Abstract
The stereocontrolled allylic alkylation of carbonyl compounds with the goal of producing the full range of stereoisomers presents an effective approach for increasing the productivity of collective natural product synthesis and the creation of chiral molecule libraries for drug exploration. The simultaneous control of regio-, diastereo-, and enantioselectivity poses a significant synthetic challenge in contemporary organic synthesis. Herein, we describe a catalytic stereodivergent α-allylation protocol applicable to both aliphatic and aromatic 2-acylimidazoles, thereby providing a practical blueprint for the divergent synthesis of important chiral building blocks. Each of the six isomeric α-allylated compounds can be readily obtained with remarkable yields and exceptional stereoselectivities, by judiciously selecting the appropriate leaving group and permutations of enantiomers adapted from nickel and iridium catalysts. The versatility of this asymmetric allylic alkylation has been successfully utilized in the enantioselective synthesis of (R)-arundic acid and (S,S)-cinamomumolide, as well as in the stereodivergent total synthesis of tapentadol.
Subject terms: Asymmetric catalysis, Synthetic chemistry methodology, Homogeneous catalysis
The simultaneous control of regio-, diastereo-, and enantioselectivity poses a significant synthetic challenge in contemporary organic synthesis. Here, the authors describe a stereodivergent allylation of 2-acylimidazoles using nickel and iridium catalysts to provide completely isomeric allylated compounds.
Introduction
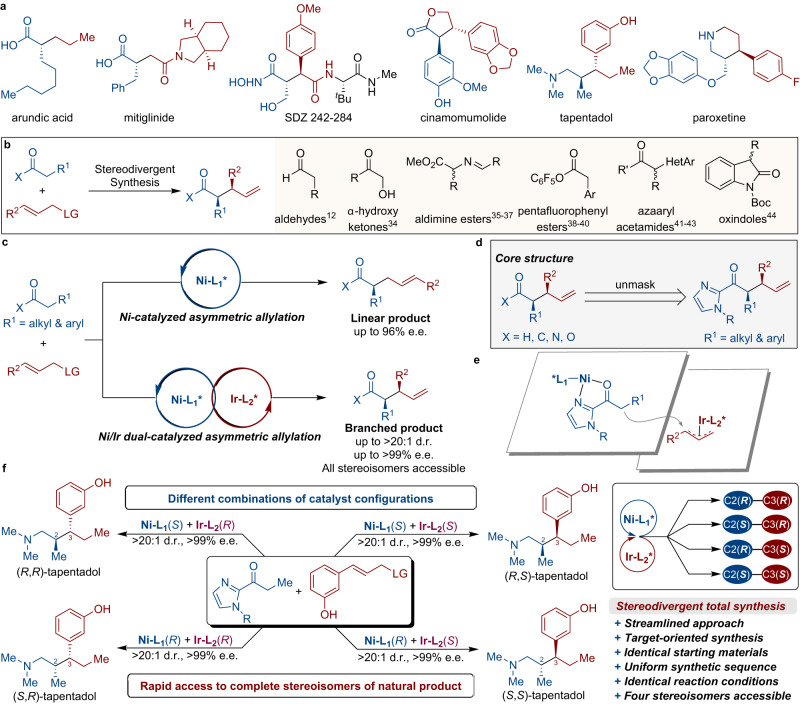
The configuration of stereocenters in organic compounds is critical in determining the intricate functionalities exhibited by vital biomacromolecules, including proteins and nucleic acids, as well as a wide range of biological compounds (Fig. 1a, e.g. arundic acid, mitiglinide, SDZ 242-484, cinamomumolide, tapentadol, and paroxetine)1. Consequently, a comprehensive understanding of the stereochemical structure-activity relationships necessitates the exploration of all potential stereoisomeric variations inherent to a given natural product or lead candidate2. The simultaneous and controlled creation of multiple stereogenic centers in a single catalytic step, ensuring both the relative and absolute configurations, remains an essential area of research3. Specifically, the enantioselective α-allylic alkylation represents a potent and straightforward approach within chemical research for the construction of carbon−carbon bonds with high enantioselectivity4. In this context, α-allylated tertiary carbonyls serve as pivotal synthetic intermediates for the synthesis of structurally diverse pharmaceuticals and natural products.
Fig. 1. Overview of regio- and stereodivergent α-allylation of 2-acylimidazoles.
a Representative chiral biologically relevant compounds. b Prior work: different carbonyl-pronucleophiles used in stereodivergent catalysis involving π-allylmetal electrophiles. c This work: catalytic stereodivergent access to all possible regio- and stereoisomers of both linear and branched α-allylated tertiary carbonyls with excellent optical purity. d Easy post-synthetic transformation of acyl-imidazoles into various carbonyl derivatives. e Ni/Ir dual-catalyzed stereodivergent α-allylation. f Target-oriented stereodivergent total synthesis of tapentadol via Ni/Ir dual catalysis.
Synergistic dual catalysis has emerged as a compelling strategy for efficiently driving contemporary synthetic processes5–11. Carreira and co-workers introduced the concept of stereodivergent dual catalysis12, wherein two distinct catalysts synergistically engage in orthogonal catalytic functions enabling the controlled formation of two new stereogenic centers (Fig. 1b)13–23. Remarkable advancements have been made in this domain through the synergistic interplay of discrete catalytic processes to achieve stereodivergent product formation24–33. Various nucleophilic substrates, including α-hydroxy ketones34, aldimine esters35–37, pentafluorophenyl esters38–40, azaaryl acetamides41–43, and oxindoles44 have been successfully employed in these dual-catalyzed stereodivergent synthesis. The ability to access all possible stereoisomers of both linear and branched α-allylated tertiary carbonyls through catalytic stereodivergence presents a fascinating avenue in terms of atom and step economy (Fig. 1c)45–55. Recently, Zheng, Wu and coworkers reported a seminal Ni/Pd dual-catalysis for the asymmetric alkylation of 2-acylimidazoles to generate linear allylated products56. Despite extensive research efforts, the constrained applicability of conventional alkyl-substituted carboxylic acid derivatives as nucleophilic reagents within the realm of catalytic stereodivergent synthesis, primarily due to the inherent intricacies associated with enolization, impedes the exhaustive exploration of all potential isomeric configurations exhibited by structurally diverse natural products57–59.
In continuation of our previous work concerning dual catalysis for achieving stereodivergent total synthesis59, we postulated that a synergistic dual catalyst system comprising a chiral Lewis acid and a chiral iridium complex would present a highly efficient strategy for the stereodivergent α-allylations of 2-acylimidazoles. Several potential advantages of this approach warrant highlighting: (1) the bidentate coordination of 2-acylimidazoles with a chiral Lewis acid enhances the nucleophilicity of the carbon atom while exhibiting commendable stereocontrol (Fig. 1d)60, 61; (2) the configurations of the two contiguous tertiary carbon stereocenters in the resulting product, each originating from the same starting 2-acylimidazole and allyl species, can be controlled by the chiral Lewis acid in conjunction with the chiral iridium complex (Fig. 1e). Consequently, manipulating the configuration of the two chiral catalysts will readily afford the four stereochemically distinct isomers of the branched products8; (3) direct synthetic approaches afford impressive enantioselectivities and allow precise regulation of the regioselectivity (branched versus linear products), effectively facilitating the creation of the complete array of isomers in a divergent manner; and (4) facile post-functionalization of the acylimidazole to yield common carbonyl analogs (e.g. aldehydes, ketones, esters, amides, alcohols, etc.) can be achieved. Remarkably, this post-functionalization can be accomplished while simultaneously preserving the enantioselectivity61, thereby facilitating collective stereodivergent total synthesis.
Herein, we present a highly selective approach for regio- and stereodivergent allylic alkylation reactions, facilitating the synthesis of diverse α-allylated acylimidazoles with exceptional regio-, diastereo-, and enantioselectivity. The simplicity and efficiency of this approach open up avenues for the enantioselective synthesis of (R)-arundic acid and (S,S)-cinamomumolide, as well as the total synthesis of all four tapentadol stereoisomers (Fig. 1f).
Results
Optimization studies
Our preliminary investigations into the divergent synthesis of linear and branched α-allylated acylimidazoles began with the utilization of 2-acyl imidazole (1a) as the nucleophile and allylic derivatives (2) as the electrophile (Table 1). In the presence of 10 mol% of nickel acetate and the chiral diamine ligand (S,S)-5a, the linear allyl substitution reaction of 1a and 2a occurred with 22% yield and 28% e.e. (entry 1). Other chiral ligands were subsequently examined (entries 2 and 3), and the use of (S,S)-5c resulted in the enantioenriched synthesis of linear product 3a in 80% yield and excellent e.e. (entry 3)56. Having established an asymmetric methodology for the synthesis of linear homoallylic 2-acylimidazoles, we attempted to address the synthesis of branched regioisomers with precise control over stereoselectivity. The combination of 2 mol% of iridium catalyst [Ir(COD)Cl]2/(S,S)-6 with nickel catalyst led to a regioselectivity switch in the allylation reaction, and the branched product 4a was obtained in good stereoselectivity (entry 4). Subsequently, the evaluation of the leaving group (LG) of the allylic substrates (entries 5 and 6) led to the identification of the optimal conditions using substrate 2c, providing 4a in 85% yield, >20:1 d.r., and >99% e.e. (entry 6). Control experiments verified that both metal catalysts remained inert in the absence of additional ligands (entries 7 and 8), and further investigations confirmed the indispensability of each component within the integrated catalytic system (entries 9 and 10).
Table 1.
Optimization studies
 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Entry | 2 | [Ni] | [Ir] | Yield (%)a | 3a/4a | e.e. of 3ab | d.r. of 4ac | e.e. of 4a (%)b |
| 1 | 2a | Ni(OAc)2/(S,S)-5a | – | 22 | >20:1 | 28 | – | – |
| 2 | 2a | Ni(OAc)2/(S,S)-5b | – | 30 | >20:1 | 60 | – | – |
| 3 | 2a | Ni(OAc)2/(S,S)-5c | – | 80 | >20:1 | 95 | – | – |
| 4 | 2a | Ni(OAc)2/(S,S)-5c | [Ir(COD)Cl]2/(S,S)-6 | 83 | 1:7 | 78 | 2:1 | 85 |
| 5d | 2b | Ni(OAc)2/(S,S)-5c | [Ir(COD)Cl]2/(S,S)-6 | 83 | <1:20 | – | >20:1 | >99 |
| 6d | 2c | Ni(OAc)2/(S,S)-5c | [Ir(COD)Cl]2/(S,S)-6 | 85 | <1:20 | – | >20:1 | >99 |
| 7d | 2c | Ni(OAc)2 | [Ir(COD)Cl]2/(S,S)-6 | NR | – | – | – | – |
| 8d | 2c | Ni(OAc)2/(S,S)-5c | [Ir(COD)Cl]2 | NR | – | – | – | – |
| 9d | 2c | – | [Ir(COD)Cl]2/(S,S)-6 | NR | – | – | – | – |
| 10d | 2c | Ni(OAc)2/(S,S)-5c | – | NR | – | – | – | – |
Unless otherwise specified, all the reactions were carried out by using 1a (0.1 mmol), 2 (0.15 mmol), Ni(OAc)2 (10 mol%), 5 (10 mol%), [Ir(COD)Cl]2 (2 mol%), 6 (4 mol%), and Cs2CO3 (0.2 mmol) in dichloromethane (DCM) (2.0 mL) at 10 °C for 48 h. aIsolated yields after chromatography are shown. bEnantiomeric excesses (e.e.) were determined by HPLC analysis. cDetermined by 1H-NMR spectroscopy of the crude reaction mixtures. dWithout Cs2CO3, with TBD (10 mol%) at 20 °C. TBD, 1,5,7-triazabicyclo[4.4.0]dec-5-ene. NR, no reaction.
Substrate scope
We extended the application of our developed methodology to enable the selective synthesis of six distinct isomers of the α-allylated product in a divergent manner (Fig. 2). Initially, we explored the asymmetric allylation of 2-acyl imidazole (1a) with cinnamyl bromide (2a) via nickel catalysis, and both enantiomers of 3a could be achieved in excellent results through the utilization of chiral ligands with distinct configurations (Fig. 2a). To assess the stereodivergence potential of the cooperative Ni/Ir-catalytic system, we carried out the branched-selective alkylation reactions of 2-acyl imidazole (1a) with cinnamyl phenyl carbonate (2c) (Fig. 2b). Notably, all four possible stereoisomers of 4a were successfully obtained with comparable efficiency, with both stereogenic centers being fully controlled under the independent guidance of the nickel and iridium catalysts, respectively.
Fig. 2. Regio- and stereodivergent allylic alkylations.
a Asymmetric synthesis of (S)−3a and (R)−3a. b Stereodivergent allylations to access four stereoisomers by simply employing the enantiomer of one of the catalysts. a1a (0.3 mmol), 2c (0.9 mmol), Cs2CO3 (1.0 equiv.), in DCE (1.0 mL).
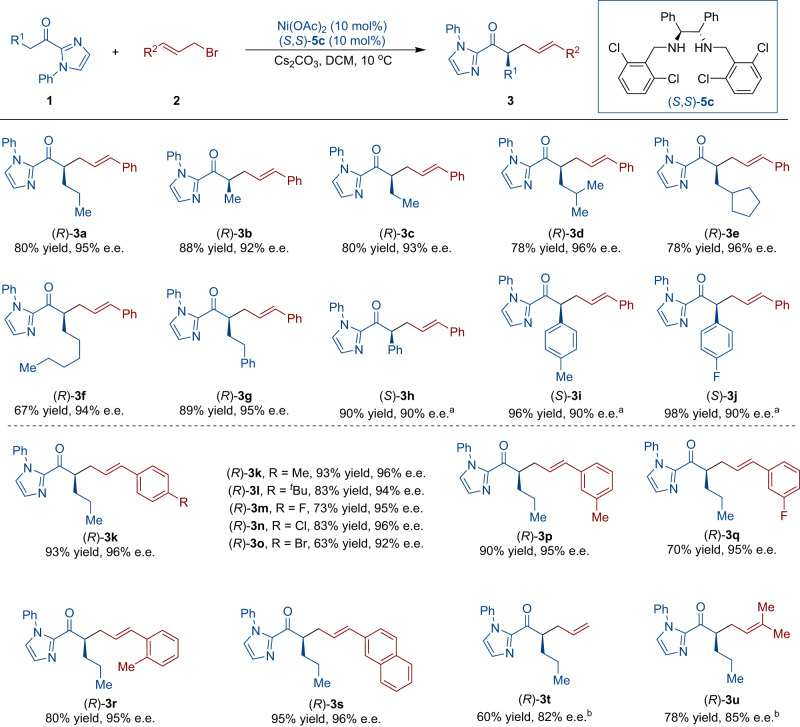
With the optimized conditions for linear allylic alkylation (Table 1, entry 3), we explored the scope with respect to the 2-acyl imidazoles (1) and allylic bromide derivatives (2). As summarized in Fig. 3, diverse alkyl substrates (1a–1g) were successfully applied to this reaction efficiently and gave the desired products in great yields (3a–3g, 67–89%) and with excellent e.e. values (92-96%). Subsequently, we investigated α-aryl substituents on 2-acyl imidazoles (1) and observed that all of these substrates reacted efficiently under modified reaction conditions, affording favorable yields and 90% e.e. for compounds 3h, 3i, and 3j. Furthermore, we focused on investigating the scope of allylic bromide derivatives 2. A wide variety of aryl groups incorporating electron-withdrawing or electron-donating substituents were found to participate in the reaction, giving the corresponding products 3k–3r in good to high yields with excellent enantioselectivities (92–96% e.e.). The utilization of naphthyl allylic bromide exhibited similar reactivity ultimately leading to the desired product in 95% yield with 96% e.e. (3s). Alkyl-substituted allylic bromides were also compatible with this reaction (3t and 3u).
Fig. 3. Scope of linear allylic alkylation.
Unless otherwise specified, all the reactions were carried out by using 1 (0.1 mmol), 2 (0.15 mmol), Ni(OAc)2 (10 mol%), (S,S)−5c (10 mol%), and Cs2CO3 (0.2 mmol) in dichloromethane (DCM) (2.0 mL) at 10 °C for 1-4 days. aWith Cs2CO3 (0.1 mmol) at 0 °C. bWith 2 (0.3 mmol).
We explored the reaction scope of cis-selective branched coupling of various 2-acyl imidazoles (1) with cinnamyl phenyl carbonates (2) catalyzed by (S,S)-5c-Ni/(S,S)-6-Ir (Fig. 4). 2-Acyl imidazoles bearing alkyl substituents in the α-position adjacent to the carbonyl group underwent stereoselective couplings in 58-86% yield with excellent d.r. values and enantioselectivities (4a-4h). The incorporation of a 2-phenoxy group in a 2-acyl imidazole proved to be a suitable substrate leading to excellent results (4i). The substrate scope of aromatic substituted acyl imidazole was investigated. Both electron-donating groups and electron-withdrawing groups proved to be compatible at the para-positon of the phenyl group, resulting in high yields and high enantioselectivities (4j-4l, 93-98% yield, >20:1 d.r., up to >99% e.e.). The introduction of the heterocyclic and alkyl substituents into the nitrogen atom of imidazole ring all gave the desired products (4m-4p) with good yields and exquisite selectivity. An X-ray crystal structure of 4n was obtained to assign the absolute configuration of the products. The imidazole moiety was also successfully replaced by benzimidazole (4q), thiazole (4r), and pyridine (4s), affording the corresponding products in good yields and excellent stereoselectivities. Remarkably, the reaction exhibited notable tolerance towards both electron-donating and electron-withdrawing groups positioned differently on the phenyl ring of cinnamyl phenyl carbonates (2), producing compounds 4t-4aa with enantioselectivities ranging from 98% to >99% e.e. Substrates with aromatic and heteroaromatic rings, like 2-naphthlalene and 2-thiophene undergo efficient and selective couplings (4ab and 4ac). Notably, allylic carbonate containing methyl group was also tolerated (4ad and 4ae).
Fig. 4. Substrate scope of branched allylic alkylation.
Unless otherwise specified, all the reactions were carried out by using 1 (0.1 mmol), 2 (0.15 mmol), Ni(OAc)2 (10 mol%), (S,S)−5c (10 mol%), [Ir(COD)Cl]2 (2 mol%), (S,S)−6 (4 mol%), and TBD (10 mol%) in dichloromethane (DCM) (2.0 mL) at 20 °C for 1-7 days. Branched/linear = >20:1 for all. aAt 0 °C. bbranched/linear = 4.6:1.
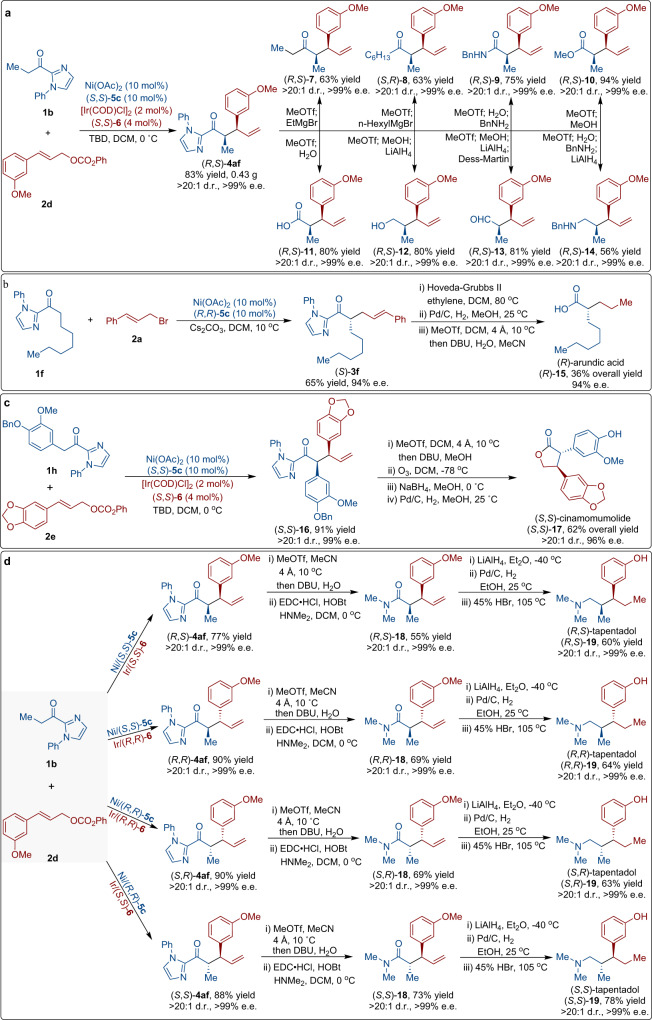
Synthetic application
To demonstrate synthetic utility of this methodology, a large scale reaction between 1b and 2d was conducted, providing the alkylation product 4af in 83% yield with maintained stereoselectivity (Fig. 5a). Further post-functionalization of the acylimidazole group of 4af to a series of useful synthetic building blocks is illustrated in Fig. 5a. The removal of the N-phenylimidazole moiety by the reaction with MeOTf followed with Grignard reagents gave the corresponding ketones 7 and 8 in good yields and with excellent enantiopurity. In addition, the imidazole moiety was readily removed to generate amide 9, ester 10, and acid 11 in high yields and with complete retention of the stereochemical information. 4af could also be transformed to alcohol 12 and aldehyde 13 under reductive conditions with the same e.e. A tandem deprotection/reduction amination sequence smoothly converted 4af to amine 14. To highlight the utility of this asymmetric allylic reaction, we applied our protocol to the synthesis of anti-Alzheimer agent arundic acid (Fig. 5b)62, 63. Direct alkylation of 2-acyl imidazole 1f with allylic bromide 2a gave 3f in 65% yield and 94% e.e. Subsequently, a concise three-step sequence afforded (R)-arundic acid in 36% overall yield without loss of enantioselectivity (94% e.e.).
Fig. 5. Synthetic utility.
a Product derivation. b Asymmetric synthesis of (R)-arundic acid. c Asymmetric synthesis of (S,S)-cinamomumolide. d Stereodivergent total synthesis of tapentadol.
Our dual catalytic strategy was successful applied to the asymmetric total synthesis of cinnamomumolide, a naturally occurring and biologically active compound which is isolated from the dried tender stems of Cinnamomum cassia (Fig. 5c)64. The Ni((S,S)-5c)/Ir((S,S)-6) catalyzed allylation of 2-acyl imidazole 1h with allyl aryl carbonate 2e delivered (S,S)-16 in 91% yield, >20:1 d.r., and 99% e.e. The subsequent synthetic sequence was initiated by a deprotection and esteration of 16 to give the corresponding ester. The subsequent sequences, which involved oxidation, reduction-cyclization, and deprotection steps, successfully yielded (S,S)-cinnamomumolide 1765.
The stereodivergence of the Ni/Ir dual-catalyzed allylic substitution reactions was demonstrated in the collective stereodivergent total synthesis of tapentadol66, 67. Under otherwise identical conditions, the enantioselective alkylation of 2-acyl imidazole 1b with allyl aryl carbonate 2d was carried out using four different pairs of enantiomers of the nickel catalyst and the iridium catalysts (Fig. 5d). By simple permutations of the enantiomers of the two catalysts, four stereoisomers of the desired products 4af were generated in good yields with excellent diastereo- and enantioselectivity from the same set of starting materials. Removal of the N-phenylimidazole moiety of 4af followed by amination with dimethylamine delivered the corresponding amide 18. Through a sequential reduction of the amide and alkene functional groups, followed by a demethylation step, we achieved successful synthesis of tapentadol 19 as the final product. Thus, we have developed a collectively stereodivergent asymmetric total synthesis of tapentadol with all four stereoisomers accessible68.
In summary, we have demonstrated the diastereodivergent allylic alkylation of 2-acylimidazoles, allowing efficient access to α-allylated tertiary carbonyls with a broad scope, high efficiency, and consistent stereoselectivity. This broadly applicable allylic alkylation reaction is now highly enantioselective and stereodivergent, enabling the synthesis of both linear and branched allylated products that feature privileged frameworks commonly found in biologically relevant molecules and natural products. Moreover, the innovative stereodivergent Ni/Ir dual catalysis provides a unified route to access all four stereoisomers of corresponding branched products with high diastereoselectivity and enantioselectivity by simple permutations of the enantiomers of the nickel and iridium complexes. Remarkably, the value of this operationally simple protocol has been highlighted through the collective synthesis of (R)-arundic acid and (S,S)-cinamomumolide, as well as the stereodivergent total synthesis of tapentadol.
Methods
General procedure A for Ni-catalyzed asymmetric allylation to generate linear product 3
To a flame-dried and argon-purged Schlenk tube were added 2-acyl imidazoles 1 (0.1 mmol, 1.0 equiv.), allylic bromide derivatives 2 (0.15 mmol, 1.5 equiv.), Ni catalyst (0.01 mmol, 0.1 equiv.), Cs2CO3 (0.2 mmol, 2.0 equiv.), and DCM (2 mL). The reaction mixture was stirred at 10 °C until complete consumption of the substrates (monitored by TLC). The solution was diluted with dichloromethane and then filtered with celite. The residue was purified by flash column chromatography on silica gel to afford the desired product 3.
General procedure B for Ni/Ir dual-catalyzed stereodivergent allylation to generate branched product 4
To a flame-dried and argon-purged Schlenk tube were added [Ir(COD)Cl]2 (0.002 mmol, 0.02 equiv.), 6 (0.004 mmol, 0.04 equiv.), TBD (0.01 mmol, 0.1 equiv.), and DCM (1 mL), and the resulting solution was stirred at 25 °C for 10 min. Then, 2-acyl imidazoles 1 (0.1 mmol, 1.0 equiv.), allylic carbonates 2 (0.15 mmol, 1.5 equiv.), Ni catalyst (0.01 mmol, 0.1 equiv.), and DCM (1 mL) were added successively. The resulting solution was stirred at 20 °C. After the reaction was complete (monitored by TLC), the residue was purified by flash column chromatography on silica gel to afford the desired product 4.
Supplementary information
Acknowledgements
The authors acknowledge financial support from the National Natural Science Foundation of China (grant no. 21971227, 22222113), CAS Project for Young Scientists in Basic Research (YSBR-054), and the Fundamental Research Funds for the Central Universities (WK9990000090, WK9990000111).
Author contributions
C.G. conceived and designed the study and wrote the paper. R.L. and Q.Z. performed the experiments and analyzed the data. R.L. performed the stereodivergent total synthesis. All authors discussed the results and commented on the manuscript.
Peer review
Peer review information
Nature Communications thanks Changwu Zheng, and the other, anonymous, reviewers for their contribution to the peer review of this work
Data availability
Crystallographic data for the structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre, under deposition number CCDC 2266877 ((S,S)-4n). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. All other data supporting the findings of this study, including experimental procedures and compound characterization, NMR, and HPLC are available within the Article and its Supplementary Information or from the authors. Source data are provided with this paper. NMR data in a mnova file format and HPLC traces are available at Zenodo at https://zenodo.org/records/10050924, under the Creative Commons Attribution 4.0 International license.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Ruimin Lu, Qinglin Zhang.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-023-43986-6.
References
- 1.Jacobsen, E. N., Pfaltz, A. & Yamamoto, H. Comprehensive Asymmetric catalysis (Springer, Berlin, New York, 1999).
- 2.Beletskaya IP, Nájera C, Yus M. Stereodivergent catalysis. Chem. Rev. 2018;118:5080–5200. doi: 10.1021/acs.chemrev.7b00561. [DOI] [PubMed] [Google Scholar]
- 3.Romiti F, et al. Different strategies for designing dual-catalytic enantioselective processes: from fully cooperative to non-cooperative systems. J. Am. Chem. Soc. 2019;141:17952–17961. doi: 10.1021/jacs.9b05464. [DOI] [PubMed] [Google Scholar]
- 4.Cheng Q, et al. Iridium-catalyzed asymmetric allylic substitution reactions. Chem. Rev. 2019;119:1855–1969. doi: 10.1021/acs.chemrev.8b00506. [DOI] [PubMed] [Google Scholar]
- 5.Du Z, Shao Z. Combining transition metal catalysis and organocatalysis-an update. Chem. Soc. Rev. 2013;42:1337–1378. doi: 10.1039/C2CS35258C. [DOI] [PubMed] [Google Scholar]
- 6.Afewerki S, Córdova A. Combinations of aminocatalysts and metal catalysts: a powerful cooperative approach in selective organic synthesis. Chem. Rev. 2016;116:13512–13570. doi: 10.1021/acs.chemrev.6b00226. [DOI] [PubMed] [Google Scholar]
- 7.Chen D-F, Han Z-Y, Zhou X-L, Gong L-Z. Asymmetric organocatalysis combined with metal catalysis: concept, proof of concept, and beyond. Acc. Chem. Res. 2014;47:2365–2377. doi: 10.1021/ar500101a. [DOI] [PubMed] [Google Scholar]
- 8.Arndtsen, B. A. & Gong, L.-Z. Asymmetric organocatalysis combined with metal catalysis, Springer, Topics in Current Chemistry (2019).
- 9.Huo X, Li G, Wang X, Zhang W. Bimetallic catalysis in stereodivergent synthesis. Angew. Chem. Int. Ed. 2022;61:e202210086. doi: 10.1002/anie.202210086. [DOI] [PubMed] [Google Scholar]
- 10.Wei L, Wang C-J. Synergistic catalysis with azomethine ylides. Chin. J. Chem. 2021;39:15–24. doi: 10.1002/cjoc.202000380. [DOI] [Google Scholar]
- 11.Wei L, Wang C-J. Asymmetric transformations enabled by synergistic dual transition-metal catalysis. Chem. Catal. 2023;3:100455. doi: 10.1016/j.checat.2022.10.031. [DOI] [Google Scholar]
- 12.Krautwald S, Sarlah D, Schafroth MA, Carreira EM. Enantio- and diastereodivergent dual catalysis: α-allylation of branched aldehydes. Science. 2013;340:1065–1068. doi: 10.1126/science.1237068. [DOI] [PubMed] [Google Scholar]
- 13.Krautwald S, Schafroth MA, Sarlah D, Carreira EM. Stereodivergent α-allylation of linear aldehydes with dual iridium and amine catalysis. J. Am. Chem. Soc. 2014;136:3020–3023. doi: 10.1021/ja5003247. [DOI] [PubMed] [Google Scholar]
- 14.Sandmeier T, Krautwald S, Zipfel HF, Carreira EM. Stereodivergent dual catalytic α-allylation of protected α-amino- and α-hydroxyacetaldehydes. Angew. Chem. Int. Ed. 2015;54:14363–14367. doi: 10.1002/anie.201506933. [DOI] [PubMed] [Google Scholar]
- 15.Krautwald S, Carreira EM. Stereodivergence in asymmetric catalysis. J. Am. Chem. Soc. 2017;139:5627–5639. doi: 10.1021/jacs.6b13340. [DOI] [PubMed] [Google Scholar]
- 16.Cruz FA, Dong VM. Stereodivergent coupling of aldehydes and alkynes via synergistic catalysis using Rh and Jacobsen’s amine. J. Am. Chem. Soc. 2017;139:1029–1032. doi: 10.1021/jacs.6b10680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singha S, Serrano E, Mondal S, Daniliuc CG, Glorius F. Diastereodivergent synthesis of enantioenriched α,β-disubstituted γ-butyrolactones via cooperative N-heterocyclic carbene and Ir catalysis. Nat. Catal. 2020;3:48–54. doi: 10.1038/s41929-019-0387-3. [DOI] [Google Scholar]
- 18.Kim B, Kim Y, Lee SY. Stereodivergent carbon–carbon bond formation between iminium and enolate intermediates by synergistic organocatalysis. J. Am. Chem. Soc. 2021;143:73–79. doi: 10.1021/jacs.0c11077. [DOI] [PubMed] [Google Scholar]
- 19.Wang H, Zhang R, Zhang Q, Zi W. Synergistic Pd/amine-catalyzed stereodivergent hydroalkylation of 1,3-dienes with aldehydes: reaction development, mechanism, and stereochemical origins. J. Am. Chem. Soc. 2021;143:10948–10962. doi: 10.1021/jacs.1c02220. [DOI] [PubMed] [Google Scholar]
- 20.Hu Q, He Z, Peng L, Guo C. Combining nickel and squaramide catalysis for the stereodivergent α-propargylation of oxindoles. Nat. Synth. 2022;1:322–331. doi: 10.1038/s44160-022-00050-3. [DOI] [Google Scholar]
- 21.Wen Y-H, Zhang Z-J, Li S, Song J, Gong L-Z. Stereodivergent propargylic alkylation of enals via cooperative NHC and copper catalysis. Nat. Commun. 2022;13:1344. doi: 10.1038/s41467-022-29059-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.You C, Shi M, Mi X, Luo S. Asymmetric α-allylic allenylation of β-ketocarbonyls and aldehydes by synergistic Pd/chiral primary amine catalysis. Nat. Commun. 2023;14:2911. doi: 10.1038/s41467-023-38488-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dai J, et al. Construction of acyclic all-carbon quaternary stereocenters and 1,3-nonadjacent stereoelements via organo/metal dual catalyzed asymmetric allenylic substitution of aldehydes. Angew. Chem. Int. Ed. 2023;62:e202300756. doi: 10.1002/anie.202300756. [DOI] [PubMed] [Google Scholar]
- 24.Xu S-M, et al. Stereodivergent assembly of tetrahydro-γ-carbolines via synergistic catalytic asymmetric cascade reaction. Nat. Commun. 2019;10:5553. doi: 10.1038/s41467-019-13529-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He R, et al. Stereodivergent Pd/Cu catalysis for the dynamic kinetic asymmetric transformation of racemic unsymmetrical 1,3-disubstituted allyl acetates. J. Am. Chem. Soc. 2020;142:8097–8103. doi: 10.1021/jacs.0c02150. [DOI] [PubMed] [Google Scholar]
- 26.Zhang J, et al. Enantio- and diastereodivergent construction of 1,3-nonadjacent stereocenters bearing axial and central chirality through synergistic Pd/Cu catalysis. J. Am. Chem. Soc. 2021;143:12622–12632. doi: 10.1021/jacs.1c05087. [DOI] [PubMed] [Google Scholar]
- 27.Zhu M, Zhang Q, Zi W. Diastereodivergent synthesis of β-amino alcohols by dual-metal-catalyzed coupling of alkoxyallenes with aldimine esters. Angew. Chem. Int. Ed. 2021;60:6545–6552. doi: 10.1002/anie.202014510. [DOI] [PubMed] [Google Scholar]
- 28.Xiao L, Wei L, Wang C-J. Stereodivergent synthesis of enantioenriched γ-butyrolactones bearing two vicinal stereocenters enabled by synergistic copper and iridium catalysis. Angew. Chem. Int. Ed. 2021;60:24930–24940. doi: 10.1002/anie.202107418. [DOI] [PubMed] [Google Scholar]
- 29.Peng Y, Huo X, Luo Y, Wu L, Zhang W. Enantio- and diastereodivergent synthesis of spirocycles through dual-metal-catalyzed [3+2] annulation of 2-vinyloxiranes with nucleophilic dipoles. Angew. Chem. Int. Ed. 2021;60:24941–24949. doi: 10.1002/anie.202111842. [DOI] [PubMed] [Google Scholar]
- 30.Le TP, Tanaka S, Yoshimura M, Sato K, Kitamura M. Stereodivergent dehydrative allylation of β-keto esters using a Ru/Pd synergistic catalyst. Nat. Commun. 2022;13:5876. doi: 10.1038/s41467-022-33432-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu Y, et al. Stereodivergent total synthesis of rocaglaol initiated by synergistic dual-metal-catalyzed asymmetric allylation of benzo furan−3[2H]-one. Chem. 2022;8:2011–2022. doi: 10.1016/j.chempr.2022.04.006. [DOI] [Google Scholar]
- 32.Wang H, Xu Y, Zhang F, Liu Y, Feng X. Bimetallic palladium/cobalt catalysis for enantioselective allylic C−H alkylation via a transient chiral nucleophile strategy. Angew. Chem. Int. Ed. 2022;61:e2021157. doi: 10.1002/anie.202115715. [DOI] [PubMed] [Google Scholar]
- 33.Wang W, Zhang F, Liu Y, Feng X. Diastereo- and enantioselective construction of vicinal all-carbon quaternary stereocenters via iridium/europium bimetallic catalysis. Angew. Chem. Int. Ed. 2022;61:e2022088. doi: 10.1002/anie.202208837. [DOI] [PubMed] [Google Scholar]
- 34.Huo X, He R, Zhang X, Zhang W. An Ir/Zn dual catalysis for enantio- and diastereodivergent α-allylation of α-hydroxyketones. J. Am. Chem. Soc. 2016;138:11093–11096. doi: 10.1021/jacs.6b06156. [DOI] [PubMed] [Google Scholar]
- 35.Huo X, Zhang J, Fu J, He R, Zhang W. Ir/Cu dual catalysis: enantio- and diastereodivergent access to α,α-disubstituted α-amino acids bearing vicinal stereocenters. J. Am. Chem. Soc. 2018;140:2080–2084. doi: 10.1021/jacs.8b00187. [DOI] [PubMed] [Google Scholar]
- 36.Wei L, Zhu Q, Xu S-M, Chang X, Wang C-J. Stereodivergent synthesis of α,α-disubstituted α-amino acids via synergistic Cu/Ir catalysis. J. Am. Chem. Soc. 2018;140:1508–1513. doi: 10.1021/jacs.7b12174. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Q, et al. Stereodivergent coupling of 1,3-dienes with aldimine esters enabled by synergistic Pd and Cu catalysis. J. Am. Chem. Soc. 2019;141:14554–14559. doi: 10.1021/jacs.9b07600. [DOI] [PubMed] [Google Scholar]
- 38.Jiang X, Beiger JJ, Hartwig JF. Stereodivergent allylic substitutions with aryl acetic acid esters by synergistic iridium and Lewis base catalysis. J. Am. Chem. Soc. 2017;139:87–90. doi: 10.1021/jacs.6b11692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu M, Wang P, Zhang Q, Tang W, Zi W. Diastereodivergent aldol-type coupling of alkoxyallenes with pentafluorophenyl esters enabled by synergistic palladium/chiral Lewis base catalysis. Angew. Chem. Int. Ed. 2022;61:e202207621. doi: 10.1002/anie.202207621. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Q, Zhu M, Zi W. Synergizing palladium with Lewis base catalysis for stereodivergent coupling of 1,3-dienes with pentafluorophenyl acetates. Chem. 2022;8:2784–2796. doi: 10.1016/j.chempr.2022.07.014. [DOI] [Google Scholar]
- 41.Jiang X, Boehm P, Hartwig JF. Stereodivergent allylation of azaaryl acetamides and acetates by synergistic iridium and copper catalysis. J. Am. Chem. Soc. 2018;140:1239–1242. doi: 10.1021/jacs.7b12824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.He Z-T, Jiang X, Hartwig JF. Stereodivergent construction of tertiary fluorides in vicinal stereogenic pairs by allylic substitution with iridium and copper catalysts. J. Am. Chem. Soc. 2019;141:13066–13073. doi: 10.1021/jacs.9b04440. [DOI] [PubMed] [Google Scholar]
- 43.Yang S-Q, Wang Y-F, Zhao W-C, Lin G-Q, He Z-T. Stereodivergent synthesis of tertiary fluoride-tethered allenes via copper and palladium dual catalysis. J. Am. Chem. Soc. 2021;143:7285–7291. doi: 10.1021/jacs.1c03157. [DOI] [PubMed] [Google Scholar]
- 44.Han J, Liu R, Lin Z, Zi W. Stereodivergent construction of Csp3−Csp3 bonds bearing vicinal stereocenters by synergistic palladium and phase-transfer catalysis. Angew. Chem. Int. Ed. 2023;62:e202215714. doi: 10.1002/anie.202215714. [DOI] [PubMed] [Google Scholar]
- 45.Trost BM, Lehr K, Michaelis DJ, Xu J, Buckl AK. Palladium-catalyzed asymmetric allylic alkylation of 2-acylimidazoles as ester enolate equivalents. J. Am. Chem. Soc. 2010;132:8915–8917. doi: 10.1021/ja103771w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwarz KJ, Amos JL, Klein JC, Do DT, Snaddon TN. Uniting C1-ammonium enolates and transition metal electrophiles via cooperative catalysis: the direct asymmetric α-allylation of aryl acetic acid esters. J. Am. Chem. Soc. 2016;138:5214–5217. doi: 10.1021/jacs.6b01694. [DOI] [PubMed] [Google Scholar]
- 47.Saito A, Kumagai N, Shibasaki M. Cu/Pd synergistic dual catalysis: asymmetric α-allylation of an α-CF3 amide. Angew. Chem. Int. Ed. 2017;56:5551–5555. doi: 10.1002/anie.201702113. [DOI] [PubMed] [Google Scholar]
- 48.Spoehrle SSM, West TH, Taylor JE, Slawin AMZ, Smith AD. Tandem palladium and isothiourea relay catalysis: enantioselective synthesis of α-amino acid derivatives via allylic amination and [2,3]-sigmatropic rearrangement. J. Am. Chem. Soc. 2017;139:11895–11902. doi: 10.1021/jacs.7b05619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schwarz KJ, Pearson CM, Cintron-Rosado GA, Liu P, Snaddon TN. Traversing steric limitations by cooperative Lewis base/palladium catalysis: an enantioselective synthesis of α-branched esters using 2-substituted allyl electrophiles. Angew. Chem. Int. Ed. 2018;57:7800–7803. doi: 10.1002/anie.201803277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schwarz KJ, Yang C, Fyfe JWB, Snaddon TN. Enantioselective α-benzylation of acyclic esters using π-extended electrophiles. Angew. Chem. Int. Ed. 2018;57:12102–12105. doi: 10.1002/anie.201806742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang T-C, Fan L-F, Shen Y, Wang P-S, Gong L-Z. Asymmetric allylic C–H alkylation of allyl ethers with 2-acylimidazoles. J. Am. Chem. Soc. 2019;141:10616–10620. doi: 10.1021/jacs.9b05247. [DOI] [PubMed] [Google Scholar]
- 52.Pearson CM, Fyfe JWB, Snaddon TN. A regio- and stereodivergent synthesis of homoallylic amines by a one-pot cooperative-catalysis-based allylic alkylation/hofmann rearrangement strategy. Angew. Chem. Int. Ed. 2019;58:10521–10527. doi: 10.1002/anie.201905426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hutchings-Goetz LS, Yang C, Fyfe JWB, Snaddon TN. Enantioselective syntheses of Strychnos and Chelidonium alkaloids through regio- and stereocontrolled cooperative catalysis. Angew. Chem. Int. Ed. 2020;59:17556–17564. doi: 10.1002/anie.202005151. [DOI] [PubMed] [Google Scholar]
- 54.Bitai J, Nimmo AJ, Slawin AMZ, Smith AD. Cooperative palladium/isothiourea catalyzed enantioselective formal (3+2) cycloaddition of vinylcyclopropanes and α,β-unsaturated esters. Angew. Chem. Int. Ed. 2022;61:e202202621. doi: 10.1002/anie.202202621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lin H-C, et al. A Pd−H/isothiourea cooperative catalysis approach to anti-aldol motifs: enantioselective α-alkylation of esters with oxyallenes. Angew. Chem. Int. Ed. 2022;61:e202201753. doi: 10.1002/anie.202201753. [DOI] [PubMed] [Google Scholar]
- 56.Wu F, et al. Synergistic Ni/Pd catalysis for asymmetric allylic alkylation of 2-acyl imidazoles. Org. Lett. 2023;25:5448–5453. doi: 10.1021/acs.orglett.3c01726. [DOI] [PubMed] [Google Scholar]
- 57.Wright TB, Evans PA. Catalytic enantioselective alkylation of prochiral enolates. Chem. Rev. 2021;121:9196–9242. doi: 10.1021/acs.chemrev.0c00564. [DOI] [PubMed] [Google Scholar]
- 58.Schafroth MA, Zuccarello G, Krautwald S, Sarlah D, Carreira EM. Stereodivergent total synthesis of Δ9-tetrahydrocannabinols. Angew. Chem. Int. Ed. 2014;53:13898–13901. doi: 10.1002/anie.201408380. [DOI] [PubMed] [Google Scholar]
- 59.He Z, Peng L, Guo C. Catalytic stereodivergent total synthesis of amathaspiramide D. Nat. Synth. 2022;1:393–400. [Google Scholar]
- 60.Zhang Q, Liang K, Guo C. Enantioselective nickel-catalyzed electrochemical radical allylation. Angew. Chem. Int. Ed. 2022;61:e202210632. doi: 10.1002/anie.202210632. [DOI] [PubMed] [Google Scholar]
- 61.Liang K, Zhang Q, Guo C. Nickel-catalyzed switchable asymmetric electrochemical functionalization of alkenes. Sci. Adv. 2022;8:eadd7134. doi: 10.1126/sciadv.add7134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fernandes RA, Ingle AB. Arundic acid a potential neuroprotective agent: biological development and syntheses. Curr. Med. Chem. 2013;20:2315–2329. doi: 10.2174/0929867311320180003. [DOI] [PubMed] [Google Scholar]
- 63.Xu N, Kong Z, Wang JZ, Lovinger GJ, Morken JP. Copper-catalyzed coupling of alkyl vicinal bis(boronic esters) to an array of electrophiles. J. Am. Chem. Soc. 2022;144:17815–17823. doi: 10.1021/jacs.2c02817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu C, Zhong S-M, Chen R-Y, Wu Y, Zhu X-J. Two new compounds from the dried tender stems of Cinnamomum cassia. J. Asian Nat. Prod. Res. 2009;11:845–849. doi: 10.1080/10286020903185942. [DOI] [PubMed] [Google Scholar]
- 65.Chen J-P, Ding C-H, Liu W, Hou X-L, Dai L-X. Palladium-catalyzed regio-, diastereo-, and enantioselective allylic alkylation of acylsilanes with monosubstituted allyl substrates. J. Am. Chem. Soc. 2010;132:15493–15495. doi: 10.1021/ja106703y. [DOI] [PubMed] [Google Scholar]
- 66.Kranthikumar R, Mainkar PS, Sukumar G, Chegondi R, Chandrasekhar S. Tetrahydrothiopyran-4-one as five-carbon source for scalable synthesis of (±)-tapentadol. Org. Process Res. Dev. 2019;23:1369–1373. doi: 10.1021/acs.oprd.9b00121. [DOI] [Google Scholar]
- 67.Wang F, et al. Asymmetric transfer hydrogenation of α-substituted-β-keto carbonitriles via dynamic kinetic resolution. J. Am. Chem. Soc. 2021;143:2477–2483. doi: 10.1021/jacs.0c13273. [DOI] [PubMed] [Google Scholar]
- 68.Wen Y-H, et al. Diastereodivergent desymmetric annulation to access spirooxindoles: chemical probes for mitosis. J. Am. Chem. Soc. 2023;145:4199–4207. doi: 10.1021/jacs.2c12648. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Crystallographic data for the structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre, under deposition number CCDC 2266877 ((S,S)-4n). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. All other data supporting the findings of this study, including experimental procedures and compound characterization, NMR, and HPLC are available within the Article and its Supplementary Information or from the authors. Source data are provided with this paper. NMR data in a mnova file format and HPLC traces are available at Zenodo at https://zenodo.org/records/10050924, under the Creative Commons Attribution 4.0 International license.