Abstract
Background:
Veliparib is a poly-ADP-ribose polymerase (PARP) inhibitor, and it has clinical activity with every 3 weeks carboplatin and paclitaxel. In breast cancer, weekly paclitaxel is associated with improved overall survival. We aimed to determine the maximum tolerated dose (MTD) and recommended phase 2 dose (RP2D) of veliparib with weekly carboplatin and paclitaxel as well as safety, pharmacokinetics, and preliminary clinical activity in triple negative breast cancer (TNBC).
Methods:
Patients with locally advanced/metastatic solid tumors and adequate organ function were eligible. A standard 3 + 3 dose escalation design was followed by a TNBC expansion cohort. Veliparib doses ranging from 50-200 mg orally bid were tested with carboplatin (AUC 2) and paclitaxel (80 mg/m2) given weekly in a 21-day cycle. Adverse events (AE) were evaluated by CTCAE v4.0, and objective response rate (ORR) was determined by RECIST 1.1.
Results:
Thirty patients were enrolled, of whom 22 had TNBC. Two dose-limiting toxicities were observed. The RP2D was determined to be 150 mg PO bid veliparib with weekly carboplatin and paclitaxel 2 weeks on, 1 week off, based on hematologic toxicity requiring dose reduction in the first 5 cycles of treatment. The most common grade 3/4 AEs included neutropenia, anemia, and thrombocytopenia. PK parameters of veliparib were comparable to single-agent veliparib. In 23 patients with evaluable disease, the ORR was 65%. In 19 patients with TNBC with evaluable disease, the ORR was 63%.
Conclusions:
Veliparib can be safely combined with weekly paclitaxel and carboplatin, and this triplet combination has promising clinical activity.
Keywords: Triple negative breast cancer, phase 1 study, veliparib, pharmacokinetics, solid tumors, PARP, DNA damage
INTRODUCTION
Triple negative breast cancer (TNBC) makes up approximately 20% of all breast cancer diagnoses, and this subtype is associated with more aggressive disease and worse prognosis than hormone receptor positive breast cancers [1]. Approximately 20% of patients with TNBC harbor BRCA1/2 mutations, particularly BRCA1 [2, 3]. The association between TNBC and BRCA-mutated cancers provides an important rationale to include TNBC patients in clinical studies of agents that target DNA repair mechanisms [4]. In addition to mutational loss, BRCA1 can be inactivated via promoter methylation [5–8]. Mutational, epigenetic, and post-translational inactivation of BRCA function may identify patients likely to benefit clinically from poly (ADP-ribose) polymerase (PARP) inhibition [9]. It has, therefore, been proposed that methylation of the BRCA1 promoter region may help to predict response to PARP inhibitors [10].
The PARP family of enzymes is responsible for several important cellular processes including differentiation, gene regulation, protein degradation, replication, transcription, and overall maintenance of genomic stability [11, 12]. Of note, PARP1 expression has also been shown to be upregulated in TNBC [13]. Cancer cells generally express higher levels of PARP, which confers resistance to various chemotherapeutic agents and other forms of genotoxic stress [14].
Olaparib and talazoparib are PARP inhibitor compounds that are currently FDA-approved for metastatic breast cancer as monotherapy in patients with germline BRCA1/2 mutations [15, 16]. Veliparib (ABT-888) is an oral, potent small molecule inhibitor of PARP1 and PARP2. There have been several phase 1 and 2 studies evaluating veliparib as monotherapy or in combination with chemotherapy [17–28]. As a single agent, it is relatively well tolerated and has clinical activity in advanced solid malignancies [17, 18]. The rationale for combining PARP inhibitors with chemotherapy is that PARP inhibition delays the repair of DNA damage induced by chemotherapy [29]. With respect to platinum analogs, carboplatin promotes the formation of platinum-DNA adducts and subsequent DNA damage [30]. With inhibition of PARP by veliparib, the co-administration of veliparib and carboplatin is hypothesized to enhance DNA damage and result in greater antitumor activity than what is observed with either agent alone [31]. Early studies of PARP inhibitors in combination with platinum chemotherapy in metastatic TNBC have been challenging due to worsening of myelosuppression, which may be the result of PARP trapping [32, 33]. Veliparib is a potent PARPi but associated with minimal PARP trapping, which may allow for a more tolerable combination with platinum chemotherapy [32, 33].
Based on the wider use of weekly taxanes in the breast cancer population, the goal of the present study was to assess the feasibility, safety, and clinical activity associated with the addition of veliparib to weekly carboplatin and paclitaxel in patients with advanced solid tumors. For this study, patients were enrolled on this study regardless of BRCA status.
MATERIALS AND METHODS
Study Design
This was an open-label, multicenter, single-arm phase 1, dose-escalation trial with expansion cohort, National Cancer Institute (NCI) / Cancer Therapy Evaluation Program (CTEP) sponsored combination study of veliparib and weekly paclitaxel and carboplatin in patients with advanced solid tumors (NCI# 8620). The NCI Division of Cancer Treatment and Diagnosis supplied veliparib under a Collaborative Research and Development Agreement (CRADA) with Abbott Laboratories, which was transferred to Abbvie following separation from Abbott Laboratories.
The dose-escalation stage of the study used a standard 3+3 design to evaluate 4 possible doses of oral veliparib (50, 100, 150, and 200 mg bid) in combination with fixed standard intravenous doses of carboplatin (AUC 2 over 30 min) and paclitaxel (80 mg/m2 over 1 h) in 21-day treatment cycles (Figure 1). In the dose-escalation stage, patients received veliparib on days 1-5, 8-12, and 15-19, and carboplatin and paclitaxel on days 3, 10, and 17. This study was initially modeled after a every 3 week paclitaxel and carboplatin study with veliparib where the veliparib was given days 1-7 and carboplatin on day 3 [26]. Due to significant hematologic toxicities observed in the dose-escalation phase requiring dosing modification and delay, a protocol amendment was implemented on December 12, 2012 (Figure 1). In the dose-escalation cohort, after 4 completed cycles (12 weeks) and in the dose expansion cohort from cycle 1, paclitaxel and carboplatin were administered on days 3 and 10 every 21 days (2 weeks on, 1 week off schedule); veliparib was dosed continuously in the expansion cohort, based on data from additional ongoing studies. The determination of the recommended phase II dose (RP2D) was based on all-cycle tolerability of the regimen.
Figure 1.
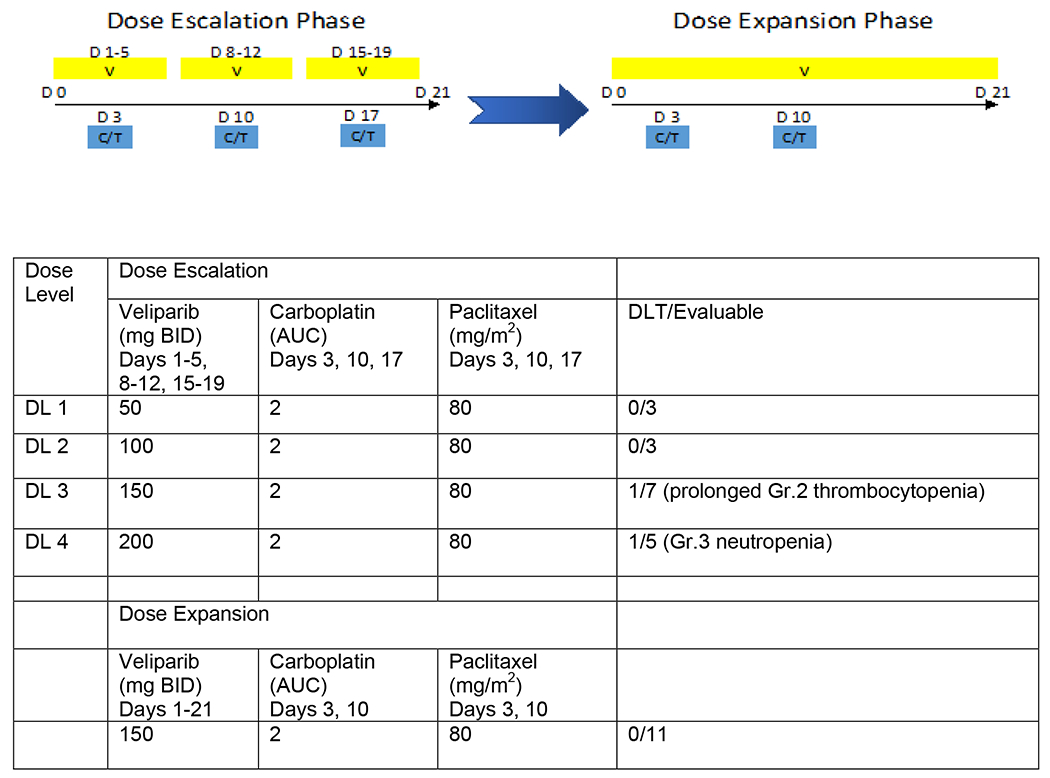
Administration schedule of veliparib (V), carboplatin (C) and paclitaxel (T) in the dose-escalation and dose-expansion phase. D=day. The blue arrow in the center represents the protocol amendment. Doses of veliparib, carboplatin, and paclitaxel at each dose level (DL) in the dose-escalation and in the dose- expansion phase. AUC=area under the curve; DL=dose level; BID=twice daily.
The protocol design and conduct complied with all applicable regulations, guidance, and local policies. ClinicalTrials.gov identifier: NCT01281150.
Patient Selection
Patients were enrolled between January 2011 and October 2014, and all patients completed treatment by April 2015. Informed consent was obtained from all individual participants included in the study. Eligible patients were at least 18 years old; had an Eastern Cooperative Oncology Group (ECOG) performance status of 0-1; had a histologically or cytologically confirmed solid tumor diagnosis for which no additional effective standard treatment was available; had a life expectancy of at least 2 months; and had adequate hematologic, renal function (defined as serum creatinine within normal institutional limits or creatinine clearance > 60 mL/min/1.73m2 for patients with creatinine levels above institutional normal), and liver function (defined as total bilirubin ≤ 1.5 times the institutional upper limit of normal and serum AST(SGOT) and ALT(SGPT) <2.5 times the institutional upper limit of normal). Any number of lines of prior treatment were allowed, including prior carboplatin, taxane, or PARP inhibitor therapy, but not all three in combination. Patients enrolled in the dose-expansion stage were required to have inoperable, recurrent, or metastatic TNBC (defined as estrogen receptor staining <10%; progesterone receptor staining <10%; HER-2 negative by IHC (staining at 0-2+) or FISH (ratio of < 2.2) and no more than 3 prior lines of therapy for metastatic disease. Concurrent treatment with hormonal therapy (tamoxifen, ovarian suppression with GnRH (Gonadotropin releasing hormone) agonists, aromatase inhibitors) or trastuzumab therapy was not allowed in breast cancer patients, but prostate cancer patients could continue GnRH agents. Patients with stable brain metastases were eligible. Notable exclusion criteria included active seizure or history of seizure disorder; chemotherapy within 4 weeks, radiotherapy within 2 weeks of entering the study, pregnancy, grade >1 peripheral neuropathy, or uncontrolled medical conditions. Documentation of BRCA mutation status was not required.
Safety Assessments
Safety evaluations were conducted at baseline and weekly thereafter and consisted of a medical history and physical examination, complete blood count and comprehensive metabolic profiles.
Dose-limiting toxicity (DLT) during the first cycle only and graded according to the standardized NCI Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 were defined as: grade ≥4 thrombocytopenia, grade ≥4 neutropenia for ≥7 days, febrile neutropenia, and grade ≥ 3 non-hematologic toxicity (excluding alopecia, nausea, vomiting, diarrhea, tumor pain and metabolic disturbances in patients not managed optimally). Any toxicity that resulted in a patient missing veliparib, paclitaxel, or carboplatin for longer than 1 week in cycle 1, regardless of attribution or grade, was also considered a DLT. The use of prophylactic growth factor support was not allowed in cycle 1. The MTD was defined as the dose level (DL) one level below the highest dose where ≥2 out of 6 patients experienced a DLT.
Tumor Response Assessment
Patients with measurable disease at baseline, who had received at least one cycle of therapy and who had their disease re-evaluated, were considered evaluable for objective response. Patients who exhibited objective disease progression prior to the end of cycle 1 were also considered evaluable for objective response. Patients with non-measurable disease were considered evaluable for non-target disease if they had received at least one cycle of therapy, and had their disease re-evaluated. Radiographic evaluation was performed at baseline and every 2 cycles based on RECIST version 1.1. If a patient’s tumor markers and clinical picture remained stable at the end of 4 cycles of treatment, restaging CT scans could be performed every 3 cycles at the discretion of the treating physician and principal investigator.
Pharmacokinetics
Peripheral blood samples were collected in heparinized tubes prior to and at 0.5, 1, 2, 4, and 8 h after veliparib dosing on cycle 1 day 3 for veliparib, carboplatin, and paclitaxel pharmacokinetic (PK) studies. Blood samples were centrifuged at 1,000 x g for 10 minutes and plasma was stored at ≤−70°C until analysis. Plasma concentrations of veliparib were quantitated with an LC-MS assay validated to FDA guidance [34]. Concentrations of ultrafilterable platinum were quantitated by atomic absorption spectrophotometry (AAS) as previously described [35]. Plasma concentrations of paclitaxel were quantitated with an LC-MS/MS assay as described previously utilizing [13C6]-paclitaxel (AlsaChim, Illkirch Graffenstaden, France) as the internal standard [36]. Plasma PK parameters were derived from the data by non-compartmental methods with PK Solutions 2.0™ (Summit Research Services; Montrose, CO, USA). ANOVA tests were performed on log-transformed veliparib Cmax and AUC0-12 using dose as the group, and Kruskal-Wallis tests were performed on carboplatin and paclitaxel PK to assess the impact of veliparib dose.
Statistical Analysis
SAS software (version 9.4) was used to analyze demographic, adverse events, and efficacy data. Patients enrolled on study but who did not receive any drug treatment were excluded from all analyses. Patients who received any study treatment were evaluable for toxicities. Adverse events that were possibly, probably, or definitely related to the treatment were considered. For PK analysis, Kruskal–Wallis and one-way ANOVA tests were performed with GraphPad Prism (San Diego, CA).
RESULTS
Patient Demographics
Patient demographics and baseline characteristics are presented in Table 1. A total of 30 patients were enrolled (median age, 52 years [range 30-83], and the median number of prior lines of therapy was 3 [range 0-8]. Twenty-four patients had breast cancer, and of this group, 22 patients had TNBC.
Table 1.
Patient demographics.
| All patients | TNBC patients | |
|---|---|---|
| Characteristic | N=30 | N = 22 |
| Median age (range) – years | 52 (30-83) | 50 (30-66) |
| Sex – no. (%) | ||
| Male | 3 (10) | 0 |
| Female | 27 (90) | 22 (100) |
| Race – no. (%) | ||
| White | 27 (90) | 21 (95) |
| Asian | 2 (7) | 1(5) |
| Black or African American | 1 (3) | |
| Tumor Type – no. (%) | ||
| Breast | 24 (80) | 22 (100) |
| Lung | 2 (7) | |
| Cervix | 1 (3) | |
| Gastric | 1 (3) | |
| Ovarian | 1 (3) | |
| Prostate | 1 (3) | |
| BRCA status – no. (%) | ||
| Positive* | 7 (23) | 5 (23) |
| Negative | 9 (30) | 9 (41) |
| Unknown | 14 (47) | 8 (36) |
| ECOG performance status – no. (%) | ||
| 0 | 19 (63) | 14 (64) |
| 1 | 11 (37) | 8 (36) |
| Median Number of prior chemo regimens (range) | 3 (0-8) | 3 (0-6) |
| Median Number of cycles (range) | 6 (1-38) | 6 (1-38) |
DLTs and P2RD
The initial dose-escalation phase enrolled patients receiving veliparib on days 1-5, 8-12, and 15-19, and carboplatin and paclitaxel on days 3, 10, and 17. Three patients each were treated at DL1 and DL2 without DLT. One patient treated at DL3 experienced a DLT (prolonged grade 2 thrombocytopenia), prompting the evaluation of three more patients (total 7 patients treated at DL3), with no additional DLTs. Five patients were treated at DL4 before DL4 was closed to enrollment and one patient experienced DLT of grade 3 neutropenia at DL4. Eleven of 18 patients underwent dose reduction within the first four cycles of therapy due to hematologic toxicity (grade ≥3 neutropenia and thrombocytopenia). Dose modifications for veliparib, carboplatin, and paclitaxel are described in Figure 2. Given this increased hematologic toxicity, the protocol was subsequently amended. For patients who had been enrolled under the initial protocol, the treatment was modified such that after four cycles, all patients received continuous therapy with veliparib, with carboplatin and paclitaxel administered on days 3 and 10 only, with day 17 omitted. This continuous 21-day dosing schedule of veliparib was based on results from a clinical trial in ovarian cancer [37]. Therefore, the MTD was not formally reached and enrollment to DL4 was terminated, followed by expansion into the modified schedule. Of 12 patients enrolled in the expansion cohort, eleven were evaluable with one patient discontinuing therapy in cycle 1 due to progressing brain metastases. Of the eleven evaluable patients, none experienced DLT. The RP2D for veliparib in combination with weekly paclitaxel at 80 mg/m2 and a weekly carboplatin dose of AUC 2 given 2 weeks on, 1 week off, was determined to be 150 mg PO BID daily.
Figure 2.
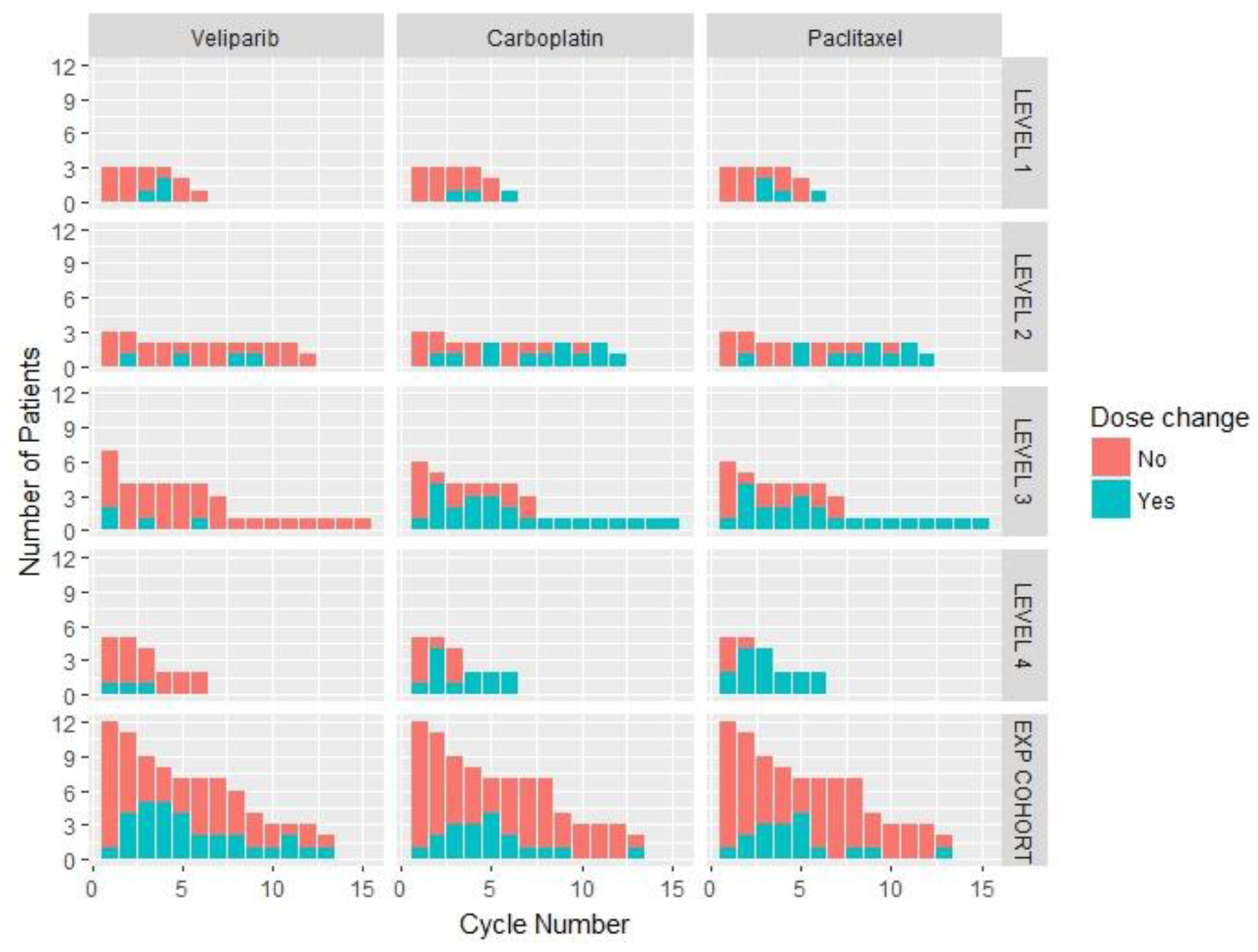
Pattern of dose modification of veliparib, carboplatin, and paclitaxel over time. Dose changes in veliparib, carboplatin, and paclitaxel across each dose level in dose escalation phase and in the dose expansion phase with the cycle numbers on the X axis and number of patients on the Y axis.
Adverse Events
Of the total 30 patients enrolled, 15 came off study for disease progression, 10 due to toxicity, 3 withdrew consent, 1 patient came off treatment for another disease, and 1 transitioned to single-agent veliparib via expanded access after cycle 38 as per investigator discretion. The most common all-grade and grade 3/4 toxicities encountered in this study across each dose level and all cycles are highlighted in Table 2. The main adverse events (AEs) included neutropenia (grade 3/4 in 18 patients [60%]), anemia (grade 3/4 in 7 patients [23%]), and thrombocytopenia (grade 3/4 in 4 patients [13%]). There was no clear relationship between veliparib dose level and either incidence or severity of adverse events.
Table 2.
Treatment-related grade ≥ 3 and all grade toxicities by dose level.
| All Patients (N = 30) |
Level 1 (n = 3) |
Level 2 (n = 3) |
Level 3 (n = 7) |
Level 4 (n = 5) |
Expansion (n = 12) |
|
|---|---|---|---|---|---|---|
| Any Grade ≥ 3 AE | 24 (80%) | 2 (67%) | 2 (67%) | 4 (57%) | 5 (100%) | 11 (92%) |
| Hematologic toxicity | ||||||
| Neutrophil count decreased | 18 (60%) | 2 (67%) | 2 (67%) | 3 (43%) | 5 (100%) | 6 (50%) |
| White blood cell decreased | 15 (50%) | - | 1 (33%) | 2 (29%) | 5 (100%) | 7 (58%) |
| Lymphocyte count decreased | 7 (23%) | - | - | 2 (29%) | 2 (40%) | 3 (25%) |
| Anemia | 7 (23%) | - | - | 2 (29%) | 2 (40%) | 3 (25%) |
| Platelet count decreased | 4 (13%) | - | - | 2 (29%) | - | 2 (17%) |
| Other | ||||||
| Nausea | 2 (7%) | - | - | - | 1 (20%) | 1 (8%) |
| Fatigue | 1 (3%) | - | - | - | 1 (20%) | - |
| Vomiting | 1 (3%) | - | - | - | 1 (20%) | - |
| Dehydration | 1 (3%) | - | - | - | - | 1 (8%) |
| Hypophosphatemia | 1 (3%) | - | - | - | - | 1 (8%) |
| Febrile neutropenia | 1 (3%) | - | - | - | 1 (8%) | |
| Any grade AE | 28 (93%) | 2 (67%) | 3 (100%) | 6 (86%) | 5 (100%) | 12 (100%) |
| Hematologic toxicity | ||||||
| Neutrophil count decreased | 24 (80%) | 2 (67%) | 3 (100%) | 4 (57%) | 5 (100%) | 10 (83%) |
| Platelet count decreased | 22 (73%) | 1 (33%) | 3 (100%) | 4 (57%) | 4 (80%) | 10 (83%) |
| Lymphocyte count decreased | 11 (37%) | - | - | 2 (29%) | 2 (40%) | 7 (58%) |
| White blood cell decreased | 22 (73%) | - | 3 (100%) | 4 (57%) | 5 (100%) | 10 (83%) |
| Anemia | 20 (67%) | - | 3 (100%) | 4 (57%) | 4 (80%) | 9 (75%) |
| Constitutional | ||||||
| Myalgia | 3 (10%) | - | 1 (33%) | - | - | 2 (17%) |
| Anorexia | 3 (10%) | - | - | 1 (14%) | - | 2 (17%) |
| Hot flashes | 4 (13%) | - | 1 (33%) | 1 (14%) | - | 2 (17%) |
| Fatigue | 18 (60%) | - | 3 (100%) | 3 (43%) | 4 (80%) | 8 (67%) |
| GI symptoms | ||||||
| Nausea | 16 (53%) | - | 3 (100%) | 2 (29%) | 1 (20%) | 10 (83%) |
| Diarrhea | 5 (17%) | - | 2 (67%) | - | - | 3 (25%) |
| Vomiting | 4 (13%) | - | - | - | 1 (20%) | 3 (25%) |
| Dysgeusia | 2 (7%) | - | 1 (33%) | 1 (14%) | - | - |
| AST increased | 3 (10%) | - | - | 1 (14%) | - | 2 (17%) |
| ALT increased | 4 (13%) | - | - | 1 (14%) | - | 3 (25%) |
| Electrolytes | ||||||
| Hypophosphatemia | 4 (13%) | - | - | 1 (14%) | - | 3 (25%) |
| Hyponatremia | 2 (7%) | - | 1 (33%) | 1 (14%) | - | - |
| Hyperglycemia | 2 (7%) | - | 1 (33%) | 1 (14%) | - | - |
| Hypocalcemia | 3 (10%) | - | 1 (33%) | 1 (14%) | - | 1 (8%) |
| Neurologic | ||||||
| Headache | 3 (10%) | - | - | 1 (14%) | - | 2 (17%) |
| Peripheral sensory neuropathy | 3 (10%) | - | - | 1 (14%) | - | 2 (17%) |
| Skin/soft tissue | ||||||
| Bruising | 2 (7%) | - | 1 (33%) | - | - | 1 (8%) |
| Rash maculo-papular | 2 (7%) | - | 1 (33%) | 1 (14%) | - | - |
Only adverse event possibly, probably, or definitely related to treatment were listed.
Clinical Activity
Of the 30 enrolled patients, 23 were evaluable for objective response. Prior treatment rates with taxane or platinum was ~88% and ~35%, respectively. The objective response rate (ORR) in the evaluable population was 65% (15/23 patients; amongst these, 14 with partial response (PR) and one patient with TNBC with complete response (CR); Figure 3). Twelve responders had TNBC; the ORR in the TNBC subgroup was 63% (12 out of 19). Within the TNBC responses, almost all had been previously treated with a taxane, but only 1 patient had prior platinum. The remaining partial responses were seen in 1 ovarian, 1 lung adenocarcinoma, and 1 gastric cancer patient. In the TNBC subgroup, the objective response rate in BRCA mutation positive and BRCA mutation negative were 67% (2 out of 3) and 75% (6/8), respectively. The objective response rate in 8 evaluable TNBC patients with unknown BRCA status was 50%. There were 5 patients with non-measurable disease and in this group, one had PD. The median progression-free survival in the entire population and in patients with TNBC was 8.4 months (95% CI, 3.9-10.0) and 7.1 months (2.4-10.6), respectively.
Figure 3.
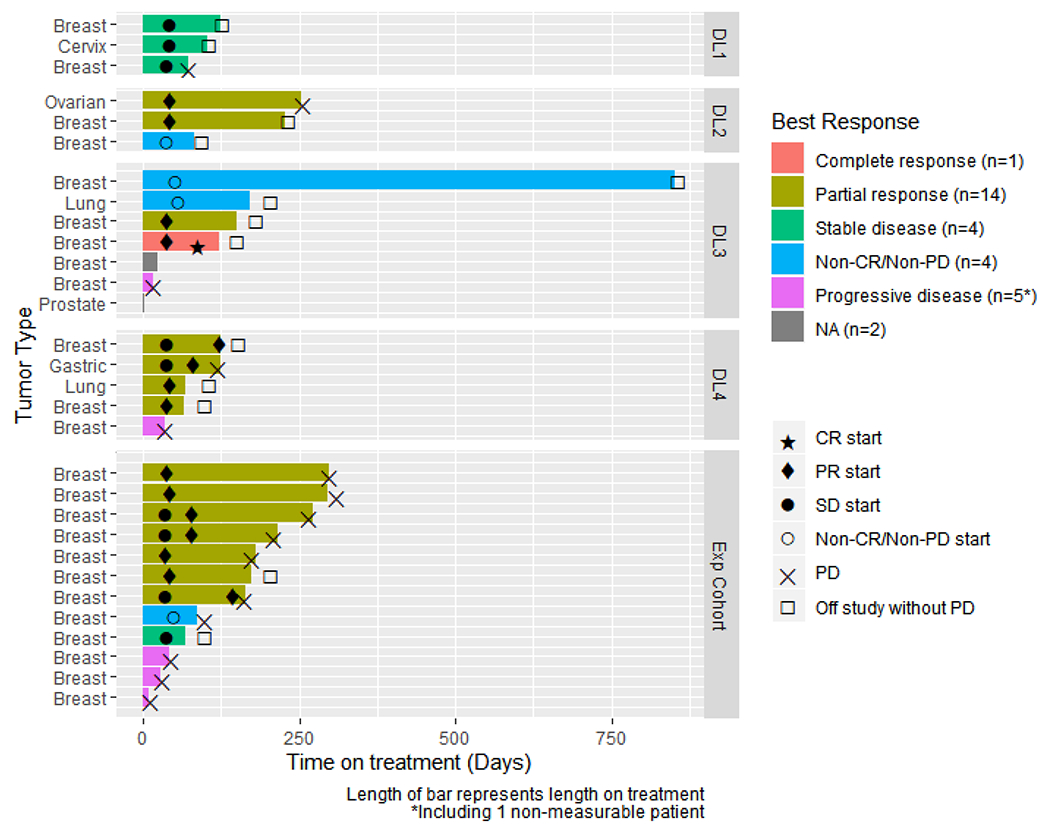
Swimmers plot representing the time on treatment and the response status. Duration of treatment and response in patients treated with veliparib combined with paclitaxel and carboplatin. Time on treatment is plotted for patients with RECIST and responses. One patient transitioned to single-agent veliparib via expanded access after cycle 38. Two patients were not evaluable (NA) for response. Symbols indicate the initiation of clinical benefit/response as indicated in the figure key. Progressive disease (PD) is indicated by an “X”, while an off-study event unrelated to PD is indicated by an open box.
Pharmacokinetics
Twenty-eight patients had evaluable veliparib PK, see Suppl.Table 1. Both AUC0-12 and Cmax appeared to increase linearly with dose, and no statistical significance was detected from either dose-normalized exposure parameter (Figures 4A and 4B). The half-life and clearance across all dose levels for all evaluable patients was 4.8 hours and 23.2 L/h, respectively. Twenty patients had evaluable carboplatin PK. Ultrafilterable carboplatin PK parameters are presented in Suppl.Table 2. Carboplatin Cmax and AUC0-∞ were not impacted by the dose of veliparib administered. (Figures 4C and 4D). Twenty-three patients had evaluable paclitaxel PK. Paclitaxel Cmax and AUC0-∞ are presented in Suppl.Table 3. Paclitaxel exposure was not altered across veliparib doses (Figure 4E and 4F). There were no differences in drug exposure (measured by AUC and Cmax) in patients who had grade 3 or 4 toxicity during cycle 1 as compared to patients who did not have grade 3 or 4 toxicity during cycle 1.
Figure 4.
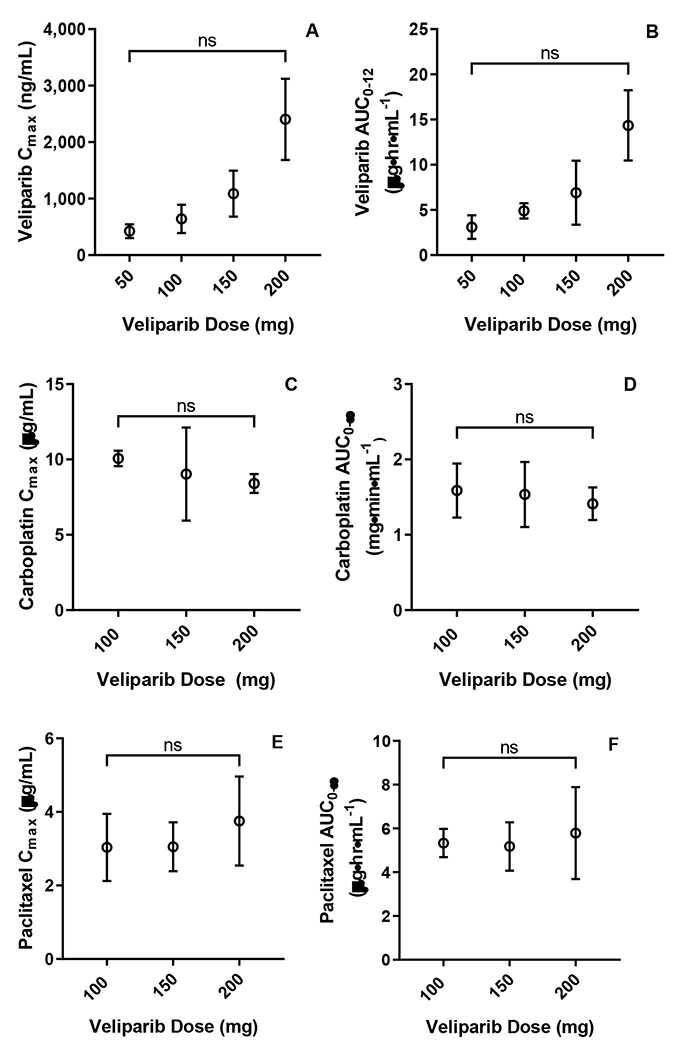
Pharmacokinetics. (A) Veliparib Cmax across investigated dose levels (p-value from ANOVA testing of dose-normalized Cmax = 0.055). (B) Veliparib AUC0-12 across investigated dose levels SD (p-value from ANOVA testing of dose-normalized AUC0-12= 0.284). (C) Carboplatin Cmax across investigated veliparib dose levels (p-value=0.670). (D) Carboplatin AUC0-∞ across investigated veliparib dose levels (p-value=0.757). (E) Paclitaxel Cmax across investigated veliparib dose levels (p-value 0.431). (F) Paclitaxel AUC0-∞ across investigated veliparib dose levels (p-value 0.554). All points represent mean PK parameter and error bars represent SD.
DISCUSSION
In this phase 1 study, we have shown that continuous dosing of veliparib at a dose of 150 mg bid combined with weekly carboplatin (AUC 2) and paclitaxel (80 mg/m2) using a 2-weeks on, 1- week off schedule is well tolerated with a manageable safety profile. The toxicity profile of veliparib in combination with carboplatin and paclitaxel is consistent with what is typically observed with carboplatin and paclitaxel doublet chemotherapy, with myelosuppression being the most common adverse event [26, 38, 39]. While there are a number of PARP inhibitors approved as single agents for the treatment of BRCA-mutated breast cancer; the role of PARP inhibition in sporadic TNBC remains uncertain. There are a few large studies that have studied veliparib in combination with chemotherapy in patients with sporadic and germline BRCA mutated TNBC. Veliparib occupies a unique space given its ability to combine with chemotherapy as well as to have single-agent efficacy (Suppl.Table 4).
The single agent phase 1 trial reported a 23% ORR in BRCA mutated patients, and response was associated with increased exposure/dose [28]. A similar ORR of 26% was observed in BRCA mutated ovarian cancer at a dose of 400 mg BID veliparib [40].
Focusing on breast cancer trials, the I-SPY-2 trial evaluated the benefit of adding veliparib (50 mg BID) and carboplatin (AUC 6) once every three weeks to standard chemotherapy with weekly paclitaxel (80 mg/m2) relative to weekly paclitaxel alone, both prior to doxorubicin (A) and cyclophosphamide(C) in the neo-adjuvant setting in early-stage TNBC patients [23]. This trial demonstrated that the addition of veliparib and carboplatin to standard chemotherapy doubled the rate of complete pathological response (cPR) rate from 26% (95% Bayesian probability interval PI, 9-43%) to 51% (95% PI, 36-66%) with veliparib and carboplatin added to paclitaxel with manageable toxicity [23]. In I-SPY-2, however, platinum and veliparib were added to paclitaxel in the experimental arm with paclitaxel alone as the control arm, and the clinical activity of carboplatin alone added to paclitaxel was not evaluated. The BROCADE 2 phase 2 trial showed that the addition of 120 mg BID to carboplatin plus paclitaxel led to improved ORR in BRCA mutated TNBC although this did not translate into a PFS benefit. The SWOG S1416 phase 2 trial investigated the combination of cisplatin with and without veliparib in patients with metastatic TNBC [41]. In this trial, the veliparib dose was 300 mg BID days 1-14 in combination with cisplatin at 75 mg/m2 every 3 weeks. There was improvement in median PFS in patients with a BRCA-like phenotype, 5.7 vs. 4.3 months and PFS at 1 year of 20% vs. 7% with veliparib, although at the cost of a significantly higher rate of grade 3/4 neutropenia (46% vs. 19%).
BrighTNess was a Phase 3 randomized, double blind, placebo-controlled clinical trial that randomized patients to one of the three arms: weekly paclitaxel 80 mg/m2 plus carboplatin (AUC 6) every 3 weeks plus veliparib 50 mg BID versus weekly paclitaxel 80 mg/m2 plus carboplatin (AUC 6) every 3 weeks plus veliparib placebo versus weekly paclitaxel 80 mg/m2 for 12 weeks plus carboplatin placebo and veliparib placebo. Patients then received 4 cycles of standard AC chemotherapy every 3 or 4 weeks [42]. The cPR rate was 53%, 58%, and 31% in these 3 arms, respectively, demonstrating no added benefit from the addition of veliparib and carboplatin to paclitaxel relative to adding carboplatin alone to paclitaxel [42]. The BROCADE 3 phase 3 trial showed that the addition of 120 mg BID to carboplatin plus paclitaxel with continuation of veliparib monotherapy led to improved PFS (14.5 vs 12.6 months) in BRCA mutated TNBC [43]. Lastly, the VELIA phase 3 trial in patients with previously untreated stage III or IV high-grade serous ovarian cancer, the addition of 150 mg veliparib BID to the frontline regimen of carboplatin (AUC=6 Q3W) and paclitaxel (175 mg/m2 Q3W or 80 mg/m2 QW) followed by veliparib maintenance resulted in significantly longer PFS (23.5 vs 17.3 months) [44].
As observed within the single agent trial [28], there appears to be a dose-response relationship demonstrated with veliparib across clinical studies, and it is conceivable that the lower dose of 50 mg veliparib BID used in BrighTNess may also have contributed to the lack of clinical activity of veliparib observed [42]. These data suggest that the dose of the PARP inhibitor should be optimized to improve efficacy.
Veliparib appears to be less toxic in combination with chemotherapy than other PARP inhibitors, which have generally exacerbated myelosuppression, limiting dose or treatment duration of the PARP inhibitor and/or chemotherapy [43–48]. Several studies suggest that olaparib with carboplatin was not tolerable [47–50], and intensified hematologic toxicity resulting in significant dose reductions [49] and schedule delays [48]. Talazoparib, which has a single agent MTD of 1000 μg, proved difficult to combine even at 250 μg with carboplatin AUC5- 6 and paclitaxel 80 mg/m2 days 1, 8, 15 of 21- day cycles. Dose modification occurred in 87-100% of patients, and the study concluded that modification of the chemotherapy component should be considered for further development of this combination [51]. One potential advantage with veliparib, therefore, appears to be the ability to use it in combination with a number of other chemotherapeutic regimens [20, 52, 53].
The challenges in the use of PARP inhibitors with chemotherapy are important, and it may be that the potential added improvement in efficacy and expansion of use in a BRCA-like sporadic TNBC population does not outweigh the toxicity. It is conceivable that studies like BrighTNess were impeded by ineffective veliparib dosing. Understanding the potential for this sporadic TNBC population to derive benefit from PARP inhibitors similar to a germline BRCA-mutated population is important, and we believe that studies like S1416 with a defined BRCA-like biomarker need to be conducted in a definitive trial. The continued subclassification of TNBC is necessary to define the subtypes of TNBC that respond to targeted therapies.
CONCLUSION
In summary, intermittent veliparib in combination with weekly paclitaxel (80 mg/m2) and carboplatin (AUC 2) was not considered tolerable for extended cycles of therapy. However, the use of continuous veliparib at 150 mg bid with weekly paclitaxel and carboplatin on a 2-week on, 1-week off schedule resulted in a significant improvement in tolerability, and we recommend this modified schedule for patients with metastatic disease. This triple combination displayed promising clinical activity, especially in the cohort of patients with TNBC.
Supplementary Material
Funding
This study was supported in part by NCI grants U01CA099168, UM1CA186690, and U24CA247643. This project used the UPMC Hillman Cancer Center Cancer Pharmacokinetics and Pharmacodynamics Facility (CPPF) and was supported in part by award P30CA047904. The project described was supported by the National Institutes of Health through Grant Number UL1TR001857. This work was also supported by a Conquer Cancer Foundation Career Development Award to Shannon Puhalla.
Competing Interests
Jan Beumer’s institute received research support from AbbVie. Leisha Emens has received research support from Aduro Biotech, AstraZeneca, Breast Cancer Research Foundation, Corvus, Department of Defense, EMD Serono, Genentech, HeritX, Inc., Maxcyte, Merck, National Cancer Institute, NSABP Foundation, Roche, Translational Breast Cancer Research Consortium, and has served as a consultant for AbbVie, Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Celgene, Genentech, Gritstone, Lilly, Macrogenics, Medimmune, Molecuvax, Novartis, Peregrine, Replimune, Roche, Syndax, and Vaccinex. She has also received royalties from Aduro Biotech. Shannon Puhalla has received research support from AbbVie, Pfizer, Lilly, Novartis, Incyte, Covance-Bayer, AstraZeneca, Genentech, Medivation and has been a consultant for AbbVie, MedImmune, Celldex, Puma, Pfizer, AstraZeneca, Esai, and Nanostring. Stacie Shepherd is a former employee of Abbott, AbbVie, and Corcept and holds Abbott, AbbVie, and Corcept stocks.
Footnotes
STATEMENTS AND DECLARATIONS
The authors would like to thank all of the participating patients and their families and the network of investigators, research nurses and study coordinators.
This trial was registered under ClinicalTrials.gov Identifier: NCT01281150
Ethics Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Data Availability
Further data are available upon reasonable request after permission of the sponsor.
REFERENCES
- 1.Sorlie T, Wang Y, Xiao C, Johnsen H, Naume B, Samaha RR, Borresen-Dale AL: Distinct molecular mechanisms underlying clinically relevant subtypes of breast cancer: gene expression analyses across three different platforms. BMC genomics 2006, 7:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foulkes WD, Brunet JS, Stefansson IM, Straume O, Chappuis PO, Begin LR, Hamel N, Goffin JR, Wong N, Trudel M et al. : The prognostic implication of the basal-like (cyclin E high/p27 low/p53+/glomeruloid-microvascular-proliferation+) phenotype of BRCA1-related breast cancer. Cancer research 2004, 64(3):830–835. [DOI] [PubMed] [Google Scholar]
- 3.Lakhani SR, Van De Vijver MJ, Jacquemier J, Anderson TJ, Osin PP, McGuffog L, Easton DF: The pathology of familial breast cancer: predictive value of immunohistochemical markers estrogen receptor, progesterone receptor, HER-2, and p53 in patients with mutations in BRCA1 and BRCA2. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2002, 20(9):2310–2318. [DOI] [PubMed] [Google Scholar]
- 4.Isakoff SJ, Mayer EL, He L, Traina TA, Carey LA, Krag KJ, Rugo HS, Liu MC, Stearns V, Come SE et al. : TBCRC009: A Multicenter Phase II Clinical Trial of Platinum Monotherapy With Biomarker Assessment in Metastatic Triple-Negative Breast Cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2015, 33(17):1902–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vos S, van Diest PJ, Moelans CB: A systematic review on the frequency of BRCA promoter methylation in breast and ovarian carcinomas of BRCA germline mutation carriers: Mutually exclusive, or not? Critical reviews in oncology/hematology 2018, 127:29–41. [DOI] [PubMed] [Google Scholar]
- 6.Zhang L, Long X: Association of BRCA1 promoter methylation with sporadic breast cancers: Evidence from 40 studies. Scientific reports 2015, 5:17869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turner N, Tutt A, Ashworth A: Hallmarks of ‘BRCAness’ in sporadic cancers. Nature reviews Cancer 2004, 4(10):814–819. [DOI] [PubMed] [Google Scholar]
- 8.Turner NC, Reis-Filho JS: Basal-like breast cancer and the BRCA1 phenotype. Oncogene 2006, 25(43):5846–5853. [DOI] [PubMed] [Google Scholar]
- 9.Schouten PC, Linn SC: Challenges in the Use of DNA Repair Deficiency As a Biomarker in Breast Cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2015, 33(17):1867–1869. [DOI] [PubMed] [Google Scholar]
- 10.Kondrashova O, Topp M, Nesic K, Lieschke E, Ho GY, Harrell MI, Zapparoli GV, Hadley A, Holian R, Boehm E et al. : Methylation of all BRCA1 copies predicts response to the PARP inhibitor rucaparib in ovarian carcinoma. Nat Commun 2018, 9(1):3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tomoda T, Kurashige T, Moriki T, Yamamoto H, Fujimoto S, Taniguchi T: Enhanced expression of poly(ADP-ribose) synthetase gene in malignant lymphoma. American journal of hematology 1991, 37(4):223–227. [DOI] [PubMed] [Google Scholar]
- 12.Tutt A, Tovey H, Cheang MCU, Kernaghan S, Kilburn L, Gazinska P, Owen J, Abraham J, Barrett S, Barrett-Lee P et al. : Carboplatin in BRCA1/2-mutated and triple-negative breast cancer BRCAness subgroups: the TNT Trial. Nature medicine 2018, 24(5):628–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ossovskaya V, Koo IC, Kaldjian EP, Alvares C, Sherman BM: Upregulation of Poly (ADP-Ribose) Polymerase-1 (PARP1) in Triple-Negative Breast Cancer and Other Primary Human Tumor Types. Genes Cancer 2010, 1(8):812–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shiobara M, Miyazaki M, Ito H, Togawa A, Nakajima N, Nomura F, Morinaga N, Noda M: Enhanced polyadenosine diphosphate-ribosylation in cirrhotic liver and carcinoma tissues in patients with hepatocellular carcinoma. Journal of gastroenterology and hepatology 2001, 16(3):338–344. [DOI] [PubMed] [Google Scholar]
- 15.Litton JK, Rugo HS, Ettl J, Hurvitz SA, Goncalves A, Lee KH, Fehrenbacher L, Yerushalmi R, Mina LA, Martin M et al. : Talazoparib in Patients with Advanced Breast Cancer and a Germline BRCA Mutation. The New England journal of medicine 2018, 379(8):753–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robson M, Im SA, Senkus E, Xu B, Domchek SM, Masuda N, Delaloge S, Li W, Tung N, Armstrong A et al. : Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. The New England journal of medicine 2017, 377(6):523–533. [DOI] [PubMed] [Google Scholar]
- 17.Nishikawa T, Matsumoto K, Tamura K, Yoshida H, Imai Y, Miyasaka A, Onoe T, Yamaguchi S, Shimizu C, Yonemori K et al. : Phase 1 dose-escalation study of single-agent veliparib in Japanese patients with advanced solid tumors. Cancer science 2017, 108(9):1834–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steffensen KD, Adimi P, Jakobsen A: Veliparib Monotherapy to Patients With BRCA Germ Line Mutation and Platinum-Resistant or Partially Platinum-Sensitive Relapse of Epithelial Ovarian Cancer: A Phase I/II Study. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society 2017, 27(9):1842–1849. [DOI] [PubMed] [Google Scholar]
- 19.Anampa J, Chen A, Wright J, Patel M, Pellegrino C, Fehn K, Sparano JA, Andreopoulou E: Phase I Trial of Veliparib, a Poly ADP Ribose Polymerase Inhibitor, Plus Metronomic Cyclophosphamide in Metastatic HER2-negative Breast Cancer. Clinical breast cancer 2018, 18(1):e135–e142. [DOI] [PubMed] [Google Scholar]
- 20.Gray HJ, Bell-McGuinn K, Fleming GF, Cristea M, Xiong H, Sullivan D, Luo Y, McKee MD, Munasinghe W, Martin LP: Phase I combination study of the PARP inhibitor veliparib plus carboplatin and gemcitabine in patients with advanced ovarian cancer and other solid malignancies. Gynecologic oncology 2018, 148(3):507–514. [DOI] [PubMed] [Google Scholar]
- 21.Isakoff SJ, Puhalla S, Domchek SM, Friedlander M, Kaufman B, Robson M, Telli ML, Dieras V, Han HS, Garber JE et al. : A randomized Phase II study of veliparib with temozolomide or carboplatin/paclitaxel versus placebo with carboplatin/paclitaxel in BRCA1/2 metastatic breast cancer: design and rationale. Future oncology 2017, 13(4):307–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nishio S, Takekuma M, Takeuchi S, Kawano K, Tsuda N, Tasaki K, Takahashi N, Abe M, Tanaka A, Nagasawa T et al. : Phase 1 study of veliparib with carboplatin and weekly paclitaxel in Japanese patients with newly diagnosed ovarian cancer. Cancer science 2017, 108(11):2213–2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rugo HS, Olopade OI, DeMichele A, Yau C, van ′t Veer LJ, Buxton MB, Hogarth M, Hylton NM, Paoloni M, Perlmutter J et al. : Adaptive Randomization of Veliparib-Carboplatin Treatment in Breast Cancer. The New England journal of medicine 2016, 375(1):23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Somlo G, Frankel PH, Arun BK, Ma CX, Garcia AA, Cigler T, Cream LV, Harvey HA, Sparano JA, Nanda R et al. : Efficacy of the PARP Inhibitor Veliparib with Carboplatin or as a Single Agent in Patients with Germline BRCA1- or BRCA2-Associated Metastatic Breast Cancer: California Cancer Consortium Trial NCT01149083. Clin Cancer Res 2017, 23(15):4066–4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stoller R, Schmitz JC, Ding F, Puhalla S, Belani CP, Appleman L, Lin Y, Jiang Y, Almokadem S, Petro D et al. : Phase I study of veliparib in combination with gemcitabine. Cancer chemotherapy and pharmacology 2017, 80(3):631–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Appleman LJ, Beumer JH, Jiang Y, Lin Y, Ding F, Puhalla S, Swartz L, Owonikoko TK, Donald Harvey R, Stoller R et al. : Phase 1 study of veliparib (ABT-888), a poly (ADP-ribose) polymerase inhibitor, with carboplatin and paclitaxel in advanced solid malignancies. Cancer chemotherapy and pharmacology 2019, 84(6):1289–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pothuri B, Brodsky AL, Sparano JA, Blank SV, Kim M, Hershman DL, Tiersten A, Kiesel BF, Beumer JH, Liebes L et al. : Phase I and pharmacokinetic study of veliparib, a PARP inhibitor, and pegylated liposomal doxorubicin (PLD) in recurrent gynecologic cancer and triple negative breast cancer with long-term follow-up. Cancer chemotherapy and pharmacology 2020, 85(4):741–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manzo J, Puhalla S, Pahuja S, Ding F, Lin Y, Appleman L, Tawbi H, Stoller R, Lee JJ, Diergaarde B et al. : A phase 1 and pharmacodynamic study of chronically-dosed, single-agent veliparib (ABT-888) in patients with BRCA1- or BRCA2-mutated cancer or platinum-refractory ovarian or triple-negative breast cancer. Cancer chemotherapy and pharmacology 2022, 89(5):721–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Q, Kassab MA, Dantzer F, Yu X: PARP2 mediates branched poly ADP-ribosylation in response to DNA damage. Nat Commun 2018, 9(1):3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu G, Chang P, Lippard SJ: Recognition of platinum-DNA damage by poly(ADP-ribose) polymerase-1. Biochemistry 2010, 49(29):6177–6183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Helleday T: The underlying mechanism for the PARP and BRCA synthetic lethality: clearing up the misunderstandings. Molecular oncology 2011, 5(4):387–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dhawan MS, Bartelink IH, Aggarwal RR, Leng J, Zhang JZ, Pawlowska N, Terranova-Barberio M, Grabowsky JA, Gewitz A, Chien AJ et al. : Differential Toxicity in Patients with and without DNA Repair Mutations: Phase I Study of Carboplatin and Talazoparib in Advanced Solid Tumors. Clin Cancer Res 2017, 23(21):6400–6410. [DOI] [PubMed] [Google Scholar]
- 33.Hopkins TA, Ainsworth WB, Ellis PA, Donawho CK, DiGiammarino EL, Panchal SC, Abraham VC, Algire MA, Shi Y, Olson AM et al. : PARP1 Trapping by PARP Inhibitors Drives Cytotoxicity in Both Cancer Cells and Healthy Bone Marrow. Molecular cancer research : MCR 2019, 17(2):409–419. [DOI] [PubMed] [Google Scholar]
- 34.Parise RA, Shawaqfeh M, Egorin MJ, Beumer JH: Liquid chromatography-mass spectrometric assay for the quantitation in human plasma of ABT-888, an orally available, small molecule inhibitor of poly(ADP-ribose) polymerase. Journal of chromatography B, Analytical technologies in the biomedical and life sciences 2008, 872(1-2):141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Colville H, Dzadony R, Kemp R, Stewart S, Zeh HJ, Bartlett DL 3rd, Holleran J, Schombert K, Kosovec JE, Egorin MJ et al. : In vitro circuit stability of 5-fluorouracil and oxaliplatin in support of hyperthermic isolated hepatic perfusion. The Journal of extra-corporeal technology 2010, 42(1):75–79. [PMC free article] [PubMed] [Google Scholar]
- 36.Parise RA, Ramanathan RK, Zamboni WC, Egorin MJ: Sensitive liquid chromatography-mass spectrometry assay for quantitation of docetaxel and paclitaxel in human plasma. Journal of chromatography B, Analytical technologies in the biomedical and life sciences 2003, 783(1):231–236. [DOI] [PubMed] [Google Scholar]
- 37.Landrum LM, Brady WE, Armstrong DK, Moore KN, DiSilvestro PA, O’Malley DM, Tenney ME, Rose PG, Fracasso PM: A phase I trial of pegylated liposomal doxorubicin (PLD), carboplatin, bevacizumab and veliparib in recurrent, platinum-sensitive ovarian, primary peritoneal, and fallopian tube cancer: An NRG Oncology/Gynecologic Oncology Group study. Gynecologic oncology 2016, 140(2):204–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kelly K, Crowley J, Bunn PA Jr., Presant CA, Grevstad PK, Moinpour CM, Ramsey SD, Wozniak AJ, Weiss GR, Moore DF et al. : Randomized phase III trial of paclitaxel plus carboplatin versus vinorelbine plus cisplatin in the treatment of patients with advanced non--small-cell lung cancer: a Southwest Oncology Group trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2001, 19(13):3210–3218. [DOI] [PubMed] [Google Scholar]
- 39.Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, Mannel RS, DeGeest K, Hartenbach EM, Baergen R et al. : Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2003, 21(17):3194–3200. [DOI] [PubMed] [Google Scholar]
- 40.Coleman RL, Sill MW, Bell-McGuinn K, Aghajanian C, Gray HJ, Tewari KS, Rubin SC, Rutherford TJ, Chan JK, Chen A et al. : A phase II evaluation of the potent, highly selective PARP inhibitor veliparib in the treatment of persistent or recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer in patients who carry a germline BRCA1 or BRCA2 mutation - An NRG Oncology/Gynecologic Oncology Group study. Gynecologic oncology 2015, 137(3):386–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodler E, Sharma P, Barlow WE, Gralow JR, Puhalla SL, Anders CK, Goldstein L, Tripathy D, Brown-Glaberman UA, Huynh T-T et al. : Cisplatin with veliparib or placebo in metastatic triple-negative breast cancer and BRCA mutation-associated breast cancer (S1416): a randomised, double-blind, placebo-controlled, phase 2 trial. The lancet oncology 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Loibl S, O’Shaughnessy J, Untch M, Sikov WM, Rugo HS, McKee MD, Huober J, Golshan M, von Minckwitz G, Maag D et al. : Addition of the PARP inhibitor veliparib plus carboplatin or carboplatin alone to standard neoadjuvant chemotherapy in triple-negative breast cancer (BrighTNess): a randomised, phase 3 trial. The lancet oncology 2018, 19(4):497–509. [DOI] [PubMed] [Google Scholar]
- 43.Dieras V, Han HS, Kaufman B, Wildiers H, Friedlander M, Ayoub JP, Puhalla SL, Bondarenko I, Campone M, Jakobsen EH et al. : Veliparib with carboplatin and paclitaxel in BRCA-mutated advanced breast cancer (BROCADE3): a randomised, double-blind, placebo-controlled, phase 3 trial. The lancet oncology 2020, 21(10):1269–1282. [DOI] [PubMed] [Google Scholar]
- 44.Coleman RL, Fleming GF, Brady MF, Swisher EM, Steffensen KD, Friedlander M, Okamoto A, Moore KN, Efrat Ben-Baruch N, Werner TL et al. : Veliparib with First-Line Chemotherapy and as Maintenance Therapy in Ovarian Cancer. The New England journal of medicine 2019, 381(25):2403–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Balmana J, Tung NM, Isakoff SJ, Grana B, Ryan PD, Saura C, Lowe ES, Frewer P, Winer E, Baselga J et al. : Phase I trial of olaparib in combination with cisplatin for the treatment of patients with advanced breast, ovarian and other solid tumors. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO 2014, 25(8):1656–1663. [DOI] [PubMed] [Google Scholar]
- 46.Dent RA, Lindeman GJ, Clemons M, Wildiers H, Chan A, McCarthy NJ, Singer CF, Lowe ES, Watkins CL, Carmichael J: Phase I trial of the oral PARP inhibitor olaparib in combination with paclitaxel for first- or second-line treatment of patients with metastatic triple-negative breast cancer. Breast Cancer Res 2013, 15(5):R88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oza AM, Cibula D, Benzaquen AO, Poole C, Mathijssen RH, Sonke GS, Colombo N, Spacek J, Vuylsteke P, Hirte H et al. : Olaparib combined with chemotherapy for recurrent platinum-sensitive ovarian cancer: a randomised phase 2 trial. The lancet oncology 2015, 16(1):87–97. [DOI] [PubMed] [Google Scholar]
- 48.van der Noll R, Jager A, Ang JE, Marchetti S, Mergui-Roelvink MWJ, Lolkema MP, de Jonge MJA, van der Biessen DA, Brunetto AT, Arkenau HT et al. : Phase I study of continuous olaparib capsule dosing in combination with carboplatin and/or paclitaxel (Part 1). Investigational new drugs 2020, 38(4):1117–1128. [DOI] [PubMed] [Google Scholar]
- 49.Lee JM, Hays JL, Annunziata CM, Noonan AM, Minasian L, Zujewski JA, Yu M, Gordon N, Ji J, Sissung TM et al. : Phase I/Ib study of olaparib and carboplatin in BRCA1 or BRCA2 mutation-associated breast or ovarian cancer with biomarker analyses. Journal of the National Cancer Institute 2014, 106(6):dju089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lampert EJ, Hays JL, Kohn EC, Annunziata CM, Minasian L, Yu M, Gordon N, Sissung TM, Chiou VL, Figg WD et al. : Phase I/Ib study of olaparib and carboplatin in heavily pretreated recurrent high-grade serous ovarian cancer at low genetic risk. Oncotarget 2019, 10(30):2855–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leal TA, Sharifi MN, Chan N, Wesolowski R, Turk AA, Bruce JY, O’Regan RM, Eickhoff J, Barroilhet LM, Malhotra J et al. : A phase I study of talazoparib (BMN 673) combined with carboplatin and paclitaxel in patients with advanced solid tumors (NCI9782). Cancer medicine 2022, 11(21):3969–3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Atrafi F, Groen HJM, Byers LA, Garralda E, Lolkema MP, Sangha RS, Viteri S, Chae YK, Camidge DR, Gabrail NY et al. : A Phase I Dose-Escalation Study of Veliparib Combined with Carboplatin and Etoposide in Patients with Extensive-Stage Small Cell Lung Cancer and Other Solid Tumors. Clin Cancer Res 2019, 25(2):496–505. [DOI] [PubMed] [Google Scholar]
- 53.Rodler ET, Kurland BF, Griffin M, Gralow JR, Porter P, Yeh RF, Gadi VK, Guenthoer J, Beumer JH, Korde L et al. : Phase I Study of Veliparib (ABT-888) Combined with Cisplatin and Vinorelbine in Advanced Triple-Negative Breast Cancer and/or BRCA Mutation-Associated Breast Cancer. Clin Cancer Res 2016, 22(12):2855–2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ramalingam SS, Blais N, Mazieres J, Reck M, Jones CM, Juhasz E, Urban L, Orlov S, Barlesi F, Kio E et al. : Randomized, Placebo-Controlled, Phase II Study of Veliparib in Combination with Carboplatin and Paclitaxel for Advanced/Metastatic Non-Small Cell Lung Cancer. Clin Cancer Res 2017, 23(8):1937–1944. [DOI] [PubMed] [Google Scholar]
- 55.Han HS, Dieras V, Robson M, Palacova M, Marcom PK, Jager A, Bondarenko I, Citrin D, Campone M, Telli ML et al. : Veliparib with temozolomide or carboplatin/paclitaxel versus placebo with carboplatin/paclitaxel in patients with BRCA1/2 locally recurrent/metastatic breast cancer: randomized phase II study. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO 2018, 29(1):154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Further data are available upon reasonable request after permission of the sponsor.


