Abstract
Mechanisms underlying interlimb transfer of adaptation to visuomotor rotations have recently been explored in depth. However, little data are available regarding interlimb transfer of adaptation to novel inertial dynamics. The present study thus investigated interlimb transfer of dynamics by examining the effect of initial training with one arm on subsequent performance with the other in adaptation to a 1.5-kg mass attached eccentrically to the forearm. Using inverse dynamic analysis, we examined the changes in torque strategies associated with adaptation to the extra mass, and with interlimb transfer of that adaptation. Following initial training with the dominant arm, nondominant arm performance improved substantially in terms of linearity and initial direction control as compared with naïve performance. However, initial training with the nondominant arm had no effect on subsequent performance with the dominant arm. Inverse dynamic analysis revealed that improvements in kinematics were implemented by increasing flexor muscle torques at the elbow to counter load-induced increases in extensor interaction torques as well as increasing flexor muscle torques at the shoulder to counter the extensor actions of elbow muscle torque. Following opposite arm adaptation, the nondominant arm adopted this dynamic strategy early in adaptation. These findings suggest that dominant arm adaptation to novel inertial dynamics leads to information that can be accessed and utilized by the opposite arm controller, but not vice versa. When compared with our previous findings on interlimb transfer of visuomotor rotations, our current findings suggest that adaptations to visuomotor and dynamic transformations are mediated by distinct neural mechanisms.
INTRODUCTION
Prior experience in one task often leads to improved performance in another. The process underlying such an improvement is referred to as generalization. Numerous studies have employed a variety of motor tasks (e.g., prism learning, mirror drawing, inverted/reversed writing, ball catching, adaptation to a rotation of visual display, adaptation to viscous force fields or Coriolis forces) to study this phenomenon and showed that generalization can occur not only within the same limb but also between different limbs (Cohen 1973; Criscimagna-Hemminger et al. 2003; Dizio and Lackner 1995; Elliott and Roy 1981; Laszlo et al. 1970; Marzi et al. 1991; Morton et al. 2001; Parlow and Kinsbourne 1989; Sainburg and Wang 2002; Taylor and Heilman 1980; Thut et al. 1996; Wang and Sainburg 2003). With regard to the direction of interlimb transfer, it seems to depend on certain factors, such as the sequence of the arms in learning the task (i.e., nondominant arm first vs. dominant arm first) and movement parameters being examined (e.g., direction error vs. endpoint error). For example, a recent study from our laboratory has shown that when subjects adapted to a 30° rotation of visual display during center-out reaching tasks, initial training with the nondominant arm improved subsequent performance with the dominant arm only in terms of trajectory direction, whereas initial training with the dominant arm improved subsequent performance with the nondominant arm only in terms of final position accuracy (Sainburg and Wang 2002). In addition to these factors, the nature of the transformations underlying the process of adaptation also seems to influence the direction of interlimb transfer. Criscimagna-Hemminger and colleagues (2003) demonstrated that in adaptation to viscous force fields, subjects showed a transfer of trajectory straightness only from the dominant to nondominant arm. These findings suggest that the nature of transformations underlying the process of adaptation is also important in determining the pattern of interlimb transfer.
A controversy exists as to whether these different types of adaptations are learned independently or not (Flanaganet al. 1999; Krakauer et al. 1999; Tong et al. 2002). Krakauer and colleagues (1999) demonstrated that learning of two novel transformations, a rotated visual display and altered intersegmental dynamics, did not interfere with each other and that they consolidated in parallel. This finding implies that these two types of adaptations involve two distinct neural processes. Flanagan and colleagues (1999) reported a similar finding, indicating lack of anterograde interference between a visuomotor rotation and a velocity-dependent force field. In contrast, Tong and colleagues (2002) showed that adaptation to a position-dependent force field interfered with adaptation to a visuomotor rotation, which was also position dependent. Based on this finding, they argued that the key determinant of interference is the kinematic variable on which the transformation depends. These authors concluded that adaptations to novel visuomotor rotations and to novel limb dynamics are not independently represented in the brain. Collectively, these findings call into question the independence of mechanisms underlying visuomotor and dynamic adaptations.
In the current study, we examined the pattern of interlimb transfer of learning after adaptation to novel inertial dynamics during reaching movements. Specifically, we examined the effect of initial training with one arm on subsequent performance with the other arm, in adaptation to a 1.5-kg mass attached eccentrically to the forearm. This manipulation substantially alters the center of mass of the forearm system, specifically manipulating the mechanical interactions between the segments (Krakauer et al. 1999; Sainburg 2002; Sainburg et al. 1999). Using inverse dynamic analysis, we examined the specific torque strategies associated with adaptation to the altered inertial condition as well as how initial training with one arm affected those strategies in the other arm during subsequent performance. Because we have previously tested the effect of opposite arm adaptation on learning a visual rotation with the other arm (Sainburg and Wang 2002), we are now able to compare the patterns of interlimb transfer of novel dynamic transformations with those of novel visuomotor transformations, in similar tasks. Our findings from the present study provide further insight into understanding the mechanisms that underlie interlimb transfer of motor learning as well as the neural processes that are involved in visuomotor and dynamic adaptations.
METHODS
Subjects
Subjects were 14 neurologically intact right-handed adults (7 female, 7 male), aged from 18 to 30 yr old. Subjects were recruited from the university community and were paid for their participation. Informed consent was solicited prior to participation. Right handedness was assessed using the 10-item version of the Edinburgh inventory (Oldfield 1971).
Apparatus
Subjects sat facing a table with either the right or left arm supported over a horizontal surface, positioned just below shoulder height, by a friction-less air jet system (Fig. 1A). A start circle, target, and cursor representing the index finger position were projected on a horizontal back-projection screen positioned above the arm (Fig. 1B). A mirror, positioned parallel and below this screen, reflected the visual display, so as to give the illusion that the display was in the same horizontal plane as the fingertip. Calibration of the display ensured that this projection was veridical. Position and orientation of each limb segment was sampled at 103 Hz using the Flock of Birds (Ascension-Technology) magnetic 6-df movement recording system. The position of the following three bony landmarks was digitized: index finger tip, the lateral epicondyle of the humerus, and the acromion, directly posterior to the acromio-clavicular joint. As sensor data were received from the Flock of Birds, the position of these landmarks was computed by our custom software. For more detailed information, see Sainburg and Wang (2002).
FIG. 1.
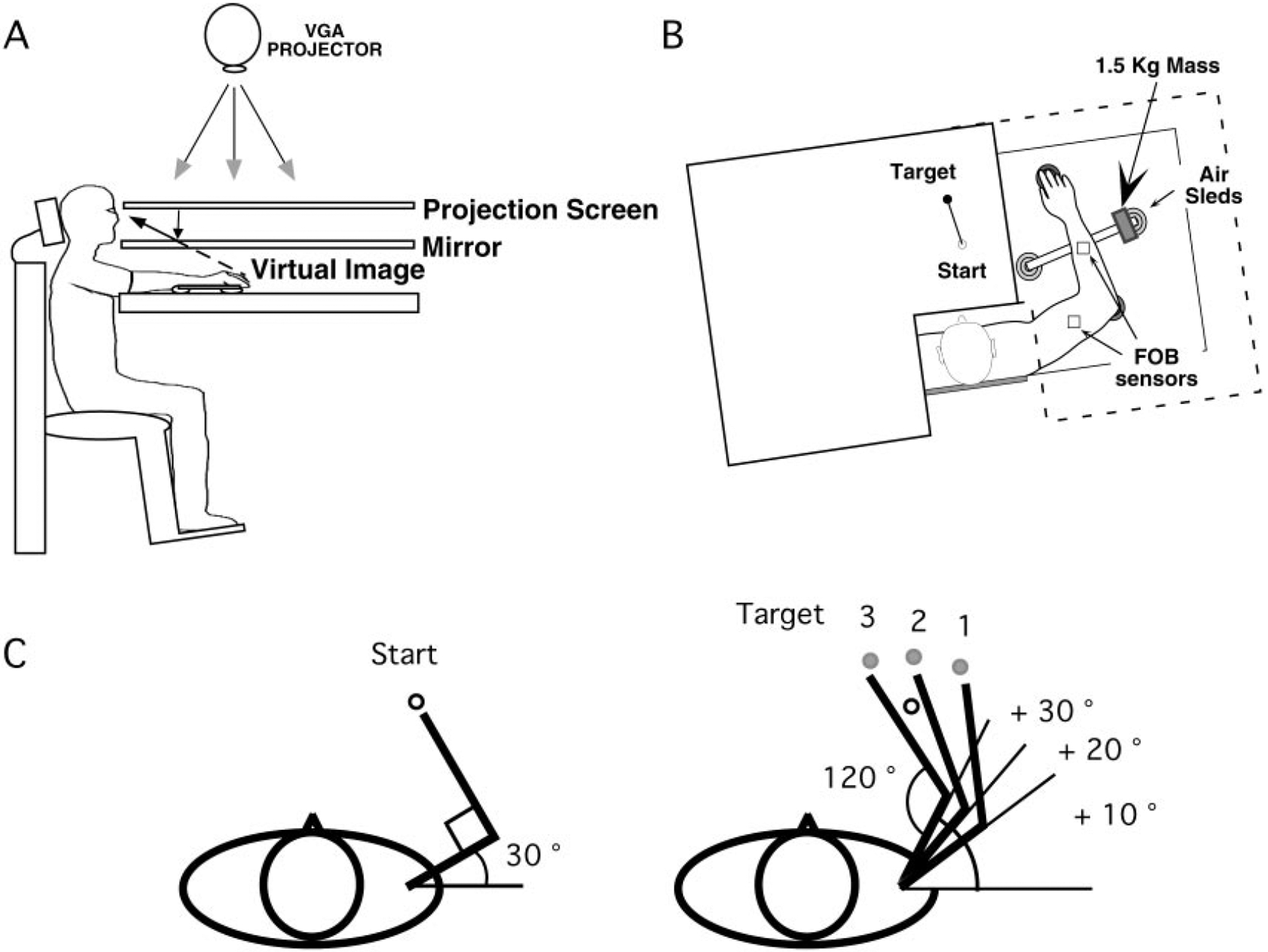
A: side view: subjects were seated in a dentist-type chair with the arm supported by an air jet system that removed the effects of friction on arm movement. Targets and the cursor representing finger position were back-projected on a screen placed above the arm. A mirror placed below this screen reflected the image, such that the projection was perceived in the plane of the arm. B: top view: the positions of the Flock of Birds sensors are shown. For the mass condition, a 1.5-kg mass was attached at the end of the arm support. C: target was randomly displayed on one of the 3 target locations. Shoulder angle varied systematically across targets while the elbow angle remained constant
Experimental design
Prior to movement, one of three targets (2 cm diam), presented in a pseudorandom sequence, was displayed on the horizontal tabletop. As illustrated in Fig. 1C, the position of the starting circle (1.5 cm diam) and the three targets was adjusted for each subject differently, such that the shoulder and elbow angles were identical across all subjects (starting circle: 30 and 90°; target 1: 40 and 120°; target 2: 50 and 120°; target 3: 60 and 120°, respectively). These angles were chosen to systematically vary the degree of shoulder excursion across different targets. A straight line was presented between the starting circle and the target. Subjects were instructed to move as straight as possible from the starting circle to the target using a single, rapid motion in response to an auditory “go” signal. No feedback was provided during the movement. At the end of each trial, knowledge of results was provided in the form of a hand-path between the starting circle and the target and by points awarded for accuracy (i.e., the distance between the target and the cursor representing the finger position): 1 point for accuracy <3 cm, 3 points for accuracy <2 cm, and 10 points for accuracy <1 cm. No points were given for movements that took longer than 600 ms.
The experiment consisted of two sessions: baseline (no mass) and exposure (mass) sessions. Subjects performed two blocks of trials in each session with each block performed with either the dominant or nondominant arm. During the exposure session, a 1.5-kg mass was placed eccentric to the long axis of the forearm as illustrated in Fig. 1B. This manipulation altered the center of mass of the forearm/load system, which substantially changed the inertia of the limb system, thereby altering the mechanical interactions between the segments (Krakauer et al. 1999; Sainburg 2002; Sainburg et al. 1999). Each block comprised 180 trials, divided into 60 cycles with each cycle containing all three of the targets. For statistical analysis, the 60 cycles of movement trials were reorganized into 20 epochs with each epoch representing the mean of three consecutive cycles. Each block of trials was separated by a 10-min break. Half the subjects performed the task with the nondominant arm first (LR), while the other half performed with the dominant arm first (RL). Table 1 shows the sequence of the experimental blocks for each group.
TABLE 1.
Experimental design
| Group | Baseline (No Mass) | Exposure (1.5-kg Mass) | ||
|---|---|---|---|---|
| LR (n = 7) | L | R | L | R |
| RL (n = 7) | R | L | R | L |
Number of trials for all conditions was 180. LR and RL, left arm first and right arm first, respectively.
Kinematic data analysis
Three measures of performance were calculated: linearity error, hand-path direction error at peak tangential arm acceleration (Amax), and final position error. Linearity error was calculated as the minor axis (the largest distance, perpendicular to the major axis, between any 2 points in the path) divided by the major axis (the largest distance between any 2 points in the path) of the hand-path. Direction error was calculated as the angular difference between the vectors defined by the target and by the hand-path position at movement start and at Amax (absolute values were used to calculate the means for each cycle/epoch). Final position error was calculated as the two-dimensional distance between the index finger at movement termination and the center of the target.
Inverse dynamic analysis
In this study, we performed inverse dynamic analysis to provide a measure of adaptation that quantifies the contributions of muscle torque to movements. This analysis was employed for three main reasons: to differentiate the passive effects of the inertial load from the active response of the neuromuscular system, to specifically detail the active compensatory mechanisms, as joint torques, which compensate the load condition, and to assess whether interlimb transfer involves adoption of joint torque patterns that reflect opposite arm adaptation. This allowed us to specifically assess the coordinate patterns associated with both adaptation and transfer.
For the purpose of this study, we assumed that the upper extremity was two interconnected rigid links (upper arm and forearm) with frictionless joints at the shoulder and elbow. The shoulder was allowed to move freely, and the torques resulting from linear accelerations of the shoulder were included in the equations of motion for each joint. To separately analyze the effects of intersegmental forces and muscle forces on the limb motion, we partitioned the terms of equations of motion at each joint into three main components: interaction torque, muscle torque, and net torque (Bagesteiro and Sainburg 2002; Sainburg et al. 1995, 1999). Interaction torque represents the rotational effect of the forces on a segment due to the rotational and linear motion of the other segment. Muscle torque represents the rotational effect of muscle forces acting on the segment. Net torque represents the inertial resistance of the segment to joint acceleration and is equal to the combined muscle and interaction torques.
These torque components were computed and analyzed for the shoulder and elbow joints [see Bagesteiro and Sainburg (2002) for the equations used for computing inverse dynamics]. The inertia and mass of the forearm support were 0.0247 kg/m2 and 0.58 kg, respectively. Limb segment inertia, mass, and center of mass were computed from regression equations using subjects’ body mass and measured limb segment lengths (Winter 1990). Shoulder and elbow flexor muscle torque profiles were also integrated between two zero crossings around peak flexor muscle torque (1 immediately prior to, and the other immediately following the peak) to obtain measures of shoulder and elbow flexor muscle torque impulses.
Statistics
A repeated-measures ANOVA was conducted with group (RL, LR) as a between-group factor and target direction (3 directions) as a within-group factor, for each arm separately. This was done for the two arms separately because the comparison between the arms was not of interest in this study (for this comparison, see Sainburg 2002). Because the purpose of this study was to examine the effect of initial training with one arm on subsequent performance with the other arm, post hoc pair-wise comparisons using Bonferroni/Dunn analysis were made between naive performance and the performance following opposite arm adaptation for the dominant arm blocks (right arm performances by LR and RL groups) and also for the nondominant arm blocks (left arm performances by LR and RL groups). This effect of opposite arm adaptation was assessed for the first epoch and for the last epoch from the exposure session, to examine initial information transfer and the extent of final adaptation, respectively. Between these two epochs, the pair-wise comparisons at the first epoch were of primary interest, because previous studies have shown substantial effects of initial training on the very first trials of the subsequent testing session (Krakauer et al. 1999; Sainburg and Wang 2002; Tong et al. 2002; Wigmore et al. 2002). The criterion for rejecting the null hypotheses (i.e., alpha level) was always 0.05 for both repeated measures ANOVA’s and post hoc tests.
RESULTS
Hand-paths
On initial exposure to the eccentric mass, subjects’ hand paths showed systematic errors in direction and linearity that varied with movement direction. Figure 2 shows typical handpaths of representative subjects during the initial three trials of the exposure session. Naive performance (dashed lines) and that following opposite arm adaptation (solid lines) are illustrated for each target separately. For both arms and conditions, movements toward target 1 are fairly straight and accurate, whereas those toward targets 2 and 3 show initial direction deviations that become progressively more lateral to the target, and movements toward target 3 are substantially curved. This progressive decrease in movement accuracy is consistent with the effect of the mass, which increased with required shoulder motion (see Sainburg et al. 1999).
FIG. 2.
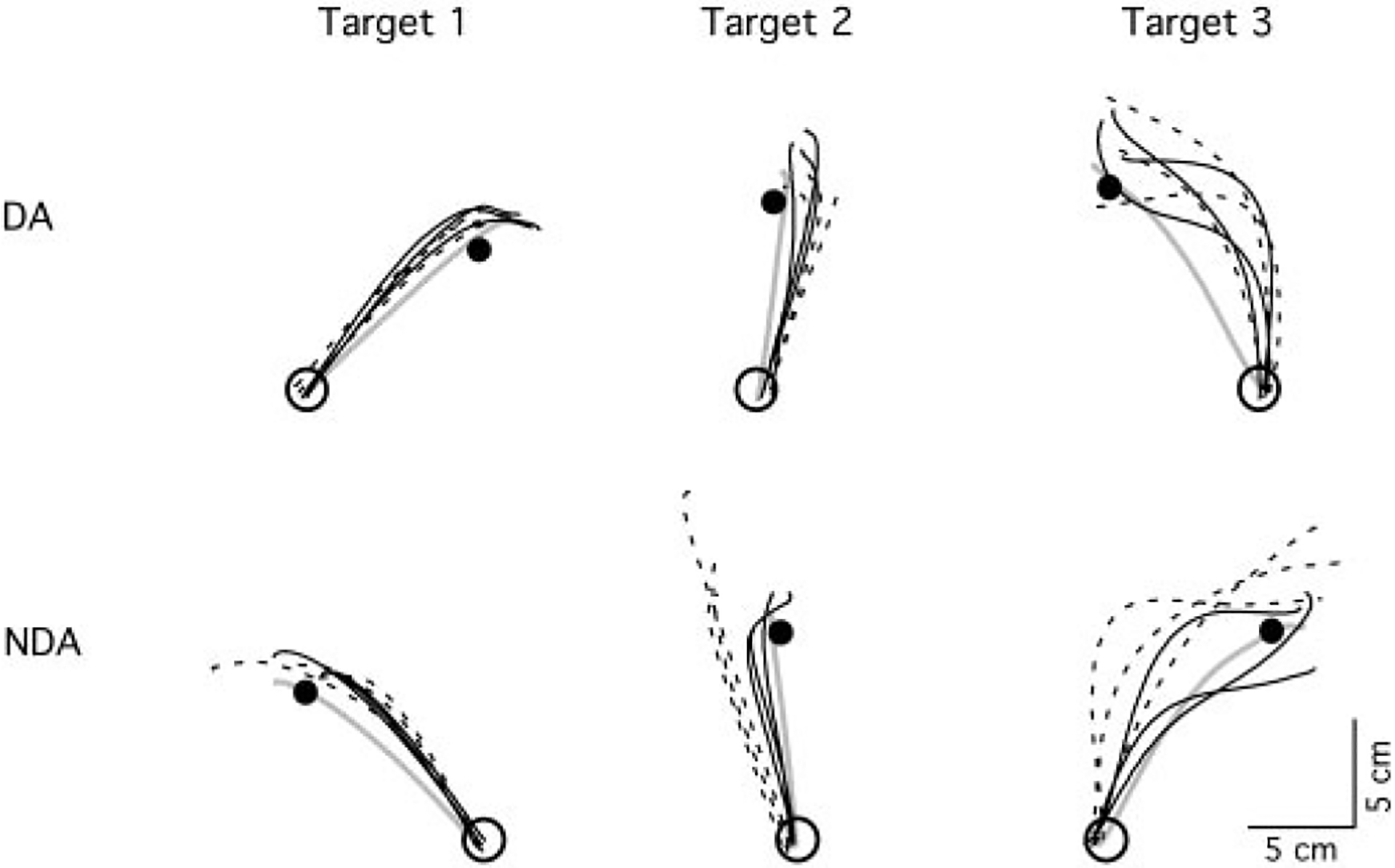
Hand-paths of representative subjects. Top: dominant hand paths; bottom: nondominant hand paths. Each column shows hand-paths of the first three trials of naive performance (broken lines) and performance following opposite arm adaptation (black solid lines) for each target direction. Hand-path after adaptation to the inertial load is represented by a gray solid line
For the dominant arm, there is no apparent effect of opposite arm adaptation on performance as reflected by the overlap of naïve trials with those following opposite arm adaptation. However, for the nondominant arm, opposite arm training had a substantial effect on subsequent performance with the nondominant arm for both targets 2 and 3 (row 2). This is reflected by the substantially more accurate initial direction of trials to targets 2 and 3, and the straighter paths of trials to target 3. For the trials toward target 3, the first trial following opposite arm exposure is similar to that of naïve performance, whereas the very next trial is quite similar in accuracy to that following final adaptation (solid gray line). We have previously shown, for adaptation to visuomotor rotations, that interlimb transfer often occurs immediately following the first trial of movement (Wang and Sainburg 2003).
Performance measures for the dominant arm
The results described in the preceding text were consistent across all subjects, as reflected by the data shown in Fig. 3, which shows the mean values (±SE) of linearity errors, direction errors and final position errors, averaged across every three consecutive trials of movements made toward each target (a single epoch) during the exposure session. These values are illustrated for naïve performance (○) and that following nondominant arm adaptation (●). As described in the preceding text, movement errors for the first epoch of each exposure session (leftward gray column for each target) increased with target, such that epoch one of target 3 showed substantially greater errors than that of target 1. Accordingly, a repeated-measures ANOVA showed a main effect of target direction for linearity and direction errors (P < 0.05). Whereas the effect of the mass on movements toward targets 1 and 2 were relatively small, movements toward target 3 showed substantial reduction in errors between epochs 1 and 20 (gray bars), reflecting adaptation. However, performance following opposite arm exposure did not show substantial differences from naïve performance as indicated by lack of the group main effect and of an interaction between group and target. Thus while the dominant arm showed substantial adaptation to the mass during both exposure sessions, no effect of opposite arm adaptation was apparent.
FIG. 3.
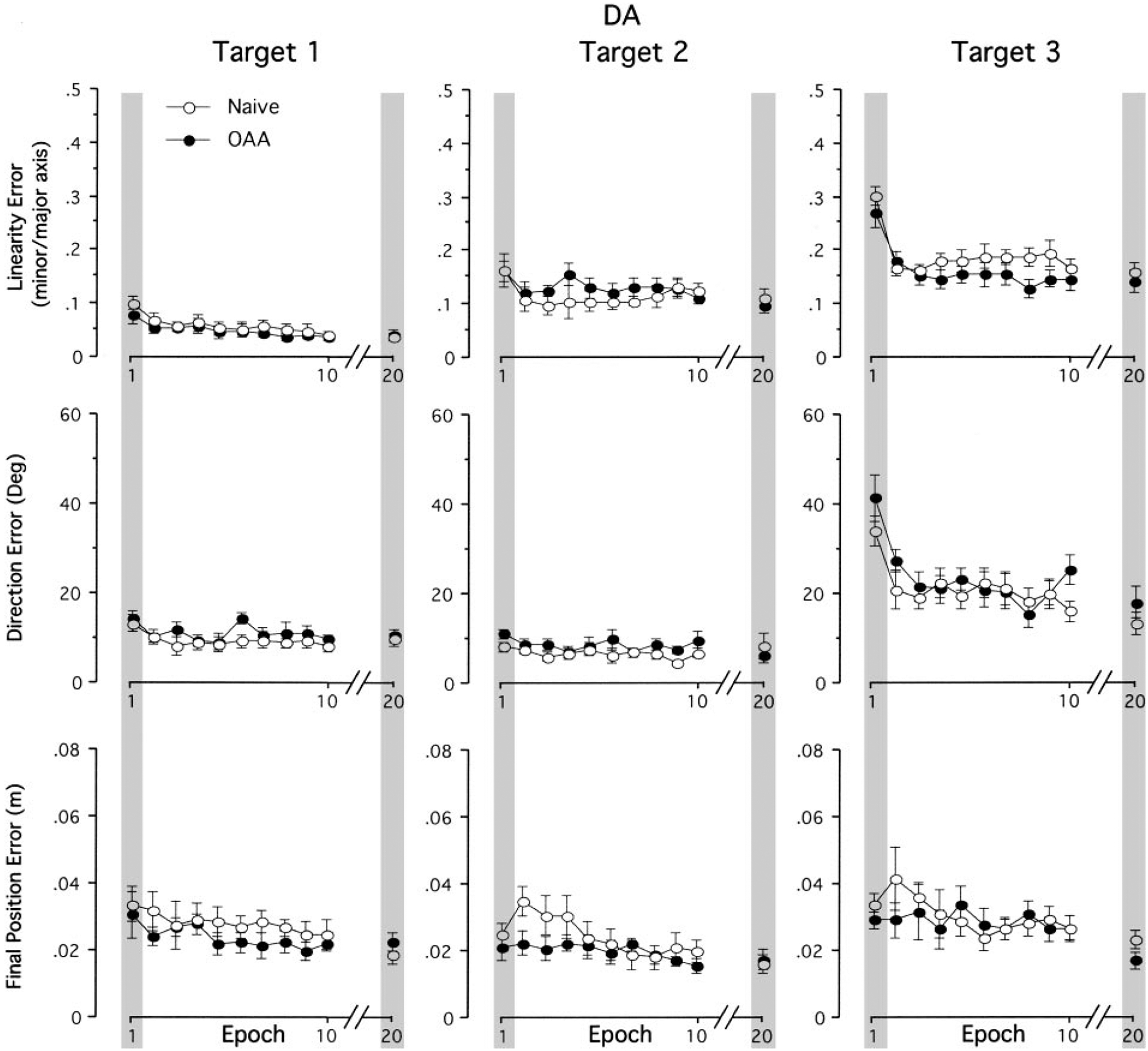
Mean performance measures of linearity error, direction error at Amax and final position error for dominant arm. Every data point shown on x axis represents the average of 3 consecutive trials for each target across all subjects (means ± SE). Performance measures for naive performance (○) and performance following opposite arm adaptation (●) are shown separately. For clarity, data between epochs 10 and 20 are removed
Performance measures for the nondominant arm
As shown in Fig. 4, performance under both exposure conditions with the nondominant arm showed the same general trends as that with the dominant arm: errors measured for the initial epoch of motion increased across targets; errors for movements toward target 1 were small throughout the session (i.e., the difference between epochs 1 and 20 were not statistically significant for either condition); and errors for movements toward targets 2 and 3 showed substantial decreases between the epochs 1 and 20 (gray bars). For the nondominant arm, a repeated-measures ANOVA revealed a significant interaction between group and target direction for linearity errors and direction errors (P < 0.05). Therefore post hoc analyses comparing between naïve performance and subsequent performance following opposite arm adaptation were conducted for each target separately. In terms of final position errors, neither main effect nor interaction effect was significant. Post hoc analyses indicated that the effect of opposite arm adaptation on the first movement epoch was significant for direction errors of target 2 movements and for both linearity and direction errors of target 3 movements (P < 0.05). For the last epoch (epoch 20), the group main effect was significant for final position errors of target 2 movements (P < 0.05). The fact that only direction errors of target 2 movements on the first epoch were affected by opposite arm adaptation, whereas both direction and linearity errors of target 3 movements were affected, is consistent with the previously described effects of the mass as detailed in describing Fig. 2.
FIG. 4.
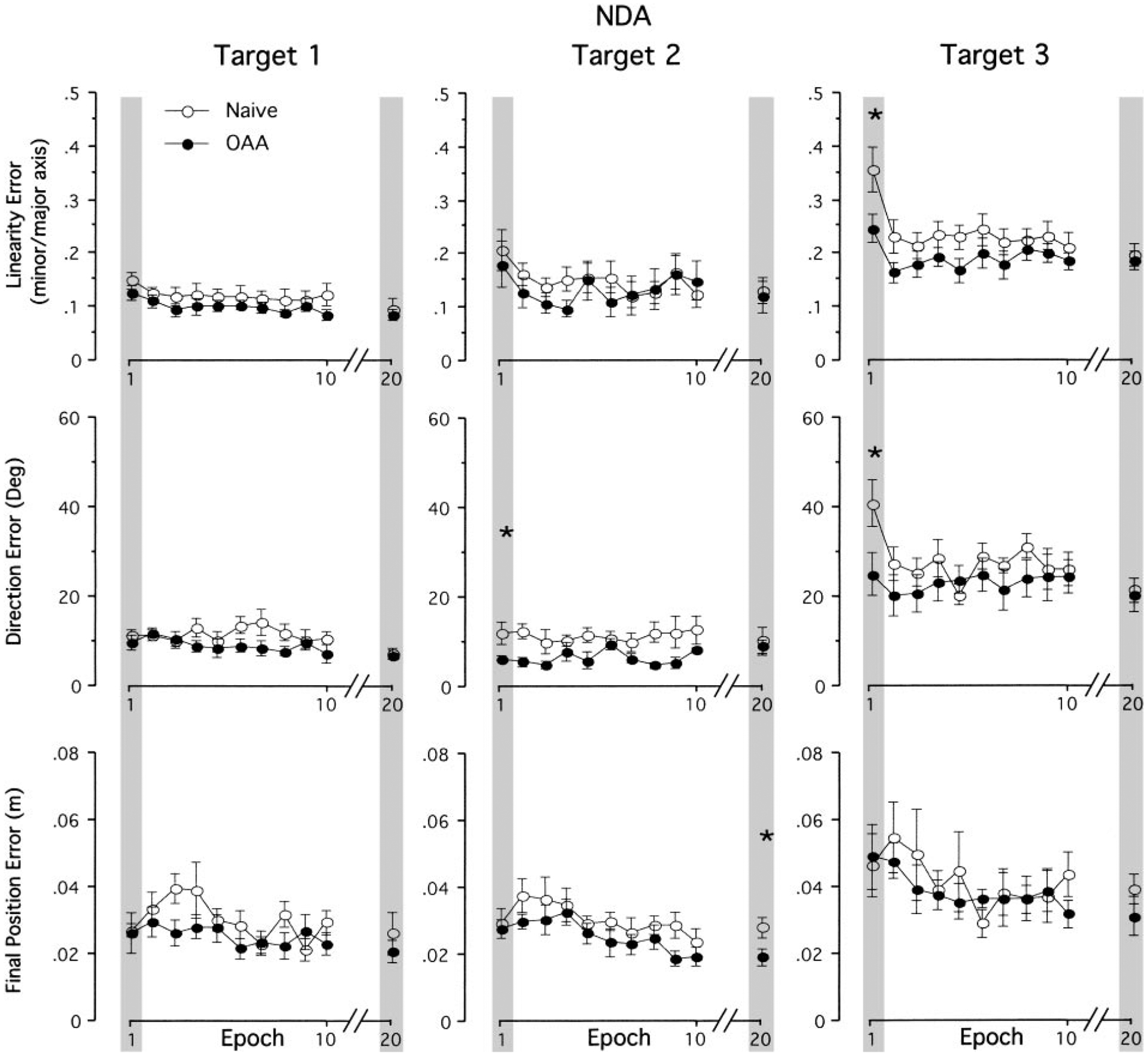
Mean performance measures of linearity error, direction error at Amax and final position error for nondominant arm. Every data point shown on x axis represents the average of 3 consecutive trials for each target across all subjects (means ± SE). Performance measures for naive performance (●) and performance following opposite arm adaptation (○) are shown separately. For clarity, data between epochs 10 and 20 are removed. *, a significant difference between naive performance and performance following opposite arm adaptation (OAA) at P < 0.05
Inverse dynamic analysis
As expected, our kinematic analysis revealed that the greatest effect of the mass occurred for movements toward target 3. Accordingly, the effects of interlimb transfer of mass adaptation were also greatest for this direction. Thus to better understand the changes in dynamic strategy required by the mass condition and how this was transferred to the nondominant from the dominant limb, we now conduct inverse dynamic analysis for movements made toward target 3 with the nondominant arm. We focus our analysis on the nondominant arm because the kinematic data analysis on the three performance measures indicated no substantial transfer of learning for the dominant arm. (For detailed analysis of adaptation to the same mass condition for dominant arm movements, see Sainburg et al. 1999.)
Figure 5A shows hand paths and velocity profiles for naive performance with the nondominant arm made under the baseline (no mass) session as well as the initial phase (1st epoch) and the final phase (last epoch) of exposure session for a representative subject from the LR group. As expected, under the baseline session, the hand path is fairly straight, and the velocity profile is roughly bell-shaped. On initial exposure to the mass, the hand path is extensively deviated laterally (counterclockwise) but curves back toward the target during the deceleration phase of motion, corresponding to a second peak in tangential hand velocity (marked with arrow). Once the subject adapted to the mass, the hand path is again fairly straight, and the velocity profile is again unimodal, although positively skewed.
FIG. 5.
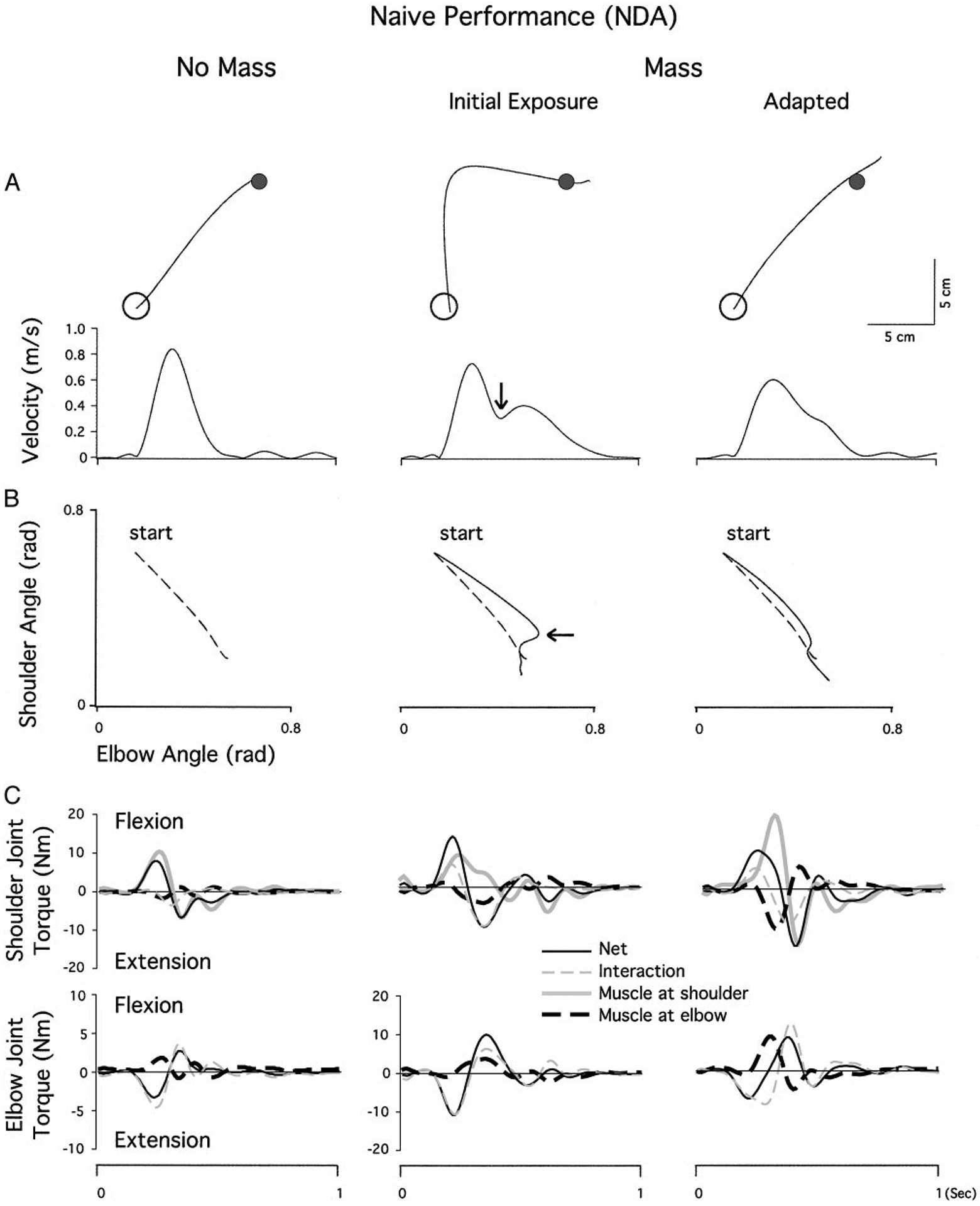
A: nondominant arm movements made toward target 3 during naïve performance. Hand paths and velocity profiles of representative subjects from the last epoch of baseline session (left), the 1st epoch of mass session (middle), and the last epoch of mass session (right). B: angle-angle plots representing the shoulder and elbow coordination patterns that correspond to the hand paths and velocity profiles shown in A. Arrows in A and B indicate the point at which a movement correction is made. C: torque patterns that correspond to the kinematic profiles shown in A and B. Top: shoulder joint torques, partitioned into net, interaction, and muscle torques; bottom: the elbow joint torques. Elbow torque profiles for the baseline session are shown at twice the scale of the mass sessions for clarity
The shoulder and elbow coordination patterns that correspond to these hand paths are illustrated in Fig. 5B as angle-angle plots. During the baseline session, the shoulder flexes forward as the elbow extends in a smooth, coordinated manner, leading to a fairly linear elbow/shoulder angle profile. On initial exposure to the mass, this profile is initially linear but with a decreased slope, indicating greater elbow excursion for a given shoulder excursion. Thus the hand moves laterally relative to the baseline trial. Later in the trial, the correction to the target (Fig. 5, arrows) is composed almost entirely of shoulder motion, resulting in a near vertical shoulder/elbow angle relationship. During the final phase of the exposure session, the slope of the initial shoulder/elbow relationship is closer to, though slightly shallower than, that of the baseline session. A slight correction, composed predominantly of shoulder flexion occurring late in the movement, is followed by a relationship that again approximates baseline performance.
The torque patterns that correspond to these kinematic profiles are shown in Fig. 5C. Net, interaction and muscle torques are shown for each joint separately. Note that the shoulder joint net torque is produced by three components, the muscle torque acting at the proximal upper arm (shoulder muscle torque), the muscle torque acting at the distal upper arm (elbow muscle torque), and the interaction torque. The elbow joint net torque is produced by only two components, elbow muscle torque and elbow interaction torque. Comparison of baseline and mass-adapted profiles (columns 1 and 3) reveals the dynamic adaptations required to produce similar kinematics under the loaded condition. During the baseline session, shoulder net torque is produced almost entirely from shoulder muscle torque and countered by interaction and elbow muscle torques, which are very small. At the elbow, extensor net torque is produced almost entirely by interaction torque, while flexor elbow muscle torques counter the acceleration produced by interaction torque.
In the mass-adapted session, kinematics are similar, but dynamics are quite different due to the mass. The eccentrically positioned mass acts to displace the center of mass of the forearm laterally, thus resulting in larger interaction torques for a given kinematic change. At the elbow, the increased extensor interaction torque is countered by a large increase in elbow flexor muscle torque, and net torque is thereby maintained smaller than interaction torque throughout the movement. At the shoulder, the large increase in elbow muscle torque must now be countered by shoulder muscle torque. Thus the adaptations associated with the mass session include a substantial increase in elbow flexor muscle torque (to counter the increased elbow extensor interaction torque) and a compensatory increase in shoulder flexor muscle torque (to counter the extensor effects of the elbow muscle torque increase).
Comparison of the Fig. 5C, middle with right (mass adapted), illustrates why, prior to these adaptaptive changes in dynamics, kinematics are erroneous. At the elbow, the extensor interaction torque is increased similarly to the mass adapted session (right). However, muscle torque does not increase, but remains similar to that of the baseline profile. This leads to a large increase in elbow net torque, which corresponds to the excessive elbow extension, characteristic of preadapted trials. This is exemplified by the substantially larger net torque under the initial exposure session (middle), as compared with the adapted condition (right). Shoulder muscle torque also remains similar to that of baseline performance, thus failing to counter the additional inertial load, which results in lower flexor acceleration (net torque). The combined effects of increased elbow extension and reduced shoulder flexion resulted in the reduced slope of the shoulder elbow graph in Fig. 5B and the excessive lateral hand motion shown in Fig. 5A.
Figure 6A shows hand paths and velocity profiles for nondominant arm movements following opposite arm adaptation under the baseline (no mass) session as well as the initial phase (1st epoch) and the final phase (last epoch) of the exposure session for a representative subject from the RL group. Movements under the baseline session (left) and the final phase of exposure session (right) are similar to those observed during naïve performance with the same arm in Fig. 5A: the hand path is fairly straight, and the velocity profile is bell-shaped under the baseline session but positively skewed under the massadapted session. However, a clear difference is observed on initial exposure to the mass (middle), where the extent of deviation in hand path is substantially smaller, and the velocity profile is unimodal. Figure 6B illustrates the shoulder and elbow coordination patterns that correspond to these hand-paths. On initial exposure to the mass (middle), this shoulder/elbow angle relationship profile is fairly linear throughout the movement, as compared with that observed during näıve performance in Fig. 5B, and is not substantially different from that of the baseline session (broken line). During the final phase of the exposure session, the shoulder/elbow relationship profile is similar to that observed during naïve performance with a slight correction comprised predominantly of shoulder flexion occurring late in the movement. Thus the effect of opposite arm adaptation is clearly observed during the initial phase of the exposure session, in that the first exposure trial following opposite arm adaptation is initiated with substantially reduced elbow extension relative to that of naïve performance.
FIG. 6.
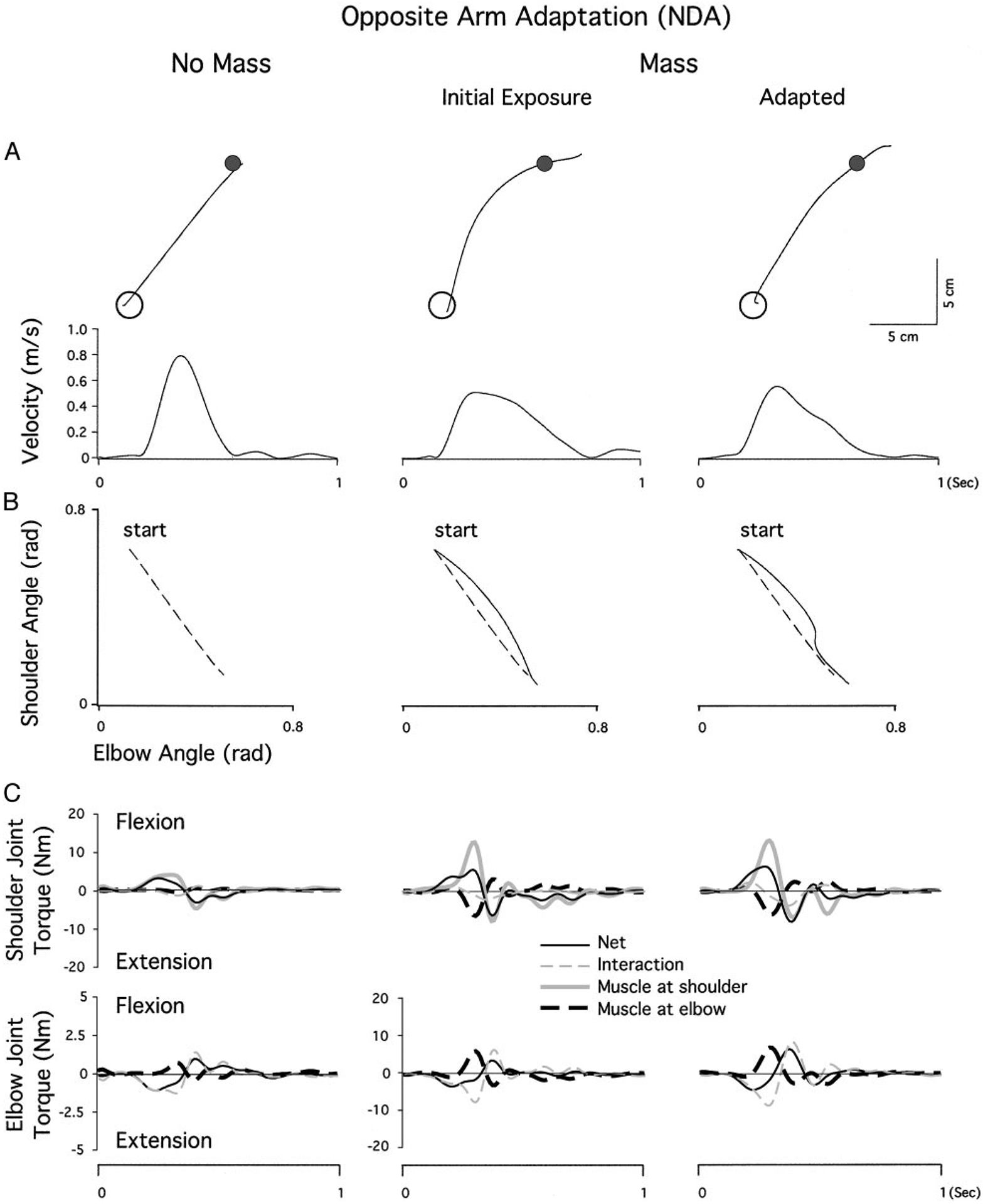
A: nondominant arm movements made toward target 3 during performance following opposite arm adaptation. Hand paths and velocity profiles of representative subjects from the last epoch of the baseline session (left), the 1st epoch of the mass session (middle), and the last epoch of the mass session (right). B: angle-angle plots representing the shoulder and elbow coordination patterns that correspond to the hand-paths and velocity profiles shown in A. Arrows in A and B indicate the point at which a movement correction is made. C: torque patterns that correspond to the kinematic profiles shown in A and B. Top: shoulder joint torques, partitioned into net, interaction, and muscle torques; bottom: the elbow joint torques. Elbow torque profiles for the baseline session are shown at 4 times the scale of the mass sessions for clarity
As expected, the torque patterns that correspond to these kinematic profiles (Fig. 6C) are similar to those shown for naïve conditions (Fig. 5C). Under the mass adapted condition, a substantial increase in elbow flexor muscle torque counters the load-induced increase in elbow extensor interaction torque. Similarly, a compensatory increase in shoulder flexor muscle torque counters the extensor effects of increased elbow muscle torque. However, in contrast to naïve conditions, this change in torque pattern is now present at the first epoch of the performance following dominant arm adaptation (middle). At the elbow, the extensor interaction torque is increased, while the flexor muscle torque shows a corresponding increase. This results in substantially reduced elbow extension, relative to that of naïve performance (Fig. 5C). Shoulder flexor muscle torque is also increased to counter the elbow flexor muscle torque. Thus the combined effects of reduced elbow extension and increased shoulder flexion resulted in the substantially reduced curvature in hand-path shown in Fig. 6A relative to that of Fig. 5A.
The effect of opposite arm adaptation was tested by calculating the shoulder and elbow flexor muscle torque impulses (see METHODS). Figure 7 illustrates these impulses for selective epochs that were normalized to the mean values of the last three epochs (epochs 18–20). During the performance following opposite arm adaptation, elbow flexor muscle torque impulse approximated 71% of the mean of the last three epochs at the very first epoch. During naïve performance it only reached 47% of the mean of the last three epochs. The difference between these two performance conditions at the first epoch was statistically significant (P < 0.05). Similarly, shoulder flexor muscle torque impulse for the opposite arm adaptation condition approximated 60% at the first epoch, while that for the naïve performance condition only reached 26%. Again, this difference was statistically significant (P < 0.05). These findings reflect the patterns exemplified in Figs. 5 and 6: opposite arm training significantly changed the initial phase of the mass exposure session in terms of both elbow and shoulder muscle torques.
FIG. 7.
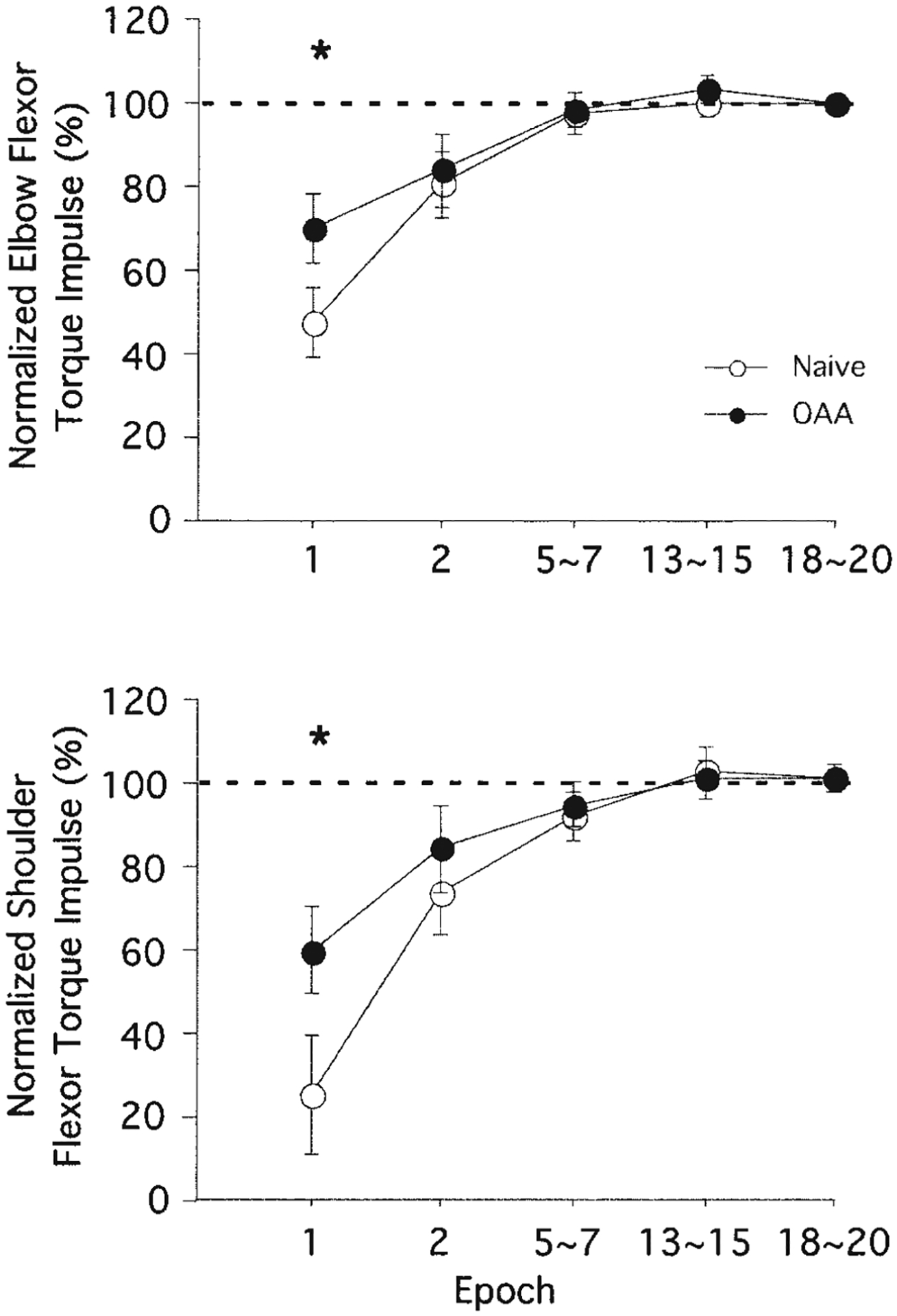
Normalized flexor muscle torque impulses from nondominant arm movements made toward target 3. Each data point at epochs 1 and 2 represents the average of 3 consecutive trials across all subjects (means ± SE), whereas every other data point represents the average of 9 consecutive trials. Performance measures for naive performance (○) and performance following opposite arm adaptation (●) are shown separately. *, a significant difference between naive performance and performance following OAA at P < 0.05
DISCUSSION
Movement direction information transfers from the dominant to nondominant arm
In this study, we tested whether initial adaptation to a novel inertial load with one arm transfers to influence subsequent performance with the other. One group learned the task with the dominant arm first, while the other group learned it with the nondominant arm first. Our results indicate asymmetrical transfer of inertial dynamics between the arms. Following initial nondominant arm adaptation, dominant arm performance did not show improvement in any performance measures we tested as compared with naïve performance with the same arm. On the other hand, dominant arm adaptation substantially improved the accuracy of subsequent movements made with the nondominant arm. Inverse dynamic analysis revealed that following the dominant arm adaptation to the extra mass, the nondominant arm immediately adopted torque strategies that were only observed at the final phase of the exposure session during naïve performance. This resulted in a hand-path that was much straighter than that of naïve performance.
Limitations in transfer, as reflected by lack of transfer to the dominant arm, can result from differential proficiencies of the arm controllers in developing internal models of the imposed dynamic conditions, as well as from restrictions in the ability of each controller to apply this model to limb specific effectors and neural circuits (see Sainburg and Wang 2002; Wang and Sainburg 2003). We expect that the instance theory of automaticity, proposed by Logan (1988), can account for the restrictions in the ability to apply an internal model to specific effectors and neural circuits. According to this theory, algorithm development accounts for the early portion of the exponential learning curve, which rapidly becomes dependent on the accumulation of specific instances of behavior. The asymptotic portion of the curve occurs when increasing the number of instances of behavior can no longer lead to substantial improvements in performance. Considering that the algorithmic phase of learning represents acquisition of an internal model of the altered inertial dynamics, we suggest that only this aspect of learning can transfer across the limbs. Continued adaptation, then, relies on practice with the neuromusculoskeletal effectors within the limb system in question. This model might explain why interlimb transfer of learning is substantially limited, as compared with intralimb transfer (Morton et al. 2001; Sainburg and Wang 2002; Wigmore et al. 2002). (For further discussion on the limitations in interlimb transfer of learning, see Wang and Sainburg 2004.)
With regard to the proficiency of the arm controllers in developing an internal model, we suggest that initial learning with the nondominant arm may have relied less on the development of an accurate model of dynamics but more on trial-and-error learning to improve performance. Even though limb-specific behavior could improve in this way, it would not occur through improving model-based algorithm development. Rather it would result from retrieval of effector-specific instances. Thus when dominant arm performance follows that of the nondominant arm, little information about the new inertial condition would be available for transfer. This explanation might account for the observed asymmetry in transfer.
The idea that the nondominant arm is less proficient at developing a model of limb dynamics is consistent with our previous findings (Bagesteiro and Sainburg 2002; Sainburg 2002; Sainburg and Kalakanis 2000). These studies have indicated that the dominant arm is more proficient in coordinating intersegmental dynamics for specifying trajectory direction and shape as compared with the nondominant arm (Bagesteiro and Sainburg 2002; Sainburg 2002; Sainburg and Kalakanis 2000). It is thus reasonable to expect that when adapting to novel inertial dynamics, dominant arm practice might lead to the development of a more accurate internal model that can readily be utilized by the nondominant arm controller in dealing with novel limb dynamics.
Another plausible explanation for this asymmetrical transfer is that the nondominant arm controller may have good access to the information obtained during dominant arm adaptation but not vice versa. According to this idea, the nondominant arm controller relies on the dominant hemisphere for specifying and controlling movement. Empirical support for this interpretation comes from electrophysiological and neural imaging studies, which have demonstrated that the motor and/or premotor cortices in the hemisphere contralateral to the dominant arm show greater activation than their nondominant counterparts during ipsilateral (Kawashima et al. 1997; Kim et al. 1993), contralateral (Dassonville et al. 1997; Kim et al. 1993; Taniguchi et al. 1998), and bilateral arm movements (Viviani et al. 1998). These findings indicate that sensorimotor access of each arm to the underlying circuitry may be asymmetrical. This is in agreement with the idea that the nondominant arm controller has better access to the memory resources in the dominant hemisphere, where the movement information obtained during dominant arm adaptation is stored. This interpretation, however, is based on an assumption that the information obtained from the two arm controllers is stored in two distinct memory resources, which are located in each brain hemisphere separately. We have previously argued, based on our findings from a study on interlimb transfer of visuomotor rotations, that working memory resources may be independent for the two arm controllers (Wang and Sainburg 2003). Further studies are needed to examine whether the memory resources used for dynamic transformations might also be independent for the two arm controllers.
Adaptations to visuomotor and dynamic transformations involve distinct neural processes
We have previously shown that in adaptation to a novel visuomotor rotation, movement direction information transferred from the nondominant to dominant arm only (Sainburg and Wang 2002). The present study, however, demonstrated an opposite direction of transfer from the dominant to nondominant arm. This finding indicates that the pattern of interlimb transfer depends on the nature of the transformations underlying the process of adaptation. This is consistent with the findings from two previous studies that examined the pattern of interlimb transfer in adaptation to viscous force fields (Criscimagna-Hemminger et al. 2003) and to Coriolis forces (DiZio and Lackner 1995), both of which reported transfer from the dominant to nondominant arm. These findings provide support to the argument made by Krakauer and colleagues (1999) that adaptations to visuomotor and dynamic transformations may be subserved by distinct neural processes. This conclusion was based on the finding that adaptations to a rotated visual display and to novel inertial dynamics do not interfere with one another and that these two types of learning appear to consolidate in parallel. In other studies, each type of adaptation has been shown to elicit a distinct pattern of intralimb generalization, such that dynamic adaptation transfers along intrinsic coordinates (Malfait et al. 2002; Sainburg et al. 1999; Shadmehr and Moussavi 2000; Shadmehr and Moussavi-Ivaldi 1994), whereas generalization of visuomotor adaptations are best described along extrinsic coordinates (Krakauer et al. 1999, 2000; Wolpert et al. 1995). The idea that the neural processes underlying these two types of adaptations are independent is supported by neural imaging studies, which indicate that visuomotor transformations (Clower et al. 1996; Ghilardi et al. 2000) and dynamic transformations (Shadmehr and Holcomb 1997) are subserved by different brain regions. It should be noted that Tong and colleagues (2002) recently questioned the independence of these mechanisms, based on the finding that adaptation to a position-dependent force field interfered with adaptation to a visuomotor rotation, which was also position dependent. However, those findings were likely due to the fact that the two instances of adaptation required exactly opposing motor responses. Thus adaptation to either condition would require competing responses, even if the underlying learning processes were independent. Our current findings support and extend this body of information by demonstrating a different pattern of interlimb transfer for adaptation to novel inertial dynamics as compared with that shown for adaptation to novel visuomotor transforms (Sainburg and Wang 2002; Wang and Sainburg 2003).
Implications for coordinate systems for representation of adaptations
As mentioned in the preceding text, multiple studies have shown that for the dominant arm, intralimb generalization following adaptation to novel velocity-dependent force fields, or to inertial dynamics, transfers best within an intrinsic coordinate system (Ghez et al. 1999; Malfait et al. 2002; Sainburg et al. 1999). Interestingly, a recent study of interlimb generalization of adaptation to velocity-dependent curl fields showed transfer along extrinsic coordinates (Criscimagna-Hemminger et al. 2003). This is particularly interesting in light of the same group’s findings demonstrating intralimb generalization of the same curl fields along intrinsic coordinates (Shadmehr and Mussa-Ivaldi 1994). In the current study, we showed that transfer of learning was most dramatic for target 3 movements, in which the required joint movements were matched between the limbs, whereas the required hand movements were oppositely directed. The effect of our experimental load was to laterally displace the center of mass of the distal limb segment. As a result, elbow interaction torque was initially increased in the extensor direction, leading to a lateral displacement of the hand path. On initial exposure, this produced a clockwise direction error in the dominant arm, but a counterclockwise direction error in the nondominant arm. In both cases, the limb segment was displaced laterally. Following adaptation with the dominant arm, the load-induced increase in extensor interaction torque was effectively countered by flexor muscle torque, preventing excessive elbow extension and the associated clockwise direction error. Interestingly, transfer of this adaptation to the nondominant arm was realized in terms of an increase in elbow flexor muscle torque, which countered the load-induced increase in extensor interaction torque, and reduced the counterclockwise direction error. It should be stressed that if adaptation was, instead, transferred in extrinsic coordinates, the clockwise direction error imposed by the load on the dominant arm would elicit a counterclockwise response in the nondominant arm, requiring extensor muscle torque. The effect would be to accentuate, rather than counter, the direction errors imposed by the load. Instead, transfer to the nondominant arm occurred as a flexor elbow muscle torque that countered the imposed load. We conclude that interlimb transfer of learning, in the current study, is best described within an intrinsic, joint-based, coordinate system.
The contrast of our findings with those of Criscimagna-Hemminger and colleagues is striking. There are two substantial differences in experimental paradigms that might be responsible for the differences in transfer patterns. First, our study employed an inertial load, which varies largely with the acceleration of the limb segments, whereas the study by Criscimagna-Hemminger and colleagues employed a force that varied with the velocity of the hand. DiZio and Lackner (1995) have also reported interlimb transfer along extrinsically consistent coordinates following learning of velocity-dependent forces. It is plausible that adaptation to these two types of forces employs different processes because of the different types of information available: segment accelerations cannot be detected directly through muscle and tendon proprioceptors, whereas changes in muscle length are directly signaled through type Ia spindle afferents (Hasan 1983; Hasan and Houk 1975; Matthews 1981). Adaptations to these two types of forces might employ two distinct neural mechanisms. The second factor that might have led to the differences in interlimb transfer patterns is the availability of haptic information about the experimental perturbation. In our experimental setup, the hand was splinted and the friction-less air jet system effectively eliminated shearing forces between the hand and splint that might otherwise provide tactile information about the load condition. Thus only proprioceptive information was available for subjects to adapt to the load in our task. In contrast, Criscimagna-Hemminger and colleagues used a setup in which the subjects learned the force field through a hand-held manipulandum, which provides ostensible haptic information about the force condition. Many previous studies have shown that haptic information is substantial in providing orientation, direction, and amplitude of body movement in space as required for control of posture and movement (Holden et al. 1994; Jeka and Lackner 1994; Lackner et al. 1999; Rabin et al. 1999; Rao and Gordon 2001). This interpretation regarding the availability of haptic information, however, needs to be considered with caution because haptic information was not available in the study conducted by DiZio and Lackner (1995). Therefore it is possible that the major differences between our findings and Cricimagna-Hemminger et al.’s findings are attributed to the combined effects of these two factors (i.e., type of forces, availability of sensory information). Further research is necessary to determine whether different types of forces (e.g., velocity dependent, acceleration dependent), different types of sensory information (e.g., haptic, vestibular, visual), and/or the combinations of these factors can lead to different patterns of interlimb generalization.
GRANTS
This research was supported by National Institutes of Health Grants R01HD-39311 and NRSA 1-F32-NS-46239-1.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Bagesteiro LB and Sainburg RL. Handedness: dominant arm advantages in control of limb dynamics. J Neurophysiol 88: 2408–2421, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clower DM, Hoffman JM, Votaw JR, Faber TL, Woods RP , and Alexander GE. Role of posterior parietal cortex in the recalibration of visually guided reaching. Nature 383: 618–621, 1996. [DOI] [PubMed] [Google Scholar]
- Cohen MM. Visual feedback, distribution of practice, and intermanual transfer of prism aftereffects. Percept Psychophys 14: 401–406, 1973. [DOI] [PubMed] [Google Scholar]
- Criscimagna-Hemminger SE, Donchin O, Gazzaniga MS , and Shadmehr R. Learned dynamics of reaching movements generalize from dominant to nondominant arm. J Neurophysiol 89: 168–176, 2003. [DOI] [PubMed] [Google Scholar]
- Dassonville P, Zhu XH, Uurbil K, Kim SG , and Ashe J. Functional activation in motor cortex reflects the direction and the degree of handedness. Proc Natl Acad Sci USA 94: 14015–14018, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dizio P and Lackner JR. Motor adaptation to Coriolis force perturbations of reaching movements: endpoint but not trajectory adaptation transfers to the nonexposed arm. J Neurophysiol 74: 1787–1792, 1995. [DOI] [PubMed] [Google Scholar]
- Elliott D and Roy EA. Interlimb transfer after adaptation to visual displacement: patterns predicted from the functional closeness of limb neural control centers. Perception 10: 383–389, 1981. [DOI] [PubMed] [Google Scholar]
- Flanagan JR, Nakano E, Imamizu H, Osu R, Yoshioka T , and Kawato M. Composition and decomposition of internal models in motor learning under altered kinematic and dynamic environments. J Neurosci (Online) 19: RC34, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghez C, Krakauer J, Sainburg RL , and Ghilardi MF. Spatial representations and internal models of limb dynamics in motor learning. In: The New Cognitive Neurosciences (2nd ed.), edited by Gazzaniga MS. Cambridge, MA: The MIT Press, 1999, p. 501–515. [Google Scholar]
- Ghilardi M, Ghez C, Dhawan V, Moeller J, Mentis M, Nakamura T, Antonini A , and Eidelberg D. Patterns of regional brain activation associated with different forms of motor learning. Brain Res 871: 127–145, 2000. [DOI] [PubMed] [Google Scholar]
- Hasan Z A model of spindle afferent response to muscle stretch. J Neurophysiol 49: 989–1006, 1983. [DOI] [PubMed] [Google Scholar]
- Hasan Z and Houk JC. Analysis of response properties of deefferented mammalian spindle receptors based on frequency response. J Neurophysiol 38: 663–672, 1975. [DOI] [PubMed] [Google Scholar]
- Holden M, Ventura J , and Lackner JR. Stabilization of posture by precision contact of the index finger. J Vestib Res 4: 285–301, 1994. [PubMed] [Google Scholar]
- Jeka JJ and Lackner JR. Fingertip contact influences human postural control. Exp Brain Res 100: 495–502, 1994. [DOI] [PubMed] [Google Scholar]
- Kawashima R, Inoue K, Sato K , and Fukuda H. Functional asymmetry of cortical motor control in left-handed subjects. Neuroreport 8: 1729–1732, 1997. [DOI] [PubMed] [Google Scholar]
- Kim SG, Ashe J, Hendrich K, Ellermann JM, Merkle H, Ugurbil K , and Georgopoulos AP. Functional magnetic resonance imaging of motor cortex: hemispheric asymmetry and handedness. Science 261: 615–617, 1993. [DOI] [PubMed] [Google Scholar]
- Krakauer JW, Ghilardi MF , and Ghez C. Independent learning of internal models for kinematic and dynamic control of reaching. Nat Neurosci 2: 1026–1031, 1999. [DOI] [PubMed] [Google Scholar]
- Krakauer JW, Pine ZM, Ghilardi MF , and Ghez C. Learning of visuomotor transformations for vectorial planning of reaching trajectories. J Neurosci 20: 8916–8924, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackner JR, DiZio P, Jeka J, Horak F, Krebs D , and Rabin E. Precision contact of the fingertip reduces postural sway of individuals with bilateral vestibular loss. Exp Brain Res 126: 459–466, 1999. [DOI] [PubMed] [Google Scholar]
- Laszlo JI, Baguley RA , and Bairstow PJ. Bilateral transfer in tapping skill in the absence of peripheral information. J Mot Behav 2: 261–271, 1970. [DOI] [PubMed] [Google Scholar]
- Logan GD. Toward an instance theory of automatization. Psychol Rev 95: 492–527, 1988. [Google Scholar]
- Malfait N, Shiller DM , and Ostry DJ. Transfer of motor learning across arm configurations. J Neurosci 22: 9656–9660, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzi CA, Bisiacchi P , and Nicoletti R. Is interhemispheric transfer of visuomotor information asymmetric? Evidence from a meta-analysis. Neuropsychologia 29: 1163–1177, 1991. [DOI] [PubMed] [Google Scholar]
- Matthews PB. Evolving views on the internal operation and functional role of the muscle spindle. J Physiol 320: 1–30, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton SM, Lang CE , and Bastian AJ. Inter- and intra-limb generalization of adaptation during catching. Exp Brain Res 141: 438–445, 2001. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia 9: 97–113, 1971. [DOI] [PubMed] [Google Scholar]
- Parlow SE and Kinsbourne M. Asymmetrical transfer of training between hands: implications for interhemispheric communication in normal brain. Brain Cogn 11: 98–113, 1989. [DOI] [PubMed] [Google Scholar]
- Rabin E, Bortolami SB, DiZio P , and Lackner JR. Haptic stabilization of posture: changes in arm proprioception and cutaneous feedback for different arm orientations. J Neurophysiol 82: 3541–3549, 1999. [DOI] [PubMed] [Google Scholar]
- Rao AK and Gordon AM. Contribution of tactile information to accuracy in pointing movements. Exp Brain Res 138: 438–445, 2001. [DOI] [PubMed] [Google Scholar]
- Sainburg RL. Evidence for a dynamic-dominance hypothesis of handedness. Exp Brain Res 142: 241–258, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg RL, Ghez C , and Kalakanis D. Intersegmental dynamics are controlled by sequential anticipatory, error correction, and postural mechanisms. J Neurophysiol 81: 1040–1056, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg RL, Ghilardi MF, Poizner H , and Ghez C. Control of limb dynamics in normal subjects and patients without proprioception. J Neurophysiol 73: 820–835, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg RL and Kalakanis D. Differences in control of limb dynamics during dominant and nondominant arm reaching. J Neurophysiol 83: 2661– 2675, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg RL and Wang J. Interlimb transfer of visuomotor rotations: independence of direction and final position information. Exp Brain Res 145: 437–447, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr R and Holcomb HH. Neural correlates of motor memory consolidation. Science 277: 821–825, 1997. [DOI] [PubMed] [Google Scholar]
- Shadmehr R and Moussavi ZM. Spatial generalization from learning dynamics of reaching movements. J Neurosci 20: 7807–7815, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr R and Mussa-Ivaldi FA. Adaptive representation of dynamics during learning of a motor task. J Neurosci 14: 3208–3224, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi M, Yoshimine T, Cheyne D, Kato A, Kihara T, Ninomiya H,Hirata M, Hirabuki N, Nakamura H , and Hayakawa T. Neuromagnetic fields preceding unilateral movements in dextrals and sinistrals. Neuroreport 9: 1497–1502, 1998. [DOI] [PubMed] [Google Scholar]
- Taylor HG and Heilman KM. Left-hemisphere motor dominance in right-handers. Cortex 16: 587–603, 1980. [DOI] [PubMed] [Google Scholar]
- Thut G, Cook ND, Regard M, Leenders KL, Halsband U , and Landis T. Intermanual transfer of proximal and distal motor engrams in humans. Exp Brain Res 108: 321–327, 1996. [DOI] [PubMed] [Google Scholar]
- Tong C, Wolpert DM , and Flanagan JR. Kinematics and dynamics are not represented independently in motor working memory: evidence from an interference study. J Neurosci 22: 1108–1113, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viviani P, Perani D, Grassi F, Bettinardi V , and Fazio F. Hemispheric asymmetries and bimanual asynchrony in left- and right-handers. Exp Brain Res 120: 531–536, 1998. [DOI] [PubMed] [Google Scholar]
- Wang J and Sainburg RL. Limitations in interlimb transfer of visuomotor rotations. Exp Brain Res 155: 1–8, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J and Sainburg RL. Mechanisms underlying interlimb transfer of visuomotor rotations. Exp Brain Res 149: 520–526, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigmore V, Tong C , and Flanagan JR. Visuomotor rotations of varying size and direction compete for a single internal model in motor working memory. J Exp Psychol Hum Percept Perform 28: 447–457, 2002. [DOI] [PubMed] [Google Scholar]
- Winter DA. Biomechanics and Motor Control of Human Movement. New York: Wiley, 1990. [Google Scholar]
- Wolpert DM, Ghahramani Z , and Jordan MI. Are arm trajectories planned in kinematic or dynamic coordinates? An adaptation study. Exp Brain Res 103: 460–470, 1995. [DOI] [PubMed] [Google Scholar]


