Abstract
Introduction:
Retrospective studies report that angiotensin-converting enzyme inhibitors (ACEIs) may reduce the severity of COVID-19, but prospective data on de novo treatment with ACEIs are limited. The RAMIC trial was a randomized, multicenter, placebo-controlled, double-blind, allocation-concealed clinical trial to examine the efficacy of de novo ramipril versus placebo for the treatment of COVID-19.
Methods:
Eligible participants were aged 18 years and older with a confirmed diagnosis of SARS-CoV-2 infection, recruited from urgent care clinics, emergency departments, and hospital inpatient wards at eight sites in the USA. Participants were randomly assigned to daily ramipril 2.5 mg or placebo orally in a 2:1 ratio, using permuted block randomization. Analyses were conducted on an intention-to-treat basis. The primary outcome was a composite of mortality, intensive care unit (ICU) admission, or invasive mechanical ventilation by day 14.
Results:
Between 27 May 2020 and 19 April 2021, a total of 114 participants (51% female) were randomized to ramipril (n = 79) or placebo (n = 35). The overall mean (± SD) age and BMI were 45 (± 15) years and 33 (± 8) kg/m2. Two participants in the ramipril group required ICU admission and one died, compared with none in the placebo group. There were no significant differences between ramipril and placebo in the primary endpoint (ICU admission, mechanical ventilation, or death) (3% versus 0%, p = 1.00) or adverse events (27% versus 29%, p = 0.82). The study was terminated early because of a low event rate and subsequent Emergency Use Authorization of therapies for COVID-19.
Conclusion:
De novo ramipril was not different compared with placebo in improving or worsening clinical outcomes from COVID-19 but appeared safe in non-critically ill patients with COVID-19.
Trial Registration: Clinicaltrials.gov NCT04366050.
Keywords: COVID-19, Coronavirus, Ramipril, Therapy
INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has infected more than 380 million people globally and caused more than 6 million deaths as of July 2023 [1]. Among patients with coronavirus disease 2019 (COVID-19), comorbidities such as hypertension, diabetes, and cardiovascular disease are associated with a higher risk of developing severe symptoms and mortality [2–4]. COVID-19 continues to exert a toll on healthcare services worldwide and continues to pose a threat to the elderly and the immunosuppressed.
The continued use of angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin II receptor blockers (ARBs) among patients with COVID-19 was initially controversial, as there were concerns that continuing ACEIs or ARBs might increase the expression of angiotensin-converting enzyme 2 (ACE2), the cellular receptor and necessary entry point for SARSCoV-2 infection [5–7]. However, multiple studies have demonstrated that there is neither an increased risk of COVID-19 infection nor a more severe clinical course among patients who previously consumed or continued ACEI/ARBs [8–14]. By contrast, some observational studies observed a potential clinical benefit among patients who consumed ACEI/ARBs [12].
Preclinical studies have demonstrated that ACE2 expression is downregulated following infection with SARS-CoV, resulting in excessive activation of the renin–angiotensin–aldosterone system and increased angiotensin II activity, causing increased inflammation, epithelial disruption, increased endothelial permeability, cell death, and fibrosis in the lungs and microcirculation [15–18]. Therefore, de novo treatment with ACEIs may be beneficial by blunting the enhanced responses to angiotensin II, thereby preventing or mitigating acute lung injury, endotheliitis, and other features of COVID-19 [19]. A randomized controlled trial investigating the de novo use of ACEIs among patients with COVID-19 has not been reported. Ramipril is an ACEI that is US Food and Drug Administration (FDA) approved for the treatment of hypertension and to reduce the risk of heart failure and death after myocardial infarction but has not been studied in patients infected with SARS-CoV-2. The suppression of the renin–angiotensin–aldosterone system pathway by ramipril suppresses ACE1 activity and may reduce the pro-inflammatory effects of COVID-19. Therefore, we conducted a randomized, multicenter, placebo-controlled, double-blind, allocation-concealed clinical trial to examine the efficacy of ramipril for the treatment of COVID-19 among participants who were hospitalized outside of the intensive care unit (ICU) or presented to the emergency department/urgent care clinic.
METHODS
Study Design
This prospective, randomized, multicenter, placebo-controlled, double-blind, allocation-concealed phase 2B trial, RAMIC, evaluated the effect of 14 days of once-daily ramipril (2.5 mg orally) versus placebo on subjects with SARS-CoV-2 infection (ClinicalTrials.gov, NCT04366050). This study was performed in accordance with the ethical principles of the Declaration of Helsinki of 1964 and its later amendments, and consistent with the International Conference on Harmonization, Good Clinical Practice, and applicable regulatory requirements. Details of the rationale and trial design have been previously described [20]. Informed consent was obtained from all participants. Subjects were consented in person, or by videoconference or telephone, which occurred in the presence of a co-signing witness. In the case of videoconference or telephone encounters, either a photograph of the signed consent was sent to the study team via secure email, or an attestation was signed by an impartial witness and the consenter indicating that the subject agreed to participate and signed the documents. Alternatively, electronic methods were used to obtain consent. These were in accordance with guidance from the FDA on the conduct of trials during the COVID-19 pandemic. This study was approved by the institutional review board for all sites (Advarra IRB approved Apr 2020, A Randomized, Doubleblind, Placebo-Controlled Trial to Evaluate the Efficacy of Ramipril to Prevent ICU Admission, Mechanical Ventilation or Death in Persons with COVID-19 [Pro00043364]).
Participants
The study population included male and female subjects aged 18 years and older with a confirmed diagnosis of SARS-CoV-2 infection, as confirmed by polymerase chain reaction, or a clinical presentation consistent with COVID-19 infection (fever, cough or shortness of breath) with positive IgM serology who presented to a COVID-19 urgent care clinic or emergency department or were hospitalized outside of the ICU.
Participants meeting any of the following criteria were excluded from the study: outpatient use of ACEI or ARB in the 7 days before screening; participation in any other clinical trial of an experimental treatment for SARS-CoV-2 infection (compassionate use of hydroxychloroquine, chloroquine, azithromycin, or remdesivir outside of a clinical trial was allowed); requirement for mechanical ventilation or ICU care at the point of screening; nonsteroidal anti-inflammatory drug use (aspirin was permitted); alanine aminotransferase (ALT) or aspartate aminotransferase (AST) >5× upper limit of normal; estimated GFR\40 mL/min; history of serum creatinine ≥ 2 mg/dl in the previous 28 days; systolic BP <100 mmHg or diastolic BP <65 mmHg at screening; known hypersensitivity to ACEI; history of angioedema; renal artery stenosis; serum potassium ≥ 5.1 mEq/L; pregnancy or breastfeeding; use of aliskiren, amifostine, lithium, or sacubitril within 7 days.
A total of 114 patients were enrolled from eight sites in the USA between 27 May 2020 and 19 April 2021 and dosed with either 2.5 mg of ramipril or a placebo daily for 14 days (randomized 2:1). The rates of death and severe COVID-19 were lower compared to what was experienced at the start of the pandemic, in part related to better supportive care. This finding along with the subsequent Emergency Use Authorization of therapies for the treatment of COVID-19 led to the decision to terminate the study early on 9 July 2021 [21, 22].
Randomization and Blinding
Participants were randomly assigned to daily ramipril 2.5 mg or placebo orally in a 2:1 ratio, using permuted block randomization in blocks of six by the University of California San Diego Investigational Drug Services. The dose of 2.5 mg ramipril was chosen to balance reliable renin–angiotensin–aldosterone system blockade for participants who were ACEI and ARB naïve and to maintain safety by minimizing the risk of acute kidney injury and hypotension in participants with COVID-19. A 2:1 design was chosen as we hypothesized that the intervention would provide clinical benefits, hence a higher proportion of treatment was chosen to provide more power to detect a difference in clinical outcomes. A double-blind randomization list was created by the study statistician, and allocation was concealed. Drug bottles were supplied to clinical sites in chronological order based on the randomization list, and sites were supplied in multiples of six. The randomization system followed Good Clinical Practice and is Part 11-compliant. All study personnel and participants were blinded. There was no provision for emergency unblinding.
Intervention and Study Assessments
All 14 doses of the study drug were dispensed in opaque bottles for nursing administration for inpatients or self-administration for outpatient participants. Participants were advised to consume the drug (ramipril 2.5 mg or placebo) in the morning after 8 hours of fasting.
All participants underwent a standardized virtual or in-person clinical evaluation at baseline, including symptom assessment through a structured tool, vital signs, laboratory investigations, and review of chest x-ray (XR) and computed tomography results, if available. The laboratory investigations included ALT, AST, total serum bilirubin, alkaline phosphatase, albumin, total protein, plasma glucose, sodium, chloride, potassium, creatinine, and C-reactive protein.
Virtual, in-person, or telephone follow-up visits were conducted with participants on days 3, 7, 14, and 28 from randomization for standardized symptom assessment, review of treatment adherence and compliance, adverse event/side effect assessment, and outcome assessment. All participants meeting grade ≥ 3 toxicity (Supplementary Material Table 1) or the primary endpoint discontinued the study drug/placebo.
Outcomes
The primary outcome was a composite of mortality, ICU admission, or use of invasive mechanical ventilation by day 14. Fourteen days was chosen on the basis of early clinical reports of a bimodal time from the onset of symptoms to the requirement for mechanical ventilation with modes at 3–4 days and 9 days [23].
The secondary outcomes were continued hospitalization at day 14; hospitalization among those who were outpatient at the time of randomization; time to discharge from the hospital; hypotension requiring vasopressor support; septic shock; acute kidney injury; a composite of mortality, ICU admission or invasive mechanical ventilator use by day 28.
Statistics
Descriptive statistics of participant demographic, clinical, and laboratory characteristics were presented at baseline and dichotomized by treatment arm, ramipril versus placebo. Baseline categorical variables were compared with chisquare or Fisher’s exact test, and continuous variables were compared using a t test or Wilcoxon two-sample test where appropriate. The primary and secondary outcomes were analyzed using a 2 × 2 contingency table (treatment by outcome) and testing via Fisher’s exact test. Analyses were done on an intention-to-treat (ITT) basis using the total number of randomly assigned participants unless otherwise specified. Sample-size estimation was performed a priori as previously described [20], and it was estimated that 510 participants (340 receiving ramipril and 170 receiving placebo) were required for sufficient power to determine the primary outcome. Statistical significance was defined as a two-tailed P value of ≤ 0.05. All statistical analyses were performed on SAS, version 9.4 (SAS Institute, Cary, North Carolina).
Role of the Funding Source
This investigator-initiated study was funded by Pfizer. The funder had no role in the design, analysis, or writing of the manuscript.
RESULTS
Characteristics of the Study Population
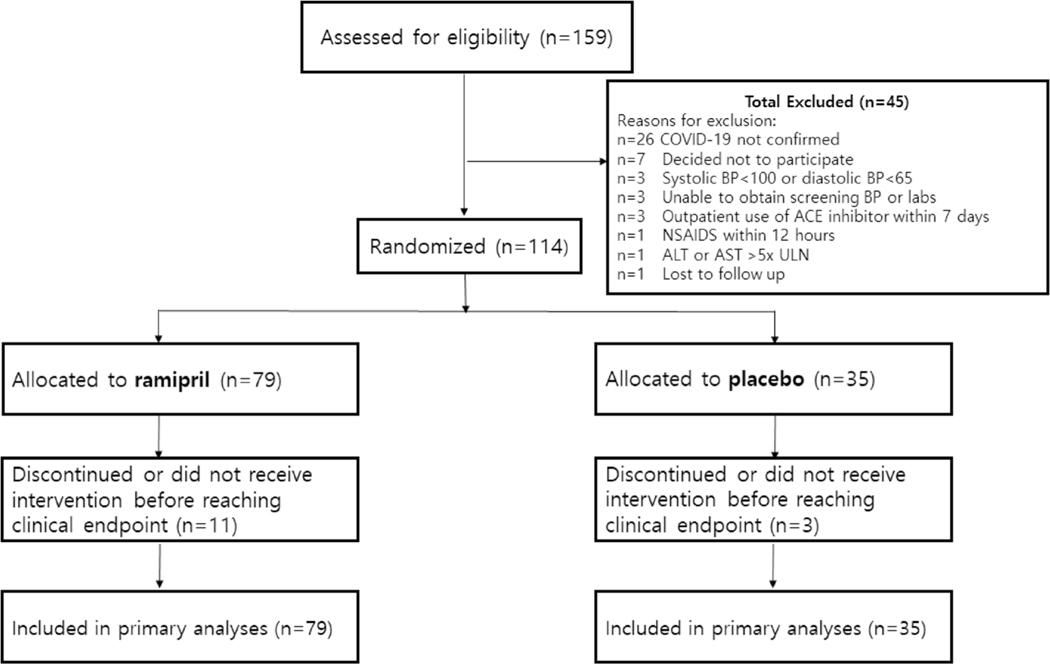
A total of 159 participants were screened at eight clinical sites in the USA and 114 participants were randomized. In total, 79 participants were randomly assigned to ramipril, and 35 participants were randomly assigned to placebo (Fig. 1). All 114 participants who were randomized were included in the ITT primary efficacy analysis.
Fig. 1.
Trial profile. COVID-19 coronavirus disease 2019, NSAIDS non-steroidal anti-inflammatory drugs, ALT alanine aminotransferase, AST aspartate aminotransferase, ULN upper limit of normal, BP blood pressure
The overall mean (SD) age was 45 (15) years, 51% of participants were female, and the mean (SD) body mass index was 33 (8) kg/m2. Overall, 81% were White, with 70% identifying as Hispanic. In the overall cohort, 59% were obese, 18% had diabetes, and 24% had hypertension. The majority (70%) of the participants were hospitalized or were in the emergency department at the time of randomization, 44% had a fever, 58% had shortness of breath, 21% had an oxygen saturation <94%, and 68% had bilateral infiltrates on chest XR.
Overall, 75% of participants received concomitant therapy for COVID-19, with a higher proportion of the ramipril group receiving azithromycin, corticosteroids, and remdesivir (Supplementary Material Table 2). Baseline demographics and disease characteristics were generally similar between the two groups (Table 1).
Table 1.
Baseline characteristics of study population, by treatment allocation
| Ramipril (N = 79) | Placebo (N = 35) | |
|---|---|---|
|
| ||
| Age in years, mean (SD) | 45 (16) | 46 (14) |
| Sex, n (%) | ||
| Male | 41 (52) | 15 (43) |
| Female | 38 (48) | 20 (57) |
| Body mass index, kg/m2 (SD) | 33 (8) | 33 (8) |
| Comorbidities, n (%) | ||
| Obesity | 47 (61) | 20 (59) |
| Diabetes | 16 (20) | 4 (11) |
| Hypertension | 21 (27) | 6 (17) |
| Hyperlipidemia | 16 (20) | 1 (3) |
| Coronary artery disease | 3 (4) | 2 (6) |
| Cancer | 4 (5) | 4 (11) |
| Chronic liver disease | 5 (6) | 1 (3) |
| Chronic kidney disease | 0 (0) | 0 (0) |
| Clinical status at randomization | ||
| Hospitalized/emergency department, n (%) | 56 (71) | 24 (69) |
| Fever, n (%) | 37 (47) | 13 (37) |
| Oxygen saturation < 94% on room air, n (%) | 18 (23) | 6 (17) |
| Shortness of breath, n (%) | 48 (61) | 18 (51) |
| Systolic blood pressure, mm Hg, mean (SD) | 132 (17) | 129 (16) |
| Diastolic blood pressure, mm Hg, mean (SD) | 81 (10) | 78 (12) |
| Heart rate, beats per min, mean (SD) | 84 (14) | 88 (17) |
| Platelets, 103 cells/μL, mean (SD) | 225 (75) | 223 (108) |
| Alanine aminotransferase (ALT), U/L, mean (SD) | 45 (40) | 42 (44) |
| Aspartate aminotransferase (AST), U/L, mean (SD) | 40 (24) | 39 (22) |
| Total bilirubin, mg/dL, mean (SD) | 0.9 (3) | 0.5 (0.2) |
| Creatinine, mg/dL, mean (SD) | 0.8 (0.2) | 0.7 (0.2) |
| C-reactive protein, mg/L, mean (SD) | 56 (78) | 63 (72) |
| Bilateral infiltrates on chest x-raya, n (%) | 42 (71) | 18 (62) |
A total of 59 patients in the ramipril group and 29 patients in the placebo group provided data for this analysis
Primary Outcome
There were no significant differences between ramipril and placebo in the primary endpoint of mortality, ICU admission, or mechanical ventilation by day 14 (3% versus 0%, p = 1.00) (Table 2). Two participants in the ramipril group required mechanical ventilation in the ICU, and one of these two participants died. None of the participants in the placebo group died or required ICU admission or mechanical ventilation.
Table 2.
Primary outcomea
| Outcome | Ramipril (N = 79) | Placebo (N = 35) | p valueb |
|---|---|---|---|
|
| |||
| Primary outcome*, n (%) | 2 (3) | 0 (0) | 1.00 |
| Components of primary outcome | |||
| ICU admission, n (%) | 2 (3) | 0 (0) | 1.00 |
| Need for mechanical ventilation, n (%) | 2 (3) | 0 (0) | 1.00 |
| Death, n (%) | 1 (1) | 0 (0) | 1.00 |
Composite of intensive care unit (ICU) admission, need for mechanical ventilation, and death, by day 14 of randomization
Fisher’s exact test
Secondary Outcomes
Comparing ramipril versus placebo, the proportion of participants that required continued hospitalization at day 14 was 1% versus 0%, the proportion with septic shock was 3% versus 0%, and the proportion that required either ICU admission or mechanical ventilation or died by day 28 was 3% versus 0% (Table 3). The proportions that required continued hospitalization at day 14, required vasopressor support, and developed acute kidney injury and time to discharge were similar between groups.
Table 3.
Secondary outcomes
| Outcome | Ramipril (N = 79) | Placebo (N = 35) |
|---|---|---|
|
| ||
| Continued hospitalization at day 14, n (%) | 1 (1) | 0 (0) |
| Time to discharge, days (SD) | 3 (3) | 3 (3) |
| Need for hospitalization among outpatient participants, n (%) | 0 (0) | 0 (0) |
| Hypotension requiring vasopressor support, n (%) | 1 (1) | 0 (0) |
| Septic shock, n (%) | 2 (3) | 0 (0) |
| Acute kidney injury, n (%) | 1 (1) | 0 (0) |
| Mortality or need for intensive care unit (ICU) admission or invasive mechanical ventilator use within 28 days, n (%) | 2 (3) | 0 (0) |
Safety
A similar proportion of participants in the ramipril and placebo groups experienced any adverse event by day 14 (27% versus 29%, p = 0.82) (Table 4). One participant in the ramipril group developed angioedema and discontinued the study drug compared with no drug discontinuations in the placebo arm due to adverse events.
Table 4.
Adverse events
| Ramipril (N = 79) | Placebo (N = 35) | |
|---|---|---|
|
| ||
| Participants who experienced any adverse event, n (%) | 21 (27) | 10 (29) |
| Total serious adverse eventsa, n (% of total adverse events) | 2 (5) | 0 (0) |
| Grade 1 adverse events, n (% of total adverse events) | 27 (73) | 15 (79) |
| Grade 2 adverse events, n (% of total adverse events) | 8 (22) | 2 (11) |
| Grade 3 adverse events, n (% of total adverse events) | 1 (3) | 0 (0) |
| Grade 4 adverse events, n (% of total adverse events) | 0 (0) | 0 (0) |
| Grade 5 adverse events, n (% of total adverse events) | 1 (3) | 0 (0) |
| Grade missing, n (% of total adverse events) | 0 (0) | 2 (11) |
| Adverse events, n | 37 | 19 |
| Allergic reaction/angioedema, n | 1 | 0 |
| Abnormal liver function test, n | 3 | 1 |
| Hyperkalemia, n | 0 | 0 |
| Acute kidney injury, n | 1 | 0 |
| Hypotension, n | 0 | 0 |
Grade ≥ 3 toxicity
DISCUSSION
Main Findings
In this randomized, multicenter, placebo-controlled, double-blind, allocation-concealed clinical trial, de novo treatment with ramipril did not improve the clinical course of participants with SARS-CoV-2 infection who were hospitalized or presented to the emergency department or urgent care clinic. There was no significant difference in the primary composite endpoint of mortality, ICU admission, or mechanical ventilation by day 14 between the ramipril and placebo groups. De novo treatment with ramipril was not associated with an improvement in any of the secondary endpoints, including continued hospitalization at day 14, time to discharge, hospitalization among outpatients, hypotension requiring vasopressor support, and acute kidney injury. A similar proportion of participants in both groups experienced an adverse event and one participant in the ramipril group discontinued treatment because of angioedema.
These findings have important clinical implications. Despite preclinical data suggesting that ACEIs may ameliorate the adverse effects of SARS-CoV-2 infection [19], the current study demonstrates that ramipril treatment did not significantly improve outcomes from COVID-19. On the basis of these data, ramipril should not be initiated specifically for the treatment of COVID-19 in adults who are not critically ill but appears to be safe.
In Context with Current Literature
Cohen et al. conducted a randomized, open-label trial that allocated hospitalized participants who were already receiving ACEIs before admission to continuing versus stopping ACEIs/ ARBs and found no overall effect on the severity of the COVID-19 disease course [24]. Puskarich et al. performed a randomized, placebo-controlled trial of de novo losartan for the treatment of mildly symptomatic outpatients with COVID-19 which demonstrated that 10 days of losartan did not reduce subsequent hospitalization rates [25]. The Puskarich study was terminated early because of the low event rate. Compared with the Puskarich study, the participants in the current study were more ill (70% were either hospitalized or required a visit to the emergency department). In addition, the current study investigated ramipril, an ACEI, rather than an ARB. Taken together, these data highlight that patients who are already consuming ACEIs/ARBs for an established indication may continue these medications after contracting SARS-CoV-2 infection, but ACEIs should not be initiated for the purpose of reducing the severity of the disease course. These findings reinforce previous studies which concluded that ACEIs are well tolerated in noncritically ill patients with COVID-19. In contrast, a recent randomized trial determined that ACEIs and ARBs in critically ill patients with COVID-19 were associated with worsened clinical outcomes [26]. Taken together, these data indicate that ACEIs should not be initiated to treat COVID-19, but may be safe in noncritically ill adults.
Limitations
This study is the first randomized, placebocontrolled trial to study de novo treatment with an ACEI for the treatment of COVID-19 and fills an important knowledge gap. However, it is not without limitations. The mortality that was expected at the start of the pandemic had changed with better supportive care, and the event rate was lower than what was experienced in the initial phase of the pandemic. This, in addition to the authorization of FDA-approved therapies for COVID-19, led to the decision to terminate the trial early. The trial was underpowered to detect any differences in the primary outcome between groups, with a post hoc analysis determining that power was 18%. The limitation in sample size and a shorter duration may have comprised the ability of this trial to provide greater diversity among participants, determine potential variations in treatment response, and potentially impact reproducibility. A priori analysis showed that the trial would need 510 patients, but only 114 patients were enrolled. However, there was a numerically higher number of ICU admissions, the requirement for mechanical ventilation, and deaths in the ramipril group, suggesting that it is unlikely that ramipril would result in a clinically meaningful benefit even with larger participant numbers. This study was conducted only in the USA; therefore, it is unclear whether its findings are generalizable to other countries/regions.
The admission criteria to ICU were based on clinical assessment and local practices of each site. We were unable to adjust for the effect of demographic factors in the analysis because of the low event rate in our study.
CONCLUSION
De novo treatment with 14 days of ramipril did not reduce mortality, ICU admission, or the need for invasive mechanical ventilation among participants who were hospitalized outside of the ICU or presented to the emergency department/urgent care clinic. As the study was terminated early because of a lower-than-expected event rate compared to the initial phase of the pandemic and subsequent Emergency Use Authorization of therapies for COVID-19, these findings require cautious interpretation. Ramipril did not significantly improve outcomes from COVID-19 but appeared safe in the setting of COVID-19 among non-critically ill adults.
Supplementary Material
Key Summary Points.
Why carry out the study?
Some observational studies have observed a potential clinical benefit among patients who consumed angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin II receptor blockers (ARBs).
What was learned from the study?
This was a multicenter, randomized, placebo-controlled, double-blind, allocation-concealed trial of de novo angiotensin-converting enzyme inhibitors for the treatment of COVID-19. The trial was terminated early because of a lowerthan-expected event rate and the approval of therapies for COVID-19.
Ramipril did not decrease the rate of COVID-19 progression as defined by the composite of intensive care unit admission, initiation of mechanical ventilation, or death.
Treatment with ramipril appeared safe in non-critically ill patients with COVID-19, with no significant difference in rates of adverse events compared to placebo.
ACKNOWLEDGEMENTS
The authors would like to thank Thuan Le and Tuo Xin from the Investigational Drug Service, University of California San Diego for assisting with the randomization process.
Funding.
This investigator-initiated study was funded by Pfizer. The journal’s Rapid Service Fee was funded by the authors.
Disclosures.
Rohit Loomba receives funding support from NIEHS (5P42ES010337), NCATS (5UL1TR001442), NIDDK (U01DK130190, U01DK061734, R01DK106419, P30DK120515, R01DK121378, R01DK124318), and NHLBI (P01HL147835). Rohit Loomba serves as a consultant to Aardvark Therapeutics, Altimmune, Anylam/Regeneron, Amgen, Arrowhead Pharmaceuticals, AstraZeneca, Bristol-Myer Squibb, CohBar, Eli Lilly, Galmed, Gilead, Glympse bio, Hightide, Inipharma, Intercept, Inventiva, Ionis, Janssen Inc., Madrigal, Metacrine, Inc., NGM Biopharmaceuticals, Novartis, Novo Nordisk, Merck,Pfizer,Sagimet,Theratechnologies,89bio, Terns Pharmaceuticals and Viking Therapeutics. In addition, his institutions received research grants from Arrowhead Pharmaceuticals, AstraZeneca, Boehringer-Ingelheim, Bristol-Myers Squibb, Eli Lilly, Galectin Therapeutics, Galmed Pharmaceuticals, Gilead, Intercept, Hanmi, Intercept, Inventiva, Ionis, Janssen, Madrigal Pharmaceuticals, Merck, NGM Biopharmaceuticals, Novo Nordisk, Merck, Pfizer, Sonic Incytes and Terns Pharmaceuticals. Co-founder of LipoNexus Inc. Daniel Huang receives funding support from Singapore Ministry of Health’s National Medical Research Council (MOH-000595-01) and the NMRC Clinician Scientist New Investigator Grant. In addition, he has served as an advisory board member for Gilead. J Pedro Teixeira serves as a consultant to Outset Medical and receives research funding from NCATS (UL1TR001449). Atul Malhotra is funded by NIH. He reports income related to medical education from Livanova, Equillium, Corvus, and Jazz. ResMed provided a philanthropic donation to UCSD. Veeral Ajmera, Christian Tomaszewski, Andrew LaFree, RickiBettencourt, WesleyKThompson, DaveyM. Smith, Ravindra L Mehta, Vaishal Tolia, Jeffrey Yin, Paul A Insel, Stone Leachman, Jinho Jung, Summer Collier, Lisa Richards, Kristin Woods, Maral Amangurbanova, Archana Bhatt, Xinlian Zhang, Oana M. Penciu, Stuart Zarich, Tamrat Retta, Michelle S. Harkins, Brian Chinnock, Netanya S Utay and Jordan E. Lake have nothing to disclose.
Footnotes
Compliance with Ethics Guidelines. This study was approved by the institutional review board for all sites (Advarra IRB approved Apr 2020, A Randomized, Double-blind, Placebo Controlled Trial to Evaluate the Efficacy of Ramipril to Prevent ICU Admission, Mechanical Ventilation or Death in Persons with COVID-19 [Pro00043364]). Consent was obtained from each patient, and was received either in-person, or by videoconference or telephone, which occurred in the presence of a co-signing witness. All authors agree with the manuscript and consent for its publication.
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s12325-023-02618-7.
Contributor Information
Daniel Q. Huang, NAFLD Research Center, Division of Gastroenterology, University of California at San Diego, La Jolla, CA, USA Department of Medicine, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore; Division of Gastroenterology and Hepatology, Department of Medicine, National University Health System, Singapore, Singapore.
Veeral Ajmera, NAFLD Research Center, Division of Gastroenterology, University of California at San Diego, La Jolla, CA, USA; Division of Gastroenterology, Department of Medicine, University of California San Diego, La Jolla, CA, USA.
Christian Tomaszewski, Department of Emergency Medicine, University of California, San Diego and the El Centro Regional, Medical Center, San Diego, CA, USA.
Andrew LaFree, Department of Emergency Medicine, University of California, San Diego and the El Centro Regional, Medical Center, San Diego, CA, USA.
Ricki Bettencourt, NAFLD Research Center, Division of Gastroenterology, University of California at San Diego, La Jolla, CA, USA.
Wesley K. Thompson, Division of Biostatistics and Bioinformatics, Department of Family Medicine and Public Health, University of California San Diego, La Jolla, CA, USA
Davey M. Smith, Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, La Jolla, CA, USA Veteran Affairs Medical Center, San Diego, CA, USA.
Atul Malhotra, Division of Pulmonary, Critical Care and Sleep Medicine, Department of Medicine, University of California San Diego, La Jolla, CA, USA.
Ravindra L. Mehta, Division of Nephrology-Hypertension, Department of Medicine, University of California San Diego, La Jolla, CA, USA
Vaishal Tolia, Department of Emergency Medicine, University of California San Diego, La Jolla, CA, USA.
Jeffrey Yin, Department of Pharmacy, University of California San Diego, La Jolla, CA, USA.
Paul A. Insel, Department of Pharmacology, University of California San Diego, La Jolla, CA, USA Division of Endocrinology and Metabolism, University of California San Diego, La Jolla, CA, USA.
Stone Leachman, NAFLD Research Center, Division of Gastroenterology, University of California at San Diego, La Jolla, CA, USA.
Jinho Jung, NAFLD Research Center, Division of Gastroenterology, University of California at San Diego, La Jolla, CA, USA.
Summer Collier, Division of Gastroenterology, Department of Medicine, University of California San Diego, La Jolla, CA, USA.
Lisa Richards, NAFLD Research Center, Division of Gastroenterology, University of California at San Diego, La Jolla, CA, USA.
Kristin Woods, Clinical & Translational Research Institute, University of California, San Diego, La Jolla, CA, USA.
Maral Amangurbanova, NAFLD Research Center, Division of Gastroenterology, University of California at San Diego, La Jolla, CA, USA.
Archana Bhatt, Clinical & Translational Research Institute, University of California, San Diego, La Jolla, CA, USA.
Xinlian Zhang, Division of Biostatistics and Bioinformatics, Herbert Wertheim School of Public Health, University of California San Diego, San Diego, CA, USA.
Oana M. Penciu, Beverly Hospital, Montebello, CA, USA
Stuart Zarich, Section of Cardiovascular Medicine, Yale New Haven Health Bridgeport Hospital, Bridgeport, CT, USA.
Tamrat Retta, Department of Internal Medicine, Howard University, Washington, DC, USA.
Michelle S. Harkins, Department of Internal Medicine, University of New Mexico, Albuquerque, NM, USA
J. Pedro Teixeira, Department of Internal Medicine, University of New Mexico, Albuquerque, NM, USA.
Brian Chinnock, Department of Emergency Medicine, University of California San Francisco–Fresno Medical Education Program, Fresno, CA, USA.
Netanya S. Utay, Division of Infectious Diseases and Geographic Medicine, Department of Internal Medicine, University of Texas Southwestern Medical Center, Dallas, TX, USA
Jordan E. Lake, Division of Infectious Diseases, Department of Internal Medicine, McGovern Medical School, University of Texas Health Science Center, Houston, TX, USA
Rohit Loomba, NAFLD Research Center, Division of Gastroenterology, University of California at San Diego, La Jolla, CA, USA; Division of Gastroenterology, Department of Medicine, University of California San Diego, La Jolla, CA, USA; Division of Epidemiology, Department of Family, Medicine and Public Health, University of California at SanDiego, San Diego, CA, USA.
Data Availability.
The datasets generated during and/or analyzed during the current study are not publicly available due to patient confidentiality. No material was used from external sources.
REFERENCES
- 1.WHO. WHO coronavirus (COVID-19) dashboard. https://covid19.who.int Accessed Feb 1, 2022.
- 2.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324(8):782–93. [DOI] [PubMed] [Google Scholar]
- 4.Li J, Huang DQ, Zou B, et al. Epidemiology of COVID-19: a systematic review and meta-analysis of clinical characteristics, risk factors, and outcomes. J Med Virol. 2020;93(3):1449–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrario CM, Jessup J, Chappell MC, et al. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111(20):2605–10. [DOI] [PubMed] [Google Scholar]
- 6.Soro-Paavonen A, Gordin D, Forsblom C, et al. Circulating ACE2 activity is increased in patients with type 1 diabetes and vascular complications. J Hypertens. 2012;30(2):375–83. [DOI] [PubMed] [Google Scholar]
- 7.Shang J, Ye G, Shi K, et al. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581(7807):221–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reynolds HR, Adhikari S, Pulgarin C, et al. Renin-angiotensin-aldosterone system inhibitors and risk of Covid-19. N Engl J Med. 2020;382(25):2441–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mancia G, Rea F, Ludergnani M, Apolone G, Corrao G. Renin-angiotensin-aldosterone system blockers and the risk of Covid-19. N Engl J Med. 2020;382(25):2431–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fosbøl EL, Butt JH, Østergaard L, et al. Association of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use with COVID-19 diagnosis and mortality. JAMA. 2020;324(2): 168–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang P, Zhu L, Cai J, et al. Association of inpatient use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res. 2020;126(12):1671–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baral R, Tsampasian V, Debski M, et al. Association between renin-angiotensin-aldosterone system inhibitors and clinical outcomes in patients with COVID-19: a systematic review and meta-analysis. JAMA Netw Open. 2021;4(3):e213594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khera R, Clark C, Lu Y, et al. Association of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers with the risk of hospitalization and death in hypertensive patients with COVID-19. J Am Heart Assoc. 2021;10(13): e018086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mackey K, King VJ, Gurley S, et al. Risks and impact of angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers on SARS-CoV-2 infection in adults: a living systematic review. Ann Intern Med. 2020;173(3):195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46(4):586–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Imai Y, Kuba K, Rao S, et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436(7047):112–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haga S, Yamamoto N, Nakai-Murakami C, et al. Modulation of TNF-α-converting enzyme by the spike protein of SARS-CoV and ACE2 induces TNF-alpha production and facilitates viral entry. Proc Natl Acad Sci U S A. 2008;105(22):7809–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sriram K, Insel PA. A hypothesis for pathobiology and treatment of COVID-19: the centrality of ACE1/ACE2 imbalance. Br J Pharmacol. 2020;177(21):4825–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuba K, Imai Y, Rao S, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11(8):875–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ajmera V, Thompson WK, Smith DM, et al. RAMIC: design of a randomized, double-blind, placebo-controlled trial to evaluate the efficacy of ramipril in patients with COVID-19. Contemp Clin Trials. 2021;103:106330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The Medical Letter. An EUA for bamlanivimab–a monoclonal antibody for COVID-19. JAMA. 2021;325(9):880–1. [DOI] [PubMed] [Google Scholar]
- 22.COVID-19 Update: FDA broadens emergency use authorization for Veklury (remdesivir) to include all hospitalized patients for treatment of COVID-19. Accessed Feb 3, 2022. https://www.fda.gov/news-events/press-announcements/covid-19-update-fda-broadens-emergency-use-authorization-vekluryremdesivir-include-all-hospitalized. 2020.
- 23.Argenziano MG, Bruce SL, Slater CL, et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ. 2020;369: m1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen JB, Hanff TC, William P, et al. Continuation versus discontinuation of renin-angiotensin system inhibitors in patients admitted to hospital with COVID-19: a prospective, randomised, open-label trial. Lancet Respir Med. 2021;9(3):275–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Puskarich MA, Cummins NW, Ingraham NE, et al. A multi-center phase II randomized clinical trial of losartan on symptomatic outpatients with COVID-19. E Clinical Medicine. 2021;37:100957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Writing Committee for the REMAP-CAP Investigators, Lawler PR, Derde LPG, et al. Effect of angiotensin-converting enzyme inhibitor and angiotensin receptor blocker initiation on organ support-free days in patients hospitalized with COVID-19: a randomized clinical trial. JAMA. 2023;329(14):1183–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available due to patient confidentiality. No material was used from external sources.