Abstract
Background
The gut microbiota may affect mood through the microbiota-gut-brain axis. The purpose of this study was to examine the effect of the gut microbiota and its metabolites, such as short-chain fatty acids (SCFAs), on prenatal depression and to determine the role of 5-hydroxytryptamine (5-HT) on prenatal depression in association with the gut microbiota and its metabolites (i.e. SCFAs).
Methods
Eighty-six pregnant women in the third trimester were recruited. Prenatal depression was determined by a score of 10 via the Edinburgh Postpartum Depression Scale. Demographic data, stool, and blood samples were collected. The gut microbiota and its metabolites SCFAs were determined by 16S rRNA gene sequencing and liquid chromatography-mass spectrometry analysis. Plasma 5-HT was determined by gas chromatography-mass spectrometry analysis.
Results
After controlling relevant covariates, our results found the higher the abundance of Candidatus_Soleaferrea, the lower the risk of prenatal depression; the higher the concentration of propanoic acid, the higher risk of prenatal depression. Our results also found the lower the plasma 5-HT, the higher the risk of prenatal depression, and 5-HT was related to unclassified_c_Clostridia and NK4A214_group. However, results of this study did not support the moderating effect of plasma 5-HT on the association of Candidatus_Soleaferrea or propionic acid with prenatal depression.
Conclusions
Results of this study supported that changes in certain gut microbiota, SCFAs, and plasma 5-HT during pregnancy were associated with prenatal depression. This finding provides new ideas for interventions based on diet or probiotics to regulate mood during pregnancy.
Keywords: Prenatal depression, 5-Hydroxytryptamine, Gut microbiota, Microbiota-gut-brain axis, Short chain fatty acids
1. Introduction
Prenatal depression is usually accompanied by multiple signs and symptoms, including low mood, fatigue, insomnia, low energy, forgetfulness, irritability, and poor physical and cognitive function (Dadi et al., 2020). Recently, the prevalence of prenatal depression was reported to range from 19.1% to 40.6% (Sulley et al., 2023; Zhang et al., 2020). Previous studies indicated that prenatal depression is associated with adverse outcomes such as postnatal depression, preeclampsia, and suicide risk (Lutsiv et al., 2015; Roomruangwong et al., 2018a; Zhang et al., 2022), as well as increasing risk of low birth weight, preterm birth, and neurodevelopmental delay in infants and children (Accortt et al., 2015; Faleschini et al., 2019; Jarde et al., 2016; Rogers et al., 2020). Therefore, understanding the influencing factors and potential pathogenesis of prenatal depression is conducive to the prevention and intervention of prenatal depression, ultimately promoting maternal and child health.
Healthy pregnancy is characterized by increased amounts of bacteria and profound changes in the composition of gut microbiota (Di Simone et al., 2020; Gosalbes et al., 2019; Koren et al., 2012). Previous studies indicated that a decrease in butyric acid-producing bacteria and individual richness and an increase in Bifidobacteria, Proteobacteria, and lactic acid-producing bacteria in the maternal gut microbiota from the first to the third trimester (Di Simone et al., 2020; Koren et al., 2012). The maternal gut microbiota has been reported to be a key determinant of offspring health, regulating offspring immunity, metabolism, and the development of brain function and behavior (Gomez-Arango et al., 2016; Jašarević and Bale, 2019; Nyangahu and Jaspan, 2019). In addition, numerous studies have shown that the gut microbiota interacts with the brain through multiple pathways in the microbiota-gut-brain axis and that these interactions play an important role in the occurrence and development of depression (Foster and Neufeld, 2013; Luna and Foster, 2015). Recently, Bernabe et al. (2020) found that Paraprevotella was enriched and Faecalibacterium was depleted in women with antenatal depression in a population study. Previous animal studies also found that germ-free mice transplanted with fecal samples from patients with “depressive microbiota” showed depressive-like behavior, which partly explained the causal role of gut microbiota in the development of depression (J. Kelly et al., 2016; Zheng et al., 2016). Short-chain fatty acids (SCFAs), which are functional metabolites produced by the fermentation of gut microbiota, can interact with the brain via the gut-brain axis (Tran and Mohajeri, 2021). Karolina et al. found that SCFAs, predominantly acetic, propionic, and caproic acids were strongly associated with depressive symptoms in Polish women (Skonieczna-Żydecka et al., 2018). Nevertheless, studies relating changes in the gut microbiota and its functional metabolites, SCFAs, in populations struggling with prenatal depression are limited, and the potential molecular mechanisms by which the gut microbiota affects prenatal depression are unclear and in need of further exploration.
Gut microbiota and its functional metabolites may regulate memory, emotion, cognition, and behavior by affecting inflammatory cytokines, hypothalamic-pituitary-adrenal (HPA) axis function, neurotransmitters, and neurons (Mohajeri et al., 2018). Previous studies indicated that serotonin 5-hydroxytryptamine (5-HT), an endogenous monoamine neurotransmitter and neurohormone formed by hydroxylation, was capable of affecting a variety of functions in the body, including emotion, behavior, and cognition (Ho and Ross, 2017; Ridaura and Belkaid, 2015). It has been suggested that 5-HT plays an important role in the onset and development of depression (Fakhoury, 2016). Some strains of gut microbiota and its functional metabolites SCFAs have also been found to affect serotonin secretion in prior studies (Clarke et al., 2014; Fukumoto et al., 2003; Ho and Ross, 2017; Ridaura and Belkaid, 2015; Valles-Colomer et al., 2019). Therefore, gut microbiota and SCFAs may influence the onset and development of depression either directly or indirectly by regulating 5-HT metabolism.
Therefore, the purpose of this study was to examine the effect of the gut microbiota and its functional metabolites, such as SCFAs, on prenatal depression, understand the correlation between 5-HT and prenatal depression, and explore the role of 5-HT as associated with prenatal depression, the gut microbiota, and its functional metabolites.
2. Methods and materials
2.1. Study type and design
A case-control study design was used in this study. Eligible participants from the obstetrics clinic at the Zhongnan Hospital of Wuhan University were recruited between October 2020 and October 2021. The inclusion criteria included: 1) pregnant women over age 20; 2) pregnant women in the third trimester who lived in Wuhan; 3) without pregnancy complications, and 4) self-reported no cognitive dysfunction. Exclusion criteria included: 1) Addiction to smoking, drinking, or other psychoactive substances; 2) obesity before pregnancy; 3) a history of mental illness or having received antipsychotic medication, 4) a history of fever within one week before the evaluation and collection of specimens. Based on the total score of the Edinburgh Postpartum Depression Scale (EPDS) (Cox et al., 1987), participants were enrolled into either the pregnant depression group (total score ≥10) or the non-depression group (total score <10). Eventually, a total of 86 pregnant women in the third trimester (43 in the pregnant depression group and 43 in the non-depression group) were included in this study, and informed consent was obtained for all participants. This study was approved by the Medical Ethics Committee of Wuhan University School of Medicine (2019YF2019).
2.2. Variables and measures
The pregnant women completed a sociodemographic characteristics questionnaire, a self-designed simple diet frequency and structure questionnaire, and the EPDS.
The sociodemographic characteristics questionnaire included age, occupation, educational background, weight before and during pregnancy, height, per capita monthly household income, gravidity, method of fertilization, planned pregnancy, relationship with their spouse, and exercise frequency before and during pregnancy. In accordance with the classification criteria recommended by the Institute of Medicine (Rasmussen and Yaktine, 2009), we divided pregnant women into the groups of insufficient growth, normal growth, and excessive growth based on their pre-pregnant weight and gestational weight gain (GWG). According to the Dietary Guidelines for Chinese Residents (Society, 2016), we designed a simple diet frequency and structure questionnaire to record the pregnant women's diet status in the past week. We also recorded the date and time for biological sample collection.
The EPDS can be used for screening prenatal and postpartum depression. The scale is a self-rating scale with 10 items. Each item is scored on a 4-point scale (0–3 points), with a total score of 0–30. The higher the total score, the more severe the level of the depression. Reliability and validity were all above 0.8 (Cox et al., 1987; Lee et al., 1998; Petrozzi and Gagliardi, 2013). An EPDS score ≥10 was considered to be the presence of depressive symptoms in pregnant women.
2.3. Fecal and blood sample collection
Fecal and blood samples were collected in their third trimester. A 30 ml sterile fecal collection tube was used for sampling by the participants themselves, who received professional training through explanation, pictures in a brochure, and a video. Two tubes of fecal samples were collected by each subject; one sample was used to measure the diversity and composition of gut microbiota and the other for SCFAs. After sampling, the samples were numbered, and then transferred to a freezer set to −80 °C.
Five ml of peripheral venous blood from each person in both groups was extracted by intravenous puncture and placed in a vacuum anticoagulant tube of sodium citrate (anticoagulant 1:9) by professional nurses for the determination of plasma 5-HT concentration. After obtaining the sample, it was immediately centrifuged (4500 rpm, 4 °C, 10 min) and processed into the required plasma sample for the experiment. Each aliquot (200 μL) of the plasma samples was numbered and stored in the freezer at −80 °C until the next step in analysis.
2.4. Extraction and sequencing for the gut microbiota
Fecal samples were subjected to genomic DNA extraction and Illumina sequencing. Primers (338F [5′-ACTCCTACGGGAGgCAGCAGCAGcag3'] and 806R [5′-GGactachVGGgTWTCtaat-3']) were used to amplify 16S rRNA V3–V4 gene regions. Polymerase chain reaction (PCR) has been described in the previous study (Wang et al., 2019). MiSeq libraries (Illumina, San Diego, CA, USA) were constructed and sequenced by using purified PCR products. The 16S RNA genetic data were spliced together and Flash (https://ccb.jhu.edu/software/FLASH/index.shtml) was used for quality control; Uparse (http://www.drive5.com/uparse/) was used to cluster sequences with high similarity (>97%) into minimum operational taxa units (OTUs). The RDP Classifier Bayesian algorithm (version 2.2 http://sourceforge.net/projects/rdp-classifier/) was used to analyze the OTU representative sequences in the SLIVA 138/16S bacterial classification database, with a classification confidence value of 0.7.
2.5. Targeted metabolomics analysis for SCFAs
Targeted metabolomics analysis of stool samples was performed by gas chromatography-mass spectrometry (GC-MS). A 25 mg stool sample was put into a 2 mL grinding tube with 1 mL water (containing 0.5% phosphoric acid). The grinding tube was placed in a cold grinding machine at 50 HZ for 3 min; it was ground twice. Next, the grinding tube stood in ice water bath for 10 min and then was centrifuged at 13000 g for 15 min at 4 °C. The supernatant was transferred to another 1.5 mL centrifuge tube and a 0.2 mL n-butanol solvent (with internal standard 2-methyl butyric acid 10 μg/mL) was added for extraction and another round of ultrasonic centrifugation. Finally, the upper organic layer was collected for analysis by injection into the GC-MS analytical instrument.
GC/MSD gas chromatograph (Agilent Technologies Inc. CA, UAS, 8890B–5977 B GC/MSD) was used as the analysis instrument. Chromatographic separation was conducted on an HP FFAP capillary column (30 m × 0.25 mm × 0.25 μm) with electron bombarded ion source (EI). High-purity helium (purity not less than 99.999%) was supplied as the carrier gas at a flow rate of 1.0 mL/min with a split ratio of 10:1; the injection volume was 1 μL. The initial temperature of the column temperature chamber was 80 °C, the temperature was programmed to 120 °C at 40 °C/min, 200 °C at 10 °C/min, and then the temperature was kept at 230 °C for 3 min. The temperatures of the chromatographic inlet, ion source, quadrupole mass, transfer line, and quadrupole mass spectrometer were regulated at 260 °C, 230 °C, 150 °C, and 230 °C, respectively.
The default parameters of Masshunter quantitative software (Agilent, USA, version number: v10.0.707.0) were used to automatically identify and integrate the ion fragments of target SCFAs and assist manual inspection. The detection concentration of each sample was calculated by a standard curve and the actual content of SCFAs in the sample was converted.
2.6. Liquid chromatography-mass spectrometry (LC-MS) analysis for 5-HT
The 200 μL of supernatant was obtained by mixing the 50 μL serum sample with 5 μL 100 ppb deuterium butyric acid and 175 μL glacial methanol, vortexed, and centrifuged at 12,000 g at 4 °C for 10 min. Then, 50 μL of supernatant was placed in a 1.5 mL centrifuge tube and processed for vortexing (with triphenylphosphine solution, Dithiodipyridine solution, and N-methyl phenyl ethylamine solution) and shaking reactions at 1500 rpm for 30 min at 40 °C. The supernatant was redissolved with 100 μL ACN/water (v/v, 0.5/9.5), vortexed for 1 min, and centrifuged (12000 g, 3 min). Finally, the 0.5 μL of supernatant was collected for analysis by injection into the LC-MS analytical instrument.
Analysis was performed on the UHPLC- ESI-MS/MS system. The chromatographic conditions were as follows: chromatographic separation was performed using an Acquity UPLC BEH C18 column (2.1 × 100 mm, 1.7 μm, Waters) at 50 °C. The mobile phase consisted of A (mixed solution of 60% acetonitrile and 40% isopropanol) and B (0.1% water formic acid). The gradient elution conditions were as follows: 0–3 min, 5% B; 3–27 min, 5%–55% B; 27–30 min 90% B; 33–35 min, 90%–5% B; 35–40 min, 5% B. The flow rate of the mobile phase was set at 0.3 mL/min. The mass spectrometry conditions were as follows: the interface voltage was 4.0 kV, and the detector voltage was set to 1.86 kV. The temperature of the desolventizing tube and heating block was set to 250 °C and 400 °C, respectively. The interface temperature was set to 300 °C; the nitrogen flow rate of the dryer and atomizer was set to 10.0 L/min and 2.0 L/min, respectively.
2.7. Statistical analysis
SPSS 20.0 was used to analyze the data. A chi-square or Fisher's exact test was used to compare the differences between the two groups of general demographic categorical variables. T-test, One-way analysis of variance (one-way ANOVA), and Mann-Whitney U were used to compare the differences between the two groups' general demographic continuous variables, SCFAs and blood 5-HT.
For gut microbiota data, core species analysis indicated that the sample size was sufficient. Community composition analysis was used to analyze the composition between the prenatal depression group and the non-depression group at phylum and genus levels. The gut microbial alpha diversity of the two groups was evaluated using Mothur software (Kozich et al., 2013). The Mann-Whitney U test was used to analyze the difference in alpha diversity between the women with prenatal depression and those without prenatal depression. Principal coordinates analysis (PCoA) and permutation multivariate analysis of variance (PERMANOVA) based on the Euclidean distance were used to analyze the association between beta diversity and prenatal depression. Linear discriminant analysis effect size (LEfSe) was used to detect the species abundance differences from phylum level to genus level between the two groups of pregnant women; significant differences and the impact of these differences (LDA value) were obtained. We screened out species whose LDA values were greater than 1 and standardized the data for further analysis. Spearman's correlation was used to evaluate the correlation between gut microbiota and its metabolic SCFAs and 5-HT. Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt) was used to predict microbial function based on the 16S rRNA data (Douglas et al., 2018). The functional genes were obtained from the Kyoto Encyclopedia of Genes and Genomes (KEGG) database (Kanehisa and Goto, 2000). The association between the predicted microbial function and prenatal depression and plasma 5-HT was analyzed based on KEGG annotation at level 2.
Logistic regression analysis was performed to assess the association between gut microbiota, SCFAs, and 5-HT, and prenatal depression. Data on genus relative abundance were transformed using central logarithmic ratios (CLR) before regression analysis. Using microbial genera related to 5-HT as the independent variable and 5-HT as the dependent variable, a stepwise linear regression was conducted to explore the relationship between genera and 5-HT. In addition, interactive items (5-HT*gut microbiota and 5-HT*SCFAs) were taken into logistic regression analyses for exploring the potential moderating effect of 5-HT in plasma between gut microbiota and SCFAs and prenatal depression. A p-value less than 0.05 was considered significant.
3. Results
3.1. Sociodemographic characteristics
In total, 86 pregnant women were enrolled in the study with 43 women in each group. All the participants were married, had not consumed alcohol during pregnancy, and had a singleton pregnancy. There was a statistical difference in the percentage of primiparous women in the antenatal depression and control groups (44.2% vs 76.7%, p = 0.007). The days between collecting the blood sample and the questionnaire (median (IQR)) in the prenatal depression group (median = 23.0, IQR = [35.0, 15.0]) was less than that in the non-depression group (median = 33.0, IQR = [44.0, 28.0]) (p = 0.009). There was no statistical significance in age, occupation, educational background, smoking, intimate relationships with their husbands, and GWG (Table 1). There was no statistically significant difference in dietary habits between the prenatal depression group and the non-depression group (Supplemental Table 1).
Table 1.
Demographic characteristic (N = 86).
| Item | Non-depression group (N = 43) | Prenatal depression group (N = 43) | t/χ2 | P |
|---|---|---|---|---|
| Occupation | NA | 0.514a | ||
| Medical | 3 (7.0%) | 5 (11.6%) | ||
| Non-medical | 34 (79.1%) | 29 (67.4%) | ||
| Unemployed | 6 (14.0%) | 9 (20.9%) | ||
| Educational background | 3.907 | 0.143 | ||
| College degree or below | 12 (27.9%) | 7 (16.3%) | ||
| Bachelor's degree | 16 (37.2%) | 25 (58.1%) | ||
| Master's degree or above | 15 (34.9%) | 11 (25.6%) | ||
| Per capita monthly household income | NA | 0.058a | ||
| <5000 yuan/month | 2 (4.7%) | 3 (7.0%) | ||
| 5000-10000 yuan/month | 28 (65.1%) | 17 (39.5%) | ||
| ≥10000 yuan/month | 13 (30.2%) | 23 (53.5%) | ||
| Gravity | 9.872 | 0.007** | ||
| 1 | 33 (76.7%) | 19 (44.2%) | ||
| 2 | 7 (16.3%) | 14 (32.6%) | ||
| ≥3 | 3 (7.0%) | 10 (23.3%) | ||
| Parity | 3.310 | 0.069 | ||
| 1 | 37 (86.0%) | 30 (69.8%) | ||
| ≥2 | 6 (14.0%) | 13 (30.2%) | ||
| Relationship with husband | 3.446 | 0.063 | ||
| Intimate | 42 (97.7%) | 36 (83.7%) | ||
| Average | 1 (2.3%) | 7 (16.3%) | ||
| GWG | 2.051 | 0.374 | ||
| Less | 22 (51.2%) | 26 (60.5%) | ||
| Adequate | 16 (37.2%) | 10 (23.3%) | ||
| Excessive | 5 (11.6%) | 7 (16.3%) |
| Item | Non-depression group (N = 43) | Prenatal depression group (N = 43) | t/χ2 | P |
|---|---|---|---|---|
| Age, years | 29.30 ± 3.08 | 30.37 ± 3.87 | −1.418 | 0.160 |
| Height, cm | 162.04 ± 4.22 | 161.86 ± 4.78 | 0.194 | 0.847 |
| Weight at present, kg | 63.68 ± 7.11 | 64.11 ± 8.09 | −0.265 | 0.792 |
| Weight before pregnancy, kg | 52.17 ± 6.23 | 53.42 ± 6.99 | −0.879 | 0.382 |
| Pregestational BMI, kg/m2 | 19.83 ± 1.95 | 20.37 ± 2.33 | −1.154 | 0.252 |
| Gestational days when completing questionnaire | 230.0 (243.0, 224.0) | 238.0 (254.0, 224.0) | NA | 0.061 |
| Gestational days when collecting stool | 250.0 (259.0, 241.0) | 254.0 (264.0, 235.0) | NA | 0.566 |
| Gestational days when collecting blood | 268.0 (277.0, 258.0) | 265.7 (274.0, 259.0) | NA | 0.601 |
| Days between collecting stool and questionnaire | 11 (31.0, 4.0) | 10 (17.0, 4.0) | NA | 0.094 |
| Days between collecting blood and questionnaire | 33.0 (44.0, 28.0) | 23.0 (35.0, 15.0) | NA | 0.009** |
| Days between collecting blood and stool | 18.0 (28.0, 6.0) | 12.0 (26.0, 5.7) | NA | 0.316 |
| Time of collecting stool, min | 95.0 (140.0, 60.0) | 110.0 (165.0, 57.0) | NA | 0.422 |
| Time of collecting blood, min | 168.0 (310.0, 110.0) | 180.0 (241.0, 90.0) | NA | 0.434 |
Note: a means using Fisher's exact method. NA means Not Applicable. * means 0.01 ≤ P < 0.05. ** means 0.001 ≤ P < 0.01. The data that were normally distributed showed in M±SD and the data that were not normally distributed showed in M (IQR).
3.2. Sequencing characteristic of gut microbiota
A total of 4,646,753 high-quality sequences were obtained from 86 women using the MiSeq Illumina sequencing. These reads were clustered into 1198 OTUs. The core OTU analysis showed that the average number of OTUs decreased with the number of samples until flat, indicating that the sequencing quantity was sufficient (Supplemental Fig. 1).
3.3. The associations of the gut microbiota and SCFAs with prenatal depression
The alpha diversity (richness and diversity) indices in the prenatal depression group were higher than those in the non-depression group, though there was no significant difference (Table 2). The beta diversity through PCoA and PERMANOVA at the OTU level showed that the bacterial structure was not separated based on prenatal depression (Fig. 1 and Supplemental Table 2). In PCoA analysis of the PC1 axis, the bacterial structure of the prenatal depression group (median = −687.84, IQR = [−1717.70, 1508.24]) was more discrete than in the non-depression group (median = −558.61, IQR = [−2085.55, 648.53]) with no significant difference.
Table 2.
Richness index, diversity index of gut microbiota and SCFAs difference between prenatal depression and non-depression group (N = 86).
| Item | Non-depression group (N = 43) | Prenatal depression group (N = 43) | P |
|---|---|---|---|
| Sobs index | 256 (307, 198) | 259 (324, 222) | 0.371 |
| Shannon index | 3.43 (3.80, 3.12) | 3.51 (3.69, 3.21) | 0.509 |
| Simpson index | 0.08 (0.10, 0.05) | 0.07 (0.09, 0.05) | 0.914 |
| Ace index | 307.76 (377.80, 245.20) | 313.78 (385.07, 261.68) | 0.566 |
| Chao index | 305.88 (377.48, 248.55) | 317.09 (398.44, 260.53) | 0.349 |
| Coverage index | 0.9981 (0.9984, 0.9976) | 0.9981 (0.9985, 0.9976) | 0.976 |
| Acetic acid, ug/mg | 0.78 (0.89, 0.58) | 0.93 (1.19, 0.68) | 0.023* |
| Propanoic acid, ug/mg | 0.55 (0.77, 0.44) | 0.78 (1.01, 0.54) | 0.005** |
| Isobutyric acid, ug/mg | 0.09 (0.13, 0.05) | 0.09 (0.13, 0.05) | 0.566 |
| Butanoic acid, ug/mg | 0.65 (0.92, 0.44) | 0.89 (1.13, 0.57) | 0.047* |
| Isovaleric acid, ug/mg | 0.08 (0.13, 0.04) | 0.08 (0.15, 0.04) | 0.390 |
| Valeric acid, ug/mg | 0.14 (0.22, 0.03) | 0.16 (0.28, 0.05) | 0.233 |
| Isohexanoic acid, ug/mg | 0.003 (0.005, 0.001) | 0.004 (0.006, 0.002) | 0.282 |
| Hexanoic acid, ug/mg | 0.003 (0.014, 0.002) | 0.005 (0.057, 0.003) | 0.082 |
Note: NA means Not Applicable. * means 0.01 ≤ P < 0.05. ** means 0.001 ≤ P < 0.01.
The data that were not normally distributed are shown in M (IQR).
Fig. 1.
PCoA on OUT level-beta diversity.
Note: The X-axis and Y-axis in Fig. 1a represent the two selected principal axes, and the percentage represents the explanatory degree value of the principal axes to the sample composition difference; the scale of X-axis and Y-axis is relative distance and has no practical significance. Points with different colors or shapes represent samples of different groups. The more scattered the two sample points are, the greater the difference in species composition between the two samples. The boxplot in Fig. 1b represents the dispersion of the distribution of different groups of samples along the PC1 axis. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
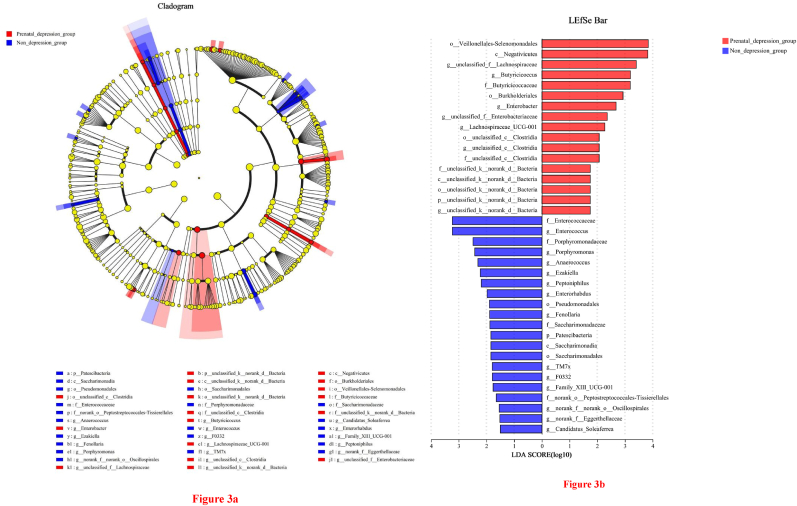
Fig. 2a–d illustrated the composition of the gut microbiota of each participant at the phylum and genus level. The top three phyla in the prenatal depression group and the non-depression group were Firmicutes, Actinobacteriota, and Bacteroidota (Fig. 2a and c). The dominant genera in both groups were Blautia, Faecalibacterium, and Bifidobacterium (Fig. 2b and d). Fig. 3 showed the gut microbiota differences from phylum to genus between the prenatal depression group and the control group. Compared to the controls, the prenatal depression group at genus level had higher abundance of unclassified_f_Lachnospiraceae, Lachnospiraceae_UCG-001, Butyricicoccus, unclassified_c_Clostridia, unclassified_f_Enterobacteriaceae, Enterobacter and unclassified_k_norank_d_Bacteria, while at genus level had lower abundance of Family_XIII_UCG-001, F0332, Anaerococcus, Ezakiella, Fenollaria, Peptoniphilus, Candidatus_Soleaferrea, Porphyromonas, norank_f_norank_o_Oscillospirales, Enterococcus, norank_f_Eggerthellaceae, Enterorhabdus, and TM7x. Other relevant results were seen in Fig. 3. All eight SCFAs levels were higher in the prenatal depression group than in the non-depression group, but only significant differences between the two groups for acetic acid (p = 0.023), propanoic acid (p = 0.005), and butanoic acid (p = 0.047) (Table 2). However, the results of the study did not find any difference in the predicted microbial functions of pregnant women in the prenatal depression group and the non-depression group (Supplemental Fig. 2).
Fig. 2.
Composition of gut microbiota of pregnant women in the prenatal depression group and non-depression group at phylum level and genus level
Note: The horizontal axis is each participant's number, the vertical axis is the proportion of species of participants, and the different colors of the column represent different species. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Fig. 3.
(a) Gut microbiota differences from phylum to genus between prenatal depression group and non-depression group; (b) The LDA discrimination results.
Note: In Fig. 3 (a), different color nodes represent the microbial groups that are significantly enriched in the corresponding groups and have significant influence on the differences between groups. The yellow nodes indicate the microbial groups that have no significant difference among different groups or have no significant effect on the difference between groups. In Fig. 3 (b), the LDA discriminant histogram counted the microbial groups with significant effects in two groups. The LDA score obtained through LDA analysis (linear regression analysis), the larger the LDA score, the greater the impact of species abundance on the difference effect. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
After controlling for covariates (i.e., gravidity and days between the collection of the blood sample and the questionnaire), all gut microbiota and SCFAs related to prenatal depression were entered into a stepwise regression to obtain the best fitting model. The results found the lower the abundance of Candidatus_Soleaferrea, the higher the risk of prenatal depression (OR = 0.370, 95% CI = [0.185, 0.738]); and the higher concentration of propanoic acid, the higher the risk of prenatal depression (OR = 8.035, 95% CI = [1.287, 50.164]) (Table 3).
Table 3.
Gut microbiota and SCFAs associated with prenatal depression.
| β | S.E. | Wald | P | OR (95% CI) | |
|---|---|---|---|---|---|
| Gravidity | 8.254 | 0.016* | |||
| Gravidity (1) | −1.566 | 0.807 | 3.766 | 0.052 | 0.209 (0.043, 1.016) |
| Gravidity (2) | 0.109 | 0.928 | 0.014 | 0.906 | 1.115 (0.181, 6.868) |
| Days between collecting blood and questionnaire | −0.043 | 0.019 | 5.420 | 0.020* | 0.958 (0.923, 0.993) |
| Lachnospiraceae_UCG_001 | 0.497 | 0.281 | 3.129 | 0.077 | 1.664 (0.948, 2.854) |
| Candidatus_Soleaferrea | −0.995 | 0.353 | 7.963 | 0.005** | 0.370 (0.185, 0.738) |
| Propanoic acid | 2.084 | 0.934 | 4.972 | 0.026* | 8.035 (1.287, 50.164) |
| Constant | 0.617 | 1.182 | 0.272 | 0.602 | 1.853 |
Note: The dependent variable was set as “prenatal depression group = 1” and “non-depression group = 0”. Gravidity (1) means “gravidity = 1” and Gravidity (2) means “gravidity = 2” which is all compared to “gravidity ≥3”. * means 0.01 ≤ P < 0.05.
3.4. The associations of plasma 5-HT with prenatal depression, gut microbiota, and SCFAs
The concentration of 5-HT was higher in the control group (median = 19.97, IQR = [24.27, 8.61] ng/ml) than in the prenatal depression group (median = 14.16, IQR = [18.08, 9.99] ng/ml); the difference of 5-HT (p = 0.032) between two groups was statistically significant. After controlling for covariates (i.e., gravidity and days between the collection of the blood sample and the questionnaire), plasma 5-HT was selected into the model for logistic regression to obtain the fitting model. The results found that the lower the 5-HT in plasma, the higher the risk of prenatal depression (OR = −0.056, 95% CI = [0.899, 0.995]) (Supplemental Table 3).
Plasma 5-HT showed positive correlations with the abundance of Phascolarctobacterium (r = 0.356, p = 0.001), unclassified_f_Enterobacteriaceae (r = 0.213, p = 0.049), NK4A214_group (r = 0.270, p = 0.012), Bilophila (r = 0.355, p = 0.001) and norank_f_norank_o_Oscillospirales (r = 0.360, p = 0.001), but it showed a negative correlation with the abundance of unclassified_c_Clostridia (r = −0.252, p = 0.019) in genus level. There was no correlation between the predicted microbial functions, SCFAs, and plasma 5-HT (Supplemental Table 4 and Supplemental Table 5). With plasma 5-HT as the dependent variable, all meaningful genera were included in the model for stepwise linear regression, and the optimal model was obtained with the normal residual distribution. The abundance of NK4A214_group (β = 3.593, p = 0.004) was positively correlated with plasma 5-HT, while the abundance of unclassified_c_Clostridia (β = −3.509, p = 0.004) was negatively correlated with plasma 5-HT (Supplemental Table 6).
3.5. Role of 5-HT in the relationship between the gut microbiota and SCFAs and prenatal depression
After controlling for the covariates, we added Candidatus_Soleaferrea, propanoic acid, and plasma 5-HT into the logistic regression model to study their effects on prenatal depression. Both Nagelkerke R2 (R2 = 0.419) and the Hosmer-Lemeshow test (χ2 = 10.493, p = 0.232) showed that the model fitted well. The concentration of plasma 5-HT (OR = 0.963, 95% CI = [0.912, 1.017]) showed no correlation with prenatal depression, while Candidatus_Soleaferrea (OR = 0.446, 95% CI = [0.229, 0.868]) and propanoic acid (OR = 6.928, 95% CI = [1.128, 42.546] were correlated with prenatal depression (Table 4). We added the interaction item (Candidatus_Soleaferre * plasma 5-HT and propanoic acid * plasma 5-HT) into the logistic regression model and found that there was no potentially moderating effect of 5-HT in plasma between Candidatus_Soleaferre, propanoic acid and prenatal depression (Supplemental Table 7).
Table 4.
The correlation of Candidatus_Soleaferrea, propanoic acid, and 5-HT and prenatal depression.
| β | S.E. | Wald | P | OR (95% CI) | |
|---|---|---|---|---|---|
| Gravidity | 7.632 | 0.022* | |||
| Gravidity (1) | −1.408 | 0.784 | 3.225 | 0.073 | 0.245 (0.053, 1.137) |
| Gravidity (2) | 0.212 | 0.913 | 0.054 | 0.817 | 1.236 (0.207, 7.391) |
| Days between collecting blood and questionnaire | −0.040 | 0.018 | 4.930 | 0.026* | 0.961 (0.927, 0.995) |
| Plasma 5-HT | −0.038 | 0.028 | 1.866 | 0.172 | 0.963 (0.912, 1.017) |
| Candidatus_Soleaferrea | −0.808 | 0.340 | 5.650 | 0.017* | 0.446 (0.229, 0.868) |
| Propanoic acid | 1.936 | 0.926 | 4.369 | 0.037* | 6.928 (1.128, 42.546) |
| Constant | 1.152 | 1.239 | 0.865 | 0.352 | 3.166 |
Note: The dependent variable was set as “prenatal depression group = 1” and “non-depression group = 0”. Gravidity (1) means “gravidity = 1” and Gravidity (2) means “gravidity = 2” which is all compared to “gravidity ≥3”. * Means 0.01 ≤ P < 0.05.
4. Discussion
Our study was the first in China to explore the relationships between prenatal depression and the gut microbiota, as well as its functional metabolites. The results of this study firstly revealed the association of certain gut microbiota and SCFAs with prenatal depression. These findings provide new ideas for interventions using diet or probiotics to regulate mood during pregnancy. In addition, the study also found a relationship between the gut microbiota and 5-HT, providing new ideas for the use of probiotics to regulate the neurotransmitter 5-HT.
Our results indicated the higher the abundance of Candidatus_Soleaferrea, the lower the risk of prenatal depression. The genus Candidatus Soleaferrea has anti-inflammatory effects by secreting metabolites and assisting in the maintenance of intestinal homeostasis, which enhances host immunity (Cao et al., 2021). Pregnancy is a pro-inflammatory and anti-inflammatory state, which depends on the stage of gestation, with a pro-inflammatory stage in the first trimester and closer to delivery and an anti-inflammatory stage in mid-gestation (Mor and Cardenas, 2010). A previous study (Zhang et al., 2016) indicated peripheral and central inflammation plays a role in the onset of depression, and anti-inflammatory drugs can ameliorate the symptoms in patients with inflammation-related depression, this may partly explain the protective role of Candidatus_Soleaferrea in the development of prenatal depression in our study. In addition, Candidatus_Soleaferrea belongs to the family of Ruminococcaceae, many studies have found that the abundance of Ruminococcaceae at family level, the upper dimension of Candidatus_Soleaferrea, declines significantly in depressed people (Knuesel and Mohajeri, 2022; Liu et al., 2020; Zheng et al., 2020). It also was found in animal experiments that Xiaoyaosan (a traditional Chinese medicine with antidepressant effects) increased the abundance of Ruminococcaceae and improved the depressed mice (Zhu et al., 2019). Therefore, the results of our study were roughly consistent with the previous findings; however, the role and function of this genus in prenatal depression should be further explored in the future.
Our study found higher concentrations of acetic acid, propanoic acid, and butanoic acid in the prenatal depression group. After controlling for covariates, the higher the concentration of propanoic acid, the higher the risk of prenatal depression. An animal study also revealed the concentration of acetic acid, propanoic acid, and valeric acid decreased in depressed mice (Wu et al., 2020). In the non-pregnant population, acetic acid and propanoic acid were negatively correlated with Beck's score in Polish women (Skonieczna-Zydecka et al., 2018), but another study reported no difference in SCFA between severely depressed patients and the control group (J. Kelly et al., 2016). Therefore, the association between SCFAs and depression remains controversial. Differences in gender, region, eating habits, and depression scales may be the reasons for the controversial results. SCFAs are usually considered to have anti-inflammatory activities that are beneficial to human health and can alleviate diseases such as colitis (Campos-Perez and Martinez-Lopez, 2021). However, previous studies have revealed that SCFAs can play a pro-inflammatory role and that in excess may adversely impact the body's immune system (Kim et al., 2013; Vinolo et al., 2009). For example, in cancer patients undergoing immunotherapy, excessive SCFAs can impair the effect of immunotherapy (Coutzac et al., 2020). Many animal experiments have also proved that propanoic acid can induce autistic behaviors in rats and mice (Kamen et al., 2019; Mirza and Sharma, 2019; Yu et al., 2020), which indicates that SCFAs may have pro-inflammatory effects. This partly supports our research results as the increase of propionic acid may be related to emotion and behavior.
However, our results found that there was no statistical difference in alpha diversity and beta diversity between the prenatal depression group and the controls, which is consistent with the findings of Zheng et al. (2016). Bernabe et al. (2020) also indicated that prenatal depression was not associated with the Shannon diversity index, but was negatively correlated with beta diversity, which was partially consistent with our results. However, some studies found that the diversity and richness of gut microbiota in depressed patients decreased (Jiang et al., 2015; J. R. Kelly et al., 2016), while other studies found that the alpha diversity in the active-MDD group increased (Jiang et al., 2015). The reason may be due to differences in diagnostic criteria for depression, grouping criteria, and methods of bacterial community detection. More studies are needed to determine the effect of prenatal depression on the diversity of gut microbiota.
Our results found that the lower the 5-HT in plasma, the higher the risk of prenatal depression, consistent with prior research. Previous studies indicated that 5-HT in the dorsal raphe nucleus, stem raphe nuclei, and hippocampus of the brain and other areas was closely associated with depression (Hesselgrave and Parsey, 2013). The decrease of 5-HT levels in brain stem raphe nuclei, hippocampus, and other areas was associated with depressive symptoms (Avraham et al., 2017). A previous study demonstrated that gut microbiota, such as Turicibacter and Clostridiacea, have been found to promote the synthesis of 5-HT by ECs (O'Mahony et al., 2015). We also found that the abundance of unclassified_c_Clostridia and NK4A214_group is negatively and positively correlated with plasma 5-HT respectively in our study. Therefore, it is speculated that gut microbiota and its metabolites may regulate depression by affecting the synthesis of 5-HT. However, we didn't find the moderating effect of plasma 5-HT in Candidatus_Soleaferre and propanoic acid on prenatal depression in our study. 5-HT cannot cross the blood-brain barrier which may partly explain why our study showed that plasma 5-HT was associated with depression, but did not show a moderating effect for the gut microbiota and SCFAs on prenatal depression (Gao et al., 2020). By contrast, tryptophan can cross the blood-brain barrier and shows great importance in reflecting the central 5-HT synthesis level, whose effects on gut microbiota and prenatal depression can be explored combined with 5-HT in the future. In addition, prenatal depression symptoms were not only associated with a single index 5-HT, but they also were associated with inflammatory cytokines in the blood, cortisol, corticosterone, dopamine, and other substances (Gilman et al., 2016; Kappelmann et al., 2022; Roomruangwong et al., 2018b). The molecular mechanisms by which gut microbiota affect prenatal depression should be further investigated in the future by expanding the sample size and combining it with other neurotransmitters or inflammatory cytokines associated with prenatal depression.
There were several limitations in our study. Firstly, we used the self-reported mood and symptoms of depression in pregnant women, rather than diagnosed depression. Secondly, this study mainly focused on physiological factors with little emphasis on the social factors related to prenatal depression; future work should further explore the impact of these social factors. Thirdly, we only collected the dietary frequency of the pregnant women, which was qualitative data, and relatively simple, specific intakes and types of intake in the diet of pregnant women could be further collected in the future. Finally, only the relationships of gut microbiota, SCFAs, neurotransmitter-5-HT, and prenatal depression were discussed. In the future, other neurotransmitters or inflammatory cytokines and metagenomes should be combined to explore the potential mechanism of prenatal depression.
5. Conclusion
Results of this study supported that changes in certain gut microbiota and its functional metabolites (i.e. SCFAs) during pregnancy were associated with prenatal depression. The higher the abundance of Candidatus_Soleaferrea and 5-HT, the lower the risk of prenatal depression; the higher the concentration of propanoic acid the higher the risk of prenatal depression. In addition, our results also found the abundance of unclassified_c_Clostridia and NK4A214_group was negatively and positively correlated with plasma 5-HT, respectively. Although plasma 5-HT may not be a moderating factor of gut microbiota and its functional metabolites SCFAs and prenatal depression in logistic regression models, our study provides evidence for further research on the mechanism of microbiota-gut-brain axis influence on the development of prenatal depression.
CRediT authorship contribution statement
Tianqu Xie: conceived the study and designed the protocol, were responsible for sample collecting and storage, Writing – original draft, verified the underlying data. Xiaoxiao Fan: conceived the study and designed the protocol, were responsible for sample collecting and storage, Writing – original draft, verified the underlying data. Hanghang Pang: conceived the study and designed the protocol. Tianzi Zang: were responsible for sample collecting and storage. Ni Wu: were responsible for sample collecting and storage. Juan Liu: were responsible for sample collecting and storage. Ziying Li: carried out liquid chromatography-mass spectrometry analysis for 5-HT. Sha Li: carried out liquid chromatography-mass spectrometry analysis for 5-HT. Quanfei Zhu: carried out liquid chromatography-mass spectrometry analysis for 5-HT. Julia Elise Slack: Writing – original draft. Jinbing Bai: Writing – original draft. Yu Xu: conceived the study and designed the protocol. Yanqun Liu: conceived the study and designed the protocol, Writing – original draft, All authors read and approved the final version of the manuscript.
Declaration of competing interest
The authors have no conflicts of interest to declare that are relevant to the content of this study.
Acknowledgments
The study was supported by the National Natural Science Foundation of China (Grant No.81903334). The funder had no role in the design and conduct of the study, collection, management, analysis, and interpretation of the data, preparation, review, or approval of the manuscript, and decision to submit the manuscript for publication. We were grateful for the technical support from Shanghai Majorbio Bio-pharm Technology Co., Ltd.
Handling Editor: Dr. John Cryan
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2023.100592.
Contributor Information
Yu Xu, Email: xuyu@whu.edu.cn.
Yanqun Liu, Email: liuyanqun1984@163.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Accortt E.E., Cheadle A.C., Dunkel Schetter C. Prenatal depression and adverse birth outcomes: an updated systematic review. Matern. Child Health J. 2015;19(6):1306–1337. doi: 10.1007/s10995-014-1637-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avraham Y., Hants Y., Vorobeiv L., Staum M., Abu Ahmad W., Mankuta D., et al. Brain neurotransmitters in an animal model with postpartum depressive-like behavior. Behav. Brain Res. 2017;326:307–321. doi: 10.1016/j.bbr.2017.01.013. [DOI] [PubMed] [Google Scholar]
- Bernabe B.P., Dowty S., Pezley L., Goel N., Hill E., Gibbons R., et al. Depression during pregnancy is associated with an altered gut microbiome in an urban diverse population [Meeting Abstract] Biol. Psychiatr. 2020;87(9) S36-S36. [Google Scholar]
- Campos-Perez W., Martinez-Lopez E. Effects of short chain fatty acids on metabolic and inflammatory processes in human health. Biochim. Biophys. Acta, Mol. Cell Biol. Lipids. 2021;1866(5) doi: 10.1016/j.bbalip.2021.158900. [DOI] [PubMed] [Google Scholar]
- Cao R.-R., He P., Lei S.-F. Novel microbiota-related gene set enrichment analysis identified osteoporosis associated gut microbiota from autoimmune diseases. J. Bone Miner. Metabol. 2021;39(6):984–996. doi: 10.1007/s00774-021-01247-w. [DOI] [PubMed] [Google Scholar]
- Clarke G., Stilling R., Kennedy P., Stanton C., Cryan J., Dinan T. Minireview: gut microbiota: the neglected endocrine organ. Mol. Endocrinol.(Baltimore, Md. 2014;28(8):1221–1238. doi: 10.1210/me.2014-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutzac C., Jouniaux J.M., Paci A., Schmidt J., Mallardo D., Seck A., et al. Systemic short chain fatty acids limit antitumor effect of CTLA-4 blockade in hosts with cancer. Nat. Commun. 2020;11(1) doi: 10.1038/s41467-020-16079-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J.L., Holden J.M., Sagovsky R. Detection of postnatal depression-development of the 10-item Edinburgh postnatal dpression scale [article] Br. J. Psychiatry. 1987;150:782–786. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- Dadi A.F., Miller E.R., Woodman R., Bisetegn T.A., Mwanri L. Antenatal depression and its potential causal mechanisms among pregnant mothers in Gondar town: application of structural equation model [Article] BMC Pregnancy Childbirth. 2020;20(1) doi: 10.1186/s12884-020-02859-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Simone N., Santamaria Ortiz A., Specchia M., Tersigni C., Villa P., Gasbarrini A., et al. Recent insights on the maternal microbiota: impact on pregnancy outcomes. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.528202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas G.M., Beiko R.G., Langille M.G.I. Predicting the functional potential of the microbiome from marker genes using PICRUSt. Methods Mol. Biol. 2018;1849:169–177. doi: 10.1007/978-1-4939-8728-3_11. [DOI] [PubMed] [Google Scholar]
- Fakhoury M. Revisiting the serotonin hypothesis: implications for major depressive disorders. Mol. Neurobiol. 2016;53(5):2778–2786. doi: 10.1007/s12035-015-9152-z. [DOI] [PubMed] [Google Scholar]
- Faleschini S., Rifas-Shiman S.L., Tiemeier H., Oken E., Hivert M.F. Associations of prenatal and postnatal maternal depressive symptoms with offspring cognition and behavior in mid-childhood: a prospective cohort study. Int. J. Environ. Res. Publ. Health. 2019;16(6) doi: 10.3390/ijerph16061007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J.A., Neufeld K.-A.M. Gut-brain: how the microbiome influences anxiety and depression. Trends Neurosci. 2013;36(5):305–312. doi: 10.1016/j.tins.2013.01.005. submitted for publication. [DOI] [PubMed] [Google Scholar]
- Fukumoto S., Tatewaki M., Yamada T., Fujimiya M., Mantyh C., Voss M., et al. Short-chain fatty acids stimulate colonic transit via intraluminal 5-HT release in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;284(5):R1269–R1276. doi: 10.1152/ajpregu.00442.2002. [DOI] [PubMed] [Google Scholar]
- Gao K., Mu C.L., Farzi A., Zhu W.Y. Tryptophan metabolism: a link between the gut microbiota and brain. Adv. Nutr. 2020;11(3):709–723. doi: 10.1093/advances/nmz127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman S.E., Cherkerzian S., Buka S.L., Hahn J., Hornig M., Goldstein J.M. Prenatal immune programming of the sex-dependent risk for major depression. Transl. Psychiatry. 2016;6 doi: 10.1038/tp.2016.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Arango L.F., Barrett H.L., McIntyre H.D., Callaway L.K., Morrison M., Dekker Nitert M. Connections between the gut microbiome and metabolic hormones in early pregnancy in overweight and obese women. Diabetes. 2016;65(8):2214–2223. doi: 10.2337/db16-0278. [DOI] [PubMed] [Google Scholar]
- Gosalbes M.J., Compte J., Moriano-Gutierrez S., Vallès Y., Jiménez-Hernández N., Pons X., et al. Metabolic adaptation in the human gut microbiota during pregnancy and the first year of life. EBioMedicine. 2019;39:497–509. doi: 10.1016/j.ebiom.2018.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselgrave N., Parsey R.V. Imaging the serotonin 1A receptor using [C-11]WAY100635 in healthy controls and major depression. Phil. Trans. Biol. Sci. 2013;368(1615) doi: 10.1098/rstb.2012.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho P., Ross D. More than a gut feeling: the implications of the gut microbiota in psychiatry. Biol. Psychiatr. 2017;81(5):e35–e37. doi: 10.1016/j.biopsych.2016.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarde A., Morais M., Kingston D., Giallo R., MacQueen G.M., Giglia L., et al. Neonatal outcomes in women with untreated antenatal depression compared with women without depression: a systematic review and meta-analysis. JAMA Psychiatr. 2016;73(8):826–837. doi: 10.1001/jamapsychiatry.2016.0934. [DOI] [PubMed] [Google Scholar]
- Jašarević E., Bale T.L. Prenatal and postnatal contributions of the maternal microbiome on offspring programming. Front. Neuroendocrinol. 2019;55 doi: 10.1016/j.yfrne.2019.100797. [DOI] [PubMed] [Google Scholar]
- Jiang H.Y., Ling Z.X., Zhang Y.H., Mao H.J., Ma Z.P., Yin Y., et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 2015;48:186–194. doi: 10.1016/j.bbi.2015.03.016. [DOI] [PubMed] [Google Scholar]
- Kamen C.L., Zevy D.L., Ward J.M., Bishnoi I.R., Kavaliers M., Ossenkopp K.P. Systemic treatment with the enteric bacterial fermentation product, propionic acid, reduces acoustic startle response magnitude in rats in a dose-dependent fashion: contribution to a rodent model of ASD. Neurotox. Res. 2019;35(2):353–359. doi: 10.1007/s12640-018-9960-9. [DOI] [PubMed] [Google Scholar]
- Kanehisa M., Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28(1):27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappelmann N., Perry B.I., Khandaker G.M. Prenatal and childhood immuno-metabolic risk factors for adult depression and psychosis. Harv. Rev. Psychiatr. 2022;30(1):8–23. doi: 10.1097/HRP.0000000000000322. [DOI] [PubMed] [Google Scholar]
- Kelly J., Borre Y., O' Brien C., Patterson E., El Aidy S., Deane J., et al. Transferring the blues: depression-associated gut microbiota induces neurobehavioural changes in the rat. J. Psychiatr. Res. 2016;82:109–118. doi: 10.1016/j.jpsychires.2016.07.019. [DOI] [PubMed] [Google Scholar]
- Kelly J.R., Borre Y., Brien C.O., Patterson E., El Aidy S., Deane J., et al. Transferring the blues: depression-associated gut microbiota induces neurobehavioural changes in the rat. J. Psychiatr. Res. 2016;82:109–118. doi: 10.1016/j.jpsychires.2016.07.019. [DOI] [PubMed] [Google Scholar]
- Kim M.H., Kang S.G., Park J.H., Yanagisawa M., Kim C.H. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology. 2013;145(2):396. doi: 10.1053/j.gastro.2013.04.056. [DOI] [PubMed] [Google Scholar]
- Knuesel T., Mohajeri M.H. The role of the gut microbiota in the development and progression of major depressive and bipolar disorder. Nutrients. 2022;14(1) doi: 10.3390/nu14010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren O., Goodrich J.K., Cullender T.C., Spor A., Laitinen K., Bäckhed H.K., et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell. 2012;150(3):470–480. doi: 10.1016/j.cell.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozich J., Westcott S., Baxter N., Highlander S., Schloss P. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl. Environ. Microbiol. 2013;79(17):5112–5120. doi: 10.1128/aem.01043-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D.T.S., Yip S.K., Chiu H.F.K., Leung T.Y.S., Chan K.P.M., Chau I.O.L., et al. Detecting postnatal depression in Chinese women - validation of the Chinese version of the Edinburgh Postnatal Depression Scale [Article] Britich J. Psychiatr. 1998;172:433–437. doi: 10.1192/bjp.172.5.433. [DOI] [PubMed] [Google Scholar]
- Liu R.T., Rowan-Nash A.D., Sheehan A.E., Walsh R.F.L., Sanzari C.M., Korry B.J., Belenky P. Reductions in anti-inflammatory gut bacteria are associated with depression in a sample of young adults. Brain Behav. Immun. 2020;88:308–324. doi: 10.1016/j.bbi.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna R.A., Foster J.A. Gut brain axis: diet microbiota interactions and implications for modulation of anxiety and depression. Curr. Opin. Biotechnol. 2015;32:35–41. doi: 10.1016/j.copbio.2014.10.007. submitted for publication. [DOI] [PubMed] [Google Scholar]
- Lutsiv O., McKinney B., Foster G., Taylor V.H., Pullenayegum E., McDonald S.D. Pregnancy complications associated with the co-prevalence of excess maternal weight and depression. Int. J. Obes. 2015;39(12):1710–1716. doi: 10.1038/ijo.2015.119. [DOI] [PubMed] [Google Scholar]
- Mirza R., Sharma B. A selective peroxisome proliferator-activated receptor-gamma agonist benefited propionic acid induced autism-like behavioral phenotypes in rats by attenuation of neuroinflammation and oxidative stress. Chem. Biol. Interact. 2019;311 doi: 10.1016/j.cbi.2019.108758. [DOI] [PubMed] [Google Scholar]
- Mohajeri M.H., La Fata G., Steinert R.E., Weber P. Relationship between the gut microbiome and brain function. Nutr. Rev. 2018;76(7):481–496. doi: 10.1093/nutrit/nuy009. [DOI] [PubMed] [Google Scholar]
- Mor G., Cardenas I. The immune system in pregnancy: a unique complexity. Am. J. Reprod. Immunol. 2010;63(6):425–433. doi: 10.1111/j.1600-0897.2010.00836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyangahu D.D., Jaspan H.B. Influence of maternal microbiota during pregnancy on infant immunity. Clin. Exp. Immunol. 2019;198(1):47–56. doi: 10.1111/cei.13331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Mahony S.M., Clarke G., Borre Y.E., Dinan T.G., Cryan J.F. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res. 2015;277:32–48. doi: 10.1016/j.bbr.2014.07.027. submitted for publication. [DOI] [PubMed] [Google Scholar]
- Petrozzi A., Gagliardi L. Anxious and depressive components of Edinburgh Postnatal Depression Scale in maternal postpartum psychological problems. J. Perinat. Med. 2013;41(4):343–348. doi: 10.1515/jpm-2012-0258. submitted for publication. [DOI] [PubMed] [Google Scholar]
- Rasmussen K.M., Yaktine A.L. National Academies Press (US; 2009. Weight Gain during Pregnancy: Reexamining the Guidelines. [PubMed] [Google Scholar]
- Ridaura V., Belkaid Y. Gut microbiota: the link to your second brain. Cell. 2015;161(2):193–194. doi: 10.1016/j.cell.2015.03.033. [DOI] [PubMed] [Google Scholar]
- Rogers A., Obst S., Teague S.J., Rossen L., Spry E.A., Macdonald J.A., et al. Association between maternal perinatal depression and anxiety and child and adolescent development: a meta-analysis. JAMA Pediatr. 2020;174(11):1082–1092. doi: 10.1001/jamapediatrics.2020.2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roomruangwong C., Anderson G., Berk M., Stoyanov D., Carvalho A.F., Maes M. A neuro-immune, neuro-oxidative and neuro-nitrosative model of prenatal and postpartum depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2018;81:262–274. doi: 10.1016/j.pnpbp.2017.09.015. [DOI] [PubMed] [Google Scholar]
- Roomruangwong C., Anderson G., Berk M., Stoyanov D., Carvalho A.F., Maes M. A neuro-immune, neuro-oxidative and neuro-nitrosative model of prenatal and postpartum depression. Prog. Neuro Psychopharmacol. Biol. Psychiatr. 2018;81:262–274. doi: 10.1016/j.pnpbp.2017.09.015. [DOI] [PubMed] [Google Scholar]
- Skonieczna-Zydecka K., Grochans E., Maciejewska D., Szkup M., Schneider-Matyka D., Jurczak A., et al. Faecal short chain fatty acids profile is changed in Polish depressive women. Nutrients. 2018;10(12) doi: 10.3390/nu10121939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skonieczna-Żydecka K., Grochans E., Maciejewska D., Szkup M., Schneider-Matyka D., Jurczak A., et al. Faecal short chain fatty acids profile is changed in polish depressive women. Nutrients. 2018;10(12) doi: 10.3390/nu10121939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Society T.C.N. 2016. Core Recommendations of Dietary Guidelines for Chinese Residents.https://www.cnsoc.org/knowledge/82170120212.html Retrieved August from. [Google Scholar]
- Sulley S., Adzrago D., Mamudu L., Odame E.A., Atandoh P.H., Tagoe I., et al. Assessment of prenatal depression among U.S. pregnant women without access to paid sick leave and regular place of care: National Health Interview Survey of U.S.-born and non-U.S.-born. Prev. Med. Rep. 2023;35 doi: 10.1016/j.pmedr.2023.102322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran S.M.S., Mohajeri M.H. The role of gut bacterial metabolites in brain development, aging and disease. Nutrients. 2021;13(3) doi: 10.3390/nu13030732. Article 732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valles-Colomer M., Falony G., Darzi Y., Tigchelaar E., Wang J., Tito R., et al. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol. 2019;4(4):623–632. doi: 10.1038/s41564-018-0337-x. [DOI] [PubMed] [Google Scholar]
- Vinolo M.A.R., Rodrigues H.G., Hatanaka E., Hebeda C.B., Farsky S.H.P., Curi R. Short-chain fatty acids stimulate the migration of neutrophils to inflammatory sites. Clin. Sci. 2009;117(9–10):331–338. doi: 10.1042/CS20080642. [DOI] [PubMed] [Google Scholar]
- Wang Y., Liu Y., Bai J., Chen X. The effect of maternal postpartum practices on infant gut microbiota: a Chinese cohort study. Microorganisms. 2019;7(11) doi: 10.3390/microorganisms7110511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M., Tian T., Mao Q., Zou T., Zhou C., Xie J., Chen J. Associations between disordered gut microbiota and changes of neurotransmitters and short-chain fatty acids in depressed mice. Transl. Psychiatry. 2020;10(1):350. doi: 10.1038/s41398-020-01038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu R.R., Wu Z.F., Wang S.H., Zhang M.C., Zhou G.L., Li B. Isolation, identification and characterization of propionic acid bacteria associated with autistic spectrum disorder. Microb. Pathog. 2020;147 doi: 10.1016/j.micpath.2020.104371. [DOI] [PubMed] [Google Scholar]
- Zhang J.C., Yao W., Hashimoto K. Brain-derived neurotrophic factor (BDNF)-TrkB signaling in inflammation-related depression and potential therapeutic targets. Curr. Neuropharmacol. 2016;14(7):721–731. doi: 10.2174/1570159x14666160119094646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Wang L., Cui S., Yuan Q., Huang C., Zhou X. Prenatal depression in women in the third trimester: prevalence, predictive factors, and relationship with maternal-fetal attachment. Front. Public Health. 2020;8 doi: 10.3389/fpubh.2020.602005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Yang Y., Li M., Zhou X., Zhang K., Yin X., Liu H. The prevalence of suicide ideation and predictive factors among pregnant women in the third trimester. BMC Pregnancy Childbirth. 2022;22(1):266. doi: 10.1186/s12884-022-04590-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng P., Yang J., Li Y.F., Wu J., Liang W.W., Yin B.M., et al. Gut microbial signatures can discriminate unipolar from bipolar depression. Adv. Sci. 2020;7(7) doi: 10.1002/advs.201902862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng P., Zeng B., Zhou C., Liu M., Fang Z., Xu X., et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host's metabolism. Mol. Psychiatr. 2016;21(6):786–796. doi: 10.1038/mp.2016.44. [DOI] [PubMed] [Google Scholar]
- Zhu H.Z., Liang Y.D., Ma Q.Y., Hao W.Z., Li X.J., Wu M.S., et al. Xiaoyaosan improves depressive-like behavior in rats with chronic immobilization stress through modulation of the gut microbiota. Biomed. Pharmacother. 2019;112 doi: 10.1016/j.biopha.2019.108621. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.