Abstract
Thioredoxin, a small, ubiquitous protein which participates in redox reactions through the reversible oxidation of its active center dithiol to a disulfide, is an essential protein in Bacillus subtilis. A variety of stresses, including heat or salt stress or ethanol treatment, strongly enhanced the synthesis of thioredoxin in B. subtilis. The stress induction of the monocistronic trxA gene encoding thioredoxin occurs at two promoters. The general stress sigma factor, ςB, was required for the initiation of transcription at the upstream site, SB, and the promoter preceding the downstream start site, SA, was presumably recognized by the vegetative sigma factor, ςA. In contrast to the heat-inducible, ςA-dependent promoters preceding the chaperone-encoding operons groESL and dnaK, no CIRCE (for controlling inverted repeat of chaperone expression) was present in the vicinity of the start site, SA. The induction patterns of the promoters differed, with the upstream promoter displaying the typical stress induction of ςB-dependent promoters. Transcription initiating at SA, but not at SB, was also induced after treatment with hydrogen peroxide or puromycin. Such a double control of stress induction at two different promoters seems to be typical of a subgroup of class III heat shock genes of B. subtilis, like clpC, and it either allows the cells to raise the level of the antioxidant thioredoxin after oxidative stress or allows stressed cells to accumulate thioredoxin. These increased levels of thioredoxin might help stressed B. subtilis cells to maintain the native and reduced state of cellular proteins.
Thioredoxins are small, heat-stable, ubiquitous proteins with a conserved pair of vicinal cysteines (-Trp-Cys-Gly-Pro-Cys-Lys-) that undergo reversible oxidation and reduction and are efficient reductants of disulfides in low-molecular-weight compounds and proteins (23). Oxidized thioredoxins are reduced at the expense of NADPH, a reaction catalyzed by thioredoxin reductase (23). Thioredoxin systems serve as hydrogen donors, for example, for ribonucleotide reductase, phosphoadenosyl phosphosulfate reductase, and methionine sulfoxide reductase (22). In Escherichia coli, thioredoxin is necessary for the assembly of filamentous phages (45) and the replication of T7 (31) but is not essential for DNA synthesis and growth (21, 34).
Furthermore, thioredoxins have been implicated in the thiol-disulfide exchange and disulfide bond formation (29, 41), which are also catalyzed by glutaredoxin or protein disulfide isomerase in the endoplasmic reticula of eukaryotes and by the Dsb proteins in the periplasmic spaces of gram-negative bacteria. Protein disulfide isomerase and DsbA have been shown to assist in the folding pathway of disulfide-containing proteins both in vitro and in vivo (39).
Thioredoxin is also believed to be involved in defense against oxidative stress through its ability to reduce hydrogen peroxide (49), by acting as a hydrogen donor for a Saccharomyces cerevisiae peroxidase (7), or by reactivation of proteins damaged by oxidative stress or other stresses which generate reactive oxygen species (14).
We are interested in the general stress response of Bacillus subtilis (18). In the course of cloning and characterization of heat-inducible promoters (52), we also sequenced the regulatory region of trxA, the coding region of which had already been cloned and sequenced by Chen et al. (8). We investigated the expression of trxA and report that trxA encodes an essential protein, which is induced by different stress conditions, including heat and salt stress or treatment with ethanol, hydrogen peroxide, or puromycin. Two different promoters, PB and PA, direct the expression of trxA, and the stress sigma factor, ςB, of B. subtilis is involved in the induction of trxA by stress.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
All bacterial strains and plasmids used in this study are listed in Table 1. The B. subtilis strains were routinely grown with vigorous agitation at 37°C in synthetic medium (50) or in complex medium. The bacteria were exposed to heat, ethanol, salt, H2O2, paraquat, cumene hydroperoxide (CHP), and puromycin according to a protocol described earlier (12, 44, 53). For the inhibition of the initiation of transcription, rifampin was added to a final concentration of 0.1 mg per ml.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype or description | Reference or source |
|---|---|---|
| E. coli | ||
| DH5α | F− Φ80dlacZΔM15 Δ(lacZYA-argF)U169 deoR recA1 endA1 hsdR17 (rK− mK+) supE44 thi-1 gyrA69 | 6 |
| RR1 | F−mcrB mrr hsdS20 (rB− mB−) ara14 proA2 lacY1 leu galK2 rpsL20 Smrxyl5 mtl1 supE44 | 46 |
| B. subtilis | ||
| IS58 | trpC2 lys-3 | 48 |
| BGH1 | trpC2 lys-3 sigB::Δ(HindIII-EcoRV)::cat | 32 |
| BSA115 | trpC2 rsbU::kan PBΔ28::PSPACrsbW313 pTet-I SPβ ctc::lacZ | 54 |
| BIG1 | trpC2 lys-3 trxA::pHV501 | pHVES1→IS58 |
| BD224 | trpC2 recE4 | 10 |
| Plasmids | ||
| pBluescript II SK(+/−) or KS(+/−) | Cloning vector, Apr | Stratagene |
| pWH703 | Promoter probe vector, XylE+ Cmr Kmr | 52 |
| pWH262 | pWH703 containing a 120-bp SauIIIA fragment carrying the 5′ region of trxA | This study |
| pHV501 | Integrative plasmid, Apr Emr | 51 |
| pHVES1 | pHV501 containing a 240-bp trxA fragment generated by PCR | This study |
| pKSES1 | pBluescript II KS containing a 459-bp fragment generated by PCR | This study |
| pSKES1 | pBluescript II SK containing a 720-bp ClaI fragment generated by inverse PCR | This study |
| pSKES2 | pBluescript II SK containing a 950-bp EcoRI fragment generated by inverse PCR | This study |
| pSKES3 | pBluescript II SK containing a 1.4-kb StyI fragment generated by inverse PCR | This study |
| pSKES4 | pBluescript II SK containing a 1.6-kb PvuII fragment generated by inverse PCR | This study |
The E. coli strains DH5α and RR1 were used for DNA manipulations.
Cloning and sequencing of the regulatory region of trxA.
Chromosomal DNA from B. subtilis IS58, isolated according to the method of Meade et al. (33), was digested with Sau3AI and cloned into the BamHI-digested promoter probe vector pWH703 (52) in front of the promoterless genes coding for catechol-2,3-oxygenase (xylE) and chloramphenicol acetyltransferase (cat). After transformation of protoplasts of B. subtilis BD224 with the ligation mixture, clones containing promoters were isolated by selection on agar plates containing kanamycin and chloramphenicol. Catechol-2,3-oxygenase-positive clones displayed a yellow color after the colonies were sprayed with catechol due to the formation of hydroxymuconic acid semialdehyde (52). Both strands of the DNA were sequenced by the dideoxy chain termination method of Sanger et al. (47) with the primers P1 (5′-CGGCACGTGACCGCGGC-3′) and P2 (5′-CCTTGTCTACAAACCCC-3′).
The DNA upstream of the promoter fragment inserted into pWH262 was cloned by inverse PCR. Chromosomal DNA from B. subtilis was digested with the restriction endonucleases StyI, EcoRI, PvuII, and ClaI, known to cut within the coding region of trxA. Purified DNA fragments of the appropriate size were ligated under conditions that favor the formation of monomeric circles. Aliquots of the ligation mixture were used for PCR with the primers PtrxIPCR1 (5′-TTCGTTCACGCTATTTTAATGC-3′) and PtrxIPCR2 (5′-TCATCATTTCACATTGGAGG-3′), and the PCR products were cloned into the vector pBluescript II SK(+) digested with EcoRV, yielding plasmids pSKES1, pSKES2, pSKES3, and pSKES4 (Fig. 1 and Table 1). Both strands were sequenced by the dideoxy chain termination method of Sanger et al. (47).
FIG. 1.
Structure and organization of the trxA region of B. subtilis. (A) Schematic representation of the trxA region. The boxes indicate the locations of the coding region of trxA and an open reading frame with homology to the arabinosidase gene xsa from B. ovatus (57). Potential terminators (T) upstream and downstream of trxA and the promoters, PB and PA, which drive the expression of trxA, are labeled. The sites of the following restriction enzymes relevant for this study are marked: C, ClaI; E, EcoRI; H, HinfI; P, PvuII; S, Sau3AI; and St, StyI. The lines represent the insertions of the plasmids used in this study. (B) Nucleotide sequence of the regulatory region of trxA. The potential −35 and −10 regions of both promoters are in boldface and underlined. The restriction sites used in this study are underlined. The potential 5′ ends of the trxA transcripts are indicated by vertical arrowheads. The horizontal arrays of arrowheads label the potential terminator just upstream of the −35 region of the ςB-dependent promoter, PB. An inverted repeat overlapping with the potential promoter PA is marked with arrows; the asterisks indicate a mismatch of the potential stem-loop structure. The consensus sequences for promoters recognized by RNA polymerase containing the vegetative sigma factor, ςA (19), or the stress sigma factor, ςB (18), are indicated, with positions critical for promoter recognition by the corresponding sigma factor in capital letters. (C) Schematic representation of the chromosomal rearrangement after integration of plasmid pHVES1 into the chromosome of B. subtilis.
Analysis of transcription.
Total RNA of the B. subtilis strains BD224 (carrying the plasmid pWH262), IS58, and BGH1 was isolated from exponentially growing or stressed cells by the acid phenol method described by Majumdar et al. (30) with some modifications described previously (53). Serial dilutions of total RNA were transferred onto a positively charged nylon membrane by slot blotting and hybridized with digoxigenin-labeled probes (Boehringer Mannheim) according to the manufacturer’s instructions. Chemiluminograms were quantified with a Personal Densitometer from Molecular Dynamics, and induction ratios were calculated by setting the value of the control to 1. The amount of xylE mRNA from B. subtilis BD224 carrying the plasmid pWH262 was determined by slot blot hybridization with a digoxigenin-labeled 1.4-kb PstI fragment, containing the xylE gene, from the plasmid pWH703 (52).
For the detection of trxA mRNA in B. subtilis IS58, a digoxigenin-labeled riboprobe was used. For the generation of the probe, the trxA gene with the potential regulatory region and the putative terminator was amplified by PCR with the primers Ptrx1 (5′-AAGCATTAAAATAGCGTGAACG-3′) and Ptrx2 (5′-TGGTTCACAATTGGCGAATA-3′). The 459-bp PCR product was blunt end ligated with the vector pBluescript II KS(+), and the orientation of the cloned fragment was verified by sequencing. The resulting plasmid, pKSES1, was linearized with BamHI and used as a template for in vitro transcription with T3 RNA polymerase. RNA synthesized from the coding strand after linearization of pKSES1 with PstI served as a negative control for the hybridization and did not yield any specific hybridization signal (data not shown).
Northern blot analysis was carried out as described earlier (56). The primer extension analysis was performed with synthetic oligonucleotides labeled at the 5′ end with [γ-32P]ATP and complementary to the N-terminal region of trxA (PtrxPE1 [5′-CAAGGTCCGCACCAAGGAGC-3′] and PtrxPE2 [5′-ATTTTTTCCTGATCACAGCCGG-3′]) and a region preceding the xylE gene (PxylPE [5′-CGGCACGTGACCGCGGC-3′]).
Construction of a conditional trxA mutant of B. subtilis.
To create a conditional mutation, a 240-bp HindIII-BamHI-clamped fragment containing the ribosome binding site and the N-terminal part of trxA was amplified by PCR with the primers Ptrx3 (5′-AAGAAGCTTCATCATTTCACATTGGAGG-3′) and Ptrx4 (5′-GGAGGATCCTTTAACACAAGAAGAGTCGG-3′) and ligated with the HindIII-BamHI-digested integration vector pHV501, generating the plasmid pHVES1. Upon transformation into B. subtilis IS58, pHVES1 should integrate into the chromosome via a Campbell-type integration, disrupt the trxA gene, and place a second copy of trxA under the control of the IPTG-inducible promoter PSPAC (Fig. 1C). Erythromycin- and lincomycin-resistant colonies were selected on agar plates containing 1 μg of erythromycin and 25 μg of lincomycin per ml in the presence of 1 mM IPTG in order to allow production of trxA from PSPAC. The integration of pHVES1 into trxA was verified by PCR (data not shown), and the resulting strain was designated BIG1.
Radioactive labeling of cultures, 2-D protein gel electrophoresis, and N-terminal microsequencing.
Cells grown in synthetic medium to an optical density at 500 nm of 0.4 were labeled for 3 min with 5 μCi of l-[35S]methionine per ml before and 10 min after exposure to different stresses according to the method of Bernhardt et al. (5). For heat shock, the cells were shifted from 37 to 48°C. The other stress conditions were achieved by exposing the cells to either 4% (wt/vol) NaCl, 4% (vol/vol) ethanol, or 100 μM paraquat. The different starvation conditions were provoked by cultivating the bacteria in a medium containing limiting amounts of glucose (0.05% [wt/vol]) or phosphate (0.3 mM). For labeling, samples were taken during the exponential or stationary growth phase. l-[35S]methionine incorporation was stopped by the addition of chloramphenicol and an excess of cold methionine as well as by transferring the culture onto ice. The cells were disrupted by sonication, and crude protein extracts were prepared as described by Bernhardt et al. (5) Two-dimensional (2-D) polyacrylamide gel electrophoresis was performed with the Investigator system of Oxford Glycosystems (Oxford, England). Carrier ampholytes at a nonlinear gradient pH of 3 to 10 were used for the first dimension, and 12% acrylamide-bisacrylamide (30:0.8) was used for the second dimension. Each gel was loaded with a crude protein extract containing 2 × 106 cpm.
For microsequencing, the Coomassie blue-stained protein spots corresponding to thioredoxin were cut out from several 2-D gels and the collected gel pieces were concentrated, blotted onto a polyvinyldifluoride membrane, stained, and sequenced as described previously (53).
General methods.
Plasmid DNA isolation, cloning of DNA fragments, restriction enzyme analysis, and agarose gel electrophoresis were performed according to standard protocols (46). Transformation of competent B. subtilis cells was carried out by the method of Hoch (20). Determination of XylE activity before (37°C) and after (48°C) heat stress was described earlier (52).
Computer analysis of sequence data.
The sequence data manipulations were performed with the Genetics Computer Group, Inc., sequence analysis software package.
Nucleotide sequence accession number.
The nucleotide sequence of the regulatory region of trxA and the gene homologous to xsa reported in this paper appear in the EMBL and GenBank nucleotide sequence databases under the accession no. X79976 and X99275.
RESULTS
Nucleotide sequence of the regulatory region of trxA.
In an attempt to analyze the heat shock response of B. subtilis, DNA fragments which confer heat induction of the promoterless reporter genes xylE and cat of the promoter probe vector pWH703 were cloned and characterized (52). One of the Sau3AI fragments contained at least part of the regulatory region, in addition to the beginning, of the coding region of trxA encoding thioredoxin (8). Measurements of the activity of the reporter enzyme catechol-2,3-dioxygenase (encoded by xylE) and slot blot analysis of mRNA prepared before and after heat shock confirmed the heat induction of xylE and cat conferred by the 120-bp Sau3AI fragment (data not shown). Inverse PCR was used to clone the whole regulatory region of trxA. The sequence of the 1.6-kb fragment revealed the presence upstream of trxA of a potential factor-independent terminator which is preceded by an open reading frame with significant similarity to the arabinosidase gene (xsa) from Bacteroides ovatus (57) (Fig. 1A and B). Since another, rho-independent potential terminator is located between trxA and uvrB (8), the sequence data suggested that trxA is transcribed as a single gene and does not form an operon with the flanking genes.
Transcriptional regulation of trxA.
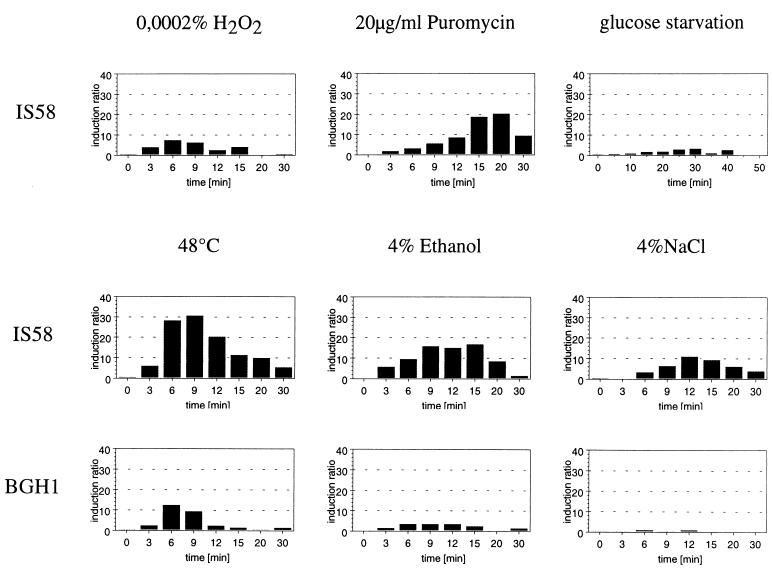
Since the initial experiments with the multicopy promoter probe vector already indicated heat induction at the trxA promoter, the effect of various stresses on the expression of trxA was measured by RNA slot blot analysis. In the wild-type strain, IS58, the level of trxA mRNA strongly increased after heat shock (30-fold), but it was also 8- to 20-fold higher after treatment with ethanol, salt, hydrogen peroxide, or puromycin (Fig. 2). Except with puromycin, the induction reached a maximum 6 to 12 min after the imposition of stress. The delay in response after treatment with puromycin might be attributed to the fact that puromycyl fragments have to accumulate before they trigger the response. Starvation for glucose resulted in only a rather weak (three- to fourfold) induction (Fig. 2).
FIG. 2.
Effect of various stresses on the trxA mRNA levels in B. subtilis IS58 (wild type) and the isogenic ςB mutant (BGH1). Serial dilutions of total RNA prepared from B. subtilis before and at different times (3, 6, 9, 12, 15, 20, and 30 min) after exposure to stress were bound to a positively charged nylon membrane and hybridized with the digoxigenin-labeled antisense RNA probes specific for the trxA gene. The hybrizidation signals were quantified with a Personal Densitometer as described in Materials and Methods. The mRNA level in the control prior to stress was set to 1, and the induction ratios are shown.
Induction by multiple stresses placed trxA in the group of general stress genes. Most of the general stress proteins of B. subtilis require the stress sigma factor, ςB, for their induction by various stresses (18). The induction of trxA in a strain with a deletion in sigB (BGH1) was reduced in response to heat and salt shock or ethanol stress (Fig. 2). Therefore, the heat, salt, and ethanol induction was at least partially controlled by ςB. Induction by hydrogen peroxide and puromycin (Fig. 2) was not altered by the deletion of sigB (data not shown).
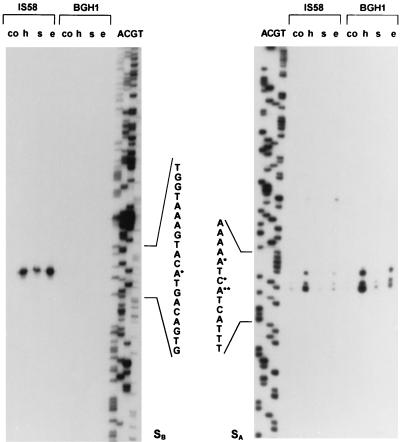
For promoter mapping, primer extension experiments were carried out with RNA from the wild-type strain (IS58) and a sigB mutant (BGH1). Two 5′ ends of the trxA mRNA separated by 219 nucleotides were found (Fig. 1B and 3). The sequence elements preceding the upstream site, SB, were very similar to known ςB-dependent promoters (Fig. 1B), e.g., promoters of sigB, ctc, gsiB, katE, and gspA (18). This putative ςB-dependent promoter, PB, was not utilized in a sigB deletion strain (BGH1), confirming the suggestion that trxA induction was at least in part ςB dependent (Fig. 3). The second, downstream 5′ end of the trxA mRNA was not uniform and displayed, in addition to the major signal, two minor signals in the immediate vicinity. The sequence upstream of these three sites (SA) resembled −10 boxes, which are recognized by RNA polymerase, containing the vegetative sigma factor, ςA (19), but the −35 region did not resemble the −35 boxes of any of the known sigma factors of B. subtilis (17).
FIG. 3.
Mapping of the 5′ end of the trxA mRNA by primer extension analysis. Equal amounts of total RNA (5 μg) isolated from B. subtilis IS58 (wild type) and the sigB mutant BGH1 before (co) and 9 min after exposure to heat shock (h), salt stress (s), and ethanol stress (e) served as templates. The 5′ ends of the transcripts, SB (left panel) and SA (right panel), are marked with asterisks. Lanes A, C, G, and T show the dideoxy sequencing ladders obtained with the same primers used in the extension analysis (PtrxPE2 in the left panel and PtrxPE1 in the right panel).
Both promoters appeared to be stress inducible. The ςB-dependent promoter was strongly induced by heat and ethanol stress, but was induced to a lower extent by salt stress and glucose starvation (Fig. 3 and 4A and B). The putative ςA-dependent promoter was strongly induced by heat stress and also by ethanol stress. In a sigB mutant only the intensity of the signals at SA increased after stress (Fig. 3). The weak signal visible upstream of the signals at SA in the wild-type strain after stress (Fig. 3) was located within the stem of the inverted repeat indicated in Fig. 1. This inverted repeat overlaps the putative promoter PA and might be involved in the stress induction at SA.
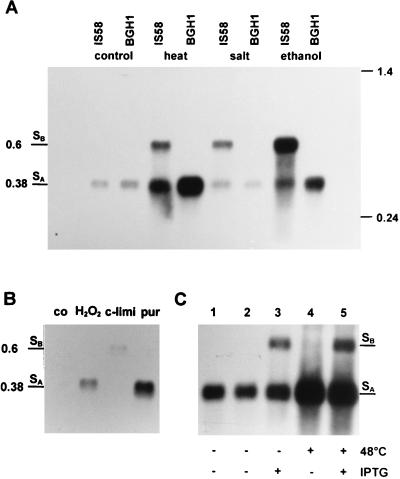
FIG. 4.
Northern blot analysis. (A) RNA was isolated from B. subtilis IS58 (wild type) and BGH1 (sigB) at 37°C (control) and 9 min after a temperature shift to 48°C (heat) or after the addition of 4% NaCl (salt) or 4% ethanol (ethanol). The RNA was separated under denaturing conditions, transferred to a nylon membrane, and hybridized with a digoxigenin-labeled RNA probe specific for trxA. The locations of molecular size standards (in kilobases) and of mRNAs with 5′ ends at SB and SA are marked. (B) Total RNA was isolated from the wild-type strain, IS58, during exponential growth (co), 9 min after exposure to 0.0002% H2O2 (H2O2) or 20 μg of puromycin (pur)/ml, or 30 min after the cells entered the stationary phase as a result of the exhaustion of glucose (c-limi). The samples were processed as described for panel A. (C) RNA was isolated from B. subtilis BSA115 before (lane 1) or 6 min after exposure (+) to 48°C heat stress (lane 4), from bacteria grown in the absence (−) (lane 2) or presence (+) (lane 3) of IPTG, and after a combination of heat shock and the addition of IPTG (lane 5).
The Northern blot analysis proved that trxA is indeed transcribed as a monocistronic mRNA (Fig. 4) and allowed good resolution of both transcripts due to the different sizes of the mRNAs originating at SB (594 bp) and SA (377 bp). These data supported the results of the primer extension analysis. For exponentially growing cells, the 5′-end of the trxA transcript was mapped to SA.
Although transcription initiating at both promoters was stress inducible, their induction profiles differed. The strongest trxA induction was mapped at the ςB-dependent promoter after ethanol treatment and heat shock, whereas heat stress was more effective than ethanol treatment in inducing transcripts with the 5′-end SA. Induction by salt stress was mostly confined to the ςB-dependent promoter, explaining the almost complete absence of salt induction in a ςB mutant (Fig. 2 and 3). In the absence of ςB (BGH1), the induction by heat shock and ethanol stress at SA was stronger than in the wild type, probably due to the lack of RNA polymerase competition for promoter binding (Fig. 4A).
Only the downstream promoter (with SA at the 5′ end), but not the ςB-dependent promoter, was induced after treatment with puromycin or hydrogen peroxide (Fig. 4B). This result explains why the same induction ratios of trxA mRNA were found in the wild type and in the ςB mutant after treatment with puromycin or H2O2 in slot blot hybridizations (data not shown).
If the activation of ςB was solely responsible for the induction of trxA at SB, production of active ςB in the absence of stress should have resulted in increased transcription from the ςB-dependent promoter. In the B. subtilis strain BSA115 (54), in which expression of sigB is controlled by PSPAC and the anti-sigma factor RsbW (4) is not produced because of a frameshift mutation in rsbW, the addition of IPTG triggers the production of active ςB molecules. In this strain only the upstream promoter, PB, was induced in response to IPTG addition (Fig. 4C). Heat shock without the addition of IPTG induced the downstream start site, SA, only, because this strain does not produce active ςB in response to stress (55). Both promoters were induced in heat-shocked cells of BSA115 treated with IPTG (Fig. 4C).
In order to exclude the possibility that the induction of trxA in response to stress was due to a stabilization of the mRNA during stress, exponentially growing cells were treated with rifampin and the influence of heat shock on the amount of trxA mRNA was analyzed. In Northern blot as well as in slot blot experiments the heat shock did not significantly change the half-life of the trxA mRNA (data not shown). The increase in the level of the trxA mRNA must therefore be due to enhanced transcriptional initiation.
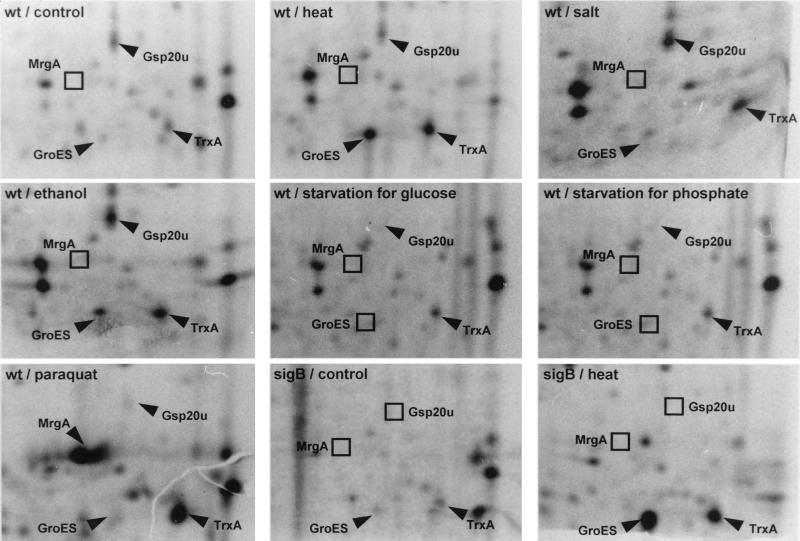
Identification of TrxA on 2-D protein gels and synthesis of thioredoxin after stress and starvation.
The determination of the N-terminal sequences of stress proteins enabled the identification of TrxA on 2-D protein gels. The N-terminal sequence MAIVKATDQSFSAETSEGVVLA perfectly matched the predicted amino acid sequence of TrxA and proved that the ATG codon marked by Chen et al. (8) is indeed the start codon of trxA. After the spot corresponding to thioredoxin on 2-D protein gels was identified, the influence of stress and starvation on the synthesis of thioredoxin was analyzed by 2-D gel electrophoresis (Fig. 5). Although synthesis of thioredoxin was already detected during growth, exposing cells to heat, salt, ethanol, or paraquat, a very effective inducer of oxidative stress proteins in B. subtilis, clearly induced the synthesis of thioredoxin. The induction of thioredoxin synthesis by glucose or phosphate starvation was less pronounced. Deleting the gene encoding the stress sigma factor, ςB, did not abolish the heat induction of thioredoxin, reinforcing the hypothesis that the second promoter is able to compensate for the loss of ςB in heat induction.
FIG. 5.
Synthesis of thioredoxin during exponential growth and after the imposition of different stresses and starvation. Wild-type bacteria (wt) and a strain with a null mutation in sigB (sigB) were grown in a synthetic medium, exposed to the indicated stimuli, and labeled prior to (control) or after stress as described in Materials and Methods. Crude protein extracts were prepared and separated by 2-D protein gel electrophoresis. The sections of the autoradiograms containing thioredoxin (TrxA) are displayed. The general stress protein Gsp20U, the oxidative stress-specific protein MrgA, and the rather heat-specific protein GroES are indicated as marker spots.
Construction of trxA mutants.
We repeatedly failed to construct an insertional mutant of trxA and decided to construct a conditional mutant by placing trxA under the control of PSPAC as described in Materials and Methods (Fig. 1C). In this strain (BIG1) the level of thioredoxin is controlled by the amount of IPTG added. The strain requires IPTG for growth, which stopped upon removal of IPTG (Fig. 6). These data argued that thioredoxin is an essential protein of B. subtilis.
FIG. 6.
Effect of variations in the level of TrxA on the growth of B. subtilis. The B. subtilis strain BIG1, in which the expression of trxA is controlled by PSPAC, was grown in the presence of 50 μM IPTG and diluted 30-fold into fresh prewarmed Luria broth with or without 50 μM IPTG. At the time indicated by the arrow different concentrations of IPTG were added to the cultures incubated without IPTG. The wild-type strain, IS58, is included as a control. ▪, IS58 control; □, BIG1 with 50 μM IPTG added immediately; •, BIG1 without IPTG; ○, BIG1 with 5 μM IPTG; ▴, BIG1 with 10 μM IPTG; ▾, BIG1 with 50 μM IPTG. OD500, optical density at 500 nm.
DISCUSSION
Thioredoxin seems to be an essential protein of B. subtilis, in contrast to E. coli (21, 34), since we failed to disrupt the trxA gene, and removal of IPTG from a strain expressing thioredoxin from an IPTG-controlled promoter resulted in growth arrest. Recently, thioredoxin has also been shown to be essential in Synechocystis (38) and Rhodobacter sphaeroides (40), and growth becomes dependent on the presence of thioredoxin in S. cerevisiae lacking glutathione reductase (37). Therefore, in B. subtilis thioredoxin might serve multiple functions in vivo, some of which in E. coli can also be catalyzed by glutathion and glutaredoxin (22), a system which has not yet been characterized in B. subtilis.
In this report we identify thioredoxin as a heat shock protein in B. subtilis. Previously, thioredoxin had been observed to induce heat shock only in human cells (24). In B. subtilis, three classes of heat-inducible proteins have been described (18). Class I genes, as exemplified by the dnaK and groE operons, are mainly induced by heat stress. Their heat induction involves a ςA-dependent promoter, an inverted repeat (CIRCE, for controlling inverted repeat of chaperone expression [TTAGCACTC-N9-GAGTGCTAA]) that is highly conserved among eubacteria, and a repressor interacting with the CIRCE element (59, 60). The activity of this repressor is modified by the GroE chaperonin machine (35).
Most of the heat-inducible genes of B. subtilis are also induced by a diverse range of stress conditions, such as salt stress, ethanol stress, and starvation for glucose, phosphate, or oxygen. These stress- or starvation-inducible proteins are called general or nonspecific stress proteins (43), the majority of which absolutely require ςB for their induction by various stresses (class II [18, 53]). Only a few genes, including lon, clpC, clpP, clpX, and ftsH, remain inducible by different stress conditions in the absence of ςB (class III [see reference 18 for a review]).
trxA remained stress inducible in the ςB mutant, although at a reduced level, and therefore belongs to the class III heat shock genes. Transcription of trxA initiates at two different promoters: the upstream promoter is ςB dependent, while the downstream promoter is presumably ςA dependent. The potentially ςA-dependent promoter might require activation by a positive regulatory protein, since it deviated in four of six positions from the consensus sequence of the −35 region recognized by ςA. Both promoters were heat and stress inducible, explaining the observation that trxA remained stress inducible even in a sigB mutant. This heat and stress induction occurring at a putatively ςA-dependent promoter in the absence of a CIRCE element is typical of the class III heat shock genes. It is interesting to note that the stress induction patterns at the two start sites differed. Both are induced by heat and ethanol stresses, although to a different extent, but only the transcription starting at the downstream start site, SA, is induced in cells treated with puromycin, paraquat, or, to a lower extent, hydrogen peroxide.
A similar double control by heat and stress involving a ςB-dependent promoter as well as a second, putatively ςA-dependent promoter was also described for clpC, a presumable chaperone and subunit of a stress protease (25). The stress induction patterns of the downstream, potentially ςA-dependent promoters of the trxA and clpC genes are quite similar. Therefore, it is tempting to speculate that ClpC and thioredoxin of B. subtilis, both of which might participate in the folding and refolding of proteins, are induced by heat and other stresses through similar mechanisms. Recently we identified a regulatory protein which might participate in the induction of the clpC operon of B. subtilis (26). The possible function of this regulator in the expression of trxA remains to be elucidated.
The thioredoxin-encoding gene trxA of B. subtilis is induced by H2O2 and paraquat at the downstream site, SA, indicating that thioredoxin is involved in the maintenance of the protein structure under oxidative stress, as suggested for thioredoxin and thioredoxin reductase in Mycobacteria (58), yeast (27, 36), and endothelial cells (14). Because any exposure of cellular proteins to oxidative stress may lead to an inappropriate formation of disulfide bonds, the protection of proteins in a functional state may require reduction of disulfides even in the otherwise very reductive cytoplasm of bacteria (9). Enhanced disulfide bond formation occurs in the cytoplasm of E. coli with a mutation in thioredoxin reductase (9). Furthermore, another repair mechanism of proteins damaged by oxidative stress is known for E. coli: the enzyme methionine sulfoxide reductase is able to recognize methionine sulfoxide and to reduce it to methionine. This repair reaction requires thioredoxin as a substrate (42).
In addition to oxidative stress, trxA is also induced by other stresses, including heat shock (Fig. 2 to 5). Heat shock and ethanol might both enhance incomplete reduction of molecular oxygen by respiration and therefore generate higher levels of peroxide anions. Although induction of trxA by heat stress or ethanol at the ςA-dependent promoter could be the result of the increased formation of reactive oxygen species, the signal responsible for induction at the ςB-dependent promoter must be different because oxidative stress does not induce the sigB regulon. Analysis of the thioredoxin function following stress might help to reveal the role of the ςB-dependent general stress response of a nongrowing cell. Although more than 40 ςB-dependent general stress proteins have been described (5, 53), there is only limited information available on the function of these proteins under stress and starvation. Recently, we obtained evidence that ςB is required for the nonspecific development of resistance to hydrogen peroxide in nongrowing, glucose-starved cells without any prior exposure to oxidative stress (13) and for the development of resistance against cumene hydroperoxide (1).
The double control at ςA-dependent and ςB-dependent promoters of genes like trxA and clpC ensures a specific induction by oxidative stress during growth as well as a nonspecific and protective induction in the nongrowing or stressed cells by ςB. The ςB-dependent general stress response of B. subtilis comprises other genes in addition to clpC (23) and trxA, such as the catalase-encoding katE (12), clpP (15), and dps (2), which are suspected to be related to oxidative stress and to protect the cell at different levels against oxidative damage. First, enzymes like catalase II (KatE) are produced and help to destroy reactive oxygen species. Secondly, ClpC and TrxA are induced at least partially in a ςB-dependent mechanism and raise the capacity of the bacteria to recover proteins damaged by oxidative stress. The risk of potential damage to the DNA is reduced by the induction of the nonspecific DNA-binding and -protecting protein Dps, which has recently been shown to play a crucial role in the development of nonspecific starvation-mediated resistance to oxidative stress (2). Finally, proteases like ClpP (15) and the chaperone and ATPase ClpC might be produced to degrade irreversibly damaged proteins and to recycle the chaperones bound to these proteins.
A similar function in the control of the expression of stationary-phase and stress genes has been assigned to RpoS in E. coli. The DNA-protecting protein Dps and a catalase (KatE), as well as a glutathion oxidoreductase (Gor), which have been shown to be subject to RpoS-dependent regulation, are essential to the oxidative stress resistance acquired in the stationary phase of growth (3, 11). In yeast cells, also, there is an increased requirement for protection from oxidative stress as the cells enter stationary phase (16). In Schizosaccharomyces pombe, glutathione reductase is not only induced by redox-cycling agents but also by high osmolarity, heat shock, or stationary phase, indicating that all these factors might provoke oxidative stress (28).
These observations raise the possibility that one of the important functions of the nonspecific stationary phase and stress response (of B. subtilis, E. coli, and probably also S. cerevisiae) may be the protection of the nongrowing or stressed cells from damage by reactive oxygen species.
ACKNOWLEDGMENTS
We are grateful to Haike Antelmann and Roland Schmid for help with the 2-D protein gel electrophoresis and for determining the N-terminal sequence of TrxA. We thank Anita Harang and Renate Gloger for excellent technical assistance and Anett Winkler for help in the characterization of the promoter fragment of pWH262.
Work in the laboratory of Michael Hecker was supported by grants from the Deutsche Forschungsgemeinschaft and the Fonds der Chemischen Industrie.
REFERENCES
- 1.Antelmann H, Engelmann S, Schmid R, Hecker M. General and oxidative stress responses in Bacillus subtilis: cloning, expression, and mutation of the alkyl hydroperoxide reductase operon. J Bacteriol. 1996;178:6571–6578. doi: 10.1128/jb.178.22.6571-6578.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antelmann H, Engelmann S, Schmid R, Sorokin A, Lapidus A, Hecker M. Expression of a stress- and starvation-induced dps/pexB-homologous gene is controlled by the alternative sigma factor ςB in Bacillus subtilis. J Bacteriol. 1997;179:7251–7256. doi: 10.1128/jb.179.23.7251-7256.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becker-Hapak M, Eisenstark A. Role of rpoS in the regulation of glutathione oxidoreductase (gor) in Escherichia coli. FEMS Microbiol Lett. 1995;134:39–44. doi: 10.1111/j.1574-6968.1995.tb07911.x. [DOI] [PubMed] [Google Scholar]
- 4.Benson A K, Haldenwang W G. Bacillus subtilis ςB is regulated by a binding protein (RsbW) that blocks its association with core RNA polymerase. Proc Natl Acad Sci USA. 1993;90:2330–2334. doi: 10.1073/pnas.90.6.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernhardt J, Völker U, Völker A, Antelmann H, Schmid R, Mach H, Hecker M. Specific and general stress proteins in Bacillus subtilis—a two dimensional protein electrophoretic study. Microbiology. 1997;143:999–1017. doi: 10.1099/00221287-143-3-999. [DOI] [PubMed] [Google Scholar]
- 6.Bolivar F, Rodrigues R L, Greener P J, Betlach M C, Heyneker H L, Boyer H W, Crosa J H, Falkow S. Construction and characterisation of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2:95–133. [PubMed] [Google Scholar]
- 7.Chae H Z, Chung S J, Rhee S G. Thioredoxin-dependent peroxide reductase from yeast. J Biol Chem. 1994;269:27670–27678. [PubMed] [Google Scholar]
- 8.Chen N-Y, Zhang J-J, Paulus H. Chromosomal location of the Bacillus subtilis aspartokinase II gene and nucleotide sequence of the adjacent genes homologous to uvrC and trx of Escherichia coli. J Gen Microbiol. 1989;135:2931–2940. doi: 10.1099/00221287-135-11-2931. [DOI] [PubMed] [Google Scholar]
- 9.Derman A I, Prinz W A, Belin D, Beckwith J. Mutations that allow disulfide bond formation in the cytoplasm of Escherichia coli. Science. 1993;262:1744–1747. doi: 10.1126/science.8259521. [DOI] [PubMed] [Google Scholar]
- 10.Dubnau D, Cirigliano C. Genetic characterization of recombination-deficient mutants of Bacillus subtilis. J Bacteriol. 1974;117:488–493. doi: 10.1128/jb.117.2.488-493.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dukan S, Touati D. Hypochlorous acid stress in Escherichia coli: resistance, DNA damage, and comparison with hydrogen peroxide stress. J Bacteriol. 1996;178:6145–6150. doi: 10.1128/jb.178.21.6145-6150.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engelmann S, Lindner C, Hecker M. Cloning, nucleotide sequence, and regulation of katE encoding a ςB-dependent catalase in Bacillus subtilis. J Bacteriol. 1995;177:5598–5605. doi: 10.1128/jb.177.19.5598-5605.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Engelmann S, Hecker M. Impaired oxidative stress resistence of Bacillus subtilis sigB mutants and the role of katA and katE. FEMS Microbiol Lett. 1996;145:63–69. doi: 10.1111/j.1574-6968.1996.tb08557.x. [DOI] [PubMed] [Google Scholar]
- 14.Fernando M R, Nanri H, Yoshitake S, Nagato-Kuno K, Minakami S. Thioredoxin regenerates proteins inactivated by oxidative stress in endothelial cells. Eur J Biochem. 1992;209:917–922. doi: 10.1111/j.1432-1033.1992.tb17363.x. [DOI] [PubMed] [Google Scholar]
- 15.Gerth, U., E. Krüger, I. Derre, T. Msadek, and M. Hecker. Stress induction of the Bacillus subtilis clpP gene encoding a homologue of the proteolytic component of the Clp protease and the role of ClpP and ClpX in stress tolerance. Mol. Microbiol., in press. [DOI] [PubMed]
- 16.Grant C M, Collinson L P, Roe J H, Dawes I W. Yeast glutathione reductase is required for protection against oxidative stress and is a target gene for yAP-1 transcriptional regulation. Mol Microbiol. 1996;21:171–179. doi: 10.1046/j.1365-2958.1996.6351340.x. [DOI] [PubMed] [Google Scholar]
- 17.Haldenwang W G. The sigma factors of Bacillus subtilis. Microbiol Rev. 1995;59:1–30. doi: 10.1128/mr.59.1.1-30.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hecker M, Schumann W, Völker U. Heat-shock and general stress response in Bacillus subtilis. Mol Microbiol. 1996;19:417–428. doi: 10.1046/j.1365-2958.1996.396932.x. [DOI] [PubMed] [Google Scholar]
- 19.Helmann J. Compilation and analysis of Bacillus subtilis ςA-dependent promoter sequences: evidence for extended contact between RNA polymerase and upstream promoter DNA. Nucleic Acids Res. 1995;23:2351–2360. doi: 10.1093/nar/23.13.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoch J A. Genetic analysis in Bacillus subtilis. Methods Enzymol. 1991;204:305–320. doi: 10.1016/0076-6879(91)04015-g. [DOI] [PubMed] [Google Scholar]
- 21.Holmgren A, Ohlsson I, Grankvist M L. Thioredoxin from Escherichia coli. Radioimmunological and enzymatic determinations in wild type cells and mutants defective in phage T7 DNA replication. J Biol Chem. 1978;253:430–436. [PubMed] [Google Scholar]
- 22.Holmgren A. Thioredoxin. Annu Rev Biochem. 1985;54:237–271. doi: 10.1146/annurev.bi.54.070185.001321. [DOI] [PubMed] [Google Scholar]
- 23.Holmgren A. Thioredoxin and glutaredoxin systems. J Biol Chem. 1989;264:13963–13966. [PubMed] [Google Scholar]
- 24.Jacquier-Sarlin M R, Polla B S. Dual regulation of heat-shock transcription factor (HSF) activation and DNA-binding activity by H2O2: role of thioredoxin. Biochem J. 1996;318:187–193. doi: 10.1042/bj3180187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krüger E, Msadek T, Hecker M. Alternate promoters direct stress-induced transcription of the Bacillus subtilis clpC operon. Mol Microbiol. 1996;20:713–723. doi: 10.1111/j.1365-2958.1996.tb02511.x. [DOI] [PubMed] [Google Scholar]
- 26.Krüger E, Msadek T, Ohlmeier S, Hecker M. The Bacillus subtilis clpC operon encodes DNA repair and competence proteins. Microbiology. 1997;143:1309–1316. doi: 10.1099/00221287-143-4-1309. [DOI] [PubMed] [Google Scholar]
- 27.Kuge S, Jones N. YAP1 dependent activation of TRX2 is essential for the response of Saccharomyces cerevisiae to oxidative stress by hydroperoxides. EMBO J. 1994;13:655–664. doi: 10.1002/j.1460-2075.1994.tb06304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee J, Dawes I W, Roe J H. Isolation, expression, and regulation of the Pgr1(+) gene encoding glutathione reductase absolutely required for the growth of Schizosaccharomyces pombe. J Biol Chem. 1997;272:23042–23049. doi: 10.1074/jbc.272.37.23042. [DOI] [PubMed] [Google Scholar]
- 29.Loferer H, Hennecke H. Expression, purification and functional properties of a soluble form of Bradyrhizobium japonicum TIpA, a thioredoxin-like protein. Eur J Biochem. 1994;223:339–344. doi: 10.1111/j.1432-1033.1994.tb18999.x. [DOI] [PubMed] [Google Scholar]
- 30.Majumdar D, Avissar Y J, Wyche J H. Simultaneous and rapid isolation of bacterial and eucaryotic DNA and RNA—a new approach for isolation DNA. BioTechniques. 1991;11:94–101. [PubMed] [Google Scholar]
- 31.Mark D F, Richardson C C. Escherichia coli thioredoxin: a subunit of bacteriophage T7 DNA polymerase. Proc Natl Acad Sci USA. 1976;73:780–784. doi: 10.1073/pnas.73.3.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maul B, Völker U, Riethdorf S, Engelmann S, Hecker M. ςB-dependent induction of gsiB by multiple stimuli in Bacillus subtilis. Mol Gen Genet. 1995;248:114–120. doi: 10.1007/BF02456620. [DOI] [PubMed] [Google Scholar]
- 33.Meade H M, Long S R, Ruvkun G B, Brown S E, Ausubel F M. Physical and genetic characterization of symbiotic and auxotrophic mutants of Rhizobium meliloti induced by transposon Tn5 mutagenesis. J Bacteriol. 1982;149:114–122. doi: 10.1128/jb.149.1.114-122.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miranda-Vizuete A, Martinez-Galisteo E, Aslund F, Lopez-Barea J, Pueyo C, Holmgren A. Null thioredoxin and glutaredoxin Escherichia coli K-12 mutants have no enhanced sensitivity to mutagens due to a new GSH-dependent hydrogen donor and high increases in ribonucleotide reductase activity. J Biol Chem. 1994;269:16631–16637. [PubMed] [Google Scholar]
- 35.Mogk A, Homuth G, Scholz C, Kim L, Schmid F X, Schumann W. The GroE chaperonin machine is a major modulator of the CIRCE heat shock regulon of Bacillus subtilis. EMBO J. 1997;16:4579–4590. doi: 10.1093/emboj/16.15.4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morgan B A, Banks G R, Toone W M, Raitt D, Kuge S, Johnston L H. The Skn7 response regulator controls gene expression in the oxidative stress response of the budding yeast Saccharomyces cerevisiae. EMBO J. 1997;16:1035–1044. doi: 10.1093/emboj/16.5.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muller E G D. A glutathione reductase mutant of yeast accumulates high levels of oxidized glutathione and requires thioredoxin for growth. Mol Biol Cell. 1996;7:1805–1813. doi: 10.1091/mbc.7.11.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Navarro F, Florencio F J. The cyanobacterial thioredoxin gene is required for both photoautotrophic and heterotrophic growth. Plant Physiol (Rockville) 1996;111:1067–1075. doi: 10.1104/pp.111.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Noiva R. Enzymatic catalysis of disulfide formation. Protein Expr Purif. 1994;5:1–13. doi: 10.1006/prep.1994.1001. [DOI] [PubMed] [Google Scholar]
- 40.Pasternak C, Assemat K, Clementmetral J D, Klug G. Thioredoxin is essential for Rhodobacter sphaeroides growth by aerobic and anaerobic respiration. Microbiology. 1997;143:83–91. doi: 10.1099/00221287-143-1-83. [DOI] [PubMed] [Google Scholar]
- 41.Pigiet V P, Schuster B J. Thioredoxin-catalyzed refolding of disulfide-containing proteins. Proc Natl Acad Sci USA. 1986;83:7643–7647. doi: 10.1073/pnas.83.20.7643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rahman M A, Nelson H, Weissbach H, Brot N. Cloning, sequencing, and expression of the Escherichia coli peptide methionine sulfoxide reductase gene. J Biol Chem. 1992;267:15549–15551. [PubMed] [Google Scholar]
- 43.Richter A, Hecker M. Heat shock proteins in Bacillus subtilis: a two-dimensional gel electrophoresis study. FEMS Microbiol Lett. 1986;36:69–71. [Google Scholar]
- 44.Riethdorf S, Völker U, Gerth U, Winkler A, Engelmann S, Hecker M. Cloning, nucleotide sequence, and expression of the Bacillus subtilis lon gene. J Bacteriol. 1994;176:6518–6527. doi: 10.1128/jb.176.21.6518-6527.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Russel M, Model P. Thioredoxin is required for filamentous phage assembly. Proc Natl Acad Sci USA. 1985;82:29–33. doi: 10.1073/pnas.82.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 47.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith I, Paress P, Cabane K, Dubnau E. Genetics and physiology of the rel system of Bacillus subtilis. Mol Gen Genet. 1980;178:271–279. doi: 10.1007/BF00270472. [DOI] [PubMed] [Google Scholar]
- 49.Spector A, Yan G Z, Huang R R C, McDermott M J, Gascoyne P R C, Pigiet V. The effect of H2O2 upon thioredoxin-enriched lens epithelial cells. J Biol Chem. 1988;263:4984–4990. [PubMed] [Google Scholar]
- 50.Stülke J, Hanschke R, Hecker M. Temporal activation of β-glucanase syntheses in Bacillus subtilis is mediated by the GTP-pool. J Gen Microbiol. 1993;39:2041–2045. doi: 10.1099/00221287-139-9-2041. [DOI] [PubMed] [Google Scholar]
- 51.Vagner V, Dervyn E, Ehrlich D. Abstracts of the 8th International Conference on Bacilli. 1995. A vector for systematic analysis of unknown genes; p. 74. [Google Scholar]
- 52.Völker U, Riethdorf S, Winkler A, Weigand B, Fortnagel P, Hecker M. Cloning and characterization of heat-inducible promoters of Bacillus subtilis. FEMS Microbiol Lett. 1993;106:287–294. doi: 10.1111/j.1574-6968.1993.tb05978.x. [DOI] [PubMed] [Google Scholar]
- 53.Völker U, Engelmann S, Maul B, Riethdorf S, Völker A, Schmid R, Mach H, Hecker M. Analysis of the induction of general stress proteins of Bacillus subtilis. Microbiology. 1994;140:714–752. doi: 10.1099/00221287-140-4-741. [DOI] [PubMed] [Google Scholar]
- 54.Völker U, Dufour A, Haldenwang W G. The Bacillus subtilis rsbU gene product is necessary for RsbX-dependent regulation of ςB. J Bacteriol. 1995;177:114–122. doi: 10.1128/jb.177.1.114-122.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Völker U, Völker A, Maul B, Hecker M, Dufour A, Haldenwang W G. Separate mechanisms activate ςB of Bacillus subtilis in response to environmental and metabolic stresses. J Bacteriol. 1995;177:3771–3780. doi: 10.1128/jb.177.13.3771-3780.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wetzstein M, Völker U, Dedio J, Löbau S, Zuber U, Schiesswohl M, Herget C, Hecker M, Schumann W. Cloning, sequencing, and molecular analysis of the dnaK locus from Bacillus subtilis. J Bacteriol. 1992;174:3300–3310. doi: 10.1128/jb.174.10.3300-3310.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Whitehead T R. Nucleotide sequences of xylan-inducible xylanase and xylosidase/arabinosidase genes from Bacteroides ovatus V975. Biochim Biophys Acta. 1995;1244:239–241. doi: 10.1016/0304-4165(95)00051-c. [DOI] [PubMed] [Google Scholar]
- 58.Wieles B, Ottenhoff T H M, Steenwijk T M, Franken K, Devries R R P, Langermans J A M. Increased intracellular survival of Mycobacterium smegmatis containing the Mycobacterium leprae thioredoxin-thioredoxin reductase gene. Infect Immun. 1997;65:2537–2541. doi: 10.1128/iai.65.7.2537-2541.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yuan G, Wong S L. Isolation and characterization of Bacillus subtilis groE regulatory mutants: evidence for orf39 in the dnaK operon as a repressor gene in regulating the expression of both groE and dnaK. J Bacteriol. 1995;177:6462–6468. doi: 10.1128/jb.177.22.6462-6468.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zuber U, Schumann W. CIRCE, a novel heat shock element involved in regulation of heat shock operon DnaK of Bacillus subtilis. J Bacteriol. 1994;176:1359–1363. doi: 10.1128/jb.176.5.1359-1363.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]