Abstract
The mass production of screen-printed electrochemical devices with integrated electrodes has facilitated the widespread adoption of electroanalytical methods. The SPEs (screen-printed electrodes) overcome some obstacles associated with the use of conventional electrochemical cells, making them accessible to untrained operators. Despite their advantages, SPEs require activation/modification of the working electrode (WE) to enhance sensitivity. Nanomaterials, with metal nanoparticles (NPs) dispersed in polymers and/or carbon NPs has gaining popularity for this purpose. In this study, we describe a modification of carbon SPEs (SPCEs) using Pt NPs and reduced graphene oxide (ERGO). The Pt-ERGO@SPCE is prepared by galvanostatic reduction of drop-casted precursors directly onto the WE surface, eliminating complex synthetic steps and high temperatures. After optimizing Pt amount and reduction extent, the modified SPCEs were tested for detecting hydroquinone (HQ) and bisphenol A (BPA). DPV results show significantly increased sensitivity for the quantification of both compounds. The modified SPCEs demonstrates promising performance: precision (5 % HQ, 8 % BPA), detection limits (1.4 μM HQ, 4.6 μM BPA), sensitivity (1688 μA mM−1 HQ, 441 μA mM−1 BPA), and recoveries (98–113 % HQ, 98–104 % BPA). This simple electrode modification holds great potential, allowing the preparation of the sensor by personnel who may lack access to well-equipped laboratories, particularly in developing countries.
Keywords: Screen-printed electrodes, Sensitive detection, Pt nanoparticles, Reduced graphene oxide, Phenolic compounds, Electrochemical sensor
Graphical abstract
Highlights
-
•
Simple electrode modification with Pt NPs and reduced GO increased SPCE sensitivity.
-
•
Efficient use of precursors via electrolysis of drop-casted Pt(IV)-GO onto SPCE surface.
-
•
Excellent sensitivity in HQ and BPA sensing.
-
•
Promising sensor for detecting phenolic water contaminants.
1. Introduction
The advent of mass production of screen-printed electrodes (SPEs) has made electroanalytical methods more accessible to untrained operators in a wide range of applications [[1], [2], [3]]. The integration of working, reference, and auxiliary electrodes in a single device, the reduced amount of solution required to assemble the electrochemical cell, portability, and lower cost are factors that have contributed to the increased popularity of SPEs. However, SPEs often suffer from reduced sensitivity due to the presence of inert constituents, such as polymeric binders, in the ink's formulation [4]. To overcome this drawback, various electrode pre-treatments and surface modifications have been devised.
Electrode pre-treatments include heat treatment in air [5], exposure to organic solvents [6], and electrochemical activation in different solutions [7], among others. These treatments have demonstrated their efficiency in removing organic constituents from the ink [8], resulting in improved kinetics of heterogeneous charge transfer. Alternatively, the modification of SPEs surfaces using nanomaterials has emerged as one of the most effective approaches to improving their performance. The combination of noble metals and carbon-based materials, such as carbon black, carbon nanotubes, and graphenes, can significantly enhance the electrochemical performance of SPEs by improving their catalytic, electrical, and interfacial properties [9].
Strategies for modifying SPEs can vary, often involving the preparation of nanomaterials/nanocomposites followed by their immobilization on the electrode surface. On the other hand, nanomaterials/nanocomposites can be prepared using a wide range of methods. Metal-containing nanomaterials can be synthesized through various methods, including chemical reduction, photochemical synthesis, microwave-assisted synthesis, electroless metallization and thermal evaporation [10]. Numerous examples in literature demonstrate increased sensitivity by incorporating noble metal nanoparticles (NPs) into screen-printed carbon electrodes (SPCEs). Pt cubes on porous Cu foam exhibited enhanced electrochemical oxidation of glucose [11]. Gold nanospike structures on SPCEs enable non-enzymatic detection of dopamine and other analytes, with a sensitivity of 0.056 μA mM−1 cm−2 and a detection limit (LOD) of 0.33 μM [12]. Dodecanethiol-stabilized Pt NPs modified SPCEs for dapsone determination, offering a LOD of 0.76 μM and sensitivity three times higher than Pt-bulk electrodes [13]. A sensitive sensor using dendritic Pt NPs coated on gold nanoparticles on SPCEs, followed by polyethyleneimine-phosphatidylcholine deposition, achieved excellent BPA detection with a LOD of 6.63 nM [14]. A highly sensitive nonenzymatic electrochemical sensor for H2O2 detection in adulterated milk was developed utilizing bimetallic Au–Pt NPs modified SPCEs [15]. Nanocomposites, including Pt–TiO2 NPs [16] and Pt–Pd nanoflakes [17] have been developed to increase the sensitivity of SPEs for significant analyte determination. A modification involving the electrodeposition of Pt NPs on a conductive layer of polyazure A-dodecyl sulphate was used for sensitive determination of hydrogen peroxide [18,19].
By combining the Pt NPs with carbon NPs, nanocomposites with enhanced electrochemical properties were developed for sensing applications. For instance, a sensor for tartrazine quantification was developed with a SPCE modified with porous reduced graphene oxide (RGO) decorated with carbon quantum dots and Pt NPs (Pt/CQDs@RGO/SPCE), achieving a limit of detection (LOD) of 7.93 nM [20]. Non-enzymatic hydrogen peroxide sensors employed nanocomposites of Pt with carbon nanotubes (CNT), graphene oxide (GO), or RGO [21]. Literature describes sensors for other biologically significant analytes, including dopamine [22] uric acid [23] and oxalic acid [24]. The incorporation of NPs, such as CdS and TiO2, into the noble metal-carbon nanohybrids enabled the development of highly sensitive sensors for natamycin [25] and H2O2 [26], respectively. Nanohybrids incorporating Pt NPs and carbon NPs are employed in enzymatic biosensors to enhance sensitivity and stability as described for the sensing of HQ, cholesterol and glucose using laccase [27], cholesterol oxidase [28], and glucose oxidase [29] respectively.
Pt NPs combined with carbon NPs are also utilized to enhance the binding strength of capture antibodies and improve the electrochemical sensitivity of immunosensors. Pt NPs deposited on single-walled CNT were employed for detection of hepatitis C virus infection [30].
In the previous studies, various modifications have been proposed to enhance the analytical performance of SPCEs. However, most of these methods involve the preparation of nanohybrid materials, which require complex procedures and specialized equipment. As a result, these important analytical chemistry tools are inaccessible to less well-equipped labs worldwide, especially in developing countries. Developing simpler methodologies for preparing these sensors is crucial to broaden the accessibility and democratize access to advanced analytical techniques. To address this limitation and promote wider accessibility to advanced analytical techniques it is crucial to develop simpler methodologies for preparing SPCE sensors.
In this work we present a novel approach where SPCEs are modified with Pt NPs and reduced GO through direct electrochemical reduction of the precursors directly drop-casted onto the working electrode. The modified SPCEs are evaluated for its potential in detecting two significant water contaminants: HQ and BPA. BPA, extensively used in the plastics industry and as a precursor in polycarbonate and epoxy resin production, raises concerns due to its role as an endocrine disruptor, capable of mimicking hormones in the body. Prolonged and intense exposure to BPA has been linked to serious health risks, including an increased likelihood of breast or prostate cancer, obesity, and diabetes [31]. Moreover, the oxidation of BPA leads to the formation of phenolic reaction intermediates, such as HQ, which is also a significant environmental pollutant, exhibiting considerable toxicity and low degradability in ecological systems [32].
By focusing on the direct electrochemical reduction method for modifying SPCEs, we aim to provide a simpler and more accessible alternative for enhancing SPCEs capabilities, particularly in regions with limited resources. This approach holds promise for the effective detection of water contaminants and contributes to addressing the environmental and health concerns associated with BPA and its by-products.
2. Materials and methods
2.1. Chemicals and solutions
Graphene oxide (2 mg/mL, dispersion in H2O), chloroplatinic(IV) acid solution (H2PtCl6, 8 % wt in water), lead(II) acetate trihydrate (99.8 %), potassium phosphate monobasic (KH2PO4, 99 %), potassium phosphate dibasic (K2HPO4, 98 %), methanol (HPLC grade, 99,5 %), hydroquinone (HQ, 99 %) and bisphenol A (BPA, 99 %) were purchased from Sigma-Aldrich and used as received without any further purification. Solutions were prepared using water of 18.2 MΩ cm resistivity obtained from a Milli-Q Gradient A10 system (Millipore).
Phosphate buffer solution (PBS) 0.10 M pH 7.0 was prepared using K2HPO4 and K2HPO4 and used as supporting electrolyte.
Working HQ and BPA solutions in 0.10 M PBS pH 7.0 were daily prepared by dilution of the corresponding stock solutions that were kept at −22 °C. The HQ stock solution (0.10 M) was prepared in water while the BPA (0.010 M) was prepared in methanol.
The platinization solution (Pt(IV) solution, 28 mM) was prepared from 1 mL chloroplatinic(IV) acid solution and 1.6 mg lead(II) acetate trihydrate added to 6.36 mL of 0.01 M PBS [33]. GO and GO-Pt(IV) suspensions (containing 1 mg/mL of GO) were prepared from the commercial GO solution (2 mg/mL) homogenized using an ultrasonic probe for 2 h (UP50H from Hielscher, maximum power of 50 W and amplitude of 60 %).
2.2. Electrodes, equipment and electrochemical assays
The screen-printed carbon electrodes (SPCEs) were purchased from DropSens, Metrohm (DRP-110). They are composed of a carbon counter electrode, an Ag pseudo-reference electrode, and a printed graphene working electrode (WE) with a diameter of 4 mm.
Electrochemical measurements were conducted by placing a drop of solution (75 μL) onto the sensor, covering all three electrodes. Voltammetric measurements and galvanostatic electrolysis were performed at room temperature using an Autolab PGSTAT30 potentiostat controlled by the GPES 4.9 software (EcoChemie). Differential pulse voltammograms were obtained with pulse amplitude of 25 mV, potential step of 5 mV, modulation time of 0.05 s and an interval time of 0.5 s.
All measurements were performed in triplicate. For HQ measurements, the sensors were rinsed thoroughly with water before use and between voltammetric measurements, achieving a good repeatability of 5 % for concentrations ranging from 5 to 20 μM, calculated as the relative standard deviation of three consecutive measurements. However, for BPA measurements, an additional cleaning step was required to ensure an adequate repeatability of 8 % for concentrations ranging from 10 to 40 μM. This involved performing 10 voltammetric cycles in a saturated Na2CO3 solution ranging from 0 to 0.6 V (vs. SPCE pseudo-reference electrode) at a scan rate of 10 mV s−1.
2.3. Modification of SPCEs
The modification of the SPCE was conducted to prepare the Ptn-ERGO(Q)@SPCE sensors. This process involved the drop-casting of 7 μL of a Pt(IV)-GO mixture onto the surface of the WE of the SPCE, followed by solvent (water) evaporation under an incandescent lamp. Subsequently, the material was subjected to galvanostatic electrolysis immersing the electrode into a 0.1 M PBS at pH 7.0. The quantity of Pt on the sensor's surface, represented as n (in nmol), was controlled by adjusting the concentration of Pt(IV) in the drop-casted solution. The extent of reduction was controlled by means of the charge used in the galvanostatic process, denoted as Q (in mC), determined by the electrolysis time using a current of −0.15 mA.
Various alternative methodologies were employed to modify the SPCEs in order to investigate the influence of different constituents and modification techniques on their performance.
The sensors denoted as Ptn@SPCE were prepared by electrodepositing Pt from a 7 mM Pt(IV) solution (diluted from the platinization solution) using a current of - 0.15 mA, with the deposition time adjusted to achieve the desired amount of Pt (n in nmol).
For the sensors labelled as GO@SPCE, 7 μL of a 1 mg/mL GO suspension were drop-casted onto the WE, followed by solvent evaporation under an incandescent lamp.
The sensors identified as GO/Ptn@SPCE underwent a two-step modification procedure. In the first step, Pt was deposited onto the WE surface following the methodology described for Ptn@SPCE. In the second step, GO was deposited onto the platinized WE surface using the procedure previously described for GO@SPCE.
To prepare the ERGO(Q)@SPCE, GO was drop-casted onto the working electrode, following the procedure described for GO@SPCE. Subsequently, a galvanostatic reduction was performed by immersing the electrode into a 0.1 M PBS at pH 7.0, with the extent of GO reduction controlled by the charge used in the galvanostatic process (−0.15 mA).
For the preparation of (ERGO-Pt)CV@SPCE, the SPCE was immersed in a platinization solution (0.5 mM) containing well-dispersed GO (0.5 mg/mL) in 0.01 M PBS, and subjected to 20 consecutive cyclic voltammetric cycles between −1.5 V and 0 V at a scan rate of 50 mV s−1 [34].
2.4. Surface characterization
The surface morphology of the working electrodes was examined using scanning electron microscopy (SEM) with a Leica Cambridge S360 apparatus. Additionally, the elemental composition of Pt12-ERGO(20)@SPCE was determined using the same microscope equipped with energy dispersive X-ray spectroscopy (EDX). X-ray photoelectron spectroscopy (XPS) characterization was performed on an ESCALAB 250 instrument with Al Kα X-rays (1489.6 eV).
3. Results
3.1. Modification of SPCEs with Pt NPs and ERGO by galvanostatic reduction
In order to enhance the sensitivity of the SPCEs response towards a model polyphenol compound, hydroquinone (HQ), several electrode modifications were undertaken using Pt(IV) and GO as precursors. Pt nanoparticles (Pt NPs) were synthetized via galvanostatic reduction [33] from either a Pt(IV) solution or a well-mixed combination of Pt(IV) and GO, which was drop-casted onto the electrode surface. Fig. 1 illustrates the voltammetric characterization of the SPCEs modified with Pt NPs in HQ solutions using different modification approaches. These approaches include galvanostatic deposition (referred as Ptn@SPCE, where n represents the quantity of Pt), galvanostatic deposition followed by deposition of GO (GO/Ptn@SPCE), and galvanostatic reduction of a Pt(IV)-GO mixture (referred as Ptn-ERGO(Q)@SPCE, where Q denotes the amount of charge employed in the galvanostatic reduction). For comparative analysis, the voltammetric response of the SPCE, the SPCE modified with GO (GO@SPCE), and the SPCE modified with electrochemically reduced graphene oxide (ERGO@SPCE), produced through galvanostatic reduction (with a charge of 15 mC) of GO drop-casted onto the working electrode of the SPCE, are also depicted in Fig. 1.
Fig. 1.
Cyclic voltammograms of modified SPCE in 0.10 M PBS pH 7.0 containing HQ. SPCE modified with: (a) GO (GO@SPCE) and with electrochemically reduced GO (ERGO@SPCE); (b) 8 nmol of Pt (Pt8@SPCE) and 16 nmol of Pt (Pt16@SPCE) by electrodeposition; (c) GO after electrodeposition of 12 nmol of Pt (GO/Pt12@SPCE) or 26 nmol of Pt (GO/Pt26@SPCE); (d) GO and 12 nmol of Pt, in a single step, followed by a galvanostatic reduction using 5 mC (Pt12-ERGO(5)@SPCE) or 20 mC (Pt12-ERGO(20)@SPCE). For reference, the response of the bare SPCE is represented in all figures. [HQ] = 0.10 mM in (a), (c), (d) and [HQ] = 1.0 mM in (b).
It is worth noting that the modifications involving Pt or GO/Pt did not yield any significant improvements in the HQ voltammograms, as shown in Fig. 1b and c. These voltammograms exhibit a reduction peak characteristic of Pt, which is also present in the blank solutions. The presence of this peak made it challenging to detect the reduction peak of HQ, and the HQ oxidation peak was less distinct in these two modified SPCEs. In contrast, the modification achieved through the galvanostatic reduction of Pt(IV)-GO drop-casted onto the SPCE (Fig. 1d) displays well-defined oxidation and reduction peaks that are closer together compared to the ones in unmodified SPCE. Although a similar effect was observed for ERGO@SPCE (Fig. 1a), a more significant increase in peak current was obtained for Pt12-ERGO(5)@SPCE (Fig. 1d), with improvements of 20 % and 68 % respectively. This outcome demonstrates a synergistic effect between the two constituents of Pt-ERGO utilized in this SPCE modification. The Pt content in the material deposited onto the SPCE surface, as well as the extent of reduction, can be easily adjusted to optimize the voltammetric response.
The two voltammograms presented in Fig. 1d clearly illustrate the significant influence of the extent of the galvanostatic reduction on the sensor response. The observed potential shift of the peaks observed in Fig. 1d is a consequence of the potential shift of the pseudo-reference electrode, which is linked to the galvanostatic reduction of the material deposited on the WE. During this process, the pseudo-reference and the secondary electrodes are interconnected and act as the counter electrode in a two-electrode configuration used in the galvanostatic assay. The Ag present in the ink used as pseudo-reference electrode material is likely to undergo oxidation during this process, affecting its potential. In addition to the potential shift, it is noteworthy that both the peak current and capacitive current show substantial variations with changes in the extent of the material reduction. This observation suggests that this variable exerts a significant impact on the overall sensor performance. The variations in peak current and capacitive current further highlight the sensitivity of the sensor to the reduction process, emphasizing the importance of optimizing this variable to achieve the desired sensor performance.
The following section describes the optimization process for both the Pt content and the charge employed in the galvanostatic reduction process.
3.2. Optimization of the sensor constitution
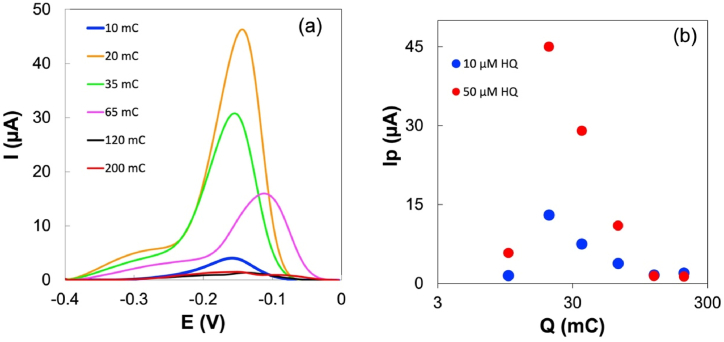
Optimizing the Pt content is essential as it directly influences the behaviour of Pt-ERGO as an electrode material. The impact of the Pt quantity was assessed through DPV voltammograms recorded from HQ solutions of 10 μM and 50 μM, using SPCE modified by drop-casting a mixture of Pt(IV)-GO with varying concentration of Pt(IV). The amount of Pt deposited onto the working electrode was calculated based on the solution's concentration and volume (7 μL). The charge utilized in the galvanostatic reduction step, Q, exceeded the requirement for reducing the quantity of drop-casted Pt(IV) by 30 mC.
The inclusion of this additional charge excess was necessary to ensure the complete reduction of Pt(IV) deposited on the surface of the WE, taking into account the relatively low reduction efficiency. It is important to note that during the reduction process, both GO and water are reduced, leading to the formation of small quantities of H2 bubbles. The incorporation of the charge excess was essential not only to ensure the complete reduction of Pt(IV) but also to facilitate and regulate the extent of GO reduction.
Fig. 2a exhibits the voltammograms obtained from the 50 μM HQ solution, while the voltammograms from the 10 μM solution can be found in the supplementary information (Fig. S4). In addition to the impact on the peak hight, the duration of the galvanostatic reduction of the electrode material significantly influences the peak position. These voltammograms reveal a shoulder to the left of the HQ peak, which is related to the presence of ERGO and is also observed in the blank response. Fig. 2b summarizes the effect of Pt quantity on peak current. The peak current values (Ip) increase with Pt content up to 12 nmol and them decrease for higher Pt quantities. The Pt content of 12 nmol was considered the optimized value for the Pt content of material on the WE. This corresponds to a precursors ratio of 1.7 mM Pt(IV) to 1 mg/mL GO in the solution used for material deposition onto the WE.
Fig. 2.
Effect of the amount of Pt(IV), n, in the GO-Pt mixture drop-casted onto the WE surface. (a) Differential pulse voltammograms obtained from a 0.10 M PBS pH 7.0 containing 50 μM HQ using different Ptn-ERGO(Q)@SPCE, where Q represents the amount of charge used in the galvanostatic reduction. (b) Representation of Ip of DPV voltammograms against the quantity of Pt for 10 and 50 μM of HQ.
Additionally, the extent of the reduction of both Pt(IV) and GO can be regulated by varying the charge employed in the galvanostatic reduction process. With the goal of determining the optimal value for this parameter and enhancing the sensor's response, experiments were conducted varying the amount of charge from 10 to 200 mC for SPCEs modified with a Pt(IV)-GO mixture containing 12 nmol of Pt(IV). Fig. 3a presents the DPV voltammograms obtained from a 50 μM HQ solution. The variation in the charge corresponds to a charge excess ranging from 5 to 195 mC regarding the charge required to reduce the Pt(IV) deposited on the SPCE surface. It is important to note that the amount of charge applied had a substantial impact both on the current and peak potential. The shift of the peaks is a consequence of changes in the potential of the pseudo-reference electrode, stemming from variations in the charge applied during the electrolysis. In this process, the pseudo-reference and the secondary electrodes are interconnected and function as the counter electrode in a two-electrode configuration. As a result, the silver in the pseudo-reference electrode material undergoes oxidation to varying degrees.
Fig. 3.
Effect of the amount of charge, Q, used for the electrochemical reduction step, Pt12-ERGO(Q)@SPCE (for 12 nmol of Pt(IV)). (a) Differential pulse voltammograms recorded in 0.1 M PBS, pH 7.0 containing 50 μM HQ. (b) Representation of Ip of DPV voltammograms against the amount of charge.
In Fig. 3b, the variation in peak current concerning the amount of charge is illustrated for both 10 and 50 μM HQ solutions. The voltammograms from the 10 μM solution can be found in the supplementary information (Fig. S5). The optimal value for the amount of charge was determined to be 20 mC (corresponding to an electrolysis duration of 133 s) for both concentrations of HQ, representing an excess of charge of 15 mC. Considering that 7 μg of GO was deposited on the surface of the WE, the ratio of charge to GO mass was calculated to be 2.1 mC μg−1.
By precisely controlling these variables, the sensor's performance can be fine-tuned, ensuring optimal sensitivity and functionality. Results from the characterization of the optimized sensor (Pt12-ERGO(20)@SPCE) are presented in the following sections.
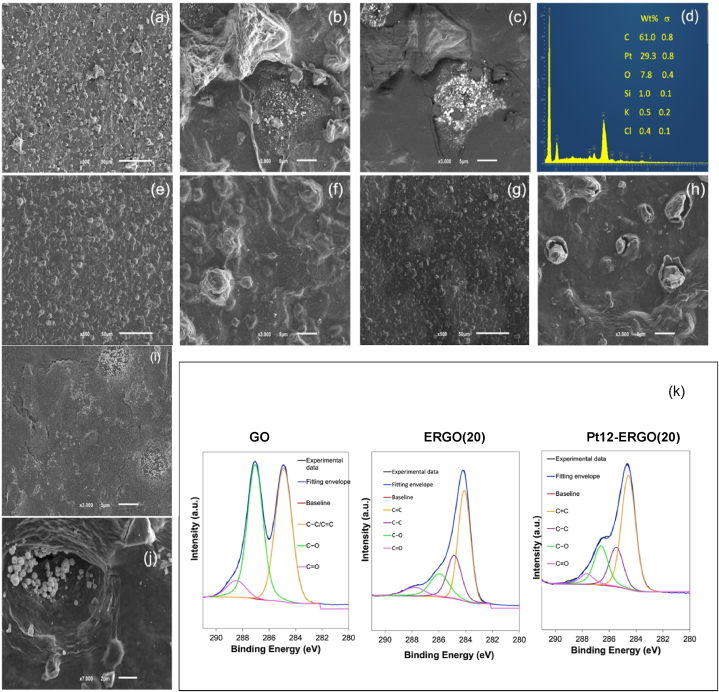
3.3. Effect of the SPCEs modifications on the surface morphology
The SPCE surface, modified with Pt NPs and ERGO, was characterized using SEM to analyse the electrode's morphology. The image displayed in Fig. 4a shows a granular structure with varying-sized granules. This granular structure bears a resemblance to that of GO (Fig. 4e) and ERGO (Fig. 4g). The larger pyramidal structures with triangular bases observed in Pt12-ERGO(20)@SPCE are better visualized in Fig. 4b, where small white dotted structures near the base of the pyramidal structure are Pt nanostructures. These Pt nanostructures appear to be confined to the triangular area surrounded by what seems to be remnants of destroyed capsule-like structures, possibly due to galvanostatic electrolysis. The role of galvanostatic electrolysis in the rupture of the granular structures is confirmed by comparing the SEM images of GO and ERGO (Fig. 4f and h). These structures are not present in any of the SEM images with similar amplification of the bare or modified SPCE with GO or Pt (as displayed in supplementary material). The rupture of the capsule-like structures might result from the reduction of water occluded between GO sheets, leading to the formation of H2 bubbles concomitantly with the galvanostatic reduction of GO and Pt. The in situ H2-generated gas bubbles have been previously used as a template to obtain a film by electrodeposition exhibiting pores and cracks [35].
Fig. 4.
SEM images from the surface of SPCE modified with: (a), (b) and (c) galvanostatic reduction (20 mC) of a mixture of the platinization solution containing 12 nmol of Pt(IV) and 7 μg of GO drop-casted on the WE surface (Pt12-ERGO(20)@SPCE); (e) and (f) drop-casting 7 μg GO (GO@SPCE); (g) and (h) galvanostatic reduction of GO (Q = 15 mC) (ERGO(15)@SPCE); (i) galvanostatic reduction (Q = 5 mC) of the platinization solution containing 12 nmol of Pt(IV) drop-casted on the WE surface; (j) galvanostatic reduction (68 mC) of a mixture of the platinization solution containing 98 nmol of Pt(IV) and 7 μg of GO drop-casted on the WE surface (Pt98-ERGO(68)@SPCE). The images were obtained with the amplifications: (a), (e) and (g) 500 × , (b), (c), (f), (h) and (i) 3000 × , (j) 7000 × . (c) Is a backscattered electron image. (d) EDX spectrum obtained for the region of Pt12-ERGO(20)@SPCE shown in images (b) and (c). (k) High-resolution XPS spectrum of C 1s for GO, ERGO(20) and Pt12-ERGO(20).
The identity of the Pt nanostructures was confirmed by the backscattered electron image and the EDX spectrum presented in Fig. 4c and d, respectively. The uneven distribution of Pt can be attributed to the deposition process used. When the Pt(IV) solution is drop-casted at the SPCE, followed by solvent evaporation and galvanostatic electrolysis in a PBS solution, Pt NPs occurs in the form of agglomerates (Fig. 4i) rather than evenly distributed, as is the case when it is deposited directly through galvanostatic electrolysis using a Pt(IV) solution (supplementary information).
To further clarify the effect of the amount of Pt and charge used in the galvanostatic electrolysis, a SPCE modified with Pt-ERGO using 98 nmol of Pt and 68 mC was characterized by SEM. Increasing both variables, n and Q, leads to the formation of larger nanostructure agglomerates with a cauliflower-like appearance (Fig. 4j), typical of Pt-black nanostructures [36]. Additionally, there is an increase in the number of destroyed capsule-like structures (supplementary information).
The surface chemical states of GO, ERGO(20), and Pt12-ERGO(20) were investigated using X-ray Photoelectron Spectroscopy (XPS). Results are displayed in Fig. 4k. In the C 1s spectrum of GO, the presence of sp3 and sp2-hybridized graphitic carbon (C–C/C C bond) is evident at 284.9 eV. The other two peaks at 287.1 eV and 288.5 eV can be assigned to C–O and C O, respectively [37,38]. Conversely, the C 1s spectrum of ERGO(20) exhibits similar peaks, with dominant sp2-hybridized graphitic carbon at 284.1 eV. The other three peaks at 284.8, 285.9, and 287.8 eV can be attributed to sp3-carbon, C–O, and C O, respectively [39,40]. The reduced intensity of oxygen-containing bonds in ERGO compared to the C 1s spectrum of GO suggests that GO has undergone a more substantial reduction, resulting in increased electrochemical activity due to a higher degree of graphitization. On the other hand, Pt12-ERGO(20) displays a slightly higher intensity peak of oxygen-containing bonds than ERGO. This was expected, as part of the galvanostatic electrolysis charge was used to reduce Pt, thereby decreasing the extent of GO reduction in Pt12-ERGO(20) compared to ERGO. Furthermore, the high-resolution XPS O 1s spectrum was divided into two peaks for all samples (Fig. S8). For GO, the peaks are centred at 531.1 eV and 532.8 eV, corresponding to O in carbonyl groups and carbonyl oxygen atoms in esters, amides, anhydrides, and oxygen atoms in hydroxyls or ethers, respectively [40]. These peaks shift to lower binding energies after the electrochemical reduction of GO to ERGO(20), indicating a greater electronic charge on the oxygen atoms [41]. Additionally, the C O peak of Pt12-ERGO(20) shifts to an intermediate binding energy between GO and ERGO(20).
Regarding the Pt 4f spectrum of Pt12-ERGO(20), no signal in the range of 70–80 eV was obtained, as the Pt content (0.02 atoms %) in Pt12-ERGO(20) is below the XPS instrument's detection limit (0.1–0.01 atoms %) [42]. Despite this, the presence of Pt in the material was confirmed by SEM and EDX. Moreover, the slightly higher intensity of oxygen-containing bonds (as shown in the C 1s spectrum) in Pt12-ERGO(20) material, compared to ERGO(20), supports the reduction of Pt in this material.
Considering these results, which indicate that Pt12-ERGO(20) possesses intermediate chemical surface properties concerning carbon and oxygen elements, it can be inferred that the enhanced sensor performance is associated with the presence of Pt and the unique surface morphology of this electrode material.
3.4. Analytical performance of the optimized sensor towards the detection of hydroquinone and bisphenol A
The analytical performance of the optimized Pt-ERGO sensor (Pt12-ERGO(20)@SPCE) was evaluated for the detection of two environmentally relevant phenolic contaminants: HQ and BPA.
Fig. 5a displays the DPV voltammograms obtained from solutions of HQ with concentrations ranging from 1 μM to 50 μM. All voltammograms exhibit a peak at approximately −0.16 V, with intensity increasing linearly up to 20 μM (Fig. 5b). The sensitivity of the determination is 1.67 μA μM−1, which is significantly higher compared to the other SPCEs modifications shown in Table 1.
Fig. 5.
(a) Differential pulse voltammograms obtained for different standard solutions of HQ. (b) Calibration curve of Pt12-ERGO(20)@SPCE. Each point corresponds to the average of at least 3 measurements. Linear curve fit equation: Ip (μA) = 1.67 [HQ] (μM) + 0.98, R2 = 0.99.
Table 1.
Comparison of the calibration curves from DPV measurements of HQ solutions obtained for different electrode modifications performed with the SPCE.
| Sensor | Electrochemical reduction | Calibration curve Ip (μA); [HQ] (μM) |
R2 |
|---|---|---|---|
| Pt12@SPCE (*) | Galv. (5 mC) | Ip = 0.44 [HQ] + 33 | 0.82 |
| GO@SPCE (*) | without | Ip = 0.066 [HQ] - 0.13 | 0.994 |
| ERGO(50)@SPCE (*) | Galv. (50 mC) | Ip = 0.46 [HQ] - 0.40 | 0.98 |
| GO/Pt25@SPCE (*) | Galv. (10 mC) | Ip = 0.024 [HQ] - 0.0071 | 0.997 |
| (Pt-ERGO)CV@SPCE (*) | CV (20 cycles, 50 mV s−1) | Ip = 0.020 [HQ] + 0.037 | 0.98 |
| Pt12-ERGO(20)@SPCE | Galv. (20 mC) | Ip = 1.67 [HQ] + 0.98 | 0.99 |
(*) The voltammograms and representation of Ip vs. [HQ] are available in the supplementary Information.
The sensor modified solely with Pt nanoparticles (Pt12@SPCE) does not show a consistent increase in response with HQ concentration. However, the SPCE modifications using either GO or ERGO lead to responses that are sensitive to variations in HQ concentration, with sensitivities of 0.066 μA μM−1 and 0.46 μA μM−1, respectively. To compare with Pt12-ERGO(20)@SPCE, two additional sensors containing Pt and GO were prepared using alternative methodologies. The GO/Pt25@SPCE was prepared by drop-casting the GO suspension onto a SPCE surface, onto which 12 nmol of Pt was previously galvanostatically deposited from a Pt(IV)-containing solution. On the other hand, the (Pt-ERGO)CV@SPCE was prepared by cyclic voltammetry using 20 cycles (from 0 V to −1.5 V) at 50 mV s−1 [34]. The sensitivities of these two sensors are significantly lower (70 times and 84 times) compared to Pt12-ERGO(20)@SPCE. This result indicates the requirement for the simultaneous presence of both sensor constituents, Pt and ERGO nanoparticles, combined with an appropriate preparation methodology in order to improve the sensor response and, therefore, its sensitivity.
The results of the optimized sensor for the detection of BPA are presented in Fig. 6. The DPV voltammograms show a single peak with potentials ranging from 0.09 V to 0.12 V. The calibration curve in Fig. 6b shows a linear relationship between the peak current and BPA concentration up to 40 μM. The quality of the sensor response can be assessed not only by the high value of the determination coefficient but also by the performance parameters listed in Table 2, along with the corresponding parameters for HQ quantification.
Fig. 6.
(a) Differential pulse voltammograms obtained for different standard solutions of BPA. (b) Calibration curve of Pt12-ERGO(20)@SPCE. Each point corresponds to the average of at least 3 measurements. Linear curve fit equation: Ip (μA) = 0.44 [BPA] (μM) + 0.28, R2 = 0.998.
Table 2.
Performance parameters of the Pt12-ERGO(20)@SPCE obtained for the determination of HQ and BPA.
| HQ | BPA | |
|---|---|---|
| Sensitivity (μA mM−1) | 1688 | 441 |
| Precision (%)a | 4.6 | 7.6 |
| Limit of detection (μM) | 1.4 | 4.6 |
| Reproducibility (inter-sensors) (%) | 5.4 | 10 |
| Recovery (%)b 10 μM | 113 | 98 |
| Recovery (%)b 20 μM | 98 | 104 |
Calculated as the method standard deviation (n = 6).
From spiked mineral water samples.
Despite the difference in response sensitivity between the two analytes, which is 38 times higher for HQ, the precision, limits of detection, and reproducibility are of the same order of magnitude, albeit consistently lower for HQ quantification. Recovery assays were carried out in commercial mineral water to assess the sensor's capability to accurately retrieve and quantify the concentration of the target analytes, HQ and BPA, which were intentionally introduced into the water samples, while specifically investigating potential interferences originating from the water matrix that might influence the accuracy of the measurements. The recoveries obtained for 10 μM and 20 μM additions to mineral water are similar for both analytes, ranging from 98 % to 113 %.
Table 3 presents the performance parameters of Pt12-ERGO(20)@SPCE alongside other electrochemical sensors used for the detection of HQ and BPA.
Table 3.
Comparison of the performance of Pt12-ERGO(20)@SPCE in the detection of HQ and BPA with literature reported sensors.
| Sensor | Technique | Sensitivity (μA mM−1) | Linear range (μM) | LOD (μM) |
|---|---|---|---|---|
| Hydroquinone | ||||
| AuNPs/Fe3O4-APTES-GO/GCE [43] | DPV | 9.0 | 3–137 | 1.1 |
| SPGrCPE [44] | CV | 9.98 | 100–5000 | 70 |
| Laccase/PB/Pt–CNF/SPCE [27] | Amp. | 2.203 × 102 (μA mM−1 cm−2) | 2.5–1450 | 0.5 |
| aSPCE-NaOH [45] | DPV | 1.12 × 103 (μA mM−1 cm−2) | 20–150 | 1.3 |
| PB-SPCE [46] | DPV | 3.016 × 102 | 4.0–90 | 0.12 |
| CSS/SPE (photoelectrochem detection) [47] | – | 69 | 3.0–23.0 | 2.7 |
| aSPE [48] | DPV | 2.8 × 102 | 0.5–50 | 0.12 |
| AuNP-CB-SPE [49] | CV | 33.01 | 100–1000 | 12 |
| Pt12-ERGO(20)@SPCEa | DPV |
1.67 × 103 1.34 × 104(μA mM−1 cm−2) |
0–20 | 1.4 |
| Bisphenol A | ||||
| PEDOT/GCE [50] | Amp. | 1.57 × 103 (μA mM−1 cm−2) | 40–410 | 22 |
| NiFe2O4 -RGO/SPE [51] | DPV | 6.132 × 102 | 0.05–25 | 0.010 |
| PEI-PC/DPNs/AuNPs/SPCE [14] | Amp. | – | 0.01–1.0 1.0–300 |
0.0066 |
| La3+/ZnO NFs/GSPE [52] | DPV | 1.48 × 102 | 0.8–300.0 | 0.25 |
| ThTAA-Fe3O4NPs/SPE [53] | DPV | 50.4 | 0.03–700.0 | 0.01 |
| GDY-cMWCNT/SPE [54] | LSV | 2.77 × 103 1.235 × 103 |
0.2–25 25–500 |
0.013 |
| aSPE [48] | DPV | 56 | 0.5–50 | 0.95 |
| Mg0.5Co2.5(PO4)2/CB/SPE [55] | DPV | 1.68 × 102 3.77 × 102 |
0.5–6.5 16.5–100 |
0.15 |
| Pt12-ERGO(20)@SPCEa | DPV |
4.41 × 102 3.50 × 103(μA mM−1 cm−2) |
5.0–40 | 4.6 |
APTES ((3-Aminopropyl)triethoxysilane); aSPCE (activated in 0.1 M NaOH with ozone bubbling); aSPE (activated flexible SPE in H2SO4); CB (carbon black); cMWCNT (carboxylated multi-walled carbon nanotubes); CNF (carbon nanofibers); CSS - carbon spherical shells; DPNs (dendritic platinum nanoparticles; GDY (Graphdiyne); GCE (Glassy carbon electrode); GSPE (graphite screen-printed electrode); NFS (nanoflowers modified); PB (Prussian blue); PEDOP (poly(3,4-ethylenedioxythiophene); PEI-PC (polyethyleneimine-phosphatidylcholine); SPGrCPE (Screen-printed graphene carbon paste electrode); ThTAA (2-(4-((3-(trimethoxysilyl)propylthio)methyl)1-H1,2,3-triazol-1-yl)acetic acid).
In this work.
For HQ detection, our developed sensor exhibits the highest sensitivity among the eight sensors tested, including seven modified SPEs. Moreover, its sensitivity surpasses that of the second most sensitive sensor, aSPCE-NaOH by an order of magnitude. In contrast, our sensor achieves a LOD, only 12 times higher than the two most sensitive sensors PB-SPCE and aSPE.
In the case of the BPA detection, our sensor's performance is compared to eight sensors, seven of which are SPEs with different modifications. The sensitivity of Pt12-ERGO(20)@SPCE is comparable to that of PEDOT/GCE and NiFe2O4-RGO/SPE sensors but is six times less sensitive than GDY-cMWCNT/SPE. The LOD of Pt12-ERGO(20)@SPCE (4.6 μM) falls between those of PEDOT/GCE (22 μM) and aSPE (0.95 μM). Among this sensors, PEI-PC/DPNs/AuNPs has the lowest LOD value (0.0066 μM). However, the strength of our sensor lies in its simplicity and ease of preparation, making it a more practical and accessible option.
Overall, the performance comparison demonstrates that Pt12-ERGO(20)@SPCE offers excellent sensitivity and a suitable LOD for the detection of HQ and BPA, outperforming other sensors in certain aspects. The simplicity and convenience of our sensor's preparation process further contribute to its favourable attributes.
4. Conclusions
The results of this study demonstrate that the proposed modification of SPCEs with Pt nanoparticles (Pt NPs) and reduced graphene oxide (ERGO) is a simple and effective method for HQ and BPA sensing. This approach allows for the preparation of a modified electrode with minimal loss of reactants, achieving a 100 % efficiency use of precursors through drop-casting and direct reduction at the working electrode surface via galvanostatic electrolysis.
The sensor preparation settings were fine-tuned to achieve optimal performance by controlling the amount of precursors and the extent of reduction. A drop-cast solution with a composition of 1 mg/mL of GO and 1.7 mM of Pt(IV) was found to be optimal, resulting in an electrode loading of 7 μg of GO and 12 nmol of Pt(IV) onto a surface area of 0.126 cm2. The optimized amount of charge was set at 20 mC (0.15 mA, 133 s), which facilitated the reduction of the drop-casted Pt(IV) and simultaneous reduction of GO (at a ratio of 2.2 mC μg−1), leading to the formation of Pt-ERGO directly on the SPCE surface.
The optimized sensor, Pt12-ERGO(20)@SPCE, exhibited excellent sensitivity and precision for HQ and BPA detection, with sensitivity values of 1688 μA mM−1 for HQ and 441 μA mM−1 for BPA, and precision levels of 5 % for HQ and 8 % for BPA. The sensor was successfully applied to the analysis of these phenolic compounds in mineral water, yielding recoveries ranging from 98 % to 113 % for HQ and 98–104 % for BPA.
The simplicity and ease of our sensor's preparation methodology make it highly promising for use in situations where access to well-equipped laboratories may be limited. This method holds great potential for effective detection of water contaminants, addressing environmental and health concerns associated with BPA and its by-products.
Data availability statement
Data associated with the study has not been deposited into a publicly available repository and data will be made available on request.
CRediT authorship contribution statement
Luís Lema: Writing – original draft, Investigation, Data curation, Conceptualization. Raquel Oliveira: Writing – original draft, Methodology, Investigation, Data curation. Isilda Amorim: Writing – review & editing, Investigation, Formal analysis, Data curation. Ana Paula Bettencourt: Writing – review & editing, Writing – original draft, Validation, Resources, Methodology, Funding acquisition, Formal analysis, Data curation, Conceptualization. Fátima Bento: Writing – review & editing, Writing – original draft, Validation, Supervision, Project administration, Methodology, Funding acquisition, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Thanks are due to Fundação para a Ciência e Tecnologia (FCT), Portugal and FEDER (European Fund for Regional Development)-COMPETE-QRENEU for financial support through the research units Chemistry Research Centre of (UID/QUI/00686/2020). L. Lema is thankful to the Angolan Government, MESCT and ISCED of Uíge for the support of PhD grant.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e22521.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Mincu N.-B., Lazar V., Stan D., Mihailescu C.M., Iosub R., Mateescu A.L. Screen-printed electrodes (SPE) for in vitro diagnostic purpose. Diagnostics. 2020;10:517. doi: 10.3390/diagnostics10080517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mohamed H.M. Screen-printed disposable electrodes: pharmaceutical applications and recent developments. TrAC, Trends Anal. Chem. 2016;82:1–11. doi: 10.1016/j.trac.2016.02.010. [DOI] [Google Scholar]
- 3.Kanoun O., Lazarević-Pašti T., Pašti I., Nasraoui S., Talbi M., Brahem A., Adiraju A., Sheremet E., Rodriguez R.D., Ben Ali M., Al-Hamry A. A review of nanocomposite-modified electrochemical sensors for water quality monitoring. Sensors. 2021;21:4131. doi: 10.3390/s21124131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Camargo J.R., Orzari L.O., Araújo D.A.G., De Oliveira P.R., Kalinke C., Rocha D.P., Luiz Dos Santos A., Takeuchi R.M., Munoz R.A.A., Bonacin J.A., Janegitz B.C. Development of conductive inks for electrochemical sensors and biosensors. Microchem. J. 2021;164 doi: 10.1016/j.microc.2021.105998. [DOI] [Google Scholar]
- 5.Wang S.C., Chang K.S., Yuan C.J. Enhancement of electrochemical properties of screen-printed carbon electrodes by oxygen plasma treatment. Electrochim. Acta. 2009;54:4937–4943. doi: 10.1016/j.electacta.2009.04.006. [DOI] [Google Scholar]
- 6.Fanjul-Bolado P., Hernández-Santos D., Lamas-Ardisana P.J., Martín-Pernía A., Costa-García A. Electrochemical characterization of screen-printed and conventional carbon paste electrodes. Electrochim. Acta. 2008;53:3635–3642. doi: 10.1016/j.electacta.2007.12.044. [DOI] [Google Scholar]
- 7.Rocha P., Vilas‐Boas Â., Fontes N., Geraldo D., Bento F. Evaluation of polyphenols in wine by voltammetric techniques with screen printed carbon electrodes. Electroanalysis. 2020;32:159–165. doi: 10.1002/elan.201900392. [DOI] [Google Scholar]
- 8.Washe A.P., Lozano-Sánchez P., Bejarano-Nosas D., Katakis I. Facile and versatile approaches to enhancing electrochemical performance of screen printed electrodes. Electrochim. Acta. 2013;91:166–172. doi: 10.1016/j.electacta.2012.12.110. [DOI] [Google Scholar]
- 9.Pandey R.R., Chusuei C.C. Carbon nanotubes, graphene, and carbon dots as electrochemical biosensing composites. Molecules. 2021;26:6674. doi: 10.3390/molecules26216674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan C., Huang X., Zhang H. Synthesis and applications of graphene-based noble metal nanostructures. Mater. Today. 2013;16:29–36. doi: 10.1016/j.mattod.2013.01.021. [DOI] [Google Scholar]
- 11.Niu X., Lan M., Zhao H., Chen C. Well-dispersed Pt cubes on porous Cu foam: high-performance catalysts for the electrochemical oxidation of glucose in neutral media. Chem. Eur J. 2013;19:9534–9541. doi: 10.1002/chem.201300234. [DOI] [PubMed] [Google Scholar]
- 12.Anshori I., Althof R.R., Rizalputri L.N., Ariasena E., Handayani M., Pradana A., Akbar M.R., Syamsunarno M.R.A.A., Purwidyantri A., Prabowo B.A., Annas M.S., Munawar H., Yuliarto B. Gold nanospikes formation on screen-printed carbon electrode through electrodeposition method for non-enzymatic electrochemical sensor. Metals. 2022;12:2116. doi: 10.3390/met12122116. [DOI] [Google Scholar]
- 13.Caetano F.R., Gevaerd A., Castro E.G., Bergamini M.F., Zarbin A.J.G., Marcolino-Junior L.H. Electroanalytical application of a screen-printed electrode modified by dodecanethiol-stabilized platinum nanoparticles for dapsone determination. Electrochim. Acta. 2012;66:265–270. doi: 10.1016/j.electacta.2012.01.100. [DOI] [Google Scholar]
- 14.Shim K., Kim J., Shahabuddin M., Yamauchi Y., Hossain MdS.A., Kim J.H. Efficient wide range electrochemical bisphenol-A sensor by self-supported dendritic platinum nanoparticles on screen-printed carbon electrode. Sensor. Actuator. B Chem. 2018;255:2800–2808. doi: 10.1016/j.snb.2017.09.096. [DOI] [Google Scholar]
- 15.Sangkaew P., Ngamaroonchote A., Sanguansap Y., Karn-orachai K. Emerging electrochemical sensor based on bimetallic AuPt NPs for on-site detection of hydrogen peroxide adulteration in raw cow milk. Electrocatalysis. 2022;13:794–806. doi: 10.1007/s12678-022-00763-1. [DOI] [Google Scholar]
- 16.Patra D.C., Deka N., Dash A., Khan S.A., Misra T.K., Mondal S.P. Noninvasive electrochemical nitric oxide detection in human saliva using Pt and TiO 2 nanoparticle composite electrodes. ACS Appl. Electron. Mater. 2023;5:832–845. doi: 10.1021/acsaelm.2c01408. [DOI] [Google Scholar]
- 17.Niu X., Lan M., Chen C., Zhao H. Nonenzymatic electrochemical glucose sensor based on novel Pt–Pd nanoflakes. Talanta. 2012;99:1062–1067. doi: 10.1016/j.talanta.2012.07.039. [DOI] [PubMed] [Google Scholar]
- 18.Jiménez-Pérez R., González-Rodríguez J., González-Sánchez M.-I., Gómez-Monedero B., Valero E. Highly sensitive H2O2 sensor based on poly(azure A)-platinum nanoparticles deposited on activated screen printed carbon electrodes. Sensors and Actuators B: Chemical. 2019;298 doi: 10.1016/j.snb.2019.126878. [DOI] [Google Scholar]
- 19.Jiménez-Pérez R., Almagro L., González-Sánchez M.I., Pedreño M.Á., Valero E. Non-enzymatic screen-printed sensor based on PtNPs@polyazure A for the real-time tracking of the H2O2 secreted from living plant cells. Bioelectrochemistry. 2020;134 doi: 10.1016/j.bioelechem.2020.107526. [DOI] [PubMed] [Google Scholar]
- 20.Mehmandoust M., Erk N., Karaman O., Karimi F., Bijad M., Karaman C. Three-dimensional porous reduced graphene oxide decorated with carbon quantum dots and platinum nanoparticles for highly selective determination of azo dye compound tartrazine. Food Chem. Toxicol. 2021;158 doi: 10.1016/j.fct.2021.112698. [DOI] [PubMed] [Google Scholar]
- 21.Chou T.-C., Wu K.-Y., Hsu F.-X., Lee C.-K. Pt-MWCNT modified carbon electrode strip for rapid and quantitative detection of H 2 O 2 in food. J. Food Drug Anal. 2018;26:662–669. doi: 10.1016/j.jfda.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li J., Huang X., Shi W., Jiang M., Tian L., Su M., Wu J., Liu Q., Yu C., Gu H. Pt nanoparticle decorated carbon nanotubes nanocomposite based sensing platform for the monitoring of cell-secreted dopamine. Sensor. Actuator. B Chem. 2021;330 doi: 10.1016/j.snb.2020.129311. [DOI] [Google Scholar]
- 23.Khumngern S., Choosang J., Thavarungkul P., Kanatharana P., Numnuam A. Flow injection enzyme-free amperometric uric acid sensor consisting of ordered mesoporous carbon decorated with 3D Pd-Pt alloy nanodendrite modified screen-printed carbon electrode. Microchem. J. 2020;157 doi: 10.1016/j.microc.2020.104923. [DOI] [Google Scholar]
- 24.Income K., Ratnarathorn N., Themsirimongkon S., Dungchai W. An oxalic acid sensor based on platinum/carbon black-nickel-reduced graphene oxide nanocomposites modified screen-printed carbon electrode. J. Electrochem. Sci. Technol. 2019;10:416–423. doi: 10.33961/jecst.2019.00206. [DOI] [Google Scholar]
- 25.Yousefi A., Babaei A., Delavar M. Application of modified screen-printed carbon electrode with MWCNTs-Pt-doped CdS nanocomposite as a sensitive sensor for determination of natamycin in yoghurt drink and cheese. J. Electroanal. Chem. 2018;822:1–9. doi: 10.1016/j.jelechem.2018.05.008. [DOI] [Google Scholar]
- 26.Frontera P., Malara A., Stelitano S., Leonardi S.G., Bonavita A., Fazio E., Antonucci P., Neri G., Neri F., Santangelo S. Characterisation and H 2 O 2 sensing properties of TiO 2 -CNTs/Pt electro-catalysts. Mater. Chem. Phys. 2016;170:129–137. doi: 10.1016/j.matchemphys.2015.12.030. [DOI] [Google Scholar]
- 27.Liu T., Xie Y., Shi L., Liu Y., Chu Z., Jin W. 3D Prussian blue/Pt decorated carbon nanofibers based screen-printed microchips for the ultrasensitive hydroquinone biosensing. Chin. J. Chem. Eng. 2021;37:105–113. doi: 10.1016/j.cjche.2021.02.017. [DOI] [Google Scholar]
- 28.Li G., Zeng J., Zhao L., Wang Z., Dong C., Liang J., Zhou Z., Huang Y. Amperometric cholesterol biosensor based on reduction graphene oxide-chitosan-ferrocene/platinum nanoparticles modified screen-printed electrode. J Nanopart Res. 2019;21:162. doi: 10.1007/s11051-019-4602-6. [DOI] [Google Scholar]
- 29.Guzsvány V., Anojčić J., Vajdle O., Radulović E., Madarász D., Kónya Z., Kalcher K. Amperometric determination of glucose in white grape and in tablets as ingredient by screen-printed electrode modified with glucose oxidase and composite of platinum and multiwalled carbon nanotubes. Food Anal. Methods. 2019;12:570–580. doi: 10.1007/s12161-018-1387-7. [DOI] [Google Scholar]
- 30.Pusomjit P., Teengam P., Chuaypen N., Tangkijvanich P., Thepsuparungsikul N., Chailapakul O. Electrochemical immunoassay for detection of hepatitis C virus core antigen using electrode modified with Pt-decorated single-walled carbon nanotubes. Microchim. Acta. 2022;189:339. doi: 10.1007/s00604-022-05400-8. [DOI] [PubMed] [Google Scholar]
- 31.Mustieles V., Pérez-Lobato R., Olea N., Fernández M.F. Bisphenol A: human exposure and neurobehavior. Neurotoxicology. 2015;49:174–184. doi: 10.1016/j.neuro.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 32.Poerschmann J., Trommler U., Górecki T. Aromatic intermediate formation during oxidative degradation of Bisphenol A by homogeneous sub-stoichiometric Fenton reaction. Chemosphere. 2010;79:975–986. doi: 10.1016/j.chemosphere.2010.03.030. [DOI] [PubMed] [Google Scholar]
- 33.Li Y., Sella C., Lemaître F., Guille Collignon M., Thouin L., Amatore C. Highly sensitive platinum-black coated platinum electrodes for electrochemical detection of hydrogen peroxide and nitrite in microchannel. Electroanalysis. 2013;25:895–902. doi: 10.1002/elan.201200456. [DOI] [Google Scholar]
- 34.Palanisamy S., Karuppiah C., Chen S.-M. 2014. Direct Electrochemistry and Electrocatalysis of Glucose Oxidase Immobilized on Reduced Graphene Oxide and Silver Nanoparticles Nanocomposite Modified Electrode. [DOI] [PubMed] [Google Scholar]
- 35.Zhang P., Chen H., Wang M., Yang Y., Jiang J., Zhang B., Duan L., Daniel Q., Li F., Sun L. Gas-templating of hierarchically structured Ni–Co–P for efficient electrocatalytic hydrogen evolution. J. Mater. Chem. A. 2017;5:7564–7570. doi: 10.1039/C7TA01716B. [DOI] [Google Scholar]
- 36.Stanca S.E., Hänschke F., Ihring A., Zieger G., Dellith J., Kessler E., Meyer H.-G. Chemical and electrochemical synthesis of platinum black. Sci. Rep. 2017;7:1074. doi: 10.1038/s41598-017-01040-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Al-Gaashani R., Najjar A., Zakaria Y., Mansour S., Atieh M.A. XPS and structural studies of high quality graphene oxide and reduced graphene oxide prepared by different chemical oxidation methods. Ceram. Int. 2019;45:14439–14448. doi: 10.1016/j.ceramint.2019.04.165. [DOI] [Google Scholar]
- 38.Meirinho S.G., Ferraria A.M., Botelho do Rego A.M., Fernandes A.J.S., Viana A.S., Oliveira M.C. Electrogenerated hydrophilic carbon nanomaterials with tailored electrocatalytic activity. Electrochim. Acta. 2019;302:402–413. doi: 10.1016/j.electacta.2019.02.025. [DOI] [Google Scholar]
- 39.Dwivedi N., Yeo R.J., Satyanarayana N., Kundu S., Tripathy S., Bhatia C.S. Understanding the role of nitrogen in plasma-assisted surface modification of magnetic recording media with and without ultrathin carbon overcoats. Sci. Rep. 2015;5:7772. doi: 10.1038/srep07772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yuan X., Ma L., Zhang J., Zheng Y. Simple pre-treatment by low-level oxygen plasma activates screen-printed carbon electrode: potential for mass production. Appl. Surf. Sci. 2021;544 doi: 10.1016/j.apsusc.2020.148760. [DOI] [Google Scholar]
- 41.Gupta B., Kumar N., Panda K., Kanan V., Joshi S., Visoly-Fisher I. Role of oxygen functional groups in reduced graphene oxide for lubrication. Sci. Rep. 2017;7 doi: 10.1038/srep45030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shard A.G. Detection limits in XPS for more than 6000 binary systems using Al and Mg Kα X-rays. Surf. Interface Anal. 2014;46:175–185. doi: 10.1002/sia.5406. [DOI] [Google Scholar]
- 43.Erogul S., Bas S.Z., Ozmen M., Yildiz S. A new electrochemical sensor based on Fe3O4 functionalized graphene oxide-gold nanoparticle composite film for simultaneous determination of catechol and hydroquinone. Electrochim. Acta. 2015;186:302–313. doi: 10.1016/j.electacta.2015.10.174. [DOI] [Google Scholar]
- 44.Duekhuntod W., Karuwan C., Tuantranont A., Nacapricha D., Teerasong S. A screen printed graphene based electrochemical sensor for single drop analysis of hydroquinone in cosmetic products. Int. J. Electrochem. Sci. 2019;14:7631–7642. doi: 10.20964/2019.08.94. [DOI] [Google Scholar]
- 45.González-Sánchez M.I., Romero-Llapa M.I., Gómez-Monedero B., Jiménez-Pérez R., Iniesta J., Valero E. A fast and simple ozone-mediated method towards highly activated screen printed carbon electrodes as versatile electroanalytical tools. Electroanalysis. 2019;31:2437–2445. doi: 10.1002/elan.201900335. [DOI] [Google Scholar]
- 46.Buleandra M., Rabinca A.A., Mihailciuc C., Balan A., Nichita C., Stamatin I., Ciucu A.A. Screen-printed Prussian Blue modified electrode for simultaneous detection of hydroquinone and catechol. Sensors and Actuators B: Chemical. 2014;203:824–832. doi: 10.1016/j.snb.2014.07.043. [DOI] [Google Scholar]
- 47.Martoni L.V.L., Gomes N.O., Prado T.M., Calegaro M.L., Jr O.N.O., Machado S.A.S., Raymundo-Pereira P.A. Carbon spherical shells in a flexible photoelectrochemical sensor to determine hydroquinone in tap water. J. Environ. Chem. Eng. 2022;10 doi: 10.1016/j.jece.2022.107556. [DOI] [Google Scholar]
- 48.de Sá A.C., Barbosa S.C., Raymundo-Pereira P.A., Wilson D., Shimizu F.M., Raposo M., Oliveira O.N. Flexible carbon electrodes for electrochemical detection of bisphenol-A, hydroquinone and catechol in water samples. Chemosensors. 2020;8 doi: 10.3390/chemosensors8040103. [DOI] [Google Scholar]
- 49.Arduini F., Zanardi C., Cinti S., Terzi F., Moscone D., Palleschi G., Seeber R. Effective electrochemical sensor based on screen-printed electrodes modified with a carbon black-Au nanoparticles composite. Sensors and Actuators B: Chemical. 2015;212:536–543. doi: 10.1016/j.snb.2015.02.051. [DOI] [Google Scholar]
- 50.Mazzotta E., Malitesta C., Margapoti E. Direct electrochemical detection of bisphenol A at PEDOT-modified glassy carbon electrodes. Anal. Bioanal. Chem. 2013;405:3587–3592. doi: 10.1007/s00216-013-6723-6. [DOI] [PubMed] [Google Scholar]
- 51.Bas S.Z., Yuncu N., Atacan K., Ozmen M. A comparison study of MFe2O4 (M: Ni, Cu, Zn)-reduced graphene oxide nanocomposite for electrochemical detection of bisphenol A. Electrochim. Acta. 2021;386 doi: 10.1016/j.electacta.2021.138519. [DOI] [Google Scholar]
- 52.Hesam Safavi Gerdin H.S., Tajik S. Determination of bisphenol A in real samples using modified graphite screen-printed electrode. Int. J. Environ. Anal. Chem. 2022;102:4986–4995. doi: 10.1080/03067319.2020.1790548. [DOI] [Google Scholar]
- 53.Peyman Mohammadzadeh Jahani Hadi Beitollahi S.T., Tashakkorian H. Selective electrochemical determination of bisphenol A via a Fe3O4 NPs derivative-modified graphite screen-printed electrode. Int. J. Environ. Anal. Chem. 2020;100:1209–1225. doi: 10.1080/03067319.2019.1651299. [DOI] [Google Scholar]
- 54.He S., Xia H., Chang F. Enzyme free electrochemical determination of bisphenol A using screen-printed electrode modified by graphdiyne and carbon nanotubes. Microchem. J. 2022;182 doi: 10.1016/j.microc.2022.107858. [DOI] [Google Scholar]
- 55.Salhi O., Oularbi L., Ez-zine T., El Attar A., Chemchoub S., Aaddane A., El Rhazi M. Smartphone-assisted electrochemical sensor based on Mg0.5Co2.5(PO4)2 and carbon black for trace bisphenol A detection. Chemelectrochem. 2023;10 doi: 10.1002/celc.202300053. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data associated with the study has not been deposited into a publicly available repository and data will be made available on request.