6 ml of pH 7.0 potassium phosphate buffer with 5 mM EDTA, 0.4% 5,5′-dithiobis-(2-nitrobenzoic acid), and 100 μl of the supernatant were used. To estimate total glutathione, the same buffer with additional components was used. GSSG content was calculated as the difference between reduced and total glutathione content.
Estimation of H2O2 and malondialdehyde (MDA)
The lyophilized biomass (100 mg) was homogenized in 2 ml potassium phosphate buffer (pH 7.0, 10 mM) and was centrifuged (8000xg) for 10 min. The reaction mixture (1.0 ml supernatant, 1 ml potassium phosphate buffer (10 mM, pH 7.0), 2 ml 1:3 v/v of potassium dichromate (5%), and glacial acetic acid) was filtered and measured absorbance at 570 nm (Mirza 1965). For MDA, 100 mg lyophilized biomass was homogenized in 2 ml of TCA (5% w/v) and the supernatant obtained after centrifugation (1000xg, 15 min) was mixed with an equal amount of TCA (20% w/v) and TBA (0.5%) were used for estimation by the method of Heath and Packer (1968).
Statistical analysis
The data obtained were analyzed by analysis of variance (ANOVA) using RStudio version 4.2.0 (RStudio, Inc., Boston, MA). The differences between mean values were compared using Tukey’s multiple comparison tests with a significance level of p ≤ 0.05. The correlation analysis was done by using Pearson’s correlation coefficient. Different letters presented in the figures as superscripts illustrate significant differences among the microalgal strains at different DAS at p ≤ 0.05.
Results
The microscopic identification of EMS (B-S, B-6, B-7, B-8, and B-18) collected from waterlogged areas of Punjab was represented in Fig. 2.
Fig. 2.
Microscopic identification of euryhaline microalgal strains (a, B-S; b, B-6; c, B-7; d, B-8; and e, B-18)
Growth-phase-dependent variation of antioxidant defense components in different EMS and FMS
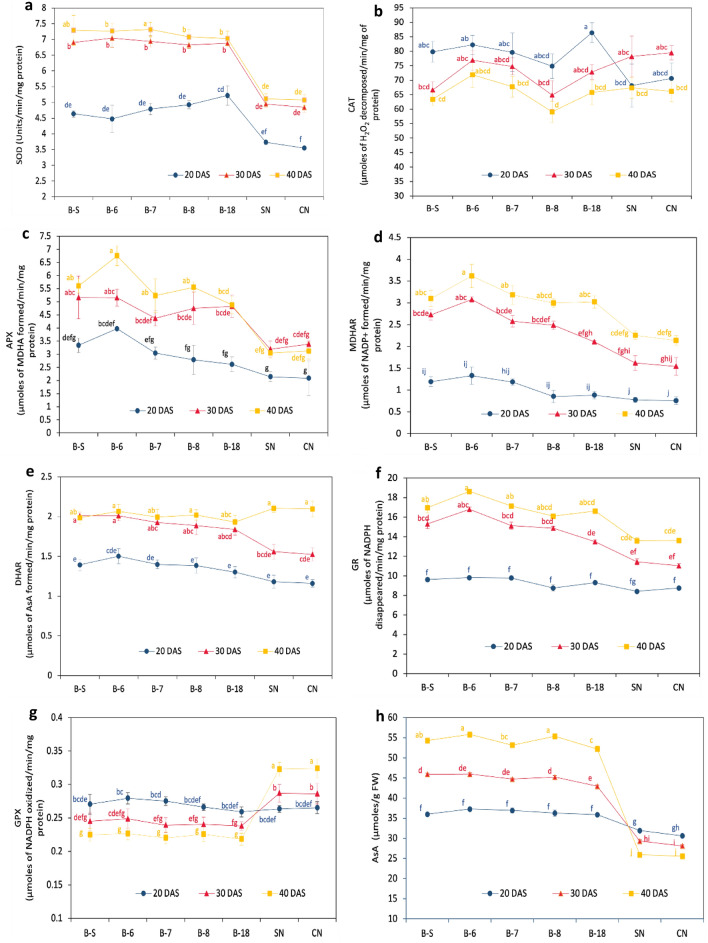
The assessment of antioxidant defense mechanisms encompassed a comprehensive evaluation of antioxidative enzyme activities and antioxidant content in the fresh biomass of five EMS (B-S, B-6, B-7, B-8, and B-18) and two FMS (S–N and C-N) at three distinct growth stages, 20 DAS, 30 DAS, and 40 DAS. This intricate analysis aimed to discern variations between marine and freshwater species while also shedding light on the impact of growth on the antioxidant defense responses. Analysis of variance exhibited the differential pattern and depicted that different EMS were significantly varied from FMS at 20DAS, 30DAS, and 40DAS (Tukey’s test, p ˂ 0.05) (Fig. 3).
Fig. 3.
The specific activities a SOD, b CAT, c APX, d MDHAR, e DHAR, f GR, and g GPX; the content of h AsA, i DHA, j GSH, k GSSG, l T. Glu; redox pair ratios m AsA/DHA and n GSH/GSSG, and stress indicators o H2O2 and p MDA in different microalgal strains (B-S, B-6, B-7, B-8, and B-18, S–N and C-N) at 20, 30, and 40 days after subculturing (DAS). Data represent the mean of triplicate values; error bars represent the SD. Different letters explain the significant differences (p ≤ 0.05) among the cultivars at 20, 30, and 40 DAS
Variation in specific activities of antioxidative enzymes
The SOD-specific activity was meaningfully higher in EMS than in FMS at 20, 30, and 40 DAS. With the progress of DAS, SOD-specific activity was significantly increased in EMS as well as FMS but the increase was much higher in EMS than FMS. At 40DAS, the increase was maximum in B-6 (62%) and B-S (57%) followed by B-7 (52%), and B-8 (43%). From 20 DAS to 30 and 40 DAS, the specific activity in EMS lay in the range of 6.88–7.31 Units/min/mg protein whereas in FMS this value corresponds to 3.26–4.31 Units/min/mg protein (Fig. 3-a). Instead, CAT-specific activity decreased significantly in EMS with DAS whereas in FMS it increased from 20 to 30 DAS and then again decreased at 40 DAS. At 40DAS, B-18 showed a CAT-specific activity of 65.799 µmoles of H2O2 decomposed/min/mg of protein with a maximum decrease of 23.8%. B-8 and B-S showed a decrease of 21.1%, and 20.5%, respectively (Fig. 3b). Whereas, in view of FMS, CAT activity initially increased at 30DAS, by 14.7% in S–N and 12.5% in C–N, however a further rise in DAS, decreased CAT activity significantly in C–N (6.2%).
A significant upsurge in the APX-specific activity was reported under a growth-phase-dependent manner in EMS and the maximum rise was detected from 20 to 40 DAS. B-8 showed a significant maximum increase of 99.1% followed by B-18 (86.4%), B-7 (71.9%), B-6 (69.7%), and B-S (67.7%). From 20 to 30 DAS the maximum specific activity was observed in B-18 (84.4%) and B-8 (70.3%). B-S, B-6, and B-7 also showed a significant rise of 54.4%, 29.6%, and 43.5%, respectively. Similarly, the specific activity was also improved in FMS at 30 DAS from 20 DAS. S–N and C–N showed an increase of 49.1 and 62.3%. However, the specific activity was further decreased with an increase in growth from 30 to 40 DAS in FMS. S–N possessed specific activity of 3.049 µmoles of MDHA formed/min/mg protein at 40 DAS which was in the lowest range (Fig. 3c). Additionally, the growth-phase-dependent significant upsurge in MDHAR-specific activity was reported in different EMS in addition to FMS. Whereas the increase was comparatively higher in EMS than in FMS. From 20 to 40 DAS, though the maximum specific activity was studied in B-6 (3.616 µmoles of NADP+ formed min−1 mg−1 protein), the maximum rise was found in B-8 (2.5-fold) and B-18 (2.4-fold), followed by B-6 (1.7-fold), B-7, and B-S (1.6-fold). While in FMS, a onefold increase was reported in S–N and C–N at 30 DAS and a 1.8–1.9-fold rise in S–N and C-N at 40 DAS (Fig. 3d). The DHAR-specific activity was augmented significantly from 20 to 40 DAS in EMS and a similar trend was observed in FMS as well. From 20 to 40 DAS, the specific activity was increased by 42% in B-S and B-7, 48.3% in B-18, 45.9% in B-18, and 37.4% in B-6. Although from 30 to 40 DAS, a significantly very less increase was reported in EMS. On the other hand, in FMS, a 31.9 and 31.4% rise was found in S–N and C–N separately from 20 to 30 DAS. Conversely, FMS showed a profound rise in DHAR-specific activity at 40 DAS from 20 DAS. S–N, and C–N showed 78.0 and 80.6% increase, respectively (Fig. 3e). The specific activity of S–N at 40 DAS lay in the highest range (2.103 µmoles of AsA formed/min/mg protein).
In all the microalgal strains either EMS or FMS, the GR-specific activity was observed to be increased with DAS and was maximum at 40 DAS. But the increase was significantly higher in EMS in contrast to FMS. At 40 DAS from 20 DAS, B-6 and B-8 showed a maximum increase by 89, and 83%, respectively. A near about 75% increase was reported in B-S and B-7. While FMS possessed the activity in the lowest range, and 61.6 and 55.4% rises were reported in S–N and C–N (Fig. 3f). The GPX-specific activity declined significantly in all the EMS, whereas the maximum decrease of 19.9 and 18.9% was reported in B-7 and B-6, respectively at 40 DAS from 20 DAS. In B-S, B-18, and B-8, a significant decrease of 16.9, 15.7, and 15.2% were reported. Contrary to it, growth-phase-dependent significant rises were observed in FMS. S–N (0.322 µmoles of NADPH oxidized min−1 mg−1 protein), and C–N (0.323 µmoles of NADPH oxidized min−1 mg−1 protein) showed the highest specific activity at 40 DAS from 20 DAS with an increase of 22.5 and 22.1%, respectively (Fig. 3-g).
Variation in AsA, DHA GSH, GSSG, and total glutathione
The AsA was increased significantly in all the EMS in a growth-phase dependent manner and was increased by 43–49% in B-7, B-18, and B-6 at 40 DAS from 20 DAS whereas the increase in B-S and B-8 laid in the highest range with increase of 50–52%. Contrary to it, the content significantly declined in FMS with a maximum decrease of 16–18% in CN at 40 DAS (Fig. 3h). While looking into DHA, the trend was differing, DHA content was increased significantly at 30 and 40 DAS in FMS, whereas a decline of 30–36% was observed in all EMS at 40 DAS which were in the lowest range (Fig. 3i). In all EMS, GSH content was significantly increased at 30 DAS and 40 DAS, whereas the highest increase was reported at 40 DAS from 20 DAS in B-7, B-8, and B-18 which were in the range of 25–31%. FMS showed a decline in GSH with an increase in growth. A decrease of 2.6–4.3% and 6–7.3% were reported at 30 DAS and 40 DAS (Fig. 3j). Contrary to it, GSSG content was significantly increased in a growth-phase-dependent manner in FMS and decreased in EMS. Near about onefold increase in GSSG was reported in C–N at 40DAS (Fig. 3k). Though the variation was found in GSH and GSSG, total glutathione was significantly similar in FMS at 20 DAS, 30 DAS, and 40 DAS while significantly varied in EMS, highest at 40 DAS and 30 DAS (Fig. 3l).
Variation in AsA/DHA and GSH/GSSG
AsA/DHA content was profoundly increased in EMS in comparison to FMS. 1.2–1.34-fold was observed in EMS at 40 DAS. In FMS, 21–26% and 36–38% decreases were observed at 30 and 40 DAS (Fig. 3m). A similar trend was observed for GSH/GSSG, where a 1.2–1.6-fold increase was found in EMS at 40 DAS and a 45–57% decrease was reported in FMS at 40 DAS, and a 20–36% decrease at 30 DAS (Fig. 3n).
Growth-phase-dependent variation of H2O2 and MDA in different EMS and FMS
A significant reduction in H2O2 content was detected in EMS at 30DAS and 40DAS. The maximum decrease was observed in B-6, B-7, and B-18 (35–36%) at 40DAS. While FMS showed an increased H2O2 content with DAS. S–N and C–N showed a rise by 7–10% and 16–17% at 30 and 40 DAS, respectively (Fig. 3o). On the other hand, a growth phase-dependent decrease was reported in all EMS. At 30 DAS a decline of 12–21% and 24–34% was reported at 30 DAS, and 40 DAS. Contrary to it, MDA content was slightly increased in FMS at 30 and 40 DAS and decreased in EMS with the progress of growth after subculturing (Fig. 3p). At 40 days after sowing (DAS), the highest observed increase of 34.9% was reported in B-7, while the remaining EMS demonstrated growth ranging from 24.5 to 27.7%. At 30 DAS, the increases ranged from 17.09 to 25.8% for the various EMS. Notably, B-S exhibited the lowest increase, measuring merely 12.5%. Whereas in FMS, a decrease by 11.3% and 17.1% were reported in S–N and C–N, respectively.
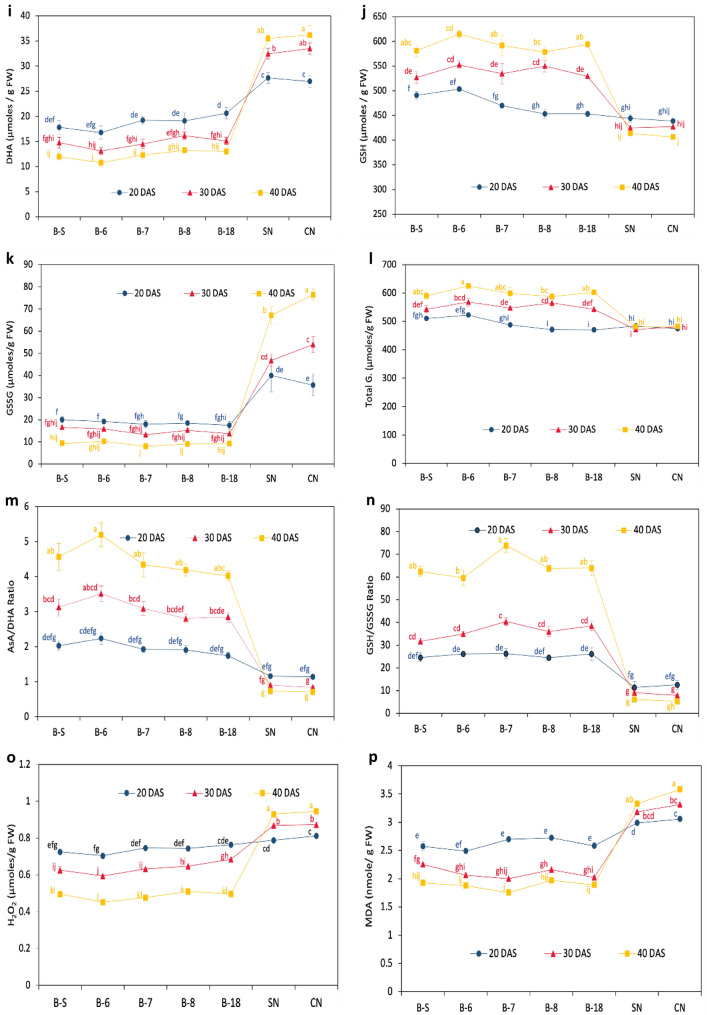
The comparison of growth-phase-dependent variation of antioxidant defense components and oxidative stress indicators between combined EMS and FMS
The synergistic effect of five different EMS (B-S, B-6, B-7, B-8, and B-18) and two FMS (S–N and C–N) on antioxidant defense system components with an increase in growth at different DAS has been represented in Fig. 4. Analysis of variance depicted that all components varied significantly between EMS and FMS and were further influenced by growth after subculturing. At 20 DAS, the specific activities of the studied antioxidative enzymes displayed noteworthy variations. MDHAR and APX exhibited the most significant differences, with EMS showing 51 and 49% higher specific activities, respectively, compared to FMS. Following this trend, DHAR showed a 19% increase, CAT showed 16% more activity, SOD had 24% higher activity, and GR possessed 9% more specific activity in EMS compared to FMS. Whereas, the GPX showed a variation of 2% which could be relatively negligible. Most significant results were obtained in the case of SOD and GR at 20DAS amongst all antioxidative enzymes (Tukey’s test, p < 0.0001) (Fig. 4a–f). While considering non-enzymatic components of AsA-GSH cycle, AsA and GSH were relatively more by 16 and 7% in EMS. In contrast, DHA and GSSG showed a significant decrease of 31 and 50% than EMS. However, total glutathione was higher only by 2% in EMS which could not be a major factor differentiating marine from freshwater strains. Whereas AsA/DHA and GSH/GSSG had a profoundly higher value of 71 and 113% in EMS depicting their vital role in the survival of EMS. H2O2 and MDA content was lessened by 7 and 13% separately in EMS as compared to FMS.
Fig. 4.
The pooled specific activities a SOD, b CAT, c APX, d MDHAR, e DHAR), f GR, and g GPX; the content of h AsA, i DHA, j GSH, k GSSG, l T. Glu; redox pair ratios m AsA/DHA and n GSH/GSSG, and stress indicators o H2O2 and p MDA in all euryhaline (EMS) and freshwater microalgal strains (FMS) at 20, 30, and 40 days after subculturing (DAS). Data represent the mean of triplicate values ± SD. Different letters explain the significant differences (p ≤ 0.05) among the cultivars at 20, 30, and 40 DAS. (*), (**), (***), (****) represent the significant difference at p < 0.05, < 0.01, < 0.001, and < 0.0001 and ns represent non-significant difference
At 30 DAS from 20 DAS, the antioxidant defense parameters showed a completely varied pattern in EMS and FMS. The SOD and CAT-specific activities were less by 7 and 9% in EMS than FMS, whereas MDHAR, APX, GR, and DHAR-specific activities were reported to be higher in EMS by 64, 47, 34, and 25%, respectively. In this stage, GPX was significantly less by 15% in EMS in comparison to FMS. SOD and CAT significantly differed in EMS and FMS (p < 0.05) (Fig. 4a–b), while APX, MDHAR, DHAR, GR, and GPX varied most significantly (p < 0.0001). AsA and GSH presented a significantly high value of 59 and 26% in EMS than FMS. Instead, oxidized forms, DHA, and GSSG profoundly decreased by 55 and 70% in EMS in relation to FMS. Being streamlined to the variation, total Glu. varied significantly by 16% more in EMS than in FMS. Among all the antioxidant parameters, redox pair ratios AsA/DHA and GSH/GSSG exhibited an overwhelming high value of 2.5 and 3.2-fold in EMS than in FMS and demonstrate their role in salinity-cum-alkalinity conditions (Fig. 4m, n). Moreover, the stress indicators H2O2 and MDA were further abridged by 26 and 35%, individually in EMS than FMS.
In the case of 40DAS, there were noteworthy variances between the components of the antioxidant defense system whereas SOD was an exemption as they had a similar decrease of 7% specific activity at 30DAS and 40DAS in EMS as compared to FMS, with an average of 5.61 Units/min/mg protein, and 6.08 Units/min/mg protein specific activities in EMS and FMS, respectively (Fig. 4a). Following the same trend, CAT-specific activity was not significantly different between EMS and FMS with an average value of 66.02 µmoles of H2O2 decomposed/min/mg of protein (Fig. 4b). Nevertheless, APX showed a high value of 81% in EMS than FMS which was quite higher than 20DAS and 30DAS. Contrary to it, the MDHAR value was higher by 44% in EMS in comparison to FMS which was a bit less than the increase difference at 30DAS (64%), whereas DHAR showed a complete diversion. EMS possessed less DHAR-specific activity by 4% relative to FMS which was opposite to the trend at 30DAS, where EMS had 25% more specific activity than FMS (Fig. 4-e). The reported increase in GR activity in EMS (25%) was comparatively smaller than the increase observed at 30 DAS (34%) in FMS. Conversely, GPX displayed a slightly higher decrease in EMS (30%) at 30 DAS as compared to FMS, where a decrease by 15% was reported. AsA showed a significant rise in EMS (1.1-Fold) than FMS, but DHA decreased comparatively by 65% in EMS (Fig. 4h–i). GSH showed an increased variation of 44% and GSSG had decreased value by 87% in EMS relative to FMS, whereas total Glu. was higher by 24% in EMS than FMS though this difference was only 16% at 30DAS (Fig. 4-j-l). While in view of redox pairs, AsA/DHA showed a very intense increase of 5.1-fold in EMS comparatively to FMS and was furthermore higher in GSH/GSSSG, as EMS showed a 10.5-fold increase in EMS than FMS. H2O2 and MDA showed reduced value in EMS which was 48 and 45% less in relation to FMS. Except for CAT and DHAR, which non-significantly differ between EMS and FMS, all other parameters showed the most significant variation (p < 0.0001) (Fig. 4).
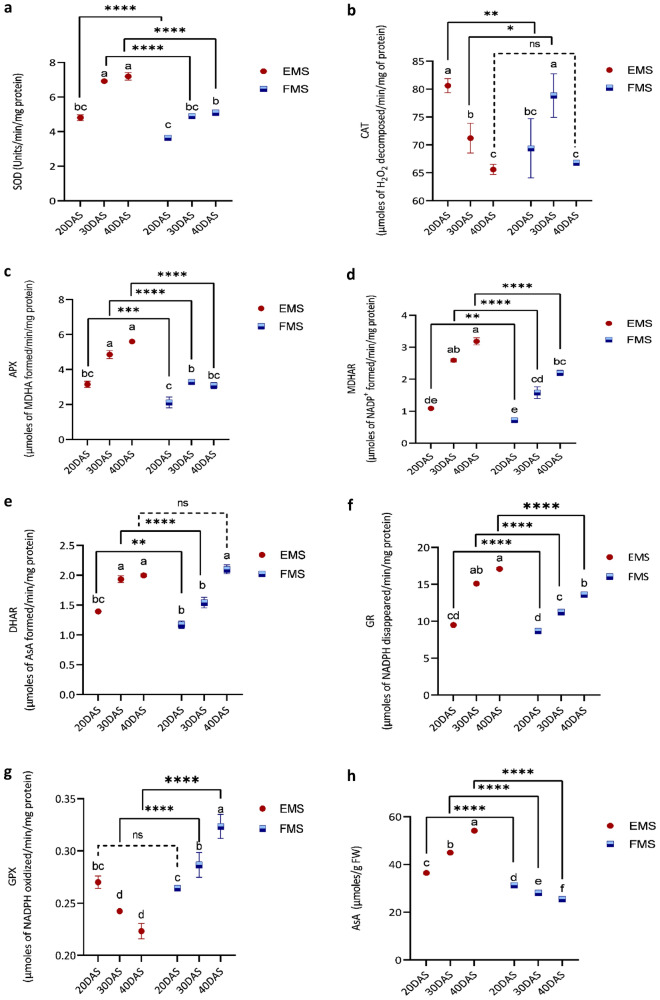
Correlation analysis amongst various antioxidant defense system components in different EMS and FMS
The impact of different antioxidant defense system components in EMS and FMS at different growth stages was evaluated using correlation analysis (Fig. 5a, b, respectively) in order to study the role of different enzymatic and non-enzymatic components of AsA-GSH cycle. In EMS, SOD was highly positively correlated to GR (r = 0.93), MDHAR (r = 0.92), DHAR (r = 0.91), and APX (0.81). While SOD was highly negatively correlated with GPX (− 0.86) and CAT (− 0.68). Instead, SOD weekly positively correlated with CAT (r = 0.12) and highly positively with GPX (r = 0.79) in FMS but other antioxidative enzymes followed the same trend in FMS like EMS. In EMS, GPX and DHA are near about equally negatively correlated with SOD (r = − 0.86 and r = − 0.82) whereas in FMS, GPX and DHA are positively correlated with SOD with r = 0.79 and r = 0.92, respectively. In comparison to the SOD trend, the correlation depicted by CAT is much weaker with little varied correlation coefficient in FMS and EMS as well. APX showed the highest constructive correlation with total glutathione (r = 0.90) in EMS whereas no correlation has been reported between them in FMS. MDHAR, GR, and DHAR are positively correlated with APX in both EMS and FMS whereas the correlation predominates in the former. Contrary to it, APX is negatively correlated with GPX (r = − 0.69) in EMS but positively in FMS (r = 0.60). Overall, MDHAR and GR are profoundly positively correlated (r = 0.99), and AsA is positively correlated with GSH (r = 0.94) in EMS. H2O2 is intensely correlated negatively with AsA (r = − 0.97) and GSH (r = − 0.96) in EMS (Fig. 5a). While in view of redox pairs, AsA/DHA is very certainly correlated with GSH and damagingly correlated with H2O2 both in EMS and FMS, while GSH/GSSG is sturdily correlated positively with AsA and negatively with GPX in EMS along with FMS highlighting their importance in maintaining the redox status. Total Glutathione and GSH/GSSG ratio depicted a highly positively correlated (r = 0.85) in EMS whereas it showed a negative correlation in FMS (r = − 0.35).
Fig. 5.
Pearson’s correlation coefficient (r) among different biochemical parameters in a EMS and b FMS. The size of the pie is relative to the percent of correlation. Red color-negative correlation, blue color-positive correlation, T.G.-total glutathione, A.-AsA/DHA, and G.-GSH/GSSG
Discussion
As the earliest investigations, it has been evident that microalgae exposed to several ecological surroundings are armed with an improved level of the antioxidant pool to alleviate organelle mutilation prompted by ROS but then the antioxidants formed will differ between species (Li et al. 2007). To identify more tolerant microalgal species that can withstand higher salt-cum-alkaline stress, one needs to figure out which antioxidative enzymes or antioxidants are primarily involved in the stress adaptation mechanism and how they function. The question could only be answered after the elucidation of effectual biochemical antioxidant pathways involved in microalgal strains already surviving under natural waterlogged areas.
SOD, CAT, APX, and GPX are significant first-line defense molecules and crucial in the complete defense stratagem of antioxidants, specifically in consideration of superoxide anion radical (*O2) that is eternally produced in ordinary metabolism. The advanced SOD-specific activity in EMS at 20 DAS than FMS might be responsible for the antioxidant defense system activation to remove (*O2) and form H2O2 and O2 molecules (Wang et al. 2018). As the growth progresses from 20 DAS, so as to satiate the accumulated (*O2), SOD-specific activity endured on the advanced side in EMS indicated from higher specific activity both at 30 DAS and 40 DAS as compared to FMS. This specified the better removal of (*O2) by SOD in EMS with the progress of growth after subculturing to surmount the effect of saline and waterlogged environments. Parallel outcomes were reported by Farghl et al. (2015) that salinity caused a persistent increase in SOD activity in C. salina. SOD activity improved significantly in salt-tolerant Antarctic microalga Chlamydomonas sp. L4 as studied by Kan et al. (2012), and salinity pre-treatment showed a five-fold rise in SOD in Anabaena PCC7120 (Srivastava et al. 2023). However, Minhas et al. (2016) reported that SOD activity exhibited a twofold increase at 1 M and a threefold increase at 2 M NaCl than 0.5 M NaCl but further rise in salinity beyond 2 M NaCl caused the decline in SOD activity was noted.
With the progress of DAS, a noteworthy variance was detected in CAT-specific activity amid the microalgal species studied. Initially, at 20 DAS, high CAT-specific activity in EMS than FMS might be accountable for maintaining redox homeostasis. A decrease in activity after 20 DAS indicated that might other biochemical pathways overtake H2O2 scavenging and CAT might not perform well in EMS with the progress of growth. Whereas, the high CAT-specific activity in FMS at 30 DAS was accountable for the effectual elimination of SOD-produced H2O2. In contrast, higher APX-specific activity in a growth-phase dependent manner in EMS might be due to the recompense of lesser CAT-specific activity and APX tried to boost the antioxidative mechanism in EMS as APX reduced H2O2 to water and DHA by consuming AsA as a reducing molecule. This is in agreement with correlation analysis that APX and CAT are negatively correlated (r = − 0.56) in EMS. Moreover, the positive correlation possessed by APX and SOD in EMS indicated the primary degeneration of produced H2O2 to sustain salinity-cum-alkalinity. This amplified specific activity of APX was also in harmony with the results of Chen et al. (2016) who reported that salt stress encouraged APX activity in C. reinhardtii at 100 mM NaCl, composed with other ascorbate-linked antioxidant enzymes including GR, which also contributes in H2O2 detoxification. However, a decrease in APX activity was noted in 200 mM NaCl salt stress (0.020 ± 0.005 U mg−1 of protein) than control (0.027 ± 0.003 U mg−1 of protein) in green microalgae Chlamydomonas reinhardtii (Fal et al. 2022).
In turn, MDHAR is sequentially accountable for generating AsA from transitory MDHA by using NADPH. Higher MDHAR-specific activities at 30 and 40 DAS in EMS resembled the high values of AsA in a growth-phase-dependent manner. This is supported by a previous study by Zhang et al. (2022) who stated that MDHAR activity in salt-tolerant oats significantly increased under 100 mM NaCl with a significantly high AsA content, and AsA/DHA ratio but limited the rise in DHA. In a similar way, it might also be accountable for providing confrontation to high salt surroundings in EMS. The high MDHAR-specific activity with advancement beyond growth in EMS indicated the continual struggle of MDHAR to regulate the MDHA generation, a free-radical formed by APX. DHAR caused DHA reduction to AsA by means of GSH as an electron donor. Therefore, DHAR along with MDHAR concerned with restoring the AsA pool and maintaining the redox state with a high AsA/DHA ratio. With the increase in DAS, the augmented MDHAR and DHAR-specific activities in EMS resulted in their coordinated action in the restoration of AsA and were highly positively correlated to each other and both displayed nearly parallel correlation patterns whereas the reverse correlation of MDHAR and DHAR with AsA was reported in FMS. In harmony, a more positive correlation of MDHAR with APX, SOD, AsA, and GSH in comparison to DHAR indicated MDHAR as a vital performer involved in reinstating the ascorbate–glutathione pool in EMS. Our results are in agreement with the study of Hossain et al. (2010) that MDHAR is perilous for preserving an optimum level of AsA. The less specific activity of DHAR in FMS at 20 and 30DAS might be because of the less oxidative stress and effective ROS quenching by nearly other antioxidative enzymes in EMS. The trend of MDHAR and DHAR-specific activities in a growth-phase-dependent manner in FMS coincided with the DHA accumulation which supported the hypothesis given by Rather et al. (2023) stated that low MDHAR and DHAR activities are linked to DHA accretion in spite of restocking ascorbate pool. The significant AsA regeneration in EMS as indicated by the profoundly increased AsA/DHA ratio depicted an effectually high rate of AsA accumulation. This renaissance was highly essential for the appropriate scavenging of accrued ROS. In disparity, MDHAR exhibited a contrasting trend, showing a decrease of 77, 76, and 82% in its activities under 15, 30, and 60 mg Cr (VI) treatments, respectively, in comparison to the control in Helianthus annuus L. as reported by Kumar et al. (2023).
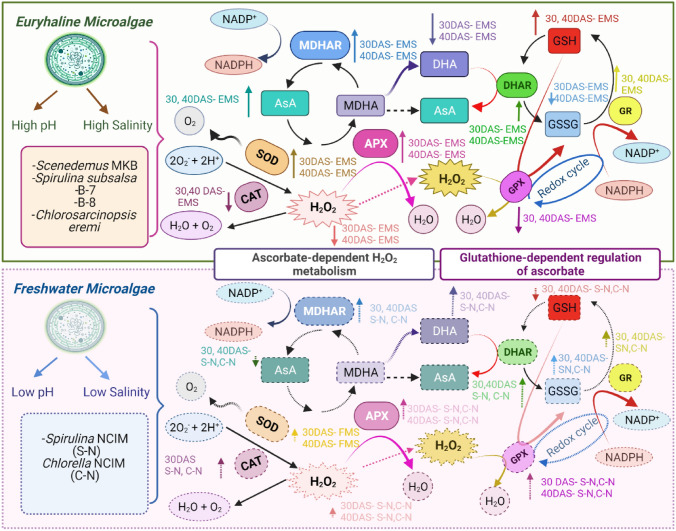
GR is accountable for continuing GSH supply which is amongst the furthermost copious reducing thiols which play vital roles in ROS control. The trend of high specific activities of GR along with APX with the progress of DAS in EMS implied that APX and GR cycle was well-organized in providing resistance to high salinity in EMS. Higher GR-specific activities correspondingly sustained GSH levels which consequently improved antioxidant potential and coupled numerous antioxidative pathways in EMS. GSH is essential for AsA regeneration from MDHA and DHA and itself gets transformed to GSSG. GR is a vital component of the AsA-GSH cycle to sustain equilibrium between the GSH/GSSG ratio under stress (Hasanuzzaman et al. 2017). Our outcomes are in harmony that GR activity increased under salinity in the salt-adapted cyanobacterium Anabaena fertilissima in 250 mM NaCl treated cells as studied by Swapnil et al. (2018). This is also supported by the study of Prajapati et al. (2023) that APX and GR activities increased with AsA/DHA and GSH/GSSG ratios, respectively in Allium cepa L. under salinity stress. GPX reduces H2O2 to H2O by using GSH and converting it into GSSG and limits its damaging effects. The less GPX-specific activity at 30 and 40 DAS in EMS might be in charge of upholding the GSH pool with a high GSH/GSSG which could efficiently help in maintaining high redox potential. Whereas in FMS, high GPX-specific activity with progress after subculturing might compensate for the decrease of APX activity in them and help in degenerating harmful H2O2 more dominantly through this pathway whereas generating more oxidized form GSSG with almost similar total glutathione content. Consequently, this could be accountable to the low GSH/GSSG ratio in freshwater in relation to EMS. An expanded GSH content and higher GR-specific activity under saltiness might be a practical system to keep a stable oxidative state. The high negative correlation of GPX with GSH might contribute to the less accumulation of GSH at 30 and 40 DAS in FMS which possessed high GPX-specific activity in order to quench the excess H2O2 formed at the expense of less APX activity. GPX and GSSG are positively correlated and consequently decreased GSH/GSSG ratio by increasing GSSG also portrayed by negative correlation amid them. This is in accordance with the fallouts of Shah et al. (2021) that karrikinolide eases salinity in wheat by increasing GSH, reducing GSSG, and maintaining a high GSH/GSSG thus regulating the redox. Similarly, the GSH/GSSG ratio showed a decline from the control to Pb treatment, Yet, salicylic acid had a beneficial effect by improving this ratio at the lethal dose of 2000 mg Pb (Agnihotri et al. 2018). In this study, the selected EMS were found to be oxidatively more stable by sustaining high redox ratios AsA/DHA and GSH/GSSG by early initiation and advanced activities of antioxidative enzymes which might be in authority for ameliorating oxidative stress under salinity (Fig. 6).
Fig. 6.
The coordinated interplay of diverse antioxidative enzymes and antioxidants in facilitating resistance to euryhaline (upper box) and freshwater (lower box) microalgal strains
The lower level of oxidative stress in EMS is further indicated by a growth-phase-dependent significant reduction of H2O2 and MDA in EMS than in FMS and is linked to specific advantages, especially in terms of enhancing resilience under conditions of waterlogging. MDA forms due to the oxidation of lipids in cell membranes caused by ROS. Likewise reported, under application of jasmonic acid, the MDA content decreased by 12, 18, and 9% for 500, 1000, and 2000 mg in Pb + jasmonic acid treatments, when compared to Pb-alone counterparts in Brassica juncea L. (Agnihotri and Seth 2020). Furthermore, Kumar and Seth (2022) reported Helianthus annuus L. demonstrated effective protection against lipid peroxidation by bolstering the synchronized functioning of enzymatic antioxidants (SOD, APX, GR) and non-enzymatic GSH, and AsA against Cr (IV) accumulation. In EMS, lower MDA levels suggest that cell membranes are subject to less oxidative harm, contributing to the preservation of membrane integrity and overall cellular well-being. This could suggest the presence of efficient strategies to counteract oxidative stress, which might play a role in their improved ability to endure stress conditions. This might be attributed to reinforced antioxidant mechanisms discussed above that remove and counteract ROS, and hinder the buildup of harmful compounds such as H2O2 and MDA.
Conclusion
The assessment of the antioxidant defense pathway underlines the role of exigent components involved in the survival of EMS. The synchronized action of the antioxidative enzymes and other components of the AsA-GSH cycle provided go-ahead stability to different EMS with growth indicated from higher specific activities of SOD, APX, MDHAR, DHAR, and GR than FMS. MDHAR outperforms in comparison to DHAR in the regeneration and accumulation of AsA, making it sufficiently available to APX for removal of excess H2O2 produced by SOD in EMS (Fig. 6). The enhanced activity of APX strained to pay off the less CAT-specific activity in EMS. Instead, high CAT-specific activity in FMS contributed to its role in the active removal of H2O2 generated by SOD. Furthermore, high redox pair ratio’s AsA/DHA and GSH/GSSG in EMS and their significant high positive correlation with APX followed by SOD, MDHAR, and GR helped in maintaining redox homeostasis. High GPX activity in FMS might be answerable for the lesser redox ratios irrespective of having near about equal glutathione like EMS. In crux, EMS exhibited higher values of AsA/DHA and GSH/GSSG than FMS through a close-knit pattern of antioxidative enzymes and metabolites involved in AsA-GSH cycle consequently marking their adaptation ability in waterlogged areas and could be a potent source of antioxidants.
Acknowledgements
The author (s) would like to convey their sincere thanks to Mr. Pawan Kumar Goel, Dr. Kiran Tewari, Chemical Resources (CHERESO), Panchkula, Haryana, India; Science & Engineering Research Board (SERB); Federation of Indian Chambers of Commerce and Industry (FICCI), India.
Abbreviations
- AsA
Ascorbic Acid
- APX
Ascorbate Peroxidase
- CAT
Catalase
- DAS
Days after Subculturing
- DHA
Dehydroascorbate
- DHAR
Dehydroascorbtae Reductase
- EMS
Euryhaline Microalgal Strains
- FMS
Freshwater Microalgal Strains
- GPX
Glutathione Peroxidase
- GR
Glutathione Reductase
- GSSG
Oxidized Glutathione
- GSH
Reduced Glutathione
- MDHAR
Monodehydroascorbtae Reductase
- ROS
Reactive Oxygen Species
- SOD
Superoxide Dismutase
- T. Glu
Total Glutathione
Author Contribution
MK: conceptualization, writing–original draft, methodology, investigation, data curation, formal analysis; SB: conceptualization, investigation, writing–review & editing, supervision; MKS: methodology, data curation, writing–review & editing, validation; UGP: conceptualization, writing–review & editing.
Funding
This work was jointly supported by the Science and Engineering Research Board, Federation of Indian Chambers of Commerce and Industry, and Chemical Resources (CHERESO) Panchkula under the Prime Minister Research Fellowship Program.
Declarations
Conflict of Interest
The authors declare they do not have any known competing financial interests or individual connections that could have seemed to impact the work described in this paper.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Agnihotri A, Seth CS. Does jasmonic acid regulate photosynthesis, clastogenecity, and phytochelatins in Brassica juncea L. in response to Pb-subcellular distribution? Chemosphere. 2020;243:125361. doi: 10.1016/j.chemosphere.2019.125361. [DOI] [PubMed] [Google Scholar]
- Agnihotri A, Gupta P, Dwivedi A, Seth CS. Counteractive mechanism (s) of salicylic acid in response to lead toxicity in Brassica juncea (L.) Czern. cv. Varuna Planta. 2018;248:49–68. doi: 10.1007/s00425-018-2867-0. [DOI] [PubMed] [Google Scholar]
- Al-Temimi AA, Al-Mossawi AEB, Al-Hilifi SA, Korma SA, Esatbeyoglu T, Rocha JM, Agarwal V. Glutathione for food and health applications with emphasis on extraction, identification, and quantification methods: a review. Metabolites. 2023;13(4):465. doi: 10.3390/metabo13040465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashir H, Ahmad J, Bagheri R, Nauman M, Qureshi MI. Limited sulfur resource forces Arabidopsis thaliana to shift towards non-sulfur tolerance under cadmium stress. Environ Exp Bot. 2013;94:19–32. doi: 10.1016/j.envexpbot.2012.05.004. [DOI] [Google Scholar]
- Chance B, Maehly AC. Assay of catalases and peroxidases. Methods Enzymol. 1955;2:764–775. doi: 10.1016/S0076-6879(55)02300-8. [DOI] [PubMed] [Google Scholar]
- Chen X, Tian D, Kong X, Chen Q, Abd Allah EF, Hu X, Jia A. The role of nitric oxide signalling in response to salt stress in Chlamydomonas reinhardtii. Planta. 2016;244:651–669. doi: 10.1007/s00425-016-2528-0. [DOI] [PubMed] [Google Scholar]
- Devkota KP, Devkota M, Rezaei M, Oosterbaan R. Managing salinity for sustainable agricultural production in salt-affected soils of irrigated drylands. Agric Syst. 2022;198:103390. doi: 10.1016/j.agsy.2022.103390. [DOI] [Google Scholar]
- Elia AC, Galarini R, Taticchi MI, Dorr AJM, Mantilacci L. Antioxidant responses and bioaccumulation in Ictalurus melas under mercury exposure. Ecotoxicol Environ Saf. 2003;55:162–167. doi: 10.1016/S0147-6513(02)00123-9. [DOI] [PubMed] [Google Scholar]
- Fal S, Aasfar A, Rabie R, Smouni A, Arroussi HE. Salt induced oxidative stress alters physiological, biochemical and metabolomic responses of green microalga Chlamydomonas reinhardtii. Heliyon. 2022;8:e08811. doi: 10.1016/j.heliyon.2022.e08811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farghl AM, Shaddad MAK, Galal HR, Hassan EA. Effect of salt stress on growth, antioxidant enzymes, lipid peroxidation, and some metabolic activities in some freshwater and marine algae. Egypt J Bot. 2015;55(1):1–15. doi: 10.21608/ejbo.2015.221. [DOI] [Google Scholar]
- Fotopoulos V, Ziogas V, Tanou G, Molassiotis A. Involvement of AsA/DHA and GSH/GSSG ratios in gene and protein expression and in the activation of defense mechanisms under abiotic stress conditions. In: Anjum NA, Chan MT, Umar S, editors. Ascorbate-glutathione pathway and stress tolerance in plants. Dordrecht: Springer; 2010. pp. 265–302. [Google Scholar]
- Hasanuzzaman M, Nahar K, Anee TI, Fujita M. Glutathione in plants: biosynthesis and physiological role in environmental stress tolerance. Physiol Mol Biol Plants. 2017;23:249–268. doi: 10.1007/s12298-017-0422-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasanuzzaman M, Raihan MRH, Masud AAC, et al. Regulation of reactive oxygen species and antioxidant defense in plants under salinity. Int J Mol Sci. 2021;22:9326. doi: 10.3390/ijms22179326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath RL, Packer L. Photoperoxidation in isolated chloroplasts: Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 1968;125:189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- Hossain MA, Fujita M. Evidence for a role of exogenous glycinebetaine and proline in antioxidant defense and methylglyoxal detoxification systems in mung bean seedlings under salt stress. Physiol Mol Biol Plants. 2010;16:19–29. doi: 10.1007/s12298-010-0003-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain M, Nakano Y, Asada K. Monodehydroascorbate reductase in spinach chloroplasts and its participation in the regeneration of ascorbate for scavenging hydrogen peroxide. Plant Cell Physiol. 1984;25:385–395. doi: 10.1093/oxfordjournals.pcp.a076726. [DOI] [Google Scholar]
- Joseph T, Vanderslice J, Gladys B. Ascorbic acid and dehydroascorbic acid content of foods-as-eaten. J Food Compos Anal. 1990;3:105–106. doi: 10.1016/0889-1575(90)90018-H. [DOI] [Google Scholar]
- Kan G, Shi C, Wang X, Xie Q, Wang M, Wang X, Miao J. Acclimatory Responses to High-Salt Stress in Chlamydomonas (Chlorophyta, Chlorophyceae) from Antarctica. Acta Oceanol Sin. 2012;31:116–124. doi: 10.1007/s13131-012-0183-2. [DOI] [Google Scholar]
- Kaur M, Bhatia S, Gupta U, et al. Microalgal bioactive metabolites as promising implements in nutraceuticals and pharmaceuticals: inspiring therapy for health benefits. Phytochem Rev. 2023 doi: 10.1007/s11101-022-09848-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyande AK, Chew KW, Rambabu K, Tao Y, Chu DT, Show PL. Microalgae: A potential alternative to health supplementation for humans. Food Sci Hum Wellness. 2019;8:16–24. doi: 10.1016/j.fshw.2019.03.001. [DOI] [Google Scholar]
- Krishan G, Rao MS, Vashisht R, Chaudhary A, Singh J, Kumar A. Isotopic assessment of groundwater salinity: a case study of the Southwest (SW) Region of Punjab. India Water. 2022;14:133. doi: 10.3390/w14010133. [DOI] [Google Scholar]
- Kumar D, Seth CS. Photosynthesis, lipid peroxidation, and antioxidative responses of Helianthus annuus L. against chromium (VI) accumulation. Int J Phytoremediation. 2022;24:590–599. doi: 10.1080/15226514.2021.1958747. [DOI] [PubMed] [Google Scholar]
- Kumar D, Dhankher OP, Tripathi RD, Seth CS. Titanium dioxide nanoparticles potentially regulate the mechanism (s) for photosynthetic attributes, genotoxicity, antioxidants defense machinery, and phytochelatins synthesis in relation to hexavalent chromium toxicity in Helianthus annuus L. J Hazard Mater. 2023;454:131418. doi: 10.1016/j.jhazmat.2023.131418. [DOI] [PubMed] [Google Scholar]
- Law MY, Charles SA, Halliwell B. Glutathione and ascorbic acid in spinach (Spinacea oleracea) chloroplasts. Biochem J. 1983;210:899–903. doi: 10.1042/bj2100899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lembi CA, Waaland JR, editors. Algae and human Affairs. Cambridge: Cambridge University Press; 1988. [Google Scholar]
- Li HB, Cheng KW, Wong CC, Fan KW, Chen F, Jiang Y. Evaluation of antioxidant capacity and total phenolic content of different fractions of selected microalgae. Food Chem. 2007;102:771–776. doi: 10.1016/j.foodchem.2006.06.022. [DOI] [Google Scholar]
- Marklund SL, Marklund G. Involvement of the superoxide anion radical in the autooxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- Minhas AK, Hodgson P, Barrow CJ, Adholeya A. A review on the assessment of stress conditions for simultaneous production of microalgal lipids and carotenoids. Front Microbiol. 2016;7:1–19. doi: 10.3389/fmicb.2016.00546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miret JA, Müller M. AsA/DHA redox pair influencing plant growth and stress tolerance. In: Hossain M, Munné-Bosch S, Burritt D, Diaz-Vivancos P, Fujita M, Lorence A, editors. Ascorbic acid in plant growth, development and stress tolerance. Cham: Springer; 2017. pp. 297–319. [Google Scholar]
- Mirza GA. Determination of potassium chromate and hydrogen peroxide in the presence of each other. Analyst. 1965;90:509–510. doi: 10.1039/AN9659000509. [DOI] [Google Scholar]
- Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22:867–880. [Google Scholar]
- Omar MM, Shitindi MJ, Massawe BJ, Fue KG, Meliyo JL, Pedersen O. Salt-affected soils in Tanzanian agricultural lands: type of soils and extent of the problem. Sustain Environ. 2023;9:2205731. doi: 10.1080/27658511.2023.2205731. [DOI] [Google Scholar]
- Prajapati GS, Rai PK, Mishra VN, Singh P, Shahi AP. Remote sensing-based assessment of waterlogging and soil salinity: a case study from Kerala. India. Results Geophysical Sci. 2021;7:100024. doi: 10.1016/j.ringps.2023.100064. [DOI] [Google Scholar]
- Prajapati P, Gupta P, Kharwar RN, Seth CS. Nitric oxide mediated regulation of ascorbate-glutathione pathway alleviates mitotic aberrations and DNA damage in Allium cepa L. under salinity stress. Int J Phytoremediation. 2023;25:403–414. doi: 10.1080/15226514.2022.2086215. [DOI] [PubMed] [Google Scholar]
- Rather BA, Sehar Z, Majid A, et al. Ethylene and cellular redox management in plants. In: Khan NA, Ferrante A, et al., editors. The Plant Hormone Ethylene. Massachusetts: Elsevier; 2023. pp. 141–170. [Google Scholar]
- Sarwer A, Hamed SM, Osman AI, et al. Algal biomass valorization for biofuel production and carbon sequestration: a review. Environ Chem Lett. 2022;20:2797–2851. doi: 10.1007/s10311-022-01458-1. [DOI] [Google Scholar]
- Shah FA, Ni J, Tang C, Chen X, Kan W, Wu L. Karrikinolide alleviates salt stress in wheat by regulating the redox and K+/Na+ homeostasis. Plant Physiol Biochem. 2021;167:921–933. doi: 10.1016/j.plaphy.2021.09.023. [DOI] [PubMed] [Google Scholar]
- Shahid SA, Zaman M, Heng L. Soil Salinity: Historical perspectives and a world overview of the problem. In: Zaman M, Shahid SA, Heng L, editors. Guideline for salinity assessment, mitigation and adaptation using nuclear and related techniques. Cham: Springer; 2018. [Google Scholar]
- Siddiki SYA, Mofijur M, Kumar PS, et al. Microalgae biomass as a sustainable source for biofuel, biochemical and biobased value-added products: An integrated biorefinery concept. Fuel. 2022;307:121782. doi: 10.1016/j.fuel.2021.121782. [DOI] [Google Scholar]
- Smith IK, Vierhaller TL, Thorne CA. Assay of glutathione reductase in crude tissue homogenates using 5,5′-dithiobis (2-nitrobenzoic acid) Anal Biochem. 1988;175:408–413. doi: 10.1016/0003-2697(88)90564-7. [DOI] [PubMed] [Google Scholar]
- Srivastava R, Kanda T, Yadav S, et al. Salinity pretreatment synergies heat shock toxicity in cyanobacterium Anabaena PCC7120. Front Microbiol. 2023;14:1061927. doi: 10.3389/fmicb.2023.1061927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swapnil P, Rai AK. Physiological responses to salt stress of salt-adapted and directly salt (NaCl and NaCl+ Na2SO4 mixture)-stressed cyanobacterium Anabaena fertilissima. Protoplasma. 2018;255:963–976. doi: 10.1007/s00709-018-1205-5. [DOI] [PubMed] [Google Scholar]
- Ullah A, Bano A, Khan N. Climate change and salinity effects on crops and chemical communication between plants and plant growth-promoting microorganisms under stress. Front Sustain Food Syst. 2021;5:618092. doi: 10.3389/fsufs.2021.618092. [DOI] [Google Scholar]
- WangY BR, Noë A, Hekimi S. Superoxide dismutases: dual roles in controlling ROS damage and regulating ROS signaling. J Cell Biol. 2018;217:1915–1928. doi: 10.1083/jcb.201708007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao M, Li Z, Zhu L, et al. The multiple roles of ascorbate in the abiotic stress response of plants: Antioxidant, cofactor, and regulator. Front Pant Sci. 2021;12:598173. doi: 10.3389/fpls.2021.598173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Li C, Yao T, Li M, Lan X, Wang Z. Plant growth–promoting Rhizobacteria Enhance salt tolerance in oat by upregulating the antioxidant system and promoting root growth. J Plant Growth Regul. 2022 doi: 10.1007/s00344-022-10821-z. [DOI] [Google Scholar]