Significance
Early-life stress (ELS) increases the risk for depression and may lower antidepressant treatment efficacy. These translational studies leveraged a combination of genome-wide data from humans and mice treated with different antidepressants, computational analyses, and in vivo pharmacological approaches to understand how molecular changes in the brain following ELS may mediate altered antidepressant response. We found that ELS alters gene expression patterns in the nucleus accumbens—a brain center of reward and mood—which correspond with patterns of antidepressant treatment failure. Transcriptional patterns predicting treatment failure were strongest among female subjects, consistent with a greater risk for depression among women. This work provides neurobiological evidence for sex- and early-life stress-related molecular adaptations in the brain that may contribute to antidepressant treatment response.
Keywords: depression, antidepressants, early-life stress/adversity, nucleus accumbens, RNA sequencing
Abstract
Individuals with a history of early-life stress (ELS) tend to have an altered course of depression and lower treatment response rates. Research suggests that ELS alters brain development, but the molecular changes in the brain following ELS that may mediate altered antidepressant response have not been systematically studied. Sex and gender also impact the risk of depression and treatment response. Here, we leveraged existing RNA sequencing datasets from 1) blood samples from depressed female- and male-identifying patients treated with escitalopram or desvenlafaxine and assessed for treatment response or failure; 2) the nucleus accumbens (NAc) of female and male mice exposed to ELS and/or adult stress; and 3) the NAc of mice after adult stress, antidepressant treatment with imipramine or ketamine, and assessed for treatment response or failure. We find that transcriptomic signatures of adult stress after a history of ELS correspond with transcriptomic signatures of treatment nonresponse, across species and multiple classes of antidepressants. Transcriptomic correspondence with treatment outcome was stronger among females and weaker among males. We next pharmacologically tested these predictions in our mouse model of early-life and adult social defeat stress and treatment with either chronic escitalopram or acute ketamine. Among female mice, the strongest predictor of behavior was an interaction between ELS and ketamine treatment. Among males, however, early experience and treatment were poor predictors of behavior, mirroring our bioinformatic predictions. These studies provide neurobiological evidence for molecular adaptations in the brain related to sex and ELS that contribute to antidepressant treatment response.
Major depressive disorder is devastating, highly prevalent, and complex. Its complexity—with 681 combinations of core and secondary symptoms—has made identification of the exact etiologies of depression and potential subtypes of depression difficult (1). Women are twice as likely to be diagnosed with depression than men, and transgender women in particular have a nearly fourfold risk for depression (2–4). Compared to other psychiatric syndromes such as bipolar disorder, which has an estimated 60 to 85% heritability, the genetic heritability of depression is approximately 35% (5). This lower rate of heritability suggests that a high proportion of risk is instead conferred by recent as well as lifelong environmental experiences and exposures.
Early-life stress (ELS) in particular increases sensitivity to stress (6) and risk for developing major depression and other mood and drug disorders by two to fourfold (7). Stress early in life may alter developmental brain trajectories, and not just steady-state processes, which has the potential to augment the long-term impact of stress. Depressed patients with a history of ELS have worse forms of depression and may constitute a unique subtype of depression. Childhood adversity predicts earlier onset of depression, more frequent episodes, and increased recurrence of depression (8). Moreover, while fewer than 50% of depressive patients achieve full remission, the efficacy of treatment with traditional antidepressant medications is even lower among those with a history of ELS (9). Interestingly, some research has suggested that depressed patients with a history of ELS may achieve a more efficacious response to subanesthetic ketamine treatment—increasingly used as a rapid-acting antidepressant—compared to traditional monoamine-targeting antidepressants (10). However, the ways in which ELS alters brain development to change the risk and course of depression and response to treatment are poorly understood.
The reward circuitry of the brain, and in particular, the nucleus accumbens (NAc), has been implicated in response to early-life and adult stress and antidepressant treatment response (11–16). The NAc integrates glutamatergic signaling from the amygdala, hippocampus, and prefrontal cortex with neuromodulatory dopamine signaling from the ventral tegmental area to regulate behavioral response to positive and negative valence stimuli (14, 17). The imbalance of these systems is strongly associated with mood and anxiety disorders (11, 18). Indeed, ELS blunts development of the ventral striatum in humans and reduces responses to rewards, which mediated the impact of ELS on depressive symptoms (19). ELS also heightens the sensitivity of neurons in NAc to experience of future stress, which may contribute to lifelong stress hypersensitivity (20). ELS alters gene expression and epigenetic regulation within NAc of both male and female mice, effects which endure into adulthood (13, 21). Moreover, transcriptional profiles of the NAc—more so than other key brain regions such as the prefrontal cortex and hippocampus—are highly associated with effective response or failure of antidepressant treatment with either the tricyclic imipramine or ketamine (12). However, it is not yet known whether enduring molecular changes in NAc following ELS are related to altered antidepressant treatment efficacy and how these vary with sex and gender (22). Understanding sex-related molecular profiles within NAc may therefore be key in linking a life history of stress with antidepressant treatment response.
We hypothesized that ELS alters the NAc at a molecular level, which contributes to reduced antidepressant treatment efficacy. Recent studies have examined genome-wide transcriptional changes associated with ELS in mice and with antidepressant treatment response or failure in humans and mice across a variety of antidepressant classes including tricyclics, serotonin and norepinephrine reuptake inhibitors, and ketamine (6, 12, 13, 23). In lieu of any human studies that have directly examined both antidepressant treatment efficacy and history of adversity, we leveraged the combination of these datasets to test whether transcriptomic patterns associated with ELS correspond with those associated with antidepressant response or nonresponse, across species and sexes. We then tested our predictions in vivo in our mouse model of ELS among both assigned-male and assigned-female mice treated with either ketamine or escitalopram. Our highly translational approach integrates both bioinformatic comparisons and behavioral pharmacology to examine how sex, life-course stress, and type of antidepressant treatment contribute to the heterogeneity of treatment response.
Results
Similarities in Transcriptional Patterns between ELS in Mice and Antidepressant Treatment Nonresponse in Human MDD Patients.
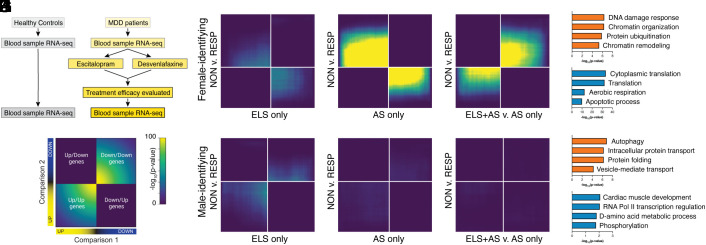
We first sought to test whether gene expression patterns—or “signatures”—in the brain resulting from ELS and/or adult stress (AS) corresponded to gene expression changes associated with treatment efficacy among human MDD patients. To test this, we leveraged two existing bulk RNA sequencing datasets. The first dataset from mouse NAc included assigned-female and assigned-male tissue after ELS alone, AS alone, or ELS+AS which was associated with the greatest rates of depression-like behavior (6, 13). ELS consisted of daily maternal separation and limited nesting from P10-17; AS consisted of chronic social defeat stress or subthreshold variable stress for male and female mice, respectively. The second dataset included white blood cell-derived RNA-seq profiles from self-identified female and male MDD patients both before and after randomly assigned treatment with either escitalopram or desvenlafaxine for eight weeks and assessment for treatment efficacy (Fig. 1A) (23). Escitalopram (brand name Lexapro) is a serotonin-selective reuptake inhibitor; desvenlafaxine (brand name Pristiq) is a serotonin-norepinephrine reuptake inhibitor. Data across both antidepressant treatments were combined for these analyses. Only genes common to both humans and mice and expressed in both tissues were kept. To assess similarities between these datasets across the entire transcriptome in a threshold-free manner, we used rank–rank hypergeometric overlap analysis (Fig. 1B). To evaluate potential sex differences, and because we found opposite patterns of gene expression response to antidepressant treatment and stress across sexes (SI Appendix, Fig. S1 A and B and refs. 15, 24, and 25), we analyzed female and male ELS and/or AS datasets separately.
Fig. 1.
Comparing transcriptomic patterns of early-life and/or adult stress in mice with antidepressant treatment efficacy in humans. (A) Study design for white blood cell (WBC)-derived RNA-seq from healthy control subjects or patients with major depressive disorder (MDD), taken before and after antidepressant treatment with either escitalopram or desvenlafaxine and assessed for treatment response or nonresponse. (B) Key for threshold-free comparisons of differentially expressed genes (DEGs) by two-sided rank–rank hypergeometric overlap (RRHO) analysis: Genes may be coregulated or oppositely regulated between two comparisons. Pixels represent the overlap between DEGs in each comparison. The significance of overlap [−log10(P-value)] of a hypergeometric test is color-coded, with a fixed maximum of 100 across all comparisons shown. Genes along each axis are sorted from most to least significantly regulated from the middle to outer corners. (C–E) RRHO’s comparing DEGs related to treatment efficacy from female WBC with DEGs related to ELS and/or AS from female mouse NAc. Gene ontology analysis of genes co-up-regulated (orange, F) or co-down-regulated (teal, G) from (E). (H–J) RRHO’s comparing DEGs related to treatment efficacy from male WBC with DEGs related to ELS and/or AS from male mouse NAc. Gene ontology analysis of genes co-up-regulated (orange, K) or co-down-regulated (teal, L) from (J). ELS-only and AS-only DEGs are vs. standard-reared control; ELS+AS DEGs are v. AS alone to assess the difference in gene expression after AS with vs. without prior ELS.
Despite differences in species and tissue type, we found correspondence among transcriptional signatures of treatment response that varied by sex and life-course history of stress. Among females, gene expression patterns in NAc after ELS alone (Fig. 1C) and AS alone (Fig. 1D) were most similar to gene expression patterns in the blood of antidepressant responders, while gene expression patterns following ELS+AS were most similar to antidepressant nonresponders (Fig. 1E). Genes co-up-regulated by ELS+AS and antidepressant nonresponse among females were enriched for functions related to responses to DNA damage, chromatin organization, and protein ubiquitination (Fig. 1F), while genes co-down-regulated were enriched for functions related to translation and apoptosis (Fig. 1G). Among males, gene expression patterns in NAc after ELS alone (Fig. 1H) and AS alone (Fig. 1I) were weakly similar to gene expression patterns in the blood of antidepressant nonresponders, while gene expression patterns following ELS+AS were not strongly associated with response to antidepressant treatment in either direction (Fig. 1J). Nevertheless, genes co-up-regulated by ELS+AS and antidepressant nonresponse among males were enriched for functions related to autophagy, intracellular protein transport, and protein folding (Fig. 1 K and L).
Similarities in Transcriptional Patterns between ELS and Antidepressant Treatment Nonresponse in Mice.
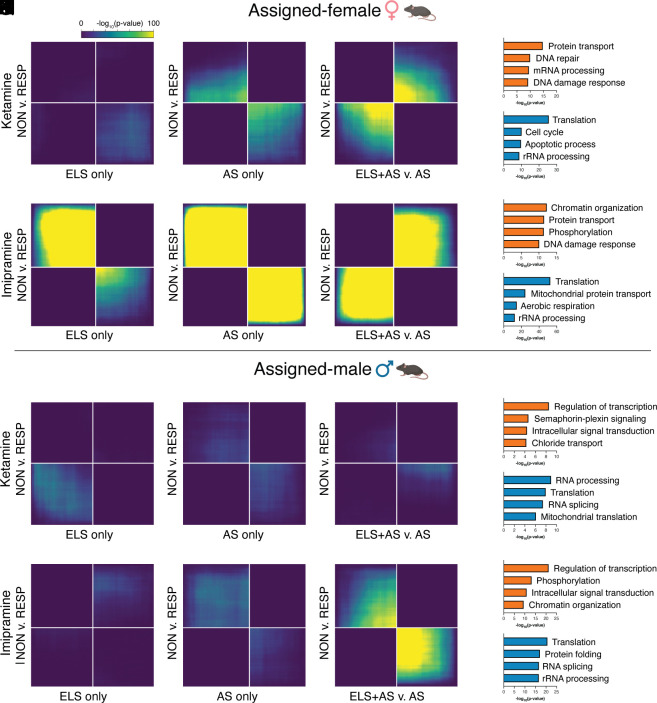
Grounded in these patient-relevant data, we next sought to determine whether gene expression signatures of stress across the lifespan correspond with antidepressant treatment response or nonresponse directly within mouse NAc in order to validate and extend the previous conclusions within a single tissue type. As above, we hypothesized that transcriptomic signatures of ELS would be most similar to those of treatment nonresponse. To test this, we integrated the RNA-seq data from ELS and/or AS NAc used above with previously published RNA-seq from male NAc after AS (chronic social defeat stress) and treatment of susceptible mice with either chronic imipramine (brand name Tofranil) or acute subanesthetic ketamine and behavioral categorization of nonresponse or response to treatment (Fig. 2 A–J) (12). Across these mouse comparisons, the strongest similarities were between female NAc and response to imipramine: Both ELS alone (Fig. 2F) and AS alone (Fig. 2G) had transcriptomic patterns most similar to imipramine responders, while ELS+AS in female mice was most similar to imipramine nonresponders (Fig. 2H). Similarly, ELS+AS among female mice was most similar to transcriptional signatures of nonresponse to ketamine (Fig. 2C), while AS alone weakly corresponded with positive ketamine treatment response (Fig. 2B), and ELS alone was not strongly associated with response to ketamine treatment in either direction (Fig. 2A). Put another way, for both ketamine and imipramine, the difference between experience of AS with prior ELS vs. AS without prior ELS predicts the difference between treatment nonresponse vs. response. Among male mice, only the transcriptional signature of the combination of ELS+AS was associated with positive imipramine response (Fig. 2 P–R). Neither ELS and/or AS was strongly associated with response to ketamine treatment in either direction (Fig. 2 K–M). We next applied gene ontology analysis to investigate whether genes co-up-regulated and co-down-regulated by ELS+AS and treatment were enriched for specific functions. Genes co-up-regulated by ELS+AS and ketamine nonresponse within female NAc were enriched for protein transport, DNA repair, mRNA processing, and DNA damage response (Fig. 2D). Genes co-up-regulated by ELS+AS and imipramine nonresponse were enriched for chromatin organization, protein transport, phosphorylation, and DNA damage response (Fig. 2I). Genes down-regulated by ELS+AS and up-regulated by ketamine response within male NAc were enriched for transcriptional regulation, semaphorin-complex signaling, intracellular signaling, and chloride transport (Fig. 2N). Finally, genes down-regulated by ELS+AS and up-regulated by imipramine response were enriched for transcriptional regulation, phosphorylation, intracellular signaling, and chromatin organization (Fig. 2S).
Fig. 2.
Comparing transcriptomic patterns of early-life and/or adult stress with antidepressant treatment efficacy within mouse NAc. Threshold-free RRHO analysis compares gene expression changes after ELS and/or AS with expression changes between antidepressant nonresponse vs. response, all within mouse NAc. Genes may be coregulated or oppositely regulated between two comparisons. Pixels represent the overlap between DEGs in each comparison, with the significance of overlap [−log10(P-value)] of a hypergeometric test color-coded, with a fixed maximum of 100 across all comparisons. RRHO comparisons were made separately for assigned-female (A–J) and assigned-male (K–T) mice against datasets for both ketamine and imipramine treatment efficacy from male mouse NAc, as indicated. Gene ontology analysis was performed for genes co-up-regulated (orange) or co-down-regulated (teal) from ELS+AS vs. AS comparisons: (D and E) correspond to C; (I and J) correspond to H; (N and O) correspond to M; and (S and T) correspond to R.
Testing Antidepressant Treatment Response in Mice.
We next sought to test the bioinformatic predictions that ELS would reduce antidepressant treatment efficacy, particularly among females. We tested both escitalopram (10 mg/kg i.p. for 21 d) to match the human comparisons and ketamine (saline for 20 d followed by a single 10 mg/kg i.p. dose) to match the mouse comparisons and as a rapid-acting antidepressant with distinct mechanisms of action (Fig. 3A).
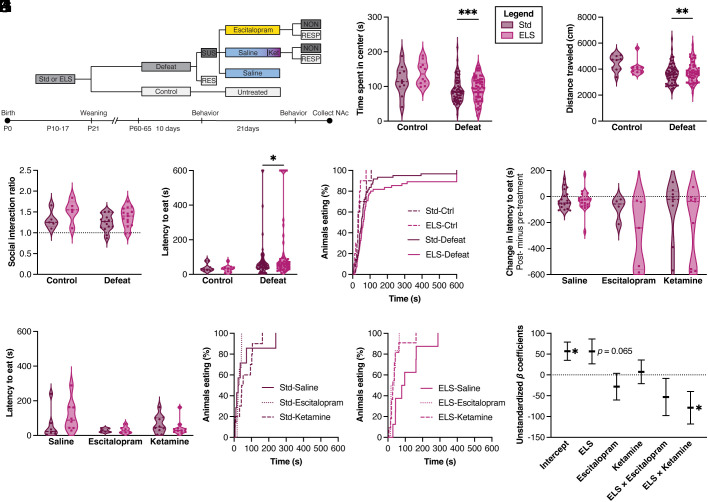
Fig. 3.
Behavioral testing of assigned-female mice. (A) Schematic of behavior and pharmacology timeline. Mice were first tested after social defeat stress and prior to pharmacological treatment (B–F; n = 10, 10, 60, 55), and again after treatment with either saline, 21 d of escitalopram, or 1 d of ketamine (G-K; n=7, 8, 6, 6, 10, 11). Open-field test: (B) Time spent in the center of an arena [main effect of social defeat stress: two-way ANOVA, F(1,132) = 17.32, P < 0.001]. (C) The total distance traveled in an arena was decreased by social defeat stress [F(1,132) = 10.40, P = 0.0016]. Social interaction: (D) Ratio of time spent in interaction zone with v. without aggressor (main effect of ELS: F(1,36) = 4.406, P = 0.0429). Novelty-suppressed feeding: (E) Pretreatment latency to eat [main effect of social defeat: F(1,131) = 3.966, P = 0.0485; main effect of ELS: F(1,30) = 7.611, P = 0.010. (F) Survival curve analysis of pretreatment latency to eat (log-rank Mantel–Cox across all groups: X2 = 17.98, P = 0.0004; between Std-Defeat and ELS-Defeat: X2 = 11.32, P = 0.0008; trend between Std-Ctrl and ELS-Ctrl: X2 = 2.846, P = 0.0916]. (G) Difference in latency to eat before and after treatment [escitalopram: two-way ANOVA, F(1,30) = 24.407, P < 0.001; ELS: F(1,30) = 7.611, P = 0.010; ELS × escitalopram interaction: F(1,30) = 9.375, P = 0.005]. (H) Posttreatment latency to eat [escitalopram v. saline: two-way ANOVA, F(1,30) = 24.407, P < 0.001]. (I and J) Survival curve of posttreatment latency (significant difference across all groups: log-rank Mantel–Cox, X2 = 16.83, P = 0.0185). (J) Among ELS mice, NSF latency differed between ELS-Def-Sal and ELS-Def-Esc groups (X2 = 7.932, P = 0.0049) and between ELS-Def-Sal and ELS-Def-Ket (X2 = 6.117, P = 0.0134). (K) Fixed effects model of posttreatment latency to eat as a function of stress and treatment [trend in overall difference across all groups: F(5,42) = 2.229, r2 = 0.2097, P = 0.0691; significant main effect of ELS: B = 56.48, t = 1.892, P = 0.0654; ELS × ketamine interaction (B = −78.94, t = 2.021, P = 0.0498), no main effect of escitalopram (B = −28.249, t = 0.880, P = 0.3837), and no ELS × escitalopram interaction (B = −53.19, P = 0.24)].
We first sought to validate that ELS and adult social defeat stress altered behavior among female (Fig. 3) and male (Fig. 4) mice. All mice were tested for exploratory and avoidant behavior in an open-field test. Among females, there was a main effect of social defeat to decrease time spent in the center of the open field (Fig. 3B), indicating that female mice are responsive to social defeat stress. However, there was no effect of ELS nor an interaction with defeat on open-field behavior among females (Fig. 3 B and C). Decreased social interaction (SI) has been a well-validated measure of response to chronic social defeat among males and to a lesser extent among females (26–29). There was a main effect of ELS but not of defeat, nor an interaction between early-life and adult stress, on SI ratio (Fig. 3D). Although female mice have been reported to have reduced SI ratio similar to males after social defeat stress (28, 30, 31), females in our study remained highly social.
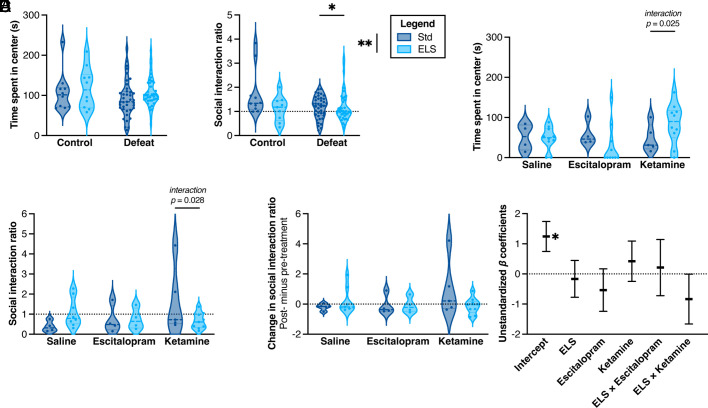
Fig. 4.
Behavioral testing of assigned-male mice. Mice were first tested after social defeat stress and prior to pharmacological treatment (A and B; n = 10, 10, 40, 38), and again after treatment with either saline, 21 d of escitalopram, or 1 d of ketamine (C–F; n = 4, 8, 4, 4, 5, 9). Open-field test: (A) Time spent in the center of an arena did not significantly differ by group. SI: (B) Ratio of time spent in interaction zone with vs. without aggressor [main effect of ELS: two-way ANOVA: F(1,94) = 7.662, P = 0.0068; main effect of social defeat: F(1,94) = 5.620, P = 0.0198; interaction of ELS × social defeat: F(1,94) = 4.401, P = 0.0386]. (C) Posttreatment time spent in the center of an arena in the open-field test [interaction of ELS × ketamine: F(1,21) = 5.841, P = 0.025]. (D) Posttreatment SI ratio [interaction of ELS × ketamine: F(1,22) = 5.546, P = 0.028]. (E) Difference in SI ratio before and after treatment [trend in the interaction of ELS × ketamine: F(1,22) = 3.479, P = 0.076]. (F) Fixed effects model of posttreatment latency to eat as a function of stress and treatment was not significant overall.
We previously found an effect of ELS and AS on novelty-suppressed feeding (NSF) behavior among females (13) and thus tested female mice in this test. We confirmed a main effect of social defeat on latency to eat (Fig. 3E). Survival curve analysis of latency to eat in the NSF also differed across groups (Fig. 3F). Post hoc analysis indicated a difference between Std-Defeat and ELS-Defeat conditions and a trend between Std-Ctrl and ELS-Ctrl. Together, the open-field and NSF tests show that female mice are sensitive to social defeat stress, and ELS exacerbates behavioral differences in an NSF test. Mice categorized as susceptible were then randomly assigned to saline, escitalopram, or ketamine treatment groups.
After treatment, female mice were tested again for behavior in the open-field and NSF tasks. There was a trend for an effect of escitalopram relative to saline on time in the center of the open field but no effect of ketamine and no interaction with ELS (SI Appendix, Fig. S2).
We next calculated behavioral difference scores for NSF latencies (posttreatment – pretreatment behavior) to determine the efficacy of treatment relative to each animal’s own initial behavior. Female mice were considered responsive to treatment if the NSF difference score was <0 (reduced latency to eat with treatment). Change in NSF latency with control saline treatment was tightly clustered around 0, indicating relatively stable NSF behavioral phenotype among both Std and ELS groups (Fig. 3G). There was a main effect of escitalopram treatment relative to saline on change in latency to eat with treatment (Fig. 3G), a main effect of ELS with ELS-mice taking longer to eat, and an interaction between ELS and escitalopram treatment. All escitalopram-treated females improved their NSF latency (difference score <0) and were considered responsive to treatment by this measure (Fig. 3G). There was also a main effect of ketamine to reduce feeding latency relative to saline (Fig. 3H) but no main effect of ELS and no interaction between ELS and ketamine treatment. Of note, 60-85% of ketamine-treated females improved their NSF latency and were considered responsive to treatment by this measure (Fig. 3G). Survival curve analyses of latency to eat in NSF were significantly different across treatment groups overall (Fig. 3 I and J). Among ELS mice, NSF latency differed between ELS-Def-Sal and ELS-Def-Esc groups and between ELS-Def-Sal and ELS-Def-Ket (Fig. 3 I and J). We also used fixed effects modeling to determine whether early experience, treatment, and their interaction predicted female NSF latency. There was an overall trend for this model, with an interaction between ELS and ketamine treatment as the strongest predictor of ameliorated female behavior (Fig. 3K). Escitalopram did not strongly predict behavior as a main effect or in interaction with ELS. In contrast, ELS was the strongest predictor of longer NSF latencies, interpreted as more depression-like behavior. Thus, in contrast to our bioinformatic predictions but in support of prior clinical observations, this model indicates that ketamine may be an efficacious treatment among females that previously experienced ELS.
Among males, there was no effect of either stress on open-field behavior prior to treatment (Fig. 4A). Whereas females were tested in the NSF task, males were tested for SI. On measures of SI, there was a main effect of ELS, a main effect of social defeat, and an interaction between ELS and social defeat on SI behavior (SI ratio) (Fig. 4B), validating previous findings that ELS sensitizes male mice to adult social defeat stress (6). Mice categorized as susceptible were then randomly assigned to treatment groups and tested again in the open-field and SI tests.
There was no effect of escitalopram relative to saline on open-field exploration, but there was a significant interaction between ELS and ketamine on open-field center time, such that ELS mice treated with ketamine increased center exploration, indicative of recovery (Fig. 4C). We again did not find an effect of escitalopram on SI behaviors (Fig. 4 D and E). However, with ketamine treatment, there was an interaction between ELS and ketamine on SI ratio (Fig. 4D) and a trend for an interaction on change in SI ratio (Fig. 4E). Treatment response was previously defined as an increase in SI behavior (12). Based on this definition, 25% of Std and ELS mice were responsive to escitalopram treatment, and 60% of Std mice and 33% of ELS mice were responsive to ketamine treatment, compared to a 25% spontaneous recovery rate among saline-treated males of both groups (Fig. 4E). However, fixed effects modeling including early experience, treatment, and their interaction did not significantly predict male SI ratio (Fig. 4F). Together across sexes, these data show little effect of escitalopram and a mixed effect of ketamine treatment, depending on behavior test: Among female mice, ketamine had a main effect on open-field behavior, while the interaction of ELS and ketamine was the strongest predictor of changes in NSF; and among males, ketamine-treated mice saw improvement in behavior in open field but not in SI. While the lack of effect of escitalopram is surprising given its efficacy in previous publications (32–34) and in females in our study (Fig. 3 G–J), this finding does mirror our bioinformatic predictions. A summary of behavioral findings across tests and treatments is in SI Appendix, Table S1.
Discussion
The inherently heterogeneous nature of depression is reflected in individual differences in antidepressant responsivity. Depressed patients with a history of ELS have worse forms of depression and may constitute a unique subtype of MDD. However, the ways in which ELS alters brain development to change the risk and course of depression and response to treatment are poorly understood. To parse the roles that sex and life-course stress play in predicting antidepressant response, we undertook a series of translational studies that leveraged peripheral and central gene biomarkers of treatment response among humans and mice treated with different antidepressants. We found that a history of ELS prior to adult stress altered patterns of gene expression in NAc which corresponded with patterns of antidepressant treatment failure from both mice and humans, across classes of antidepressants. Consistent with a greater risk for depression among individuals identifying as women, we also found transcriptional patterns predicting treatment failure to be strongest among female subjects. Among female mice susceptible to adult chronic social defeat stress and treated with either chronic escitalopram or acute ketamine, the strongest predictor of behavior was an interaction between ELS and ketamine treatment. Among males, however, early experience and treatment were poor predictors of behavior, mirroring our bioinformatic predictions.
Transcriptional Signatures of Early-Life and Adult Stress Predict Antidepressant Failure.
Despite several major caveats (different species, different tissue types in brain vs. periphery, and a lack of information on ELS history among human patients), we found transcriptomic patterns in mouse NAc after ELS+AS that corresponded with patterns of antidepressant nonresponse in human blood. The correspondence across mouse and human tissue was weaker than within the mouse brain, as would be expected based on larger transcriptomic differences across tissues (35), but these findings nonetheless support the conclusion that a history of ELS alters molecular profiles in the brain in a way that relates to human treatment efficacy. Importantly, our bioinformatic comparisons show that it is the difference between experience of adult stress with vs. without prior ELS that corresponds to the difference between treatment responders and nonresponders.
One important question that remains is whether it is a prior history of ELS specifically before additional adult stress that predicts antidepressant treatment failure at the molecular level or whether two hits of stress at any age (such as multiple adult stressors) would similarly impact the brain and treatment response. While we cannot currently make these bioinformatic predictions, clinical research suggests that timing, type, and number of stressful life events do impact the risk for MDD and treatment resistance (36–38).
Sex Differences in Depression and Antidepressant Treatment Response.
Depression disproportionately affects women wherein women are twice as likely to be diagnosed with depression than men. However, the amount of research on depression including sex as a biological variable in clinical studies and females in preclinical animal models is not commensurate with this higher prevalence. The few genome-wide studies that have directly examined sex-related factors at baseline and in stress and depression have exposed not only a lack of overlap in differentially expressed genes in other corticolimbic brain regions but also, more importantly, significantly opposite directions of and sex-specific gene expression between men and women with MDD (24, 25). Further, sex differences in behaviors and transcriptional patterns associated with depression are driven differently by gonadal vs. genetic sex (39). Within the NAc, which has been strongly associated with antidepressant response/failure across classes of antidepressants (12), there are also sex differences in transcription at baseline and in response to stress (15) indicating that sex-related molecular profiles of the brain may indeed affect treatment response. Indeed, we find clear sex differences associated with treatment response across life stress and all classes of antidepressants. However, we also find some similarities in the functions of genes altered across sexes and treatments in nonresponse. Dimension reduction by gene ontology analysis revealed an enrichment of co-up-regulated genes associated with chromatin organization with female escitalopram/desvenlafaxine response and female and male imipramine response. There was also an enrichment of co-down-regulated genes associated with translation across female escitalopram/desvenlafaxine response and female and male ketamine and imipramine response. This indicates that similar gene pathways may be engaged across sexes in treatment response despite differences in specific gene expression changes and efficacy. Overall, our results support the call for both research and medical care providers to consider the timing of stress experiences as well as sex during antidepressant treatment (40).
Sex Differences in Behavioral Pharmacology.
Our bioinformatic analysis of treatment response within the NAc of female mice exposed to two hits of stress strongly predicted treatment failure with escitalopram/desvenlafaxine and imipramine and weakly predicted failure with ketamine. Among males, transcriptomic profiles of ELS prior to adult stress weakly predicted escitalopram/desvenlafaxine treatment failure, did not show any correspondence with ketamine efficacy, and moderately predicted positive response to imipramine. In contrast, transcriptomic profiles derived from mice exposed to ELS alone (in which mice did not show depression-like behavioral profiles) and adult stress alone (with mixed depression-like behavior) largely predicted positive response to treatment among females, while again transcriptional signatures of life stress had little correspondence with treatment response among males.
Consistent with these predictions, we found significant sex-related differences in behavior, with females generally showing stronger response to treatment, both compared to saline and compared to males. To the extent that studies have examined the role of sex in antidepressant efficacy, there are indications that women may respond better to SSRI/SNRIs (such as escitalopram and desvenlafaxine) and men may respond better to tricyclics (such as imipramine) (41–45). Indeed, the only transcriptomic prediction for treatment efficacy after two hits of stress was among males with imipramine treatment. We did not treat mice with imipramine in the current study, and previous preclinical studies have not examined sex differences, but it would be interesting for future research to test these life-stress x sex predictions with imipramine, which may also show stronger treatment failure among ELS females. Although all female mice treated with escitalopram universally improved their NSF latencies, the main effect of ELS indicated that improvement was modestly but significantly stronger among standard-reared than ELS female mice. Thus, the degree of escitalopram efficacy was somewhat reduced by ELS. Somewhat surprisingly, we did not find a strong effect of either treatment among male mice susceptible to social defeat, with or without ELS. Ketamine appeared to improve anxiety-like behavior in an open field among ELS but not Std males, but improved SI behavior among Std but not ELS mice. Our findings on the mixed effects of ketamine treatment outcomes are similar to previous studies that also observed mixed effects of ketamine depending on assigned sex, degree of stress, and the behavioral test used (33, 46, 47). Although 237 mice were started in the study across two cohorts, given the complex study design, one caveat is that the treatment group size is still small. Another caveat is that only one dose of each drug was tested. Although dosages were based on literature from male mouse studies (12, 32, 34), it is possible that different doses are needed to separate the impact of ELS on treatment outcome, particularly for male mice receiving ketamine. While there are some indications of milder reduction in depression symptoms among women treated with ketamine than men, other research has indicated that female rodents have higher sensitivity to ketamine and that higher doses (e.g., 30 mg/kg) are needed for male mice (48–51).
Childhood Trauma and Ketamine as an Antidepressant.
While ketamine is primarily used as a dissociative anesthetic, it was first described as a potential antidepressant in 1973 (52). Ketamine acts acutely as a noncompetitive NMDA receptor antagonist (53, 54) and confers rapid-acting relief from depression within hours to days (55). Ketamine is long-lasting with an average duration of effect of days to weeks after a single treatment regime, with sustained effects described after multiple doses (56). In contrast, typical first-line antidepressants including tricyclics, SSRIs, and SNRIs (such as imipramine, escitalopram, and desvenlafaxine, respectively) act on monoamine systems, take several weeks for antidepressant effects to be felt, and must be taken daily. Given these contrasts, there has been increasing interest in ketamine as an antidepressant, particularly for treatment-resistant depression (1, 57). Recent research has suggested that while ELS may predispose a worse response to traditional antidepressant treatment (9), ketamine may in fact be a more efficacious treatment among individuals with high childhood trauma burden (10). Here, transcriptional signatures of ELS and/or adult stress showed low correspondence with signatures of ketamine response or nonresponse in NAc with the exception of ELS+AS in females corresponding to ketamine nonresponse (Fig. 2C). Contrary to our bioinformatic predictions but consistent with the hypothesis that ketamine could be more effective in patients with high childhood trauma (10), the strongest predictor of improved female NSF latencies in our model was the interaction between ELS and ketamine treatment (Fig. 3M). Ketamine has also been described as a potential prophylactic that can promote resilience in the face of later stress (58–61). It is therefore possible that treatment of ELS-exposed mice prior to a second hit of adult stress would be more efficacious than treatment after the second hit of stress. This possibility has translational appeal, given that children who have experienced early adversity are already identified as at risk and would therefore be better candidates for prophylactic treatment than the average population.
In Silico Predictions to Discover Novel Antidepressants.
One drawback of the current study is that while analyses predicted antidepressant nonresponse after ELS which is consistent with the literature (9), the ideal goal would be to identify treatments with greater potential efficacy in individuals with a history of ELS. Transcriptional signatures of many drugs and compounds have been generated in vitro (62). Future research may be able to screen these datasets in silico for compounds whose transcriptional signatures oppose that of ELS for additional testing in vivo. This would facilitate discovery of novel potential antidepressants with greater efficacy in this subpopulation of depressed patients who may have unique and long-lasting transcriptional signatures in the brain and need unique treatment plans.
Conclusions
This work provides translational, genome-wide evidence for sex- and early-life stress-related molecular adaptations in the brain that may contribute to antidepressant treatment response, potentially requiring unique pharmacological treatments.
Materials and Methods
Transcriptomic Datasets and Analysis.
Three independent RNA-seq datasets (SI Appendix, Materials and Methods) were analyzed for predicting the effects of ELS on antidepressant response. The first dataset includes the NAc of female- and male-assigned mice that experienced ELS and/or AS in a 2 × 2 design (GSE89692) (6, 13). The second dataset includes the NAc of adult, male-assigned mice that experienced chronic social defeat stress and were subsequently categorized as stress-naive, resilient, susceptible, and responsive/nonresponsive to either of the antidepressants imipramine or ketamine (GSE81672) (12). The primary comparisons were between imipramine/ketamine nonresponders v. responders. The third dataset (Fig. 1A) includes blood samples from healthy controls and patients with MDD before and after antidepressant treatment with escitalopram/desvenlafaxine. The primary comparisons were between nonresponders v. responders to antidepressant treatment. RNA-seq analyses for these datasets utilized the R packages “DESeq2,” “removeBatchEffect,” and “limma” (63).
RRHO analysis compared gene expression datasets in a threshold-free manner, detailed in SI Appendix, Materials and Methods (64, 65). Two-sided RRHO assessed coincident and opposite enrichment (65). Genes were ranked by −log10(P value) multiplied by fold change sign. Genes expressed (>2 base mean) in both lists were included for human-mouse comparisons. RRHO difference maps of pixels were calculated with respect to Z scores converted to P values represented by pixels. Gene ontology analysis utilized “clusterProfiler,” “org.Hs.eg.db,” and “org.Mm.eg.db” packages (66, 67).
Mice.
Subjects were C57BL/6J mice, bred and reared in-house (SI Appendix, Materials and Methods). Pups were assessed for sex at birth and assigned as “female” or “male” at weaning by their external genitalia on postnatal day P21. All experiments with animals were conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee at Princeton University.
Early-Life and Adult Stress Paradigms.
Litters were randomly assigned to ELS or Std conditions. ELS litters had limited nesting from P10 to 17, and pups were separated from their home cages for 3 to 4 h/day at random times each day (6, 13, 68). AS involved a chronic nondiscriminatory social defeat stress paradigm effective for both male and female mice (28, 69). Experimental female- and male-assigned mice were simultaneously exposed to attacks from a novel aggressor Swiss Webster mouse daily for 10 consecutive days. Mice then underwent behavioral testing for three consecutive days before tissue collection. More details are in SI Appendix, Materials and Methods.
Behavioral Testing.
Mice underwent behavioral testing beginning the day after social defeat and again after antidepressant treatment (Fig. 3A). For each round of testing, one behavioral test was conducted per day: SI, open-field, and NSF tests, fully detailed in SI Appendix, Materials and Methods. We tested male mice in the SI test and female mice primarily in the NSF test. All mice were tested on the open-field test.
Antidepressant Treatment.
Mice susceptible to social defeat stress were randomly assigned to saline, escitalopram, or ketamine treatment after the first round of behavioral testing (SI Appendix, Materials and Methods). Saline-treated mice received 0.1 mL sterile normal saline (i.p.) daily for 21 d, and escitalopram-treated mice received escitalopram (10 mg/kg, i.p.) for 21 d. The ketamine group received 0.1 mL sterile normal saline (i.p.) daily for 20 d, followed by a single dose of ketamine (10 mg/kg, i.p.) on day 21.
Statistical Analyses of Behavior.
A two-way ANOVA was used to determine main effects and interactions. A behavioral difference score was calculated for within-animal change in behavior with treatment. Differences in latency to eat in the NSF test were calculated by log-rank Mantel–Cox survival analysis. Fixed effects models were generated to examine the effects of ELS, treatment, and their interactions on latency to eat in the NSF test for females and SI ratio for males. Further details of statistical analyses are in SI Appendix, Materials and Methods.
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
This research was supported by the Brain and Behavior Research Foundation NARSAD (C.J.P.); NIH R00MH115096 (C.J.P.); NIH R01MH129643 (C.J.P.); PNI Research Innovator Award (C.J.P.); NYSCF (C.J.P.); and Robin Chemers Neustein (C.J.P.). C.J.P. is a New York Stem Cell Foundation Robertson Investigator. Some parts of the figures were created with BioRender.com. We gratefully acknowledge Dr. Annegret Falkner, Dr. Zoe Donaldson, and Dr. Deena Walker for their helpful discussions.
Author contributions
S.T.P., S.N.B., and C.J.P. designed research; S.T.P., S.N.B., C.J.C., and C.J.P. performed research; S.T.P., C.J.C., O.C.T., and C.J.P. analyzed data; L.M.F. and G.T. contributed sequencing data; and S.T.P. and C.J.P. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Data, Materials, and Software Availability
Mouse behavioral data have been deposited in figshare (10.6084/m9.figshare.22814492). Previously published data were used for this work [10.1126/science.aan4491 (GEO GSE89692) (6, 13); 10.1016/j.biopsych.2016.06.012 (GEO GSE81672) (12); 10.1371/journal.pone.0285123].
Supporting Information
References
- 1.Akil H., et al. , Treatment resistant depression: A multi-scale, systems biology approach. Neurosci. Biobehav. Rev. 84, 272–288 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoffman B., An overview of depression among transgender women. Depress. Res. Treat. 2014, 394283 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Witcomb G. L., et al. , Levels of depression in transgender people and its predictors: Results of a large matched control study with transgender people accessing clinical services. J. Affect. Disord. 235, 308–315 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Pellicane M. J., Ciesla J. A., Associations between minority stress, depression, and suicidal ideation and attempts in transgender and gender diverse (TGD) individuals: Systematic review and meta-analysis. Clin. Psychol. Rev. 91, 102113 (2022). [DOI] [PubMed] [Google Scholar]
- 5.Geschwind D. H., Flint J., Genetics and genomics of psychiatric disease. Science 349, 1489–1494 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peña C. J., et al. , Early life stress confers lifelong stress susceptibility in mice via ventral tegmental area OTX2. Science 356, 1185–1188 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jonson-Reid M., Kohl P. L., Drake B., Child and adult outcomes of chronic child maltreatment. Pediatrics 129, 839–845 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernet C. Z., Stein M. B., Relationship of childhood maltreatment to the onset and course of major depression in adulthood. Depress. Anxiety 9, 169–174 (1999). [PubMed] [Google Scholar]
- 9.Nanni V., Uher R., Danese A., Childhood maltreatment predicts unfavorable course of illness and treatment outcome in depression: A meta-analysis. Am. J. Psychiatry 169, 141–151 (2012). [DOI] [PubMed] [Google Scholar]
- 10.O’Brien B., Lijffijt M., Wells A., Swann A. C., Mathew S. J., The impact of childhood maltreatment on intravenous ketamine outcomes for adult patients with treatment-resistant depression. Pharmaceuticals 12, 133 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanson J. L., Williams A. V., Bangasser D. A., Peña C. J., Impact of early life stress on reward circuit function and regulation. Front. Psychiatry 12, 1799 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bagot R. C., et al. , Ketamine and imipramine reverse transcriptional signatures of susceptibility and induce resilience-specific gene expression profiles. Biol. Psychiatry 81, 285–295 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peña C. J., et al. , Early life stress alters transcriptomic patterning across reward circuitry in male and female mice. Nat. Commun. 10, 5098 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Russo S. J., Nestler E. J., The brain reward circuitry in mood disorders. Nat. Rev. Neurosci. 14, 609–625 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hodes G. E., et al. , Sex differences in nucleus accumbens transcriptome profiles associated with susceptibility versus resilience to subchronic variable stress. J. Neurosci. 35, 16362–16376 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ordoñes Sanchez E., et al. , Early life adversity promotes resilience to opioid addiction-related phenotypes in male rats and sex-specific transcriptional changes. Proc. Natl. Acad. Sci. U.S.A. 118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Polter A. M., Kauer J. A., Stress and VTA synapses: Implications for addiction and depression. Eur. J. Neurosci. 39, 1179–1188 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herzberg M. P., Gunnar M. R., Early life stress and brain function: Activity and connectivity associated with processing emotion and reward. Neuroimage 209, 116493 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanson J. L., Hariri A. R., Williamson D. E., Blunted ventral striatum development in adolescence reflects emotional neglect and predicts depressive symptoms. Biol. Psychiatry 78, 598–605 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balouek J.-A., et al. , Reactivation of early-life stress-sensitive neuronal ensembles contributes to lifelong stress hypersensitivity. J. Neurosci. 43, 5996–6009 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kronman H., et al. , Long-term behavioral and cell-type-specific molecular effects of early life stress are mediated by H3K79me2 dynamics in medium spiny neurons. Nat. Neurosci. 25, 667–676 (2021), 10.1038/s41593-021-00814-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parel S. T., Peña C. J., Genome-wide signatures of early-life stress: Influence of sex. Biol. Psychiatry 91, 36–42 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fuh S.-C., Fiori L. M., Turecki G., Nagy C., Li Y., Multi-omic modeling of antidepressant response implicates dynamic immune and inflammatory changes in individuals who respond to treatment. PLoS One 18, e0285123 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seney M. L., et al. , Opposite molecular signatures of depression in men and women. Biol. Psychiatry 84, 18–27 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Labonté B., et al. , Sex-specific transcriptional signatures in human depression. Nat. Med. 23, 1102–1111 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berton O., et al. , Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science 311, 864–868 (2006). [DOI] [PubMed] [Google Scholar]
- 27.Krishnan V., et al. , Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 131, 391–404 (2007). [DOI] [PubMed] [Google Scholar]
- 28.Yohn C. N., et al. , Chronic non-discriminatory social defeat is an effective chronic stress paradigm for both male and female mice. Neuropsychopharmacology 44, 2220–2229 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lopez J., Bagot R. C., Defining valid chronic stress models for depression with female rodents. Biol. Psychiatry 90, 226–235 (2021). [DOI] [PubMed] [Google Scholar]
- 30.Takahashi A., et al. , Establishment of a repeated social defeat stress model in female mice. Sci. Rep. 7, 12838 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harris A. Z., et al. , A novel method for chronic social defeat stress in female mice. Neuropsychopharmacology 43, 1276–1283 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sánchez C., et al. , Escitalopram, the S-(+)-enantiomer of citalopram, is a selective serotonin reuptake inhibitor with potent effects in animal models predictive of antidepressant and anxiolytic activities. Psychopharmacology 167, 353–362 (2003). [DOI] [PubMed] [Google Scholar]
- 33.Fitzgerald P. J., Yen J. Y., Watson B. O., Stress-sensitive antidepressant-like effects of ketamine in the mouse forced swim test. PLoS One 14, e0215554 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anwar M. J., Pillai K. K., Samad A., Vohora D., Effect of escitalopram on cardiomyopathy-induced anxiety in mice. Hum. Exp. Toxicol. 32, 632–639 (2013). [DOI] [PubMed] [Google Scholar]
- 35.Yang R. Y., et al. , A systematic survey of human tissue-specific gene expression and splicing reveals new opportunities for therapeutic target identification and evaluation. bioRxiv [Preprint] (2018). 10.1101/311563 (Accessed 6 June 2023). [DOI]
- 36.Amital D., Fostick L., Silberman A., Beckman M., Spivak B., Serious life events among resistant and non-resistant MDD patients. J. Affect. Disord. 110, 260–264 (2008). [DOI] [PubMed] [Google Scholar]
- 37.Kohn Y., et al. , Increased prevalence of negative life events in subtypes of major depressive disorder. Compr. Psychiatry 42, 57–63 (2001). [DOI] [PubMed] [Google Scholar]
- 38.McLaughlin K. A., Conron K. J., Koenen K. C., Gilman S. E., Childhood adversity, adult stressful life events, and risk of past-year psychiatric disorder: A test of the stress sensitization hypothesis in a population-based sample of adults. Psychol. Med. 40, 1647–1658 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paden W., et al. , Sex differences in adult mood and in stress-induced transcriptional coherence across mesocorticolimbic circuitry. Transl. Psychiatry 10, 59 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hodes G. E., Epperson C. N., Sex differences in vulnerability and resilience to stress across the life span. Biol. Psychiatry 86, 421–432 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berlanga C., Flores-Ramos M., Different gender response to serotonergic and noradrenergic antidepressants. A comparative study of the efficacy of citalopram and reboxetine. J. Affect. Disord. 95, 119–123 (2006). [DOI] [PubMed] [Google Scholar]
- 42.Khan A., Brodhead A. E., Schwartz K. A., Kolts R. L., Brown W. A., Sex differences in antidepressant response in recent antidepressant clinical trials. J. Clin. Psychopharmacol. 25, 318–324 (2005). [DOI] [PubMed] [Google Scholar]
- 43.Kornstein S. G., et al. , Gender differences in treatment response to sertraline versus imipramine in chronic depression. Am. J. Psychiatry 157, 1445–1452 (2000). [DOI] [PubMed] [Google Scholar]
- 44.Raskin A., Age-sex differences in response to antidepressant drugs. J. Nerv. Ment. Dis. 159, 120–130 (1974). [DOI] [PubMed] [Google Scholar]
- 45.LeGates T. A., Kvarta M. D., Thompson S. M., Sex differences in antidepressant efficacy. Neuropsychopharmacology 44, 140–154 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fitzgerald P. J., et al. , Sex- and stress-dependent effects of a single injection of ketamine on open field and forced swim behavior. Stress 24, 857–865 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johnston C. J., Fitzgerald P. J., Gewarges J. S., Watson B. O., Spencer-Segal J. L., Ketamine decreases HPA axis reactivity to a novel stressor in male but not female mice. bioRxiv [Preprint] (2021). 10.1101/2021.06.29.450387 (Accessed 3 May 2023). [DOI]
- 48.Carrier N., Kabbaj M., Sex differences in the antidepressant-like effects of ketamine. Neuropharmacology 70, 27–34 (2013). [DOI] [PubMed] [Google Scholar]
- 49.Coyle C. M., Laws K. R., The use of ketamine as an antidepressant: A systematic review and meta-analysis. Hum. Psychopharmacol. 30, 152–163 (2015). [DOI] [PubMed] [Google Scholar]
- 50.Wright K. N., Kabbaj M., Sex differences in sub-anesthetic ketamine’s antidepressant effects and abuse liability. Curr. Opin. Behav. Sci. 23, 36–41 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gerhard D. M., Duman R. S., Rapid-acting antidepressants: Mechanistic insights and future directions. Curr. Behav. Neurosci. Rep. 5, 36–47 (2018). [PMC free article] [PubMed] [Google Scholar]
- 52.Khorramzadeh E., Lotfy A. O., The use of ketamine in psychiatry. Psychosomatics 14, 344–346 (1973). [DOI] [PubMed] [Google Scholar]
- 53.Moghaddam B., Adams B., Verma A., Daly D., Activation of glutamatergic neurotransmission by ketamine: A novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J. Neurosci. 17, 2921–2927 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Autry A. E., et al. , NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 475, 91–95 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Berman R. M., et al. , Antidepressant effects of ketamine in depressed patients. Biol. Psychiatry 47, 351–354 (2000). [DOI] [PubMed] [Google Scholar]
- 56.Kim J.-W., Suzuki K., Kavalali E. T., Monteggia L. M., Bridging rapid and sustained antidepressant effects of ketamine. Trends Mol. Med. 29, 364–375 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Krystal J. H., Abdallah C. G., Sanacora G., Charney D. S., Duman R. S., Ketamine: A paradigm shift for depression research and treatment. Neuron 101, 774–778 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brachman R. A., et al. , Ketamine as a prophylactic against stress-induced depressive-like behavior. Biol. Psychiatry 79, 776–786 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McGowan J. C., et al. , Prophylactic ketamine alters nucleotide and neurotransmitter metabolism in brain and plasma following stress. Neuropsychopharmacology 43, 1813–1821 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McGowan J. C., et al. , Prophylactic ketamine attenuates learned fear. Neuropsychopharmacology 42, 1577–1589 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mastrodonato A., et al. , Acute (R, S)-Ketamine administration induces sex-specific behavioral effects in adolescent but not aged mice. Front. Neurosci. 16, 852010 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hodos R. A., Kidd B. A., Shameer K., Readhead B. P., Dudley J. T., In silico methods for drug repurposing and pharmacology. Wiley Interdiscip. Rev. Syst. Biol. Med. 8, 186–210 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Love M. I., Huber W., Anders S., Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Plaisier S. B., Taschereau R., Wong J. A., Graeber T. G., Rank-rank hypergeometric overlap: Identification of statistically significant overlap between gene-expression signatures. Nucleic Acids Res. 38, e169 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cahill K. M., Huo Z., Tseng G. C., Logan R. W., Seney M. L., Improved identification of concordant and discordant gene expression signatures using an updated rank-rank hypergeometric overlap approach. Sci. Rep. 8, 9588 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu T., et al. , clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innov. J. 2, 100141. (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yu G., Wang L.-G., Han Y., He Q.-Y., clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS 16, 284–287 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Peña C. J., Nestler E. J., Bagot R. C., Environmental programming of susceptibility and resilience to stress in adulthood in male mice. Front. Behav. Neurosci. 13, 40 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dieterich A., Liu T., Samuels B. A., Chronic non-discriminatory social defeat stress reduces effort-related motivated behaviors in male and female mice. Transl. Psychiatry 11, 125 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
Mouse behavioral data have been deposited in figshare (10.6084/m9.figshare.22814492). Previously published data were used for this work [10.1126/science.aan4491 (GEO GSE89692) (6, 13); 10.1016/j.biopsych.2016.06.012 (GEO GSE81672) (12); 10.1371/journal.pone.0285123].