Abstract
Cancer-related pain is one of the most common and incapacitating symptoms for cancer patients. Cancer pain can be caused by diagnostic or therapeutic procedures, side effects and toxicity from therapy, or the cancer itself. Uncontrolled cancer-related pain is associated with inadequate quality of life and reduced functional status. Optimal pain management during the perioperative period requires a tailored approach. Interventions that are recommended for the management of acute surgical pain include regional anesthesia, local anesthetic infusions, non-opioid analgesics (ketamine, dexmedetomidine, lidocaine, and non-steroidal anti-inflammatory drugs), and opioids. Despite continued research efforts and advances in cancer treatment, opioids remain the most effective medication to treat moderate to severe cancer-related pain; however, their role has been changing significantly due to the opioid epidemic and opioid misuse. While pre-clinical and retrospective studies have shown a negative association between opioid use and cancer outcomes, randomized control trials have failed to confirm this association. The purpose of this review is to summarize the pharmacological management of acute cancer-related pain during the perioperative period with an emphasis on cancer recurrence.
Keywords: pain, opioids, multimodal analgesia, cancer progression, cancer, progression free-survival, overall survival, ketamine, lidocaine, dexmedetomidine
Introduction
It is estimated that 20.3 million people in the United States will survive cancer in 2030, a significant increase from the 15.5 million cancer survivors reported in 2016.1 Cancer patients experience pain because of the progression of the disease and/or cancer treatment (chemotherapy, radiotherapy).2 Additionally, patients with solid tumors who undergo curative-intent or palliative surgical procedures may experience significant acute pain from the surgery itself.
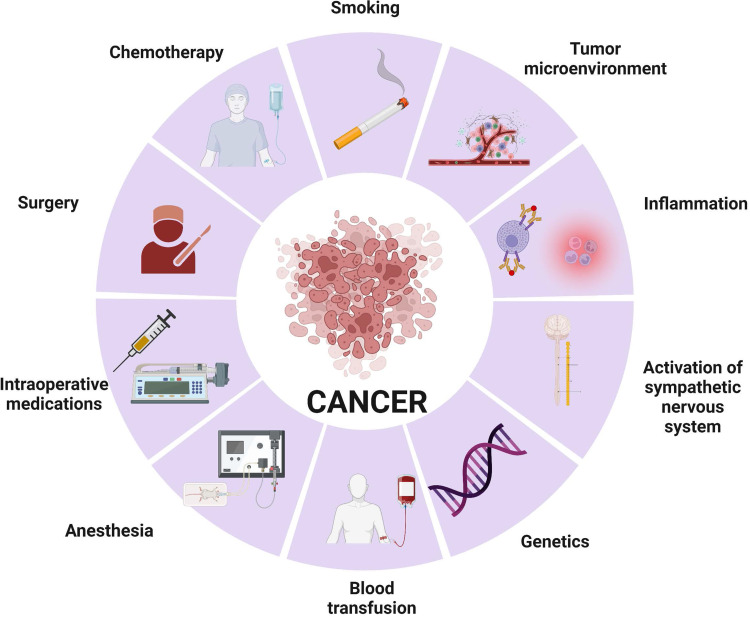
Cancer surgery continues to be a fundamental pillar for the treatment of solid tumors; however, preclinical data have suggested that cancer surgery and associated anesthesia might increase the risk of metastasis, minimal residual disease, and clinical cancer recurrence. The theories behind this finding include (Figure 1):
Surgery promotes inflammation followed by a compensatory anti-inflammatory response with immunosuppression. The inflammatory response is a necessary step for wound healing; however, the immunosuppression that follows the initial inflammation provides a window of opportunity for cancer cells to proliferate by inhibiting cell-mediated immunity. Importantly, the degree of immunosuppression is proportional to the degree of surgical trauma.3
Activation of the sympathetic nervous system: tissue trauma, inflammation, and pain activate the sympathetic nervous system and consequently modulate gene expression within cancer cells. Specifically, this activation promotes cancer cell metastasis by promoting tumor cell invasion and inhibiting cellular stress response.4
Anesthetic medications: a direct effect of opioids, volatile anesthetics, and other analgesic medications during surgery can modify intracellular signals involved in key aspects of migration, proliferation, and invasion of cancer cells.5
Figure 1.
Perioperative factors associated with cancer cell proliferation and metastasis during cancer surgery. Created with BioRender.com.
Acute post-surgical pain after oncological surgery can be severe yet challenging to manage during the perioperative period. Several factors contribute to this difficulty, including pre-existing oncological pain, opioid tolerance, or contraindications for non-opioid medications and/or regional anesthesia.6 Additionally, inadequate assessment and treatment of acute post-surgical pain in cancer patients can be associated with increased risk of opioid abuse/misuse and the development of postoperative chronic pain.7 Several risk factors for chronic post-surgical have been identified including pre-surgical and postoperative pain, type of surgery, postoperative complications, chemotherapy exposure, young age, and psychological factors (eg, anxiety and depression).8,9
Multimodal analgesia refers to the use of multiple pharmacological classes of analgesic medications to manage pain effectively.10 The primary objective of multimodal analgesia is to target different pain receptors or pathways, providing enhanced pain relief while minimizing the side effects associated with individual drug classes.11 Pharmacological treatment of acute surgical pain in cancer patients needs to be tailored to individual needs. This individualized approach may enhance outcomes and reduce cost. In the following section, we will review the pharmacological treatment of acute surgical pain and its potential impact on long-term cancer outcomes.
Methodology
We reviewed the literature from electronic database of PUBMED, COCHRANE and MEDSCAPE (2000–2023). Randomised controlled trials, retrospective studies, meta-analysis, and systematic reviews were included. The keywords for searcher were cancer, progression free-survival, overall survival, opioids, ketamine, lidocaine, dexmedetomidine, and non-steroidal anti-inflammatory drugs (NSAIDs). The articles that met the following criteria were included: a) the studies were written in English; b) the studies were performed in adults; c) the administration of opioids, ketamine, lidocaine, dexmedetomidine, and NSAIDS was perioperative in cancer patients; and d) the studies assessed the effect of the perioperative administration of opioids, ketamine, lidocaine, dexmedetomidine, and NSAIDS on cancer recurrence and overall survival.
Opioids
Opioids are the most common medication used for acute post-surgical pain in cancer patients. While they can effectively relieve pain, opioids are associated with other non-analgesic effects on cancer cell growth. This effect on tumor growth is still contradictory, as both growth-promoting and growth-inhibiting effects have been described. For instance, a high concentration of morphine (>10μM) significantly reduced cell proliferation and tumor growth in breast and colon cancer.12,13 Additionally, morphine has shown anti-apoptotic and anti-angiogenic properties.14 On the other hand, animal and retrospective clinical studies suggest that opioids could promote cancer cells proliferation and growth. The possible mechanisms involved in the opioid-induced cancer progression theory include:
Opioids induce immunosuppression: For instance, morphine administration has been shown to decrease the release of nitric oxide (NO), reactive oxygen species (ROS), cytokines IL-6, and TNF-a and reduce macrophage recruitment.15 Additionally, both morphine and fentanyl have been shown to suppress NK cell cytotoxicity, which is a critical cell with potent cytolytic activity against cancer cells.16 The immunosuppressive potential of opioids has been shown to depend on the capacity of the opioid to cross the blood–brain barrier since immunosuppression is mediated by central pathways.15,17 Thus, opioids such as codeine, morphine, methadone, fentanyl, and remifentanil, which easily cross the blood–brain barrier, have been found to possess high immunosuppressive potential, whereas buprenorphine, hydromorphone, tramadol, and oxycodone, which cross the blood–brain barrier to a lesser extent, have exhibited lesser immunosuppressive potential.
Opioids promote tumor proliferation, angiogenesis, and neovascularization on cancer cells expressing the mµ opioid receptor (MOR). For instance, in cervical cancer cells, morphine has been shown to increase cell proliferation and cell migration.18 Additionally, morphine and oxycodone increase endothelial cell tube formation and proliferation in a dose-dependent manner.19
When analysing the association between opioid administration during cancer surgery and cancer progression, the data is conflicting. For instance, intraoperative fentanyl administration has been associated with decreased overall survival in patients undergoing non-small cell lung cancer (NSCLC) resection.;20 however, in colon cancer, the use of intraoperative fentanyl did not impact recurrence or overall survival.21 Surprisingly, some authors found that intraoperative opioid administration improved recurrence-free survival in triple-negative breast cancer patients.22 Lastly, the latest meta-analysis addressing the relationship between intraoperative opioids and cancer prognosis found that intraoperative administration of opioids did not affect progression-free survival and overall survival; however, the administration of high dose of opioids post-operatively did have a negative impact on progression-free survival.23
Taken together, opioids are the most widely used medication to treat acute surgical cancer pain. It is unclear whether there is a negative or positive relationship between intraoperative opioid use and cancer progression after surgery. Unfortunately, the majority of the available data originates from preclinical studies and, evidently, clinical studies looking at this relationship during the perioperative period lack confirmatory data.
Dexmedetomidine
Dexmedetomidine is a potent and highly selective α-2 agonist. Dexmedetomidine exerts its effects through the activation of central presynaptical α-2 receptors in the locus coeruleus, which decreases the neurotransmission of norepinephrine in the synaptic cleft.24 Dexmedetomidine is used as a sedative, analgesic, anxiolytic, and sympatholytic that provides an effective technique to minimize opioid use in surgical and non-operating room anesthesia settings.25 In the context of cancer, dexmedetomidine has shown an anti-inflammatory profile. For instance, during gastric and liver cancer resections, dexmedetomidine diminished the levels of interleukin-6 (IL-6) and Tumor Necrosis Factor-α (TNF-α).26,27 Additionally, dexmedetomidine has been shown to preserve th1/th2 balance in colon cancer.28
In clinical studies, the data regarding the effect of dexmedetomidine on cancer outcomes are still scarce (Table 1). For instance, in patients with (NSCLC), the intraoperative use of dexmedetomidine was associated with worse overall survival.29 Another randomized control trial, patients with NSCLC who were also exposed to dexmedetomidine (2 mcg/kg/h for 15 minutes before anesthesia, 0.5mcg/kg/hour during surgery, and 0.25mcg/kg/hour until 24 hours after surgery) had less inflammation, improved cellular immune function, and improved quality of recovery. However, this study did not investigate cancer outcomes such as survival or recurrence-free survival.30 In uterine cancer surgery, the intraoperative administration of dexmedetomidine did not preserve the function of natural killer cells and did not affect cancer recurrence or overall mortality.31
Table 1.
Clinical Trials Assessing the Effect of Intraoperative Use of Dexmedetomidine on Cancer Outcomes
| Author | Cancer | Study Type | Dose | Cancer Outcomes |
|---|---|---|---|---|
| Cata et al29 | Lung | Retrospective | Not specified | Dexmedetomidine was associated with reduced OS but no effect in RFS. |
Abbreviations: OS, overall survival; RFS, recurrence-free survival.
In conclusion, dexmedetomidine is commonly used intraoperatively in cancer patients. It has been shown to have an anti-inflammatory profile and opioid sparing effect. However, the clinical data comparing the effect of this medication on cancer outcomes are still very limited.
Ketamine
Ketamine is a non-competitive antagonist of the N-methyl-D-aspartate (NMDA) receptor Ca2+ channel. The blockage of NMDA is the primary mechanism of action for anesthesia analgesia. Beyond its most well-known mechanism of action, ketamine also affects other receptors such as, but not limited to, dopaminergic, serotonergic, adrenergic, opioidergic, and cholinergic receptors.32
Ketamine infusions have been used regularly for the treatment of acute pain, but ketamine has gained special attention in the context of the treatment of chronic pain, opioid induce-hyperalgesia and opioid-tolerant patients.33 In subanesthetic doses, ketamine infusion allows the patient to remain awake while providing analgesia. The recommended dose for intraoperative use is 0.5–1 mg/kg bolus followed by a continuous infusion of 2–5mcg/kg/min. For palliative care, a recommended dose of 0.1 mg/kg/h with a maximum infusion rate of 0.2 mg/kg/hour.34
In chronic pain, ketamine infusion provides short-term analgesia in a dose-dependent manner.35 In opioid tolerant patients, ketamine infusions have been reported to improve analgesia in patients in uncontrolled pain receiving high doses of opioids.36 In the context of palliative care, continuous ketamine infusion at subanesthetic doses is considered an alternative to alleviate pain (including nociceptive and neuropathic pain) in patients with uncontrolled severe pain despite escalating doses of opioids.
Ketamine has been proposed to have an anti-tumoral effect. For example, in colorectal cancer cells, ketamine decreased cell migration via the NMDA receptor.37 In breast cancer, ketamine inhibits the proliferation of cancer cells.38 Similar findings have been reported in pancreatic and ovarian cancers.39,40
Clinical studies regarding the impact of subanesthetic ketamine infusion on cancer surgery and cancer outcomes are limited (Table 2). In a recent randomized controlled trial, the administration of ketamine (0.25mg/kg bolus followed by an infusion of 0.05mg/kg/hour) did not impact the inflammatory response after surgery. Most importantly, ketamine did not change cancer recurrence 2 years after surgery.31 In lung cancer surgery, intraoperative ketamine improved recurrence-specific survival in patients with early stage lung adenocarcinoma.41
Table 2.
Clinical Trials Assessing the Effect of Intraoperative Use of Ketamine on Cancer Outcomes
| Author | Cancer | Study Type | Dose | Cancer Outcome |
|---|---|---|---|---|
| Cho et al42 | Colorectal | RCT | 0.25 mg/kg followed by 0.05 mg/kg/hr infusion | No effect in 2-year cancer recurrence |
| Connolly et al41 | Lung | Retrospective | Not specified | Improved recurrence-specific survival in early stages |
| Silagy et al43 | Renal | Retrospective | Not specified | Improved RFS on multivariable analysis |
| Forget et al44 | Breast | Retrospective | 0–0.5mg/kg | No effect on RFS |
Abbreviations: OS, overall survival; RFS, recurrence-free survival; RCT, randomized controlled trial.
Taken together, subanesthetic ketamine infusion plays a vital role in treating cancer patients since it is a great alternative to alleviate severe advanced cancer pain, particularly in patients with opioid tolerance. Preclinical studies have consistently shown that ketamine decreases tumor proliferation. Unfortunately, the number of clinical studies looking at the relationship between ketamine and cancer outcomes is very small and therefore no conclusion can be drawn to change clinical practice.
Lidocaine
Lidocaine is a common local anesthetic used for regional anesthesia. When used intravenously, lidocaine has shown analgesic and anti-inflammatory properties.45
The intraoperative dose recommended is a loading dose of 1–2 mg/kg, followed by an infusion of 1–2 mg/kg/hour.46 Additionally, the perioperative use of lidocaine infusion has opioid sparing effects and can help attenuate postoperative ileus. Specifically in cancer patients, preclinical studies have shown that lidocaine is associated with anti-tumoral effects. Lidocaine suppresses invasion, proliferation, and angiogenesis and promotes apoptosis of tumor cells.47 Furthermore, lidocaine influences the immune inflammatory response (NK cells) necessary to eliminate cancer cells.48
When studying the relationship between intravenous infusion of lidocaine and cancer outcomes, the evidence is insufficient to support preclinical studies (Table 3). In pancreatic cancer, lidocaine infusion did not improve overall or disease free-survival. However, it reduced the formation of circulating neutrophil extracellular traps (a mechanism involved in cancer metastasis).49 On the other hand, in a retrospective study of ovarian cancer surgery, intravenous infusion of lidocaine (1.5mg/kg bolus followed by continuous infusion of 2mg/kg/hour) was associated with improved overall survival and disease-free survival.50 In breast cancer patients, intravenous infusion of lidocaine preserved the cellular function, reduced opioid consumption, improved post-operative analgesia, and improved the quality of recovery.51
Table 3.
Clinical Trials Assessing the Effect of Intraoperative Use of Lidocaine on Cancer Outcomes
| Author | Cancer | Study Type | Dose | Cancer Outcome |
|---|---|---|---|---|
| Zhang et al52 | Pancreatic | Retrospective | Lidocaine bolus of 1.5 mg kg−1 followed by a continuous infusion of 2 mg kg−1 h−1 intraoperatively | Prolonged OS |
| Zhang et al49 | Pancreatic | RCT | Lidocaine bolus of 1.5 mg kg−1 followed by a continuous infusion of 2 mg kg−1 h−1 intraoperatively | No effect in OS |
| Zhang et al50 | Ovarian | Retrospective | Lidocaine bolus of 1.5 mg kg−1 followed by a continuous infusion of 2 mg kg−1 h−1 intraoperatively | Improved OS and DFS |
Abbreviations: OS, overall survival; RCT, randomized controlled trial; DFS, disease free-survival.
Overall, lidocaine exhibits analgesic and anti-inflammatory properties. Additionally, it decreases opioid consumption and improves gastrointestinal function in cancer patients. Several preclinical data have shown that lidocaine could attenuate the inflammatory response to cancer surgery. The majority of available data supporting the anti-tumoral effects of intraoperative lidocaine infusion are derived from preclinical studies, and their translation into clinical practice requires further investigation.
Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)
NSAIDs are an attractive alternative to opioids when administering analgesia for cancer patients. They provide effective analgesia while avoiding side effects such as sedation, respiratory depression, and nausea/vomiting. NSAIDs (eg, naproxen, ibuprofen, indomethacin, diclofenac, and toradol) exert their analgesic properties by inhibiting cyclo-oxygenase (COX), an enzyme that is required for the metabolism of arachidonic acid to prostaglandins and thromboxanes. There are two isoforms of the COX enzyme, COX-1 and COX-2. COX-1 enzyme regulates platelet aggregation, kidney afferent arteriole vasodilation, and gastric mucosa acid protection. COX-2 enzyme is induced during the inflammatory process. Coxibs, a group of highly selective inhibitors of COX-2 (eg, celecoxib, rofecoxib), are effective analgesic medications devoid of the gastrointestinal side effects of NSAIDs. There are several clinical studies supporting the idea that chronic NSAID administration reduces the risk of breast, prostate, colon, and ovarian cancer, among others.53–55 The anti-inflammatory properties of NSAIDs are thought to contribute to their potential “protective” effect on cancer development, especially since chronic inflammation has been linked to the development of certain types of cancer. However, NSAIDs may also exert direct anti-tumoral properties through various mechanisms such as 1) inhibiting proliferation and promoting apoptosis of cancer cells, 2) inhibiting transcription factors relevant to gene expression in the inflammation and immune response, 3) inhibiting signaling pathways necessary for cancer cell migration and invasion, and 4) inhibiting angiogenesis.56
The relationship between the perioperative use of NSAIDs and long-term outcomes in cancer surgery is still controversial (Table 4). For example, in a cohort study involving 2308 patients undergoing colorectal cancer surgery, the postoperative administration of ibuprofen decreased cancer recurrence risk but showed no significant effect on mortality and disease-free survival (DFS).57 Moreover, another study in colorectal cancer patients found no association between the use of postoperative NSAIDs and recurrence-free survival, local or distant recurrence, or overall survival (OS).58
Table 4.
Clinical Trials Assessing the Effect of Intraoperative Use of NSAIDs on Cancer Outcomes
| Author | Cancer | Study Type | Dose | Cancer Outcomes |
|---|---|---|---|---|
| Retsky et al59 | Breast | Retrospective | Ketorolac Not specified |
5-fold reduction in cancer recurrence. |
| Desmedt et al60 | Breast | Retrospective | Ketorolac 20 mg in patients <60 kg; 30 mg in patients ≥60 kg | Ketorolac, but not diclofenac, decreases distant recurrences in patients with BMIs >25 kg/m2 |
| Forget et al61 | Breast | Retrospective | Ketorolac 20 mg in patients <60 kg; 30 mg in patients ≥60 kg Diclofenac 17mg |
Ketorolac and diclofenac increase DFS and OS |
| De Castro- Araujo et al62 | Melanoma | Retrospective | Tenoxicam, Parecoxib, Ketorolac (dosages unspecified) | No effect overall |
| Connolly et al63 | Lung | Retrospective | Ketorolac 15 or 30 mg | Improved RSS |
| Mao et al64 | Bladder | Retrospective | Parecoxib 40 mg | No effect on RFS or OS |
| Wuethrich et al65 | Prostate | Retrospective | Ketorolac 30 mg ketorolac q 8h | No effect on RFS or OS |
| Forget et al44 | Breast, lung, kidney | Observational | Ketorolac 20 mg in patients <60 kg; 30 mg in patients ≥60 kg Diclofenac 75mg |
Reduced recurrence rate in breast cancer patients; Reduced risk of distant metastasis in lung cancer patients |
| Forget et al66 | Prostate | Retrospective | Not specified | No effect on biochemical recurrence-free (BRF) survival |
Abbreviations: OS, overall survival; DFS, disease-free survival, RFS, recurrence-free survival; RCT, randomized controlled trial; RSS, recurrence-specific survival; BRF, biochemical-free.
The latest systematic review and meta-analysis published by Shaji et al in 2021 investigated the clinical relationship between perioperative use of NSAIDs and DFS and overall survival (OS). The study included 16 trials and 12,994 cancer patients. The author concluded that perioperative use of NSAIDs was associated with longer DFS and OS. However, it is worth mentioning that the level of certainty of the evidence is low due to the retrospective nature of the majority of the studies included. Other limitations of the studies include the heterogeneity in regard to dose, duration, timing, and type of NSAIDs.67
Overall, NSAIDs are an effective alternative to opioids for the treatment of acute postoperative pain. The use of NSAIDs during the perioperative period in cancer patients reduces opioid consumption with additional reduction in opioids-related side effects such as nausea and vomiting. Unfortunately, NSAID use during the perioperative period might be limited in this specific patient population given many cancer patients tend to be older, have pre-existing renal disease, and carry a high risk of developing coagulopathy. In terms of cancer outcomes, chronic and perioperative NSAID administration has shown in clinical studies to reduce the risk of metastasis; however, caution should be exercised when interpreting these findings due to heterogeneity among studies.
Conclusion
Cancer-related pain is one of the most common and troublesome symptoms affecting cancer patients. Cancer-related pain is secondary to the progression of the disease, diagnostic intervention, or surgery. In the context of cancer surgery, opioids remain the cornerstone of postoperative pain control. Although effective for postsurgical pain relief, opioids have limitations due to their side effects, including respiratory depression, nausea, and vomiting. There has been an increasing interest in the relationship between opioid administration during the perioperative period and cancer recurrence and metastasis. However, there is no conclusive clinical evidence to support opioid avoidance with the goal of minimizing the risk of cancer recurrence.
Multimodal analgesia is a strategy that reduces the reliance on opioids through the use of non-opioids analgesics with different mechanisms of action. Various interventions, such as regional anesthesia, local anesthetic infusions, and non-opioid analgesics (ketamine, dexmedetomidine, lidocaine, and non-steroidal anti-inflammatory drugs), have shown benefits in postoperative pain management and a reduction in opioid-related adverse events. While preclinical data has supported the potential advantages of intraoperative use of these medications on cancer outcomes, the data that originates from clinical studies are insufficient to support this finding. More importantly, the heterogenecity of pain control among studies increases the challenges to properly assess the impact of every single medication on long-term cancer outcomes. Prospective randomized control trials are needed to properly assess the clinical utility of these medications during cancer surgery and long-term cancer outcomes. Abundant pre-clinical data suggest the use of multimodal analgesia in patients undergoing cancer surgery may be associated with favorable cancer-related outcomes. However, the challenge of establishing the effects of multiple anesthetic or analgesic modalities on numerous oncological pathologies have precluded any conclusive clinical evidence of such benefit. Perhaps a different approach is required to establish whether anesthetic drugs and analgesic agents do indeed affect cancer recurrence.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Siegel RL, Miller KD, Wagle NS, et al. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48. doi: 10.3322/caac.21763 [DOI] [PubMed] [Google Scholar]
- 2.Nestvold K, Lundar T, Blikra G, et al. Head injuries during one year in a central hospital in Norway: a prospective study. Epidemiologic features. Neuroepidemiology. 1988;7(3):134–144. doi: 10.1159/000110147 [DOI] [PubMed] [Google Scholar]
- 3.Angele MK, Faist E. Clinical review: immunodepression in the surgical patient and increased susceptibility to infection. Crit Care. 2002;6(4):298–305. doi: 10.1186/cc1514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cole SW, Nagaraja AS, Lutgendorf SK, et al. Sympathetic nervous system regulation of the tumour microenvironment. Nat Rev Cancer. 2015;15(9):563–572. doi: 10.1038/nrc3978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benzonana LL, Perry NJS, Watts HR, et al. Isoflurane, a commonly used volatile anesthetic, enhances renal cancer growth and malignant potential via the hypoxia-inducible factor cellular signaling pathway in vitro. Anesthesiology. 2013;119(3):593–605. doi: 10.1097/ALN.0b013e31829e47fd [DOI] [PubMed] [Google Scholar]
- 6.Cata JP, Corrales G, Speer B, et al. Postoperative acute pain challenges in patients with cancer. Best Pract Res Clin Anaesthesiol. 2019;33(3):361–371. doi: 10.1016/j.bpa.2019.07.018 [DOI] [PubMed] [Google Scholar]
- 7.McGreevy K, Bottros MM, Raja SN. Preventing chronic pain following acute pain: risk factors, preventive strategies, and their efficacy. Eur J Pain Suppl. 2011;5(2):365–372. doi: 10.1016/j.eujps.2011.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hill BL, Lefkowits C. Strategies for optimizing perioperative pain management for the cancer patient. Surg Oncol Clin N Am. 2021;30(3):519–534. doi: 10.1016/j.soc.2021.02.011 [DOI] [PubMed] [Google Scholar]
- 9.Rosenberger DC, Pogatzki-Zahn EM. Chronic post-surgical pain - update on incidence, risk factors and preventive treatment options. BJA Educ. 2022;22(5):190–196. doi: 10.1016/j.bjae.2021.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Neill A, Lirk P. Multimodal analgesia. Anesthesiol Clin. 2022;40(3):455–468. doi: 10.1016/j.anclin.2022.04.002 [DOI] [PubMed] [Google Scholar]
- 11.Helander EM, Menard BL, Harmon CM, et al. Multimodal analgesia, current concepts, and acute pain considerations. Curr Pain Headache Rep. 2017;21(1):3. doi: 10.1007/s11916-017-0607-y [DOI] [PubMed] [Google Scholar]
- 12.Tegeder I, Grösch S, Schmidtko A, et al. G protein-independent G1 cell cycle block and apoptosis with morphine in adenocarcinoma cells: involvement of p53 phosphorylation. Cancer Res. 2003;63(8):1846–1852. [PubMed] [Google Scholar]
- 13.Yeager MP, Colacchio TA. Effect of morphine on growth of metastatic colon cancer in vivo. Arch Surg. 1991;126(4):454–456. doi: 10.1001/archsurg.1991.01410280056007 [DOI] [PubMed] [Google Scholar]
- 14.Gach K, Wyrębska A, Fichna J, et al. The role of morphine in regulation of cancer cell growth. Naunyn Schmiedebergs Arch Pharmacol. 2011;384(3):221–230. doi: 10.1007/s00210-011-0672-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roy S, Ninkovic J, Banerjee S, et al. Opioid drug abuse and modulation of immune function: consequences in the susceptibility to opportunistic infections. J Neuroimmune Pharmacol. 2011;6(4):442–465. doi: 10.1007/s11481-011-9292-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alam A, Rampes S, Patel S, et al. Anesthetics or anesthetic techniques and cancer surgical outcomes: a possible link. Korean J Anesthesiol. 2021;74(3):191–203. doi: 10.4097/kja.20679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hernandez MC, Flores LR, Bayer BM. Immunosuppression by morphine is mediated by central pathways. J Pharmacol Exp Ther. 1993;267(3):1336–1341. [PubMed] [Google Scholar]
- 18.Yu Z, Jin S, Tian S, et al. Morphine stimulates cervical cancer cells and alleviates cytotoxicity of chemotherapeutic drugs via opioid receptor-dependent and -independent mechanisms. Pharmacol Res Perspect. 2022;10(5):e01016. doi: 10.1002/prp2.1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feng T, Zeng S, Ding J, et al. Comparative analysis of the effects of opioids in angiogenesis. BMC Anesthesiol. 2021;21(1):257. doi: 10.1186/s12871-021-01475-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cata JP, Keerty V, Keerty D, et al. A retrospective analysis of the effect of intraoperative opioid dose on cancer recurrence after non-small cell lung cancer resection. Cancer Med. 2014;3(4):900–908. doi: 10.1002/cam4.236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tai YH, Wu H-L, Chang W-K, et al. Intraoperative fentanyl consumption does not impact cancer recurrence or overall survival after curative colorectal cancer resection. Sci Rep. 2017;7(1):10816. doi: 10.1038/s41598-017-11460-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Montagna G, Gupta HV, Hannum M, et al. Intraoperative opioids are associated with improved recurrence-free survival in triple-negative breast cancer. Br J Anaesth. 2021;126(2):367–376. doi: 10.1016/j.bja.2020.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng J, He J, Wang W, et al. The impact of pain and opioids use on survival in cancer patients: results from a population-based cohort study and a meta-analysis. Medicine. 2020;99(9):e19306. doi: 10.1097/MD.0000000000019306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee S. Dexmedetomidine: present and future directions. Korean J Anesthesiol. 2019;72(4):323–330. doi: 10.4097/kja.19259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weerink MAS, Struys MMRF, Hannivoort LN, et al. Clinical pharmacokinetics and pharmacodynamics of dexmedetomidine. Clin Pharmacokinet. 2017;56(8):893–913. doi: 10.1007/s40262-017-0507-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dong W, Chen M-H, Yang Y-H, et al. The effect of dexmedetomidine on expressions of inflammatory factors in patients with radical resection of gastric cancer. Eur Rev Med Pharmacol Sci. 2017;21(15):3510–3515. [PubMed] [Google Scholar]
- 27.Wang ZX, Huang CY, Hua YP, et al. Dexmedetomidine reduces intestinal and hepatic injury after hepatectomy with inflow occlusion under general anaesthesia: a randomized controlled trial. Br J Anaesth. 2014;112(6):1055–1064. doi: 10.1093/bja/aeu132 [DOI] [PubMed] [Google Scholar]
- 28.Wang K, Li C. Effects of dexmedetomidine on inflammatory factors, T lymphocyte subsets and expression of NF-kappaB in peripheral blood mononuclear cells in patients receiving radical surgery of colon carcinoma. Oncol Lett. 2018;15(5):7153–7157. doi: 10.3892/ol.2018.8205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cata JP, Singh V, Lee B, et al. Intraoperative use of dexmedetomidine is associated with decreased overall survival after lung cancer surgery. J Anaesthesiol Clin Pharmacol. 2017;33(3):317–323. doi: 10.4103/joacp.JOACP_299_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ren B, Cheng M, Liu C, et al. Perioperative lidocaine and dexmedetomidine intravenous infusion reduce the serum levels of NETs and biomarkers of tumor metastasis in lung cancer patients: a prospective, single-center, double-blinded, randomized clinical trial. Front Oncol. 2023;13:1101449. doi: 10.3389/fonc.2023.1101449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cho JS, Seon K, Kim M-Y, et al. Effects of perioperative dexmedetomidine on immunomodulation in uterine cancer surgery: a randomized, controlled trial. Front Oncol. 2021;11:749003. doi: 10.3389/fonc.2021.749003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clements JA, Nimmo WS. Pharmacokinetics and analgesic effect of ketamine in man. Br J Anaesth. 1981;53(1):27–30. doi: 10.1093/bja/53.1.27 [DOI] [PubMed] [Google Scholar]
- 33.Loftus RW, Yeager MP, Clark JA, et al. Intraoperative ketamine reduces perioperative opiate consumption in opiate-dependent patients with chronic back pain undergoing back surgery. Anesthesiology. 2010;113(3):639–646. doi: 10.1097/ALN.0b013e3181e90914 [DOI] [PubMed] [Google Scholar]
- 34.Loveday BA, Sindt J. Ketamine protocol for palliative care in cancer patients with refractory pain. J Adv Pract Oncol. 2015;6(6):555–561. [PMC free article] [PubMed] [Google Scholar]
- 35.Orhurhu V, Orhurhu MS, Bhatia A, et al. Ketamine infusions for chronic pain: a systematic review and meta-analysis of randomized controlled trials. Anesth Analg. 2019;129(1):241–254. doi: 10.1213/ANE.0000000000004185 [DOI] [PubMed] [Google Scholar]
- 36.Kapural L, Kapural M, Bensitel T, Sessler DI. Opioid-sparing effect of intravenous outpatient ketamine infusions appears short-lived in chronic-pain patients with high opioid requirements. Pain Physician. 2010;13(4):389–394. doi: 10.36076/ppj.2010/13/389 [DOI] [PubMed] [Google Scholar]
- 37.Duan W, Hu J, Liu Y. Ketamine inhibits colorectal cancer cells malignant potential via blockage of NMDA receptor. Exp Mol Pathol. 2019;107:171–178. doi: 10.1016/j.yexmp.2019.02.004 [DOI] [PubMed] [Google Scholar]
- 38.Li H, Liu W, Zhang X, et al. Ketamine suppresses proliferation and induces ferroptosis and apoptosis of breast cancer cells by targeting KAT5/GPX4 axis. Biochem Biophys Res Commun. 2021;585:111–116. doi: 10.1016/j.bbrc.2021.11.029 [DOI] [PubMed] [Google Scholar]
- 39.Malsy M, Gebhardt K, Gruber M, et al. Effects of ketamine, s-ketamine, and MK 801 on proliferation, apoptosis, and necrosis in pancreatic cancer cells. BMC Anesthesiol. 2015;15(1):111. doi: 10.1186/s12871-015-0076-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li T, Yang J, Yang B, et al. Ketamine inhibits ovarian cancer cell growth by regulating the lncRNA-PVT1/EZH2/p57 axis. Front Genet. 2020;11:597467. doi: 10.3389/fgene.2020.597467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Connolly JG, Tan KS, Mastrogiacomo B, et al. Intraoperative opioid exposure, tumour genomic alterations, and survival differences in people with lung adenocarcinoma. Br J Anaesth. 2021;127(1):75–84. doi: 10.1016/j.bja.2021.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cho JS, Young KN, Jae-Kwang S, et al. The immunomodulatory effect of ketamine in colorectal cancer surgery: a randomized-controlled trial. Can J Anaesth. 2021;68(5):683–692. doi: 10.1007/s12630-021-01925-3 [DOI] [PubMed] [Google Scholar]
- 43.Silagy AW, Hannum ML, Mano R, et al. Impact of intraoperative opioid and adjunct analgesic use on renal cell carcinoma recurrence: role for onco-anaesthesia. Br J Anaesth. 2020;125(5):e402–e404. doi: 10.1016/j.bja.2020.06.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Forget P, Machiels J-P, Coulie PG, et al. Neutrophil: lymphocyte ratio and intraoperative use of ketorolac or diclofenac are prognostic factors in different cohorts of patients undergoing breast, lung, and kidney cancer surgery. Ann Surg Oncol. 2013;20(Suppl 3):S650–S660. doi: 10.1245/s10434-013-3136-x [DOI] [PubMed] [Google Scholar]
- 45.Masic D, Liang E, Long C, et al. Intravenous lidocaine for acute pain: a systematic review. Pharmacotherapy. 2018;38(12):1250–1259. doi: 10.1002/phar.2189 [DOI] [PubMed] [Google Scholar]
- 46.Chu R, Umukoro N, Greer T, et al. Intravenous lidocaine infusion for the management of early postoperative pain: a comprehensive review of controlled trials. Psychopharmacol Bull. 2020;50(4 Suppl 1):216–259. [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang C, Xie C, Lu Y. Local anesthetic lidocaine and cancer: insight into tumor progression and recurrence. Front Oncol. 2021;11:669746. doi: 10.3389/fonc.2021.669746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramirez MF, Tran P, Cata JP. The effect of clinically therapeutic plasma concentrations of lidocaine on natural killer cell cytotoxicity. Reg Anesth Pain Med. 2015;40(1):43–48. doi: 10.1097/AAP.0000000000000191 [DOI] [PubMed] [Google Scholar]
- 49.Zhang H, Qu M, Guo K, et al. Intraoperative lidocaine infusion in patients undergoing pancreatectomy for pancreatic cancer: a mechanistic, multicentre randomised clinical trial. Br J Anaesth. 2022;129(2):244–253. doi: 10.1016/j.bja.2022.03.031 [DOI] [PubMed] [Google Scholar]
- 50.Zhang H, Gu J, Qu M, et al. Effects of intravenous infusion of lidocaine on short-term outcomes and survival in patients undergoing surgery for ovarian cancer: a retrospective propensity score matching study. Front Oncol. 2021;11:689832. doi: 10.3389/fonc.2021.689832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wei Q, Xia M, Zhang Q, et al. Effect of intravenous lidocaine infusion on perioperative cellular immunity and the quality of postoperative recovery in breast cancer patients: a randomized controlled trial. Gland Surg. 2022;11(3):599–610. doi: 10.21037/gs-22-134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang H, Yang L, Zhu X, et al. Association between intraoperative intravenous lidocaine infusion and survival in patients undergoing pancreatectomy for pancreatic cancer: a retrospective study. Br J Anaesth. 2020;125(2):141–148. [DOI] [PubMed] [Google Scholar]
- 53.Harris RE, Chlebowski RT, Jackson RD, et al. Breast cancer and nonsteroidal anti-inflammatory drugs: prospective results from the Women’s Health Initiative. Cancer Res. 2003;63(18):6096–6101. [PubMed] [Google Scholar]
- 54.Vidal AC, Howard LE, Moreira DM, Castro-Santamaria R, Andriole GL, Freedland SJ. Aspirin, NSAIDs, and risk of prostate cancer: results from the REDUCE study. Clin Cancer Res. 2015;21(4):756–762. doi: 10.1158/1078-0432.CCR-14-2235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ruder EH, Laiyemo AO, Graubard BI, et al. Non-steroidal anti-inflammatory drugs and colorectal cancer risk in a large, prospective cohort. Am J Gastroenterol. 2011;106(7):1340–1350. doi: 10.1038/ajg.2011.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kolawole OR, Kashfi K. NSAIDs and cancer resolution: new paradigms beyond cyclooxygenase. Int J Mol Sci. 2022;23(3):1432. doi: 10.3390/ijms23031432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schack A, Fransgaard T, Klein MF, et al. Perioperative use of nonsteroidal anti-inflammatory drugs decreases the risk of recurrence of cancer after colorectal resection: a cohort study based on prospective data. Ann Surg Oncol. 2019;26(12):3826–3837. doi: 10.1245/s10434-019-07600-8 [DOI] [PubMed] [Google Scholar]
- 58.Grahn O, Lundin M, Lydrup M-L, et al. Postoperative non-steroidal anti-inflammatory drug use and oncological outcomes of rectal cancer. BJS Open. 2021;5(1). doi: 10.1093/bjsopen/zraa050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Retsky M, Rogers R, Demicheli R, et al. NSAID analgesic ketorolac used perioperatively may suppress early breast cancer relapse: particular relevance to triple negative subgroup. Breast Cancer Res Treat. 2012;134(2):881–888. doi: 10.1007/s10549-012-2094-5 [DOI] [PubMed] [Google Scholar]
- 60.Desmedt C, Demicheli R, Fornili M, et al. Potential benefit of intra-operative administration of ketorolac on breast cancer recurrence according to the Patient’s Body Mass Index. J Natl Cancer Inst. 2018;110(10):1115–1122. doi: 10.1093/jnci/djy042 [DOI] [PubMed] [Google Scholar]
- 61.Forget P, Bentin C, Machiels J-P, et al. Intraoperative use of ketorolac or diclofenac is associated with improved disease-free survival and overall survival in conservative breast cancer surgery. Br J Anaesth. 2014;113(Suppl 1):i82–i87. doi: 10.1093/bja/aet464 [DOI] [PubMed] [Google Scholar]
- 62.de Castro Araujo BL, de Oliveira JL, Rezende JFN, et al. Impact of non-steroidal anti-inflammatory drugs on recurrence and survival after melanoma surgery: a cohort study. Cancer Invest. 2020;38(7):415–423. doi: 10.1080/07357907.2020.1793351 [DOI] [PubMed] [Google Scholar]
- 63.Connolly JG, Scarpa JR, Gupta HV, et al. Intraoperative ketorolac may interact with patient-specific tumour genomics to modify recurrence risk in lung adenocarcinoma: an exploratory analysis. Br J Anaesth. 2021;127(3):e82–e85. doi: 10.1016/j.bja.2021.05.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mao S, Wu Y, Wang R, et al. Intraoperative use of single dose of nonsteroidal anti-inflammatory drugs was not associated with cancer recurrence and mortality after bladder cancer surgery: a retrospective study. Ann Palliat Med. 2020;9(1):8–18. doi: 10.21037/apm.2019.11.27 [DOI] [PubMed] [Google Scholar]
- 65.Wuethrich PY, Hsu Schmitz S-F, Kessler TM, et al. Potential influence of the anesthetic technique used during open radical prostatectomy on prostate cancer-related outcome: a retrospective study. Anesthesiology. 2010;113(3):570–576. doi: 10.1097/ALN.0b013e3181e4f6ec [DOI] [PubMed] [Google Scholar]
- 66.Forget P, Tombal B, Scholtès J-L, et al. Do intraoperative analgesics influence oncological outcomes after radical prostatectomy for prostate cancer? Eur J Anaesthesiol. 2011;28(12):830–835. doi: 10.1097/EJA.0b013e32834b7d9a [DOI] [PubMed] [Google Scholar]
- 67.Shaji S, Smith C, Forget P. Perioperative NSAIDs and long-term outcomes after cancer surgery: a systematic review and meta-analysis. Curr Oncol Rep. 2021;23(12):146. doi: 10.1007/s11912-021-01133-8 [DOI] [PMC free article] [PubMed] [Google Scholar]