Abstract
Penicillin-resistant isolates of Streptococcus pneumoniae generally contain mosaic genes encoding the low-affinity penicillin-binding proteins (PBPs) PBP2x, PBP2b, and PBP1a. We now present evidence that PBP2a and PBP1b also appear to be low-affinity variants and are encoded by distinct alleles in β-lactam-resistant transformants of S. pneumoniae obtained with chromosomal donor DNA from a Streptococcus mitis isolate. Different lineages of β-lactam-resistant pneumococcal transformants were analyzed, and transformants with low-affinity variants of all high-molecular-mass PBPs, PBP2x, -2a, -2b, -1a, and -1b, were isolated. The MICs of benzylpenicillin, oxacillin, and cefotaxime for these transformants were up to 40, 100, and 50 μg/ml, respectively, close to the MICs for the S. mitis donor strain. Recruitment of low-affinity PBPs was accompanied by a decrease in cross-linked muropeptides as revealed by high-performance liquid chromatography of muramidase-digested cell walls, but no qualitative changes in muropeptide chemistry were detected. The growth rates of all transformants were identical to that of the parental S. pneumoniae strain. The results stress the potential for the acquisition by S. pneumoniae of high-level β-lactam resistance by interspecies gene transfer.
Since the first reports of the phenomenon in the 1970s, penicillin-resistant isolates of Streptococcus pneumoniae have been reported with increasing frequency worldwide (3, 25, 43). New bacterial clones emerge via intra- and interspecies gene transfer involving the penicillin resistance determinants, the penicillin-binding proteins (PBPs) (20, 48). The gene pool which is accessible to the pneumococcus includes a variety of commensal streptococci, similar to the situation which exists for another naturally transformable bacterial group, the neisseriae (1, 32, 35, 49).
Penicillin resistance in clinical isolates of S. pneumoniae is due to altered PBPs with a reduced affinity to penicillin, i.e., a higher antibiotic concentration is required to inhibit their in vivo function. Such low-affinity PBP variants are encoded by mosaic genes in which sequence blocks are replaced by homologous genes that diverge approximately 20% from the DNA sequence of susceptible isolates (12, 31, 34). PBP2b and PBP2x constitute primary resistance determinants and confer low-level β-lactam resistance (15, 19, 29). PBP2b does not interact with expanded-spectrum cephalosporins (23) and thus is not involved in resistance to this group of β-lactams (9, 36). PBP1a is required for high-level β-lactam resistance (15, 36, 41). Although the role of these three PBPs in β-lactam resistance has been clearly established, it is not certain whether the other two high-Mr PBPs, PBP2a and -1b, also participate in this process. Some penicillin-resistant clinical isolates contain a low-affinity PBP2a (31), and PBP2a is also affected in cefotaxime-resistant laboratory mutants (27). Since the DNA sequences of these genes were not previously available, investigations on the molecular level have been lacking.
Interspecies gene transfer of a variety of antibiotic resistance markers has been documented between different streptococcal species, all of which are naturally transformable (5, 39). Identical or closely related DNA sequences of PBP genes occur in penicillin-resistant S. pneumoniae, Streptococcus mitis, Streptococcus oralis, and Streptococcus sanguis (10, 14, 37, 38, 40), and transformation of penicillin resistance from one Streptococcus sp. to another accompanied by changes in PBPs has been reported on several occasions (6, 8, 47). S. oralis and S. mitis have been implicated as potential origins for PBP2x and/or PBP2b, which act as resistance determinants in S. pneumoniae (11, 47).
In 1994, two S. mitis strains, B6 and B7, were isolated in Germany that had unusually high resistance levels to a variety of β-lactam antibiotics, i.e., the MICs of cefotaxime and benzylpenicillin was >32 μg/ml (unpublished data). The strains have identical properties and probably belong to one clonal group. A combination of MICs as high as those for the S. mitis isolates has not been reported for S. pneumoniae but would be disastrous from a therapeutic standpoint. In the study described here we have investigated whether and to what degree resistance to cefotaxime, benzylpenicillin, and oxacillin could be transferred into a penicillin-susceptible laboratory S. pneumoniae strain by successive transformations using chromosomal DNA from S. mitis B6, and we have analyzed alterations in PBPs and the murein of resistant transformants. High-level β-lactam-resistant transformants were obtained that showed successive alterations in up to five PBPs. Transfer of pbp2a and pbp1b alleles into pneumococci during selection with cefotaxime could be documented for the first time.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
S. pneumoniae R6 is a nonencapsulated derivative of the Rockefeller University strain R36A (2). S. mitis B6 was isolated from a hospital in Bochum, Germany, and will be described elsewhere (unpublished data). Streptococci were grown in liquid culture in a casein-based semisynthetic medium supplemented with 0.2% yeast extract (26). MICs of β-lactam antibiotics were tested by agar dilution on blood agar plates (3% sheep blood). Since differences between individual transformants can easily escape detection, when twofold dilutions are used, a narrow range of antibiotic concentrations was used especially for high concentrations of antibiotics. Escherichia coli INVαF′ was used for cloning (TA Cloning Kit; Invitrogen, Leek, The Netherlands) the PBP-PCR product. SOC medium (42) was used during the cloning procedure; for glycerol stocks and plasmid preparation, cells were grown in Luria broth containing 100 μg of ampicillin/ml.
Transformation experiments.
S. pneumoniae R6 was transformed essentially according to published procedures by 30 min of incubation in the presence of DNA at 30°C followed by a 2-h phenotypic expression period at 37°C (29, 50). Purification of chromosomal DNA from S. pneumoniae (29) and oral streptococci (47) has been previously described. Transformants were selected in blood agar plates at the antibiotic concentrations specified in Results. The transformants were named according to the selective antibiotics (C, cefotaxime; O, oxacillin; B, benzylpenicillin; P, piperacillin). For example, R6T-CCP is a third-step transformant obtained after three successive rounds of transformation and selection with cefotaxime (the first two steps) and piperacillin. Individual transformants are specified by number.
Amplification and sequencing of PBP genes.
PBP genes from S. mitis B6 and the S. pneumoniae R6 transformants were amplified with the indicated oligonucleotide primers as follows: pbp2x, Pn2xUP and Pn2xDOWN (to amplify the region between codon 85 and the 3′ end of the gene [31]); pbp2b, 5′-TTAAGTTAGAAATGAGAC and 5′-TTTCCTTTCTAGTTCATTGG (13); pbp1a, 5′-GAGAGCAAATTAGTCGCAAC and 5′-ACAAACATTTCATCTGGAGCTAC (33); pbp2a, 5′-GTGAACTAGAGGACTCTG and 5′-GAAATAGATTGACTATCG; pbp1b (from S. pneumoniae R6), 5′-GTGGTATAATAGATAAAGTGAGG and either 5′-CCCTTGAAGAAGAAGGTCG (for S. pneumoniae R6) or 5′-CCCTTGACATCAACACCC (for the transformant R6T5-CCC). Sequences of the pbp1b and pbp2a genes were obtained from a collaboration between Hoffmann-La Roche and Human Genome Sciences Inc., Rockville, Md. Amplification of chromosomal DNA with PCR was carried out in a biomed Thermocycler for 30 cycles of denaturation at 96°C for 30 s, annealing at 54°C for 1 min, and extension at 72°C for 1 min, followed by a 3-min delay period at 72°C after the last cycle. The reaction mixture (100 μl) contained 10 pmol of each oligonucleotide primer and 2.5 U of Taq polymerase (Perkin-Elmer, Norwalk, Conn.). DNA fragments were purified with the QIAEX II gel extraction kit (Qiagen, Hilden, Germany) according to the manufacturer’s recommendations. PCR-derived DNA fragments were cloned into the PCR II vector (Invitrogen). DNA sequences were determined for at least two clones. In cases of discrepancies between the two sequences, PCR-amplified chromosomal DNA was directly sequenced by using the ABI PRISM dye terminator cycle sequencing ready reaction kit (Perkin-Elmer). Automatic cycle sequencing was conducted with an ABI 377 sequencer.
Preparation of peptidoglycan.
In contrast to the methodology used previously where the peptide network of S. pneumoniae was analyzed after amidase digestion (17), we isolated the murein sacculi with its saccharide backbone and used cellosyl, a muramidase for enzymatic hydrolysis (kindly provided by R. Marquardt, Hoechst AG, Frankfurt, Germany). A 500-ml culture of an exponentially growing culture (80 nephelometry units) was quickly chilled in an ethanol-ice bath. Cells were collected by centrifugation, resuspended in H2O, and immediately boiled in 4% sodium dodecyl sulfate (SDS). Cell walls were washed in H2O–1 M NaCl to remove the SDS and then purified by incubation with 200 μg of pronase/ml for 16 h, which was followed by the addition of 200 μg of trypsin/ml for 16 h. The mixture was then washed three times with 1% SDS, 8 M LiCl, and 100 mM EDTA and then was washed at least twice with H2O. Teichoic acid was removed with HF (7% [vol/vol], final concentration) for 36 h at 4°C prior to digestion with cellosyl (0.3 mg/ml) for at least 24 h at 37°C. Undigested cell wall material was removed by centrifugation (30 min, 40,000 rpm, SW50.1 Ti rotor in a Beckman ultracentrifuge); at least 95% of the material remained soluble.
Separation and analysis of muropeptides.
Samples were mixed with equal volumes of borate buffer (0.5 M, pH 9) and reduced with sodium borohydride for 15 min at room temperature. Excess borohydride was destroyed by the addition of H3PO4 (20%, wt/vol) up to a pH of 4. Samples were kept at −20°C. Separation of the digested cell wall components was performed according to the procedure of Glauner (24) with some modifications. The high-performance liquid chromatography (HPLC) system consisted of a Merck L6200A pump and a L4250 UV detector, a Waters 717 autosampler, and a Merck D2500 chromato-integrator. Samples were applied to an Interchim (250 by 4.6 mm) reverse-phase column (ODS hypersil C18, 3 μM). The column was eluted at a low rate of 0.5 ml/min for 10 min with 0.05% (vol/vol) trifluoroacetic acid in water and subsequently with a 90-min linear acetonitrile gradient (0 to 20%, vol/vol) in 0.035% trifluoroacetic acid. The column temperature was maintained at 30°C, and the eluted compounds were detected by absorption at 210 nm.
Identification of the peaks.
Liquid chromatography-mass spectrometry was performed using a Waters 600 MSHPLC pump system and a Waters model PD1991 with a diode array detector system liquid chromatograph coupled to a Finnigam (San Jose, Calif.) TSQ7000 triple quadrupole mass spectrometer. Data acquisition was performed between 300 and 2,500 Da with scan times on the order of 2 to 3 s. The fact that molecules of higher mass were multiply charged allowed identification of these structures. The muropeptide structures were deduced from mass spectrometry data taking in account the previous described structures and their elution time (17, 46).
Detection of PBPs.
Cells of exponentially growing cultures were collected by centrifugation and resuspended in 10 mM Na phosphate buffer (pH 7.2)–0.1% (wt/vol) Triton X-100; in the case of S. mitis, 0.5 mg of cellosyl/ml and 0.8 mg of lysozyme/ml were added (40). PBPs were labeled with 10 μl of a cell suspension corresponding to 107 cells with 3[H]propionylampicillin for 30 min at 37°C (22); routinely, approximately 1 μCi of radioactive β-lactam was used per sample. The specific radioactivity of 3[H]propionylampicillin was not determined directly and was estimated to be almost that of the 3[H]succinimidylpropionate (90 to 100 Ci/mmol; Amersham, Buckinghamshire, England) used for its synthesis. Proteins were separated by polyacrylamide gel electrophoresis. The final acrylamide concentration of the separation gel was 7.5% with a ratio of acrylamide:bisacrylamide of 30:1.1, as described for optimal separation of PBP2x, -2a, and -2b (22). PBPs were visualized after fluorography. Alternatively, proteins were blotted onto nitrocellulose filter, and PBPs were detected after immunostaining with rabbit antisera and mouse monoclonal antibodies directed against S. pneumoniae PBP1a, -2b, or -2x as described previously (21, 30).
Nucleotide sequence accession numbers.
The PBP sequences have been deposited in the EMBL and GenBank databases under the following accession numbers: S. pneumoniae R6 pbp2a, AJ002292; S. pneumoniae R6 pbp1b, AJ002291; S. mitis pbp2b, AJ002289; S. mitis pbp2x, AJ002288; S. mitis pbp1a (fragment), AJ002290; S. mitis pbp1b (fragment), AJ002293; and S. mitis pbp2a (fragment), AJ002294.
RESULTS
Cefotaxime-resistant transformants of S. pneumoniae R6.
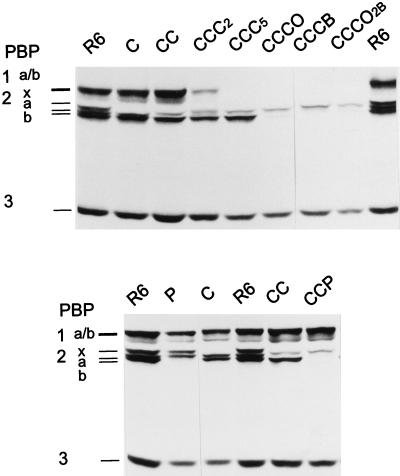
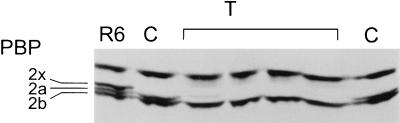
With chromosomal DNA from S. mitis B6 and the penicillin-susceptible laboratory S. pneumoniae strain R6, three successive transformations were required to obtain transformants which required cefotaxime MICs of 16 μg/ml (Table 1). PBP profiles of the transformants obtained after the first, second, and third selection steps (R6T-C, R6T-CC, and R6T-CCC, respectively) were investigated, and examples are shown in Fig. 1.
TABLE 1.
Susceptibilities to β-lactam antibiotics of S. pneumoniae transformants obtained with S. mitis B6 DNA
| Strain or transformanta | Selective antibioticb (μg/ml) | PBP changedc | MIC (μg/ml)
|
|||
|---|---|---|---|---|---|---|
| Cefotaxime | Oxacillin | Benzylpenicillin | Piperacillin | |||
| S. pneumoniae R6 | 0.02 | 0.08 | 0.02 | 0.04 | ||
| S. mitis B6 | 50 | 120 | 50 | 64 | ||
| R6T-C | C (0.2) | 2x | 0.2–0.25 | 0.2–0.5 | 0.03 | <0.2 |
| R6T3-C | C (0.2) | 2x | 0.2–0.25 | 0.2–0.5 | 0.03 | <0.2 |
| R6T-CC | C (2) | 2x, 2a | 1.5–2 | 0.5 | 0.02 | <0.2 |
| R6T2-CC | C (2) | 2x, 2a | 1.5–2 | 0.5 | 0.02 | <0.2 |
| R6T-CCC | C (6) | 2x, 2a, 1a | 12–16 | 1–2 | 0.05–0.06 | <0.2 |
| R6T5-CCC | C (6) | 2x, 2a, 1a, 1b | 12–16 | 0.5–1 | 0.05 | <0.2 |
| R6T-CCCO | O (200) | 2x, 2a, 1a, 1b, 2b | 25–30 | 60–80 | 1–3 | 2–4 |
| R6T-CCCO2b | O (20) | 2x, 2a, 1a, 1b, 2b | 25–35 | 60 | 1–2 | 2–4 |
| R6T-CCCB | B (10) | 2x, 2a, 1a, 1b, 2b | 50 | 80–100 | 40 | 16 |
| R6T-CCP | P (0.1) | 2x, 2a, 2b | 1–2 | 4–16 | 0.05–0.12 | 0.5–4 |
| R6T-P | P (0.1) | 2b | 0.02 | 0.1 | 0.02 | 0.1–0.15 |
Transformants were obtained with chromosomal DNA or in the case of R6T-CCCO2b with the cloned PBP2b gene of S. mitis B6. Between three and eight transformants were tested. Boldface indicates the transformants used as recipients in successive transformations. Thus, R6T5-CCC is a third-step transformant obtained after three successive selections with increasing concentrations of cefotaxime and is the parental strain of (i) R6T-CCCO transformants obtained via oxacillin selection with chromosomal S. mitis B6 DNA, (ii) R6T-CCCO2b (oxacillin selection by using cloned PBP2b of S. mitis B6 as donor), and (iii) R6T-CCCB (transformation with chromosomal DNA and selection with benzylpenicillin).
Antibiotics used for selection are listed in the order of the transformation steps used. The concentrations used at the last selection steps are indicated. C, cefotaxime; P, piperacillin; O, oxacillin; B, benzylpenicillin.
2x, PBP2x; 2a, PBP2a; 1a, PBP1a; 1b, PBP1b; 2b, PBP2b.
FIG. 1.
PBP profiles of β-lactam-resistant S. pneumoniae transformants. PBPs in whole-cell lysates were labeled with [3H]propionylampicillin and separated on sodium dodecyl sulfate-polyacrylamide gels followed by fluorography. PBPs of the parental S. pneumoniae R6 are indicated on the left. C, CC, and CCC, respective transformants obtained after one, two, and three successive transformations and selections with cefotaxime; other transformants were obtained after selection with P (piperacillin), O (oxacillin) or B (benzylpenicillin). All transformants were obtained with chromosomal DNA except for the transformants CCCO2B, where cloned PBP2b of B6 was used as donor DNA. CCC2 (R6T2-CCC) and CCC5 (R6T5-CCC) were isolated from the same transformation experiment.
So far, only the pbp2x and pbp1a genes, which could even be transferred simultaneously, have been documented to be sufficient for cefotaxime resistance in S. pneumoniae (9, 36). As expected, all first-level transformants contained a low-affinity PBP2x (Fig. 1 and 2). All eight second-level transformants, however, also contained a low-affinity PBP2a, whereas PBP1a could still be perfectly labeled (Fig. 1 and 2). Even after a third transformation with 4 μg of cefotaxime/ml for selection, four of the eight transformants analyzed still contained only low-affinity PBP2x and PBP2a, suggesting that either not all changes relevant for resistance of these PBP genes had been transferred before or that another non-PBP gene may contribute to resistance; the other four transformants contained low-affinity PBP1a as well (not shown). Only with 6 μg of cefotaxime/ml as the selective concentration at the third step did all eight R6T-CCC transformants analyzed have a low-affinity PBP1a; surprisingly, one of them (R6T5-CCC) contained a low-affinity PBP1b as well (Fig. 1 and Table 1). None of the cefotaxime-resistant transformants contained an apparent low-affinity PBP2b, since it is not a target for this class of cephalosporins (23).
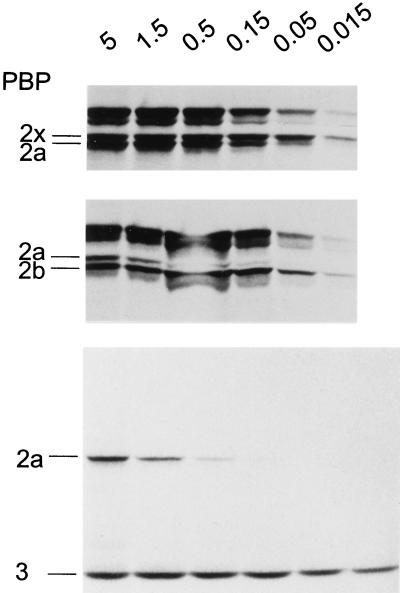
FIG. 2.
Affinities of PBPs in β-lactam-resistant transformants of S. pneumoniae R6. Cell lysates of the transformants were incubated with the relative [3H]propionylampicillin concentrations (in microcuries) indicated at the top. PBPs of the transformants are indicated to the left. (Top) R6T1-P with a low-affinity PBP2b; (middle) R6T2-CC with a low-affinity PBP2x; (bottom) R6T4-CCCB (all high-Mr PBPs are low-affinity variants and only the low-Mr PBP3 is labeled at all concentrations).
The cefotaxime resistance increased from 0.02 μg/ml for the sensitive recipient roughly 10-fold in the first-step transformants, and the cefotaxime MIC for the second-step transformants with low-affinity PBP2x and -2a was already up to 2 μg/ml, i.e., values which are generally associated with high-level penicillin-resistant clinical isolates (Table 1). MICs for the third-step transformants, 12 to 16 μg/ml, are very high values for S. pneumoniae, but they are still below those of the donor S. mitis strain. The differences between the transformants and the donor strain were still enormous for oxacillin (MIC of 0.5 to 1 versus 120 μg/ml) and benzylpenicillin (MIC of <0.06 versus 50 μg/ml).
Penicillin-resistant transformants.
Since PBP2b plays a major role in resistance to penicillin antibiotics (4, 15, 41), we tested whether selection with penicillins can be used to transfer the S. mitis pbp2b into R6. Low-affinity PBP2b variants can be selected in the S. pneumoniae R6 background with low concentrations of piperacillin but not with benzylpenicillin or oxacillin (19). Selection of S. pneumoniae R6 transformants with chromosomal S. mitis B6 DNA was possible between 0.05 and 0.1 μg of piperacillin/ml. The three transformants analyzed contained a low-affinity PBP2b (Fig. 1 and 2). Two of them contained a low-affinity PBP2x as well (not shown). The MIC of piperacillin increased from 0.04 μg/ml for S. pneumoniae R6 to 0.15 μg/ml for the transformants, whereas the oxacillin MIC increased only marginally and those of cefotaxime and benzylpenicillin did not change at all (Table 1).
After demonstrating that the gene encoding the S. mitis PBP2b could be transferred into S. pneumoniae R6, transformants were isolated containing the low-affinity PBP2b in different combinations with other low-affinity PBPs by using different cefotaxime-resistant transformants as recipients as follows: R6T2-CC, which contained low-affinity PBP2x and -2a, and R6T5-CCC, with low-affinity PBP2x, -2a, -1a, and -1b (Table 1).
With R6T2-CC as recipient, transformation with B6 DNA and selection with piperacillin resulted in R6T-CCP transformants, which contained low-affinity PBP2x, -2a, and -2b (Fig. 1). A marked increase in the piperacillin MIC became evident for all four transformants analyzed. The piperacillin MIC varied between 0.5 and 4 μg/ml, and the oxacillin MIC increased up to eightfold to 16 μg/ml (Table 1). The cefotaxime resistance remained basically unchanged. The cefotaxime, oxacillin, and piperacillin MICs for the R6T-CCP transformants were in the concentration range considered to be high resistance, and it should be noted that despite these high values, no changes in the affinity of PBP1a were apparent. In contrast, benzylpenicillin MICs were still rather low and did not exceed 0.15 μg/ml.
In order to construct transformants with affinity changes in all high-Mr PBPs, the R6T5-CCC transformant affected in PBP2x, -2a, -1a, and -1b was used as recipient. First, oxacillin was used as the selective antibiotic. Very high concentrations between 200 and 500 μg/ml were required for selection, probably due to the relative instability of the drug. The oxacillin MIC for the five transformants analyzed increased from ≤1 to 60 to 80 μg/ml. The same MIC range was obtained when the cloned pbp2b gene was used as donor DNA, demonstrating that it is indeed PBP2b alone that was responsible for the resistance increase (R6T-CCCO2b in Fig. 1 and Table 1). However, in all cases resistance to benzylpenicillin was still low compared to that of the donor strain. Therefore, this compound was used to select another set of transformants, R6T-CCCB, for which MICs of benzylpenicillin were 40 μg/ml and MICs of cefotaxime and oxacillin were also higher compared to those of the oxacillin-selected transformants.
The affinity for the radioactive β-lactam was dramatically reduced in all high-Mr PBPs of the R6T-CCCB transformants, and only PBP2a could be labeled at high antibiotic concentrations (Fig. 1 and 2). In summary, introduction of S. mitis-derived low-affinity PBP2x, -2a, -2b, -1a, and -1b resulted in a 1,000- to 4,000-fold increase in β-lactam resistance in S. pneumoniae.
PBP genes of β-lactam-resistant S. pneumoniae transformants.
The unusually high resistance levels and the fact that all five PBPs, PBP1a, -1b, -2x, -2a, and -2b, appeared as low affinity in the S. pneumoniae transformants suggested the transfer of all five PBP genes. In order to clarify this, the DNA sequences of the PBPs of the S. pneumoniae transformants containing the PBP variants were examined and compared to those of S. pneumoniae R6 and S. mitis B6. The pbp2x, pbp2b, and pbp2a genes could be amplified with primers designed according to known sequences of penicillin-sensitive S. pneumoniae. The 3′ ends of the pbp1a and pbp1b genes could not be amplified, probably because the sequences were too divergent to allow priming of the oligonucleotides.
PBP2x.
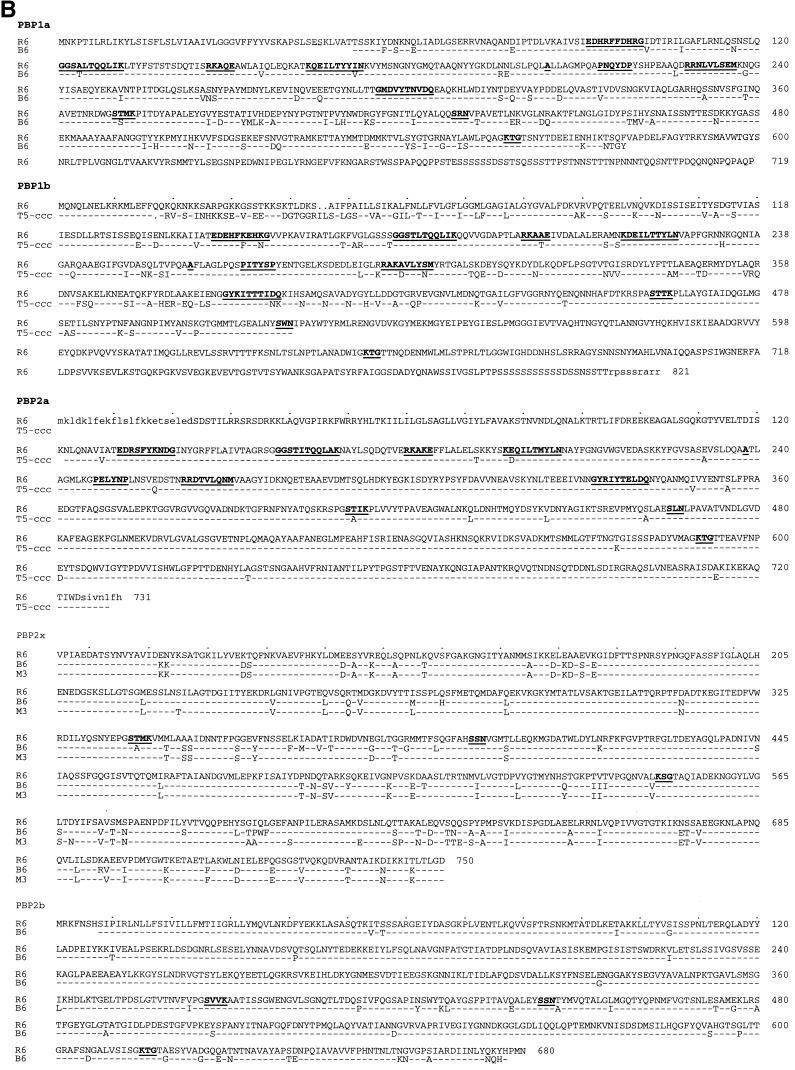
The pbp2x gene of the high-level resistance transformant R6T-CCCB was identical to the pbp2x gene of the S. mitis donor strain. It diverged from the S. pneumoniae R6 pbp2x by 19.5%. However, it was closely related to the pbp2x gene of penicillin-sensitive S. oralis M3 (2.5% divergence), which is similar to a major class of pbp2x mosaic genes of penicillin-resistant S. pneumoniae (47). Only the short region between codons 182 and 197 was almost identical to the R6 pbp2x gene; another block (codons 584 to 637) that differed by 31.5% from the M3 gene was not related to any known pbp2x gene (Fig. 3). The pbp2x gene of the first-step transformant R6T2-C contained R6 sequences between codons 182 and 278, indicating that two recombination events had occurred in this particular strain (Fig. 3A). The high-level resistance transformant R6T4-CCCB contained the entire S. mitis pbp2x gene, documenting that during another transformation step pbp2x sequences must have been introduced.
FIG. 3.
PBPs of S. mitis B6. (A) Schematic representation of the mosaic gene structure. White box indicates sequence that is closely related to the S. pneumoniae R6 gene. Black, gray, and hatched boxes mark divergent sequences with the percentages of divergence indicated below. Dashed boxes at the 5′ and 3′ ends indicate regions in the genes of the B6 strain (pbp2x, -2b, and -1a) or the R6 transformant (pbp2a and pbp1b) that were not sequenced. pbp2x and pbp2b genes were also examined in the transformant R6T-CCCB; the S. mitis sequences present in the S. pneumoniae R6 transformants R6T2-C (pbp2x) and R6T1-P (pbp2b) are indicated by black lines underneath the pbp2x and pbp2b sequences. pbp2a and pbp1b sequences were obtained from the transformant R6T5-CCC. Arrowheads indicate the positions of the three active-site motifs in each penicillin-binding domain. Amino acid residues that are discussed in the text are shown. (B) Amino acid sequences. Positions at which the S. mitis sequences differ from the R6 sequences are shown (dashes indicate identical residues). The PBP2x of the penicillin-sensitive S. oralis M3 is included for comparison. The terminal regions which were not determined in S. pneumoniae R6 are in lowercase letters. The three active-site motifs in each penicillin-binding domain are underlined and in boldface. In PBP1a, -2a, and -1b, the conserved regions in the N-terminal transglycosylase domains of the high-Mr PBPs of class A are also underlined and in boldface. T5-ccc, R6T5-CCC.
PBP2b.
The transformant R6T-CCCB also contained the pbp2b gene of S. mitis B6. It contained two sequence blocks that diverge from S. pneumoniae R6 pbp2b by 15 and 22%, respectively (Fig. 3). For this transformant it was also the case that the entire pbp2b gene was not necessarily transformed in one transformation step: the transformant R6T1-P contained only the first divergent sequence block, which included the active-site serine and the SSN (Ser-Ser-Asn) box (Fig. 3A). The gene was distinct from other known pbp2b sequences.
PBP1a.
The entire pbp1a gene could not be amplified, and only partial sequence information was obtained between codons 45 and 385 for the determination of whether it is related to any of the known pbp1a genes (Fig. 3). The B6 pbp1a gene had a novel mosaic structure: whereas the 5′ end of the gene was closely related to the corresponding region of a variety of pbp1a genes, including South African and European penicillin-resistant S. pneumoniae (34), the region that includes the penicillin-binding domain after codon 361 was distinct from known pbp1a sequences.
PBP2a.
PBP2a belongs to the class A high-Mr PBP. In order to see whether the low-affinity pbp2a is due to a point mutation or differs from the S. pneumoniae R6 gene considerably, the DNA sequence of pbp2a of the transformant R6T5-CCC was determined. The pbp2a gene differed by 6% from the R6 sequence, and the changes were scattered throughout the sequenced region. The S. mitis B6 pbp2a, which was sequenced between codons 349 and 524, was identical to that of the transformant, demonstrating that the altered pbp2a sequences must have been introduced into R6 via transformation.
PBP1b.
PBP1b is also a class A high-Mr PBP. The entire pbp1b gene of the R6T5-CCC transformant could not be amplified with the primers used for the R6 pbp1b gene, indicating that the sequence is very different. A DNA fragment covering the 5′ region of the gene was obtained, and the sequence up to codon 520 that included part of the penicillin-binding domain clearly demonstrated a high degree of divergence compared to that of the R6 strain (Fig. 3). In the N-terminal putative transglycosylase domain, one amino acid deletion (Gln17) and one insertion were found (Ile-Leu between Ser44 and Ala45). The divergence from the S. pneumoniae gene was 25% on the nucleotide level, corresponding to almost 19% amino acid substitution (Fig. 3B).
PBP2a as resistance determinant.
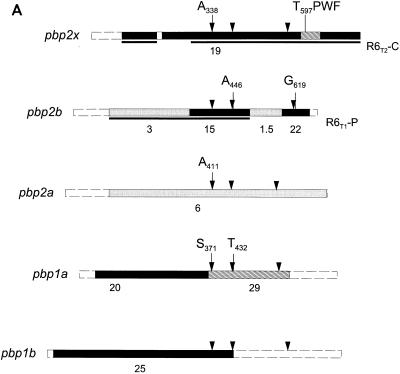
The fact that all transformants from the second selection step with cefotaxime contained a low-affinity PBP2a but no apparent changes in PBP1a indicated that PBP2a plays a role as a resistance determinant. Indeed, when the cloned pbp2a gene was used as donor DNA instead of chromosomal DNA from S. mitis B6, transformants of the R6T-C recipient strain could be selected with cefotaxime. Twenty transformants were isolated and the cefotaxime MICs for them ranged between 1.2 and 1.8 μg/ml, similar to those for the R6T-CC transformants obtained with chromosomal DNA. PBP profiles were investigated in four transformants, and all of them contained a low-affinity PBP2a (Fig. 4), demonstrating that the pbp2a gene was indeed transferred and contributed to cefotaxime resistance. Similar to the situation with pbp1a, it could not be selected in the susceptible R6 background.
FIG. 4.
PBP profiles of cefotaxime-resistant transformants. The pbp2a gene of the transformant R6T5-CCC was used to transform R6T-C to increased cefotaxime resistance. PBP profiles of three transformants (T) are compared to PBP profiles in the R6T-C recipient (C) and the R6 strain. PBP2x, -2a, and -2b of S. pneumoniae R6 are indicated on the left.
Amino acid changes in low-affinity PBPs.
The deduced amino acid sequence of S. mitis PBP2x between residues 198 and 583 is only 2.8% divergent from that of the S. oralis M3 gene, which does not confer relevant cefotaxime resistance in S. pneumoniae. In contrast, the amino acid sequence differed by 4.7% from that of the M3 PBP, i.e., 56% of the nucleotide changes resulted in an amino acid substitution. The region between codons 338 (immediately after the active-site Ser337) and 417 is particularly remarkable in that each of the nine nucleotide changes results in an amino acid alteration, strongly suggesting that this is the result of mutation and selection (Fig. 3b). There are three sites which are potentially relevant for resistance: (i) the Thr338 to Ala change directly after the active-site serine, which change is involved in β-lactam resistance (25a), (ii) the Ile366 to Met change, which is also present in PBP2x of high-level cefotaxime-resistant S. mitis and S. oralis but not in penicillin-resistant S. pneumoniae (40), and (iii) the region between Ser596 and Gly601, which is highly altered in the S. mitis B6 PBP2x and has been implicated in cefotaxime resistance in laboratory mutants (28).
The S. mitis PBP2b contained the Thr446 to Ala change, which is present in PBP2b of all resistant streptococci analyzed (11) and which is responsible for both low-level resistance and a reduced lysis rate (19). The second block of the S. mitis PBP2b which has been introduced in the higher-level transformants includes the conserved motif K615TG. There is a change from Ala619 to Gly, and similar substitutions in PBP2x Thr550 to Ala or to Gly were shown to be significant in β-lactam resistance (9, 19).
It is remarkable that in the low-affinity PBP2a, a Thr411 to Ala change after the active-site serine occurred as in the case of PBP2x, and PBP1a also contained a change at the corresponding site (Thr371 to Ser), suggesting that these changes indeed may account for altered enzymatic activities of the PBPs.
Effects of low-affinity PBPs.
The replacement of five important enzymes involved in biosynthesis of a crucial cellular component, peptidoglycan, seems to be critical for the cell, especially if the homologs have altered activities at least in terms of their enzymatic interaction with an inhibitor. Two parameters were investigated to see whether the low-affinity PBPs have any effect on the S. pneumoniae transformants. Cellular growth was monitored, and the cell wall biochemistry was analyzed in two transformants which contained different sets of low-affinity PBPs: the transformant R6T3-CCP, containing low affinity PBP2x, -2a, and -2b; and the transformant R6T5-CCC, containing low-affinity PBP2x, -2a, -1a, and -1b.
Cellular growth of the transformants.
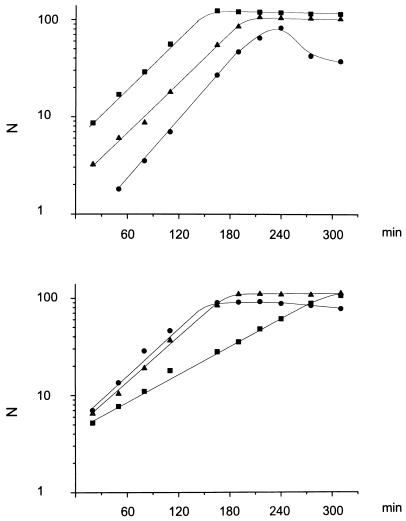
S. mitis B6 had a longer generation time (65 min) compared to S. pneumoniae R6 (36 min) (Fig. 5). All transformants, independent of the number and the combination of altered PBPs, grew with generation times of 35 to 38 min, which is identical to that of the parental R6 strain. Only in the case of the R6T1-P transformant, which contained a low-affinity PBP2b, was an enhanced lysis rate observed as soon as the transformant reached the end of the exponential growth phase. This was not seen in another transformant, R6T2-P or in higher-resistant transformants of the CCP or CCCO class with a low-affinity PBP2b (Fig. 5). This particular transformant, R6T1-P, contained only part of the pbp2b gene (Fig. 3). Lysis has been correlated with inhibition (i.e., nonfunction) of PBP2b (19), and thus the phenotype in the transformant may indicate that in this case the encoded protein is not functioning sufficiently well.
FIG. 5.
Growth of S. pneumoniae R6 transformants in liquid medium. Cells of an exponentially growing culture were diluted 1:50 in prewarmed C medium, and growth was monitored by nephelometry (N, nephelometry units). (Top) ▪, S. pneumoniae R6; •, R6T-P; ▴, R6T-CCCO. (Bottom) ▪, S. mitis B6; •, R6T-CCP; ▴, R6T5-CCC.
Composition of muropeptides in transformants.
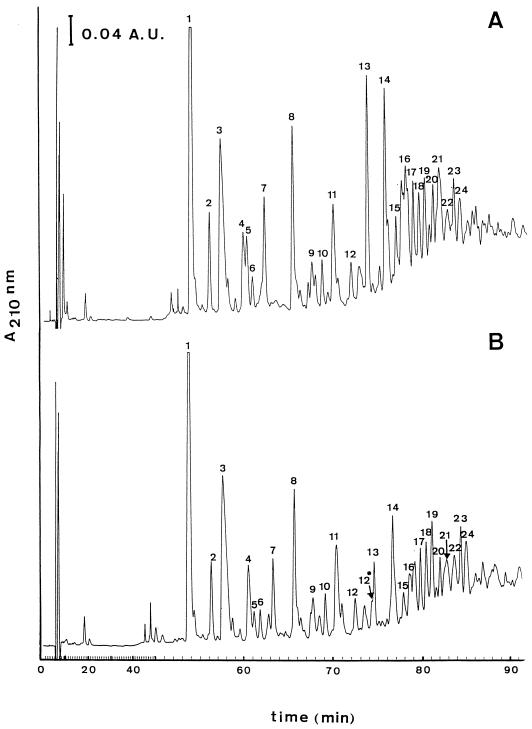
We chose the transformant R6T3-CCP, with altered PBP2x, PBP2a, and PBP2b and the R6T5-CCC transformant with altered PBP1a, -1b, -2a, and -2x for examination of the muropeptide composition. Isolated cell walls were digested with a muramidase, and muropeptides were separated by HPLC analysis. The pattern of muropeptides obtained from the transformants compared to that of the R6 strain revealed no qualitative differences (not shown). Muropeptides from R6T3-CCP were analyzed in more detail (Fig. 6). The removal of teichoic acid with HF was necessary for optimal separation of the peaks, which allowed quantitative determination and identification of the muropeptides by mass spectrometry. As before, the transformant contained no muropeptides that did not exist in the R6 strain. The proportion of disaccharide-tripeptide monomers, however, was significantly higher (63 versus 54% of the total muropeptides), but the direct cross-linked dimers were lower (8 versus 14%) (Table 2). We cannot rule out some variation in the multimeric fractions; however, they constituted only a minor portion (<3%) of the recovered material.
FIG. 6.
HPLC analysis of cellosyl-digested cell walls of S. pneumoniae R6 and the transformant R6T-CCP. HPLC chromatography of cellosyl-digested cell walls was performed as described in the text. The peaks are numbered according to their position during elution (see Table 2 for analytical data). (Top) S. pneumoniae R6; (bottom) transformant R6T3-CCP.
TABLE 2.
Molecular mass and composition of the muropeptides of S. pneumoniae R6 and transformant R6T3-CCP after separation by HPLC
| Peak no.a | Structureb |
Mr
|
Size of peak/ total size of all peaks (%)
|
||
|---|---|---|---|---|---|
| Observed | Calculated | R6 | R6T3CCP | ||
| 1 | DS-tri | 825.4 | 825.3 | 19.01 | 20.81 |
| 2 | DS-tri(OH) | 826.4 | 826.3 | 3.18 | 3.28 |
| 3 | DS-tri(−42) | 783.4 | 783.3 | 12.44 | 15.11 |
| 4 | DS-tri(OH)(−42) | 784.4 | 784.3 | 3.15 | 4.40 |
| 5 | DS-tetra | 897.2 | 896.4 | 2.67 | 1.18 |
| 6 | DS-S-tri | 912.2 | 912.4 | 0.81 | 1.03 |
| 7 | DS-penta | 967.4 | 967.3 | 4.75 | 3.52 |
| 8 | DS-A-S-tri | 983.6 | 983.5 | 7.70 | 7.12 |
| 9 | DS-penta(−42) | 925.7 | 925.5 | 0.92 | 0.60 |
| 10 | DS-A-A-tri | 967.6 | 967.5 | 1.38 | 1.63 |
| 11 | DS-A-S-tri(−42) | 941.6 | 941.2 | 5.16 | 6.21 |
| 12 | DS-A-S-tri(OH)(−42) | 942.5 | 942.2 | 1.62 | 1.73 |
| 12* | DS-A-A-tri(−42) | 925.8 | 925.5 | 0 | 1.96 |
| 13 | BisDS-tetra-tri | 1704.4 | 1703.8 | 6.65 | 2.46 |
| 14 | BisDS-tetra-A-S-tri | 1862.7 | 1862 | 6.1 | 4.13 |
| 15 | BisDS-A-S-tetra-tri | 1862.8 | 1862 | 1.66 | 1.15 |
| 16 | BisDS-tetra-tri(−42) | 1662.6 | 1662.9 | 3.05 | 3.16 |
| 17 | BisDS-A-S-tetra-A-S-tri | 2020.4 | 2020.1 | 2.58 | 2.15 |
| BisDS-A-A-tetra-A-S-tri | 2004.3 | 2004.1 | |||
| 18 | BisDS-tetra-A-S-tri(−42) | 1820 | 1820 | 2.21 | 2.72 |
| 19 | BisDS-A-S-tetra-tri(−42) | 1820.8 | 1820.1 | 2.71 | 3.46 |
| 20 | BisDS-A-A-tetra-A-S-tri(OH) | 2005.2 | 2005.1 | 1.80 | 1.26 |
| 21 | BisDS-tetra-tri(−42x2) | 1620.5 | 1619.7 | 4.54 | 2.89 |
| 22 | BisDS-tetra-A-A-tri(−42) | 1804.8 | 1804.8 | 1.62 | 2.47 |
| 23 | BisDS-A-S-tetra-A-S-tri(−42) | 1978.6 | 1978.1 | 2.31 | 2.50 |
| 24 | BisDS-tetra-A-S-tri(−42x2) | 1778.2 | 1777.9 | 1.98 | 3.07 |
Peaks are shown in Fig. 6.
DS, disaccharide (GlcNAc-MurNAc); bis, dimeric form; tri, tripeptide (l-Ala-d-Gln-l-Lys); tetra, tetrapeptide (l-Ala-d-Gln-l-Lys-d-Ala); penta, pentapeptide (l-Ala-d-Gln-l-Lys-d-Ala-d-Ala). OH indicates the presence of Glu instead of Gln. S, serine; A-A, Ala-Ala; A-S, Ala-Ser; these amino acids are branched to the ɛ amino group of the Lys residue and are also present in the interpeptide bridge. Peak 17 contained two compounds as described which could not be separated. −42, the mass that was obtained after cleavage of an acetyl radical from the GlcNAc moiety of the DS during preparation of the peptidoglycan; −42x2, two acetyl radicals were missing, one on each DS of the dimer.
DISCUSSION
We demonstrate here that all high-Mr PBPs, PBP1a, -1b, -2a, -2x, and -2b, can be changed into variants with low affinity to penicillin by successive transformation with chromosomal DNA of an S. mitis with high resistance levels to β-lactam antibiotics. Previous reports clearly demonstrated that, at least in the particular clinical isolates investigated, the combination of only pbp2x, pbp2b, and pbp1a are required for penicillin and cefotaxime resistance (9, 36). In contrast, the setting here differs in important respects: (i) the S. mitis B6 isolate used as donor is extremely resistant to a variety of β-lactams, and (ii) it contains pbp2x, pbp1a, and pbp2b genes that, although clearly homologs to the S. pneumoniae genes, have novel mosaic structures and contain sequences not described so far.
The low-affinity PBP2a appeared in all transformants analyzed prior to acquisition of a low-affinity PBP1a, suggesting it may be a prerequisite for transfer of this particular pbp1a. The pbp2a gene from the transformant encoding a low-affinity PBP2a variant could easily be transformed into a low-level resistant strain with an altered pbp2x, confirming its potential role as resistance determinant. Unlike pbp2x and pbp2b, however, pbp2a did not confer resistance in the susceptible R6 background, i.e., it is not a primary resistance determinant (19).
The role of the pbp1b gene in resistance was not analyzed since only the 5′ end (which does not cover the entire penicillin-binding domain where relevant mutations are to be expected) could be amplified from the transformant. Thus, it is not clear whether pbp1b was incidentally transferred into this particular transformant or whether it represents indeed another resistance determinant. PBP1b variants with reduced affinity have been described before in interspecies transformations to penicillin resistance (7), and a low-affinity PBP2a was noted in several penicillin-resistant clinical isolates of S. pneumoniae (31) as well as in cefotaxime-resistant transformants obtained with chromosomal DNA from S. mitis and S. oralis (40, 41), suggesting that alterations in the respective genes may be more common although not necessarily present in all penicillin-resistant isolates.
The fact that high-level oxacillin resistance in the R6T-CCCO transformants does not correlate with high-level resistance to benzylpenicillin is curious. Low-level oxacillin-resistant strains that are not penicillin resistant have been described (15), but it is not clear whether the same mechanism, changes in pbp2x, applies for the high resistance levels as well.
The generation time of the S. pneumoniae transformants was identical to that of the parental S. pneumoniae R6, independent of the combination of low-affinity PBPs present, indicating that all PBP variants from the S. mitis donor strain are functioning sufficiently well in the different genetic background. The only effect noted, an early onset of stationary-phase lysis, concerned one particular transformant, R6T1-P, with an altered PBP2b.
No qualitative changes in the biochemistry of murein, the substrate and product of PBP activity, were detected in the pneumococcal transformants containing S. mitis low-affinity PBP variants. Substantial muropeptide changes have been reported for penicillin-resistant Hungarian and South African S. pneumoniae isolates (18, 46). S. pneumoniae R6 derivatives have been obtained that also produced an altered cell wall after transfer of penicillin resistance from such isolates into the R6 background (16). Our results indicate that such changes do not automatically occur during transfer of resistance determinants from one species to another. It is possible that the different biochemistry of the peptidoglycan is an intrinsic property of the clones analyzed, and the recent report that penicillin resistance and concomitant PBP changes could be separated from the cell wall changes indicates that indeed, other non-PBP genes could be responsible for this effect (44), similar to our findings. An increase in the ratio of monomeric versus cross-linked peptides has also been observed for laboratory mutants with altered high-Mr PBPs (45), but it cannot be deduced from the analyses which one of the PBPs is responsible for this effect.
The ease with which all altered high-Mr PBPs, being after all essential enzymes, could be transferred between two species is remarkable. It is equally remarkable that all five PBPs contain almost a completely identical number of codons, and this property may contribute significantly to their ability to be exchangeable in total, or in parts, between different species. The experiments described here were done under laboratory conditions that are feasible in vivo, giving rise to a frightful perspective of future therapeutic problems in geographic areas where penicillin-resistant pneumococci are still rare.
ACKNOWLEDGMENTS
We thank R. Marquardt from Hoechst AG for providing cellosyl, Werner Fischer for his advice in teichoic acid hydrolysis, and Richard Reinhard for his help in automated DNA sequencing.
This work was supported by the BMBF (grant no. 01KI9402), an EMBO fellowship to R.H., and by INSERM (CRI 95061).
REFERENCES
- 1.Achtman M, Hakenbeck R. Recent developments regarding the evolution of pathogenic bacteria. In: Hormaeche C, Penn C W, Smyth C J, editors. Molecular biology of bacterial infection: current status and future perspectives. Cambridge, United Kingdom: Cambridge University Press; 1992. pp. 13–31. [Google Scholar]
- 2.Avery O T, MacLeod C M, McCarty M. Studies on the chemical nature of the substance inducing transformation of pneumococcal types. J Exp Med. 1944;79:137–158. doi: 10.1084/jem.79.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baquero F. Pneumococcal resistance to β-lactam antibiotics. Microb Drug Resist. 1995;1:115–120. doi: 10.1089/mdr.1995.1.115. [DOI] [PubMed] [Google Scholar]
- 4.Barcus V A, Ghanekar K, Yeo M, Coffey T J, Dowson C G. Genetics of high level penicillin resistance in clinical isolates of Streptococcus pneumoniae. FEMS Microbiol Lett. 1995;126:299–303. doi: 10.1111/j.1574-6968.1995.tb07433.x. [DOI] [PubMed] [Google Scholar]
- 5.Bracco R M, Krauss M R, Roe A S, McLeod C M. Transformation reactions between pneumococcus and three strains of streptococci. J Exp Med. 1957;106:247–259. doi: 10.1084/jem.106.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chalkley L, Schuster C, Potgieter E, Hakenbeck R. Relatedness between Streptococcus pneumoniae and viridans streptococci: transfer of penicillin resistance determinants and immunological similarities of penicillin-binding proteins. FEMS Microbiol Lett. 1991;90:35–41. doi: 10.1016/0378-1097(91)90642-n. [DOI] [PubMed] [Google Scholar]
- 7.Chalkley L J, Koornhof H J. Intra- and interspecific transformation of Streptococcus pneumoniae to penicillin resistance. J Antimicrob Chemother. 1990;26:21–28. doi: 10.1093/jac/26.1.21. [DOI] [PubMed] [Google Scholar]
- 8.Chalkley L J, Koornhof H J. Transformation of clinical isolates of Streptococcus pneumoniae to increased penicillin resistance using donor DNA from viridans streptococci. S Afr J Sci. 1991;87:314–317. [Google Scholar]
- 9.Coffey T J, Daniels M, McDougal L K, Dowson C G, Tenover F C, Spratt B G. Genetic analysis of clinical isolates of Streptococcus pneumoniae with high-level resistance to expanded-spectrum cephalosporins. Antimicrob Agents Chemother. 1995;39:1306–1313. doi: 10.1128/aac.39.6.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coffey T J, Dowson C G, Daniels M, Spratt B G. Horizontal spread of an altered penicillin-binding protein 2B gene between Streptococcus pneumoniae and Streptococcus oralis. FEMS Microbiol Lett. 1993;110:335–340. doi: 10.1111/j.1574-6968.1993.tb06345.x. [DOI] [PubMed] [Google Scholar]
- 11.Dowson C G, Coffey T J, Kell C, Whiley R A. Evolution of penicillin resistance in Streptococcus pneumoniae: the role of Streptococcus mitis in the formation of a low affinity PBP2B in S. pneumoniae. Mol Microbiol. 1993;9:635–643. doi: 10.1111/j.1365-2958.1993.tb01723.x. [DOI] [PubMed] [Google Scholar]
- 12.Dowson C G, Hutchison A, Spratt B G. Extensive re-modelling of the transpeptidase domain of penicillin-binding protein 2B of a penicillin-resistant South African isolate of Streptococcus pneumoniae. Mol Microbiol. 1989;3:95–102. doi: 10.1111/j.1365-2958.1989.tb00108.x. [DOI] [PubMed] [Google Scholar]
- 13.Dowson C G, Hutchison A, Spratt B G. Nucleotide sequence of the penicillin-binding protein 2B of Streptococcus pneumoniae. Nucleic Acids Res. 1989;17:7518. doi: 10.1093/nar/17.18.7518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dowson C G, Hutchison A, Woodford N, Johnson A P, George R C, Spratt B G. Penicillin-resistant viridans streptococci have obtained altered penicillin-binding protein genes from penicillin-resistant strains of Streptococcus pneumoniae. Proc Natl Acad Sci USA. 1990;87:5858–5862. doi: 10.1073/pnas.87.15.5858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dowson C G, Johnson A P, Cercenado E, George R C. Genetics of oxacillin resistance in clinical isolates of Streptococcus pneumoniae that are oxacillin resistant and penicillin susceptible. Antimicrob Agents Chemother. 1994;38:49–53. doi: 10.1128/aac.38.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia-Bustos J F, Chait B, Tomasz A. Altered peptidoglycan structure in a pneumococcal transformant resistant to penicillin. J Bacteriol. 1988;170:2143–2147. doi: 10.1128/jb.170.5.2143-2147.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia-Bustos J F, Chait B T, Tomasz A. Structure of the peptide network of pneumococcal peptidoglycan. J Biol Chem. 1987;262:15400–15405. [PubMed] [Google Scholar]
- 18.Garcia-Bustos J, Tomasz A. A biological price of antibiotic resistance: major changes in the peptidoglycan structure of penicillin-resistant pneumococci. Proc Natl Acad Sci USA. 1990;87:5415–5419. doi: 10.1073/pnas.87.14.5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grebe T, Hakenbeck R. Penicillin-binding proteins 2b and 2x of Streptococcus pneumoniae are primary resistance determinants for different classes of β-lactam antibiotics. Antimicrob Agents Chemother. 1996;40:829–834. doi: 10.1128/aac.40.4.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hakenbeck R. Target mediated resistance to β-lactam antibiotics. Biochem Pharmacol. 1995;50:1121–1127. doi: 10.1016/0006-2952(95)00158-v. [DOI] [PubMed] [Google Scholar]
- 21.Hakenbeck R, Briese T, Chalkley L, Ellerbrok H, Kalliokoski R, Latorre C, Leinonen M, Martin C. Variability of penicillin-binding proteins from penicillin-sensitive Streptococcus pneumoniae. J Infect Dis. 1991;164:307–312. doi: 10.1093/infdis/164.2.307. [DOI] [PubMed] [Google Scholar]
- 22.Hakenbeck R, Briese T, Ellerbrok H, Laible G, Martin C, Metelmann C, Schier H-M, Tornette S. Targets of β-lactams in Streptococcus pneumoniae. In: Actor P, Daneo-Moore L, Higgins M L, Salton M R J, Shockman G D, editors. Antibiotic inhibition of bacterial cell surface assembly and function. Washington, D.C: American Society for Microbiology; 1988. pp. 390–399. [Google Scholar]
- 23.Hakenbeck R, Tornette S, Adkinson N F. Interaction of non-lytic β-lactams with penicillin-binding proteins in Streptococcus pneumoniae. J Gen Microbiol. 1987;133:755–760. doi: 10.1099/00221287-133-3-755. [DOI] [PubMed] [Google Scholar]
- 24.Halter R, Pohlner J, Meyer T F. Mosaic-like organization of IgA protease genes in Neisseria gonorrhoeae generated by horizontal genetic exchange in vivo. EMBO J. 1989;8:2737–2744. doi: 10.1002/j.1460-2075.1989.tb08415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klugman K P, Koornhof H J. Worldwide increase in pneumococcal antibiotic resistance. Lancet. 1989;ii:444. doi: 10.1016/s0140-6736(89)90617-x. [DOI] [PubMed] [Google Scholar]
- 25a.Krauß, J., and R. Hakenbeck. Unpublished data.
- 26.Lacks S A, Hotchkiss R D. A study of the genetic material determining an enzyme activity in pneumococcus. Biochim Biophys Acta. 1960;39:508–517. doi: 10.1016/0006-3002(60)90205-5. [DOI] [PubMed] [Google Scholar]
- 27.Laible G, Hakenbeck R. Penicillin-binding proteins in β-lactam-resistant laboratory mutants of Streptococcus pneumoniae. Mol Microbiol. 1987;1:355–363. doi: 10.1111/j.1365-2958.1987.tb01942.x. [DOI] [PubMed] [Google Scholar]
- 28.Laible G, Hakenbeck R. Five independent combinations of mutations can result in low-affinity penicillin-binding protein 2x of Streptococcus pneumoniae. J Bacteriol. 1991;173:6986–6990. doi: 10.1128/jb.173.21.6986-6990.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laible G, Hakenbeck R, Sicard M A, Joris B, Ghuysen J-M. Nucleotide sequences of the pbpX genes encoding the penicillin-binding protein 2x from Streptococcus pneumoniae R6 and a cefotaxime-resistant mutant, C506. Mol Microbiol. 1989;3:1337–1348. doi: 10.1111/j.1365-2958.1989.tb00115.x. [DOI] [PubMed] [Google Scholar]
- 30.Laible G, Keck W, Mottl H, Frère J-M, Jamin M, Hakenbeck R. Penicillin-binding protein 2x of Streptococcus pneumoniae expression in Escherichia coli and purification of a soluble enzymatically active derivative. Eur J Biochem. 1992;207:943–949. doi: 10.1111/j.1432-1033.1992.tb17128.x. [DOI] [PubMed] [Google Scholar]
- 31.Laible G, Spratt B G, Hakenbeck R. Inter-species recombinational events during the evolution of altered PBP 2x genes in penicillin-resistant clinical isolates of Streptococcus pneumoniae. Mol Microbiol. 1991;5:1993–2002. doi: 10.1111/j.1365-2958.1991.tb00821.x. [DOI] [PubMed] [Google Scholar]
- 32.Maiden M C J, Malorny B, Achtman M. A global gene pool in the neisseriae. Mol Microbiol. 1996;21:1297–1298. doi: 10.1046/j.1365-2958.1996.981457.x. [DOI] [PubMed] [Google Scholar]
- 33.Martin C, Briese T, Hakenbeck R. Nucleotide sequences of genes encoding penicillin-binding proteins from Streptococcus pneumoniae and Streptococcus oralis with high homology to Escherichia coli penicillin-binding protein 1A and 1B. J Bacteriol. 1992;174:4517–4523. doi: 10.1128/jb.174.13.4517-4523.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin C, Sibold C, Hakenbeck R. Relatedness of penicillin-binding protein 1a genes from different clones of penicillin-resistant Streptococcus pneumoniae isolated in South Africa and Spain. EMBO J. 1992;11:3831–3836. doi: 10.1002/j.1460-2075.1992.tb05475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maynard Smith J, Dowson C G, Spratt B G. Localized sex in bacteria. Nature. 1991;349:29–31. doi: 10.1038/349029a0. [DOI] [PubMed] [Google Scholar]
- 36.Muñóz R, Dowson C G, Daniels M, Coffey T J, Martin C, Hakenbeck R, Spratt B G. Genetics of resistance to third-generation cephalosporins in clinical isolates of Streptococcus pneumoniae. Mol Microbiol. 1992;6:2461–2465. doi: 10.1111/j.1365-2958.1992.tb01422.x. [DOI] [PubMed] [Google Scholar]
- 37.Potgieter E, Chalkley L J. Relatedness among penicillin-binding protein 2b genes of Streptococcus mitis, Streptococcus oralis, and Streptococcus pneumoniae. Microb Drug Resist. 1995;1:35–42. doi: 10.1089/mdr.1995.1.35. [DOI] [PubMed] [Google Scholar]
- 38.Potgieter E, Koornhof H J, Chalkley L J. Penicillin-binding proteins in Streptococcus mitis. Curr Microbiol. 1992;24:289–294. [Google Scholar]
- 39.Ravin A W, De Sa J D H. Genetic linkage of mutational sites affecting similar characters in pneumococcus and streptococcus. J Bacteriol. 1964;87:86–96. doi: 10.1128/jb.87.1.86-96.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reichmann P, König A, Liñares J, Alcaide F, Tenover F C, McDougal L, Swidsinski S, Hakenbeck R. A global gene pool for high-level cephalosporin resistance in commensal Streptococcus spp. and Streptococcus pneumoniae. J Infect Dis. 1997;176:1001–1012. doi: 10.1086/516532. [DOI] [PubMed] [Google Scholar]
- 41.Reichmann P, König A, Marton A, Hakenbeck R. Penicillin-binding proteins as resistance determinants in clinical isolates of Streptococcus pneumoniae. Microb Drug Resist. 1996;2:177–181. doi: 10.1089/mdr.1996.2.177. [DOI] [PubMed] [Google Scholar]
- 42.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 43.Schutze G E, Kaplan S L, Jacobs R F. Resistant pneumococcus: a worldwide problem. Infection. 1994;22:233–237. doi: 10.1007/BF01739904. [DOI] [PubMed] [Google Scholar]
- 44.Severin A, Figueiredo A M S, Tomasz A. Separation of abnormal cell wall composition from penicillin resistance through genetic transformation of Streptococcus pneumoniae. J Bacteriol. 1996;178:1788–1792. doi: 10.1128/jb.178.7.1788-1792.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Severin A, Pato M V V, Figueiredo A M S, Tomasz A. Drastic changes in the peptidoglycan composition of penicillin resistant laboratory mutants of Streptococcus pneumoniae. FEMS Microbiol Lett. 1995;130:31–35. doi: 10.1111/j.1574-6968.1995.tb07694.x. [DOI] [PubMed] [Google Scholar]
- 46.Severin A, Tomasz A. Naturally occurring peptidoglycan variants of Streptococcus pneumoniae. J Bacteriol. 1996;178:168–174. doi: 10.1128/jb.178.1.168-174.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sibold C, Henrichsen J, König A, Martin C, Chalkley L, Hakenbeck R. Mosaic pbpX genes of major clones of penicillin-resistant Streptococcus pneumoniae have evolved from pbpX genes of a penicillin-sensitive Streptococcus oralis. Mol Microbiol. 1994;12:1013–1023. doi: 10.1111/j.1365-2958.1994.tb01089.x. [DOI] [PubMed] [Google Scholar]
- 48.Spratt B G. Resistance to β-lactam antibiotics. In: Ghuysen J-M, Hakenbeck R, editors. Bacterial cell wall. Amsterdam, The Netherlands: Elsevier Science B. V.; 1994. pp. 517–534. [Google Scholar]
- 49.Spratt B G, Bowler L D, Zhang Q-Y, Zhou J, Maynard Smith J. Role of interspecies transfer of chromosomal genes in the evolution of penicillin resistance in pathogenic and commensal Neisseria species. J Mol Evol. 1992;34:115–125. doi: 10.1007/BF00182388. [DOI] [PubMed] [Google Scholar]
- 50.Tiraby J-G, Fox M S. Marker discrimination and mutagen-induced alterations in pneumococcal transformation. Genetics. 1974;77:449–458. doi: 10.1093/genetics/77.3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]