Summary
Background
Chikungunya disease (CHIKD) is a threat to global health, as it impairs the quality of life of an infected individual ranging from months to years. A systematic evaluation of the serological, virological, and immunological aspects of the circulating viruses and their impact on the host response is imperative for better understanding of the evolving disease dynamics.
Methods
Serum samples were collected from 196 acute CHIKD patients from ten tertiary care hospitals across India during 2016–2021. Out of 196 patients, paired convalescent samples were collected from 51 patients (one-month post-onset of symptoms). The serum samples were profiled for cytokines and neutralisation capacity. Further, chikungunya virus (CHIKV) was isolated from the acute sera and the replication kinetics of the clinical isolates was evaluated.
Findings
Serological analysis indicated that neutralisation could be correlated to seroconversion in the convalescent phase but not found significant in acute phase. In the acute phase samples, there was a correlation between elevated serum levels of IFN-γ, IP-10, MCP-1 and MIG and disease severity. During convalescent phase, pro-inflammatory markers such as IL-6, IL-1β, IL-9 and IP-10 were found to be elevated with a corresponding decline in the secretion of anti-inflammatory cytokines such as IL-4 and IL-10, which correlated with persistent arthralgia. Analysis of replication of the clinical isolates revealed that 68.4% of viruses were fast-growing in the Vero cells (cytopathic effect [CPE] observed within 24 h post-infection), and their corresponding acute serum samples showed an elevated secretion of IFN-α, IL-1RA, IL-17F, IL-9, MCP-1 and MIP-1α.
Interpretation
This study provides an important overview of neutralisation capabilities and cytokine responses along with virus pathogenesis associated with CHIKV infections in India.
Funding
Biotechnology Industry Research Assistance Council (BIRAC).
Keywords: Chikungunya virus, Chikungunya disease, Virus replication, Inflammatory cytokine, Virus neutralization, Arthralgia, Acute infection
Research in context.
Evidence before this study
Initial viral load and host immune response are critical factors in determining the outcome of chikungunya disease (CHIKD) with respect to severe and long-term consequences. A differential pathogenicity amongst the genotypes of chikungunya virus (CHIKV) is also well documented. The innate immune response against CHIKV involves the induction of a local cytokine response and recruitment of inflammatory cells at the site of viral replication. The pro-inflammatory immune response against viral infection results in persistent symptoms, whereas the anti-inflammatory response appears to act as a checkpoint for progression from acute to chronic disease. Previous studies have reported the involvement of cytokines such as tumor necrotic factor (TNF)-α, interleukin-1 (IL-1), IL-6 and IL-18 and other inflammatory mediators in disease resolution and progression.
Added value of this study
Increased serum levels of IFN-γ, IP-10, MCP-1 and MIG were associated with disease severity. Pro-inflammatory markers such as IL-6, IL-1β, IL-9 and IP-10 were elevated with a corresponding decline in the secretion of anti-inflammatory cytokines such as IL-4 and IL-10, which correlated with persistent arthralgia. The replication kinetics of the largest number of clinical isolates of CHIKV in a single study was carried out, and the fast-growing isolates were more pathogenic with increased levels of IFN-α, IL-1α, IL-1RA and MIP-1α.
Implications of all the available evidence
Our study showed that 57 clinical isolates circulating in India have different replication pattern; the fast-growing isolates began to show higher viral load and pronounced cytopathic effect (CPE) at 24 h earlier than the slow-growing isolates suggesting differences in the pathogenic potential of clinical isolates. Our study has provided important insights pertaining to circulating CHIKV strains during outbreaks and host responses during disease progression in CHIKD. Constant surveillance and characterisation of the circulating viruses will be critical in devising effective control measures and mitigating the severity of disease outbreaks.
Introduction
Chikungunya disease (CHIKD) is an infection caused by chikungunya virus (CHIKV) and is transmitted by Aedes mosquitoes. It was first reported in Tanzania in 1952, and several epidemics were reported in different parts of Africa and Asia.1 The cases dwindled during 1970s until 2004, when it re-emerged in the Indian Ocean Islands and has expanded to over 60 countries across the globe.1 In 1963, CHIKV first appeared in India and a few epidemics occurred till 1973. For 32 years, CHIKV was not reported until it re-appeared in 2005–2006 and since then, several outbreaks are being reported in different Indian states1 across the country. CHIKD is characterised by an abrupt onset of fever, headache, diffused body rash, myalgia, nausea, and debilitating arthralgia. The febrile phase of the disease, also referred to as the acute phase, lasts 7–14 days. After the acute phase, the symptoms resolve within 3–4 weeks. However, in a subset of affected individuals, the disease may progress to a chronic phase of debilitating and recurring arthritis that may take months or years to resolve. Those underlying mechanisms that decide disease resolution vs progression are poorly understood.2
Existing information vis-à-vis CHIKD outcome indicates that viral load and host immune response are the critical factors determining the fate of CHIKV infection for severe and long-term sequelae.3, 4, 5 Likewise, the interplay of virus replication and host immunity is reported to determine the pathogenic outcome of CHIKD.6 Innate immune response against CHIKV involves the induction of a local cytokine response that recruits the inflammatory cells at the site of viral replication, such as macrophages, dendritic cells (DCs) and natural killer cells (NKs).7 It is also known that CD4+ T cells are the major pro-inflammatory mediators during the peak of the febrile phase of CHIKV infection,8 while antibodies remain the key player for viral resolution from early convalescent phase onward.8,9 The pro-inflammatory immune response against the viral infection results in the persisting symptoms and the host’s anti-inflammatory response seems to act as a checkpoint from the acute to chronic disease progression.2 Studies reported that specific cytokines and inflammatory mediators might be linked to disease resolution and progression.10
India presents with a unique profile with respect to CHIKD transmission and is considered the hub for CHIKV global transmission along with frequent reintroduction of strains from other countries.11 Although CHIKV incidences in India have been increasing since its re-emergence in 2005,12 reports on viral pathogenesis and immune responses associated with the circulating strains remain scanty. To address some of these concerns, CHIKV patient sera samples (acute phase and convalescent phase) were used to investigate seroconversion, antibody neutralisation capacity, and cytokine profiles. Also, replication kinetics of the clinical isolates were evaluated, to check a possible correlation of the findings with clinical features and disease progression.
Methods
Clinical sites
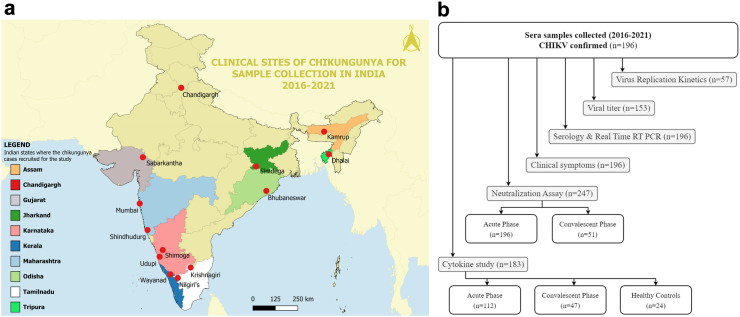
The present study was conducted as part of two consortia involving thirteen clinical sites across India (Fig. 1a). The sites were healthcare centres (either community health centres or general hospitals or tertiary care hospitals) in the following states or Union Territory: Tamil Nadu (Krishnagiri and Nilgiri’s), Karnataka (Shimoga and Udupi), Kerala (Wayanad), Gujarat (Sabarkantha), Tripura (Dhalai), Assam (Kamrup), Maharashtra (Mumbai and Sindhudurgh), Odisha (Bhubaneswar), Punjab (Chandigarh), and Jharkhand (Simdega). Written informed consents were obtained from all participants before the clinical samples were collected following ethical clearances from the participating institutes (Reference: MAHE EC/004/2020, UEC/32/2013-14, ICGEB/IEC/2019/17, version 3, PGIMER PGI/IEC/2019/000011, TNMC IEC/24/2020, and AIIMS Bhubaneswar T/EMF/Micro/19/09).
Fig. 1.
The clinical sites and the study design for the current study. (a). The map depicts the 13 clinical sites located in ten Indian states involved in the recruitment of chikungunya cases (2016–2021). (b). The flow chart representing the study design including the number of samples available and the assay performed on the samples.
Study design and sample collection
All patients recruited in this study were CHIKD cases confirmed through real-time reverse transcriptase PCR (qRT-PCR) and/or IgM ELISA. Details of their medical and clinical history of arthralgia were collected via a questionnaires and paired serum samples (acute and one-month follow-up) were drawn for laboratory investigations and further analyses. The serum samples used in this study were collected between 2016 and 2021. Other clinical-epidemiological data, including age, gender, temperature, Clinical Disease Activity Index (CDAI) score, the onset of symptoms, signs and symptoms, co-morbidities, total leucocyte count, platelet count, haemoglobin and travel history were collected (Table 1). All serum samples collected in this study were aliquoted into multiple vials to prevent freeze and thaw and stored at −80 °C. Aliquots were used to carry out the immunological and virological assays.
Table 1.
Demography and clinical features of chikungunya cases recruited in the study (n = 196).
| General observations | |||
|---|---|---|---|
| Age, range (median) | 12–70 years (35.5) | ||
| Males (n [%]) | 91 (46.43) | ||
| Females (n [%]) | 105 (53.57) | ||
| Clinical features | |||
| Temperature range (median) | 98–103.9 °F (99.2) | ||
| Days of onset of fever—range (median) | 1–13 days (3) | ||
| Fever (n [%]) | 196/196 (100) | ||
| Headache (n [%]) | 181/196 (92.35) | ||
| Rashes (n [%]) | 2/196 (1.02) | ||
| Cough (n [%]) | 53/196 (27.04) | ||
| Photophobia (n [%]) | 78/196 (39.80) | ||
| Retro-orbital pain (n [%]) | 88/196 (44.90) | ||
| Red Eye present (n [%]) | 60/196 (30.61) | ||
| Myalgia (n [%]) | 178/196 (90.82) | ||
| Morning stiffness (n [%]) | 48/85 (56.47) | ||
| Abdominal pain (n [%]) | 41/196 (20.92) | ||
| Nausea (n [%]) | 85/196 (43.37) | ||
| Vomiting (n [%]) | 56/196 (28.57) | ||
| Diarrhoea (n [%]) | 9/196 (4.59) | ||
| Lymphadenopathy (n [%]) | 26/196 (13.27) | ||
| Oedema (n [%]) | 15/196 (7.65) | ||
| Conjunctivitis (n [%]) | 14/196 (7.14) | ||
| Joint Pain (n [%]) | 180/196 (91.84) | ||
| Joint pain location (n [%]) | Both joints | 139/180 (77.22) | |
| Large joints | 38/180 (21.11) | ||
| Small joints | 1/180 (0.56) | ||
| CDAI score (n [%]) | High score (22.1–76) | Severe | 52/77 (67.53) |
| Remission (0–2.8) | Non-severe | 0/77 (0.00) | |
| Low score (2.9–10) | 12/77 (15.58) | ||
| Moderate score (10.1–22) | 13/77 (16.88) | ||
CDAI - clinical disease activity index.
The samples that were collected within the first two weeks of the onset of symptoms were classified as “Acute samples” and follow-up samples collected after a month of the onset of symptoms were classified as “Convalescent samples”. The severity of the disease was assessed using the CDAI and categorised as (i) Severe (CDAI: 22.1–76) and non-severe (CDAI: 0–22) in acute phase (Table 1), (ii) Persistent arthralgia (CDAI >1) and recovered (CDAI = 0) in convalescent phase.
Healthy controls were included in the study to achieve a baseline for the serum levels of cytokines. Blood samples were obtained from 24 consenting individuals (Supplement Table S2) with no clinical symptoms of febrile illness six months before the sample collection, free of chronic conditions and negative for CHIKV RNA, anti-CHIKV IgM and IgG antibodies. The summary of the study design is depicted in (Fig. 1b).
Laboratory diagnosis of CHIKV cases
Serum samples were tested using qRT-PCR and IgM antibody ELISA for confirming chikungunya infection. CHIKV E1 gene was amplified by Titanium One-Step RT-PCR Kit (Clontech) using a previously published protocol.13 Anti-IgM antibodies against CHIKV were detected using CHIKjj Detect™ IgM ELISA (InBios International, Inc. USA) according to the manufacturer’s instructions. Additionally, to detect midpoint and endpoint titres of anti-CHIKV IgG and IgM antibodies, indirect ELISA using purified, inactivated CHIKV as antigen was performed as previously reported.3
Plaque reduction neutralisation test (PRNT)
The neutralisation capacity of the patient’s sera was analysed by PRNT in Vero cells using previously published protocols.3 Briefly, starting with the 1:20 dilutions, up to 10 dilutions, two-fold serially diluted sera were mixed with 50 plaque-forming units (PFU) of a lab adapted CHIKV strain isolated from a patient during an outbreak in India in 2010 (Chikungunya virus isolate IND/2010/DEL/01, ECSA genotype, Accession: MH124570.1), in Dulbecco’s Modified Eagle Medium (DMEM), and incubated for 60 min at 37 °C. Dilutions were then added to the Vero cell monolayer and incubated for 2 h at 37 °C. Two percent carboxymethyl cellulose in DMEM (with 10% fetal bovine serum) was used for overlay and cells were incubated for 72 h at 37 °C and 5% CO2. The cells were washed and fixed with 4% paraformaldehyde, and visualized plaques were counted. Results were analysed in GraphPad Prism 6, and PRNT endpoint titres were expressed as the maximum serum dilution inducing a 50% reduction (PRNT50) in plaque counts.
CHIKV growth kinetic study
Samples for replication kinetics were selected based on day of onset of symptoms in the patients. Specifically, samples collected between day 2–5 were selected for further processing. Viruses were isolated from patients’ sera after propagation in C6/36 cells. Aliquots of clinical sera samples were thawed on ice; 30 μl sample was filtered and added to C6/36 cells (2 × 106 cells) in 24-well plates. Cells were grown in 2% DMEM for 72 h at 28 °C, supernatant was harvested, centrifuged at 10,000 rpm for 10 mins and stored at −80 °C. Standard plaque assay was performed to estimate the viral titre and multiplicity of infection (MOI). For CHIKV growth kinetics, Vero cells were seeded in 24-well plates (80,000 cells per well) to 80–90% confluency and inoculated with the CHIKV isolates at the MOI of 0.01 pfu/well, kept for 2 h at 37 °C then washed and added DMEM supplemented with 2% FBS. Uninfected cells served as control. Supernatants were harvested every 24 h for three consecutive days. The efficiencies of replication of isolates were determined by standard plaque assay.
Bead-based multiplex immunoassay (Luminex)
To quantify the levels of cytokines in the CHIKV-infected serum samples, a magnetic bead-based multiplex immunoassay was performed using a MAGPIX® (Luminex Corporation, USA) instrument system. A human cytokine magnetic 35-plex panel (Invitrogen™, Vienna, Austria) was utilized to determine the immune parameters such as; (i) Pro-inflammatory cytokines (IL-2, IL-9, IL-15, IL-7, IL-5, IL-6, IL-12/IL-23 p40, GM-CSF, IFN-α, IFN-γ, TNF-α, IL-17A, IL-1α, IL-1β, IL-2r, IL-8, IL-17F and IL-22), (ii) Anti-inflammatory cytokines (IL-4, IL-13, IL-1ra, and IL-10), (iii) Chemokines (MCP-1, MIP-1α, MIP-1β, RANTES, EOTAXIN, MIG, IP-10 and G-CSF) and (iv) Growth-factors (EGF, FGF basic, HGF, VEGF and IL-3) following the manufacturer’s instructions.
Statistical analysis
The Spearman rank test was used to determine the correlation between virus-neutralising capacity of serum antibodies. Analysis of neutralisation capacity of antibodies during acute and convalescent phases was done using paired t-test. The differences in the levels of cytokines, chemokines and growth factors of CHIKV cases were analysed using non-parametric analyses such as the Kruskal–Wallis test and Dunn’s multiple comparison test (to analyse the differences within the groups) by using statistical software R. A p value ≤ 0.05 was considered statistically significant for the analysis.
Role of the funding source
The funding agency had no role in study design, data collection, data analysis, interpretation, or writing of the report.
Results
Clinical features and case classification of CHIKV patients
All the recruited cases (n = 196) were laboratory-confirmed as CHIKV positive by qRT-PCR and/or IgM ELISA (Table 1). Only 47 samples were PCR positive, 116 were both PCR and IgM positive and 33 were only IgM positive. Clinical features of all the confirmed CHIKV cases are summarised in Table 1. The frequently observed clinical features during the first two weeks of the infection included joint pain (91.84%), myalgia (90.82%), headache (92.35%), morning stiffness (56.47%). Other less frequently observed symptoms recorded were retro-orbital pain, nausea, abdominal pain rashes, cough, photophobia, diarrhoea, vomiting, oedema, red eye and conjunctivitis (Table 1). Owing to inclusion of patients from two different studies, there was minor variation in the collection of clinical information, and the estimates were derived from varied denominators depending on the number of patients who answered the question (Table 1). The CHIKV confirmed cases were further classified using the CDAI score wherever available, and it was observed that at the time of recruitment, 67% of the cases (n = 52/77) had a severe acute phase of CHIKD.
A subset of the 196 patients were followed up (n = 47) and their samples were collected one month post the onset of symptoms. The clinical features of these subset of patients, particularly the CDAI scores, were documented. The CDAI scores were used to categorise disease progression in the patients. Cases with a “0” CDAI score in the convalescent phase were categorised as recovered while the cases with >1 CDAI score were categorised as persistent arthralgia group. It was observed that 74.5% (n = 35/47) of patients had persistent arthralgia (>1 CDAI score) in the convalescent phase; of these 35 cases with persistent arthralgia, 74.29% (26/35) were found to have had severe symptoms (CDAI: 22.1–76) in their acute phase. The remaining convalescent patients (n = 12) exhibited a CDAI score = 0 and were categorised as recovered.
Neutralisation correlated to sero-conversion in convalescent samples
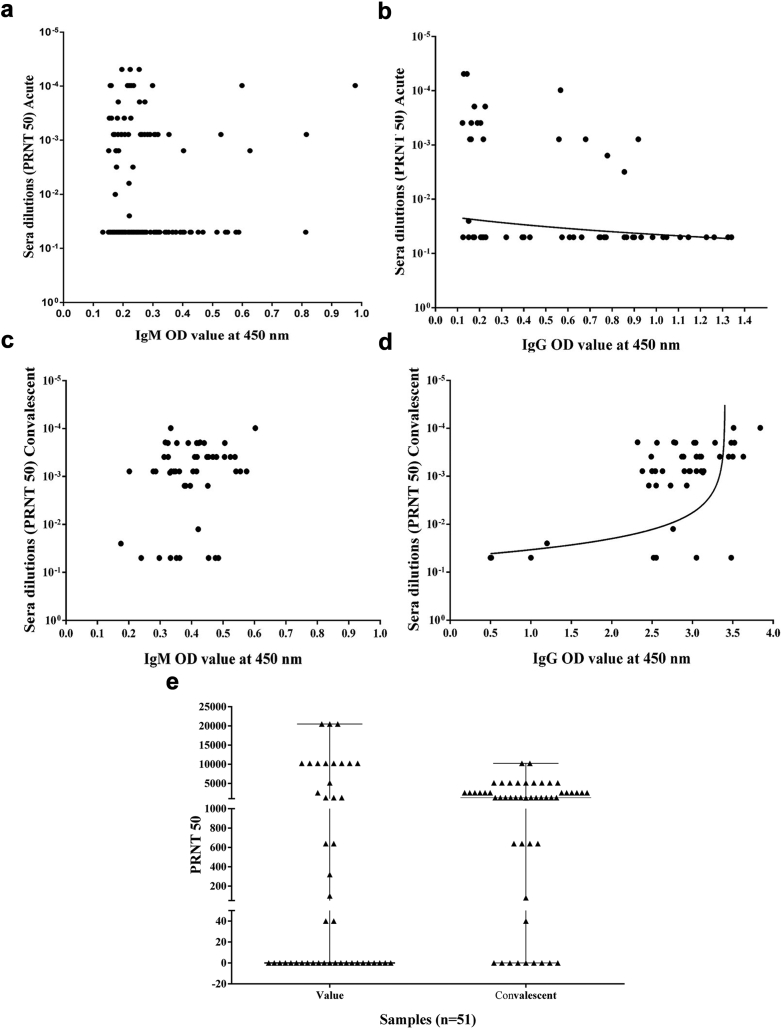
Levels of IgM and IgG were quantified from all the 196 samples in acute phase; and 145 and 56 were found to be IgM and IgG positive respectively (Supplementary Fig. S1a and b). Out of 51 convalescent samples all 51 were positive for IgM and 47 were positive for IgG (Supplementary Fig. S1c and d). Paired analysis between acute and convalescent samples revealed that 9 samples that tested negative for IgM antibody in acute phase seroconverted to IgM positive in the convalescent phase. Similarly, 34 samples that were IgG negative in acute phase tested positive for IgM and IgG during the convalescent phase.
The neutralising capacity of the binding antibodies, in the acute phase samples was found to be 34% (50/145) among the IgM-positive samples and 33% (19/56) among the IgG-positive samples. The control antibody titre level was 0.152 (OD value) for IgM and 0.207 (OD value) for IgG (Fig. 2a–d); a significant correlation was observed with IgG antibody titre (p < 0.0001). We then compared the neutralising capacity between acute and convalescent group and observed an increase in the neutralising capacity in convalescent phase samples that was statistically significant (p = 0.0186) (Fig. 2e). It was observed that 27 samples did not have neutralisation capacity at acute phase, whereas 20 of these samples showed neutralisation at convalescent phase. It was further observed that seven samples did not exhibit any neutralising capacity at both acute and convalescent phase. Upon analysing the clinical information of these samples, we found that six of these seven cases had non-severe symptoms during the first disease presentation. The remaining one case presented with severe symptoms at acute and which had resolved at convalescence. These results indicate that the neutralisation can be correlated to seroconversion, mostly in the convalescent samples.
Fig. 2.
Neutralisation status of Anti-chikungunya virus antibodies at acute and convalescent phase. IgM and IgG antibody was quantified for 196 acute and 51 convalescent samples after one month follow-up and their neutralisation status were analysed. (a) Neutralisation status of IgM Antibody in acute phase were showing 34% (50/144 IgM positive) neutralising capacity but did not showed a significant correlation with IgM titre (b) Neutralisation status of IgG Antibody in acute phase were showing 33% (19/56 IgG positives) neutralisation capacity which is significantly correlated with IgG titre (p < 0.0001). (c) Neutralisation status of IgM Antibody in convalescent phase showing 100% (51/51 IgM positive) and a significant correlation was not observed with IgM titre. (d) Neutralisation status of IgG Antibody in convalescent phase were showing 87% (41/47 IgG positive) neutralisation and a significant correlation was observed with IgG titre (p = 0.0013). € Neutralisation capacity of acute and convalescent samples. The comparison of neutralising capacity between acute and convalescent group showed a significant difference (p 0.0186). At acute phase, 27 samples did not have neutralisation capacity; however, 20 of these samples showed neutralisation at convalescent phase.
Modulations of serum cytokine levels correlated with disease progression parameters
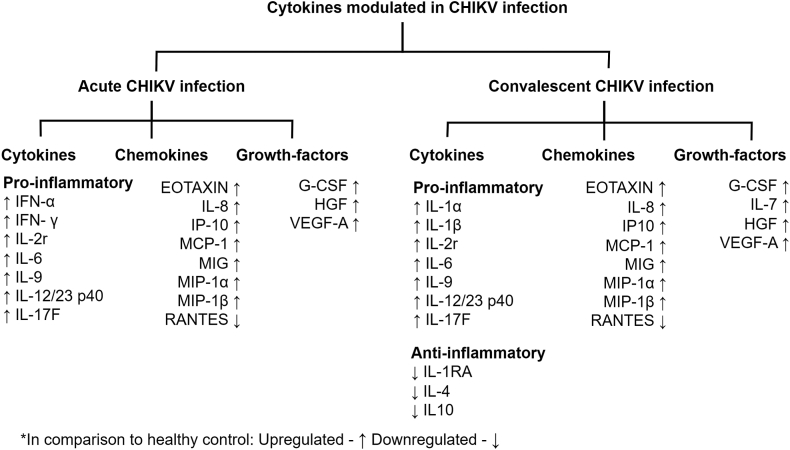
To understand the modulation of the serum cytokine levels during disease progression of CHIKD, a panel of 35 cytokines was estimated in 112 acute and 47 convalescent samples in comparison to 24 healthy controls (Supplement Table S1). Of the 35 cytokines that were analysed, 24 have been significantly modulated (p < 0.05) either in the acute or in the convalescent phase of the infection (cytokines [n = 12], chemokines [n = 8] and growth factors [n = 4]) (Fig. 3 and Supplementary Figs. S2–S6).
Fig. 3.
Cytokines, chemokines and growth factors response in Chikungunya virus-infected patients are segregated into acute and convalescent phases. Further cytokine responses have been classified into pro-inflammatory and anti-inflammatory responses. Each cytokine, chemokine and growth factor response are represented by either an upward or downward arrow indicating increased or decreased response, respectively, compared to healthy controls.
The comparative modulations of the cytokine among acute and convalescent phases were assessed using 47 paired serum samples. The pro-inflammatory cytokines, chemokines, and growth factors were upregulated in both phases of the infection (Supplementary Fig. S4). In the acute samples, pro-inflammatory cytokines IFN-α, IFN-γ, IL-6, IL-9, IL-12/23 p40 IL17F, and IL-2r were found to be upregulated causing mild to severe inflammations (p < 0.05). In the convalescent sample, other pro-inflammatory cytokines, such as IL-1α and IL-1β, were found to be upregulated, whereas a significant downregulation of anti-inflammatory cytokines (IL-4, IL-10 and IL-1RA) were also observed (p < 0.05). Chemokines MIP-1α, IL-8, MIP-1β, IP-10, MCP-1, and EOTAXIN; and the growth factors IL-7, G-CSF, HGF and VEGF-A were found to be consistently upregulated in both the phases (p < 0.05). However, RANTES levels were seen to be downregulated in both phases (Supplementary Fig. S4).
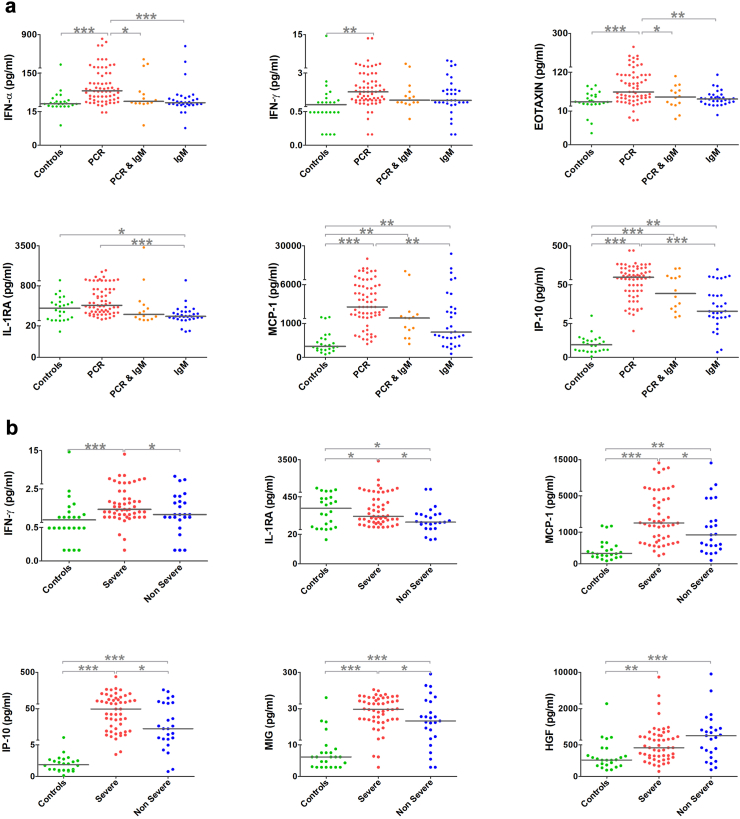
Further, to understand the chronological modulations of pro-inflammatory cytokines in the acute phase, the samples were categorised into “early viraemic stage” (only PCR positive), “late viraemic stage” (both PCR and IgM positive) and “post viraemic stage” (only IgM positive) (Supplementary Fig. S2). It was observed that upregulation of IL-1RA, IP-10 and MCP-1 decreased from the early to post viraemic stage while the levels of IL-8, IL-2R and MIP-1β increased towards the post viraemic stage (p < 0.05). Interestingly, the levels of IFN-α, IFN-γ and EOTAXIN were elevated (p < 0.05) only during the early viraemic stage (Fig. 4a), though there was no statistical significance when EOTAXIN grouped together as acute samples.
Fig. 4.
Scatter plots of significant modulations in serum cytokines, chemokines, and growth factors of prognostic importance in Chikungunya virus infection. (a) Upregulation of serum cytokine levels during the viraemic phase of chikungunya infection; (b) Modulation of cytokines among the severe chikungunya; and (c) Upregulation of proinflammatory and downregulation of anti-inflammatory cytokines among convalescent serum samples of persistent arthralgia cases. The statistical significance was estimated using Kruskal–Wallis and Dunn’s multiple comparison tests (p-value presented in the plots: <0.05-∗, <0.01-∗∗ and <0.001-∗∗∗).
The cytokine levels of the acute phase samples, when categorised into severe and non-severe (based on CDAI score), revealed that the levels of pro-inflammatory cytokines IFN-γ, the chemokines IP-10, MCP-1 and MIG; and the growth factor HGF were upregulated (p < 0.05) in the severe group (Fig. 4b and Supplementary Fig. S3). Furthermore, the levels of anti-inflammatory cytokine IL-RA were downregulated in both the severe and non-severe cases (Fig. 4b and Supplementary Fig. S3).
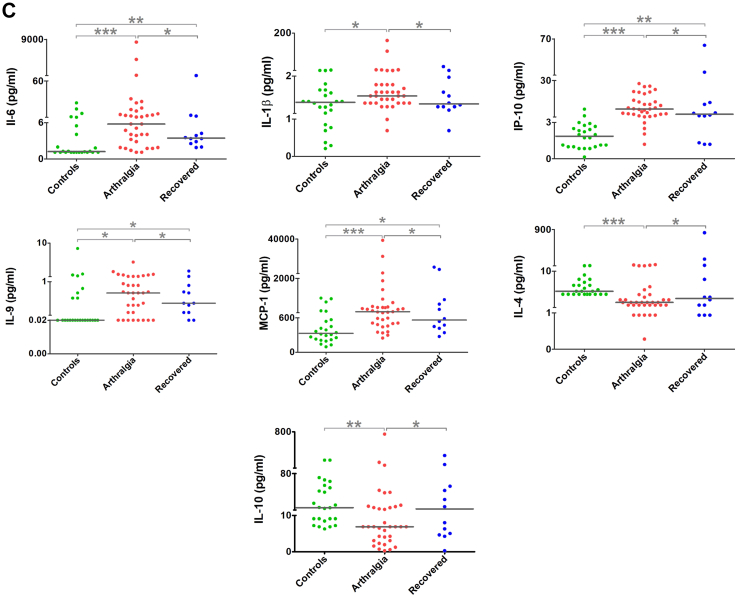
The samples of the convalescent phase (n = 47) were grouped into persistent arthralgia (n = 35) and recovered (n = 12) based on their disease progression. Patients with persistent arthralgia had higher levels of pro-inflammatory cytokines such as IL-1β, IL-6 and IL-9; and the chemokines IP-10, MCP-1 and MIG compared to the recovered group with a simultaneous reduction in the anti-inflammatory cytokines IL-4 and IL-10 (p < 0.05) (Fig. 4c and Supplementary Fig. S5).
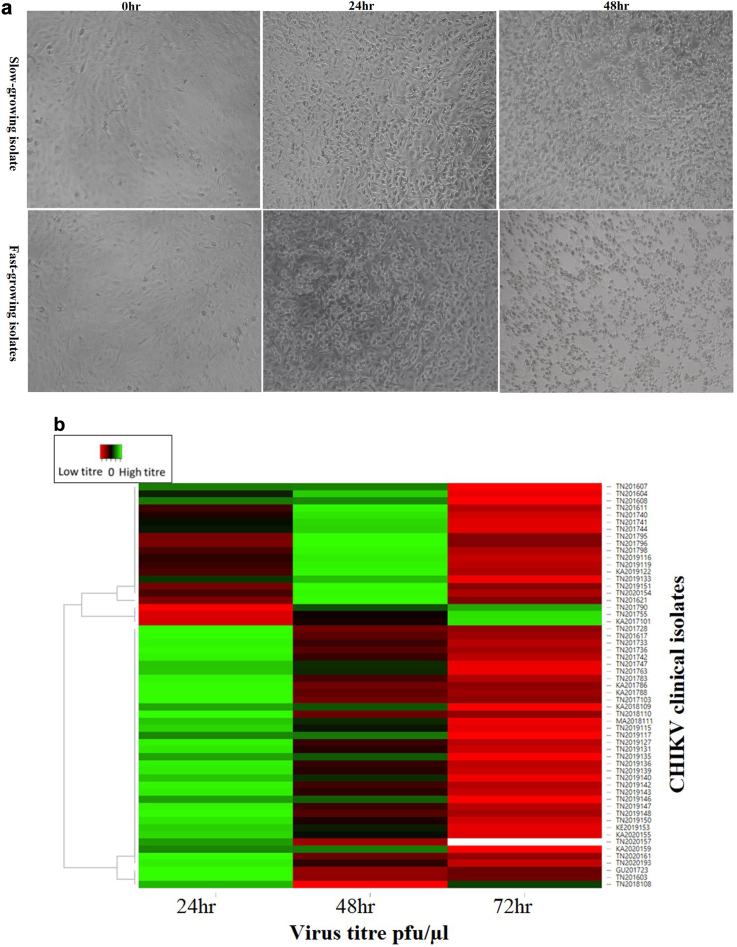
Replication kinetics of viral isolates correlated with pro-inflammatory markers
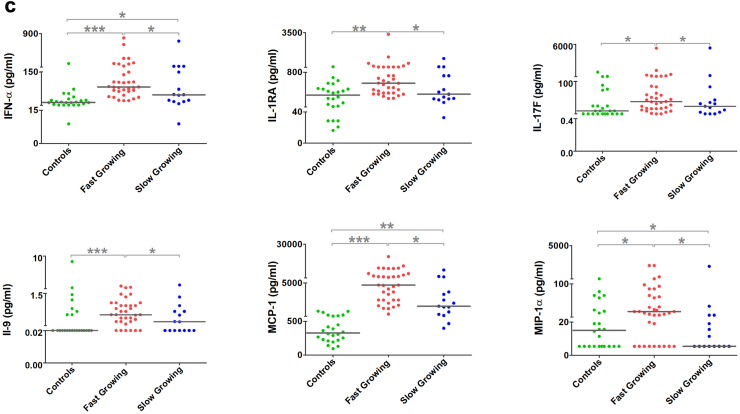
A virus replication kinetics study was performed from samples from different geographical locations over the study and collected between Day 2–5 since the onset of symptoms; 57 samples fulfilled these criteria. Based on the cytopathic effect (CPE) and the viral titre of the viruses over 72 h post infection (hpi), the isolates were categorised as either ‘fast-growing’ or ‘slow-growing’ (Fig. 5a). 30/57 virus isolates showed the highest viral titre at 24 hpi (7.73 × 101 to 8.79 × 103 pfu/ul) and 9/57 isolates showed high titre at both 24 and 48 hpi categorised as fast-growing 18/57 isolates showed the highest viral titre at 48hpi are slow-growing isolates (1.3 × 102to 4.14 × 103) (Fig. 5b). Analysing the patients' clinical symptoms at the time of recruitment with the replication kinetics did not reveal any statistically significant difference in clinical presentation. Additionally, the virus kinetics groups were analysed for the cytokine profiles in the acute serum samples, and it was observed that the pro-inflammatory cytokines were upregulated in the fast-growing virus isolate group and the levels of IFN-α, IL-1RA, IL-17F, IL-9, MCP-1 and MIP-1α were significantly higher in the fast-growing isolates (Fig. 5c).
Fig. 5.
Replication kinetics of viral isolates and their correlation with proinflammatory markers. (a) Cytopathic effect on Vero cells infected with CHIKV clinical isolates: Panel A shows the slow growing isolates at 0 h, 24 h, and 48 h post infection timepoint, similarly Panel B shows post infection timepoint of fast-growing isolates at 0 h, 24 h, and 48 h. (b) CHIKV clinical isolates (n = 57) growth kinetics represented by a heatmap the peak of virus titre at 24 h, 48 h, and 78 h clustered with the green bars and low titter is represented by red colour (c) Association of serum cytokine levels to the CHIKV replication kinetics. CHIKV – chikungunya virus.
Discussion
The present study reports the virological, seroconversion, neutralisation potential, and host immune responses in the CHIKD cohort of 196 acute samples and a subset of 51 paired convalescent samples collected between 2016 and 2021. One of the study's highlights was the robust sample collection during the acute phase of infection; more than 75% of the samples were PCR-positive at the time of collection. This facilitated a detailed analysis of seroconversion and its neutralisation potential. The IgG antibodies of acute samples showed a robust neutralising capacity, similar to earlier reports.3,14,15 Additionally, the IgM antibodies of the convalescent samples exhibited a stronger neutralising capacity than their paired acute samples. Previous studies have shown that IgM antibodies display a stronger neutralising capacity during the early phase of CHIKV infection16 and the IgG presents a stronger efficacy with passage of time since the onset of disease.17
With respect to the clinical presentation of the patients enrolled in this study, we did not observe much variation as compared to already published information.3,18 Joint pain, myalgia, headache, morning stiffness were the most frequently observed clinical features during the acute phase and the other less frequently observed symptoms such as retro-orbital pain, nausea, abdominal pain, rashes, cough, photophobia, diarrhoea, vomiting, oedema, red eye and conjunctivitis. The study recruited more severe cases (67%) at the acute phase by screening tertiary care hospitals. During follow-up with patients for sample collection, we were able to perform correlation study by evaluating disease progression starting from acute phase. At the time of first sample collection, 67% of interviewed patients showed severe disease based on their CDAI scores. Upon interviewing these patients during the follow-up, it was observed 74% of those patients among the severe disease converted to persistence arthralgia. Whereas in the remaining patients the symptoms appeared to be resolved and based on the convalescent information. Such information is critical in understanding disease dynamics at a given point in time as these aspects may have a direct bearing to the DALY caused by CHIKD especially during outbreak conditions.
While it is well established that the cell-mediated immune system plays critical roles in viral clearance in CHIKD, the role of T-cell effector molecules, (especially the role of cytokines in disease progression/resolution) has been well documented in CHIKV immunopathology.2 In the present study, we assessed several pro-inflammatory and anti-inflammatory cytokines, chemokines and growth factors that may contribute to the progression or resolution of CHIKD. We observed that most of the effector molecules were elevated during the acute phase and remained so even during convalescence except for IFN-α and IFN- γ. Notably, analysis of the cytokines at convalescent phase revealed a significant elevation of the inflammatory mediators such as IL-6, IL-1β and MCP-1 with a corresponding downregulation of anti-inflammatory markers such as IL-4 and IL-10 in patients with persistent arthralgia; emphasising the crucial role of these markers in disease severity.
Similarly, among the diverse profile of circulating cytokine responses, unique cytokine responses included IL-1α, IL-1β, IL-1RA, HGF, VEGF and IL-7. It is well known that IL-1 family of ligands and receptors is primarily associated with inflammation. In this study, IL-1α and IL-1β are elevated in the convalescent phase. Interestingly, the counterpart IL-1RA, a competitive anti-inflammatory mediator, also synchronously downregulated in the convalescent phase. However, a previous report by Chaaitanya and colleagues showed elevated levels of IL-1 in acute patients.19 Further, higher production of IL-1RA was associated with protection against chronic CHIKV infection.10 With respect to growth factors, HGF and VEGF were markedly increased among the growth factors in the acute phase of our samples corroborating with previously published reports.20,21 Cellular infiltration is an important phenomenon observed in CHIKD and the increase of expression of growth factors such as VEGF can indicate such infiltration. A similar increased expression of VEGF was observed in synovial biopsies of CHIKV patients where the phenomenon was attributed to angiogenesis and cellular.22 Additionally, IL-7 was markedly elevated in convalescent phase. A similar observation was made in CHIKV patients with neurological complications.20
Chemokines are also reported to play diverse roles in CHIKD progression. Several studies have reported increased levels of chemokines such as eotaxin, MIP-1α and MIP-1β in both acute and chronic phase of CHIKD and often associated with disease severity.19,20,23 Previous studies have associated chemokines such as eotaxin with neurological complications in CHIKD.20 However, another study by Chow and colleagues reported that patients who fully recovered had high levels of eotaxin.21 In our study, we report a marked increase in the expression of eotaxin in the acute that peaked in the convalescent samples. MIP-1α and MIP-1β peaked in the convalescent phase, corroborating previously published data. Other chemokines such as IP-10, MIG and MCP-1 were elevated in the acute phase, as mentioned in a few other studies.10,23 Molecules such as MCP-1 have been reported to regulate CHIKV viral load along with IP-1024 and along with MIG, found to be associated with persistent arthralgia10 and neurological complications.20,25 Of interest in the present study was RANTES, which was found to be downregulated in acute and became further significantly downregulated in the convalescent phase. This molecule has been reported to have a dynamic expression profile in earlier studies. Several reports suggested elevation of RANTES in both serum20,25 and cerebrospinal fluid.25 Conversely, RANTES was reported to be downregulated in severe patients as compared to non-severe patients.10
Characterising viruses from clinical samples and identifying correlates to host response is a crucial—yet often ignored—aspect while using patient samples to understand disease dynamics. In this study, we addressed this caveat and evaluated the viral growth kinetics of more than 50 clinical CHIKV isolates, one of the most extensive sets of samples to be thus studied. We found that circulating viruses exhibited a diverse replication pattern, some peaking their titres sooner than others. The fast-growing isolates started displaying CPE at 24 h and lysed the Vero cells by 48 hpi; implying the viruses to be more pathogenic than the slow-growing isolates (started showing CPE at 48 hpi). Furthermore, these results also suggest fast-growing isolates to be more virulent. Examining earlier reports on CHIKV circulating in India and Thailand revealed that these viruses exhibited different patterns of infection and virulence.26,27 While another study comparing two CHIKV genotypes i.e., Asian and ECSA circulating in Malaysia also showed different phenotypic and genotypic characteristics, ECSA displayed a more epidemic potential than the Asian genotype.28 Different CHIKV strains have also presented different permissiveness in muscle cells and induced different levels of inflammatory response.29
Further exploration of viral determinants and the pathogenesis of virus isolates with different replication patterns would be beneficial but was beyond the scope of the present study. However, analysis of the immunogenic outcome associated with slow and fast-growing viral isolates in this study revealed that the levels of IFN-α, IL-1α, IL-1RA and MIP-1α were significantly elevated among the samples belonging to fast-growing isolates. Increased levels of IFN-α, and IL-1Rα have been previously correlated with higher viremia.21 In contrast, IFN-α, IL-1α, IL-1RA and MIP-1α are found to be associated with chronic infection.19
Our study is a cross-sectional investigation of a cohort of patients recruited over a period of five years in two different consortia addressing febrile illnesses. While one consortium was devoted to CHIKD, the other was addressing all acute febrile illnesses and therefore, the information regarding arthritis was not exhaustive in these recruited patients. Owing to this, we could not have the information pertaining to the CDAI scores in the acute phase in some patients and the samples from these patients were mostly used for virus characterizations. Varied aspects were being investigated this study and served to provide a bird’s eye view of the virological, serological and immunological aspects of CHIKD in the selected population and thereby provide direction for multiple new studies instead of dwelling into every aspect in greater depth. For instance, we could not explore the cellular source from which the respective cytokines were secreted. Additionally, evaluating the neutralisation capacity in acute and convalescent phase of the samples correlated with higher CDAI scores and thereby disease progression; however, more in-depth analysis is required to explain the underlying mechanism. Also, identifying those genetic variations that could contribute to the circulating viruses’ diverse growth kinetics was not within the scope of the current study. We postulate that distinct genetic determinants may play roles in deciding the pathogenicity of the clinical isolates and the interaction of these determinants with the host immune system may contribute to deciding the course of the disease.
In conclusion, the overall findings of this study have provided crucial insights into several aspects of CHIKD. The study is among the first to examine convalescence in a large number of paired samples from India. Most importantly, the immunological findings of the paired samples suggest that some immune markers may be utilized to measure disease severity. To the best of our knowledge, this is first study to report viral growth kinetics of such a large number of clinical isolates and our analysis reveal that circulating CHIKV isolates have different growth pattern that suggests collecting more detailed information about the virus which might play a role in virus pathogenicity. Overall, the immunological and virological findings provide direction to engage in more detailed follow-ups and association studies leading to better public health management prognoses.
Contributors
AJ and SS conceptualised and designed the study. NB, AnuJ, US, RS collated the clinical data, performed flow cytometry and cytokine analyses. SM, GM, and SAI performed IgM, IgG, neutralisation assays, viral kinetics experiments and data analyses. JSS, SA, MB, RPK, PPM and AJ provided reagents and supervised clinical sample collection and their processing. AJ and SS provided reagents for the experiments and supervised the study. NB and SM wrote the draft with inputs from AnuJ, SAI, GM, PSK, ShS, SSP. AJ, SS and SC refined the draft and gave final approval.
Data sharing statement
The corresponding author can provide the raw data that support the findings and study protocol on reasonable request.
Editor note
The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Declaration of interests
None. The current study was funded by Biotechnology Industry Research Assistance Council (BIRAC) (BT/NBM0101/02/18).
Acknowledgements
Authors acknowledge their sincere gratitude to the state and district Government health officials, healthcare workers and the families of the case from Tamil Nadu (Krishnagiri and Nilgiri’s), Karnataka (Shimoga and Udupi), Kerala (Wayanad), Gujarat (Sabarkantha), Tripura (Dhalai), Assam (Kamrup), Maharashtra (Mumbai and Sindhudurgh), Odisha (Bhubaneswar), Punjab (Chandigarh) and Jharkhand (Simdega) for their immense support in this study. We are thankful to all the tertiary care hospitals (KH Manipal, PGIMER Chandigarh, TNMC Mumbai and AIIMS Bhubaneswar) that participated in the study for providing their facilities, resources, and support to investigators. We would like to acknowledge the AFI surveillance study funded by CDC, Atlanta under the GHSA scheme for providing the archived CHIKV samples and clinical information for the current study.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lansea.2023.100269.
Contributor Information
Anitha Jagadesh, Email: anitha.j@manipal.edu.
Sujatha Sunil, Email: sujatha@icgeb.res.in.
Appendix A. Supplementary data
References
- 1.Manzoor K.N., Javed F., Ejaz M., et al. The global emergence of Chikungunya infection: an integrated view. Rev Med Virol. 2022;32(3) doi: 10.1002/rmv.2287. [DOI] [PubMed] [Google Scholar]
- 2.Srivastava P., Kumar A., Hasan A., et al. Disease resolution in Chikungunya—what decides the outcome? Front Immunol. 2020;11:695. doi: 10.3389/fimmu.2020.00695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jain J., Nayak K., Tanwar N., et al. Clinical, serological, and virological analysis of 572 chikungunya patients from 2010 to 2013 in India. Clin Infect Dis. 2017;65(1):133–140. doi: 10.1093/cid/cix283. [DOI] [PubMed] [Google Scholar]
- 4.Jain J., Kaur N., Haller S.L., et al. Chikungunya outbreaks in India: a prospective study comparing neutralisation and sequelae during two outbreaks in 2010 and 2016. Am J Trop Med Hyg. 2020;102(4):857. doi: 10.4269/ajtmh.19-0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nayak K., Jain V., Kaur M., et al. Antibody response patterns in chikungunya febrile phase predict protection versus progression to chronic arthritis. JCI insight. 2020;5(7) doi: 10.1172/jci.insight.130509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silva L.A., Dermody T.S. Chikungunya virus: epidemiology, replication, disease mechanisms, and prospective intervention strategies. J Clin Invest. 2017;127(3):737–749. doi: 10.1172/JCI84417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Labadie K., Larcher T., Joubert C., et al. Chikungunya disease in nonhuman primates involves long-term viral persistence in macrophages. J Clin Invest. 2010;120(3):894–906. doi: 10.1172/JCI40104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hawman D.W., Stoermer K.A., Montgomery S.A., et al. Chronic joint disease caused by persistent Chikungunya virus infection is controlled by the adaptive immune response. J Virol. 2013;87(24):13878–13888. doi: 10.1128/jvi.02666-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lum F.-M., Teo T.-H., Lee W.W., Kam Y.-W., Rénia L., Ng L.F. An essential role of antibodies in the control of Chikungunya virus infection. J Immunol. 2013;190(12):6295–6302. doi: 10.4049/jimmunol.1300304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chirathaworn C., Chansaenroj J., Poovorawan Y. Cytokines and chemokines in Chikungunya virus infection: protection or induction of pathology. Pathogens. 2020;9(6):415. doi: 10.3390/pathogens9060415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaudhary S., Jain J., Kumar R., et al. Chikungunya virus molecular evolution in India since its re-emergence in 2005. Virus Evol. 2021;7(2) doi: 10.1093/ve/veab074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Translational Research Consortia (TRC) for Chikungunya Virus in India Current status of chikungunya in India. Front Microbiol. 2021;12 doi: 10.3389/fmicb.2021.695173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shrinet J., Jain S., Sharma A., et al. Genetic characterization of Chikungunya virus from New Delhi reveal emergence of a new molecular signature in Indian isolates. Virol J. 2012;9(1):1–8. doi: 10.1186/1743-422X-9-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kam Y.W., Lum F.M., Teo T.H., et al. Early neutralizing IgG response to Chikungunya virus in infected patients targets a dominant linear epitope on the E2 glycoprotein. EMBO Mol Med. 2012;4(4):330–343. doi: 10.1002/emmm.201200213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoon I.-K., Alera M.T., Lago C.B., et al. High rate of subclinical chikungunya virus infection and association of neutralizing antibody with protection in a prospective cohort in the Philippines. PLoS Neglected Trop Dis. 2015;9(5) doi: 10.1371/journal.pntd.0003764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chua C.-L., Sam I.-C., Chiam C.-W., Chan Y.-F. The neutralizing role of IgM during early Chikungunya virus infection. PLoS One. 2017;12(2) doi: 10.1371/journal.pone.0171989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kam Y.-W., Simarmata D., Chow A., et al. Early appearance of neutralizing immunoglobulin G3 antibodies is associated with chikungunya virus clearance and long-term clinical protection. J Infect Dis. 2012;205(7):1147–1154. doi: 10.1093/infdis/jis033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaur N., Jain J., Kumar A., et al. Chikungunya outbreak in Delhi, India, 2016: report on coinfection status and comorbid conditions in patients. New Microbes New Infect. 2017;20:39–42. doi: 10.1016/j.nmni.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chaaithanya I.K., Muruganandam N., Sundaram S.G., et al. Role of proinflammatory cytokines and chemokines in chronic arthropathy in CHIKV infection. Viral Immunol. 2011;24(4):265–271. doi: 10.1089/vim.2010.0123. [DOI] [PubMed] [Google Scholar]
- 20.Almeida R.S., Ferreira M.L.B., Sonon P., et al. Cytokines and soluble HLA-G levels in the acute and recovery phases of arbovirus-infected Brazilian patients exhibiting neurological complications. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.582935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chow A., Her Z., Ong E.K., et al. Persistent arthralgia induced by Chikungunya virus infection is associated with interleukin-6 and granulocyte macrophage colony-stimulating factor. J Infect Dis. 2011;203(2):149–157. doi: 10.1093/infdis/jiq042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bedoui Y., Septembre-Malaterre A., Giry C., et al. Robust COX-2-mediated prostaglandin response may drive arthralgia and bone destruction in patients with chronic inflammation post-chikungunya. PLoS Negl Trop Dis. 2021;15(2) doi: 10.1371/journal.pntd.0009115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alves de Souza T.M., Fernandes-Santos C., Araújo da Paixão de Oliveira J., et al. Increased indoleamine 2, 3-Dioxygenase 1 (IDO-1) activity and inflammatory responses during chikungunya virus infection. Pathogens. 2022;11(4):444. doi: 10.3390/pathogens11040444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sánchez-Arcila J.C., Badolato-Correa J., de Souza T.M.A., et al. Clinical, virological, and immunological profiles of DENV, ZIKV, and/or CHIKV-infected Brazilian patients. Intervirology. 2020;63(1–6):33–45. doi: 10.1159/000510223. [DOI] [PubMed] [Google Scholar]
- 25.Kashyap R.S., Morey S., Bhullar S., et al. Determination of Toll-like receptor-induced cytokine profiles in the blood and cerebrospinal fluid of Chikungunya patients. Neuroimmunomodulation. 2014;21(6):338–346. doi: 10.1159/000358240. [DOI] [PubMed] [Google Scholar]
- 26.Kumar A., Mamidi P., Das I., et al. A novel 2006 Indian outbreak strain of Chikungunya virus exhibits different pattern of infection as compared to prototype strain. PLoS One. 2014;9(1) doi: 10.1371/journal.pone.0085714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chalaem P., Chusri S., Fernandez S., et al. Characterization of a Chikungunya virus strain isolated from banked patients’ sera. Virol J. 2016;13(1):1–12. doi: 10.1186/s12985-016-0606-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sam I.-C., Loong S.-K., Michael J.C., et al. Genotypic and phenotypic characterization of Chikungunya virus of different genotypes from Malaysia. PLoS One. 2012;7(11) doi: 10.1371/journal.pone.0050476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lohachanakul J., Phuklia W., Thannagith M., Thongsakulprasert T., Smith D.R., Ubol S. Differences in response of primary human myoblasts to infection with recent epidemic strains of Chikungunya virus isolated from patients with and without myalgia. J Med Virol. 2015;87(5):733–739. doi: 10.1002/jmv.24081. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.