Abstract
Background
Hip and knee replacement are common operative procedures to improve mobility and quality of life. Adequate pain relief is essential in the postoperative period to enable ambulation and initiation of physiotherapy. Lumbar epidural analgesia is a common modality for pain relief following these procedures. As the use of epidural analgesia may delay the initiation of anticoagulant thromboprophylaxis due to the potential risk of epidural hematoma, a synthesis of the evidence is necessary to determine whether or not alternative analgesic modalities are worse, equivalent, or better than epidural analgesia.
Objectives
Is lumbar epidural analgesia more efficacious than systemic analgesia or long‐acting spinal analgesia for postoperative pain relief in patients after elective hip or knee replacement?
Search methods
MEDLINE, EMBASE, CINAHL, LILACS, and the CENTRAL were searched from their inception to June 2001.
Selection criteria
A study was included if it was a randomized or pseudo randomized controlled clinical trial (RCT) of patients undergoing hip or knee replacement, in which postoperative lumbar epidural analgesia was compared to other methods for pain relief. Study selection was performed unblinded in duplicate.
Data collection and analysis
Data were collected unblinded in duplicate. Information on patients, methods, interventions, outcomes (pain relief, postoperative function, length of stay) and adverse events were recorded. Methodological quality was assessed using a validated 5‐point scale. Meta‐analysis was conducted when sufficient data existed from two or more studies. Heterogeneity testing was performed using the Breslow‐Day method. The fixed‐effect model was used unless heterogeneity was present, in which case, a random‐effects model was used. Continuous data were summarized as weighted mean differences (WMD) or standardized mean differences (SMD) with 95% confidence intervals (CI). Dichotomous data were summarized as odds ratios (OR) and numbers‐needed‐to‐treat‐to‐benefit (NNT) or numbers‐needed‐to‐treat‐to‐harm (NNH) with their respective 95% CI.
Main results
In the first four to six hours after surgery, patients receiving epidural analgesia had less pain at rest, based on visual analog scores (VAS), than patients receiving systemic analgesia (SMD ‐0.77; 95% CI ‐1.24 to ‐0.31). This effect was not statistically significant by 18 to 24 hours (SMD ‐0.29; 95% CI ‐0.73 to 0.16). These observations were based only on studies evaluating populations consisting of total knee replacements alone or mixed populations of total hip or total knee replacements. For pain relief with movement after surgery, patients receiving epidural analgesia reported lower pain scores than patients receiving systemic analgesia in all four studies examining these outcomes. The choice of epidural agents may also influence the extent to which epidural analgesia differs from systemic analgesia. The differences between epidural analgesia and systemic analgesia in the frequency of nausea and vomiting (OR 0.95; 95% CI 0.60 to 1.49) or depression of breathing (OR 1.07; 95% CI 0.45 to 2.54) were not statistically significant. Sedation occurred less frequently with epidural analgesia (OR 0.30; 95% CI 0.09 to 0.97) with a number‐needed‐to‐harm of 7.7 (95% CI 3.5 to 42.0) patients for the systemic analgesia group. Retention of urine (OR 3.50, 95% CI 1.63 to 7.51; NNH 4.5, 95% CI 2.3 to 12.2), itching (OR 4.74, 95% CI 1.76 to 12.78; NNH 6.8, 95% CI 4.4 to 15.8), and low blood pressure (OR 2.78, 95% CI 1.15 to 6.72; NNH 6.7, 95% CI 3.5 to 103) were more frequent with epidural analgesia compared to systemic analgesia. There were insufficient numbers to draw conclusions on the effect of epidural analgesia on serious postoperative complications, functional outcomes, or length of hospital stay.
Authors' conclusions
Epidural analgesia may be useful for postoperative pain relief following major lower limb joint replacements. However, the benefits may be limited to the early (four to six hours) postoperative period. An epidural infusion of local anaesthetic or local anaesthetic‐narcotic mixture may be better than epidural narcotic alone. The magnitude of pain relief must be weighed against the frequency of adverse events. The current evidence is insufficient to draw conclusions on the frequency of rare complications from epidural analgesia, postoperative morbidity or mortality, functional outcomes, or length of hospital stay.
Plain language summary
Epidural analgesia (a form of pain control) for the pain relief following hip and knee replacement
Epidural analgesia may give good pain relief after hip or knee replacement surgery, but this benefit must be weighed against the possibility of adverse effects and complications. Hip and knee replacements are common operations to improve mobility and quality of life. After surgery, good pain relief is essential to enable patients to start walking again. Epidurals (pain medicine injected into the spinal canal) are commonly used. However, this pain relief method may delay the start of blood thinners, which prevent life‐threatening blood clot formation (thrombosis) in veins, because there is also a risk of bleeding at the epidural injection site if blood thinners are used at the same time. This review found that an epidural comprising local anaesthetic with or without a strong opioid might give better pain relief than an epidural with only strong opioids; the benefit may be felt only in the first four to six hours after surgery. Aside from pain relief, there was insufficient information to draw conclusions on other benefits or harms arising from epidural analgesia.
Background
Hip and knee replacement are common operative procedures that relieve pain and improve mobility and quality of life in individuals with various rheumatological conditions, especially osteoarthritis (Harris 1990). Adequate pain relief is essential in the immediate postoperative period to enable the patient to undertake physiotherapy that will facilitate movement of the joints and tissues. Inadequate pain relief may, therefore, prevent early mobility and delay discharge from hospital.
Lumbar epidural analgesia is a common modality for pain relief following hip or knee replacement. Some physicians assert that epidural analgesia provides better pain relief than other postoperative analgesic modalities. However, no systematic review of the evidence to support or refute this impression has been performed.
A synthesis of this information is urgently needed due to the potential implications of co‐administered epidural analgesia and anticoagulant prophylaxis after hip or knee replacement. In the absence of anticoagulant prophylaxis, hip or knee replacements are associated with 40 to 70% risk of deep vein thrombosis (DVT) and 1 to 2% risk of fatal pulmonary embolism (Clagett 1998). On the other hand, co‐administered anticoagulant prophylaxis and epidural analgesia is associated with an increased risk of spinal epidural hematoma, a devastating complication that can result in permanent neurological impairment even after prompt neurosurgical decompression (Lawton 1995). In the past, unfractionated heparin was used for thromboprophylaxis in this population. Evidence from large, randomized clinical trials (RCTs) indicates that low molecular weight heparins (LMWH) are more efficacious than unfractionated heparin after hip arthroplasty (Planes 1988, Levine 1991, Leyvraz 1991) and more effective than warfarin after knee arthroplasty (Hull 1993, Leclerc 1996). Consequently, LMWH is the anticoagulant prophylaxis of choice in patients undergoing lower limb joint replacement. However, in recent years, there have been over 50 reports of spinal epidural hematoma occurring in association with LMWH use in patients receiving spinal anesthesia or epidural analgesia. The absolute risk of epidural hematoma with co‐administered LMWH and epidural analgesia is not known but may be as high as 1 in 3200 or as low as 1 in 150,000 cases (Schroeder 1998).
Ideally, the start of LMWH should be delayed until an epidural catheter is removed. However, clinicians may be concerned about the risk of DVT if LMWH prophylaxis is delayed for two to three days after surgery because of coincident epidural analgesia. On the other hand, avoiding epidural analgesia to allow early initiation of LMWH prophylaxis may result in suboptimal joint movement and pain control. If alternative methods of pain control, which avoid the risk of epidural hematoma, are as efficacious as epidural analgesia, then these alternatives would help to resolve the management of patients who require effective thromboprophylaxis and pain relief after hip or knee replacement surgery.
Against this background, we critically reviewed the evidence regarding the efficacy of epidural analgesia compared to other analgesic modalities for postoperative pain relief following hip and knee replacement surgery.
Objectives
Our objective is to examine the efficacy and effectiveness of epidural analgesia for postoperative pain control following hip or knee replacement surgery based on the evidence from controlled clinical trials. In particular, we wish to answer the primary question: "Is lumbar epidural analgesia more efficacious than systemic analgesia or long‐acting intrathecal (spinal) analgesia for postoperative pain relief in patients after hip or knee replacement?"
Methods
Criteria for considering studies for this review
Types of studies
Included studies were prospective, controlled clinical trials in which allocation was achieved in a randomized or pseudo‐randomized (health card number or sequential alternation) manner. Given the nature of our comparators, we expected that some trials would not be blinded for ethical reasons. These trials were included. Concurrent cohort studies and observational studies were excluded.
Types of participants
The study population consisted of patients of any age, who underwent elective hip or knee replacement surgery. The procedure could be either a primary (first time) replacement, or removal of initial hardware and secondary (repeat) replacement.
Emergency joint replacements or joint replacements for treatment of acute fractures were excluded.
In trials where both unilateral and bilateral procedures or hip and knee replacements were included, attempts were made to conduct subgroup analyses according to number and type of joint replaced. We anticipated that there would be trials with insufficient details to permit stratification; these studies were included and evaluated separately.
Types of interventions
Included studies had at least one arm in which epidural agents were used to provide postoperative pain relief after lower limb joint replacement surgery. Epidural agents were included if they were:
long acting agents (for example, morphine) given in the intraoperative period, or
agents given as boluses or infusions in the postoperative period.
As our intent was to compare clinically relevant modalities of analgesia, acceptable comparators were systemic (intravenous or intramuscular) analgesics administered in the postoperative period or long‐acting intrathecal analgesics administered in the intraoperative period.
We anticipated that patients may have received other analgesics as co‐interventions. (Co‐interventions are described in the 'Characteristics of included studies' table). However, we expected heterogeneity in the use of other analgesics between studies due to the uncontrolled nature of these co‐interventions. Studies were not excluded because of the co‐interventions administered, and adjustment for these co‐interventions was not made in our analysis.
Types of outcome measures
Our efficacy analysis was based on the extent to which pain is relieved. Whenever possible, our inferences on this outcome were based on pain measurements using the visual analog score (VAS) to obtain a continuous variable. When trials did not use the VAS but utilized a discrete pain measurement, conversion to a dichotomous variable with 50% pain reduction was performed and inferences were made based on this variable.
For analysis of effectiveness, we evaluated functional outcomes, side effects, and length of hospital stay. All measurements of functional status were recorded as we anticipated that functional outcome measures would be heterogeneous between trials; however, we preferred to analyze measurements based on validated, prospective scales. The incidences of cognitive, neurological, respiratory, cardiovascular, and gastrointestinal adverse events were recorded if relevant data were available from the trials. Adverse events were included if they occurred during the time period in which the intervention occurred. The frequencies of deep vein thrombosis (DVT) and pulmonary embolism (PE) were assessed if relevant data were available. The length of hospital stay was also recorded.
Search methods for identification of studies
The MEDLINE, EMBASE, CINAHL, and LILACS databases and the Cochrane Controlled Trials Register (CCTR) were searched from their inception to June 2001 using the strategies listed below. The reference lists of selected studies and review articles were reviewed for additional citations. No language restrictions were applied. Unpublished studies were not sought. Please see Appendix 1 for search startegy.
Data collection and analysis
Eligibility
Review authors were not blinded to authors, institutions, journal of publication, or study results. Two review authors (MB, PC) independently evaluated the titles and abstracts of trials identified in the literature search for their eligibility. Disagreements were resolved through discussion.
Data extraction
Data were extracted from included trials by two independent review authors (JD, JS). Disagreements were resolved through a third review author (PC). Information on the patients, methods, interventions, outcomes (pain relief, postoperative function, length of stay) and adverse events were recorded. Authors were not contacted for missing information or unpublished data.
For continuous data with a normal distribution (e.g. VAS), the mean value ± standard deviation for each group was derived (if necessary) and recorded for each study arm. For continuous data without a normal distribution (e.g. length of stay data), median values and range were recorded for each study arm if possible. Data that did not permit extraction (or derivation) of the median value were excluded from analysis.
Only functional outcome scores based on prospective validated scales were used for analysis. As different trials were likely to use different scales, data from functional outcome scores were converted to dichotomous outcomes with the cut‐off point dependent on the value representing a clinically significant change for each individual scale.
For dichotomous outcomes, such as adverse events, absolute numbers were expressed as fractions (for example, four patients with outcome of interest /ten patients in study arm) to permit calculations of absolute risk reductions (ARR) and numbers‐needed‐to‐treat‐to‐benefit (NNT) or numbers‐needed‐to‐treat‐to‐harm (NNH). If the dichotomous outcomes were expressed as a proportion, the data were converted into the original fraction. If dichotomous data were expressed in an ordinal manner (e.g. 0 to 25% / 26 to 50%/51 to 75%/76 to 100%), attempts were made to convert this information into a dichotomous form that would represent a threshold for a clinically significant outcome. If this was not possible, the categorical data were excluded from analysis.
Assessment of methodological quality
The internal validity of the included trials was evaluated by two reviewers (JD, JS) using the 5‐point scale devised by Jadad et al (Jadad 1996). Possible scores range from 0 to 5, with higher scores indicating higher methodological quality. Allocation concealment was rated as adequate (A), unclear (B), or inadequate (C).
Summarizing the results
Meta‐analysis was conducted when sufficient data existed from two or more studies. Heterogeneity testing was performed using the Breslow‐Day method (Breslow 1980). The fixed‐effect model was used unless heterogeneity was present, in which case, the random effects model of DerSimonian and Laird (DerSimonian 1986) was used. For continuous data with normal distributions, the weighted mean difference (WMD) with 95% confidence intervals (CI) was used if studies used an identical measurement scale (e.g. all studies used a 100 mm VAS) and the standardized mean difference (SMD) with 95% CI was used if studies used different measurement scales (e.g. some studies used a 50 mm VAS and other studies used a 100 mm VAS). Dichotomous data were summarized as odds ratios (OR) and NNT or NNH with their respective 95% CI. Sensitivity analyses were performed to assess the effects of methodological quality (randomized versus pseudo randomized), type of joint (hip versus knee), and type of medication in the epidural (local anesthetics versus opioids versus combined local anesthetics‐opioids versus other agents).
Results
Description of studies
Fifty‐eight articles were identified in the literature search. Of these, 41 studies did not meet the inclusion criteria and three studies (Nielsen 1990; Williams‐Russo 1992; D'Ambrosio 1998) were duplicate publications. One study (Lopes 1999) was unavailable through our library or inter‐library loans. The details of the 44 excluded studies are described in the "Characteristics of excluded studies" table. Fourteen studies met the inclusion criteria. Inter‐rater reliability for the study selection was 0.46.
The thirteen included studies varied extensively in the intraoperative co‐interventions. Spinal anaesthesia, epidural anaesthesia, general anaesthesia, and epigeneral anaesthesia was used for all groups in three, two, one, and two studies respectively. Three studies compared epidural anaesthesia and analgesia with general anaesthesia and systemic analgesia. Two studies compared epigeneral anaesthesia and epidural analgesia with general anaesthesia and systemic analgesia.
Four studies evaluated patients undergoing total hip replacement, six studies evaluated patients undergoing total knee replacement, and three studies evaluated patients undergoing either types of joint replacement.
The timing of measurements varied between one hour after surgery to seven days after surgery for pain VAS scores, which were measured in 11 studies. Two studies reported baseline preoperative pain scores; one study reported baseline postoperative pain scores. Thirteen studies reported adverse events. Three studies evaluated functional outcomes. Five studies reported lengths of stay.
The details of the included studies are described in the "Characteristics of included studies" table. Table 1 and Table 2 in the Additional Tables section categorize the studies by type of intraoperative anaesthetic received and the type of epidural agents given for epidural analgesia.
1. Classification of studies by type of joint replacement and anaesthesia.
| Anaesthetic | Hip | Knee | Hip or knee |
| All patients received neuraxial blockade | Bertini 1995; Gustafsson 1986 | Sharrock 1994; Hendolin 1996; Klassen 1999 | Weller 1991; Homeril 1994 |
| Neuraxial blockade vs general anaesthesia | D'Ambrosio 1999; Wulf 1999 | Jorgensen 1991; Singelyn 1998 | Moiniche 1994 |
| All patients received general anaesthesia | Capdevila 1999 |
2. Classification of studies by type of postoperative epidural analgesia.
| Epidural analgesia | Study |
| Local anesthetic | Jorgensen 1991; Wulf 1999 |
| Narcotic | Gustafsson 1986; Weller 1991; Hommeril 1994; Hendolin 1996; Klasen 1999 |
| Local anesthetic‐narcotic | Moiniche 1994; Sharrock 1994; Bertini 1995; Singelyn 1998; Capdevila 1999; D'Ambrosio 1999 |
Risk of bias in included studies
In general, the methodological quality of the included studies was poor. Although all studies stated that patients were randomized, only five studies reported the method of randomization. Five studies reported double‐blinding, but only one study reported the method. In two other studies in which double‐blinding was reported, patients in the systemic analgesia groups did not receive placebo epidural catheters; thus, the likelihood of differentiating patients in epidural versus systemic analgesia groups was high. Seven studies reported the number of withdrawals. The Jadad score ranged from one to four with a median score of two. Only four studies (Gustafsson 1986, Hommeril 1994, Klasen 1999, Sharrock 1994) were considered of high methodological quality (Jadad score of three or greater). In general, for any given outcome, there were too few studies of high methodological quality to perform sensitivity analyses based on methodological quality.
Effects of interventions
Post operative pain relief
Eleven of the thirteen studies evaluated pain relief:
three studied patients undergoing total hip replacement (Gustafsson 1986; Bertini 1995; Wulf 1999);
five studied patients undergoing total knee replacement (Sharrock 1994; Hendolin 1996; Singelyn 1998; Capdevila 1999; Klasen 1999);
three studied patients undergoing either joint replacements (Weller 1991; Hommeril 1994; Moiniche 1994).
Three studies (Gustafsson 1986; Moiniche 1994; Capdevila 1999; Wulf 1999) reported resting pain VAS scores as medians and three studies (Sharrock 1994; Bertini 1995; Hendolin 1996) did not report standard deviations; therefore, the resting pain VAS scores were amenable for pooling from only four studies.
Four studies (Moiniche 1994; Bertini 1995; Singelyn 1998; Wulf 1999) reported dynamic pain scores; only one (Singelyn 1998) reported scores as means with standard deviations. Thus, results were pooled only for early (four to six hours) and late (18 to 24 hours) postoperative pain relief at rest. Because studies used different ranges for the VAS scores, the standardized mean difference was used to summarize the treatment effect.
For early postoperative pain relief at rest, epidural analgesia appears to be superior to systemic analgesia (SMD ‐0.77; 95% CI ‐1.24 to ‐0.31). This effect is not statistically significant by 18 to 24 hours (SMD ‐0.29; 95% CI ‐0.73 to 0.16). These observations are based only on studies evaluating populations consisting of total knee replacements alone or mixed populations of total hip or total knee replacements.
The choice of epidural agents may also influence the extent to which epidural analgesia differs from systemic analgesia. Two (Gustafsson 1986; Klasen 1999) of five studies that compared epidural narcotics to systemic analgesics found lower VAS scores in the systemic analgesic groups. No study that compared epidural local anaesthetic alone or local anaesthetic‐narcotic mixtures to systemic analgesics found lower VAS scores in the systemic analgesic groups.
For both early and late postoperative pain relief during leg movement or ambulation, patients receiving epidural analgesia reported lower VAS scores than patients receiving systemic analgesia in all four studies examining these outcomes. None of these studies used epidural narcotics alone. A quantitative estimate of the treatment effect could not be calculated due to the variations in reporting of VAS scores.
Adverse events
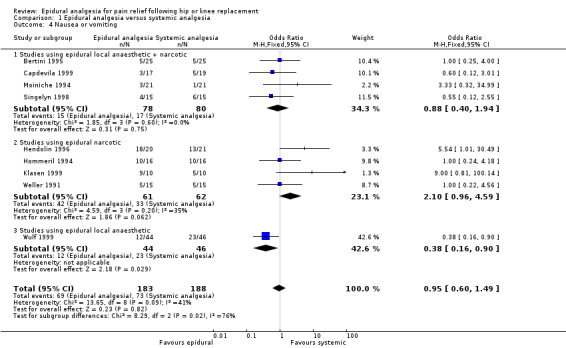
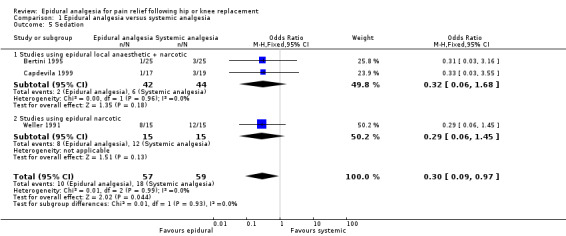
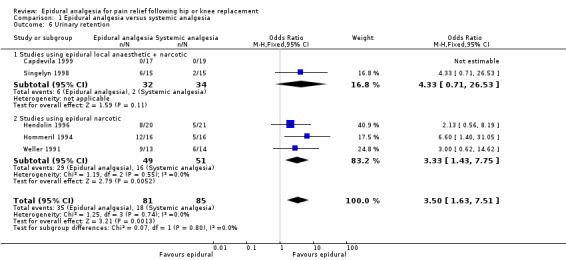
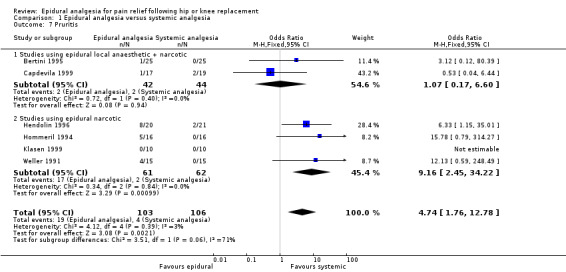
Nausea and vomiting, sedation, urinary retention, pruritis, respiratory depression, and hypotension were reported in a number of studies. The data are summarized in the 'Comparisons and Data' section of this review (Analysis 1.4 Nausea and Vomiting; Analysis 1.5 Sedation; Analysis 1.6 Urinary Retention; Analysis 1.7 Pruritis; Analysis 1.8 Respiratory Depression; Analysis 1.9Hypotension).
1.4. Analysis.
Comparison 1 Epidural analgesia versus systemic analgesia, Outcome 4 Nausea or vomiting.
1.5. Analysis.
Comparison 1 Epidural analgesia versus systemic analgesia, Outcome 5 Sedation.
1.6. Analysis.
Comparison 1 Epidural analgesia versus systemic analgesia, Outcome 6 Urinary retention.
1.7. Analysis.
Comparison 1 Epidural analgesia versus systemic analgesia, Outcome 7 Pruritis.
1.8. Analysis.
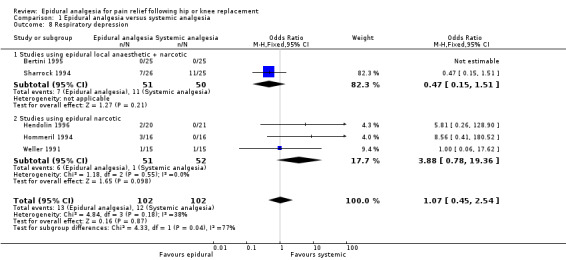
Comparison 1 Epidural analgesia versus systemic analgesia, Outcome 8 Respiratory depression.
1.9. Analysis.
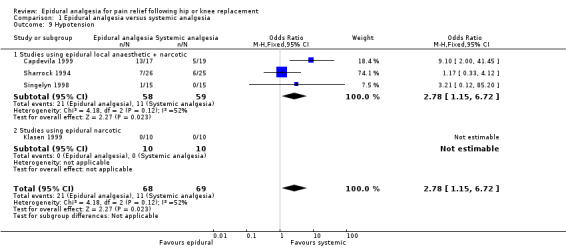
Comparison 1 Epidural analgesia versus systemic analgesia, Outcome 9 Hypotension.
The differences between epidural analgesia and systemic analgesia in the frequency of nausea and vomiting (OR 0.95; 95% CI 0.60 to 1.49) or respiratory depression (OR 1.07; 95% CI 0.45 to 2.54) were not statistically significant. Sedation occurred less frequently with epidural analgesia (OR 0.30; 95% CI 0.09 to 0.97) with a number‐needed‐to‐harm of 7.7 (95% CI 3.5 to 42.0) patients for the systemic analgesic group. Urinary retention (OR 3.50, 95% CI 1.63 to 7.51; NNH 4.5, 95% CI 2.3 to 12.2), pruritis (OR 4.74, 95% CI 1.76 to 12.78; NNH 6.8; 95% CI 4.4 to 15.8), and hypotension (OR 2.78, 95% CI 1.15 to 6.72; NNH 6.7, 95% CI 3.5 to 103) were more frequent with epidural analgesia compared to systemic analgesia.
Ten studies reported on serious life‐threatening postoperative complications. Six of these studies found no serious complications (Weller 1991; Bertini 1995; Singelyn 1998; Capdevila 1999; D'Ambrosio 1999; Klasen 1999). Sharrock 1994 observed one myocardial infarction and two serious dysrhythmias in their epidural analgesic group (n = 26) and no serious complications in their systemic analgesic group (n = 25). In contrast, Hendolin 1996 observed three serious atrial dysrhythmias in their systemic analgesic group (n=21) and no serious complications in their epidural analgesic group (n =2 0). Hommeril 1994 noted that all three patients who experienced respiratory depression in their epidural analgesic group required naloxone but did not require mechanical ventilation. In one study (Jorgensen 1991) that evaluated the frequency of DVT and PE, a lower frequency of DVT was observed with epidural anesthesia and analgesia compared to general anesthesia and systemic analgesia (3 / 17 vs 13 / 22; P = 0.02). One patient in the systemic analgesic group had a non‐fatal PE. Wulf 1999 reported serious complications "typical ... of surgery" in both the epidural (5/44) and systemic (3/46) analgesic groups but did not provide further details.
Although the use of thromboprophylaxis and the risk of epidural hematoma are important considerations with epidural analgesia in hip and knee replacement surgery, no study reported details on the use of warfarin, heparin, or low molecular weight heparins for thromboprophylaxis nor did any study report epidural hematoma as an outcome.
Functional measures
Three studies (Sharrock 1994; Singelyn 1998; Capdevila 1999) evaluated the effect of epidural versus systemic analgesia on rehabilitation following total knee replacement. The functional measures varied between all three studies; Table 3 in the Additional Tables section summarizes the results. Sharrock 1994 found no differences in rehabilitative outcomes between patients receiving epidural or systemic analgesia. Both Singelyn 1998 and Capdevila 1999 found statistically significant (P < 0.05) increases in the degree of knee flexion achieved by patients receiving epidural analgesia compared to those receiving systemic analgesia; however, the clinical significance of the observed differences was unclear. All three studies used mixtures of local anaesthetic and narcotic as their epidural agents.
3. Summary of results on functional outcomes.
| Study | Outcome Measure | Results |
| Sharrock 1994 | Number of days to reach seven rehabilitation milestones: ability to dangle legs over the side of bed unsupported, transfer from bed to walker unassisted, use a walker unassisted, use canes unassisted, climb stairs unassisted, achieve 90 degrees of knee flexion, and discharge from rehabilitation | No differences were seen between patients receiving epidural analgesia and patients receiving intravenous analgesia. |
| Singelyn 1998 | Degree of knee flexion; number of days to reach 90 degrees of knee flexion | Degree of knee flexion, measured daily until discharge, was significantly (P<0.001) greater in patients receiving epidural analgesia compared to patients receiving intravenous analgesia. A statistically significant difference (P=0.03) in favour of the epidural analgesia group persisted at six weeks after knee replacement. There was no difference in degree of knee flexion between the two groups 3 months after knee replacement. Similarly, degree of knee flexion, measured daily until discharge, was significantly (P<0.001) greater in patients receiving 3‐in‐1 block compared to patients receiving intravenous analgesia. A statistically significant difference (P=0.03) in favour of the 3‐in‐1 block group persisted at six weeks after knee replacement. There was no difference in degree of knee flexion between the two groups 3 months after knee replacement. Epidural analgesia or 3‐in‐1 block permitted patients to reach 90 degrees of knee flexion faster than intravenous analgesia (P<0.001; 8+/‐5 days [epidural], 11+/‐6 days [3‐in‐1 block]; 17+/‐7 days [intravenous]). |
| Capdevila 1999 | Maximal knee flexion at 5 days, 7 days (discharge), 1 month, and 3 months after knee replacement | Continuous epidural analgesia or continuous femoral block both resulted in greater degrees of knee flexion compared to intravenous patient controlled analgesia (P<0.05) at 5 days and 7 days after knee replacement. No differences were seen between the three groups 1 month and 3 months after knee replacement. |
Length of stay
Five studies (Moiniche 1994; Sharrock 1994; Singelyn 1998; Capdevila 1999; Wulf 1999) reported length of hospital stay. Table 4 in the Additional Tables section summarizes the data.
4. Summary of results on length of stay.
| Study | Results |
| Moiniche 1994 | No statistically significant differences in median length of hospital stay were observed in knee replacement (epidural 12 days; intravenous 13 days) or hip replacement (epidural 9 days; intravenous 9 days) patients. |
| Sharrock 1994 | No statistically significant difference in mean length of hospital stay was observed between patients receiving epidural analgesia (16.7+/‐3.8 days) and patients receiving intravenous analgesia (15.6+/‐2.1 days). |
| Singelyn 1998 | Compared to epidural analgesia (mean 16 days, SD 4 days) or 3‐in‐1 block (mean 17 days, SD 3 days), intravenous analgesia resulted in longer length of stay (mean 21 days, SD 3 days; p<0.001). |
| Capdevila 1999 | Compared to continuous epidural analgesia (median 37 days, range 30 to 45 days) or continuous femoral block (median 40 days, range 31 to 60 days), intravenous PCA resulted in longer length of stay (median 50 days, range 30 to 80 days; p<0.05) in the rehabilitation center using objective discharge criteria. |
| Wulf 1999 | No statistically significant difference in mean length of hospital stay was observed between patients receiving epidural analgesia (19+/‐5 days) and patients receiving intravenous analgesia (22+/‐10 days). |
Discussion
Despite strict selection criteria for this systematic review, the randomised controlled trials (RCTs) comparing epidural analgesia and systemic analgesia in patients undergoing hip or knee replacements vary in terms of the patient populations, interventions, and outcome measures. These differences across studies, coupled with the small patient sample sizes, limit the strength of the conclusions that may be drawn. At rest, epidural analgesia may be superior to systemic analgesia in the early (4 to 6 hours) postoperative period but the difference is no longer significant by 18 to 24 hours. For pain relief with leg movement or ambulation, the evidence suggests epidural analgesia may be superior to systemic analgesia; however, the data could not be pooled to provide a quantitative estimate of the effect.
No statistically significant differences were seen in frequency of nausea and vomiting or respiratory depression between epidural analgesia and systemic analgesia; however, epidural analgesia results in lower frequency of sedation and greater frequencies of urinary retention, pruritis, and hypotension. Rare complications of epidural analgesia, such as epidural hematoma or epidural abscess, were not reported. The total numbers of events for each group were small (<200); therefore, the results should be interpreted with caution.
The current evidence does not demonstrate differences in life‐threatening postoperative complications. Again, the total number of patients in the included studies is too small to draw conclusions on infrequent events. Other systematic reviews of postoperative epidural analgesia compared to systemic analgesia suggest that the former modality may reduce postoperative respiratory complications (Ballantyne 1998) and cardiac complications (Beattie 2001). Rodgers et al (Rodgers 2000) reviewed 141 RCTs of neuraxial blockade compared to general anesthesia and found a reduction in mortality with intraoperative neuraxial blockade (OR 0.70; 95% CI 0.54 to 0.90) but not with perioperative neuraxial blockade (OR 0.68, 95% CI 0.43 to 1.08). Intraoperative neuraxial blockade appeared to be associated with a decreased frequency of DVT (OR 0.56; 95% CI 0.43 to 0.72) with over 80% of the data coming from orthopedic trials (Rodgers 2000). Similar risk reductions were seen for PE (OR 0.45; 95% CI 0.29 to 0.69; Rodgers 2000). The extent to which postoperative epidural analgesia affects the frequency of DVT and PE was not reported. In general, additional adequately‐powered RCTs are needed using relevant clinical endpoints before conclusions can be drawn on the effect of postoperative epidural analgesia on postoperative complications.
Despite the clinical importance of functional outcomes, few studies have evaluated functional measures. No study has used validated functional measures. The effect of postoperative analgesics on function is still uncertain. Similarly, the effect of postoperative analgesics on length of hospital stay is unclear. Length of stay can be influenced by many factors, including factors unrelated to the patient's medical or rehabilitation status. Use of strict discharge criteria would be helpful in controlling for factors influencing length of stay. Furthermore, it would be useful if triallists reported the median as the statistic of central tendency, rather than the mean, given the skewed nature of length of stay data.
Authors' conclusions
Implications for practice.
Epidural analgesia may be useful for pain relief after hip or knee replacement surgery; however, the benefits may be limited to the early (four to six hours) postoperative period. An epidural infusion of local anesthetic or local anesthetic‐narcotic mixture may be better than epidural narcotic alone. The magnitude of pain relief must be weighed against the frequency of adverse events. The current evidence is insufficient to draw conclusions on the frequency of rare complications from epidural analgesia, postoperative morbidity or mortality, functional outcomes, or length of hospital stay.
Implications for research.
Large well‐designed randomised clinical trials are still needed to answer questions on the effect of epidural analgesia, compared to systemic analgesia, on important clinical outcomes in patients undergoing hip or knee replacement. Measurements of pain need to be standardised to permit pooling of results. Important clinical and rehabilitative outcomes need to be incorporated into future studies. The required sample sizes will necessitate concerted efforts by collaborative research groups in multicentre trials. To reflect clinical practice, current thromboprophylactic practices need to be incorporated, in a standardised fashion, as part of the interventions. Serious adverse events, such as epidural hematoma, should be included to enable clinicians and patients to weigh the risks and benefits of epidural analgesia.
What's new
| Date | Event | Description |
|---|---|---|
| 30 January 2014 | Amended | The former authors of this review (Choi 2003) have decided not to continue with the topic. A new team has taken the review over. The new authors have decided to change the scope of the review. They have registered a title "Nerve blocks or no nerve blocks for elective hip replacement (arthroplasty) surgery". When that new protocol is published, the current review (Choi 2003) will be withdrawn from The Cochrane Library. |
History
Protocol first published: Issue 3, 2001 Review first published: Issue 3, 2003
| Date | Event | Description |
|---|---|---|
| 6 March 2013 | Amended | This review has been transferred from the Cochrane Pain, Palliative & Supportive Care Review Group to the Cochrane Anaesthesia Group. The lead author's contact details also updated. |
| 9 November 2009 | Amended | Contact details updated. |
| 29 October 2008 | Amended | Further revisions with respect to RM5 |
| 9 July 2008 | Amended | Converted to new review format. |
Notes
March 6th 2013: This review has been transferred from the Cochrane Pain, Palliative & Supportive Care Review Group to the Cochrane Anaesthesia Group. The lead author's contact details were also updated.
January 2014: The former authors of this review (Choi 2003) have decided not to continue with the topic. A new team has taken the review over. The new authors have decided to change the scope of the review. They have registered a title "Nerve blocks or no nerve blocks for elective hip replacement (arthroplasty) surgery". When that new protocol is published, the current review (Choi 2003) will be withdrawn from The Cochrane Library.
Acknowledgements
The authors wish to thank the members of the Department of Anesthesia, St. Joseph's Healthcare Hamilton, for their financial support of this study.
Appendices
Appendix 1. search strategy
MEDLINE (1966 to present) and CINAHL (1982 to present) strategies:
1 exp randomized controlled trials/ 2 randomized controlled trial.pt. 3 exp random allocation/ 4 exp double blind method/ 5 exp single blind method/ 6 exp clinical trials/ 7 clinical trial.pt. 8 controlled clinical trial.pt. 9 randomi$ 10 random$ near2 (assign$ or allocate$) 11 clin$ near trial$ 12 (singl$ or doubl$ or trebl$ or tripl$) near (blind$ or mask$) 13 or / 1‐12 14 exp arthroplasty, replacement, hip/ 15 exp hip prosthesis 16 exp hip joint/su 17 exp arthroplasty, replacement, knee/ 18 exp knee prosthesis 19 exp knee joint/su 20 hip:.tw. 21 knee:.tw. 22 or / 14‐21 23 exp analgesia, patient‐controlled/ 24 pca.tw. 25 parenteral analg:.tw. 26 intravenous analg:.tw. 27 intramusc: analg:.tw. 28 patient controlled analg:.tw. 29 systemic analg:.tw. 30 or / 23‐29 31 exp analgesia, epidural/ 32 cea.tw. 33 cse.tw. 34 epid: analg:.tw. 35 extradur: analg:.tw. 36 peridur: analg:.tw. 37 spinal epid:.tw. 38 spinal analg:.tw. 39 or / 31‐38 40 and / 22,30,39 41 (animal not human).sh 42 40 not 41 43 and / 13,42
EMBASE (January 1980 to present):
1 randomi* (in ti,ab,kwds) 2 [(singl* or doubl* or trebl* or tripl* (in ti,ab,kwds)) and (blind* or mask* (in ti,ab,kwds))] 3 randomised controlled trial (in kmajor,kminor) 4 or / 1‐4 5 exp hip arthroplasty 6 exp hip prosthesis 7 exp total hip prosthesis 8 exp acetabuloplasty 9 exp knee arthroplasty 10 exp total knee replacement 11 exp knee prosthesis 12 hip (in ti,ab,kwds) 13 knee (in ti,ab,kwds) 14 or / 5‐13 15 exp patient controlled analgesia 16 exp postoperative analgesia 17 pca (in ti,ab,kwds) 18 parenteral analg* (in ti,ab,kwds) 19 intraven* analg* (in ti,ab,kwds) 20 intramuscular analg (in ti,ab,kwds) 21 systemic analg* (in ti,ab,kwds) 22 or / 15‐21 23 exp epidural anesthesia 24 cea (in ti,ab,kwds) 25 cse (in ti,ab,kwds) 26 epid* analg* (in ti,ab,kwds) 27 extradur* analg* (in ti,ab,kwds) 28 peridur* analg* (in ti,ab,kwds) 29 spinal analg* (in ti,ab,kwds) 30 spinal epid* (in ti,ab,kwds) 31 or / 23‐30 32 and / 4,14,22,31
LILACS (January 1982 to present):
1 random$ and allocat$ 2 randomi$ 3 randoni$ 4 randomizacion 5 duplecego 6 (singl$ or doubl$ or trebl$ or tripl$) and (blind$ or mask$) 7 (simpl$ or dupl$ or doble or tripl$) and (cego or ciego) 8 single‐masked study/ 9 double‐masked study 10 clinical and trial$ 11 (clinico and control$) and (ensaio$ or etud$ or experimento$) 12 prophylactic controlled trial/ 13 or / 1‐12 14 hip/ 15 knee/ 16 (hip or cadera) and (replacement or reemplazo) 17 (knee or rodilla) and (replacement or reemplazo) 18 OR / 14‐17 19 analg$ and control$ 20 parenteral and analg$ 21 intraven$ and analg$ 22 intramusc$ and analg$ 23 (system$ or sistem$) and analg$ 24 OR / 19‐23 25 (epidural or extradural or peridural) and analg$ 26 spinal and epidural 27 (intrathec$ or intratec$ or spinal or espinal) and analg$ 28 OR / 25‐27 29 AND / 18,24,28 30 Ct animal AND NOT (Ct human and Ct animal) 31 AND NOT / 29,30 32 AND / 13,31
Data and analyses
Comparison 1. Epidural analgesia versus systemic analgesia.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Early (4‐6 hours) postoperative pain relief at rest | 7 | 236 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.77 [‐1.24, ‐0.31] |
| 1.1 Studies evaluating total hip replacements only | 1 | 50 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Studies evaluating total knee replacement only | 4 | 122 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.66 [‐1.30, ‐0.02] |
| 1.3 Studies evaluating total hip or total knee replacements | 2 | 64 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.90 [‐1.57, ‐0.22] |
| 2 Late (18‐24 hours) postoperative pain relief at rest | 6 | 182 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.29 [‐0.73, 0.16] |
| 2.1 Studies evaluating total hip replacements only | 1 | 50 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Studies evaluating total knee replacements only | 4 | 102 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.47 [‐1.04, 0.10] |
| 2.3 Studies evaluating total hip or total knee replacements | 1 | 30 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.72, 0.72] |
| 3 Early (4‐6 hours) postoperative dynamic pain relief | 2 | 60 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐2.45 [‐3.43, ‐1.48] |
| 3.1 Studies evaluating total hip replacements only | 1 | 30 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Studies evaluating total knee replacements only | 1 | 30 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐2.45 [‐3.43, ‐1.48] |
| 4 Nausea or vomiting | 9 | 371 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.60, 1.49] |
| 4.1 Studies using epidural local anaesthetic + narcotic | 4 | 158 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.40, 1.94] |
| 4.2 Studies using epidural narcotic | 4 | 123 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.10 [0.96, 4.59] |
| 4.3 Studies using epidural local anaesthetic | 1 | 90 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.16, 0.90] |
| 5 Sedation | 3 | 116 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.09, 0.97] |
| 5.1 Studies using epidural local anaesthetic + narcotic | 2 | 86 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.06, 1.68] |
| 5.2 Studies using epidural narcotic | 1 | 30 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.06, 1.45] |
| 6 Urinary retention | 5 | 166 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.50 [1.63, 7.51] |
| 6.1 Studies using epidural local anaesthetic + narcotic | 2 | 66 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.33 [0.71, 26.53] |
| 6.2 Studies using epidural narcotic | 3 | 100 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.33 [1.43, 7.75] |
| 7 Pruritis | 6 | 209 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.74 [1.76, 12.78] |
| 7.1 Studies using epidural local anaesthetic + narcotic | 2 | 86 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.17, 6.60] |
| 7.2 Studies using epidural narcotic | 4 | 123 | Odds Ratio (M‐H, Fixed, 95% CI) | 9.16 [2.45, 34.22] |
| 8 Respiratory depression | 5 | 204 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.45, 2.54] |
| 8.1 Studies using epidural local anaesthetic + narcotic | 2 | 101 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.15, 1.51] |
| 8.2 Studies using epidural narcotic | 3 | 103 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.88 [0.78, 19.36] |
| 9 Hypotension | 4 | 137 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.78 [1.15, 6.72] |
| 9.1 Studies using epidural local anaesthetic + narcotic | 3 | 117 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.78 [1.15, 6.72] |
| 9.2 Studies using epidural narcotic | 1 | 20 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
1.1. Analysis.
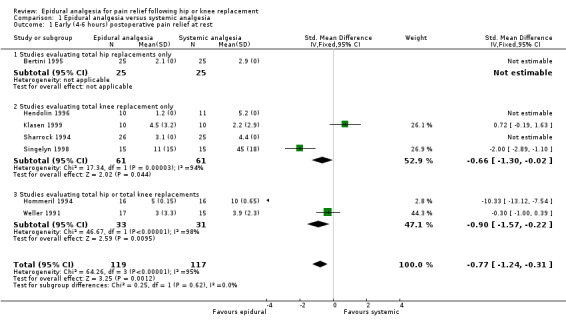
Comparison 1 Epidural analgesia versus systemic analgesia, Outcome 1 Early (4‐6 hours) postoperative pain relief at rest.
1.2. Analysis.
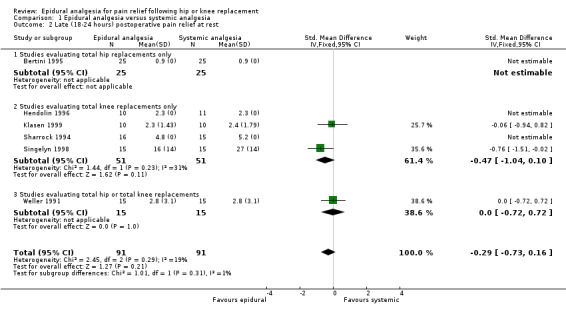
Comparison 1 Epidural analgesia versus systemic analgesia, Outcome 2 Late (18‐24 hours) postoperative pain relief at rest.
1.3. Analysis.
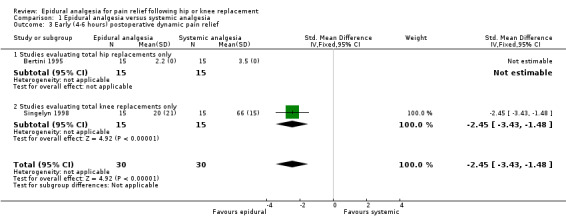
Comparison 1 Epidural analgesia versus systemic analgesia, Outcome 3 Early (4‐6 hours) postoperative dynamic pain relief.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bertini 1995.
| Methods | Randomization stated, but not described. Double‐blinding not reported. Withdrawals (none) reported. Jadad score=2 |
|
| Participants | Total hip replacement (n=50) ASA II‐III, mean age 63.7 yrs, 27 females Country: Italy |
|
| Interventions | All patients received combined spinal epidural anaesthesia (intrathecal bupivicaine 15.8 +/‐ 0.6 mg) then Group E (n=25): 24h PCEA bupivicaine 0.125% + morphine 40 mcg/mL, 1 mL bolus, 10 min lockout, 25 mL / 4 h max and 3 mL/h infusion Group S (n=25): 24h IV PCA ketorolac 0.9 mg + morphine 0.3 mg bolus, 5 min lockout, ketorolac 36 mg + morphine 12 mg / 4h max and ketorolac 1.8‐3.6 mg/h + morphine 0.6‐1.2 mg/h infusion |
|
| Outcomes | Mean resting and dynamic pain VAS (0‐4) scores and TOTPAR at 6h, 12h, 18h, and 24h postoperatively Adverse effects: sedation, nausea / vomiting, respiratory depression, pruritis |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Capdevila 1999.
| Methods | Randomization stated, but not described. Not double‐blind for evaluation of pain or adverse effects; functional outcomes assessed by blinded surgeon. Withdrawals not reported. Jadad score=1 |
|
| Participants | Total knee replacement (n=56) ASA I‐II, age 18‐75 yrs, 28 females Country: France |
|
| Interventions | All patients received balanced general anaesthesia then Group E (n=17): 5 mL epidural boluses of lidocaine 2% + epinephrine 5 mcg/mL mixture until T10 block reached + morphine 2 mg epidural then 48 h epidural infusion of lidocaine 1% + 2 mcg/mL clonidine + 0.03 mg/mL morphine @ 0.1 mL/kg/h Group F (n=20): 25 mL lidocaine 2% + epinephrine 5 mcg/mL + morphine 2 mg femoral nerve block then 48 h femoral catheter infusion of same mixture as group E @ 0.1 mL/kg/h [results not recorded in this table] Group S (n=19): 48 h IV PCA morphine 1 mg bolus, 7 min lockout, 30 mg / 4h max |
|
| Outcomes | Mean resting pain VAS (0‐100 mm) score at 24h and 48h postoperatively Adverse effects: numbness, sedation, nausea / vomiting, urinary retention, hypotension (decrease BP > 20% preop), pruritis, catheter complications Functional measures: maximal knee flexion (degrees) at 5d and 7d postoperatively Mean length of stay in rehabilitation center (all patients discharged from surgery on postoperative day 7) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
D'Ambrosio 1999.
| Methods | Randomization stated, but not described. Double‐blinding stated, but not described. Withdrawals not reported. Jadad score=2 |
|
| Participants | Total hip replacement (n=60) ASA I‐II, 29 females Country: Italy |
|
| Interventions | All patients received balanced general anaesthesia and Group E1 (n=15): epidural anaesthesia with 2 mL/10 kg bolus of bupivicaine 0.25% then postoperative 48 h epidural infusion of bupivicaine 0.25% + buprenorphine 0.0006% @ 3 mL/hr; aprotinin 500,000 KIU bolus before incision + 500,000 KIU/h infusion until skin closure Group E2 (n=15): epidural anaesthesia and analgesia as per group E1; no aprotinin Group S1: prn systemic opioids; aprotinin as per group E1 Group S2: prn systemic opioids; no aprotinin |
|
| Outcomes | Mean postoperative blood loss at 1h, 2h, 3h, 4h, and 24h postoperatively and total perioperative blood loss | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Gustafsson 1986.
| Methods | Randomization stated, but not described. Double‐blinding stated and described (masked solutions prepared by anaesthetist not involved in study). Withdrawals not reported. Jadad score=3 |
|
| Participants | Total hip replacement (n=21) Mean age 66 yrs Country: Sweden |
|
| Interventions | All patients received epidural anaesthesia to T4‐5 with mepivicaine‐epinephrine 1.5% + prn diazepam 5 mg IM then, when patients experienced severe postoperative pain (1‐3 h postoperatively) Group E1 (n=7): 1 dose of epidural pethidine 60 mg in 10 mL 0.9% saline + IM 0.9% saline 0.02 mL/kg Group E2 (n=7): 1 dose of epidural pethidine 20 mg in 10 mL 0.9% saline + IM 0.9% saline 0.02 mL/kg Group S (n=7): 1 dose of epidural 0.9% saline 10 mL + IM pethidine 0.02 mL/kg |
|
| Outcomes | Median resting pain VAS (0‐100 mm) score at time of administration and at 0.25h, 0.5h, 1h, 1.5h, 2h, 2.5h, 3h, 4h, 6h, 8h, 10h, and 18h after administration | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Hendolin 1996.
| Methods | Randomization stated, but not described. Double‐blinding stated, but not described. Withdrawals not reported. Jadad score=2 |
|
| Participants | Total knee replacement (n=41) 36 females Country: Finland |
|
| Interventions | All patients received combined spinal epidural anaesthesia with intrathecal 3 mL isobaric bupivicaine 0.5%, and IV PCA fentanyl 50 mcg bolus, 5 min lockout, as well as Group E1 (n=10): IM morphine 0.14 mg/kg 1h preop + epidural morphine 4 mg immediately postop and 3 mg 10h postop Group E2 (n=10): IM saline 1 h preop + epidural morphine 4 mg immediately postop and 3 mg 10h postop Group S1 (n=10): IM morphine 0.14 mg/kg 1 h preop + epidural saline immediately postop and 10h postop Group S2 (n=11): IM saline 1 h preop + epidural saline immediately postop and 10h postop |
|
| Outcomes | Mean resting pain VAS (0‐10) score hourly after surgery, except at night, up to 20h postoperatively. Adverse effects: nausea / vomiting, urinary retention, dysrhythmia, respiratory depression, pruritis |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Hommeril 1994.
| Methods | Randomization with random numbers table. Double‐blinding stated, but not described. Withdrawals (none) reported. Jadad score=4 |
|
| Participants | Total hip (n=16) or total knee (n=16) replacements ASA I‐II, age 44‐83 yrs, 22 females Country: France |
|
| Interventions | All patients received epigeneral anaesthesia with epidural 10 mL lidocaine 2% and balanced general anaesthesia then Group E (n=16): epidural morphine 4 mg in 0.9% saline 8 mL + IV 0.9% saline during first 13.5h postop Group S (n=16): epidural 0.9% saline 8 mL + IV ketoprofen 200 mg during first 30 min postop, then 12.5 mg/h over next 13h (total 365 mg) |
|
| Outcomes | Mean resting pain VAS (0‐50 mm?) score immediately postoperatively (0h), 1h postoperatively, then q2h until 13h postoperatively. Adverse effects: vomiting, epigastric discomfort, urinary retention, respiratory depression, pruritis |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Jorgensen 1991.
| Methods | Randomization stated, but not described. Double‐blinding not reported. Withdrawals (n=9) reported. Jadad score=2 |
|
| Participants | Total knee replacement (n=48 randomized; 39 completed study) Age 38‐87 yrs, 28 females Country: Denmark |
|
| Interventions | Group E (n=17): epidural 11‐18 mL mepivicaine 2% anaesthesia + epidural 5 mL/h bupivicaine 0.25% postoperative analgesia Group S (n=22): balanced general anaesthesia + postoperative IM ketobemidone 5‐7.5 mg prn pain or PO ketobemidone 5‐10 mg prn pain + postoperative PO paracetamol 1 g prn pain |
|
| Outcomes | Adverse effects: symptomatic deep vein thrombosis or pulmonary embolus, venographic deep vein thrombosis | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Klasen 1999.
| Methods | Randomization with sealed envelopes. Double‐blinding not reported. Withdrawals (n=7) reported. Jadad score=3 |
|
| Participants | Total knee replacement (n=37 randomized; 30 completed study) ASA II‐IV, 29 females Country: Germany |
|
| Interventions | All patients received spinal anaesthesia with intrathecal 3.2‐4 mL bupivicaine 0.5% then Group E (n=10): epidural morphine 2.5 mg in 0.9% saline 10 mL 1postop prn pain, repeated once 30 min later prn pain, then q4h prn pain up to 24h postoperatively Group I (n=10): intraarticular morphine 1 mg in 0.9% saline 20 mL after joint closure, then IV PCA morphine 2.5 mg bolus, 15 min lockout, 20 mg / 4 h max up to 24 h postoperatively Group S (n=10): 24 h IV PCA morphine 2.5 mg bolus, 15 min lockout, 20 mg / 4 h max |
|
| Outcomes | Mean resting pain VAS (0‐10 cm) score at 4h, 8h, 12h, and 24h postoperatively Adverse effects: dizziness, headache, nausea and vomiting, hypotension, pruritis |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Moiniche 1994.
| Methods | Randomization with sealed envelopes. Double‐blinding not reported. Withdrawals not reported. Jadad score=2 |
|
| Participants | Total hip (n=22) and total knee (n=20) replacements ASA I‐II, age 61‐85 yrs, 32 females Country: Denmark |
|
| Interventions | Group E (n=11 THR + 10 TKR): epidural anaesthesia with 20 mL bupivicaine 0.75% + morphine 2 mg, then postoperative epidural analgesia with 0.0625% bupivicaine + morphine 0.05 mg/mL infusion @ 4 mL/h for 48 h (THR) or 0.125% bupivicaine + morphine 0.05 mg/mL infusion @ 4 mL/h for first 24h postoperative and 0.0625% bupivicaine+/ morphine 0.05 mg/mL infusion @ 4 mL/h for second 24h postoperatively (TKR) Group S (n=11 THR + 10 TKR): balanced general anaesthesia then postoperative analgesia, as needed, with morphine 5 mg IV, morphine 0.125 mg/kg IM, or acetaminophen |
|
| Outcomes | Median resting and dynamic pain VAS (0‐100) scores preoperatively, and 24h, 30h, 48h, 54h, and daily after 72h postoperatively; median resting pain VAS scores also measured at 4h, 8h, and 12h postoperatively Adverse effects: nausea and vomiting, "fatigue" Median length of hospital stay |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Sharrock 1994.
| Methods | Randomization with random numbers table. Double‐blinding not reported. Withdrawals (n=3) reported. Jadad score=3 |
|
| Participants | Bilateral total knee replacements (n=54 randomized; 51 completed study) ASA I‐III, mean age 68 yrs, 28 females Country: United States |
|
| Interventions | All patients received epidural anaesthesia with 20 mL bupivicaine 0.75% and intravenous sedation, then Group E (n=26): >36h postoperative epidural bupivicaine 0.5% + fentanyl 10 mcg/mL infusion @ 3‐5 mL/h Group S (n=25): >36h postoperative IV fentanyl 10 mcg/mL infusion @ 10 mL/h |
|
| Outcomes | Mean resting pain VAS (0‐10 cm) scores preoperatively, 1h after resolution of epidural anaesthesia, then daily for 7 days postoperatively Adverse effects: delirium, ileus, urinary tract infections, MI, dysrhythmia, hypotension, SpO2<95%, PCO2>45 mmHg Functional measures: number of days needed to reach specific rehabilitation goals (see text) Mean length of hospital stay |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Singelyn 1998.
| Methods | Randomization with computer‐generated random numbers. Double‐blinding not reported. Withdrawals not reported. Jadad score=2 |
|
| Participants | Total knee replacement (n=45) ASA II‐III Country: Belgium |
|
| Interventions | All patients received balanced general anaesthesia then Group E (n=15): epidural 13 mL bupivicaine 0.25% + epinephrine 5 mcg/mL loading then 48 h epidural bupivicaine 0.125% + sufentanil 0.1 mcg/mL + clonidine 1mcg/mL infusion @ 10 mL/h Group F (n=15): 3‐in‐1 block with 37 mL bupivicaine 0.25% + epinephrine 5 mcg/mL loading then 48 h femoral sheath catheter infusion with same mixture as group E @ 10 mL/h Group S (n=15): 48 h IV PCA morphine 1.5 mg, 8 min lockout |
|
| Outcomes | Mean resting and dynamic pain VAS (0‐100 mm) scores at 4h, 24h, and 48h postoperatively Adverse effects: nausea and vomiting, urinary retention, hypotension, catheter‐related problems Functional measures: degree of knee flexion (twice daily until discharge), number of days to reach 90 degrees of knee flexion Mean length of hospital stay |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Weller 1991.
| Methods | Randomization reported but not described. Double‐blinding not reported. Withdrawals (n=2) reported. Jadad score=2 |
|
| Participants | Total hip (n=17 randomized) and total knee (n=13 randomized) replacements Age 34‐78 yrs, 17 females Country: United States |
|
| Interventions | All patients received epidural anaesthesia with bupivicaine 0.5% or 0.75% with or without general anaesthesia then Group E (n=15): epidural 3 mL morphine 0.1% 1 h before end of surgery and postoperative epidural 2 mL morphine 0.1% in recovery room (if pain VAS score >7 out of 10) and epidural 3‐5 mL morphine 0.1% 12 h later Group S (n=15): postoperative IV PCA morphine 1‐1.5 mg, 10 min lockout |
|
| Outcomes | Mean resting pain VAS (0‐10) score at 6h, 18h, and 24h postoperatively Adverse effects: sedation, nausea and vomiting, urinary retention, respiratory depression, pruritis |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Wulf 1999.
| Methods | Randomization reported but not described. Double‐blinding not reported. Withdrawals (n=20 reported. Jadad score=2 |
|
| Participants | Total hip replacement (n=90 randomized; 88 completed study) ASA I‐III, 46 females Country: Germany |
|
| Interventions | Group E (n=44): epidural anaesthesia with 12‐15 mL ropivicaine 0.1% then postoperative epidural ropivicaine 0.2% @ 4‐6 mL/h + 6 mL ropivicaine 0.2% boluses q30 min prn breakthrough pain in first 24 h, then no continuous infusion + 10 mL ropivicaine 0.2% boluses in second 24 h. Group S (n=46): balanced general anaesthesia then postoperative 48 h IV PCA morphine 1‐1.5 mg, 5 min lockout |
|
| Outcomes | Median resting pain VAS (0‐100 mm) scores at 0h, 10h, 24h, and 48h postoperatively Adverse effects: nausea and vomiting Mean length of hospital stay |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Allen 1986 | RCT compared several doses of epidural morphine (wrong intervention) for postoperative pain relief after femoral‐popliteal bypass or total knee replacement. |
| Allen 1998 | RCT compared spinal anaesthesia vs femoral 3‐in‐1 block (wrong intervention) in patients undergoing total knee replacement. |
| Banning 1986 | RCT compared epidural morphine vs. oral morphine in patients undergoing knee arthrotomy (wrong population) |
| Bennett 1994 | Article was a review article of lumbar plexus block for acute pain management; therefore did not meet any inclusion criteria. |
| Carabine 1992 | RCT compared postoperative epidural clonidine, epidural morphine, or both (wrong intervention) in patients undergoing total hip replacement. |
| Cooper 1993 | RCT compared postoperative epidural bupivicaine, epidural fentanyl, or both (wrong intervention) in patients undergoing total hip or total knee replacements. |
| D'Ambrosio 1998 | Duplicate publication of D'Ambrosio 1999. |
| Dahn 1999 | RCT compared regional anaesthesia vs general anaesthesia but did not study postoperative analgesia (wrong intervention) in patients undergoing total hip replacement. |
| Dalldorf 1994 | Case‐control study (wrong study design) compared epidural anaesthesia vs general anaesthesia (wrong intervention) in patients undergoing total hip replacement. |
| Dauphin 1997 | RCT compared epidural vs epigeneral anaesthesia in patients undergoing total hip replacement; all patients received systemic analgesics after surgery (wrong intervention). |
| Erskine 1994 | RCT compared spinal vs general (halothane or isoflurane) anaesthesia in patients undergoing total hip replacement but did not study postoperative analgesia (wrong intervention). Study examined neutrophil biocidal activity (wrong outcome). |
| Feller 1992 | RCT compared mini‐dose warfarin vs adjusted dose warfarin (wrong intervention) in patients undergoing total hip replacement. |
| Fogarty 1993 | RCT compared intrathecal clonidine, morphine, or placebo during spinal anaesthesia in patients undergoing total hip replacement; all patients received intravenous morphine after surgery (wrong intervention). |
| Grace 1995 | RCT compared epidural tramadol vs morphine during epigeneral anaesthesia in patients undergoing total knee replacement; all patients received systemic analgesics after surgery (wrong intervention). |
| Kampe 1999 | RCT compared epidural vs epigeneral anaesthesia in patients undergoing total hip replacement; all patients received epidural analgesia after surgery (wrong intervention). |
| Kohro 1998 | RCT compared epidural vs general anaesthesia in patients undergoing total knee replacement but did not study postoperative analgesia (wrong intervention). Study examined blood coagulability and fibrinolysis (wrong outcome). |
| Kopacz 1999 | RCT compared levobupivicaine, fentanyl, or both for postoperative patient‐controlled epidural analgesia (wrong intervention) in patients undergoing total hip or total knee replacement. |
| Lauretti 1999a | RCT compared epidural neostigmine or placebo during combined spinal epidual anaesthesia in patients undergoing minor knee procedures (wrong population); all patients received systemic analgesics after surgery (wrong intervention). |
| Lauretti 1999b | RCT compared intrathecal sufentanil or placebo with or without transdermal nitroglycerin during spinal anaesthesia in patients undergoing lower limb arthroscopy or meniscectomy (wrong population). |
| Markel 1997 | RCT compared fentanyl with or without bupivicaine for postoperative epidural analgesia (wrong intervention) in patients undergoing total hip replacement. |
| Mauerhan 1997 | RCT compared intraarticular morphine vs intraarticular bupivicaine for postoperative analgesia (wrong intervention) in patients undergoing total knee replacement. |
| McBeath 1995 | Retrospective chart audit (wrong study design) compared epidural analgesia vs patient controlled analgesia in patients undergoing total hip or total knee replacements. |
| Mitchell 1991 | RCT compared epidural vs general anaesthesia in patients undergoing total knee replacement; all patients received systemic analgesics after surgery (wrong intervention). |
| Modig 1976 | Prospective cohort study (wrong study design) compared epidural vs systemic analgesia in patients undergoing total hip replacement. |
| Mollmann 1999 | RCT compared continuous spinal vs continuous epidural analgesia (wrong intervention) in patients undergoing total hip replacement. |
| Murphy 1984 | RCT compared epidural vs intramuscular analgesia in patients undergoing total hip replacement or surgical repair of femoral neck fracture; data on population of interest could not be extracted. |
| Nielsen 1990 | Duplicate publication of Jorgensen 1991 |
| Nielson 1990 | RCT compared spinal vs general anaesthesia in patients undergoing total knee replacement; all patients received systemic analgesics after surgery (wrong intervention). |
| Niemi 1994 | RCT compared epidural vs spinal postoperative analgesia (wrong intervention) in patients undergoing total hip replacement. |
| Pati 1994 | Retrospective cohort study (wrong study design) compared epidural vs intravenous analgesia in patients undergoing total knee replacement. |
| Raj 1987 | Prospective cohort study (wrong study design) compared epidural vs systemic analgesia in patients undergoing total knee replacement. |
| Segstro 1991 | RCT compared rectal indomethacin vs placebo in patients receiving intravenous morphine (wrong intervention) following total hip replacement. |
| Sharrock 1992 | Study compared epidural vs general anaesthesia (wrong intervention) and examined fibrinolysis (wrong outcome). |
| Sharrock 1997 | RCT compared epidural vs general anaesthesia in patients undergoing total knee replacement but did not study postoperative analgesia (wrong intervention). Study examined thrombin generation and fibrinolysis (wrong outcome). |
| Silvasti 2000 | RCT compared epidural vs systemic postoperative analgesia in patients undergoing anterior cruciate ligament repair (wrong population). |
| Singelyn 1999 | Prospective cohort study (wrong study design) compared epidural analgesia vs patient controlled analgesia vs femoral 3‐in‐1 block for postoperative analgesia in patients undergoing total hip replacement. |
| Tsueda 1998 | RCT compared morphine vs fentanyl for postoperative patient‐controlled epidural analgesia (wrong intervention) in patients undergoing total hip or total knee replacement. |
| Turner 1996 | RCT compared varying doses of postoperative epidural ropivicaine 0.2% analgesia in patients undergoing total hip or total knee replacement or cruciate ligament repair (wrong population) but all groups also received PCA narcotics (wrong intervention). Data on population of interest could not be extracted. |
| Weir 1998 | RCT compared three bupivicaine‐ketamine epidural mixtures for anaesthesia and analgesia (wrong intervention) in patients undergoing total knee replacement. |
| Wilder‐Smith 1998 | RCT compared epidural tramadol vs placebo during epidural anaesthesia in patients undergoing total hip or total knee replacement or cruciate ligament repair; all patients received intravenous tramadol after surgery (wrong intervention). Data on population of interest could not be extracted. |
| Williams‐Russo 1992 | Duplicate data of Sharrock 1994, which reports additional outcomes. |
| Wong 1997 | RCT compared epidural vs general anaesthesia in patients undergoing total knee replacement; all patients received epidural + patient‐controlled intravenous analgesia after surgery (wrong intervention). |
| Wright 1992 | RCT compared fluid loading, ephedrine, or methoxamine (wrong intervention) to prevent hypotension (wrong outcome) during epidural or epigeneral anaesthesia in patients undergoing total knee replacement. |
| Zayas 1999 | RCT compared three doses of mepivicaine for combined spinal anaesthesia in patients undergoing knee arthroscopy (wrong population) and did not study postoperative analgesia (wrong intervention). |
Contributions of authors
Peter Choi wrote the protocol, performed the literature search and study selection, tabulated the results, performed the data analysis, and wrote the results and discussion of this systematic review.
Mohit Bhandari performed the literature search and study selection, performed the data analysis, and contributed extensively to the writing and revision of this review.
James Douketis formulated the original research question, wrote the protocol, assessed the methodological quality of each study, extracted data from each study, and contributed extensively to the writing and revision of this review.
Julia Scott assessed the methodological quality of each study, extracted data from each study, and contributed extensively to the writing and revision of this review.
Sources of support
Internal sources
St. Joseph's Hospital (Hamilton) Anesthesiologists' Research Fund, Canada.
External sources
No sources of support supplied
Declarations of interest
The authors of this Cochrane review have never received any honoraria or consulting fees from pharmaceutical companies that produce anticoagulants or analgesics. The authors do not have any commercial interest in anticoagulant thromboprophylaxis or postoperative analgesia and they do not hold any patents related to these fields.
Edited (no change to conclusions)
References
References to studies included in this review
Bertini 1995 {published data only}
- Bertini L, Tagariello V, Molino FM, Posteraro CM, Mancini S, Rossignoli L. [Patient‐controlled postoperative analgesia in orthopedic surgery: epidural PCA versus intravenous PCA]. [Italian]. Minerva Anestesiologica 1995;61(7‐8):319‐28. [PubMed] [Google Scholar]
Capdevila 1999 {published data only}
- Capdevila X, Barthelet Y, Biboulet P, Ryckwaert Y, Rubenovitch J, d'Athis F. Effects of perioperative analgesic technique on the surgical outcome and duration of rehabilitation after major knee surgery [see comments]. Anesthesiology 1999;91(1):8‐15. [DOI] [PubMed] [Google Scholar]
D'Ambrosio 1999 {published data only}
- D'Ambrosio A, Borghi B, Damato A, D'Amato G, Antonacci D, Valeri F. Reducing perioperative blood loss in patients undergoing total hip arthroplasty. International Journal of Artificial Organs 1999;22(1):47‐51. [PubMed] [Google Scholar]
Gustafsson 1986 {published data only}
- Gustafsson LL, Johannisson J, Garle M. Extradural and parenteral pethidine as analgesia after total hip replacement: Effects and kinetics. A controlled clinical study. European Journal of Clinical Pharmacology 1986;29(5):529‐34. [DOI] [PubMed] [Google Scholar]
Hendolin 1996 {published data only}
- Hendolin H, Nuutinen L, Kokki H, Tuomisto L. Does morphine premedication influence the pain and consumption of postoperative analgesics after total knee arthroplasty?. Acta Anaesthesiologica Scandinavica 1996;40(1):81‐85. [DOI] [PubMed] [Google Scholar]
Hommeril 1994 {published data only}
- Hommeril JL, Bernard JM, Gouin F, Pinaud M. Ketoprofen for pain after hip and knee arthroplasty. British Journal of Anaesthesia 1994;72(4):383‐87. [DOI] [PubMed] [Google Scholar]
Jorgensen 1991 {published data only}
- Jorgensen LN, Rasmussen LS, Nielsen PT, Leffers A, Albrecht BE. Antithrombotic efficacy of continuous extradural analgesia after knee replacement [see comments]. British Journal of Anaesthesia 1991;66(1):8‐12. [DOI] [PubMed] [Google Scholar]
Klasen 1999 {published data only}
- Klasen JA, Opitz SA, Melzer C, Thiel A, Hempelmann G. Intraarticular, epidural, and intravenous analgesia after total knee arthroplasty. Acta Anaesthesiologica Scandinavica 1999;43(10):1021‐26. [DOI] [PubMed] [Google Scholar]
Moiniche 1994 {published data only}
- Moiniche S, Hjortso NC, Hansen BL, Dahl JB, Rosenberg J, Gebuhr P, et al. The effect of balanced analgesia on early convalescence after major orthopaedic surgery. Acta Anaesthesiologica Scandinavica 1994;38(4):328‐35. [DOI] [PubMed] [Google Scholar]
Sharrock 1994 {published data only}
- Sharrock NE, Urquhart BL, Ganz S, Williams‐Russo PG. Epidural infusions of bupivacaine and fentanyl do not improve rehabilitation following one‐stage bilateral total knee arthroplasty. Annals of the Academy of Medicine, Singapore 1994;23(6 Suppl):3‐9. [PubMed] [Google Scholar]
Singelyn 1998 {published data only}
- Singelyn FJ, Deyaert M, Joris D, Pendeville E, Gouverneur JM. Effects of intravenous patient‐controlled analgesia with morphine, continuous epidural analgesia, and continuous three‐in‐one block on postoperative pain and knee rehabilitation after unilateral total knee arthroplasty. Anesthesia & Analgesia 1998;87(1):88‐92. [DOI] [PubMed] [Google Scholar]
Weller 1991 {published data only}
- Weller R, Rosenblum M, Conard P, Gross JB. Comparison of epidural and patient‐controlled intravenous morphine following joint replacement surgery. Canadian Journal of Anaesthesia 1991;38(5):582‐86. [DOI] [PubMed] [Google Scholar]
Wulf 1999 {published data only}
- Wulf H, Biscoping J, Beland B, Bachmann‐Mennenga B, Motsch J. Ropivacaine epidural anesthesia and analgesia versus general anesthesia and intravenous patient‐controlled analgesia with morphine in the perioperative management of hip replacement. Ropivacaine Hip Replacement Multicenter Study Group. Anesthesia & Analgesia 1999;89(1):111‐16. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Allen 1986 {published data only}
- Allen PD, Walman T, Concepcion M. Epidural morphine provides postoperative pain relief in peripheral vascular and orthopedic surgical patients: A dose‐response study. Anesthesia & Analgesia 1986;65(2):165‐70. [PubMed] [Google Scholar]
Allen 1998 {published data only}
- Allen JG, Denny NM, Oakman N. Postoperative analgesia following total knee arthroplasty: a study comparing spinal anesthesia and combined sciatic femoral 3‐in‐1 block [see comments]. Regional Anesthesia & Pain Medicine 1998;23(2):142‐6. [PubMed] [Google Scholar]
Banning 1986 {published data only}
- Banning AM, Schmidt JF, Chraemmer JB, Risbo A. Comparison of oral controlled release morphine and epidural morphine in the management of postoperative pain. Anesthesia & Analgesia 1986;65(4):385‐88. [PubMed] [Google Scholar]
Bennett 1994 {published data only}
- Bennett LP. Lumbar plexus block for the management of acute pain. Orthopaedic Nursing 1994;13(3):9‐14. [DOI] [PubMed] [Google Scholar]
Carabine 1992 {published data only}
- Carabine UA, Milligan KR, Mulholland D, Moore J. Extradural clonidine infusions for analgesia after total hip replacement. British Journal of Anaesthesia 1992;68(4):338‐43. [DOI] [PubMed] [Google Scholar]
Cooper 1993 {published data only}
- Cooper DW, Turner G. Patient‐controlled extradural analgesia to compare bupivacaine, fentanyl and bupivacaine with fentanyl in the treatment of postoperative pain. British Journal of Anaesthesia 1993;70(5):503‐7. [DOI] [PubMed] [Google Scholar]
D'Ambrosio 1998 {published data only}
- D'Ambrosio A, Borghi B, Damato A, D'Amato G, Antonacci D, Grossi P. Interactions between epidural analgesia and antifibrinolytics. Anaesthesia 1998;53 Suppl 2:57‐58. [DOI] [PubMed] [Google Scholar]
Dahn 1999 {published data only}
- Dahn J, Oster M, Moltner A, Wohrle C, Ratzer FA, Ackern K, et al. [Anesthesia in geriatric patients. The determination of physiological variables for cognitive function in geriatric patients after regional or general anesthesia]. [German]. Anaesthesist 1999;48(6):379‐86. [DOI] [PubMed] [Google Scholar]
Dalldorf 1994 {published data only}
- Dalldorf PG, Perkins FM, Totterman S, Pellegrini VD, Jr. Deep venous thrombosis following total hip arthroplasty. Effects of prolonged postoperative epidural anesthesia [see comments]. Journal of Arthroplasty 1994;9(6):611‐16. [DOI] [PubMed] [Google Scholar]
Dauphin 1997 {published data only}
- Dauphin A, Raymer KE, Stanton EB, Fuller HD. Comparison of general anesthesia with and without lumbar epidural for total hip arthroplasty: effects of epidural block on hip arthroplasty. Journal of Clinical Anesthesia 1997;9(3):200‐3. [DOI] [PubMed] [Google Scholar]
Erskine 1994 {published data only}
- Erskine R, Janicki PK, Neil G, James MF. Spinal anaesthesia but not general anaesthesia enhances neutrophil biocidal activity in hip arthroplasty patients. Canadian Journal of Anaesthesia 1994;41(7):632‐38. [DOI] [PubMed] [Google Scholar]
Feller 1992 {published data only}
- Feller JA, Parkin JD, Phillips GW, Hannon PJ, Hennessy O, Huggins RM. Prophylaxis against venous thrombosis after total hip arthroplasty. Australian & New Zealand Journal of Surgery 1992;62(8):606‐10. [DOI] [PubMed] [Google Scholar]
Fogarty 1993 {published data only}
- Fogarty DJ, Carabine UA, Milligan KR. Comparison of the analgesic effects of intrathecal clonidine and intrathecal morphine after spinal anaesthesia in patients undergoing total hip replacement. British Journal of Anaesthesia 1993;71(5):661‐64. [DOI] [PubMed] [Google Scholar]
Grace 1995 {published data only}
- Grace D, Fee JP. Ineffective analgesia after extradural tramadol hydrochloride in patients undergoing total knee replacement. Anaesthesia 1995;50(6):555‐58. [DOI] [PubMed] [Google Scholar]
Kampe 1999 {published data only}
- Kampe S, Weigand C, Kaufmann J, Klimek M, Konig DP, Lynch J. Postoperative analgesia with no motor block by continuous epidural infusion of ropivacaine 0.1% and sufentanil after total hip replacement. Anesthesia & Analgesia 1999;89(2):395‐98. [DOI] [PubMed] [Google Scholar]
Kohro 1998 {published data only}
- Kohro S, Yamakage M, Arakawa J, Kotaki M, Omote T, Namiki A. Surgical/tourniquet pain accelerates blood coagulability but not fibrinolysis. British Journal of Anaesthesia 1998;80(4):460‐63. [DOI] [PubMed] [Google Scholar]
Kopacz 1999 {published data only}
- Kopacz DJ, Sharrock NE, Allen HW. A comparison of levobupivacaine 0.125%, fentanyl 4 mu g/ml, or their combination for patient‐controlled epidural analgesia after major orthopedic surgery. Anesthesia & Analgesia 1999;89(6):1497‐1503. [DOI] [PubMed] [Google Scholar]
Lauretti 1999a {published data only}
- Lauretti GR, Oliveira R, Reis MP, Juliao M‐DC, Pereira NL. Study of three different doses of epidural neostigmine coadministered with lidocaine for postoperative analgesia. Anesthesiology 1999;90(6):1534‐38. [DOI] [PubMed] [Google Scholar]
Lauretti 1999b {published data only}
- Lauretti GR, Oliveira R, Reis MP, Mattos AL, Pereira NL. Transdermal nitroglycerine enhances spinal sufentanil postoperative analgesia following orthopedic surgery. Anesthesiology 1999;90(3):734‐39. [DOI] [PubMed] [Google Scholar]
Markel 1997 {published data only}
- Markel DC, Urquhart B, Derkowska I, Salvati EA, Sharrock NE. Effect of epidural analgesia on venous blood flow after hip arthroplasty. Clinical Orthopaedics & Related Research 1997;334:168‐74. [PubMed] [Google Scholar]
Mauerhan 1997 {published data only}
- Mauerhan DR, Campbell M, Miller JS, Mokris JG, Gregory A, Kiebzak GM. Intra‐articular morphine and/or bupivacaine in the management of pain after total knee arthroplasty. Journal of Arthroplasty 1997;12(5):546‐52. [DOI] [PubMed] [Google Scholar]
McBeath 1995 {published data only}
- McBeath DM, Shah J, Sebastian L, Sledzinski K. The effect of patient controlled analgesia and continuous epidural infusion on length of hospital stay after total knee or total hip replacement. CRNA ‐ the Clinical Forum for Nurse Anesthetists 1995;6(1):31‐6. [PubMed] [Google Scholar]
Mitchell 1991 {published data only}
- Mitchell D, Friedman RJ, Baker JD, III, Cooke JE, Darcy MD, Miller MC, III. Prevention of thromboembolic disease following total knee arthroplasty. Epidural versus general anesthesia. Clinical Orthopaedics & Related Research 1991;269:109‐12. [PubMed] [Google Scholar]
Modig 1976 {published data only}
- Modig J. Respiration and circulation after total hip replacement surgery. A comparison between parenteral analgesics and continuous lumbar epidural block. Acta Anaesthesiologica Scandinavica 1976;20(3):225‐36. [DOI] [PubMed] [Google Scholar]
Mollmann 1999 {published data only}
- Mollmann M, Cord S, Holst D, Auf‐der LU. Continuous spinal anaesthesia or continuous epidural anaesthesia for post‐operative pain control after hip replacement?. European Journal of Anaesthesiology 1999;16(7):454‐61. [DOI] [PubMed] [Google Scholar]
Murphy 1984 {published data only}
- Murphy DF, MacGrath P, Stritch M. Postoperative analgesia in hip surgery. A controlled comparison of epidural buprenorphine with intramuscular morphine. Anaesthesia 1984;39(2):181‐3. [DOI] [PubMed] [Google Scholar]
Nielsen 1990 {published data only}
- Nielsen PT, Jorgensen LN, Albrecht BE, Leffers AM, Rasmussen LS. Lower thrombosis risk with epidural blockade in knee arthroplasty. Acta Orthopaedica Scandinavica 1990;61(1):29‐31. [DOI] [PubMed] [Google Scholar]
Nielson 1990 {published data only}
- Nielson WR, Gelb AW, Casey JE, Penny FJ, Merchant RN, Manninen PH. Long‐term cognitive and social sequelae of general versus regional anesthesia during arthroplasty in the elderly. Anesthesiology 1990;73(6):1103‐9. [DOI] [PubMed] [Google Scholar]
Niemi 1994 {published data only}
- Niemi L, Pitkanen M, Tuominen M, Rosenberg PH. Technical problems and side effects associated with continuous intrathecal or epidural post‐operative analgesia in patients undergoing hip arthroplasty. European Journal of Anaesthesiology 1994;11(6):469‐74. [PubMed] [Google Scholar]
Pati 1994 {published data only}
- Pati AB, Perme DC, Trail M, Henry PK, Bryan WJ. Rehabilitation parameters in total knee replacement patients undergoing epidural vs. conventional analgesia. Journal of Orthopaedic & Sports Physical Therapy 1994;19(2):88‐92. [DOI] [PubMed] [Google Scholar]
Raj 1987 {published data only}
- Raj PP, Knarr DC, Vigdorth E, Denson DD, Pither CE, Hartrick CT, et al. Comparison of continuous epidural infusion of a local anesthetic and administration of systemic narcotics in the management of pain after total knee replacement surgery. Anesthesia & Analgesia 1987;66(5):401‐6. [DOI] [PubMed] [Google Scholar]
Segstro 1991 {published data only}
- Segstro R, Morley‐Forster PK, Lu G. Indomethacin as a postoperative analgesic for total hip arthroplasty. Canadian Journal of Anaesthesia 1991;38(5):578‐81. [DOI] [PubMed] [Google Scholar]
Sharrock 1992 {published data only}
- Sharrock NE, Go G. Fibrinolytic activity following total knee arthroplasty under epidural or general anesthesia. Regional Anesthesia 1992;17(Supplement 3):94‐4. [PubMed] [Google Scholar]
Sharrock 1997 {published data only}
- Sharrock NE, Go G, Williams RP, Haas SB, Harpel PC. Comparison of extradural and general anaesthesia on the fibrinolytic response to total knee arthroplasty [see comments]. British Journal of Anaesthesia 1997;79(1):29‐34. [DOI] [PubMed] [Google Scholar]
Silvasti 2000 {published data only}
- Silvasti M, Pitkanen M. Continuous epidural analgesia with bupivacaine‐fentanyl versus patient‐controlled analgesia with i.v. morphine for postoperative pain relief after knee ligament surgery. Acta Anaesthesiologica Scandinavica 2000;44(1):37‐42. [DOI] [PubMed] [Google Scholar]
Singelyn 1999 {published data only}
- Singelyn FJ, Gouverneur JM. Postoperative analgesia after total hip arthroplasty: i.v. PCA with morphine, patient‐controlled epidural analgesia, or continuous "3‐in‐1" block?: a prospective evaluation by our acute pain service in more than 1300 patients. Journal of Clinical Anesthesia 1999;11(7):550‐4. [DOI] [PubMed] [Google Scholar]
Tsueda 1998 {published data only}
- Tsueda K, Mosca PJ, Heine MF, Loyd GE, Durkis DA, Malkani AL, et al. Mood during epidural patient‐controlled analgesia with morphine or fentanyl. Anesthesiology 1998;88(4):885‐91. [DOI] [PubMed] [Google Scholar]
Turner 1996 {published data only}
- Turner G, Blake D, Buckland M, Chamley D, Dawson P, Goodchild C, et al. Continuous extradural infusion of ropivacaine for prevention of postoperative pain after major orthopaedic surgery. British Journal of Anaesthesia 1996;76(5):606‐10. [DOI] [PubMed] [Google Scholar]
Weir 1998 {published data only}
- Weir PS, Fee JPH. Double‐blind comparison of extradural block with three bupivacaine‐ketamine mixtures in knee arthroplasty. British Journal of Anaesthesia 1998;80(3):299‐301. [DOI] [PubMed] [Google Scholar]
Wilder‐Smith 1998 {published data only}
- Wilder‐Smith CH, Wilder‐Smith OH, Farschtschian M, Naji P. Preoperative adjuvant epidural tramadol: the effect of different doses on postoperative analgesia and pain processing. Acta Anaesthesiologica Scandinavica 1998;42(3):299‐305. [DOI] [PubMed] [Google Scholar]
Williams‐Russo 1992 {published data only}
- Williams‐Russo RP, Urquhart BL, Sharrock NE, Charlson ME. Post‐operative delirium: Predictors and prognosis in elderly orthopedic patients. Journal of the American Geriatrics Society 1992;40(8):759‐67. [DOI] [PubMed] [Google Scholar]
Wong 1997 {published data only}
- Wong CS, Lu CC, Cherng CH, Ho ST. Pre‐emptive analgesia with ketamine, morphine and epidural lidocaine prior to total knee replacement. Canadian Journal of Anaesthesia 1997;44(1):31‐7. [DOI] [PubMed] [Google Scholar]
Wright 1992 {published data only}
- Wright PM, Fee JP. Cardiovascular support during combined extradural and general anaesthesia. British Journal of Anaesthesia 1992;68(6):585‐9. [DOI] [PubMed] [Google Scholar]
Zayas 1999 {published data only}
- Zayas VM, Liguori GA, Chisholm MF, Susman MH, Gordon MA. Dose response relationships for isobaric spinal mepivacaine using the combined spinal epidural technique. Anesthesia & Analgesia 1999;89(5):1167‐71. [PubMed] [Google Scholar]
References to studies awaiting assessment
Lopes 1999 {published data only}
- Lopes MB, Sousa LR, Porsani DF, Amaral AGV, Boas‐AV J, Birchta SR. Association of intra‐articular bupivacaine and morphine for arthroscopic knee surgery postoperative analgesia. Revista Brasiliera de Anestesiologia 1999;49(3):165‐8. [Google Scholar]
Additional references
Ballantyne 1998
- Ballantyne JC, Carr DB, deFerranti S, Suarez T, Lau J, Chalmers TC, et al. The comparative effects of postoperative analgesic therapies on pulmonary outcome: cumulative meta‐analyses of randomized, controlled trials [see comments]. Anesthesia & Analgesia 1998;86(3):598‐612. [DOI] [PubMed] [Google Scholar]
Beattie 2001
- Beattie WS, Badner NH, Choi P. Epidural analgesia reduces postoperative myocardial infarction: A meta‐analysis. Anesthesia & Analgesia 2001;93(4):853‐8. [DOI] [PubMed] [Google Scholar]
Breslow 1980
- Breslow NE, Day NE. Statistical Methods in Cancer Research: The Analysis of Case‐Control Studies. Vol. 1, Lyons: International Agency for Research on Cancer, 1980. [PubMed] [Google Scholar]
Choi 2003
- Choi P, Bhandari M, Scott J, Douketis JD. Epidural analgesia for pain relief following hip or knee replacement. Cochrane Database of Systematic Reviews 2003, Issue 3. [DOI: 10.1002/14651858.CD003071] [DOI] [PMC free article] [PubMed] [Google Scholar]
Clagett 1998
- Clagett GP, Anderson FA, Jr, Geerts W, Heit JA, Knudson M, Lieberman, JR, et al. Prevention of venous thromboembolism [see comments]. [Review] [424 refs]. Chest 1998;114(5 Suppl):531S‐60S. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Harris 1990
- Harris WH, Sledge CB. Total hip and total knee replacement (1). [Review] [76 refs]. New England Journal of Medicine 13‐9‐1990;323(11):725‐31. [DOI] [PubMed] [Google Scholar]
Hull 1993
- Hull R, Raskob G, Pineo G, Rosenbloom D, Evans W, Mallory T, Anquist, K, Smith F, Hughes G, Green D. A comparison of subcutaneous low‐molecular‐weight heparin with warfarin sodium for prophylaxis against deep‐vein thrombosis after hip or knee implantation [see comments]. New England Journal of Medicine 4‐11‐1993;329(19):1370‐1376. [DOI] [PubMed] [Google Scholar]
Jadad 1996
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17(1):1‐12. [DOI] [PubMed] [Google Scholar]
Lawton 1995
- Lawton MT, Porter RW, Heiserman JE, Jacobowitz R, Sonntag VK, Dickman, CA. Surgical management of spinal epidural hematoma: relationship between surgical timing and neurological outcome [see comments]. Journal of Neurosurgery 1995;83(1):1‐7. [DOI] [PubMed] [Google Scholar]
Leclerc 1996
- Leclerc JR, Geerts WH, Desjardins L, Laflamme GH, L'Esperance B, Demers C, Kassis J, Cruickshank M, Whitman L, Delorme F. Prevention of venous thromboembolism after knee arthroplasty. A randomized, double‐blind trial comparing enoxaparin with warfarin [see comments]. Annals of Internal Medicine 1‐4‐1996;124(7):619‐26. [DOI] [PubMed] [Google Scholar]
Levine 1991
- Levine MN, Hirsh J, Gent M, Turpie AG, Leclerc J, Powers PJ, Jay RM, Neemeh J. Prevention of deep vein thrombosis after elective hip surgery. A randomized trial comparing low molecular weight heparin with standard unfractionated heparin [see comments]. Annals of Internal Medicine 1‐4‐1991;114(7):545‐51. [DOI] [PubMed] [Google Scholar]
Leyvraz 1991
- Leyvraz PF, Bachmann F, Hoek J, Buller HR, Postel M, Samama M, et al. Prevention of deep vein thrombosis after hip replacement: randomised comparison between unfractionated heparin and low molecular weight heparin [published erratum appears in BMJ 1991 Nov 16;303(6812);1243] [see comments]. BMJ 7‐9‐1991;303(6802):543‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Planes 1988
- Planes A, Vochelle N, Mazas F, Mansat C, Zucman J, Landais A, et al. Prevention of postoperative venous thrombosis: a randomized trial comparing unfractionated heparin with low molecular weight heparin in patients undergoing total hip replacement. Thrombosis & Haemostasis 22‐12‐1988;60(3):407‐10. [PubMed] [Google Scholar]
Rodgers 2000
- Rodgers A, Walker N, Schug S, McKee A, Kehlet H, Zundert A, et al. Reduction of postoperative mortality and morbidity with epidural or spinal anaesthesia: results from overview of randomised trials. BMJ 2000;321:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schroeder 1998
- Schroeder DR. Statistics: detecting a rare adverse drug reaction using spontaneous reports [see comments]. Regional Anesthesia & Pain Medicine 1998;23(6 Suppl 2):183‐9. [DOI] [PubMed] [Google Scholar]


