Abstract
It is unclear which patients with ER-positive, HER2-negative breast cancer benefit from extended endocrine therapy beyond 5 years. Prognostic factors for late-recurring breast cancer postrelapse survival have been reported. We retrospectively analyzed data from 892 patients with ER-positive and HER2-negative invasive breast cancer who were disease-free after completing a 5-year adjuvant endocrine therapy. Patients were then classified as high-risk (positive lymph nodes, large tumor size, high tumor grade) or low-risk. High-risk patients were divided into extended endocrine therapy and stop groups. Comparisons were made using propensity score matching, and the benefits of extended endocrine therapy for high-risk patients and prognostic factors for postrelapse survival were assessed. The high- and low-risk groups comprised 444 and 448 patients, respectively. The 10-year distant disease-free survival (DDFS) rates were 96.3 % (95 % confidence interval [CI] 0.912–0.985) and 86.5 % (95 % CI 0.798–0911) in the extended and stop groups, respectively (P = 0.00382). Cox proportional hazards model revealed that extended endocrine therapy promoted greater reduction in distant metastasis risk than 5-year endocrine therapy in high-risk populations (hazard ratio [HR] 0.27; 95 % CI 0.11–0.68; P = 0.0054). Postrelapse survival was significantly different in patients with DDFS ≥7 years (HR 0.24; 95 % CI 0.072–0.81; P = 0.021) and those with better response to first-line treatment (HR 0.072; 95 % CI, 0.058–0.90; P = 0.041). Patients with risk factors for late recurrence should be considered for extended endocrine therapy. Longer DDFS and response to first-line treatment may be a prognostic factor for postrelapse survival after late recurrence.
Keywords: Breast cancer, Receptors, Estrogen, Risk factor, Prognostic factor
Highlights
-
•
We analyzed 892 patients with ER+/HER2− breast cancer after 5-year adjuvant endocrine therapy.
-
•
Risk factors for late distant recurrence were lymph node involvement, large tumor size, high tumor grade, and locoregional recurrence.
-
•
Patients at high-risk for late recurrence benefited from extended endocrine therapy.
-
•
Longer DDFS and response to first-line treatment may affect postrelapse survival after late recurrence.
1. Introduction
Approximately 70 % of breast cancers are estrogen receptor (ER)-positive and are predominantly treated with at least 5 years of adjuvant endocrine therapy. In ER-positive disease, 5 years of treatment with tamoxifen reduced the risk of recurrence by approximately 50 % [1]. However, it was reported that the risk of recurrence continues up to 20 years postoperatively in patients with ER-positive, human epidermal growth factor 2 (HER2)-negative breast cancer and that at least half of the recurrences occur at >5 years postoperatively [2]. Extended endocrine therapy, after 5 years of tamoxifen or aromatase inhibitor (AI) treatment, reduced the risk of recurrence and overall mortality [3,4]. Therefore, it is important to identify patients in whom extended endocrine therapy should reduce the risk of recurrence and those in whom this approach would be unnecessary.
The first-line treatment for patients with ER-positive, HER2-negative metastatic breast cancer is endocrine therapy. Several prognostic factors have been reported for metastatic breast cancer. Disease-free survival (DFS) of ≥2 years from primary breast cancer after completion of treatment is more favorable overall survival (OS) than a shorter time to relapse [5,6]. Patients who respond better to first-line treatment following primary diagnosis also have improved odds of survival after relapse [7,8]. However, the implications of treatment response and other prognostic factors are not well-understood in patients with late recurrence, occurring more than several years after completion of postoperative endocrine therapy.
The primary objective of this study is to determine the risk factors for late distant recurrence in patients who have completed 5 years of endocrine therapy and are relapse-free. The secondary objective is to determine prognostic factors for OS after late recurrence in patients with ER-positive, HER2-negative breast cancer.
2. Material and methods
2.1. Patients
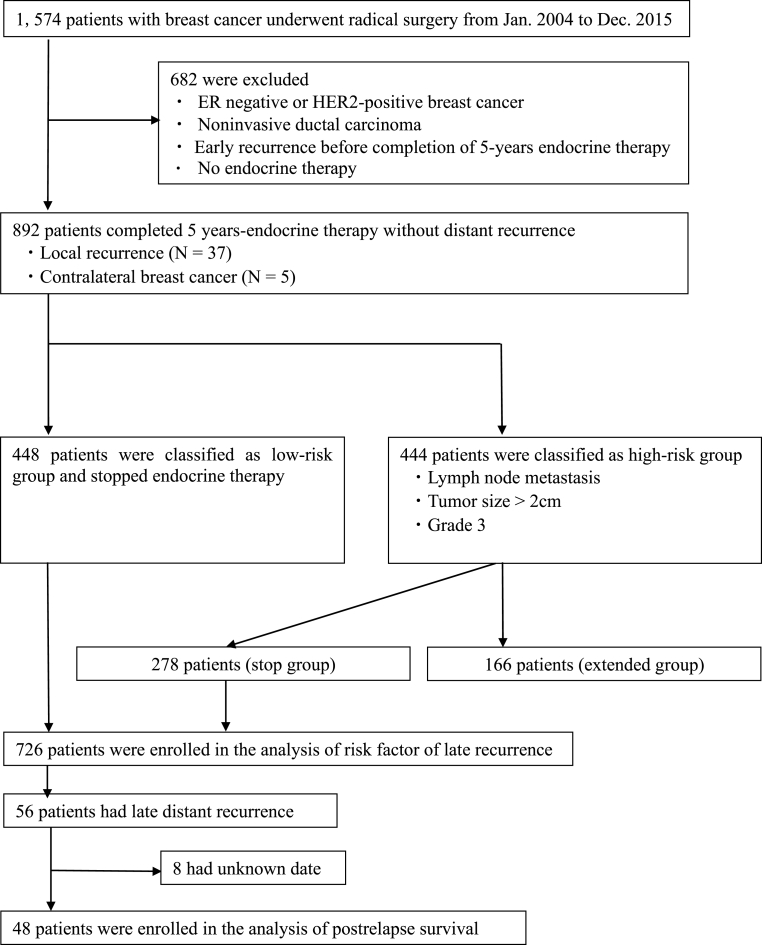
We retrospectively analyzed data from 1574 patients with breast cancer who underwent radical surgery at Tohoku Kosai Hospital between 2004 and 2015. We identified 892 ER-positive, HER2-negative patients without distant metastasis after completing 5 years of adjuvant endocrine therapy. These included 37 patients with locoregional recurrence and five patients who developed contralateral breast cancer. Patients with ER-negative or HER2-positive breast cancer, noninvasive ductal carcinoma, or recurrence within 5 years were excluded. Patients with positive lymph nodes, tumor size of >2 cm, and high tumor grade were classified into the high-risk group for late recurrence. Those not fulfilling these criteria were classified into the low-risk group. Hence, the low-risk group included 448 patients who stopped endocrine therapy, whereas the high-risk group included 444 patients. In the high-risk group, 166 patients received extended endocrine therapy (extended group) and 278 patients stopped endocrine therapy (stop group). A total of 726 patients in the 5-year endocrine therapy group were included in the analysis of risk factor for late recurrence. To assess whether patients with risk factors for late recurrence would benefit from extended endocrine therapy, those from the extended group were matched with those from the stop group using propensity score matching to balance the characteristics of both groups. Among those in the 5-year endocrine therapy group, 56 experienced late recurrence, defined as distant metastatic recurrence occurring after 5 years of endocrine therapy. Of the 56 patients who experienced late recurrence, 48 had records of treatment details that could be confirmed and analyzed (Fig. 1).
Fig. 1.
Flowchart of patient selection.
Distant disease-free survival (DDFS) was defined as the duration from surgical removal of the primary breast tumor to the first report of distant recurrence. OS was defined as the time from the first confirmed recurrence to the date of death from any cause. Progression-free survival (PFS) during first-line treatment was analyzed, and patients were assigned to long (>12 months) or short (<12 months) PFS groups. The cutoff value was set at 12.5 months, based on the median PFS.
ER and progesterone receptor (PR) expressions were defined as positive when the positivity rate was ≥1 %; positivity was classified as either 1%–10 % or ≥10 %. HER2-negativity was defined as score 0 and 1 plus. Score 2 plus was confirmed as negative by fluorescence in situ hybridization. Tumor size and lymph node metastasis definitions were based on the Union for International Cancer Control, 8th Edition. Postoperative endocrine therapy includes selective ER modulators such as tamoxifen and toremifene as well as AIs, such as exemestane, letrozole, and anastrozole. This study was approved by the institutional review board of our hospitals and all enrolled patients provided informed consent.
2.2. Statistical analyses
We conducted a landmark analysis in which only patients without distant metastasis until the landmark time were included in the analyses of data from these time points. The landmark time was 5 years after the initiation of endocrine treatment. We used the chi-square and Mann–Whitney U tests to compare clinicopathologic characteristics and treatments among patients with late recurrence. Logistic regression analysis was used for the analysis of significant variables in univariate chi-square test. The estimation of DDFS and postrelapse OS was performed using the Kaplan–Meier method; differences between survival curves were assessed using the log-rank test. Propensity score matching was performed using the following variables: age, menopausal status, tumor size, lymph node involvement, and tumor grade. To reduce bias due to these potential confounding factors, 1:1 matching of the two groups was performed using the nearest neighbor method with a caliper width equal to 0.20 of the standard deviation of the logit of the propensity scores. Prognostic factors of postrelapse OS were analyzed by univariate and multivariate Cox proportional hazard model. All statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), a graphical user interfaces for R 2.13.0 (R Foundation for Statistical Computing, Vienna, Austria) [9]. A P value of <0.05 was considered statistically significant.
3. Results
3.1. Risk factors for late recurrence after 5-year endocrine therapy
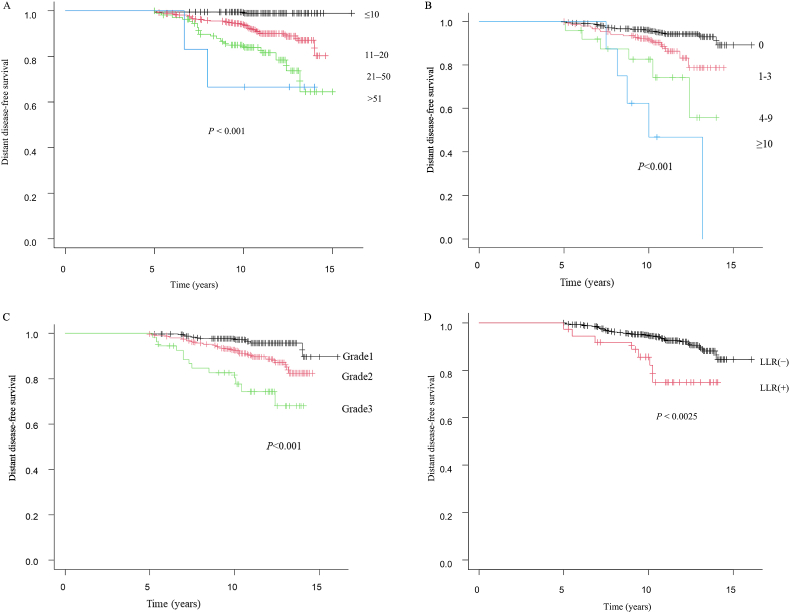
Table 1 summarizes the clinicopathologic characteristics of this study population. The median follow-up periods were 122 (range 60–193) months. The median DDFS was 94.5 (range 60–168) months at the late recurrence. Of the 37 local recurrences observed, 8 (14.3 %) developed distant metastases. During the follow-up period, 20 deaths occurred. In the univariate analysis, tumor size (P < 0.001), lymph node involvement (P < 0.001), tumor grade (P < 0.001), whether or not the patient received chemotherapy (P < 0.001), and locoregional recurrence (P = 0.005) were significantly related to late recurrence. Using the multivariate logistic regression analysis, a statistically significant difference was observed in tumor size (odds ratio [OR] 2.82; 95 % confidence interval [CI] 1.47–5.44; P = 0.0019), lymph node involvement (OR 2.39; 95 % CI 1.27–4.51; P = 0.0070), tumor grade (OR 3.77; 95 % CI 1.79–7.94; P < 0.001), and locoregional recurrence (OR 4.88; 95 % CI 1.96–12.2; P < 0.001) in patients with late recurrence (Table 2). The tumor size (P < 0.001) was larger in patients with late recurrence than in patients without (Fig. 2A). Lymph node metastasis (P < 0.001) (Fig. 2B), high grade (P < 0.001) (Fig. 2C), locoregional recurrence (P < 0.0025) (Fig. 2D) were associated with late recurrence.
Table 1.
Clinicopathologic characteristics with and without late recurrence.
| Characteristics | No distant recurrence |
Late recurrence |
|---|---|---|
| n = 670 | n = 56 | |
| Age (median) | 54.0 (27.0–85.0) | 53.5 (27.0–80.0) |
| Menopausal status | ||
| Premenopausal | 321 (47.9 %) | 27 (48.2 %) |
| Postmenopausal | 349 (52.1 %) | 29 (51.8 %) |
| Tumor size, mm | ||
| ≤10 | 239 (35.7 %) | 2 (3.6 %) |
| 11–20 | 335 (50.0 %) | 31 (55.4 %) |
| 21–50 | 92 (13.7 %) | 21 (37.5 %) |
| >50 | 4 (0.6 %) | 2 (3.6 %) |
| Lymph node involvement | ||
| 0 | 508 (76.8 %) | 27 (48.2 %) |
| 1–3 | 140 (20.9 %) | 18 (32.1 %) |
| 4–9 | 19 (2.8 %) | 6 (10.7 %) |
| ≥10 | 3 (0.4 %) | 5 (8.9 %) |
| Histology | ||
| IDC | 585 (87.3 %) | 50 (89.3 %) |
| ILC | 38 (5.7 %) | 3 (5.4 %) |
| Mucinous ca | 28 (4.2 %) | 1 (1.8 %) |
| IMPCa | 11 (1.6 %) | 1 (1.8 %) |
| Others | 8 (1.1 %) | 1 (1.8 %) |
| Grade | ||
| 1 | 342 (51.4 %) | 12 (21.4 %) |
| 2 | 287 (42.8 %) | 31 (55.4 %) |
| 3 | 41 (6.1 %) | 13 (23.2 %) |
| Type of surgery | ||
| BCT | 423 (64.1 %) | 23 (41.1 %) |
| Mastectomy | 246 (36.8 %) | 33 (58.9 %) |
| ER (%) | ||
| <10 | 10 (1.5 %) | 1 (1.8 %) |
| ≥10 | 660 (98.5 %) | 55(98.2 %) |
| PR (%) | ||
| <10 | 64 (9.6 %) | 6 (10.7 %) |
| ≥10 | 606 (90.4 %) | 50 (89.3 %) |
| Endocrine therapy | ||
| SERM | 400 (59.8 %) | 29 (51.8 %) |
| AI | 269 (40.2 %) | 27 (48.2 %) |
| Chemotherapy | ||
| Yes | 72 (10.7 %) | 20 (35.7 %) |
| No | 598 (89.3 %) | 36 (64.3 %) |
| Radiotherapy | ||
| Yes | 460 (68.7 %) | 37 (66.1 %) |
| No | 210 (31.3 %) | 19 (33.9 %) |
| Locoregional recurrence | ||
| Yes | 29 (4.3 %) | 8 (14.3 %) |
| No | 641 (95.7 %) | 48 (85.7 %) |
ER, estrogen receptor; PR, progesterone receptor, AI, aromatase inhibitor.
Table 2.
Univariate (chi-square) and multivariate (logistic regression) analysis for clinicopathologic factors associated with late recurrence.
| Variables | Univariate |
Multivariate |
|---|---|---|
| P value | OR 95 % CI P value |
|
| Age | 0.759 | 0.99 0.95–1.04 0.724 |
| Menopausal status | 1.00 | 1.39 0.53–3.65 0.502 |
| Tumor size (≤2 cm vs. >2 cm) | <0.001 | 2.82 1.47–5.44 0.0019 |
| Lymph node involvement | <0.001 | 2.39 1.27–4.51 0.0070 |
| Grade (I, II vs. III) | <0.001 | 3.77 1.79–7.94 <0.001 |
| PR (<10 % vs. ≥10 %) | 0.813 | 1.09 0.41–2.89 0.856 |
| Chemotherapy | <0.001 | 1.68 0.92–3.06 0.088 |
| Locoregional recurrence | 0.005 | 4.88 1.96–12.2 <0.001 |
OR, odds ratio; CI, confidence interval; PR, progesterone receptor.
Fig. 2.
Kaplan-Meier plots disease-free survival as a clinicopathologic factor. (2A) Tumor size (mm), (2B) Lymph node involvement, (2C) Grade, (2D) Locoregional recurrence (LLR).
The high-risk group consisted of 278 patients, and the low-risk group consisted of 448 patients. The 10-year DDFS rates were 87.4 % for the high-risk group and 97.9 % for the low-risk group (hazard ratio [HR] 0.16; 95 % CI 0.09–0.31; P < 0.001) (Fig. 3).
Fig. 3.
Comparison of Kaplan–Meier plots for patients with low-risk group versus high-risk group.
3.2. Benefits of extended endocrine therapy in patients at high risk for late recurrence
Table 3 summarizes the baseline characteristics of the high-risk group before and after propensity score matching. After matching the 310 selected patients, the stop and extended groups included 155 patients each. The 10-year DDFS rate was 96.3 % (95 % CI 0.912–0.985) for the extended group and 86.5 % (95 % CI 0.798–0911) for the stop group (P = 0.00382) (Fig. 4). The multivariate Cox proportional hazards model to predict DDFS of late recurrence after propensity score matching indicated that, compared with 5-years endocrine therapy, the extended endocrine therapy reduced the risk of distant metastasis in high-risk populations (HR 0.27; 95 % CI 0.11–0.68; P = 0.0054). Additionally, we found that tumor size of >2 cm (HR 2.24; 95 % CI 1.25–4.02; P = 0.0064), lymph node involvement (HR 1.52; 95 % CI 1.02–2.27; P = 0.035), and high tumor grade (HR 1.91; 95 % CI 1.05–3.45; P = 0.032) increased the risk of late distant recurrence (Table 4).
Table 3.
Characteristics of patients before and after propensity score matching.
| Characteristics | All-patients population (n = 444) |
Propensity-matched population (n = 310) |
||||
|---|---|---|---|---|---|---|
| Stop group (n = 278) | Extended group (n = 166) | P value | Stop group (n = 155) | Extended group (n = 155) | P value | |
| Age, years | 53.5 (27–85) | 49.0 (29–86) | 0.003 | 50 (27–85) | 50 (29–86) | 0.670 |
| Menopausal status | 0.014 | 1.00 | ||||
| Premenopausal | 133 (47.8 %) | 100 (60.2 %) | 92 (59.4 %) | 91 (58.7 %) | ||
| Postmenopausal | 145 (52.2 %) | 66 (39.8 %) | 63 (40.6 %) | 64 (41.3 %) | ||
| Tumor size, mm | 0.272 | |||||
| ≤10 | 26 (9.4 %) | 17 (10.2 %) | 10 (6.5 %) | 17 (11 %) | ||
| 11–20 | 133 (47.8 %) | 72 (43.4 %) | 77 (49.7 %) | 69 (44.5 %) | ||
| 21–50 | 113 (40.6 %) | 67 (40.4 %) | 65 (41.9 %) | 61 (39.4 %) | ||
| ≥51 | 6 (2.2 %) | 10 (6.0 %) | 3 (1.9 %) | 8 (5.1 %) | ||
| Lymph node involvement | 0.025 | 0.613 | ||||
| 0 | 87 (31.3 %) | 31 (18.7 %) | 38 (24.5 %) | 31 (20 %) | ||
| 1–3 | 158 (56.8 %) | 108 (65.1 %) | 92 (59.4 %) | 100 (64.5 %) | ||
| 4–9 | 25 (9.0 %) | 21 (12.7 %) | 17 (11 %) | 19 (12.3 %) | ||
| ≥10 | 8 (2.9 %) | 6 (3.6 %) | 8 (5.2 %) | 5 (3.2 %) | ||
| Grade | 0.139 | 0.613 | ||||
| 1 | 92 (33.1 %) | 42 (25.3 %) | 47 (30.3 %) | 41 (26.5 %) | ||
| 2 | 132 (47.5 %) | 94 (56.6 %) | 78 (50.3 %) | 85 (54.8 %) | ||
| 3 | 54 (19.4 %) | 30 (18.1 %) | 30 (19.4 %) | 29 (18.7 %) | ||
Fig. 4.
Comparison of distant disease-free survival between extended group and stop group after propensity score matching.
Table 4.
Multivariate Cox proportional hazard models for predicting distant disease-free survival of late recurrence after propensity score matching.
| Characteristics | Multivariate analysis |
|
|---|---|---|
| HR (95 % CI) | P value | |
| Age, years | 1.01 (0.95–1.06) | 0.711 |
| Menopausal status (postmenopausal) | 1.32 (0.44–3.96) | 0.615 |
| Tumor size >2 cm | 2.24 (1.25–4.02) | 0.0064 |
| Lymph node involvement | 1.52 (1.02–2.27) | 0.035 |
| High grade | 1.91 (1.05–3.45) | 0.032 |
| Extended endocrine therapy | 0.27(0.11–0.68) | 0.0054 |
HR, hazard ratio; CI, confidence interval.
3.3. Characteristics of patients with late recurrence
Patients experiencing late recurrence exhibited predominantly single organ (37 out of 48 patients, 77.1 %), bone (24 out of 48 patients, 50.0 %), and lung metastases (22 out of 48 patients, 45.8 %). 77.1 % of cases with late recurrence occurred more than 7 years after the initial diagnosis (more than 2 years after completion of endocrine therapy). Approximately 77 % of the patients began the first-line treatment for tumor recurrence with endocrine therapy (Table 5). The median PFS after first-line treatment was 12.5 months overall and was 25 months (range 14–84) in the long PFS group and 6 months (range 3–11) in the short PFS group (Table 6). The median duration of hormone therapy was 40 months (range 14–93) in the long PFS group and 6.5 months (range 0–41) in the short PFS group (Table 6).
Table 5.
Univariate analysis of postrelapse survival of late recurrence.
| Characteristics | Late recurrence |
Univariate |
P value |
|---|---|---|---|
| (n = 48) | HR (95 % CI) | ||
| Menopausal status | 1.05 (0.392–2.84) | 0.913 | |
| Premenopausal | 23 (47.9 %) | ||
| Postmenopausal | 25 (52.1 %) | ||
| Tumor size, mm | 1.97 (0.83–4.7) | 0.122 | |
| ≤10 | 1 (2.1 %) | ||
| 11–20 | 25 (52.1 %) | ||
| 21–50 | 20 (41.7 %) | ||
| ≥51 | 2 (4.2 %) | ||
| Lymph node involvement | 1.04 (0.66–1.65) | 0.839 | |
| 0 | 21 (43.8 %) | ||
| 1–3 | 16 (33.3 %) | ||
| 4–9 | 6 (12.5 %) | ||
| ≥10 | 5 (10.4 %) | ||
| Grade | 1.32 (0.59–2.94) | 0.493 | |
| 1 | 12 (25.0 %) | ||
| 2 | 28 (58.3 %) | ||
| 3 | 8 (16.7 %) | ||
| ER (%) | 0.998 | ||
| <10 | 1 (2.1 %) | ||
| ≥10 | 47 (97.9 %) | ||
| PR (%) | 0.38 (0.10–1.38) | 0.144 | |
| <10 | 6 (12.5 %) | ||
| ≥10 | 42 (87.5 %) | ||
| Locoregional recurrence | 1.50 (0.42–5.3) | 0.525 | |
| Yes | 6 (12.5 %) | ||
| No | 42 (87.5 %) | ||
| Number of distant metastasis | 1.00 (0.28–3.59) | 0.991 | |
| Single | 37 (77.1 %) | ||
| Multiple | 11 (22.9 %) | ||
| Site of distant metastasis | 1.09 (0.86–1.38) | 0.462 | |
| Bone | 24 (50.0 %) | ||
| Lung | 22 (45.8 %) | ||
| Liver | 7 (20.8 %) | ||
| Other | 3 (6.2 %) | ||
| Distant disease-free survival | 0.23 (0.08–0.65) | 0.0052 | |
| ≥7 years | 37 (77.1 %) | ||
| <7 years | 11 (22.9 %) | ||
| First-line therapy | 1.85 (0.50–6.78) | 0.351 | |
| Endocrine therapy | 37 (77.1 %) | ||
| Chemotherapy | 10 (20.8 %) | ||
| Radiation therapy | 1 (2.1 %) | ||
| PFS (month) | 12.5 (3–84) | 0.854 (0.761–0.949) | 0.0035 |
| Response to the first-line treatment | 0.034 (0.0044–0.268) | 0.0012 | |
| Long PFS group (months) | 24 (50 %) | ||
| Short PFS group (months) | 24 (50 %) |
HR, hazard ratio; CI, confidence interval; ER, estrogen receptor; PR, progesterone receptor; PFS, progression-free survival.
Table 6.
Comparison of characteristics of patient with late recurrence according to response to the first-line therapy.
| Short PFS group | Long PFS group | ||
|---|---|---|---|
| n = 24 | n = 24 | P value | |
| Age, years | 54 (35–80) | 53 (36–75) | 0.41 |
| Menopausal status | 1.00 | ||
| Premenopausal | 7 (46.7 %) | 16 (48.5 %) | |
| Postmenopausal | 8(53.3) | 17 (51.5 %) | |
| ER (%) | 1.00 | ||
| <10 | 0 (0 %) | 1 (3.0 %) | |
| ≥10 | 15 (100 %) | 32 (97.0 %) | |
| PR (%) | 0.66 | ||
| <10 | 3 (20 %) | 3 (9.1 %) | |
| ≥10 | 12 (80 %) | 30 (90.9 %) | |
| Number of distant metastasis | 1.00 | ||
| Single | 12 (80 %) | 25 (75.8 %) | |
| Multiple | 3 (20 %) | 8 (24.2 %) | |
| Site of distant metastasis | 0.46 | ||
| Bone | 7 (46.6 %) | 17 (51.5 %) | |
| Lung | 6 (40.0 %) | 16 (48.4 %) | |
| Liver | 1 (6.6 %) | 6 (18.1 %) | |
| Other | 1 (6.6 %) | 1 (3.0) | |
| Distant disease-free survival | 0.036 | ||
| <7 years | 9 (37.5 %) | 2 (8.3 %) | |
| ≥7 years | 15 (62.5 %) | 22 (91.7 %) | |
| First-line treatment | 0.724 | ||
| Chemotherapy | 4 (17.4 %) | 6 (25.0 %) | |
| Endocrine therapy | 18 (78.3 %) | 18 (75.0 %) | |
| Radiation therapy | 1 (4.3 %) | 0 (0 %) | |
| Duration of HT | 6.5 months (0–41) | 40 months (14–93) | <0.001 |
| PFS | 6 months (3–11) | 25 months (14–84) | <0.001 |
PFS. Progression-free survival; ER, estrogen receptor; PR, progesterone receptor.
3.4. Clinical factors correlate with OS
In the univariate analysis, significant differences were observed in OS between ≥7 years and <7 years (HR 0.23; 95 % CI 008–0.65; P = 0.0052), PFS after first-line treatment (HR 0.854; 95 % CI 0.761–0.949; P = 0.0035), and response to first-line treatment (HR 0.034; 95 % CI 0.0044–0.268, P = 0.0012) (Table 5). Using the multivariate Cox proportional hazards model, a statistically significant difference was observed in OS between ≥7 years and <7 years (HR 0.24; 95 % CI 0.072–0.81; P = 0.021), and response to first-line treatment in the long PFS group (HR 0.072; 95 % CI 0.0058–0.90; P = 0.041) (Table 7).
Table 7.
Multivariate Cox proportional hazard models for predicting overall survival of late recurrence.
| Characteristics | Multivariate analysis |
|
|---|---|---|
| HR (95 % CI) | P value | |
| Distant disease-free survival (≥7 years) | 0.24 (0.072–0.81) | 0.021 |
| PFS | 0.96 (0.89–1.04) | 0.396 |
| Response to first-line treatment (Long PFS group) | 0.072(0.0058–0.90) | 0.041 |
HR, hazard ratio; CI, confidence interval; PFS, progression-free survival.
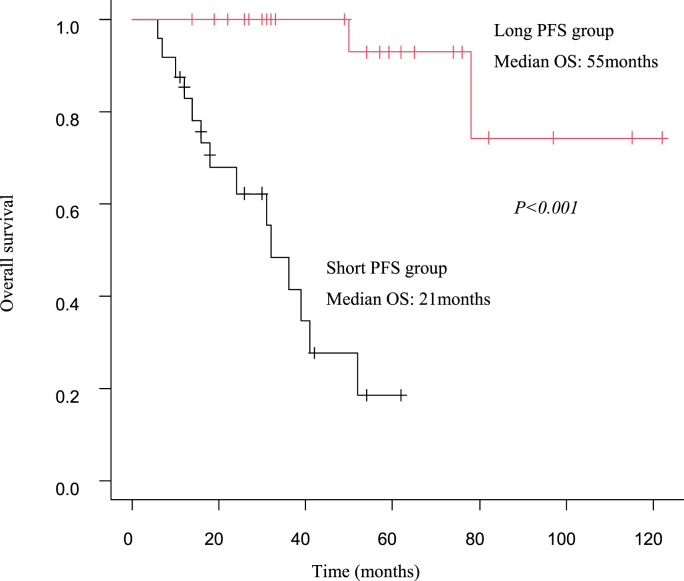
The long PFS group had a median survival of 55 months, whereas the short PFS group had a median survival of 21 months, indicating a significantly better prognosis (P < 0.001) (Fig. 5).
Fig. 5.
Comparison of overall survival between Long PFS group versus Short PFS group. Abbreviation: PFS, progression-free survival, OS, overall survival.
3.5. Factors associated with response to first-line treatment
Comparing the long PFS group and short PFS group, significant differences were observed in DDFS (≥7 years vs. <7 years), duration of endocrine therapy in univariate analysis (Table 6). Patients in the long PFS group were more likely to have had DDFS of over 7 years (91.7 %) than did those in the short PFS group (62.5 %) before recurrence. Patients in the long PFS group had a longer duration of endocrine therapy than did those in the short PFS group initially (40 vs. 6.5 months).
Multivariate logistic regression analysis of clinicopathologic factors also showed that DDFS ≥7 years after first-line treatment was associated with long PFS after recurrence (Table 8).
Table 8.
Odds ratio of long progression-free survival according to patient and tumor characteristics (logistic regression).
| Characteristics | OR | 95 % CI | P value |
|---|---|---|---|
| Distant disease-free survival ≥7 years | 8.97 | 1.44–55.9 | 0.018 |
| Age at first-line treatment | 0.98 | 0.92–1.05 | 0.596 |
| multiple distant metastasis | 1.27 | 0.10–15.9 | 0.85 |
| Site of distant metastasis | 1.13 | 0.64–1.96 | 0.67 |
| First-line endocrine therapy | 0.43 | 0.09–2.06 | 0.29 |
OR, odds ratio; CI, confidence interval.
4. Discussion
In this study, we conducted a landmark analysis to predict risk factors for late distant recurrence among patients who were recurrence-free after completing 5 years of adjuvant endocrine therapy. We demonstrated that lymph node involvement, large tumor size, high tumor grade, and locoregional recurrence were factors associated with late distant recurrence in patients with ER-positive, HER2-negative invasive breast cancer who received 5 years of endocrine therapy. Furthermore, this study showed that patients with high-risk factors for late recurrence (large tumor size, lymph node involvement, and high tumor grade) showed a reduced risk for late distant recurrence with extended administration after 5 years of endocrine therapy.
In previous literature, risk factors vary by the report, with most studies reporting lymph node metastases and large tumor sizes as risk factors for late recurrence. Some studies have reported that high grade and low PR expression are associated with early recurrence (within 5 years) [2,[10], [11], [12], [13]]. Pan et al. reported a 20-year risk of breast cancer recurrence, with higher tumor grade indicating a cumulative increase in recurrence beyond 5 years, making tumor grade an ongoing risk factor for both early and late recurrence [2]. Locoregional recurrence is also associated with a high-risk of developing distant metastasis. In a Danish Breast Cancer Group study, 50 % of patients who underwent mastectomy and experienced locoregional recurrence developed distant metastasis within 2 years [14]. In our study, 20.0 % of cases with locoregional recurrence after receiving mastectomy developed distant metastasis. Our study excluded cases who developed early distant metastasis within 5 years, which may have resulted in lower locoregional recurrence rates than previously reported. In a National Surgical Adjuvant Breast and Bowel Project trial, among breast-conserving therapy patients who experienced ipsilateral breast cancer recurrence, 5-year DDFS was 67 % for node-negative patients and 51 % for node-positive patients [15,16]. Among our patients who underwent breast-conserving surgery and experienced locoregional recurrence, those who were node-negative and node-positive had a DDFS of 71.4 % and 66.7 %, respectively, showing a similar trend.
The Clinical Treatment Score post-5 years (CTS5) is a clinicopathologic tool used to estimate residual risk of distant recurrence after 5 years of endocrine therapy. This tool uses an algorithm including four clinicopathologic variables (nodes, age, tumor size, and grade), which have been reported to predict late DR [17]. Variables of the CTS5 score include nodes, tumor size, and grade, suggesting that these variables are important predictors of late distant recurrence. Therefore, patients with large tumor size, lymph node involvement, and high tumor grade are at high risk of late distant recurrence, with the present study demonstrating that these patients may benefit from extended endocrine therapy for >5 years to reduce the risk of recurrence. On the contrary, patients without high-risk factors showed a good 10-year DDFS of 97.9 % after 5 years of endocrine therapy. These patients may experience a lower benefit from extended endocrine therapy.
In addition to clinicopathologic data, some researchers have attempted to predict late recurrence in terms of tumor biology using multigene assays. Oncotype DX [18], MammaPrint [19], Breast Cancer Index (BCI) [20], EndoPredict [21], and PAM 50 [22] describe risk scores based on gene signature analysis established and validated in clinical trials. BCI is a gene expression-based biomarker developed using a cohort of tamoxifen-treated patients from the randomized prospective Stockholm trial; it has been shown to provide an individual risk of late breast cancer recurrence based on a continuous risk model [23]. Patients with high BCI had an increased risk of late recurrence and benefited from extended endocrine therapy, whereas those with low BCI had a low-risk of recurrence and a low benefit from extended endocrine therapy [24]. The National Comprehensive Cancer Network (NCCN) and American Society of Clinical Oncology (ASCO) updated their guidelines regarding the use of BCI to determine the need for extended postoperative adjuvant endocrine therapy, indicating that patients with a high BCI benefit from extended endocrine therapy, whereas patients with a low BCI have a lower risk of recurrence and receive less benefit from extended endocrine therapy [25,26].
Several clinicopathologic prognostic factors for metastatic breast cancer have been reported. Liver metastases tend to have shorter PFS and OS, and poorer prognosis, than bone metastases [6]. In general, the bone is the most common site of metastasis in ER-positive breast cancer; visceral metastasis locations, such as the liver, are less common [27]. In this study, the first site of late recurrence was characterized by a high incidence of single organ metastases, mostly bone and lung, with a low incidence of liver metastases. No significant difference in OS was found between the different metastatic sites. The fact that there were only seven cases of liver metastases and only one death event may have affected the results.
When it comes to post-recurrence survival of metastatic breast cancer, DFS of ≥24 months after primary breast cancer is more favorable than a shorter time to relapse [5,6]. We demonstrated that longer response to first-line treatment, whether endocrine therapy or chemotherapy, was associated with better OS for late recurrence. Patients with DDFS ≥7 years tended to be more responsive to the first-line endocrine therapy and have longer PFS after recurrence. Patients with long PFS had a median duration of endocrine therapy of 40 months. Those with short PFS had a shorter duration of response to endocrine therapy and a poorer prognosis. This suggests that patients with short PFS may have a high proportion of tumors resistant to endocrine therapy. Causes of resistance to endocrine therapy include biologic changes at metastatic sites, like estrogen receptor 1 (ESR1) mutations and phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit alpha (PIC3CA) mutations. Approximately 15 % of metastatic cancer may have discordant ER measurement compared with primary cancer [28]. Therefore, it is important to confirm the biology of metastatic sites when initiating treatment for metastatic breast cancer. ESR1 mutation is thought to be related to resistance to AIs; cell-free circulating DNA analyses show that approximately 40 % patients with advanced ER-positive, HER2-negative breast cancer have detectable ESR1 mutations in their blood after progression on an AI [29]. The phosphoinositide 3-kinase/protein kinase B/mechanistic target of rapamycin (PI3K/AKT/mTOR) signaling pathway plays a critical role in mediating cell growth, survival, and angiogenesis. Aromatase inhibitor-resistant tumors have been reported to activate PI3K/AKT/mTOR signaling pathways, and mutations in PIK3CA are detected in over 40 % of ER-positive breast cancers [30]. The PI3K inhibitor alpelisib has shown efficacy when combined with fulvestrant in patients with PIK3CA mutation-positive, ER-positive breast cancer [31].
This study has several limitations. It is a retrospective design and is limited to patients from a single institution. In addition, the total number of patients with late recurrence is small. Since the risk of late recurrence is believed to persist for at least 20 years, a longer observation period may be needed.
5. Conclusions
Patients with risk factors of late recurrences, such as lymph node involvement, large tumor size, and high tumor grade should be considered for extended endocrine therapy. Conversely, patients without risk factors of late recurrence may not require it. Our study suggests that response to first-line treatment is a prognostic factor. Patients with DDFS ≥7 years can be expected to have a better response to endocrine therapy and a longer positive prognosis. Patients with PFS less than 12 months after first-line endocrine treatment may have endocrine resistance, and ESR1 and PIC3CA mutations should be investigated and a change to chemotherapy should be considered.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The author would like to thank Dr. Hisashi Hirakawa for his cooperation with the patient data.
References
- 1.Davies C., Godwin J., Gray R., Clarke M., Cutter D., Darby S., et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378:771–784. doi: 10.1016/S0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pan H., Gray R., Braybrooke J., Davies C., Taylor C., McGale P., et al. 20-year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N Engl J Med. 2017;377:1836–1846. doi: 10.1056/NEJMoa1701830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goss P.E., Ingle J.N., Pritchard K.I., Robert N.J., Muss H., Gralow J., et al. Extending aromatase-inhibitor adjuvant therapy to 10 years. N Engl J Med. 2016;375:209–219. doi: 10.1056/NEJMoa1604700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davies C., Pan H., Godwin J., Gray R., Arriagada R., Raina V., et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381:805–816. doi: 10.1016/S0140-6736(12)61963-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swenerton K.D., Legha S.S., Smith T., Hortobagyi G.N., Gehan E.A., Yap H.Y., et al. Prognostic factors in metastatic breast cancer treated with combination chemotherapy. Cancer Reserach. 1979;39:1552–1562. [PubMed] [Google Scholar]
- 6.Hortobagyi G.N., Smith T.L., Legha S.S., Swenerton K.D., Gehan E.A., Yap H.Y., et al. Multivariate analysis of prognostic factors in metastatic breast cancer. J Clin Oncol: official journal of the American Society of Clinical Oncology. 1983;1:776–786. doi: 10.1200/JCO.1983.1.12.776. [DOI] [PubMed] [Google Scholar]
- 7.Gennari A., Stockler M., Puntoni M., Sormani M., Nanni O., Amadori D., et al. Duration of chemotherapy for metastatic breast cancer: a systematic review and meta-analysis of randomized clinical trials. J Clin Oncol: official journal of the American Society of Clinical Oncology. 2011;29:2144–2149. doi: 10.1200/JCO.2010.31.5374. [DOI] [PubMed] [Google Scholar]
- 8.Yamamura J., Kamigaki S., Tsujie M., Fujita J., Osato H., Higashi C., et al. Response to first-line recurrence treatment influences survival in hormone receptor-positive, HER2-negative breast cancer: a multicenter study. In Vivo. 2019;33:281–287. doi: 10.21873/invivo.11473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48:452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sestak I., Dowsett M., Zabaglo L., Lopez-Knowles E., Ferree S., Cowens J.W., Cuzick J. Factors predicting late recurrence for estrogen receptor-positive breast cancer. J Natl Cancer Inst. 2013;105:1504–1511. doi: 10.1093/jnci/djt244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brewster A.M., Hortobagyi G.N., Broglio K.R., Kau S.W., Santa-Maria C.A., Arun B., et al. Residual risk of breast cancer recurrence 5 years after adjuvant therapy. J Natl Cancer Inst. 2008;100:1179–1183. doi: 10.1093/jnci/djn233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamashita H., Ogiya A., Shien T., Horimoto Y., Masuda N., Inao T., et al. Clinicopathological factors predicting early and late distant recurrence in estrogen receptor-positive, HER2-negative breast cancer. Breast Cancer. 2016;23:830–843. doi: 10.1007/s12282-015-0649-0. [DOI] [PubMed] [Google Scholar]
- 13.Lee E.S., Han W., Kim M.K., Kim J., Yoo T.K., Lee M.H., et al. Factors associated with late recurrence after completion of 5-year adjuvant tamoxifen in estrogen receptor positive breast cancer. BMC Cancer. 2016;16:430. doi: 10.1186/s12885-016-2423-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nielsen H.M., Overgaard M., Grau C., Jensen A.R., Overgaard J. Loco-regional recurrence after mastectomy in high-risk breast cancer--risk and prognosis. An analysis of patients from the DBCG 82 b&c randomization trials. Radiotherapy and Oncology. journal of the European Society for Therapeutic Radiology and Oncology. 2006;79:147–155. doi: 10.1016/j.radonc.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 15.Wapnir I.L., Anderson S.J., Mamounas E.P., Geyer C.E., Jr., Jeong J.H., Tan-Chiu E., et al. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in five National Surgical Adjuvant Breast and Bowel Project node-positive adjuvant breast cancer trials. J Clin Oncol: official journal of the American Society of Clinical Oncology. 2006;24:2028–2037. doi: 10.1200/JCO.2005.04.3273. [DOI] [PubMed] [Google Scholar]
- 16.Anderson S.J., Wapnir I., Dignam J.J., Fisher B., Mamounas E.P., Jeong J.H., et al. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in patients treated by breast-conserving therapy in five National Surgical Adjuvant Breast and Bowel Project protocols of node-negative breast cancer. J Clin Oncol: official journal of the American Society of Clinical Oncology. 2009;27:2466–2473. doi: 10.1200/JCO.2008.19.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dowsett M., Sestak I., Regan M.M., Dodson A., Viale G., Thurlimann B., et al. Integration of clinical variables for the prediction of late distant recurrence in patients with estrogen receptor-positive breast cancer treated with 5 years of endocrine therapy: CTS5. J Clin Oncol: official journal of the American Society of Clinical Oncology. 2018;36:1941–1948. doi: 10.1200/JCO.2017.76.4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paik S., Shak S., Tang G., Kim C., Baker J., Cronin M., et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 19.van de Vijver M.J., He Y.D., van't Veer L.J., Dai H., Hart A.A., Voskuil D.W., et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347:1999–2009. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- 20.Sotiriou C., Wirapati P., Loi S., Harris A., Fox S., Smeds J., et al. Gene expression profiling in breast cancer: understanding the molecular basis of histologic grade to improve prognosis. J Natl Cancer Inst. 2006;98:262–272. doi: 10.1093/jnci/djj052. [DOI] [PubMed] [Google Scholar]
- 21.Filipits M., Rudas M., Jakesz R., Dubsky P., Fitzal F., Singer C.F., et al. A new molecular predictor of distant recurrence in ER-positive, HER2-negative breast cancer adds independent information to conventional clinical risk factors. Clin Cancer Res: an official journal of the American Association for Cancer Research. 2011;17:6012–6020. doi: 10.1158/1078-0432.CCR-11-0926. [DOI] [PubMed] [Google Scholar]
- 22.Gnant M., Filipits M., Greil R., Stoeger H., Rudas M., Bago-Horvath Z., et al. Predicting distant recurrence in receptor-positive breast cancer patients with limited clinicopathological risk: using the PAM50 Risk of recurrence score in 1478 postmenopausal patients of the ABCSG-8 trial treated with adjuvant endocrine therapy alone. Ann Oncol: official journal of the European Society for Medical Oncology/ESMO. 2014;25:339–345. doi: 10.1093/annonc/mdt494. [DOI] [PubMed] [Google Scholar]
- 23.Jerevall P.L., Ma X.J., Li H., Salunga R., Kesty N.C., Erlander M.G., et al. Prognostic utility of HOXB13:IL17BR and molecular grade index in early-stage breast cancer patients from the Stockholm trial. Br J Cancer. 2011;104:1762–1769. doi: 10.1038/bjc.2011.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noordhoek I., Treuner K., Putter H., Zhang Y., Wong J., Meershoek-Klein Kranenbarg E., et al. Breast cancer index predicts extended endocrine benefit to individualize selection of patients with HR(+) early-stage breast cancer for 10 years of endocrine therapy. Clin Cancer Res: an official journal of the American Association for Cancer Research. 2021;27:311–319. doi: 10.1158/1078-0432.CCR-20-2737. [DOI] [PubMed] [Google Scholar]
- 25.Gradishar W.J., Moran M.S., Abraham J., Aft R., Agnese D., Allison K.H., et al. NCCN Guidelines® insights: breast cancer, version 4.2021. J Natl Compr Cancer Netw: J Natl Compr Cancer Netw. 2021;19:484–493. doi: 10.6004/jnccn.2021.0023. [DOI] [PubMed] [Google Scholar]
- 26.Andre F., Ismaila N., Allison K.H., Barlow W.E., Collyar D.E., Damodaran S., et al. Biomarkers for adjuvant endocrine and chemotherapy in early-stage breast cancer: ASCO guideline update. J Clin Oncol: official journal of the American Society of Clinical Oncology. 2022;40:1816–1837. doi: 10.1200/JCO.22.00069. [DOI] [PubMed] [Google Scholar]
- 27.Kennecke H., Yerushalmi R., Woods R., Cheang M.C., Voduc D., Speers C.H., et al. Metastatic behavior of breast cancer subtypes. J Clin Oncol: official journal of the American Society of Clinical Oncology. 2010;28:3271–3277. doi: 10.1200/JCO.2009.25.9820. [DOI] [PubMed] [Google Scholar]
- 28.Lindström L.S., Karlsson E., Wilking U.M., Johansson U., Hartman J., Lidbrink E.K., et al. Clinically used breast cancer markers such as estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 are unstable throughout tumor progression. J Clin Oncol: official journal of the American Society of Clinical Oncology. 2012;30:2601–2608. doi: 10.1200/JCO.2011.37.2482. [DOI] [PubMed] [Google Scholar]
- 29.Brett J.O., Spring L.M., Bardia A., Wander S.A. ESR1 mutation as an emerging clinical biomarker in metastatic hormone receptor-positive breast cancer. Breast Cancer Res. 2021;23:85. doi: 10.1186/s13058-021-01462-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cancer Genome Atlas Network Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.André F., Ciruelos E., Rubovszky G., Campone M., Loibl S., Rugo H.S., et al. Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer. N Engl J Med. 2019;380:1929–1940. doi: 10.1056/NEJMoa1813904. [DOI] [PubMed] [Google Scholar]