Abstract
The aim of this study was to establish a new method for the determination of homoisoflavanones (Ⅲ, Ⅳ, V) in polygonatum odoratum(POD) by combination of thin layer chromatography (TLC) and chemical derivative resonance Raman spectroscopy (RRS). The twice chromatography method of TLC was used to improve the specificity of the component to be tested; the method of the relative Rf was used to reduce the use of the reference substance of the component to be tested; the chemical derivatization method was used to improve the signal intensity of Raman spectrum for the component to be tested in POD, so as to obtain a trace amount fingerprint structure information of the measured component. The method exhibits robust specificity, high sensitivity, and reliable stability, there by offering a novel reference approach for the identification and evaluation of homoisoflavanones (Ⅲ, Ⅳ, V) in POD.
Keywords: Polygonatum odoratum, Homoisoflavanones, Twice chromatography, Relative Rf, Resonance Raman spectrum(RRS)
1. Introduction
Polygonatum odoratu(POD) is dry rhizome of Polygonatum odoratum(Mill.) Druce [1]. The medicinal and edible homology of POD in the root genus. It has a long application history and it is one of the well-known traditional Chinese medicines [2]. POD has vast resources, and its active components vary greatly due to different biotope. The 2020 edition of Chinese Pharmacopoeia only includes "the total amount of polysaccharides shall not be less than 6.0 %" "the total amount of alcohol extract shall not be less than 50.0 %", the quality of POD can not be reflected and controlled in an all-round way.
According to modern scientific analysis and research, POD is rich in bioactive components [[3], [4], [5], [6]], and about 30 kinds of homoisoflavanones have been extracted. Among them, homoisoflavones(III, IV, V)(Fig. 1(b, c, d))are relatively high in content, and have strong anticancer, antibacterial and nonenzymatic glycosylation activities for proteins [7,8]. Homoisoflavones(III, IV, V) are used as identification index components, which is one of the effective methods to further control the quality of POD. In the current edition of Chinese Pharmacopoeia, most Chinese medicinal materials, Chinese herbal pieces and Chinese patent medicines are identified by traditional TLC. Due to the complex composition of traditional Chinese medicine, its specificity is greatly affected and limited [[9], [10], [11]]. for purpose of improve the specificity of the existing TLC method of traditional Chinese medicine, the new methods reported in the literature include the method of combining TLC with in situ enrichment Raman spectroscopy [[12], [13], [14], [15]] and the method of combining TLC with surface enhanced Raman spectroscopy(SERS) [[16], [17], [18], [19]]. The specificity has been improved at different degrees, but the above methods need the reference substance of the components to be tested. Only a few milligrams of homoisoflavones(III, IV, V) can be purified from 50 kg of POD medicinal material, so the reference substance is extremely rare. Raman spectrum is a scattering spectrum which is caused by inelastic collision between matter and photon. Raman spectrum is capable of reflecting the unique fingerprint information associated with the compound's structural characteristics, albeit with limited sensitivity [[20], [21], [22]], but its sensitivity is low. In order to reduce the use of reference substance and improve the sensitivity of Raman spectrum, the aim of this study was to establish a novel method for the determination of homoisoflavanones(Ⅲ, Ⅳ, V) in POD by combining the twice chromatography method and relative Rf method of TLC with Raman scattering of the chemical derivatization, which provides a novel reference method for the identification and evaluation of homoisoflavones(III, IV, V) in POD.
Fig. 1.
Structures of three homoisoflavanones[(b): Homoisoflavanone(Ⅲ); (c): Homoisoflavanone(Ⅳ); (d): Homoisoflavanone(Ⅴ)], methylophiopogonanone B(e) and their mother nucleus(a).
2. Material and methods
2.1. Materials and reagents
TLC plates made of aluminum (Merck KGaA, Darmstadt, Germany) was composed of high-performance silica gel 60 F254, and the layer thickness was 0.25 ± 0.03 mm, the particle size of silica gel powder was 8±2μm80 %. These panels contain a fluorescent additive (F254), and spots invisible to the naked eye can be observed by irradiation with 254 nm UV light.
Homoisoflavanones(Ⅲ, Ⅳ, V) reference substances were self-made, and the contents were 99.4 %, 99.3 %, 99.3 % respectively. Methylophiopogonanone B(Fig. 1(e)) reference substance(A1180-100 mg) was purchased from Chengdu mansite Biotechnology Co., Ltd. (Chengdu, China). Dichloromethane, methanol, formic acid, ethanol, petroleum ether(60 °C–90 °C) and ethyl acetate were purchased from Tianjin Kaitong Chemical Reagent Co., Ltd.(Tianjin, China). The basic information of 18 batches of POD medicinal materials and decoction pieces was shown in Table S1, and the quality was in accord with the provisions of part I of the Chinese Pharmacopoeia(2020 Edition).
2.2. Apparatus
Microinjector of 25 μL (Shanghai Gaoge industry and Trade Co., Ltd. Shanghai, China) and SC-5 three purpose ultraviolet analyzer(Beijing Jinjianzhiguang Pharmaceutical Information Technology Center. Beijing, China) were used to identify the isolated spots under 254 nm UV light. DxRxi Micro - Raman Imaging spectrometer(Thermo Fisher Scientifific, Waltham, MA, USA) with light source wavelength 780 nm and 532 nm was used to detect the Micro-Raman spectrum. KQ5200DE ultrasonic cleaner (Kunshan ultrasonic electronic equipment Co., Ltd. Kunshan, China), YGC-12TERMOVAP SAMPLE CONCENTRATOR (Zhengzhou Baojing Electronic Technology Co., Ltd. Zhengzhou, China) and C18–H solid phase extraction column(4000 mg/20 mL, BESEP, Germany) were used to prepare samples.
2.3. Preparation of solutions
2.3.1. Preparation of standard solutions
Dissolving the standard powder of homoisoflavanones(Ⅲ, Ⅳ, V) in ethanol to prepare a standard solution of homoisoflavanones(Ⅲ, Ⅳ, V) with a concentration of 0.5 mg mL−1, and sealing it in a brown bottle for future use. Similarly, preparing a standard solution of methylophiopogonanone B with a concentration of 1.0 mg mL−1.
Dissolving all standard powders(homoisoflavanones(Ⅲ, Ⅳ, V)) together in ethanol to prepare a mixed reference solution with a concentration of 0.5 mg mL−1.
2.3.2. Preparation of POD sample solutions
The dried medicinal materials or decoction pieces of POD were crushed and sifted through No. 4 sieve. The 3.3 g of powder was weighed accurately and put into a 100 mL flat bottom flask, then the powder was extracted with 14 mL ethanol with ultrasound (250 W, 50 KH) for 1h and heated to reflux in a water bath(80 °C) taking 2 h.The supernatant was took.The precipitate was extracted again as above. The two supernatants were combined and filtered while hot. The filtrate was transferred to an evaporation dish and was evaporated into extractum in a water bath(80 °C). The extractum was made into a turbid liquid with 7 mL of water and transferred to C18–H solid phase extraction column. Then, the filtrate from the column was discarded and the column was eluted with 7 mLwater and the eluent from the column was discarded agian. The above C18–H solid phase extraction column was slowly eluted with 10 mL methanol and the eluent was evaporated in an evaporation dish to about 1 mL in a water bath(80 °C) and transferred to a brown injection bottle of 1.5 mL and dried with nitrogen, and then the solid was fixed to constant volume with 100 μL methanol. The sample solutions of POD should be preserved.
2.3.3. Preparation of FeCl3 solution
It was prepared according to Chinese Pharmacopoeia (2020 Edition) by dissolving 9 g FeCl3 in distilled water to form 100 mL solution and mixing well.
2.4. Establishment of detection method
2.4.1. Twice chromatography method and relative Rf method of TLC
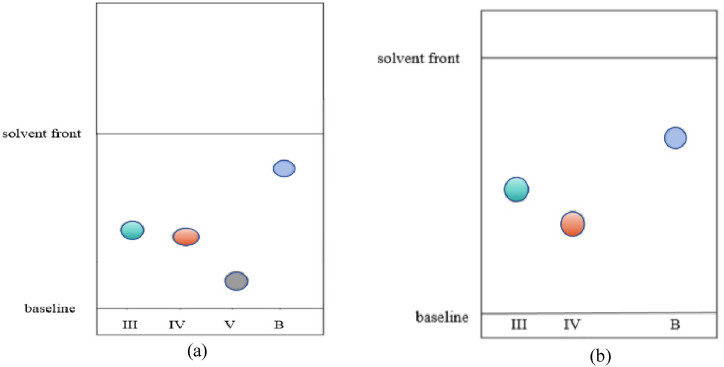
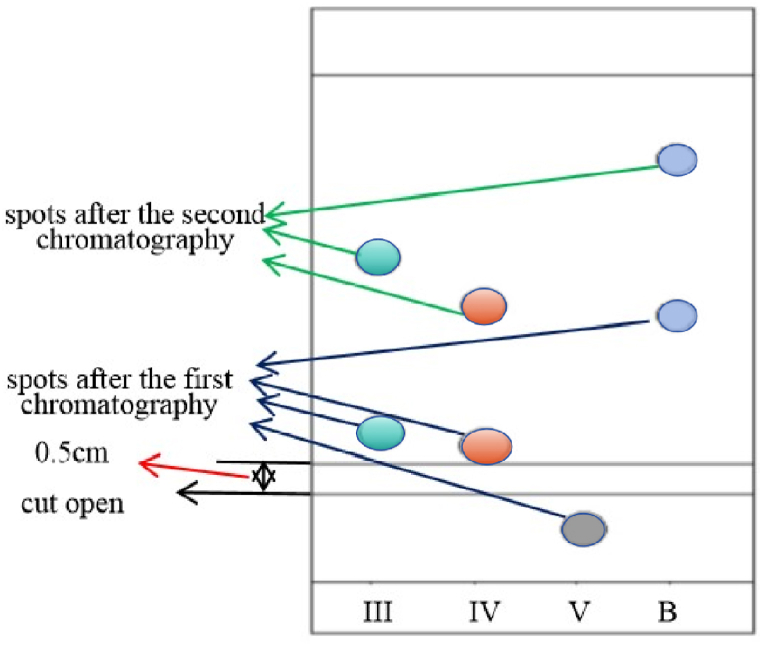
10 μL of methylophiopogonanone B and homoisoflavanones (Ⅲ, Ⅳ, V) standard solutions and sample solutions were respectively spotted on the same silica gel 60F254 aluminum plate using microinjector of 25 μL, then were eluted in a chromatographic tank with the optimum mobile phase of dichloromethane-methanol-formic acid(150:5:3, v/v/v) and inspected at 254 nm. The relative Rf(the ratio of Rf [15] of sample spot to Rf of standard reference substance) of homoisoflavanones(Ⅲ, Ⅳ, V) to methylophiopogonanone B on TLC plate was calculated(Fig. 2(a)). Then a parallel line to the bottom about 0.5 cm below the main spots of homoisoflavanones (Ⅲ, Ⅳ) was drew and the TLC plate was cut into two parts along this line(Fig. 3). Then, the upper part was eluted with petroleum ether-ethyl acetate(3:2, v/v), and immediately inspected at 254 nm. The relative Rf of homoisoflavanones(Ⅲ, Ⅳ) to methylophiopogonanone B on TLC plate was calculated(Fig. 2(b)). At last the FeCl3 solution was spotted on the spots of the four components on the TLC plate to make it react to produce colored substances(derivatives) and the Raman spectrum was detected.
Fig. 2.
Sketch map of the relative Rf method ((a): the first chromatography; (b): the second chromatography; III: homoisoflavanone(III); IV: homoisoflavanone(IV); c: homoisoflavanone(V); B: methylophiopogonanone B).
Fig. 3.
Sketch map of twice chromatography of TLC(III: homoisoflavanone(III); IV: homoisoflavanone(IV); c:homoisoflavanone(V); B: methylophiopogonanone B).
2.4.2. Raman spectrum acquisition method and conditions
The light source of the Micro -Raman imaging spectrometer was directly aligned with the spots of the derivatives of the TLC plate, and the spots were tested in situ using Raman spectrometer.The Raman spectra of the main spots of the homoisoflavanones(Ⅲ, Ⅳ, V) reference substance and the main spots of the samples were compared with the derivatives produced by the reaction of FeCl3 solution, and the characteristic peaks should be consistent.
Raman Scattering Spectra were gotten by using a DxRxi Micro - Raman Imaging spectrometer(Thermo Fisher Scientifific, Waltham, MA, USA). The detection conditions included light source wavelength, light source power, exposure time, scan times, magnification of microscope, scan range, scan mode, and confocal pinhole aperture, which were respectively 532 nm, 10.0 mW, 0.05000 s, 30, 10X, 100-3300 cm−1, region scanning, and 50 μm. The data acquisition and analysis software was OMNICxi, the mapping software was OriginPro2021. In order to avoid the influence of intensity and facilitate comparison, individual spectra were normalized.
3. Results and discussion
3.1. Twice chromatography method and relative Rf method of TLC
Homoisoflavones(III, IV, V) of POD have similar structure (Fig. 1(a)). In TLC, if the mixed solution of dichloromethane-methanol-formic acid(150:5:3, v/v/v) is used as the developing agent, homoisoflavanone(III) and homoisoflavanone(IV) cannot be effectively separated, as shown in Fig. 4(a); If the mixed solution of petroleum ether-ethyl acetate(3:2, v/v) is used as the developing agent, it cannot effectively separate homoisoflavanone(IV) and homoisoflavanone(V), as shown in Figu.4(b). Therefore, the twice chromatographymethod of TLC is required to effectively separate homoisoflavanones(Ⅲ, Ⅳ, V). First, the mixed solution of dichloromethane-methanol-formic acid (150:5:3, v/v/v) is used as the developing agent, homoisoflavanone(V) could be separated(Fig. 4(a)), then according to twice chromatography method and relative Rf method of TLC under "2.4.1″, the mixed solution of petroleum ether-ethyl acetate(3:2, v/v) is used as the developing agent, homoisoflavanone(III) and homoisoflavanone(IV) could be effectively separated(Fig. 4(c)).
Fig. 4.
TLC of three kinds of hyperisoflavanones under different development systems (a): dichloromethane-methanol-formic acid(150:5:3, v/v/v); (b): petroleum ether-ethyl acetate (3:2, v/v); (c): petroleum ether-ethyl acetate (3:2, v/v); III: homoisoflavanone(III); IV: homoisoflavanone(IV); V: homoisoflavanone(V); B: methylophiopogonanone B; M: mixed solution of four components).
Results of the first chromatographic Rf(Fig. 4(a)): RfⅢ = 0.33, RfⅣ = 0.28, RfⅤ = 0.13, RfB = 0.72. Results of the second chromatographic Rf(Fig. 4(c)): RfⅢ = 0.73, RfⅣ = 0.60, RfB = 0.96. Calculation formula of Relative Rf (RRf): RRf= Rfi/RfB(i = Ⅲ, Ⅳ, Ⅴ). Results of the first chromatographic RRf: RRfⅢ = 0.45, RRfⅣ = 0.38,RRfⅤ = 0.18. Results of the second chromatographic RRf: RRfⅢ = 0.76, RRfⅣ: 0.63.
Because a few milligrams of homoisoflavones(III, IV, V) can be purified from 50 kg of POD medicinal material, so the reference substance is extremely rare. In order to reduce the use of reference substance, a relative Rf method was proposed to preliminarily judge whether the sample contained three target components of homoisoflavones(III, IV, V) based on the relative Rf (the ratio of Rf of each spot in the sample to Rf of methylopogonone B) in the absence of Homoisoflavanones(Ⅲ, Ⅳ, V) reference substances.The reason for choosing methylophiopogonanone B as the reference material is based on methylophiopogonanone B(Fig. 1(e)) with similar structure of homoisoflavones(III, IV, V).
3.2. Derivatization with FeCl3 solution and choice of light source wavelength of Raman spectroscopy
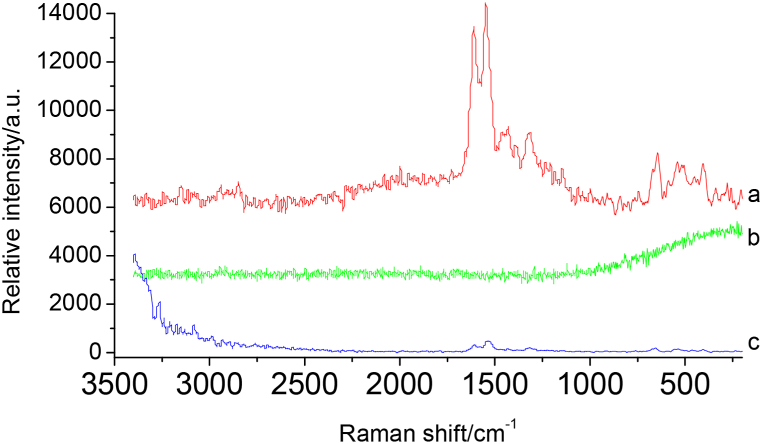
When the deposition amount of spot sample was 5 μg, the Raman spectrum was tested under 532 nm light source after TLC separation, no Raman spectrum signal was found(Fig. 5(b)). In consideration of the component containing enol form structure(Fig. 1), it can be chemically derivatized with FeCl3 solution to form colored substances, the following experiments have been conducted in this work. The FeCl3 solution was spotted on the spot of the component on the TLC plate to make it react to produce colored substances(derivatives),then the Raman spectrum was detected with 532 nm laser. It was found that the signal of Raman spectrum after derivatization at 532 nm was very strong(Fig. 5(a)), this may be because the maximum absorption wavelength(λmax) of the generated derivative is in the visible region, when the excitation wavelength coincides with or approaches the λmax of the derivatives to be measured, surface plasmons amplify Raman detection signals through resonance, so as to realize the resonance Raman spectroscopy(RRS) detection, and there was no interference at Raman background spectrum of the FeCl3 solution on the TLC plate(Fig. 8). The laboratory currently has 532 nm and 780 nm lasers as light sources for Micro-Raman Spectrometer. Firstly, using a 780 nm laser light source for Raman spectroscopy, it was found that the signal of Raman spectrum after derivatization was very weak(Fig. 5(c)), therefore, 532 nm was chosen as the laser light source for Raman spectroscopy in this study.
Fig. 5.
Raman spectra of homoisoflavanone(IV) mixed FeCl3 solution at different wavelengths (a: homoisoflavanone(IV) mixed FeCl3 solution under 532 nm; b: homoisoflavanone(IV) under 532 nm; c: homoisoflavanone(IV) mixed FeCl3 solution under 780 nm).
Fig. 8.
Raman spectrum of derivatives of homoisoflavanones(Ⅲ、Ⅳ、Ⅴ) and FeCl3.
3.3. Study on the similarities and differences of Raman spectra of three kinds of homoisoflavanones
Three kinds of homoisoflavanones(Ⅲ,Ⅳ,Ⅴ) with relatively high content, wide and strong biological activity in POD are difficult to distinguish from each other only by single chemical method and ultraviolet spectroscopy(UV) method because of their similar structures(Fig. 1). However, the Raman spectra of the three kinds of homoisoflavanones are significantly different, which can provide the fingerprint structure information of homoisoflavanones(Ⅲ,Ⅳ,Ⅴ) respectively. According to the Raman spectrum acquisition conditions under "2.4.2″, the Raman spectra of standard substance powder of the above three homoisoflavanones and methylophiopogonanone B were respectively measured. The results could been seen in Fig. 6 and Table 1.
Fig. 6.
Raman spectra of standard substance powder of homoisoflavanones(Ⅲ, Ⅳ, Ⅴ) and methylophiopogonanone B.
Table 1.
Characteristic peak and attribution of Raman spectra of standard substance powder of homoisoflavanones(Ⅲ、Ⅳ、Ⅴ)and methylophiopogonanone B.
| Analytes | Structure | Raman shift/(cm−1) | Attribution |
|---|---|---|---|
| mother nucleu | 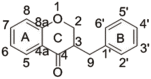 |
||
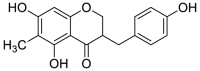
| homoisoflavanone(Ⅲ) |  |
3062 (s) 2928 (s) 1634 (shoulder peak) 1611 (s),1539(w) 1380(m) 1289(s) 853 (m) |
ν = CH ν-CH3 ν-C O of C ring νC C of benzene rings A and B δ-CH of position 6 and position 8 of A ring β-CH at positions 6 and 8 of A ring νC-O-C |
| homoisoflavanone(Ⅳ) |  |
3062 (s) 2935 (s) 1636 (shoulder peak) 1620(s),1593(m) 1378(m) 1255 (s) 858 (w) |
ν = CH ν-CH3 ν-C O of C ring νC C of benzene rings A and B δ-CH of position 6 of A ring β-CH at position 8 of A ring νC-O-C |
| continued Table 1 homoisoflavanone(Ⅴ) |
 |
3058 (s) 2932(s) 1638 (shoulder peak) 1608(s) 1385(w) 855 (s) |
ν = CH ν-CH ν-C O of C ring νC C of benzene rings A and B δ-CH of position 6 of A ring νC-O-C |
| methylophiopogonanone B | 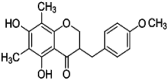 |
1638(shoulder peak) 1615(s) 1370(m) 1283(s) 850(w) 356(s) |
ν-C
O of C ring νC C of benzene rings A and B δ-CH of position 6 and position 8 of A ring β-CH produced by 4′ methoxy group νC-O-C deformation vibrationm of methoxy group of B ring |
The commonality analysis of the spectra is as follows: we can see from Fig. 6 and Table 1 that the peak around 1610 cm−1 is from ν C C of benzene rings A and B in the structure, which is the strongest scattering peak in the Raman spectra of three kinds of homoisoflavanones(III, IV, V). The scattering peak of methylophiopogonanone B at 1610 cm−1 is slightly weaker than that of three kinds of homoisoflavanones. The moderately strong peak (shoulder peak) near 1636 cm−1 is ν- C O of C ring. Near 1380 cm−1, there is δ- CH from - CH3 of position 6 and position 8 of A ring, and there is an obvious peak of νC-O-C near 853 cm−1. The ν-OH of phenolic hydroxyl has almost no signal in the Raman spectrum [23].
The difference analysis of the spectra is as follows: we can see from Fig. 6 and Table 1 that there are five = CH and three - CH of –CH3 in the structure of homoisoflavanone(V), so the peak produced by ν= CH at 3060 cm−1 are higher than the peak produced by ν- CH at 2930 cm−1, which can identify high isoflavanone(V).There are four = CH and six –CH of –CH3 in the structure of homoisoflavanone(Ⅲ), so the peak produced by ν= CH at 3062 cm−1 are lower than the peak produced by ν- CH at 2928 cm−1, and there is a strong peak at 1289 cm−1(from β- CH of two - CH3 at positions 6 and 8 of A ring), whose peak intensity is about 95 % of the peak at 1610 cm-1,which can identify high isoflavanone(Ⅲ). In the Raman spectra, there are a pair of peaks with the similar intensity near 1319 cm−1 and 1255 cm−1, whose peak intensity is about 80 % of the peak at 1610 cm−1 of homoisoflavanone(Ⅳ) and 100 % of the peak at 1610 cm−1 of methylophiopogonanone B,which can be distinguished from homoisoflavanone(Ⅳ), methylophiopogonanone B and homoisoflavanones(III, V). Because methylophiopogonanone B has a strong peak near 356 cm−1, it may be produced by 4′ methoxy group, which can distinguish homoisoflavanone(Ⅳ) from methylophiopogonanone B.
3.4. Comparison of Raman spectrum of FeCl3 derivatives and reference substance powder
The principle of forming derivatives of homoisoflavanones(Ⅲ、Ⅳ、Ⅴ) and FeCl3 is to use oxygen on 6 phenolic hydroxyl groups to form a complex with Fe3+, and other structures are basically unaffected. According to the operation of "2.4″, the Raman spectrum of FeCl3 derivative in TLC and reference substance powder of homoisoflavanones(Ⅲ、Ⅳ、Ⅴ) were measured and compared. As shown in Fig. 7. It could be seen that the Raman spectrum of the derivatives produced by the trace amount of homoisoflavanones (Ⅲ, Ⅳ, V) and FeCl3 solution after TLC separation was similar from the characteristic peaks of the Raman spectrum of its powder, but quite different about relative intensity, indicating that the derivative reaction had already occurred. It was found that the signal of Raman spectrum after derivatization was very stronger(Fig. 7(a) and (b)) around 1610 cm−1 and 1550 cm−1 from ν C C of benzene rings than reference substance powder, probable cause was accumulation of benzene rings after the formation of complex.The peaks produced by ν= CH at 3060 cm−1 and ν- CH at 2930 cm−1, 2870 cm−1 were weaker after derivatization(Fig. 7(a) and (b)), but they were stronger after derivatization(Fig. 7(c)).
Fig. 7.
Comparison of Raman spectrum of FeCl3 derivative and standard substance powder.
we can see from Fig. 8 and Table 1 that there are five = CH and three - CH of –CH3 in the structure of homoisoflavanone(V), so the peak produced by ν= CH at 3060 cm−1 are higher than the peak produced by ν- CH at 2930 cm−1, which can identify derivative of homoisoflavanone(V). The peak around 1610 cm−1 and 1550 cm−1 are from ν C C of benzene rings A and B in the structure, which are the strongest scattering peak in the Raman spectra of FeCl3 derivatives of homoisoflavanones(III, IV, V). The scattering peak of FeCl3 derivatives of homoisoflavanones(V) at 1550 cm−1 is relatively weaker than that of FeCl3 derivatives of homoisoflavanones(III, IV). Near 1380 cm−1, there is δ- CH from - CH3 of position 6 and position 8 of A ring in FeCl3 derivatives of homoisoflavanones(III, IV), the peak is very week in derivative of homoisoflavanones(V),which maybe because it has just a methyl group. There is two common peaks from νC-O-C near 850 cm−1 and B ring vibration near 739 cm−1 and there is an obvious peaknear 1180 cm−1 from δ- CH of para-position substituted phenyl in derivative of homoisoflavanones(V). The differences of three derivatives were obvious, which could be distinguished, the background of FeCl3 solution in TLC plate had no interference(Fig. 8).
3.5. Analysis of specificity
The commercial sample S8 (Item No.:20190904; Place of origin:Hebei; Place of sale:Tongzhou, China; Preparation method: decoction pieces) without homoisoflavanones(Ⅲ, Ⅳ, Ⅴ)(which was tested by HPLC) was selected as negative sample. 3.3 g of the negative sample powder in two copies was weighed accurately, according to the sample preparation method, which was prepared into extractum and heated in a water bath(80 °C) for 5 h, other operations were the same as "2.3.2″,and the negative sample solution was gotten.
A portion of the above negative sample solution was taken and respectively added 0.1 mL of homoisoflavanones(Ⅲ, Ⅳ, Ⅴ) standard solutions(equivalent to 50 μg of Ⅲ, Ⅳ and V respectively), and dried with nitrogen, and then the solid was fixed to constant volume with 100 μL methanol, the desired simulated positive sample solution was gotten.
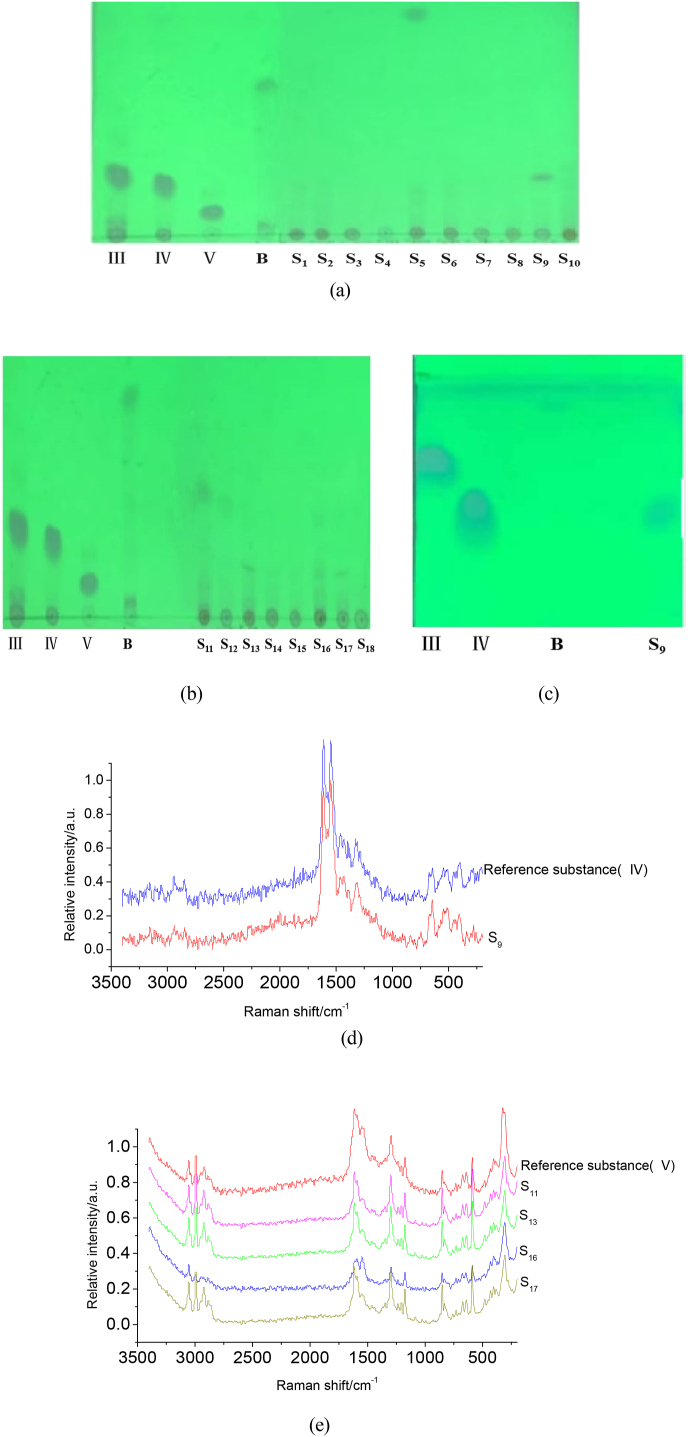
The homoisoflavanones(Ⅲ, Ⅳ, V) standard solutions, simulated positive sample solution and the negative sample solution were detected based on twice chromatography derivatization to investigate specificity. The results of TLC (Fig. 9(a and b)) showed that the location and appearance of spots on TLC plate of homoisoflavanones(Ⅲ, Ⅳ, Ⅴ) standard substance, simulated positive samples had spots with similar color and location, while negative samples had no spots at the same Rf position, which showed that homoisoflavanones(Ⅲ, Ⅳ, Ⅴ) could be preliminarily separated from other components in POD.
Fig. 9.
TLC(a,b) and Raman spectra(c-f) of homoisoflavanones(Ⅲ, Ⅳ, V) after derivatization from simulated positive sample((a): the first chromatography, (b): the second chromatography III, IV, V, and B: homoisoflavanones(Ⅲ, Ⅳ, V), methylophiopogonanone B respectively; -: negative sample; +: simulated positive sample)).
For the sake of further improving the specificity of the test of homoisoflavanones(Ⅲ, Ⅳ, Ⅴ) in POD, the main spots of the homoisoflavanones(Ⅲ, Ⅳ, Ⅴ) standard substance and the simulated positive sample were labeled with color by FeCl3 solution in the TLC of Fig. 9(a and b), and the negative samples were labeled at the same Rf. According to the operation of "2.4″, Raman spectra of the spots in situ after derivatization were detected, the results were shown in Fig. 9(c–f) respectively. It could be seen from Fig. 9(c–f) that the peak position and the peak shape of homoisoflavanones(Ⅲ, Ⅳ, V) in TLC was the same as that of the simulated positive sample at the identical Rf, while the negative sample had almost no Raman similar signal at the wave number (2000–600 cm−1), indicating that other components in POD had no effect on the detection of homoisoflavanones(Ⅲ, Ⅳ, V), therefore, this method had strong selectivity for detecting homoisoflavanones(Ⅲ, Ⅳ, V).
3.6. Examination of limit of Detection(LOD)
Diluting homoisoflavanones(Ⅲ, Ⅳ, V) standard solutions (0.5 mg. mL−1) to five concentrations(0.1, 0.2, 0.3, 0.4, 0.5 mg mL−1), and sequentially spotting on the same silica gel 60F254 aluminum plate with the sample amount of 10 μL(equivalent to 1.0 μg, 2.0 μg, 3.0 μg, 4.0 μg and 5.0 μg of homoisoflavanone). The hyperisoflavanones(Ⅲ, Ⅳ) were eluted with the optimum mobile phase of dichloromethane-methanol-formic acid(150:5:3,v/v/v) and the hyperisoflavanone(V) was eluted with petroleum ether-ethyl acetate(3:2, v/v), which were inspected at 254 nm (Fig. 10(a, c, e)), and then the Raman spectra of derivatives of homoisoflavanones(Ⅲ, Ⅳ, Ⅴ) and FeCl3 in different sample amounts were respectively detected. Referring to the calculation method of LOD(S/N = 3) by the International Union of Pure and Applied Chemistry (IUPAC), the LOD of homoisoflavanones(Ⅲ, Ⅳ, V) in this method was calculated to be respectively 1.0 μg(0.1 mg mL−1), 1.0 μg(0.1 mg mL−1), 2.0 μg(0.2 mg mL−1)(Fig. 10(b, d, f)), which was converted to 3.0 mg kg−1, 3.0 mg kg−1, 6.0 mg kg−1, respectively, on the basis of the preparation method of POD sample solution. The method of LC-MS for determining homoisoflavanones (Ⅲ, Ⅳ, V) was used in literature [24], the lower limit of quantitation of homoisoflavanones(Ⅲ, Ⅳ, V) was calculated to be respectively 0.2 ng mL−1, 0.2 ng mL−1, 0.0875 ng mL−1, which is lower than that of the current method, but the current method can meet qualitative needs and it is simple, inexpensive, and does not require a high level of professional proficiency from operators, which can be regarded as an alternative method.
Fig. 10.
The LOD of TLC(a, c, e) and Raman spectra(b, d, f) after derivatization of homoisoflavanones (Ⅲ, Ⅳ, V)(1–5: 1 μg, 2 μg, 3 μg, 4 μg, 5 μg respectively).
3.7. Detection of real samples
The 18 batches of commercial POD samples(Table S1) were produced in different places and were detected respectively with homoisoflavanones(Ⅲ, Ⅳ, V) as the target components. The results were shown in Fig. 11. Fig. 11(a and b) showed TLC of the first chromatogram. It could be seen from Fig. 11(a) that S21 presented at the same Rf as homoisoflavanones(Ⅲ, Ⅳ), but it was impossible to confirm which component of homoisoflavanones(Ⅲ, Ⅳ). Fig. 11(c) showed TLC of secondary chromatogram of Fig. 11(a), so it was preliminarily determined that the spot was homoisoflavanone(Ⅳ). As could be seen from Fig. 11(b), four samples (S11, S13, S16, S17) showed the same spots at Rf as homoisoflavanone(V). Then FeCl3 solution was dropped at the Rf position of the parallel position that of homoisoflavanones(Ⅲ, Ⅳ, V), and the Raman spectrum was detected(Fig. 11(d and e)). According to the Raman spectra of Fig. 11 d, It was found that the peak shape and position of the Raman spectrum of S9 after derivatization were consistent with that of homoisoflavanone(Ⅳ), which indicated that the S9 contains the component of homoisoflavanone(Ⅳ); according to the Raman spectra in Fig. 11(e), the Raman spectra of four samples(S11, S13, S16, S17) after derivatization were consistent with the peak shape and position of the Raman spectra after derivatization of homoisoflavanone(V). Therefore, it could be determined that these samples contained the component of homoisoflavanone(V).
Fig. 11.
TLC(a-c) and Raman spectra(d, e) after derivatization of samples(III: homoisoflavanone(III); IV: homoisoflavanone(IV); V: homoisoflavanone(V); B: methylophiopogonanone B; S: sample).
4. Conclusion
In this study, based on the study of twice chromatography derivatization of three kinds of homoisoflavanones(Ⅲ, Ⅳ, V) in POD, a method for the identification of homoisoflavanones(Ⅲ, Ⅳ, V) by TLC and Raman spectroscopy was established, which was used for the identification of POD samples.
The specificity of the traditional TLC method used to identify the index components is limited. This method made use of combining the merit of simple operation, fast separation speed of TLC method and the characteristics that Raman spectroscopy can express the structural fingerprint information of compounds. The combination of the two methods greatly improves the specificity of the analysis method while complementing each other's advantages. In this study, the relative shift value method was used for the first time to detect three kinds of homoisoflavanones(Ⅲ, Ⅳ, V) in POD by twice chromatography of TLC, which reduced the use of homoisoflavanone reference substance. At the same time, the sensitivity of the analytical method was further improved by main spot of TLC in situ chemical derivatization method, and the identification method of Raman spectrum of homoisoflavanones (Ⅲ, Ⅳ, V) and FeCl3 derivatization was used for the first time. The sensitivity of in situ detection by Raman spectroscopy was indirectly improved. This method has strong specificity, high sensitivity and this method also reduces detection costs. It can be used as a new reference method for identifying and evaluating homoisoflavanones(Ⅲ, Ⅳ, V) in POD.
Data availability statement
Not applicable.
CRediT authorship contribution statement
Tao Xu: Writing – original draft, Visualization, Investigation. Qian Li: Writing – original draft, Visualization, Investigation, Formal analysis. Feng Xu: Writing – review & editing, Project administration, Funding acquisition. Li Li: Writing – review & editing, Resources, Methodology. Shuang Li: Investigation. Yanli Dong: Formal analysis, Data curation. Xin Liang: Investigation. Hongguang Zhang: Formal analysis. Peng Hou: Formal analysis, Data curation. Ge Sun: Investigation. Meng Cao: Investigation. Hao Dou: Investigation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This research was funded by Science and Technology Plan Project (grant no. LSFGG-2022060) of Qiqihar Science and Technology Bureau of Heilongjiang Province and Project(grant no. QMSI2022Z-03) of Qiqihar Academy of Medical Sciences of Heilongjiang Province of China.
Footnotes
This research was funded by Science and Technology Plan Project of Qiqihar Science and Technology Bureau of Heilongjiang Province (grant no. LSFGG-2022060) and Project of Qiqihar Academy of Medical Sciences of Heilongjiang Province of China (grant no. QMSI2022Z-03).
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e22441.
Contributor Information
Tao Xu, Email: harvey-333@163.com.
Qian Li, Email: liqian67@126.com.
Feng Xu, Email: 15845205504@qmu.edu.cn.
Li Li, Email: lilianlinsuo@163.com.
Shuang Li, Email: lishuang1220@163.com.
Yanli Dong, Email: qudw1225@163.com.
Xin Liang, Email: liangxin@qmu.edu.cn.
Hongguang Zhang, Email: zhanghg@qmu.edu.cn.
Peng Hou, Email: houpeng1982@163.com.
Ge Sun, Email: sunge691220@126.com.
Meng Cao, Email: 19917731195@163.com.
Hao Dou, Email: aq990714@163.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Zhou X.L., Zhang Y.P., Zhao H.D., Liang J.S., Zhang Y., Shi S.Y. Antioxidant homoisoflavonoids from polygonatum odoratum. Food Chem. 2015;186:63–68. doi: 10.1016/j.foodchem.2015.02.058. [DOI] [PubMed] [Google Scholar]
- 2.Wang Y., Fei Y.Q., Liu L.R., Xiao Y.H., Pang Y.L., Kang J.H., wang Z. Polygonatum odoratum polysaccharides modulate gut microbiota and mitigate experimentally induced obesity in rats. Int. J. Mol. Sci. 2018;19:3587. doi: 10.3390/ijms19113587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quan L.T., Wang S.C., Zhang J. Chemical constituents from polygonatum odoratum. BioChem. Syst. Ecol. 2015;58:281–284. doi: 10.1016/j.bse.2014.12.019. [DOI] [Google Scholar]
- 4.Bai H., Li W., Zhao H.X., Anzai Y., Li H.M., Kato F., Koike K. Isolation and structural elucidation of novel cholestane glycosides and spirostane saponins from polygonatum odoratum. Steroids. 2014;80:7–14. doi: 10.1016/j.steroids.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 5.Zhang S.X., Shi Y.Y., Huang L.Q., Wang C.K., Zhao D.R., Ma K.L., Wu J.W., Peng D.Y. Comparative transcriptomic analysis of rhizomes, stems, and leave of Polygonatum odoratum(Mill.) Druce reveals candidate genes associated with polysaccharide synthesis. Gene. 2020 doi: 10.1016/j.gene.2020.144626. [DOI] [PubMed] [Google Scholar]
- 6.Pang X., Zhao J.Y., Wang Y.J., Zheng W., Zhang J., Chen X.J., Cen S., Yu L.Y., Ma B.P. Steroidal glycosides, homoisoflavanones and cinnamic acid derivatives from polygonatum odoratum and their inhibitory effects against influenza a virus. Fitoterapia. 2020;146 doi: 10.1016/j.fitote.2020.104689. [DOI] [PubMed] [Google Scholar]
- 7.Ning D.L., Jin M., Tao X., Sun J.K., Min L. Homoisoflavanone-1 isolated frompolygonatum odoratumarrests the cell cycle and induces apoptosis in a549 cells. Oncol. Lett. 2018;16:3545–3554. doi: 10.3892/ol.2018.9085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang D.M., Li D.W., Zhu W., Peng P. A new c-methylated homoisoflavanone and triterpenoid from the rhizomes of polygonatum odoratum. Nat. Prod. Res. 2009;23:580–589. doi: 10.1080/14786410802560633. [DOI] [PubMed] [Google Scholar]
- 9.Yang X.G., Chen S.B., Chen S.L., Yang D.J., Liu T.S. Studies on TLC fingerprint of flavonoids in rhizome of Polygonatum odoratum. China J. Chin. Mater. Med. 2005;30:104–106. doi: 10.3321/j.issn:1001-5302.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Sobstyl E., Szopa A., Ekiert H., Gnat S., Typek R., Choma I.M. Effect directed analysis and tlc screening of schisandra chinensis fruits. J. Chromatogr., A. 2020;1618 doi: 10.1016/j.chroma.2020.460942. [DOI] [PubMed] [Google Scholar]
- 11.Ghodke S.S., Sathiyanarayanan L., Chopade S.S., Mahadik K.R. Validated HPTLC method for simultaneous estimation of dihydroartemisinin and piperaquine phosphate in pharmaceutical dosage form. Pharm. Chem. J. 2022;55:1254–1260. doi: 10.1007/S11094-022-02567-5. [DOI] [Google Scholar]
- 12.Li X., Tan L.L., Liu J.C., Li L., Jia S.S. Rapid detection of four chemical components added illegally in slimming health food with TLC Situ Raman spectroscopy. Spectrosc. Spectr. Anal. 2018;38:830–836. doi: 10.3964/j.issn.1000-0593(2018)03-0830-07. [DOI] [Google Scholar]
- 13.Xu F., Li X.X., Li L., Liu J.C. Rapid detection of three chemical components added illegally into antihypertensive health food by TLC concentration in situ combined with Micro-Raman spectroscopy method. Chem. J. Chin. Univ. 2018;39:1172–1177. doi: 10.7503/cjcu20170735. [DOI] [Google Scholar]
- 14.Li L., Cui Y.H., Dong Y., Wang C.Z., Lang X., Liu X. The identification of adrenal corticosteroid preparations by combing TLC with Spot-Concentrated Raman Scattering. Chin. Hosp. Pharm. J. 2019;39:1259–1264. doi: 10.13286/j.cnki.chinhosppharmacyj.2019.12.09. [DOI] [Google Scholar]
- 15.Li L., Lang X., Xu T., Xu F., Dong W. Rapid detection of six glucocorticoids added illegally to dietary supplements by combining TLC with spot-concentrated Raman scattering. Molecules. 2018;23:1504. doi: 10.3390/molecules23071504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang S.H., Fan Q.Z., Guo J.Q., Jiao X.H., Kong X.H., Yu Q. Surface-enhanced Raman spectroscopy tandem with derivatized thin-layer chromatography for ultra-sensitive on-site detection of histamine from fish. Food Control. 2022;138 doi: 10.1016/j.foodcont.2022. 108987. [DOI] [Google Scholar]
- 17.Hou X., Sivashanmugan K., Zhao Y., Zhang B., Wang A.X. Multiplex sensing of complex mixtures by machine vision analysis of TLC-SERS images. Sensor. Actuator. B Chem. 2022;357 doi: 10.1016/j.snb.2021.131355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shen Z.D., Wang H.Y., Yu Q., Li Q., Lu X.M., Kong X.M. On-site separation and identification of polycyclic aromatic hydrocarbons from edible oil by TLC-SERS on diatomite photonic biosilica plate. Microchem. J. 2021;160 doi: 10.1016/j.microc.2020.105672. [DOI] [Google Scholar]
- 19.Li J.H., Cheng N.N., Liu J.C., Li L., Jia S.S. Rapid on-site TLC-SERS detection of four sleep problems drugs used as adulterants in health-care food. Spectrosc. Spectr. Anal. 2018;38:1122–1128. doi: 10.3964/j.issn.1000-0593. (2018)04-1122-07. [DOI] [Google Scholar]
- 20.Hegna T.A., Czaja A.D., Christopher R.D. Raman spectroscopic analysis of the composition of the clam-shrimp carapace (Branchiopoda: laevicaudata, Spinicaudata, Cyclestherida): a dual calcium phosphate-calcium carbonate composition. J. Crustac Biol. 2020;6:756–760. doi: 10.1093/jcbiol/ruaa078. [DOI] [Google Scholar]
- 21.Nichols N.A., Lednev I.K. Raman spectroscopy for forensic identification of body fluid traces: method validation for potential false negatives caused by blood-affecting diseases. Am. J. Anal. Chem. 2022;13:1–8. doi: 10.4236/ajac.2022.131001. [DOI] [Google Scholar]
- 22.Varnasseri M., Xu Y., Goodacre R. Rapid detection and quantification of the adulteration of orange juice with grapefruit juice using handheld Raman spectroscopy and multivariate analysis. Anal. Methods: Adv. Meth. Appl. 2022;14:1663–1670. doi: 10.1039/D2AY00219A. [DOI] [PubMed] [Google Scholar]
- 23.Zhu Z.Y., Gu R.A., Lu T.H. first ed. Northeastern University Press; Shenyang,China: 1998. Application of Raman Spectroscopy in Chemistry; pp. 295–301. [Google Scholar]
- 24.Qu Z.J. Harbin University of Commerce; 2020. Pharmacokinetics of Homoisoflavanones from Polygonati Odorati Rhizoma by LC-MS [D] [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.