Abstract
Learned associations about salient experiences (e.g. drug exposure, stress) and their associated environmental stimuli are mediated by a minority of sparsely distributed, behaviourally activated neurons coined ‘neuronal ensembles’. For many years, it was not known whether these neuronal ensembles were involved in mediating learned behaviours. However, in the last several years the ‘Daun02 inactivation technique’ in Fos-lacZ transgenic rats has proved very useful in establishing causal links between neuronal ensembles that express the activity-regulated protein ‘Fos’ and learned behaviours. Activated, Fos-expressing neurons in these rats also express the bacterial protein β-galactosidase (β-gal)1. When the prodrug Daun022 is injected into the brains of these rats 90 min after a behaviour (e.g. drug-seeking) or cue exposure, then the Daun02 is converted into daunorubicin by β-gal that inactivates the Fos and β-gal-expressing neurons.3, 4 This unit presents protocols for breeding the Fos-lacZ rats and conducting appropriate Daun02 inactivation experiments.
Keywords: neuronal ensemble inactivation, Daun02 inactivation, Fos-lacZ rodents
INTRODUCTION
For many decades, neuroscientists have been busy deciphering the neurobiological basis of learned behaviours by mapping the activity of neurons. Early studies from the 1970’s using in vivo electrophysiology tools have observed that only a minority of neurons were activated during learned behaviours.5 Starting in the late 1980’s, researchers complemented these electrophysiology findings using immediate early gene (IEG) markers of strong neuronal activity6, 7. The most commonly used IEG marker was Fos mRNA and its protein product Fos that have been used to perform large-scale activity mapping of many brain areas in behaviour. Today, it is known that various stimuli induce Fos in motivationally relevant brain areas such as the prefrontal cortex, nucleus accumbens8. These stimuli range from stressful stimuli, drugs of abuse (e.g. cocaine), ingestion of palatable foods, and cues associated with drug and natural rewards 9. Although interesting, these findings were purely correlative and did not demonstrate whether these activated neurons played a direct causal role in the learned behaviours. Until recently, tools did not exist to selectively silence the small number of activated neurons without disrupting activity of the surrounding majority of neurons.
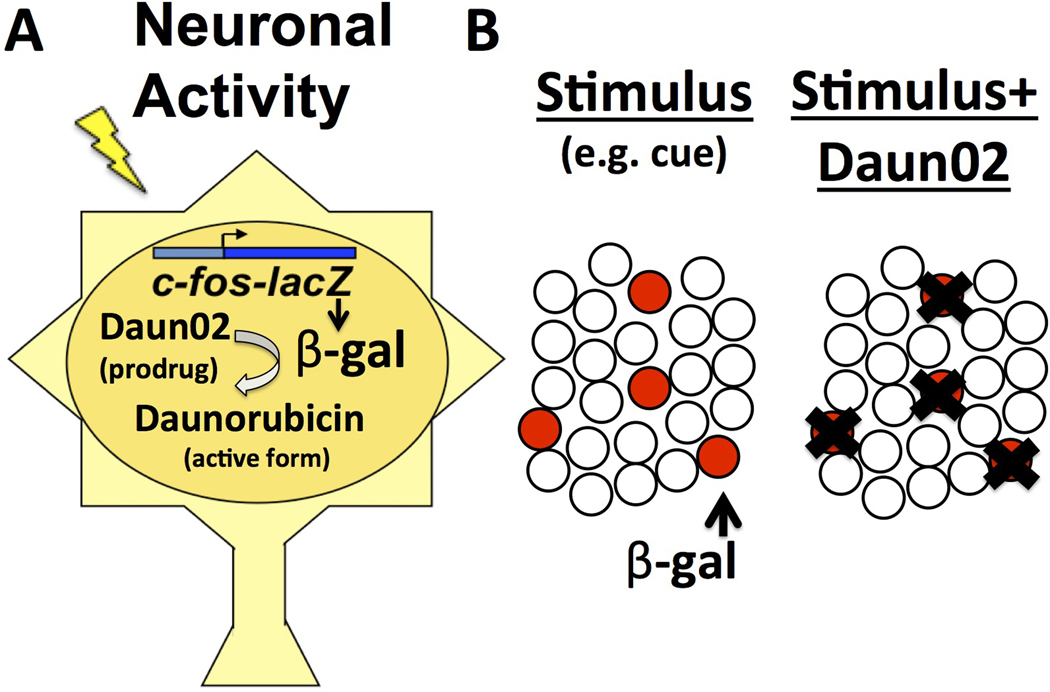
To overcome this challenge, we developed a tool, coined the ‘Daun02 inactivation procedure’, to selectively silence Fos-expressing neurons (Fig. 1). In this inactivation procedure, Fos-lacZ transgenic rats undergo some form of learning, and then are exposed to external stimuli (e.g. reward-related cues) that induce expression of the activity-dependent transgene lacZ and its protein product β-galactosidase (β-gal) in activated Fos-expressing neurons. When β-gal levels are maximal, the inactive prodrug Daun02 is injected directly into the brain area of interest. Daun02 is converted by β-gal into daunorubicin to reduce cellular excitability10 and induce apoptosis and cell death4. Thus, activated β-gal expressing neurons are selectively and persistently silenced, while the surrounding neurons are unaffected. The behavioural consequences of inactivating the Fos/β-gal-expressing neuronal ensembles can then be examined.
Figure 1.
Daun02-mediated selective inactivation of behaviourally activated neurons A) Fos-lacZ rat or mouse neurons express β-gal when activated. After Daun02 is locally injected in the target brain area, β-gal converts Daun02 into its active form, daunorubicin, which persistently reduces neuronal excitability and/or lesions neurons. B) Selective inactivation of behaviourally-activated neurons following Daun02 application.
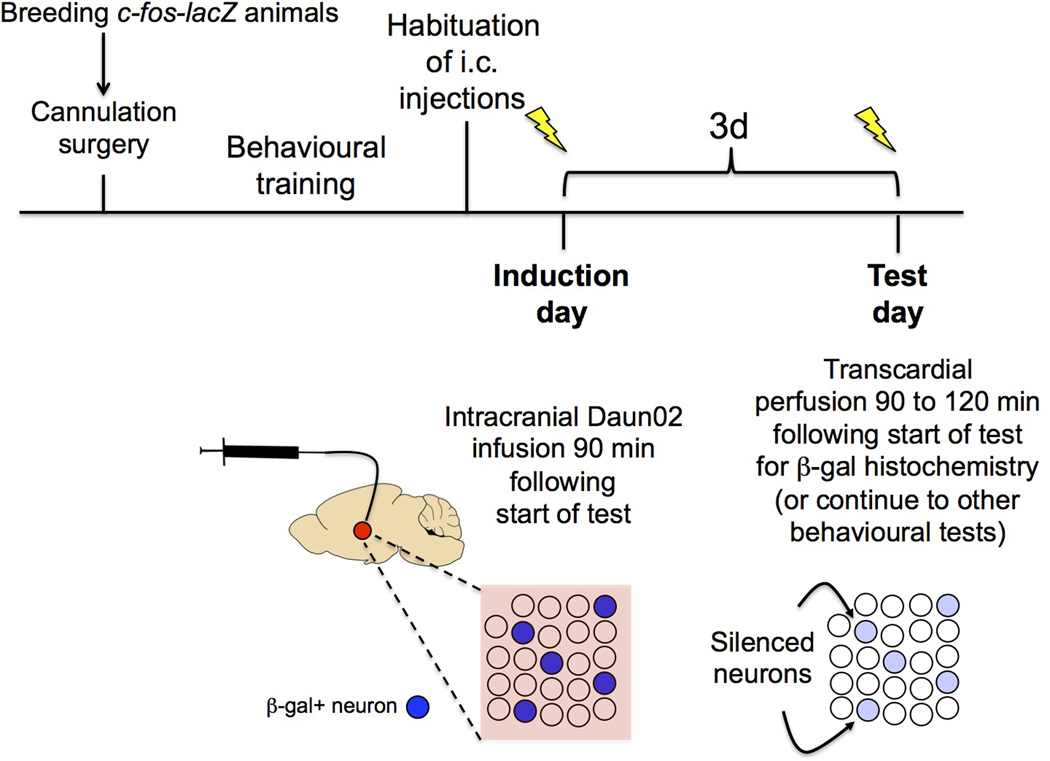
This unit describes the Daun02 inactivation procedure that allows inactivation of behaviourally activated, β-gal (and Fos)–expressing neurons in Fos-lacZ rats and mice. There are multiple Basic Protocols in this unit with several key steps described in each Protocol, and they are written in the order of the events you will encounter from start to finish. Figure 2 provides a general overview of the major steps.
Figure 2.
A general overview of the experimental procedures beginning with breeding the Fos-lacZ animals and finishing with behavioural testing.
Note: All protocols using live animals must be reviewed first by institutional ethical committees and must follow officially approved procedures for care and use of laboratory animals.
BASIC PROTOCOL 1
Breeding and genotyping of Fos-lacZ transgenic rats or mice
Fos-lacZ rats are bred on a Sprague-Dawley background, where as the Fos-lacZ mice are bred on a hybrid B6C3F1 x B6D2 background. Use only hemizygous Fos-lacZ rats and mice. We had found that homozygous Fos-lacZ rats express high basal levels of β-gal that will interfere with selective Daun02 inactivation of only the behaviourally activated neurons (unpublished observations). An animal heterozygous for the Fos-lacZ gene (male or female) should be mated with an outbred wild-type animal (male or female). Approximately 50% of the offspring will be transgene positive. As a general rule of thumb, 11–12 breeding pairs will produce approximately 28–32 positive male and female offspring per month. For the purposes of genotyping, either ear punches or tail snips can be obtained. The PCR protocol for detecting the Fos-lacZ gene with the specific primer sets is provided below, but the protocol can be modified depending on the primer set.
Materials
Ear punch collector (for ear punches)
1.5mL microfuge tubes
Vortex device
Sodium hydroxide solution 10.0M (Cat no. 72068–100ML, Sigma)
Trizma® hydrochloride solution 1M (Tris-HCl) pH 8 (Cat no., T2694–1L, Sigma)
OneTaq DNA polymerase mastermix (Cat no. M0482S, New England Biolabs)
Oligos (IDT DNA Technologies, standard synthesis, and desalt prepration)
PCR primers forward sequence: GTTGCAGTGCACGGCAGATACACTTGCTGA ; reverse sequence GCCACTGGTGTGGGCCATAATTCAATTCGC
100bp DNA ladder (Cat no. N3231S, New England Biolabs)
Ultra pure 10x TAE buffer (Cat no. 15558–026, ThermoFisher Scientific)
Agarose
Sub-Cell® Model 96 Cell and PowerPac™ Universal Power Supply (Bio-rad)
Protocol steps
Place the biopsy sample in a 1.5ml microfuge tube.
Add 300 µL of 50mM NaOH
Incubate tubes at 95°C for 60 minutes.
Vortex tubes on medium power setting for 5 seconds.
Quick spin the tubes to bring down the condensation.
Neutralize each sample by adding 30 µL of 1M Tris-HCl (pH 8).
Vortex tubes on medium power setting for 5 seconds.
Quick spin the tubes to bring down the condensation. The undigested remnant of the sample or debris may remain visible at the bottom of the tube. Some debris is to be expected, and make sure to only take the supernatant (what we refer to as ‘genomic DNA lysate’) when setting the PCR reaction.
Prepare the PCR reaction mix as indicated below:
| 1 reaction | |
|---|---|
| (µL) | |
| 2x One Taq mastermix | 5.5 |
| 5 µM Forward Oligo | |
| 5 µM Reverse Oligo | 1.1 |
| Water | 3.4 |
|
| |
| Total Volume | 10 |
Dispense 10µL per tube and then add 1µL of genomic DNA lysate.
Transgene-specific oligos for OTTC126 (lacZ gene):
| Forward Oligo | LacZ F2538 | GTTGCAGTGCACGGCAGATACACTTGCTGA |
| Reverse Oligo | LacZ R2926 | GCCACTGGTGTGGGCCATAATTCAATTCGC |
These oligos produce a 388 base pair amplicon that was previously tested using an annealing gradient that indicates good amplification between 58°C and 68°C (ideally between 64° to 68 °C). This allows the use of the two-step PCR program described below.
-
0.
94 °C – HOLD (hot start)
-
1.
94 °C – 2 min
-
2.
94 °C – 30 sec
-
3.
68 °C – 1 min
-
4.
go to step 2, repeat 40x
-
5.
4 °C – HOLD (end of program)
The samples should be analyzed on a 2% agarose gel in 1x TAE buffer.
BASIC PROTOCOL 2
Preparation of Daun02
Daun02 (MW 884.79) is an anthracyline-based ‘prodrug’ that is initially biologically inactive. Daun02 becomes biologically active when it is converted into daunorubicin (also known as daunomycin) by the bacterial protein β-gal. In the first study, Daun02 was solubilized in 50% DMSO and 50% artificial cerebrospinal fluid (aCSF), which worked well for nucleus accumbens injections3. For reasons unknown, this vehicle produces more neuronal damage in cortical areas, and thus we and others routinely use 4 µg/µL Daun02 in 5% DMSO, 6% Tween-80 in PBS, which can be prepared using the protocol below.
Note: The Daun02 compound should be stored at −20°C in a sealed container using desiccant. As with all anthracyline-related compounds it should be handled in a well-ventilated area using appropriate safety measures (e.g. gloves). Also, when dissolving Daun02 make sure to use containers using DMSO compatible material (e.g. LDPE, HDPE, polypropylene, PPCO, polymethylpentene) that tend to have an opaque colour, as non-compatible materials (e.g. polysulfone, PVC, polycarbonate) with a clear appearance, will be dissolved by DMSO.
Materials
Dimethyl sulfoxide (DMSO) (Cat no. D8418–50mL, Sigma)
Tween-80 (Cat no. P4780–100mL, Sigma)
10x Phosphate buffered saline (PBS) (Cat no. P5493–1L, Sigma)
Artificial cerebral spinal fluid (aCSF) (Cat No. 3525, Tocris)
Dissolve Daun02 in 100% DMSO in a microfuge tube at a concentration of 80 µg/µL by gently pipetting up and down, followed by vortexing. This stock solution may be stored at −20°C for up to 1 year (or at −80°C for several years).
On the day of use, fully thaw the frozen aliquot to room temperature or if freshly prepared, directly dissolve 2.5 µL of 80 µg/µL Daun02 in DMSO (or 2.5 µL DMSO to make vehicle) for every 15 µL of 20% Tween-80. Make 9 µL aliquots and store at −20°C for up to 1 year or −80°C for several years.
On the day of use, fully thaw frozen Daun02 (or vehicle) aliquot to room temperature. Then add 16.5 µL of sterile PBS or aCSF and mix well to make a 4 µg/µL Daun02 solution in 5% DMSO, 6% Tween-80 vehicle. Daun02 will precipitate irreversibly if PBS or aCSF is added before the Daun02 stock solution has warmed to room temperature. It is important to make the vehicle control for injections using the same solution. Quick spin both the Daun02 and vehicle to bring solution to the bottom of the microfuge tube. Keep this solution at room temperature until use, and discard the remaining solution.
BASIC PROTOCOL 3
Surgical procedures for guide cannula implantation for rat
In order to exert its neuronal inactivation functions in a region-specific manner, Daun02 must be directly injected into the target brain areas. Thus, Fos-lacZ animals must be intracranially implanted with guide cannula prior to Daun02 infusions. This protocol describes this cannula implantation. For a useful video guide, please see Fornari et al., 2012.11
Materials
Any anesthesia (e.g. ketamine and xylazine) that will last for the duration of surgery (approximately 1 hour).
Atropine sulfate
Betadine disinfectant
70% ethanol
Dental acrylic (Plastics one)
Stereotaxic atlas for rat or mouse
Stereotaxic frame equipped with carrier for guide cannula (David Kopf Instruments, Stoelting)
26-G internal cannula (Plastics one)
Shaver
Scalpel
Serrefine forceps or hemostats
Cotton Swabs
Spatula
Dental drill
3/16 inch bone screws, 0 to 80 stainless steel (Plastics one)
Jeweler’s forceps and screwdriver
Protocol steps
-
1
Using a stereotaxic atlas such as Paxinos 198612, determine the stereotaxic coordinates for the target brain areas. Make sure to order the appropriate guide cannulas and injector needles that extend 0.3–0.5 mm below the cannula. A commonly used landmark on the skull is the ‘bregma’ where the major fissures of the skull form a plus-like symbol. This examination should be done after the anesthetized animal has been inserted into the stereotaxic frame. Pilot studies are highly recommended for accurate guide cannula placement.
-
2
Once the rat is anesthetized, mount the rat in the stereotaxic apparatus. Then expose the skull, by first making an incision using a sterile scalpel and scrape off the dura matter using a metal spatula, and clean off any excess blood using cotton swabs. Lower the guide cannula such that it is centered on the bregma, which is the intersection of the coronal and sagittal sutures. Read the anterior-posterior (AP), lateral (L), and dorsal-ventral (DV) coordinates from the scale, and add or subtract from the stereotaxic coordinates.
-
3
Before surgery, please ensure that all surgical instruments are properly sterilized (via autoclave or chemical sterilization) .
-
4
Once the rat is calm and well-handled, inject intraperioteneally (i.p.) with an anesthetic that will last for the duration of surgery (e.g. ketamine and xylazine). If necessary, administer atropine to relieve respiratory distress. The efficacy of the anesthesia should be monitored by observing the breathing (which should mostly be abdominal) and test for pain reflexes by pinching on of the feet with forceps. If a response is observed after 10 min, a supplemental dose of anesthetic should be administered.
-
5
Shave the rat’s head and mount it into the stereotaxic instrument. Make sure to mount the rat first on the ear bars of the stereotax.
-
6
Read the coordinates of each ear bar and center the animal between the bars. Open the rat’s mouth and insert the upper incisors over the incisor bar. Adjust the nose clamp to firmly hold the rat’s head.
-
7
Disinfect the head using Betadine, followed by 70% ethanol, and then Betadine again. Make an incision around the midline of the head using a scalpel using one smooth stroke. Afterwards the skin should be retracted with hemostats or serrefine forceps.
-
8
Using cotton swabs, scrape away connective tissue on skull. Dry the skull area using a cotton sponge. Identify the bregma (intersection of the coronal suture with midline) and lambda (intersection of the occipital suture with the midline).
Implantation of the guide cannula
-
9
Using a hand drill with a bit that is sized similarly with the diameter of the guide cannula, drill two holes bilaterally into the skull where the guide cannula are to be inserted. The drilling point may be marked with using a fine-tipped felt pen. Drill until the dura is broken, but do not damage the brain tissue by drilling too deep.
-
10
Using jeweler’s forceps and a screwdriver, insert the 3/16-inch bone screws into the 2 holes. These screws will anchor the dental cement.
-
11
Attach the guide cannula into the stereotaxic holder and straighten. Carefully lower the guide cannula into the target area until the cannula base rests on the skull.
-
12
Dry the skull again if necessary and remove the skin retractors. Mix dental acrylic and liquid component until the mixture is wet. While it is wet, apply this mixture to the skull around the base of the guide cannula and screws.
-
13
Once the acrylic is dry, remove the stereotax holder. If there are any jagged edges, make sure to smooth them out by applying additional dental acrylic mixture. The jagged edges will irritate the rat.
-
14
Remove the rat from the stereotaxic holder, and implant a dummy cannula into the guide cannula. Allow the rat to rest in its home cage and use a heating blanket and thermometer to maintain proper body temperature.
-
15
The rats should be allowed to recover for 7 days (or longer if necessary) before commencing any behavioural experiments. Make sure to unblock the guide cannula for debris every 2 days by repeatedly inserting the dummy cannula up and down.
Alternate protocol 1
Guide cannula implantation for mouse
Intracranial infusions of Daun02 can also be performed in the Fos-lacZ mouse. Similar surgical procedures are used, except due to the smaller body size, either adapters or a stereotaxic apparatus designed for mice must be utilized. Below is the protocol for guide cannula implantation, as similar procedures are used as the rat, many of the steps have been condensed.
Materials
Anesthetic: Any anesthetic that will last during the duration of the surgery (e.g. ketamine and xylazine) which will last about one hour.
Bonding resin (dental acrylic, Denmat)
Shaver
Stereotaxic frame adapted for use for mice
Stage to elevate body of mouse to level of ear bars (if necessary)
Blunt forceps
Surgical instruments for mice
Hand drill
Miniature pin vice
Guide cannula (Plastics one)
Stereotaxic atlas for mouse (e.g. Paxinos and Franklin, 200113)
Placing the mouse in the stereotaxic frame
-
1
Anesthetize the mouse and shave the top of its head.
-
2
Insert the mouse into the stereotaxic frame. Lock one ear onto the ear bar until stationary and then place the temporal mandibular joint of the mouse firmly against it. Move the second ear onto the other ear bar and tighten the ear cup fittings.
-
3
Insert the upper incisors over the incisor bar and gently close the nose bar.
Performing surgery and implant guide cannula
-
4
Make a midline incision to expose the skull, and then clean the skull by scraping off the fascia. Ensure that the skull is dry using cotton swabs, so that the skull’s fissures are clearly visible.
-
5
Adjust head so that the skull position is flat.
-
6
Mark the skull where the guide cannulae are supposed to be inserted. Then using a hand drill, drill holes into the skull without damaging the brain.
-
7
Place a straight guide cannula into the cannula holder and lower it into the target region. Apply wet dental acrylic around the cannula shaft, which allows the dental cement to connect with the bone. Apply sufficient amount of dental cement to properly secure the cannula. Once the acrylic is dry remove the cannula holder.
BASIC PROTOCOL 4
Intracranial infusions of Daun02 and subsequent behavioural testing
Behavioural training of the rats or mice may commence approximately a week after surgery. Many Daun02 studies to date had silenced neurons that were activated during different motivated behaviours, and then rats were tested 3 days later to observe whether the behaviour of interest was disrupted. This protocol describes how and when to intracranially infuse Daun02 to silence the neuronal ensembles of interest. It is highly advisable to perform pilot experiments to see if the target brain area expresses β-gal and characterise the degree of neuronal activation (i.e. counting the number of Fos or β-gal expressing neurons) during the behaviour of interest, to gain better insight about the nature of the neuronal ensemble that is recruited.
Materials
Fos-lacZ rat or mouse with implanted guide cannula
Daun02 (prepared from previous protocol, 4µg/µL at room temperature)
Vehicle (prepared from previous protocol)
Flexible tubing
Hamilton syringes (10µL, with 30 G needle)
Infusion pump (Item 702001; Harvard Apparatus)
Two to three days before infusing Daun02, use dummy injector needles that are shorter than the guide cannula to practice a sham infusions session. The purpose of this step is to habituate the animals to the infusion procedure.
On the day of Daun02 infusions, prepare the Daun02 as described in protocol 2 step 3 for a final concentration of 4 µg/µL. Attach plastic tubing to 2 Hamilton syringes, then attach 2 injector needles. Make sure that the tubing and needles are cleaned first with 70% ethanol, then water. Push out excess water, and then aspirate 10µL of Daun02 or vehicle using the Hamilton syringes. Then use the pump to push the Daun02 or vehicle solution until it is slightly dripping from the injector needles. Wipe off excess fluid with sterile gauze.
- Initiate the *behavioural test. β-gal expression is at its peak level 90 min after initiation of this behavioural test. At this time, gently restrain the rat and insert the injector needles bilaterally. Place the rat in a comfortable cage with bedding and infuse 0.5µL of Daun02 or vehicle at a rate of 1µL/min. Once the infusions are over, the injector needles should remain in the animal for an additional 1 min. For mice, it is advisable to perform this infusion procedure after briefly anesthetizing the animal using isoflurane.To date, many behavioural studies have used relatively short behavioural tests (e.g. 15–30 min) since they utilized procedures in which extinction learning took place (e.g. lever-pressing for a drug-associated cue).14–16 The duration was kept short in order prevent the behavior of interest from being fully extinguished, such that the behaviour could still be observed in the control group if animals were to be subjected to the same behavioural test.
The behavioural test is performed 3 days later to test the effects of Daun02 versus vehicle injections. Recent evidence suggests that this time point allows Daun02 to lesion β−gal-expressing neurons. Although we and others normally test the effects of Daun02 three days following its infusion, it is likely that Daun02 is efficacious at time points earlier or later than 3 days. Users may want to perform pilot experiment to examine whether this is the case.
90–120 min after initiating the behavioural test, users may choose to transcardially perfuse the animals using 4% paraformaldehyde for X-gal staining or immunohistochemistry of brain sections to assess Daun02-mediated decreases of activated neurons using X-Gal staining of β−gal (see next protocol) or immunohistochemistry for Fos protein. Alternatively, since Daun02 has been shown to permanently silence neurons (via lesioning), one may also continue to test the animals on other behavioural tests to examine the effects of this inactivation.
BASIC PROTOCOL 5
Transcardial perfusions and X-gal staining (or β-gal immunohistochemistry) following the final behavioural test.
In previous Daun02 studies, the number of β-gal-expressing neurons were quantified following the final behavioural test in order to determine the degree of neuronal inactivation3, 16. The protocol below describes how to transcardially perfuse a Fos-lacZ rat or mouse following a behavioural test to prepare the brain tissue for X-gal staining or β-gal (or Fos) immunohistochemistry. Although both types of staining can be used to detect changes in the number of strongly activated neurons, the advantage of X-gal staining is that it has a very high signal to noise ratio.
Materials
Peristaltic pump attached to an IV line with 16 gauge blunt end needles at end (for rat)
Paraformaldehyde (PFA, for making 4% PFA) (Cat no. P6148–1KG, Sigma)
Sucrose (for making 30% sucrose solution in PBS)
10x PBS pH 7.4 for making (1x PBS) (Cat no. P5493-1L, Sigma)
Sodium hydroxide solution 10.0 M (Cat no. 72068-100ML, Sigma)
Sodium phosphate monobasic monohydrate (Cat no. S9638–25G, Sigma)
Sodium Chloride (Cat no. S7653–1kg, Sigma)
Ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA) (Cat no. E3889–25G)
Magnesium chloride solution, 1.0M (Cat no. M1028–100mL, Sigma)
Triton X-100 (Cat no. 93443–100mL, Sigma)
Potassium ferricyanide (III) K3Fe(CN)6 (Cat no. 244023–100G, Sigma)
Potassium hexa-cyanoferrate (II) trihydrate K4Fe(CN)6 • 3H2O (Cat no. P3289–100g, Sigma)
5-Bromo-4-chloro-3-indolyl-β-D-galactoside (X-gal) (Cat no., B71800–1.0, RPI corporation)
N,N-Dimethylformamide (DMF) (Cat no., D4551–250mL, Sigma)
Corning® Costar® cell culture plates 6 well, (Cat no. CLS3516–50EA, Sigma)
Corning® Netwells® inserts, membrane diam. 24 mm (Cat no. CLS3480–48EA, Sigma)
Staining racks and handles (Cat. No. 70312–24, 70312–25, Electron microscopy services)
6 Staining dishes (Cat. No 70312–20, Electron microscopy services)
CitriSolv™ Solvent and Clearing Agent (Cat no. 89426–268, VWR)
(In the UK use Histoclear III (Cat no. HS-204–1 gal, AGTC Bioproducts)
Fisher Chemical Permount™ Mounting Medium (Cat no. SP15–500, Fisher)
(In the UK use Histomount (Cat no. HS-103–100mL, AGTC Bioproducts)
Incubator or waterbath
Any anesthetic that will produce deep anesthesia (e.g. isoflurane).
Protocol steps
Transcardial perfusions
-
1
Prepare 1x PBS, 4% Paraformaldehyde (PFA), and 30% sucrose (in PBS) solutions. These should be set up together with the perfusion equipment before the behavioural testing begins. We will use approximately 500mL of 4% PFA per rat (200 mL per mouse).
-
2
90–120 min following initiation of the behavioural test, deeply anesthetize the animal. After confirming that the animal is deeply anesthetized by checking for the pain reflex (e.g. following pressure application on the footpad), use a large sharp scalpel blade to cut through the skin and muscle of the thorax along the mid-axillary line, extending from the axilla to the inferior margin of the rib cage. Make the incision at the level of the xiphisternum.
-
3
Grasp the xiphoid process with a large hemostat and elevate the anterior body wall. Using medium-sized scissors, cut through the abdominal muscles along the inferior margin of the ribs.
-
4
Cut through the diaphragm along its attachment to the ventral and lateral margins of the rib cage.
-
5
Cut through the lateral part of the rib cage on both sides. Use the hemostat attached to the xiphoid process to retract the anterior part of the rib cage and hold it out of the field.
-
6
Open the pericardial sac using a small pair of sharp scissors. Cut open the right atrium.
-
7Using scissors, make a transverse cut through the posterior left aspect of the heart to open left ventricle. While stabilizing the heart using forceps, insert the perfusion needle through the opening in the left ventricle into the ascending aorta. Clamp the cannula in place using a hemostat across the superior part of the ventricles.For the mouse, it is possible to gently pierce the 26G perfusion needle directly into the left ventricle since the ascending aorta is very small.
-
8After confirming the placement of the needle tip, turn on the perfusion pump and perfuse PBS into the animal at a flow rate of 60–100 and 3–4 mL/min for rats and mice, respectively. Once PBS has replaced the blood in the animal’s vascular system, and the fluid escaping the atrium is clear, then transfer the pump input line from the PBS to 4% PFA solution.Note: If fluid is coming out of the nostrils the perfusion pressure may be too high.
-
9
Once the line transfer is complete, begin pumping the 4% PFA solution. As the fixative solution enters the body, the body should rapidly begin to stiffen. Perfuse the desired volume, turn off the pump and remove the cannula from the heart.
-
10
Clear the line of 4% PFA and air, and then refill it with PBS again to commence the next perfusion.
-
11Remove the brain and post-fix in 4% PFA for 1–4 hours. Then transfer to 30% sucrose solution made in PBS in a 50 mL conical tube (or other appropriate container) at 4°C. The brains should remain in sucrose until they sink to the bottom (which may take 2–3 days). Once sunk, the brains can be frozen in hammer-crushed dry ice for at least 45 min, then wrapped in aluminum foil (to prevent dehydration), and stored at −80°C overnight (or until further use).It is important not to overfix the brain (e.g. overnight in 4% PFA) as this might decrease the action of β-gal enzyme and hence the efficacy of the X-gal staining procedure (that is dependent on β-gal enzymatic activity).17
-
12
Mount the brain onto a cryostat and cut 30–40 µm brain sections in cryostat and collect in PBS (with 0.02% w/v Sodium azide) at RT. While slicing, pay close attention to the presence of the injection sites and make sure to collect these sections. Sections may be stored at 4°C for 1–2 weeks or may be used immediately for X-gal staining. Alternatively, sections may be stored in cryopreservant at −80 °C until further use.
X-gal histochemistry
-
13
Wash sections 3 × 10 min in PBS using 6 well plates and net wells.
-
14
Incubate sections in X-gal solution in a 6 well-plate in an incubator (or 1.5 mL tubes in water bath away from light) for 2–3 hours (or over night if β-gal is to be detected in naïve animals) at 37°C while gentle shaking.
-
15
Wash sections 3 × 10 min in PBS
-
16
Mount onto chrom-alum coated slides and dry over night.
-
17
Place slides in increasing concentrations of ethanol (2 min each at 70%, 80%, 95%, 100%, 100% ethanol, followed by 10 min of CitriSolv™ Solvent or Histoclear solvent). Then remove excess solvent and coverslip using mounting medium (Permount or Histomount). Allow the mounting medium to dry over night before imaging.
-
18
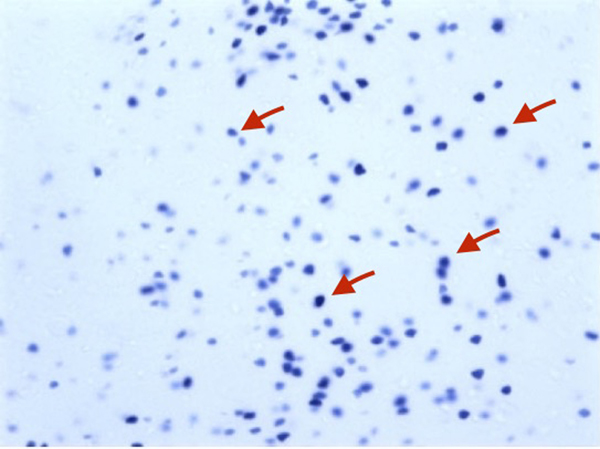
Observe β-gal labeling using a bright field microscope. Due to the extremely low background the tissue may appear almost translucent and it might be difficult to determine some major anatomical landmarks in X-gal stained brained sections to properly identify the region of interest. This problem may be overcome by 1) Illuminating your sample using a fluorescent light source; 2) Creating more contrast in the section by adjusting the amount of light coming in using the iris. Both methods help visualize the brain section by providing more contrast and thus making major anatomical landmarks more visible. Properly labeled nuclei should have a dark blue appearance (see Fig. 3) In order to determine Daun02-mediated decreases in activated neurons, count β-gal+ cells 200–300 µm around the injection site from 2–3 sections from Daun02 and vehicle-injected animals.
Figure 3.
X-gal staining of Fos-lacZ rat brain. Note the dark blue β-gal expressing nuclei (red arrows).
REAGENTS AND SOLUTIONS
Use deionized, distilled water in all recipes.
4% (w/v) Paraformaldehyde solution
Recipe for 1L: In 600–700 mL water, dissolve 4 g sodium hydroxide pellets, and then dissolve 40 g of PFA (this might take more than 20 min). Once the PFA is dissolved then add sodium phosphate monobasic until dissolved. Bring up to 1 L with water. Then filter the PFA using a vacuum or gravity filter to get rid of any residual PFA granules. Final pH should be about 7.4–7.5. Store at 4°C for 3–4 days.
30% (w/v) sucrose in PBS
Recipe for 1L: Dissolve 300 g of sucrose in 600 mL of 1X PBS. Then bring up to 1 L total volume with 1x PBS. Store at 4°C for 3 months.
20% Triton X-100
Recipe for 500 mL: Dissolve 100 mL of Triton X-100 to 400 mL of water. Store at RT for 6 months.
100 mM EGTA
Recipe for 500 mL: Add 19 g EGTA to about 200 mL of distilled water and add 10 M NaOH to dissolve. Then bring the volume up to 500 mL with water. Store at RT for 1 year.
Cryopreservant
Recipe for 1 L: Add 100 mL of 10x PBS into 400 mL water. Then add 200 mL glycerol and 20 mL DMSO. Then bring the volume up to 1 L with water. Store at 4°C for 1 year.
2x β-gal buffer
Recipe for 1 L:
-
-
Add 27.6 g of NaH2PO4•H2O to 500 mL of distilled water (200 mM final). Use 10 M sodium hydroxide solution (about few mL) to bring pH to 7.4.
-
-
Once dissolved add 11.7 g of sodium chloride (200 mM final).
-
-
Then add 100 mL of 100 mM EGTA solution, followed by 4 mL of 1.0 M magnesium chloride solution (final concentration 4mM), and then add 20 mL of 20% Triton X-100.
-
-
Bring the volume up to 1 L with water. Final pH should be 7.4–7.5. Store at RT for 1 year.
Note: The pH of this stock solution buffer is critical to successful X-gal staining. If the pH is out of this range, the X-gal staining may not work. Make sure that the pH meter is calibrated properly.
X-gal solution
Recipe for 100mL, mix the following:
-
-
50 mL of 2x β-gal buffer.
-
-
2.5 mL of 200 mM K3Fe(CN)6 (0.165 g of K3Fe(CN)6 in 2.5 mL water)
-
-
2.5 mL of 200 mM K4Fe(CN)6 • 3H2O (0.211 g of K4Fe(CN)6 in 2.5 mL water)
-
-
2.0 mL of 50 mg/mL X-gal dissolved in DMF (100 mg X-gal in 2 mL DMF)
-
-
43 mL of water
-
-
This makes a solution with final concentrations of:
2.4 mM X-gal
100 mM sodium phosphate
100 mM sodium chloride
5 mM EGTA
2 mM MgCl2
0.2% Triton X-100
5 mM K3Fe(CN)6
5 mM K4Fe(CN)6
-
-
Store away from light at 4 °C for 3–4 days or at −20 °C for 1 year.
Note: The final solution should have a yellowish color. Make sure to use DMF compatible tubes (opaque plastic).
COMMENTARY
Background Information
For many years neuroscientists have hypothesized that neuronal ensembles that were activated during a particular behaviour played a direct role in mediating that behaviour. However, techniques such as generalized lesioning or pharmacological neuronal inactivation methods lacked the ability to selectively silence only the behaviourally activated neurons. Daun02 inactivation allows us to determine whether Fos-expressing neuronal ensembles are necessary for many types of learned and motivated behaviours that are controlled by specific stimuli (typically conditioned stimuli that are associated with salient events such as drug-taking).
In many Daun02 studies performed so far, rats undergo behavioural training to associate two distinct sets of stimuli (e.g. drug reward and drug context ‘A’) via operant learning (e.g. lever pressing for a drug in a certain context).14, 16 After several conditioning sessions, the animals form a learned association (e.g. drug and drug context), and it is thought that this association is stored in neuronal ensembles in various brain areas.9 Some days after the last training session on what has been referred to as ‘induction day’ (Fig. 2), the animals are exposed to the conditioned stimulus (e.g. drug-associated context ‘A’) to activate a neuronal ensemble that encodes the learned association and modulates the associated behaviour (e.g. drug-seeking). Daun02 is injected into the brain area of interest to silence this ensemble, and then 3 days later on ‘Test day’ the effects of Daun02 inactivation are examined by re-testing the animals for the same behaviour that was observed on induction day. We have previously tested the idea that repeated presentations of the same stimuli activate the same neuronal ensemble.3, 14–16 As such, if the animal is subsequently (e.g. 3 days later) presented with the same stimulus (e.g. drug-associated context ‘A’), the associated behaviour is disrupted. Moreover, if Daun02 is injected following presentation of stimuli that is unrelated to the training context (e.g. a saline-associated context ‘B’ or a novel context ‘C’), and then the animal is tested in context ‘A’, then drug-seeking is not disrupted. This result implies that Daun02 presumably silenced a neuronal ensemble (activated by the non-training context) that is unrelated to the ensemble that normally triggers the behaviour of interest. Thus, on test day the neuronal ensemble that drives drug-seeking is spared. This latter context or cue control is critical for demonstrating ensemble-specific inactivation and its effects on behavior. Of note, a novel context ‘C’ often induces very high levels of Fos or β−gal expression in neurons unrelated to context A, which makes it a good control for demonstrating that it is the specific pattern of neurons that mediate behavior, not the number of Fos or βgal-expressing neurons. 14, 15
Overall, conducting Daun02 experiments requires 1) Breeding sufficient numbers of Fos-lacZ rats or mice. 2) β−gal expression in the brain area of interest following the behaviour of interest. 3) Successful guide cannula placement and Daun02 injections on ‘induction day’. 4) Re-testing of the animals for altered behaviour on ‘test day’. A general overview of the Daun02 experimental timeline is shown in Figure 2.
Breeding Fos-lacZ rats and mice
As previously mentioned Fos-lacZ rats have been bred onto a Sprague-Dawley genetic background strain and Fos-lacZ mice onto a B6C3F1 x B6D2 background strain. If one wishes to use e.g. Long-Evans background or C57/Bl6 to produce Fos-lacZ animals that genetically resemble another strain, then it is possible to backcross the transgenic animals onto these strains over time. Using a conventional backcrossing procedure, it will take up to 2–2.5 years to produce near 100% genetic resemblance. However, companies such as Charles River can assist one with ‘speed congenics’ services that will require less time (1.5 years) to reach the this near 100% resemblance by performing a through genetic screen of your transgenic progeny In practice, due to the extensive time it takes to generate a congenic animal, many researchers simply backcross for a few generations and utilize animals that are more than <90% genetically identical to the strain of interest. It is critical in these situations that pilot experiments are performed in order to assess whether these backcrossed animals phenotypically (e.g. on a behavioural level) resemble the strain of interest.
Describing the many different breeding protocols is beyond the scope of this protocol, and one may want to consult or leave this task to a breeding specialist at the animal unit in one’s institute. But in short, a male or female that is heterozygous for the Fos-lacZ gene is crossed with an outbred wild-type male or female. Usually, 50% of the offspring (males and females) are heterozygous for the transgene, where as 50% are not. If studies are performed on only one sex, then only 25% of the offspring are useable for the Daun02 studies. As with all transgenic breeding procedures, excellent recordkeeping of the positive and negative offspring is crucial. Ideally, a specialist should manage the colony, genotyping, sorting positive and negative offspring, and record-keeping, as these tasks become rather time-consuming when the colony sizes grow.
Critical Parameters
Designing the Daun02 experiment
Since Daun02 silences β-gal expressing neurons, it is critical to check if the brain area(s) of interest expresses β−gal in the Fos-lacZ rats or mice. We and others have observed that β−gal labeling is very weak or non-existent in many thalamic nuclei in Fos-lacZ rats, and these areas are not suitable for Daun02 inactivation. To date, many researchers have successfully used Daun02 in the prefrontal cortex and the striatum3, 4, 14, 16. We have not tested this method in other brain areas that express high levels of β-gal such as the hippocampus, basolateral and central amygdala.
When designing the behavioural experiments it is important to include two main groups. In the first group, the aim is to induce β-gal using a ‘known’ stimulus (to induce the behaviour of interest) to silence the neuronal ensemble of interest. For the second group, the aim is to induce β-gal in a different neuronal ensemble using a stimulus with different properties (e.g. visual, auditory) than the ‘known’ stimulus. Using these groups will allow one to determine whether the same stimulus repeatedly activates the same neuronal ensemble, and to determine whether distinct sets of stimuli activate different sets of ensembles.
Troubleshooting
X-gal staining
X-gal staining of brain tissue is easily influenced by the pH of the β-gal buffer, and hence it is important the pH probe is working optimally and/or the pH meter is properly calibrated. If the pH is incorrect (especially if it is acidic), then it is possible to see blue-stained blood vessels (possibly reflecting conversion of X-gal by mammalian β-gal in vessels) with little to no staining of β-gal+ neurons. If blood-vessel staining is observed, then remake the β-gal buffer. One can also use immunohistochemistry for Fos or β−gal protein expression.
Exclusion of animals from study
Although it is rare, PCR-based genotyping may produce false positives, i.e. a Fos-lacZ negative animals may be labeled genotype-positive. In such cases, Daun02 will have no effect. Fortunately, these animals can be excluded by performing a X-gal stain or via β-gal immunohistochemistry. Also, as with any intracranial injection procedure, a small minority of injection sites may contain necrotic tissue. These animals should also be excluded.
Breeding pairs are not producing any transgene positive offspring
In such cases, re-genotype the transgene positive parent, and cull this animal if it turns out to be transgene negative. However, usually such cases can be avoided by taking two or more tissue samples and checking to see if there’s consistency in the PCR results.
Anticipated Results
If Daun02 has silenced the neuronal ensemble of interest, then on ‘test day’ the associated behaviour of interest will be altered compared to animals injected with vehicle or another group injected with Daun02 following exposure to a stimulus that should not activate the neuronal ensemble of interest (e.g. novel cue). If there is no Daun02-mediated behavioural alteration, there are several possibilities: 1) The neuronal ensemble in question does not directly mediate the behaviour, although could still play a general modulatory role (e.g. arousal) in the behaviour. 2) Several neuronal ensembles exist in the brain that encode the learned association, and inactivation one of these ensembles may leave the behaviour largely intact.
Time Considerations
A considerable amount of time is required for breeding the Fos-lacZ rat or mouse colony. Each breeding cycle will take about 12 weeks for mice and 15 weeks for rats. This time frame includes time to reach sexual maturity (6–8 and 8–12 weeks, respectively for mice and rats), gestation period (3 weeks for mice and rats). Animals can be weaned usually at 3 weeks old, but for the purposes of implanting guide cannula mice and rats should be approximately 9–10 and 16 weeks old, respectively, in order for their skulls to be thick enough to properly hold the cannulae. Also, the animals should recover for a week post-surgery. Thus, users should consider these time periods when planning experiments.
It would be advisable to consider about 4–6 months until a usable size colony is obtained. Also, a typical Daun02 experiment may require n=18–20 per group for 4 groups. As with all experiments requiring cannula, it is likely that 15–20% of animals will be excluded due to injection sites falling out of the target area. Our previous experience informs us that completion of a typical behavioural experiment with a 2×2 design may easily take up to 1 to 1.5 years including breeding, surgeries, conducting behavioural experiments, performing X-gal staining, etc. Of course, this process may be sped up if the size of the breeding colony is expanded and/or multiple people simultaneously perform the surgeries and behavioural experiments.
ACKNOWLEDGEMENT
(mandatory for NIH, optional for all others)
Eisuke Koya is funded by BBSRC BB/M009017/1. Bruce Hope is funded by the NIDA Intramural Research Program, NIH.
Footnotes
INTERNET RESOURCES (none)
LITERATURE CITED
- 1.Kasof GM, Smeyne RJ, Curran T & Morgan JI. Developmental expression of Fos-lacZ in the brains of postnatal transgenic rats. Brain Res Dev Brain Res 93, 191–7 (1996). [DOI] [PubMed] [Google Scholar]
- 2.Farquhar D et al. Suicide gene therapy using E. coli beta-galactosidase. Cancer Chemother Pharmacol 50, 65–70 (2002). [DOI] [PubMed] [Google Scholar]
- 3.Koya E et al. Targeted disruption of cocaine-activated nucleus accumbens neurons prevents context-specific sensitization. Nat Neurosci 12, 1069–73 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pfarr S et al. Losing Control: Excessive Alcohol Seeking after Selective Inactivation of Cue-Responsive Neurons in the Infralimbic Cortex. J Neurosci 35, 10750–61 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Umemoto M & Olds ME. Effects of chlordiazepoxide, diazepam and chlorpromazine on conditioned emotional behaviour and conditioned neuronal activity in limbic, hypothalamic and geniculate regions. Neuropharmacology 14, 413–25 (1975). [DOI] [PubMed] [Google Scholar]
- 6.Morgan JI & Curran T. Calcium as a modulator of the immediate-early gene cascade in neurons. Cell Calcium 9, 303–11 (1988). [DOI] [PubMed] [Google Scholar]
- 7.Morgan JI & Curran T. Stimulus-transcription coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun. Annu Rev Neurosci 14, 421–51 (1991). [DOI] [PubMed] [Google Scholar]
- 8.Cruz FC, Javier Rubio F & Hope BT. Using c-fos to study neuronal ensembles in corticostriatal circuitry of addiction. Brain Res (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cruz FC et al. New technologies for examining neuronal ensembles in drug addiction and fear. Nature Reviews Neuroscience Nov;14(11):743–54. (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engeln M et al. Selective Inactivation of Striatal FosB/DeltaFosB-Expressing Neurons Alleviates L-Dopa-Induced Dyskinesia. Biol Psychiatry (2014). [DOI] [PubMed] [Google Scholar]
- 11.Fornari RV et al. Rodent stereotaxic surgery and animal welfare outcome improvements for behavioral neuroscience. J Vis Exp, e3528 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paxinos GAWC The rat brain in stereotactic coordinates. (Academic, New York, U.S.A., 1986). [Google Scholar]
- 13.Paxinos G & Franklin K The Mouse Brain in Stereotaxic Coordinates (Academic Press, San Diego, CA, 2001). [Google Scholar]
- 14.Cruz FC et al. Role of nucleus accumbens shell neuronal ensembles in context-induced reinstatement of cocaine-seeking. J Neurosci 34, 7437–46 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fanous S et al. Role of orbitofrontal cortex neuronal ensembles in the expression of incubation of heroin craving. J Neurosci 32, 11600–9 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bossert JM et al. Ventral medial prefrontal cortex neuronal ensembles mediate context-induced relapse to heroin. Nat Neurosci 14, 420–2 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma W, Rogers K, Zbar B & Schmidt L. Effects of different fixatives on beta-galactosidase activity. J Histochem Cytochem 50, 1421–4 (2002). [DOI] [PubMed] [Google Scholar]